Abstract
Objective
To determine the therapeutic effects of dietary supplementation on C. difficile infection (CDI).
Background
With limited treatment options, the rise of Clostridium difficile-associated disease has spurred on the search for novel therapies. Recent data define a role for the aryl hydrocarbon receptor (AHR) and diet-derived AHR ligands in mucosal immunity. We investigated the efficacy of indole-3-carbinol (I3C), a dietary supplement and AHR precursor ligand, in a murine model of CDI.
Methods
C57BL/6 (B6), AHR−/− and AHR+/− mice were placed on either grain-based or semi-purified diets with or without I3C prior to and during CDI. Mice were followed clinically for a minimum of 6 days, or euthanized between days 0 and 4 of inoculation for analysis of the inflammatory response and microbiota.
Results
B6 mice fed an AHR ligand-deficient, semi-purified diet have significantly increased disease severity (p < 0.001) and mortality (p<0.001) compared to mice fed diet containing I3C. The addition of I3C to the diet of AHR null mice had less of an impact than in AHR heterozygous littermates, although some protection was seen. Mice on semi-purified I3C-diet had increased cecal Tregs, ILC3s, and γδ T cells as well as an increased neutrophilic response without increased inflammation or bacterial translocation compared to controls.
Conclusions
I3C is a powerful treatment to reduce impact of CDI in mice. The findings indicate I3C may be acting through both AHR-dependent and -independent mechanisms in this model. Dietary supplementation with I3C is a potential new therapy for prevention and amelioration of C. difficile disease.
INTRODUCTION
Clostridium difficile infection (CDI) is a major health concern in the United States accounting for over 29,000 deaths per year and costing the healthcare system greater than $1 billion a year1. Disruption of the gut microbiome following the use of antibiotics is a major risk factor for C. difficile-associated disease (CDAD) in humans2. Other risk factors include age, acid suppression, immunosuppression, gastrointestinal surgery and inflammatory bowel disease3–5. Primary treatment for acute infections is antibiotics. However, up to 20 percent of patients experience recurrence of CDI that may be resistant to subsequent treatment with antibiotics6. For these patients, novel therapies such as probiotics and fecal microbiota transplant are being tested7, 8. The efficacy of probiotics in preventing infection has recently been brought into question9, leaving the medical community with no options for disease prevention that are supported by data, other than minimizing antibiotic use and acid suppression. Fecal transplantation for recurrent/resistant disease has shown some promise8, 10, but barriers to its adoption include difficulty in quality control, infectious issues, and dosing.
While murine models have demonstrated the importance of microbiome integrity in minimizing CDI11, host immune cells and their responses have also been shown to be critical. This includes the presence of group 3 innate lymphoid cells (ILC3s12) and the expression of IL-2213. The aryl hydrocarbon receptor (AHR) is critical for the development and maintenance of ILC3s as well as intra-epithelial lymphocytes (IELs) of the intestine14, 15, and mice placed on semi-purified diet that is free of phytochemicals (containing minimal AHR ligands) show a decline in IEL and ILC3 cell number compared to mice on standard chow. Addition of the AHR precursor ligand indole-3-carbinol (I3C) to purified diet reverses this cell loss14, 15. Both IELs and ILC3s are major sources of IL-22 in the gut16, 17 and the AHR is vital for the expression of IL-22 in these lymphocyte subsets as well as conventional T cells18, 19. In addition to immune cell maintenance, dietary- and microbiome-derived AHR ligands have been shown to be effective in treating/ameliorating a different infectious disease (vaginal candidiasis) at mucosal surfaces in mice in an IL-22-dependent fashion20.
Using this information, we hypothesized that the inclusion of I3C in the diet could reduce the severity of CDI. To test this hypothesis, we utilized a well-established murine model of CDAD21. If this is indeed the case, such a finding would suggest that the addition of a simple and safe dietary supplement begun shortly before planned antibiotics or other interventions that increase risk of CDI may have efficacy in reducing morbidity from this highly prevalent and severely debilitating disease.
METHODS
Mice
6–8 week old male C57BL/6j mice (B6) were purchased from Jackson Laboratory (Bar Harbor ME). For some experiments, B6 mice were placed on either a grain-based chow or custom chow prepared to contain 1000 ppm I3C throughout the experiments. For those experiments where a diet low in AHR ligands was desired, mice were placed on either “semi-purified diet” (AIN-76A Semi-Purified Diet; TestDiet, St. Louis, Mo.) or “I3C diet” (AIN-76A containing 1000 ppm I3C) for the remainder of the study. AHR heterozygotes (AHR+/−) mice, which have AHR expression equal to that of wildtype B6 mice, and AHR null (AHR−/−) mice on a C57BL/6J background22 were bred and maintained under specific pathogen-free conditions. Animal experiments were carried out according to institutional guidelines. All procedures were approved by the University of Wisconsin School of Medicine and Public Health IACUC.
