Abstract
Kava is one of the most widely sold herbal dietary supplements in the United States. It has been reported that, besides exhibiting hepatotoxicity, kava also possesses photosensitivity and induces dermopathy in humans. In this study, we determined that UVA irradiation of kava in the presence of a lipid, methyl linoleate, generated lipid peroxidation which was mediated by singlet oxygen generated during photoirradiation. The six major kavalactones (yangonin, 7,8-dihydrokawa in, kawain, 7,8-dihydromethysticin, methysticin, and 5,6-dehydrokawain) were also studied in parallel; only 5,6-dehydrokawain and yangonin-induced a low level of lipid peroxidation. UVA irradiation of kava in human HaCaT skin keratinocytes induced cytotoxicity which was mediated by oxidative stress, led to DNA strand cleavage, and produced 8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct. Study by the electron spin resonance (ESR) method revealed that UVA irradiation of kava produced singlet oxygen and carbon-centered radicals. The overall results suggest that kava is photocytotoxic and photogenotoxic, both mediated by free radicals generated during photoirradiation.
Keywords: Kava Extract, Photosensitivity, UVA Light, Reactive Oxygen Species, Lipid Peroxidation, HaCaT Keratinocytes, 8-OHdG
Introduction
Since the United States Congress passed the Dietary Supplement Health and Education Act (DSHEA) in 1994, the consumer use of herbal products has been rapidly growing in the United States. In 2004 the American Herbal Products Association estimated that there were about 3000 species of plants, in as many as 50,000 different products, sold as herbal supplements in the United States (Zurer and Hanson, 2004). However, to date, safety concerns about the hepatotoxic, genotoxic and tumorigenic ingredients in many raw herbal plants and herbal products, including herbal dietary supplements, still remained (Fu et al., 2009). To ensure consumer health protection, the quality and safety of dietary supplement preparations have to be determined (Yamaguchi et al., 2011).
Kava, prepared from the rhizome of the kava tropical shrub plant Piper methysticum Forst F., has been consumed as a traditional beverage in the South Pacific. During recent years, kava has been used in the United States as a natural alternative to anti-anxiety drugs and sleeping pills. In Europe it has been used for treatment of anxiety and nervous disorders such as stress and restlessness (Fu et al., 2008; Johnson et al., 2011; NTP, 2011). Through this use, millions of consumers using anti-anxiety preparations are potentially exposed to kava (NTP, 2011). Meanwhile, the association between the use of kava products and cause of hepatic injury in humans has also been reported, which has prompted many countries, including Germany, Switzerland, France, Canada, and the United Kingdom, to take regulatory actions. While the kava-containing products remain popular in the United States and continue to be sold in health food stores and ethnic markets, the US National Toxicology Program (NTP) has completed a two-year chronic tumorigenicity assay and determined that kava extract induced liver cancer in mice (NTP, 2011).
In addition, it has also been reported that kava also possesses photosensitivity (Wooltorton, 2002) and induces dermopathy (Ruze, 1990; Norton and Ruze, 1994; Norton, 1998). To date, the mechanism of kava-induced skin damage is not yet clear. In this paper, we will study kava-induced phototoxicity under UVA light. The substituted 4-methoxy-5,6-dihydro-alpha-pyrones or kava pyrones, commonly called kavalactones, are kava chemical constituents that possess the highest purported pharmacological activities of kava (Fu et al., 2008). In this study, the phototoxicities of kava extract and its six major kavalactones (i.e. yangonin, 7,8-dihydrokawain, kawain, 7,8-dihydromethysticin, methysticin, and 5,6-dehydrokawain) were determined. We found that UVA irradiation of kava extract induced singlet oxygen and carbon-centered free radicals, which mediated lipid peroxidation, caused DNA strand cleavage, and generated 8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct in human HaCaT skin keratinocytes. We propose that this might be the underlying mechanism(s) by which kava induces dermopathy and skin cancer.
