Abstract
Background
Geriatric nutritional risk index (GNRI) is a validated nutritional assessment method, and lower GNRI values are closely associated with adverse clinical outcomes in dialysis patients. This study investigated the impact of changes in GNRI during the first year of dialysis on cardiovascular outcomes in incident peritoneal dialysis (PD) patients.
Methods
We reviewed medical records in 133 incident PD patients to determine GNRI at the start of PD and after 12 months. Patients were categorized into improved (delta GNRI > 0) and worsening/stationary (delta GNRI ≤ 0) groups. The primary outcome was major adverse cardiac and cerebrovascular events (MACCEs).
Results
During a mean follow-up of 51.1 months, the primary outcome was observed in 42 patients (31.6%). The baseline GNRI at PD initiation was not significantly associated with MACCEs (log-rank test, P = 0.40). However, the cumulative event-free rate was significantly lower in the worsening or stationary GNRI group than in the improved group (log-rank test, P = 0.004). Multivariate Cox analysis revealed that a worsening or stationary GNRI was independently associated with higher risk for MACCEs (hazard ratio, 2.47; 95% confidence interval, 1.15–5.29; P = 0.02). In subgroup analysis, patients with worsening or stationary GNRI were at significantly greater risk for MACCEs in both the lower (P = 0.04) and higher (P = 0.01) baseline GNRI groups.
Conclusion
Baseline GNRI was not associated with MACCEs, but patients with deteriorating or stationary nutritional status were at significantly greater risk for MACCEs, suggesting that serial monitoring of nutritional status is important to stratify cardiovascular risk in incident PD patients.
Keywords: Dialysis, Geriatric nutritional risk index, Major adverse cardiac and cerebrovascular events, Peritoneal dialysis, Protein-energy wasting
Introduction
Cardiovascular disease is the most common cause of death in patients with end-stage renal disease (ESRD) treated with dialysis [1]. In addition to traditional risk factors, protein-energy wasting is an established risk factor for cardiovascular disease in dialysis patients [2–4]. Furthermore, it is well known that protein-energy wasting worsens quality of life and clinical outcomes in these patients [2–8]. Therefore, identification and risk stratification of protein-energy wasting are clinically relevant to the management of dialysis patients.
To date, many nutritional assessment methods have been developed and investigated in patients with ESRD [9–15]. The geriatric nutritional risk index (GNRI), originally developed to examine the nutritional status of elderly hospitalized patients, consists of three objective nutritional variables: height, body weight, and serum albumin concentration [16]. Lower GNRI is a significant risk factor for nutrition-related morbidity and mortality in elderly hospitalized patients [16]. In patients with ESRD, GNRI is a useful nutritional indicator [13,17]. The prognostic value of GNRI has also been studied in patients on hemodialysis (HD) [18–23] and peritoneal dialysis (PD) [24]. In HD, GNRI is a significant predictor of all-cause mortality in Korean [19], Japanese [18,23], and Caucasian [21,25] populations. GNRI is also a significant predictor of cardiovascular mortality in Japanese patients on maintenance HD [22]. In Korean PD patients, Kang et al [24] demonstrated that GNRI was independently associated with all-cause mortality.
However, there are limited data on the association between changes in GNRI and clinical outcomes [20]. Because nutritional status constantly changes, the prognostic value of GNRI can also change over time. Moreover, changes in nutritional status might be more prominent in patients initiating dialysis than in patients on chronic dialysis. With this in mind, the current study investigates the impact of changes in GNRI during the first year of PD on cardiovascular outcomes in incident PD patients.
Methods
Patients
The medical records of all patients with ESRD over 18 years of age who started PD at Yonsei University Health System or CHA Bundang Medical Center between January 2005 and December 2008 were initially reviewed for this study. Among 323 incident PD patients, patients were excluded if they had malignancy (n = 12), active infection (n = 3), or decompensated liver disease (n = 3) or if they did not maintain PD during the first three months (n = 54). Since this study was designed to investigate the effects of changes in GNRI during the first year of PD, 45 patients who discontinued PD within the first year were excluded. Twenty-seven patients died within the first year of PD, 10 patients changed dialysis modality to HD, and 8 patients underwent kidney transplantation. In addition, 73 patients who maintained PD over one year but did not have one-year follow-up data (including demographics and laboratory variables) were excluded. Finally, 133 patients were analyzed in the present study. This study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University Health System Clinical Trial Center (IRB No. 4-2016-0932) and CHA Bundang Medical Center Clinical Trial Center (IRB No. 2016-11-017).