Antibiotic administration and infection with Clostridium difficile
The model was adopted from the methods of Chen21. Mice were monitored/scored twice daily for signs of clinical disease and weighed every morning. The experiment was terminated when all mice were euthanized based on the following criteria: clinical scores > 13, weight loss > 20% or at day 7 after inoculation. All scoring was done by investigators blinded to the experimental group. Cecal histology was examined and scored by a single blinded histopathologist as described in the supplement.
Tissue and stool collection
A modified version of a previously published protocol23 was used to prepare the cecum for histology and RT-PCR. Feces from mice were collected and stored at −80°C until further processing and microbiome analysis.
Flow cytometry
Cecal cells were collected using a modified version of a previously published protocol24. IEL and lamina propria (LM) cells were analyzed by flow cytometry.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. For evenly distributed continuous variables, Fisher’s exact t-test was used to compare means. For unevenly distributed data sets, a Welch’s correction was applied or a Mann-Whitney U test was applied. To analyze survival, groups were compared using Kaplan Meier survival curves. The p values < 0.05 were considered significant. Additional detail to the methods can be found in the online supplemental materials.
RESULTS
Dietary I3C improves outcome in C. difficile infection
To investigate the impact I3C could have on CDAD prevention, B6 mice were placed on either a grain-based chow or chow with 1000 ppm I3C. After two weeks on diet, mice received antibiotic treatment (ABx) followed by inoculation with 105 C. difficile spores (Fig. 1A). Mice on I3C-supplemented diet had a significant increase in survival (66.7%) compared to control (20%; Fig. 1B). I3C supplementation resulted in significantly less weight loss on day 3 post-inoculation and reduced disease scores on days 1, 2 and 5 (Fig. 1C, D). Both groups presented with peak mean clinical score on day 3 post-inoculation.
Figure 1. Addition of dietary I3C reduces CDI-related mortality and morbidity.
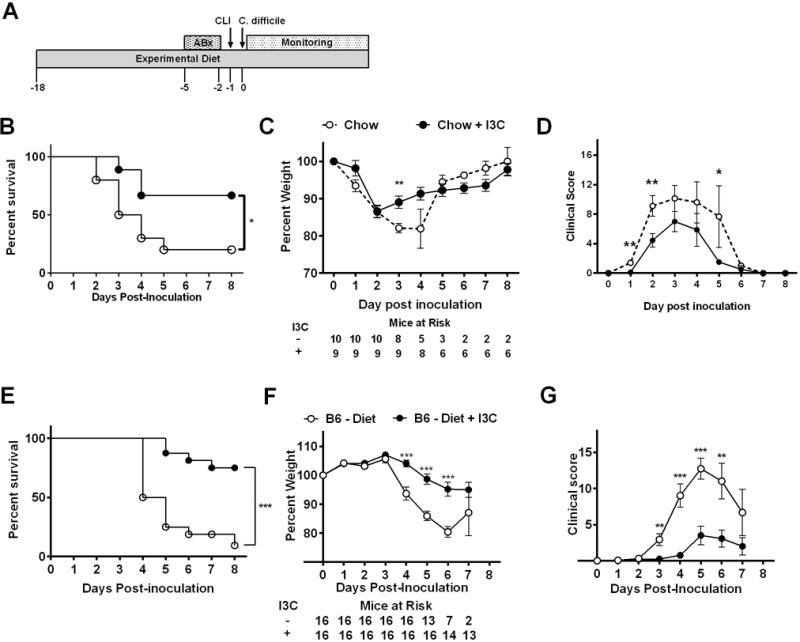
(A) Diagram of CDI model testing efficacy of dietary I3C on disease progression. Treatments include addition of antibiotics (ABx) to drinking water, i.p injection of Clindamycin (CLI) and oral gavage with C. difficile spores. (B) Percent survival, (C) mean percent weight +/− SEM and (D) mean clinical score +/− SEM after inoculation with 105 VPI 10463 C. difficile of male B6 mice fed standard chow or chow + I3C (1000 ppm) starting two weeks prior to ABx treatment. Data was pooled from two independent experiments. (E) Percent survival, (F) mean percent weight +/− SEM and (G) mean clinical score +/− SEM after inoculation with 104 VPI 10463 C. difficile of male B6 mice fed semi-purified diet (diet) or semi-purified diet supplemented with I3C (1000 ppm) starting two weeks prior to ABx treatment. Data was pooled from three independent experiments. Daily mice at risk are indicated in panels B and F. Log-rank test was used for survival analysis, two-tailed Student’s t-test was used for comparing daily percent weight and Mann-Whitney U test was used for comparing daily clinical score. * P ≤ 0.05; ** P ≤ 0.01; *** P < 0.001.