Materials and Methods
Chemicals
Methyl linoleate, sodium azide (NaN3), superoxide dismutase (SOD), diethylene-triaminepentaacetic acid (DTPA), and 2,2,6,6-tetramethyl-piperidine (TEMP), α-(4-pyridyl-1-oxide)-N-tert-butylnitrone (POBN), 2′,7′-dichlorofluorescin diacetate (DCFDA), dimethyl sulphoxide (DMSO), ammonium iron (II) sulfate hexahydrate, and hydrogen peroxide were purchased from Sigma-Aldrich (St. Louis, MO). The nitrone spin trap, 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO), was purchased from Applied Bioanalytical Labs (Sarasota, FL). Dulbecco’s modified Eagle’s medium (DMEM), phosphate buffered saline (PBS, pH 7.4), fetal bovine serum (FBS), trypsin, penicillin, and streptomycin were purchased from Invitrogen Inc. (Grand Island, NY). MTS assay kit (CellTiter 96® Aqueous) was purchased from Promega Co. (Madison, WI). Supercoiled ΦX174 phage DNA (about 90% supercoiled form, 27 μM in base pairs) was purchased from Fermentas Life Science (Glen Burnie, MD). The six kavalactones, yangonin, 7,8-dihydrokawain, kawain, 7,8-dihydromethysticin, methysticin, and 5,6-dehydrokawain, were purchased from LKT Laboratories, Inc. (St. Paul, MN). All other reagents were obtained through commercial sources. All solvents were HPLC grade.
The same kava extract used for the NTP 14-week toxicity bioassays and for the NTP 2-year chronic tumorigenicity bioassays (NTP, 2011) was used for this study. This kava extract (Lot No. 082203) was prepared from the rhizome of the kava tropical shrub plant, Piper methysticum Forst F. by extraction with methanol. The previously reported high-performance liquid chromatography with ultraviolet detection (HPLC/UV) analysis showed that the kava extract contained 30% kavalactones, of which 96% were a mixture of yangonin, demethoxyyangonin, methysticin, 7,8-dihydromethysticin, kawain, and 7,8-dihydrokawain. Liquid chromatography/mass spectrometry and gas chromatography/mass spectrometry identified and quantified the six major components as yangonin (3.62%), 7,8-dihydrokawain (6.90%), kawain (6.55%), 7,8-dihydromethysticin (3.05%), methysticin (3.11%), and 5,6-dehydrokawain (2.15%) (NTP, 2011).
Light Sources
The UVA light box was custom made using 4 UVA lamps (National Biologics, Twinsburg, OH) (Xia et al., 2006). The irradiance of the light box was determined using an Optronics OL754 Spectroradiometer (Optronics Laboratories, Orlando, FL), and the light dose was routinely measured using a Solar Light PMA-2110 UVA detector (Solar Light Inc., Philadelphia, PA). The maximum emission of the UVA light box was determined to be between 340 and 355 nm. The light intensities at wavelengths below 320 nm (UVB light) and above 400 nm (visible light) are approximately two orders of magnitude lower than the maximum in the 340–355 nm spectral regions.
In this study, the UVA-irradiation dose was from 14–70 J/cm2, approximately 46 to 230 min exposure at the dose rate of 5 mW/cm2. Ten J/cm2 of UVA equates to about 2 h of exposure at the noon time of sunny days during the summer around world. This estimation is based upon observations of UVA intensity of 3.6 mW/cm2 in Jackson, MS, USA in August (Yu et al., 2001), 5.4 mW/cm2 in Paris, France in July (Jeanmougin and Civatte, 1987), and 6.6 mW/cm2 in Coimbatore, India in July (Balasaraswathy et al., 2002).