Demographics and biochemical data collection
Baseline demographics and biochemical data were collected from medical record reviews and were recorded by a well-trained examiner at the time of PD initiation. Traditional cardiovascular risk factors of age, hypertension, diabetes mellitus, smoking history, and cardiovascular disease history were recorded. Cardiovascular disease was defined as a history of coronary artery disease, peripheral artery disease, congestive heart failure, or cerebrovascular accident. Coronary artery disease was defined as history of angioplasty, coronary artery bypass grafting, myocardial infarction, or angina. Peripheral artery disease was defined as history of claudication, ischemic limb loss and/or ulceration, or peripheral revascularization procedure. A cerebrovascular accident was defined as history of transient ischemic attack, stroke, or carotid endarterectomy.
Blood was drawn after a 12-hour overnight fast, and the following laboratory data were measured from blood samples: hemoglobin, blood urea nitrogen, creatinine, calcium, phosphorous, albumin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, intact parathyroid hormone, and high-sensitivity C-reactive protein (hs-CRP) concentrations. Residual kidney function and urine volume were determined through 24-hour urine collection. Kt/Vurea was determined from the total loss of urea nitrogen in the spent dialysate using PD Adequest 2.0 for Windows software (Baxter Healthcare, Deerfield, IL, USA).
Assessment of the GNRI
The GNRI was calculated with an equation validated in dialysis patients [13,17–19,21–26]: GNRI = [1.489 × albumin (g/L)] + [(41.7 × (body weight/ideal body weight)]. Ideal body weight was calculated from the Lorentz equation [16,17,24]. If a patient’s body weight was above the ideal body weight, the ratio of body weight to ideal body weight was replaced with 1, in accordance with previous studies [16,17,24,26]. In our study, the dry body weight was used as body weight and was determined by the patient’s nephrologist in the context of hypotension, blood pressure, peripheral edema, or pulmonary edema on chest X-ray. To reflect the actual status of patients, usual overnight dialysate volumes or glucose concentrations were not altered for this study. After complete emptying of the PD dialysate, patients were weighed in light clothing, and their heights were measured while they were not wearing shoes.
Outcome measurement
Patients were regularly followed up at the PD clinic until December 2015. All deaths and hospitalizations were recorded in a serious adverse events database. All events were carefully reviewed for this study. The primary outcome was development of a major adverse cardiac and cerebrovascular event (MACCE), which was defined as death or hospitalization from acute coronary syndrome, stable angina requiring coronary revascularization by percutaneous coronary intervention or coronary artery bypass grafting, congestive heart failure, or cerebrovascular accident. Loss to follow-up, kidney transplantation, and dialysis modality change to HD after the first year of PD were censored at the end of PD treatment.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics for Windows version 20.0 (IBM Co., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation or median (interquartile range [IQR]), and categorical variables were expressed as number (percentage). Baseline characteristics were compared with Student’s t test, Mann-Whitney U test, or chi-square test between patients who did and did not experience MACCE. In the first analysis, 133 patients were divided into two groups of higher baseline GNRI (≥ 96.7) and lower baseline GNRI (< 96.7), according to median baseline GNRI value. The effect of baseline GNRI on MACCE risk was tested by Kaplan-Meier analysis and time-varying Cox regression analysis. In the second analysis, the change in GNRI (delta GNRI) was calculated by subtracting the baseline GNRI from GNRI one year after PD initiation to determine the prognostic value of GNRI change over the first year. Patients were categorized into two groups according to changed GNRI value: improved (delta GNRI > 0) and worsening or stationary (delta GNRI ≤ 0) groups. Cumulative survival curves were generated by the Kaplan-Meier method, and between-group survival was compared by a log-rank test. The independent prognostic value of worsening or stationary GNRI in determining MACCE risk was ascertained by Cox’s regression analysis. Furthermore, subgroup analysis was performed according to baseline GNRI group to explore whether the impact of changes in GNRI on MACCE risk was affected by baseline nutritional status. A P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Patient baseline characteristics are shown in Table 1. The mean age was 50.8 ± 11.9 years, and 69 patients (51.9%) were men. The mean GNRI was 96.2 ± 8.3 (median 96.7, IQR 92.0–101.2). During a mean follow-up duration of 51.1 months, MACCEs were observed in 42 patients (31.6%). The mean age, proportion of patients with cardiovascular disease history, and hs-CRP level were significantly higher in patients who experienced MAC-CEs than in those who did not. High-density lipoprotein cholesterol concentrations were significantly lower in patients who had MACCEs than in those who did not. Baseline GNRI values did not differ significantly between patients with and without MACCEs.
Table 1.