Given that grain-based diet likely contains naturally occurring AHR ligands25, similar experiments were performed utilizing semi-purified diet manufactured with and without I3C. Semi-purified diet lacks the majority of AHR ligands found in regular chow25. After two weeks on diet, mice underwent CDI induction, receiving 104 spores. This dose of spores was chosen as it consistently gave CDAD in these mice, whereas in experiments with regular chow, a higher dose (105) was needed for consistent disease (data not shown). Mice in both groups showed peak mean clinical score occurring on day 5 post-inoculation. Mice on semi-purified diet alone developed severe disease with an overall mortality of 87.5% (Fig. 1E). Interestingly, I3C-fed mice had significantly improved survival, with an overall mortality of only 12.5%. Additionally, I3C-fed mice had less weight loss and lower clinical disease severity scores (Fig. 1F, G) when compared to mice on semi-purified diet.
To investigate the role of the AHR in CDAD, AHR−/− mice and AHR+/− litter mate controls on standard chow were treated with ABx followed by inoculation with 105 C. difficile spores. As shown in Fig. 2A, AHR+/− mice had 100% survival at 5 days post-inoculation. However, AHR−/− mice had reduced survival (67%; p = 0.052). Peak mean clinical score was day 3 post-inoculation. Further measures of clinical disease severity, including percent weight loss and disease severity score (Fig. 2B, C) demonstrated that AHR−/− mice had significantly increased disease compared to AHR+/− mice. Thus, loss of AHR expression resulted in more severe C. difficile infection and disease. Similar to what was seen in wild-type B6 mice, AHR+/− mice on I3C-supplemented semi-purified diet displayed increased survival and reduced weight loss and clinical score compared to mice on control diet (Fig. 2D, E, F). The significant protection in survival seen in I3C-supplemented mice was lost in littermate AHR−/− mice, although they did trend towards non-significant improvement in survival (Fig. 2G). Significant differences between the semi-purified diet AHR−/− mice vs the I3C-diet AHR−/− mice were found for both weight loss and clinical score (Fig. 2H, I) at some time points. The peak mean clinical score for control diet AHR−/− mice was day 5 versus day 7 for I3C-fed AHR−/− mice. Mortality was also delayed in I3C-fed AHR−/− mice. Thus, CDAD was delayed by I3C supplementation in AHR−/− mice, and had less amelioration than seen in wild-type mice. These data suggest that some of the I3C-protective effects require the AHR, but I3C also has protective effects in this model that do not require the AHR.
Figure 2. Protective effects of dietary I3C are both AHR dependent and independent.
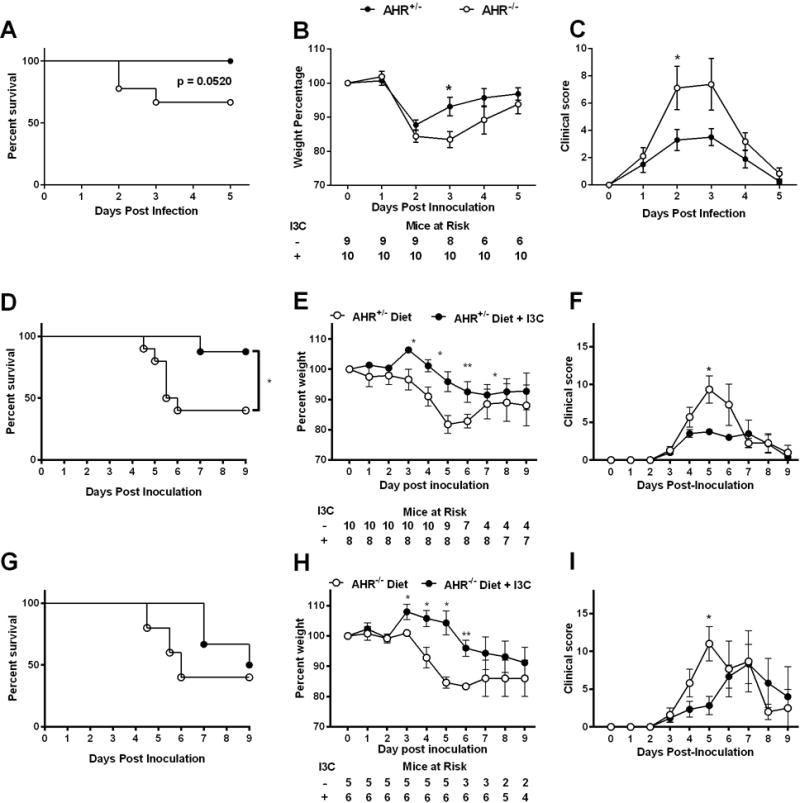
(A) Percent survival, (B) mean percent weight +/− SEM and (C) mean clinical score +/− SEM after inoculation with 105 VPI 10463 C. difficile of male AHR +/− and AHR−/− mice fed standard chow. Data was pooled from two independent experiments. (D,G) Percent survival, (E,H) mean percent weight +/− SEM and (F,I) mean clinical score +/− SEM after inoculation with 104 VPI 10463 C. difficile of male AHR+/− (D – F) or male AHR−/− (G – I) mice fed semi-purified diet (Diet) or semi-purified diet supplemented with I3C (Diet + I3C) starting two weeks prior to ABx treatment. Data was pooled from two independent experiments. Daily mice at risk are indicated in panels B, E, H. Log-rank test was used for survival analysis, two-tailed Student’s t-test was used for comparing daily percent weight and Mann-Whitney U test was used for comparing daily clinical score. * P ≤ 0.05; ** P ≤ 0.01.