Baseline characteristics of patients according to incidence of MACCE
| Characteristic | All (n = 133) | Patients with MACCE (n = 42) | Patients without MACCE (n = 91) | P value |
|---|---|---|---|---|
| Age (yr) | 50.8 ± 11.9 | 55.1 ± 10.5 | 48.7 ± 12.0 | 0.004 |
| Sex, men | 69 (51.9) | 21 (50.0) | 48 (52.7) | 0.85 |
| Diabetes mellitus | 20 (15.0) | 8 (19.0) | 12 (13.2) | 0.44 |
| CVD* | 18 (13.5) | 10 (23.8) | 8 (8.8) | 0.03 |
| Smoker | 40 (30.1) | 11 (26.2) | 29 (31.9) | 0.55 |
| SBP (mmHg) | 134.3 ± 21.1 | 136.2 ± 20.2 | 133.4 ± 21.5 | 0.48 |
| Height (cm) | 162.1 ± 8.1 | 160.8 ± 8.3 | 162.7 ± 8.0 | 0.21 |
| Body weight (kg) | 59.8 ± 10.2 | 60.3 ± 9.7 | 59.6 ± 10.4 | 0.70 |
| Hemoglobin (g/L) | 107 ± 15 | 108 ± 15 | 106 ± 12 | 0.40 |
| BUN (mmol/L) | 21.3 ± 6.7 | 20.1 ± 5.9 | 21.8 ± 7.0 | 0.19 |
| Creatinine (μmol/L) | 875 ± 327 | 831 ± 344 | 892 ± 344 | 0.30 |
| Albumin (g/L) | 37 ± 5 | 37 ± 4 | 37 ± 5 | 0.81 |
| Glucose (mmol/L) | 5.3 ± 1.9 | 5.7 ± 3.1 | 5.1 ± 0.9 | 0.12 |
| Calcium (mmol/L) | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.51 |
| Phosphorus (mmol/L) | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.63 |
| iPTH (ng/L) | 167.0 (76.0–325.9) | 159.6 (71.8–284.5) | 189.0 (80.0–356.0) | 0.51 |
| Total cholesterol (mmol/L) | 4.7 ± 1.0 | 4.6 ± 0.9 | 4.7 ± 1.0 | 0.41 |
| HDL-C (mmol/L) | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.3 ± 0.4 | 0.04 |
| LDL-C (mmol/L) | 2.7 ± 0.8 | 2.7 ± 0.7 | 2.8 ± 0.9 | 0.41 |
| Triglycerides (mmol/L) | 1.6 ± 1.1 | 1.7 ± 1.3 | 1.5 ± 1.0 | 0.28 |
| hs-CRP (mg/L) | 1.2 (0.7–2.1) | 1.5 (0.9–2.8) | 1.0 (0.7–2.1) | 0.04 |
| Medications | ||||
| RAS blockers | 125 (94.0) | 40 (95.2) | 85 (93.4) | 0.9 |
| Beta-blockers | 91 (68.4) | 26 (61.9) | 65 (71.4) | 0.32 |
| Calcium channel blockers | 104 (78.2) | 34 (81.0) | 70 (76.9) | 0.66 |
| Diuretics | 23 (17.3) | 6 (14.3) | 17 (18.7) | 0.63 |
| Lipid lowering therapy | 65 (48.9) | 20 (47.6) | 45 (49.5) | 0.85 |
| Weekly peritoneal Kt/Vurea | 1.4 ± 0.5 | 1.4 ± 0.3 | 1.4 ± 0.5 | 0.10 |
| RKF (mL/min/1.73m2) | 2.1 (0.1–5.5) | 0.5 (0.1–5.0) | 2.5 (0.1–5.8) | 0.21 |
| Urine volume (L/d) | 0.50 (0.05–1.10) | 0.20 (0.03–0.94) | 0.60 (0.03–0.10) | 0.27 |
| GNRI | 96.2 ± 8.3 | 96.2 ± 7.3 | 96.2 ± 8.7 | 0.9 |
Values are presented as mean ± standard deviation, number of patients (percentage), or median (interquartile range).
BUN, blood urea nitrogen; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; CVD, cardiovascular disease; GNRI, geriatric nutritional risk index; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; iPTH, intact parathyroid hormone; LDL-C, low-density lipo-protein cholesterol; MACCE, major adverse cardiac and cerebrovascular events; PAD, peripheral artery disease; RAS, renin-angiotensin system; RKF, residual kidney function; SBP, systolic blood pressure.
CVD: Composite of CAD, PAD, CVA, and CHF.
No significant association between baseline GNRI and MACCEs
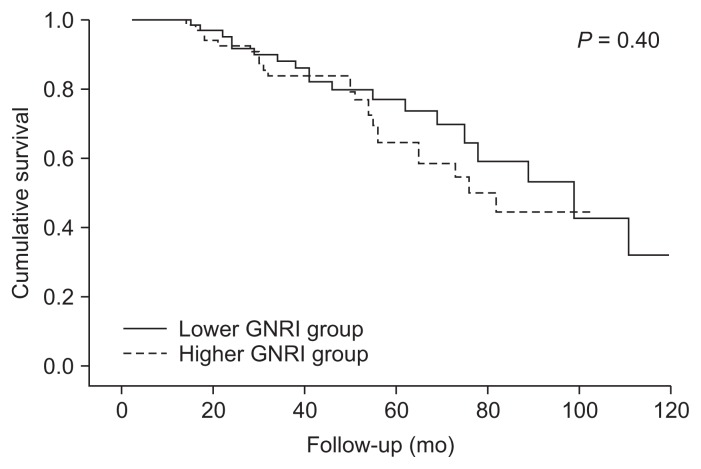
Patients were divided into two groups according to the median value of baseline GNRI, which was 96.7. In the lower baseline GNRI group (GNRI < 96.7, n = 63), MAC-CEs were observed in 19 patients (30.2%). In the higher baseline GNRI group (GNRI ≥ 96.7, n = 70), MACCEs were observed in 23 patients (32.9%). Kaplan-Meier analysis indicated that event-free survival rates did not differ between the two groups (log-rank test, P = 0.40) (Fig. 1). Univariate time-varying Cox regression analysis revealed that patients in the lower baseline GNRI group were not at greater risk for MACCEs (hazard ratio [HR]=1.31, 95% confidence interval [CI] = 0.70–2.43, P = 0.40) than those in the higher baseline GNRI group.
Figure 1. Kaplan-Meier analysis of baseline GNRI groups for MACCE.
Baseline GNRI group was not significantly associated with incidence of MACCE (log-rank test, P = 0.40).
GNRI, geriatric nutritional risk index; MACCE, major adverse cardiac and cerebrovascular event.
Worsening or stationary GNRI one year after PD initiation as an independent risk factor for MACCEs
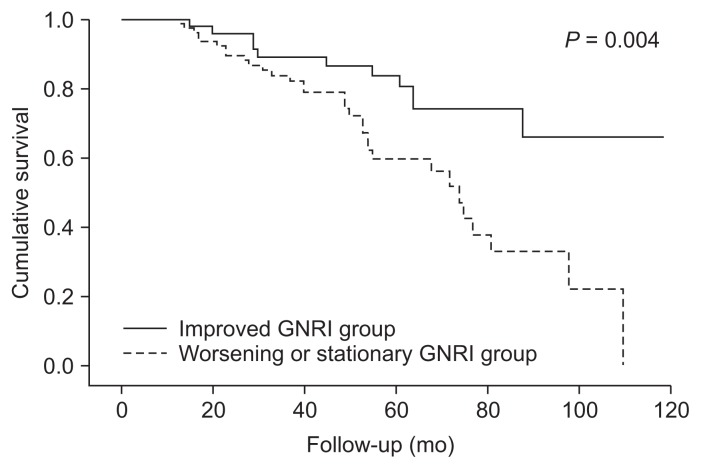
The mean GNRI value at one year after PD initiation was 94.9 ± 7.0 (median 94.0, IQR 90.8–99.8). When patients were dichotomized according to the median value of follow up GNRI, Kaplan-Meier analysis demonstrated that event-free survival rates were significantly lower in the lower GNRI group than in the higher group (log-rank test, P = 0.03). To evaluate the prognostic value of GNRI changes, the delta GNRI was calculated from GNRI values at baseline and one year after PD initiation. Patients were classified according to baseline GNRI values and changes in GNRI value (Table 2). According to delta GNRI, patients were categorized into improved (delta GNRI > 0) and worsening or stationary (delta GNRI ≤ 0) groups. Fifty-two patients were in the improved GNRI group, and 81 patients were in the worsening or stationary GNRI group. Forty-eight patients (76.2%) exhibited worsening or stationary GNRI in the lower baseline GNRI group, while 33 patients (47.1%) exhibited worsening or stationary GNRI in the higher baseline GNRI group. The cumulative event-free survival rate was significantly lower in the worsening or stationary GNRI group (log-rank test, P = 0.004) than in the improved GNRI group (Fig. 2). Multivariate Cox analysis showed that worsening or stationary GNRI was an independent predictor of MACCEs (HR, 2.47; 95% CI, 1.15–5.29; P = 0.02) (Table 3).
Table 2.
Proportion of patients according to baseline GNRI value and GNRI changes during one year after PD initiation
| Lower baseline GNRI group (GNRI < 96.7, n = 63) | Higher baseline GNRI group (GNRI ≥ 96.7, n = 70) | |
|---|---|---|
| Improved GNRI group (delta GNRI > 0, n = 52) | 15 (23.8) | 37 (52.9) |
| Worsening or stationary group (delta GNRI ≤ 0, n = 81) | 48 (76.2) | 33 (47.1) |
Values are presented as number of patients (percentage).