Alteration in mucosal immune cells in mice fed semi-purified diet
Mechanistic experiments focused on mice maintained on semi-purified diet, as these mice had the poorest outcome with CDI and received the most benefit from dietary supplementation. As anticipated based on previous findings26, I3C-fed mice had significantly increased cecal Cyp1a1 mRNA expression compared to mice on semi-purified diet suggesting greater AHR activation (Fig. 3A). Importantly, Cyp1a1 levels were significantly elevated both pre- and post-antibiotic exposure in I3C-fed mice, suggesting that the majority of AHR activation was due to diet-derived ligands as opposed to microbial-derived AHR ligands. Mice on I3C diet also had higher levels of FoxP3 mRNA both before and after CDI (Fig. 3B). This finding was confirmed by flow cytometric measurement of FoxP3+ CD4+ T cells from the cecal lamina propria (cLP) on the day of C. difficile inoculation (Fig. 3C, D). At this same time point, we also found a higher proportion of ILC3s in the cLP of I3C-fed mice (Fig. 3C, E). Additionally, upon evaluation of cecal IELs, mice on semi-purified diet were found to have significantly less γδ T cells than their I3C-fed counterparts (Fig. 3C, F). Together, the decreased numbers of these gut immune cells in mice on semi-purified diet may leave the host more susceptible to infection and increase the risk of an overly robust and pathologic immune response.
Figure 3. Lack of dietary AHR ligands alters gut immune cells prior to inoculation with C. difficile.
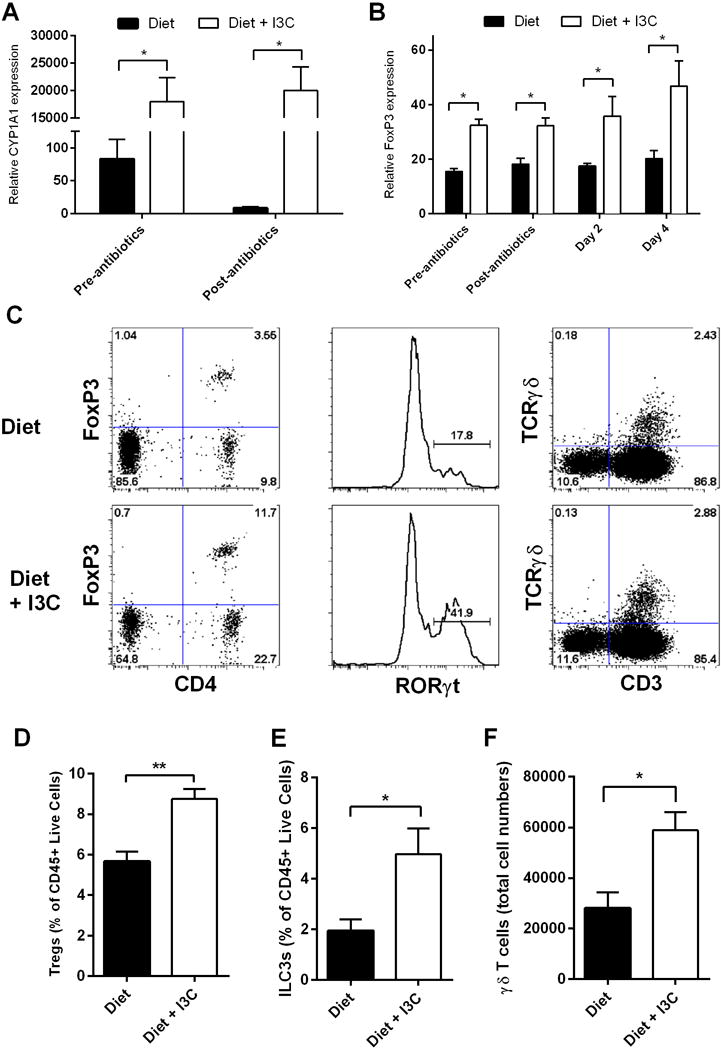
(A and B) Relative Cyp1a1 and FoxP3 expression as measured by RT-PCR in semi-purified diet and I3C mice compared to levels of β-actin. (C) Representative plots of isolated cecal cells with percent parent population. (D–F) Graphs of semi-purified diet and I3C mice at day 0 demonstrating frequency of cLP Tregs (Live/CD45+/CD11b−/Ly6G−/CD3+/CD4+/FoxP3+; D), ILC3s (Live/CD45+/CD11b−/Ly6G−/CD3−/CD4−/RORγt+; E), and number of IEL γδ T cells (Live/CD45+/CD11b−/Ly6G−/TCRβ−/CD3+/ TCRγδ+; F). All data are representative of n = 4 per group, with error bars showing SEM, repeated in 2 independent experiments. * P ≤ 0.05; ** P ≤ 0.01. Two-tailed Student’s t-test or Mann-Whitney U test used for comparing averages.
We did consider the possibility that the different diets could lead to differences in the microbiome that could account for differences in survival after CDI. As seen in Supplemental Figure 1, the difference in loss of diversity between the semi-purified diet and I3C-supplemented diet (before or after antibiotics) is small, and likely does not account for the dramatic improvement in survival with I3C-supplementation.
Dietary supplementation with I3C increases neutrophil response but reduces inflammation
Despite significant differences in the clinical course of CDAD in diet- versus diet + I3C-fed mice, no difference in colony-forming units of C. difficile in the cecum of mice at day two and day four was found between the two dietary groups (Fig. 4A). As studies have demonstrated both a protective and pathologic role for the host immune system in response to infection, and bacterial clearance of pathogens other than C. difficile by immune cells may be important for survival in this model13, the neutrophil response to CDI was examined in both sets of mice. Prior to CDI, mice on semi-purified and I3C diet had similar levels of cLP neutrophils (Fig. 4B, C). However, by day 3 of CDI, I3C mice had significantly more cLP neutrophils than semi-purified diet mice. Despite this increase in neutrophils, I3C mice did not show more cecal inflammation than semi-purified diet mice when analyzed by histopathology (Fig. 4 D, E). To further examine the immune response to CDI, cytokine production in the cecum was examined. We hypothesized that IL-22 in particular would be more significantly elevated in mice on I3C supplementation compared to those without I3C supplementation, given the close association of this cytokine with AHR activation and its known protective effect in colitis. We did find a significant elevation of IL-22 in the I3C-supplemented group on day 4 after infection, as predicted. However, a similar elevation of IL-22 was seen in mice on semi-purified diet without I3C-supplementation when analyzed on day 4 after infection (Fig. 4F, G). Differences in IL-17 and IFN-γ were also examined, and were not different between mice on the two diets (Fig. 4 F).
Figure 4. Mice fed I3C supplemented diet display increased neutrophil response to C. difficile without increased cecal inflammation.
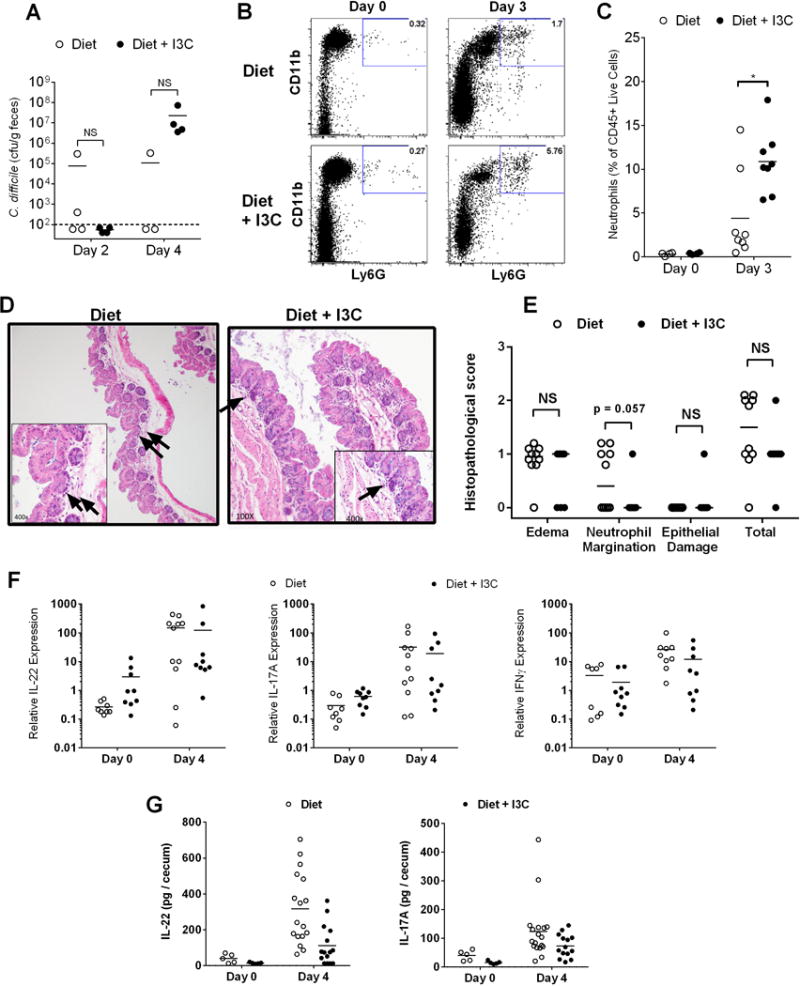
(A) Cecal C. difficile CFU/g feces in diet and diet + I3C mice at day 2 and 4 (n=4 per group). Limit of detection indicated by dash-line. Determination of the presence or absence of C. difficile cfu for each day determined by Chi-square analysis. (B and C) Representative flow plots and % of cLP neutrophils per CD45+ cells on day 0 (n=4 per group) and day 3 (n=8 per group) after inoculation in diet and diet + I3C mice. (D and E) Representative histology at 100x and 400x in semi-purified diet and I3C mice at day 3 (arrows depict neutrophils) and corresponding histopathological scoring (n=10 per group). (F) Relative IL-22, IL-17, and IFN-γ expression at day 0 and 4 as measured by RT-PCR in semi-purified diet and diet + I3C mice normalized to β-actin (n=4–10 per group). (G) Measurement of total IL-22 and IL-17A protein content per cecum by ELISA.