GNRI, geriatric nutritional risk index; PD, peritoneal dialysis.
Figure 2. Kaplan-Meier analysis of GNRI change groups for MACCE.
The worsening or stationary GNRI (delta GNRI ≤ 0) group showed significantly higher risk of MACCE compared to the improved GNRI (delta GNRI > 0) group (long-rank test, P = 0.004).
GNRI, geriatric nutritional risk index; MACCE, major adverse cardiac and cerebrovascular event.
Table 3.
Uni- and multivariate Cox regression analyses of GNRI change groups for MACCE
| Variable | Unadjusted | *Adjusted | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (yr) | 1.05 (1.02–1.08) | 0.001 | 1.06 (1.02–1.10) | 0.002 |
| Women (vs. men) | 0.89 (0.48–1.63) | 0.70 | 0.80 (0.42–1.52) | 0.50 |
| Diabetes mellitus | 2.25 (1.01–5.01) | 0.04 | 1.04 (0.41–2.49) | 0.9 |
| Cardiovascular disease | 2.21 (1.08–4.54) | 0.03 | 1.67 (0.76–3.66) | 0.21 |
| Ca × P (per mmol2/L2) | 1.03 (0.98–1.03) | 0.78 | 1.02 (0.99–1.05) | 0.10 |
| hs-CRP (per mg/L) | 1.58 (0.80–3.11) | 0.19 | 0.80 (0.45–1.44) | 0.46 |
| Urine volume (L/day) | 0.99 (0.99–1.03) | 0.39 | 1.00 (0.99–1.00) | 0.38 |
| GNRI change groups | ||||
| Improved group | Reference | Reference | ||
| Worsening or stationary group | 2.69 (1.34–5.39) | 0.01 | 2.47 (1.15–5.29) | 0.02 |
Adjusted for age, sex, diabetes mellitus, history of cardiovascular disease, Ca × P products, hs-CRP, and residual urine volume.
Improved group included 52 patients with delta GNRI > 0; Worsening or stationary group included 81 patients with delta GNRI ≤ 0.
Ca, calcium; CI, confidence interval; GNRI, geriatric nutritional risk index; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; MACCE, major adverse cardiac and cerebrovascular events; P, phosphorus.
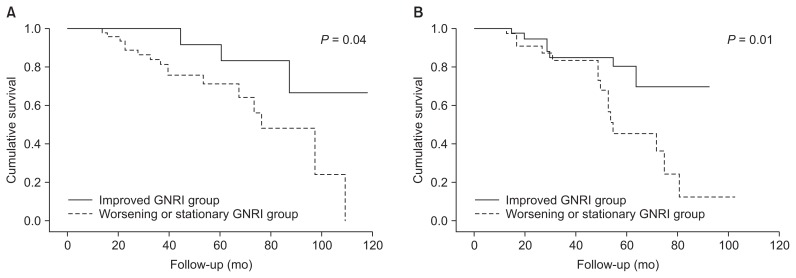
In addition, to investigate whether the impact of changes in GNRI on MACCE risk was affected by baseline nutritional status, subgroup analyses were performed according to baseline GNRI group. Kaplan-Meier analysis revealed that the worsening or stationary GNRI group was at greater risk for MACCEs in both the lower and higher baseline GNRI groups (log-rank test, P = 0.04 and P = 0.01, respectively) (Fig. 3). Multivariate Cox regression analysis showed that worsening or stationary GNRI was independently associated with a higher risk of MACCEs (HR, 3.90; 95% CI, 1.45–10.53; P = 0.01) in patients with higher baseline GNRI values. In patients with lower baseline GNRI values, the risk of MACCEs was also higher in the worsening or stationary GNRI group than in the improved group, but the difference was not statistically significant (HR, 3.33; 95% CI, 0.90–12.31; P = 0.06) (Table 4).
Figure 3. Kaplan-Meier analysis of GNRI change groups for MACCE according to baseline GNRI group.
The worsening or stationary GNRI group had higher risk of MACCE compared to the improved group in both (A) lower and (B) higher baseline GNRI groups (log-rank test, P = 0.04 and 0.01, respectively).
GNRI, geriatric nutritional risk index; MACCE, major adverse cardiac and cerebrovascular event.
Table 4.