Addition of I3C to the diet decreases bacterial translocation during CDI
Bacterial translocation has been identified as a main contributor to disease severity in mice deficient in IL-22 or in neutrophil influx13, 27. To examine whether supplementation of diet with I3C maintains epithelial integrity during CDI and decreases bacterial translocation, tissue was harvested from four sites from semi-purified and I3C-fed mice on day 4 post-CDI and viable bacterial counts were determined using standard methods13. While both groups exhibited measureable bacterial loads in liver and lung with little to no measureable bacterial loads in kidney, I3C-fed mice had significantly less bacterial counts in spleens and a trend towards lower counts in lungs compared to control mice (Fig. 5 A–D). The possibility that reduction of translocation is a mechanism of protection in this model was tested by treating mice during CDI with the oral antibiotic ciprofloxacin, an antibiotic with no action against this strain of C. difficile but with efficacy against many other translocating bacteria27. When mice on semi-purified diet were treated with ciprofloxacin beginning after inoculation, survival, weight loss, and clinical score were all dramatically improved (Fig. 5 E–G). Together, these findings suggest that dietary I3C may contribute to prevention of bacterial translocation after CDI.
Figure 5. I3C added to the diet reduces bacterial translocation during CDI.
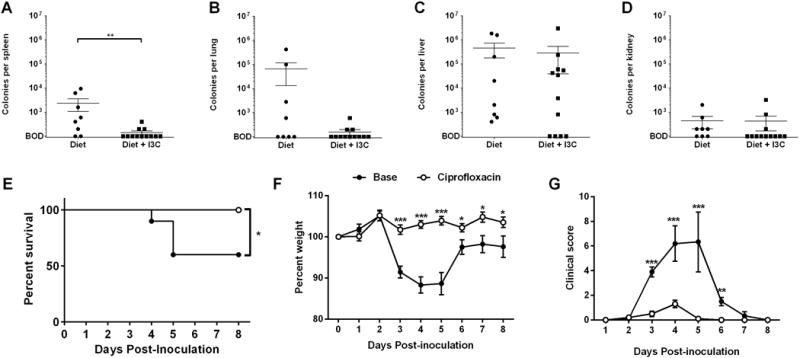
Bacterial counts from tissue homogenates of (A) spleen, (B) lung, (C) liver and (D) kidney on day 4 following CDI in mice fed diet or diet + I3C. (E) Survival, (F) weight loss, and (G) clinical scores of mice on semi-purified diet treated with plain drinking water or drinking water with 0.125 mg/mL ciprofloxacin beginning 1 day after inoculation with 104 VPI 10463 C. difficile (n = 10 per group). All error bars show SEM. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001. Log-rank test used for survival analysis and two-tailed Student’s t-test or Mann-Whitney U test used for comparing averages.
DISCUSSION
In this study, we have demonstrated that the addition of I3C to either grain-based standard chow or phytochemical-free semi-purified diet reduced the morbidity and mortality associated with CDI in a murine model. This finding has important clinical ramifications as the addition of I3C to the diet of patients scheduled for future procedures that include peri-procedural antibiotics, or to any patient population known to be at increased risk for CDAD, may have efficacy in reducing the incidence and/or severity of CDAD. Furthermore, the semi-purified diet may better represent the population in humans that fare the worst with CDI, many of who are malnourished or NPO (nil per os) while in the hospital. In addition, the potential exists that for patients who have previously had CDI, the addition of I3C to their diet might reduce the incidence of recurrence. The impact of these findings is strengthened by the fact that I3C is a safe dietary supplement that has already gone through clinical trials in humans28–30, is present in food, and could be added to the diet of patients at risk without the need for extensive testing or approval.