Multivariate Cox regression analysis of GNRI change groups for MACCE according to baseline nutritional status
| Unadjusted | *Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Lower baseline GNRI group (n = 63) | ||||
| Improved group | Reference | Reference | ||
| Worsening or stationary group | 3.46 (0.98–12.37) | 0.06 | 3.33 (0.90–12.31) | 0.06 |
| Higher baseline GNRI group (n = 70) | ||||
| Improved group | Reference | Reference | ||
| Worsening or stationary group | 2.98 (1.25–7.11) | 0.01 | 3.90 (1.45–10.53) | 0.01 |
Adjusted for age, sex, presence of diabetes mellitus, previous cardiovascular disease, and high sensitivity C-reactive protein.
Improved group included patients with delta GNRI > 0; Worsening or stationary group included patients with delta GNRI ≤ 0.
CI, confidence interval; GNRI, geriatric nutritional risk index; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events.
Discussion
In the current study, we investigated the prognostic value of changes in GNRI during the first year of dialysis in determining MACCE risk for incident PD patients. Baseline GNRI was not significantly associated with MACCEs. However, patients with worsened or unchanged GNRI value were at 2.47-fold greater risk for MACCEs than those with improved GNRI values. Moreover, the increased MACCE risk in the worsening or stationary group was significant, irrespective of the baseline GNRI group. These findings suggest that regular assessment of nutritional status using the GNRI could be useful for stratifying cardiovascular risk in incident PD patients.
Protein-energy wasting is a well-known risk factor for cardiovascular disease and unfavorable clinical outcomes, including mortality and impaired quality of life, in patients with ESRD treated with dialysis [2–8]. A number of studies have evaluated protein-energy wasting and its prognostic impact. The GNRI was developed primarily for elderly patients [16], and Yamada et al [13] first investigated the usefulness of GNRI as a nutritional indicator in patients on chronic HD. When malnutrition was defined as malnutrition-inflammation score (MIS) ≥ 6, the area under the receiver operating characteristic curve (AUC) of the GNRI was larger than that of four other nutritional indices—the mini nutritional assessment-short form (MNA-SF), nutritional risk score (NRS), malnutrition universal screening tool (MUST), and malnutrition screening tool (MST) [13]. Similarly, GNRI and change in GNRI correlated significantly with MIS, a 7-point subjective global assessment (SGA), and their changes in PD patients [17]. In terms of clinical outcomes, the GNRI was found to significantly predict all-cause mortality in several HD studies [18,19,22] and PD [24] patients. The GNRI also predicted cardiovascular mortality in HD patients [22]. However, to date, there have been few studies investigating the effects of GNRI changes on clinical outcomes in patients with ESRD [20]. In a study of 75 HD patients, the GNRI, MIS, dietary energy intake, and body composition were measured at baseline and at 6, 12, and 18 months after enrollment. The GNRI and MIS correlated with changes in nutritional biomarkers, inflammatory cytokine (interleukin-6) levels, and body composition parameters [20]. Moreover, both GNRI and MIS were significantly associated with hospitalization, but only MIS was associated with mortality.
In the present study, baseline GNRI at PD initiation was not significantly associated with MACCEs, but changes in GNRI over one year did affect the incidence of MAC-CEs. Patients with worsening or stationary GNRI values were at greater risk for MACCEs than those with improved GNRI values. In contrast, a study of 486 Korean PD patients indicated that the lowest tertile of baseline GNRI was significantly associated with higher all-cause mortality compared to the middle or higher tertiles [24]. Although we cannot clarify the reason for conflicting findings, differences in study outcomes and subjects are possible explanations. Compared with a previous study, the incidence of the study outcome was lower in our study (all-cause mortality, 50.8% vs. MACCE, 31.6%). Because we only included patients who maintained PD for at least one year, we may have underestimated the effect of baseline GNRI on study outcome. Other previous studies also showed that low baseline GNRI was a significant predictor of adverse outcomes in patients with ESRD [18,19,21–23]. Although the exact reason is unclear, we presume that differences in dialysis modality (HD or PD), dialysis vintage (prevalent or incident dialysis patients), study outcomes (all-cause mortality, cardiovascular mortality, or cardiovascular events), and study design (retrospective or prospective) contributed to the discrepancies between our findings and previous findings. Because this study included only incident dialysis patients, the prognostic impact of changes in nutritional status can be more prominent in such patients than in patients on chronic dialysis. Furthermore, a previous study by our group demonstrated that changes in nutritional status as assessed by the SGA, not baseline nutritional status, were significantly associated with all-cause mortality in incident dialysis patients in a nationwide prospective cohort [15], supporting the results of the present study.
Although we did not completely elucidate the mechanisms by which changes in GNRI are associated with MACCEs, the associations of nutritional status with inflammation and physical activity are possible explanations. In patients with ESRD, protein-energy wasting is associated with inflammation and atherosclerosis, suggesting that malnutrition can increase the risk of cardiovascular mortality in these patients [3]. In our study, a significantly greater increase in hs-CRP concentration between baseline and one year after PD initiation was observed in the worsening or stationary GNRI group compared to the improved group (log delta hs-CRP, worsening or stationary group vs. improved group: 0.1 ± 3.3 vs. −1.4 ± 2.4 mg/L; P = 0.03). Based on these results, progression of inflammation accompanied by a worsening nutritional status can explain the higher incidence of MACCEs in the worsening or stationary GNRI group. The significant association between nutritional status and physical activity is another possible explanation for our findings. In 48 prevalent PD patients, a low GNRI was associated with reduced physical activity, assessed by the daily number of steps and daily energy expenditure for 9 days [26]. Given that malnutrition and low physical activity are independent predictors of hospitalization, morbidity, and mortality in patients with ESRD [27,28], reduced physical activity might have contributed to the increased MACCE risk in patients in the worsening or stationary GNRI group. Unfortunately, we did not measure physical activity indices such as the number of daily steps or gait speed in our study. A comprehensive study with nutritional parameters and physical activity indices could be helpful to verify the association of nutritional status and physical activity with cardiovascular disease in patients with ESRD.
This study has several limitations. First, it included a small number of subjects. Because the stationary group was too small to analyze separately, we could not clarify the independent effect of stationary GNRI on the primary outcome. Although Kaplan-Meier analysis demonstrated that the risk of MACCEs was significantly elevated in the worsening or stationary GNRI group in both lower and higher baseline GNRI groups, the HR in the lower baseline GNRI subgroup did not reach statistical significance in multivariate Cox regression analysis. Because there were very few improved patients in the lower baseline GNRI group (n = 15) and only three patients demonstrated the primary outcome, the negative results of Cox regression analysis might have been due to lack of statistical power. Furthermore, sensitivity analysis was not performed according to various subgroups, such as diabetes mellitus. Thus, a future large-scale prospective investigation should be conducted to confirm our results. Second, only patients who maintained PD for over one year and had one-year follow-up data were included, resulting in selection bias. In particular, 73 patients who maintained PD for over one year but did not have follow-up data were excluded. To mitigate the effect of selection bias, we first analyzed the difference in baseline characteristics between the 133 included patients and 73 excluded patients. There were no significant differences in baseline characteristics between the two groups except for hs-CRP (Supplementary table 1). In addition, survival analysis for the prognostic value of baseline GNRI was performed in 206 patients including the 73 excluded patients. Kaplan-Meier analysis demonstrated that there was no significant difference in MACCE-free survival rates between the higher and lower baseline GNRI groups (log-rank test, P = 0.9), in accordance with the primary result (Supplementary fig. 1). Nevertheless, 133 patients might not be representative of all incident PD patients, leading to potential selection bias. Third, only GNRI was used as a nutritional indicator. However, when the GNRI was compared with other nutritional indices, the AUC of the GNRI was the largest, suggesting that GNRI is superior for identifying nutritional risk in patients with ESRD [13]. Along with GNRI, the SGA and MIS are also validated nutritional assessment tools in dialysis patients [9–12,15], but both the SGA and MIS are based on subjective assessment. Therefore, these methods require a skilled examiner and are prone to inter-observer variability. In contrast, the GNRI is calculated from three objective variables: height, body weight, and serum albumin concentration. Taken together, these findings indicate that the GNRI is a simple nutritional assessment method and is suitable for large-scale population-based research. Fourth, there is no definite GNRI cutoff value for malnutrition in PD patients. We dichotomized patients by the median value of the GNRI (96.7). This value was similar to that previously reported for Korean PD patients [24]. In 486 PD patients, the cutoff value for time-averaged GNRI over one year for a diagnosis of a decline in lean mass was 96.4 [24]. In addition, when we tested different cutoff values, our results did not change. Baseline GNRI was not significantly associated with MACCEs when we used a cutoff value of 90 (log-rank test, P = 0.16) [18] or 92 (P = 0.36) [23]. Fifth, we determined the GNRI only two times per patient—at PD initiation and after one year. There are no definite guidelines for the frequency of nutritional assessment; however, a 4- to 6-month interval is suggested for patients with stable ESRD [15]. Studies with more frequent follow-up over a longer period of time would be worthwhile. Finally, because this was a retrospective study, residual confounding effects of other risk factors such as previous history of cardiovascular disease cannot be totally excluded and could not provide evidence for intervention to reduce the risk of MACCEs.
In conclusion, the current study demonstrated that there was no association between baseline GNRI and MACCEs. However, incident PD patients whose nutritional status deteriorated or remained unchanged were at significantly greater risk for MACCEs than patients whose nutritional status improved. These findings suggest that serial monitoring of nutritional status is important to stratify cardiovascular risk in incident PD patients.