I3C is a weak AHR ligand31, 32 and a metabolite of glucobrassicin, a natural product derived from members of the cabbage family26. Following consumption, I3C is converted by acid-mediated catalysis into various byproducts including the strong AHR ligands diindolylmethane (DIM) and indole[3,2-b] carbazole (ICZ). To test whether the effects on CDAD seen in mice fed I3C were mediated through the AHR, we utilized AHR null mice plus their heterozygous AHR+/− littermates. Mice lacking the AHR developed worse CDAD, as measured by weight loss, clinical score, and mortality compared to littermate controls. This finding was predicted given the effects on mucosal immune cells that has been described in AHR−/− mice15. When mice were placed on either semi-purified diet or I3C-supplemented diet, I3C-fed AHR heterozygous mice demonstrated improved clinical outcomes as measured by increased survival, and reduced CDAD, similar to that seen in the wild-type B6 mice. I3C-fed AHR−/− mice did not have significantly improved survival compared to nulls on semi-purified diet, but they did show reduced clinical scores and weight loss with supplementation, suggesting that I3C may be acting through AHR-dependent and independent mechanisms. While it is not surprising that I3C might have mechanisms of protection that are AHR-independent, the global AHR null is imperfect as a control for the role of the AHR in gut disease. AHR null mice are known to have a patent ductus venosus33. This is relevant to a colitis model, as the patent ductus prevents gut-derived blood from interacting with immune cells and detoxifying properties of the liver, and could have an effect on the microbiome of these mice as well. This phenotypic change in AHR−/− mice may lessen the utility of the global null as a control for the role of the AHR in gut disease in mice. Future studies using cell-specific AHR deletion will better define the role of the AHR in our model. Regardless, our data would suggest that the effects include AHR dependent and independent mechanisms.
Our studies examining the mechanism(s) of I3C-mediated control of CDAD utilized the semi-purified phytochemical-free diet with and without I3C. The most striking difference in the phenotype of mice on these diets was found in the gut immune cells. Specifically, mice supplemented with I3C had more Tregs, ILC3s, and γδ T cells in the gut than mice on ligand deficient diet. The increase in Tregs may limit the immune response to C. difficile at the site of infection and decrease host derived pathology. Indeed, in other models of colitis, Tregs have played a critical role in preventing disease34, 35. ILC3s play a crucial role in host resistance to intestinal pathogens, are important sources of IL-22 in the gut, and are implicated in preventing CDAD in mice12. Finally, although γδ T cells have not been specifically examined in C. difficile, they are known to play a critical role in maintaining gut immunity through the production of IL-22, IL-17, and other protective cytokines18, and loss of these cells leads to bacterial overgrowth15.
Our data support that the protection from morbidity/mortality in C. difficile infection was secondary to a modulation of the immune response, as opposed to a reduction of C. difficile proliferation and growth. We did consider the possibility that the diet deficient of AHR ligands led to more dysbiosis and left these mice more susceptible to CDAD secondary to microbiome changes. There were some differences in the bacterial profile in I3C-supplemented mice, which may play some role in the protection from CDAD seen in this model. But in general, mice on both diets had more similarities than differences, and the mild changes seen are unlikely to fully explain the dramatic improvement in outcome with I3C supplementation. A previous study did identify that oral supplementation of the gut enzyme intestinal alkaline phosphatase (IAP) during antibiotic treatment protects mice from CDAD, possibly by preserving the normal intestinal flora36. As this did not appear to be the mechanism of protection in I3C supplementation, it may be interesting to consider supplementation of both IAP and I3C for more robust protection in future studies.
In response to infection, mice supplemented with AHR ligands demonstrated early influx of cecal neutrophils without an increase in inflammation or decrease in total number of C. difficile CFU. We hypothesize that this increase in recruitment of neutrophils may be mediated in part by the increase in both ILC3s and γδ T cells and their associated cytokines37. Given the finding that the increased neutrophil infiltrate does not increase the total amount of inflammation seen on histology, and the fact that the total number of C. difficile bacteria is not different between the diets despite dramatically different survival, we hypothesize that the neutrophils prevent overwhelming translocation of commensal gut bacteria that would cause the host to die from sepsis. Indeed, I3C-supplemented mice have a reduced bacterial presence in the spleen compared to control mice. While the identity of the translocating bacteria remains to be determined, the importance of bacterial translocation is supported by the finding that ligand-deficient mice can be rescued from C. difficile disease by treatment with ciprofloxacin. As ciprofloxacin has no efficacy against this strain of C. difficile but is effective against other commensal bacteria, the translocating bacteria are not likely to be C. difficile.
Current treatment strategies of C. difficile are directed at patients who have already presented with symptoms, an approach that can be too late to reverse severe disease. Some practitioners are attempting to manipulate the host microbiome to prevent CDAD, primarily with probiotic supplementation, but results have been disappointing9. Although the role of dietary AHR ligands in preventing CDAD in humans has not yet been studied, a number of risk factors for CDAD have been identified. Patients on tube feeding carry an increased rate of CDAD38, and while the mechanism of this is unclear, it is known that commonly used elemental feedings are absorbed in the small intestine and do not reach the colon. These patients may be at risk for a similar lack of AHR ligands present in their colon as seen in mice on ligand deficient diet. Furthermore, those most at risk for developing CDAD are elderly, moribund, institutionalized, and antibiotic-exposed patients. These patients are at high risk for having decreased exposure to AHR ligands in their gut from both dietary and microbial sources, potentially leading to a poorly-maintained gut immune system. Our study offers powerful new insights in the prevention of CDAD, where simple dietary supplementation of readily available AHR ligands could have profound impacts on rates and severity of CDAD in high risk patients. In addition, given that mice on regular diet also saw protection from CDAD with supplementation, it would make sense to consider dietary supplementation as little as two weeks prior to an elective treatment that requires antibiotics, which is known to increase the risk of CDAD. This would apply to any patient that is scheduled to undergo a procedure or operation that requires perioperative antibiotics, scenarios where CDI can be more expensive, morbid, and even life-threatening than the procedure itself. Another area of intervention may be patients at risk for recurrent disease, which can affect as many as 25% of patients and is particularly morbid for those afflicted. Future experiments will directly test the efficacy of oral supplementation in a model of disease recurrence. In summary, this report represents the first strong evidence that we are aware of that simple addition of a safe oral supplement already found in our diets may maintain gut integrity and minimize the onset and morbidity of a severely morbid iatrogenic disease that has truly become an epidemic in our hospitalized patients. Given the burden of CDAD, dietary supplementation represents an exciting new treatment paradigm that could have a major impact in healthcare in the imminent future.
Supplementary Material
Acknowledgments
We would like to thank Megan Duster for her assistance preparing the C. difficile inoculum.
References
- 1.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313(4):398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547–53. doi: 10.1053/j.gastro.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surawicz CM. Clostridium difficile infection: risk factors, diagnosis and management. Curr Treat Options Gastroenterol. 2015;13(1):121–9. doi: 10.1007/s11938-014-0038-3. [DOI] [PubMed] [Google Scholar]
- 4.Regnault H, Bourrier A, Lalande V, et al. Prevalence and risk factors of Clostridium difficile infection in patients hospitalized for flare of inflammatory bowel disease: a retrospective assessment. Dig Liver Dis. 2014;46(12):1086–92. doi: 10.1016/j.dld.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Biswal S. Proton pump inhibitors and risk for Clostridium difficile associated diarrhea. Biomed J. 2014;37(4):178–83. doi: 10.4103/2319-4170.128002. [DOI] [PubMed] [Google Scholar]
- 6.Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–70. doi: 10.1056/NEJMc1505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao K, Safdar N. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. J Hosp Med. 2015 doi: 10.1002/jhm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–8. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 9.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249–57. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 10.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 11.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiger TL, Abt MC, Gasteiger G, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211(9):1723–31. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa M, Yada S, Liu MZ, et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41(4):620–32. doi: 10.1016/j.immuni.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334(6062):1561–5. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Basu R, O’Quinn DB, Silberger DJ, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37(6):1061–75. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlfors H, Morrison PJ, Duarte JH, et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol. 2014;193(9):4602–13. doi: 10.4049/jimmunol.1401244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin B, Hirota K, Cua DJ, et al. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–92. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt JV, Su GH, Reddy JK, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geem D, Medina-Contreras O, Kim W, et al. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp. 2012;(63):e4040. doi: 10.3791/4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Zaatari M, Chang YM, Zhang M, et al. Tryptophan catabolism restricts IFN-gamma-expressing neutrophils and Clostridium difficile immunopathology. J Immunol. 2014;193(2):807–16. doi: 10.4049/jimmunol.1302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Chen C, Satoh J, et al. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117(7):1940–50. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjeldanes LF, Kim JY, Grose KR, et al. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88(21):9543–7. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa M, Kamada N, Jiao Y, et al. Protective role of commensals against Clostridium difficile infection via an IL-1beta-mediated positive-feedback loop. J Immunol. 2012;189(6):3085–91. doi: 10.4049/jimmunol.1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik R, Nixon S, Lopes A, et al. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2006;16(2):786–90. doi: 10.1111/j.1525-1438.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 29.Bradlow HL, Michnovicz JJ, Halper M, et al. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev. 1994;3(7):591–5. [PubMed] [Google Scholar]
- 30.Minich DM, Bland JS. A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr Rev. 2007;65(6 Pt 1):259–67. doi: 10.1301/nr.2007.jun.259-267. [DOI] [PubMed] [Google Scholar]
- 31.Peter Guengerich F, Martin MV, McCormick WA, et al. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch Biochem Biophys. 2004;423(2):309–16. doi: 10.1016/j.abb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci. 2011;124(2):327–38. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahvis GP, Lindell SL, Thomas RS, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A. 2000;97(19):10442–7. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 36.Alam SN, Yammine H, Moaven O, et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg. 2014;259(4):715–22. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. 2014;260(1):129–44. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bliss DZ, Johnson S, Savik K, et al. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129(12):1012–9. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