Supplementary Data
Acknowledgments
This work was supported by the Brain Korea PLUS 21 Project for Medical Science, Yonsei University, by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2011-0030711), and by a grant of the Korea Healthcare Technology R&D Project through the Korean Heath Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1129).
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr. 1997;65:1544–1557. doi: 10.1093/ajcn/65.5.1544. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 4.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW NECOSAD Study Group. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 5.Han SH, Han DS. Nutrition in patients on peritoneal dialysis. Nat Rev Nephrol. 2012;8:163–175. doi: 10.1038/nrneph.2012.12. [DOI] [PubMed] [Google Scholar]
- 6.Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis. 1994;24:1002–1009. doi: 10.1016/S0272-6386(12)81075-4. [DOI] [PubMed] [Google Scholar]
- 7.Bergström J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol. 1995;6:1329–1341. doi: 10.1681/ASN.V651329. [DOI] [PubMed] [Google Scholar]
- 8.Dong J, Li Y, Xu Y, Xu R. Daily protein intake and survival in patients on peritoneal dialysis. Nephrol Dial Transplant. 2011;26:3715–3721. doi: 10.1093/ndt/gfr142. [DOI] [PubMed] [Google Scholar]
- 9.Enia G, Sicuso C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant. 1993;8:1094–1098. [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Kleiner M, Dunne E, Lee GH, Luft FC. A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant. 1999;14:1732–1738. doi: 10.1093/ndt/14.7.1732. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87:106–113. doi: 10.1093/ajcn/87.1.106. [DOI] [PubMed] [Google Scholar]
- 14.Beberashvili I, Azar A, Sinuani I, et al. Objective Score of Nutrition on Dialysis (OSND) as an alternative for the malnutrition-inflammation score in assessment of nutritional risk of haemodialysis patients. Nephrol Dial Transplant. 2010;25:2662–2671. doi: 10.1093/ndt/gfq031. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YE, Kee YK, Yoon CY, et al. Change of nutritional status assessed using subjective global assessment is associated with all-cause mortality in incident dialysis patients. Medicine (Baltimore) 2016;95:e2714. doi: 10.1097/MD.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 17.Szeto CC, Kwan BC, Chow KM, Law MC, Li PK. Geriatric nutritional risk index as a screening tool for malnutrition in patients on chronic peritoneal dialysis. J Ren Nutr. 2010;20:29–37. doi: 10.1053/j.jrn.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi I, Ishimura E, Kato Y, et al. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. 2010;25:3361–3365. doi: 10.1093/ndt/gfq211. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Kim SB, Shin HS, Jung YS, Rim H. Geriatric nutritional risk index may be a significant predictor of mortality in Korean hemodialysis patients: a single center study. Ther Apher Dial. 2012;16:121–126. doi: 10.1111/j.1744-9987.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 20.Beberashvili I, Azar A, Sinuani I, et al. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:443–451. doi: 10.2215/CJN.04980512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panichi V, Cupisti A, Rosati A, et al. Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: data from the Riscavid cohort. J Nephrol. 2014;27:193–201. doi: 10.1007/s40620-013-0033-0. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Ito Y, Ishii H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol. 2014;64:32–36. doi: 10.1016/j.jjcc.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu M, Okazaki M, Tsuchiya K, Kawaguchi H, Nitta K. Geriatric nutritional risk index is a simple predictor of mortality in chronic hemodialysis patients. Blood Purif. 2015;39:281–287. doi: 10.1159/000381798. [DOI] [PubMed] [Google Scholar]
- 24.Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Geriatric Nutritional Risk Index as a prognostic factor in peritoneal dialysis patients. Perit Dial Int. 2013;33:405–410. doi: 10.3747/pdi.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beberashvili I, Azar A, Sinuani I, et al. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin Nutr. 2016;35:1522–1529. doi: 10.1016/j.clnu.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Wakamiya A, Hiraki K, Hotta C, et al. Poor nutritional status is associated with low physical activity in patients undergoing peritoneal dialysis. Int J Cardiol. 2015;187:648–650. doi: 10.1016/j.ijcard.2015.03.387. [DOI] [PubMed] [Google Scholar]
- 27.Cupisti A, Capitanini A, Betti G, D’Alessandro C, Barsotti G. Assessment of habitual physical activity and energy expenditure in dialysis patients and relationships to nutritional parameters. Clin Nephrol. 2011;75:218–225. doi: 10.5414/CNP75218. [DOI] [PubMed] [Google Scholar]
- 28.Ikizler TA. A patient with CKD and poor nutritional status. Clin J Am Soc Nephrol. 2013;8:2174–2182. doi: 10.2215/CJN.04630513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.