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of Kava Extract or Kavalactones
Experiments were conducted using a solution of: (i) 100 mM methyl linoleate; (ii) 100 mM methyl linoleate and 1 or 2 mg/ml kava extract in water; and (iii) 100 mM methyl linoleate and 100 μM kavalactone (7,8-dihydrokawain, kawain, methysticin, 7,8-dihydromethysticin, 5,6-dehydrokawain, or yangonin) in methanol. Samples were placed in a UV-transparent cuvette and irradiated with 0, 14, 35 or 70 J/cm2 of UVA light. After irradiation, the levels of lipid peroxidation were expressed as the amount of methyl linoleate hydroperoxides determined by HPLC peak area by monitoring the elution at 235 nm (Tokita and Morita, 2000). Methyl linoleate hydroperoxides and the recovered substrate (kava or kava lactones) were separated by HPLC using a Prodigy 5 μ ODS column (4.6 × 250 mm, Phenomenex, Torrance, CA) eluted isocratically with 10% water in methanol (v/v) at 1 ml/min, and conducted on an Alliance HPLC system consisting of Waters 2695 Separations Module and a Waters 2996 photodiode array detector.
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of Kava Extract in the Presence of a Free Radical Scavenger or Enhancer
Experiments were carried out as described above in the presence of NaN3. The concentration of NaN3 was 20 mM. It has been established that the lifetime of singlet oxygen is longer in a deuterated solvent such as deuterated water (D2O) or deuterated methanol (CH3OD), than in a protic solvent (Ogilby and Foote, 1983). The effect on the levels of lipid peroxide formation induced from UVA photoirradiation of kava extract and kavalactones with CH3OH and CH3OD was conducted similarly.
Detection of Free Radicals Formed Following Photoexcitation of Kava Extract Sample
Electron spin resonance spectroscopy (ESR) with spin trapping was used to detect singlet oxygen, superoxide, and carbon-centered radicals (C·) formed during UVA photoexcitation of the kava extract sample. The specific ESR spin trapping agent of singlet oxygen, TEMP (Rinalducci et al., 2004), was employed to investigate whether the UVA irradiation of kava extract generates singlet oxygen species. ESR instrument settings were as follows: 20 mW microwave power, 100 G sweep width, 1 G field modulation amplitude, and 100 kHz modulation frequency.
Detection of superoxide radicals was conducted with 1 mg/ml kava extract in distilled water with 50 mM spin trap BMPO concomitantly photoirradiated with UVA light at wavelength 320 nm for 0.5, 3, and 5 min.
For detection of carbon-centered radicals (C·), a sample containing 0.36 g/ml kava extract and 50 mM POBN in 70% ethanol in water or distilled water was transferred to a quartz capillary tube placed into the microwave cavity of a Bruker EMX ESR Spectrometer (Billerica, MA). The sample was irradiated at 275 nm and 350 nm for 2, 10 and 20 min, respectively in the microwave cavity using light emitted from a 500 watt Xe arc lamp directed through a McPherson monochromator, model DM200 (Chelmsford, MA). ESR spectra were collected during irradiation times at 2 min through nine scans.
Similar experiments were conducted for the samples containing 25 mM BMPO and 0.36 g/ml kava extract in distilled water irradiated at 340 nm for 2 min. The ESR spectrum of Fenton reaction without photoexcitation was recorded at 2 min through one scan. All ESR measurements were carried out at ambient temperature (25°C) using the following settings for detection of the spin adduct between BMPO and carbon-centered free radicals (BMPO/C·): 20 mW microwave power, 100 G scan range and 1 G field modulation.
All experiments were performed in duplicate. The data were obtained with error of less than 10%.
The computer simulation was performed using WINSIM, a computer program developed in NIEHS/NIH (Duling, 1994).
Cell Culture
The human HaCaT skin keratinocytes were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and were grown in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and glutamine in a humidified atmosphere at 37°C and 5% CO2.
MTS Assay for Cytotoxicity and Phototoxicity
The cell viability was assayed by the CellTiter 96® Aqueous kit according to the manufacture’s manual. Cells with 104/well were seeded in 96-well plate for 24 h. The cells were treated with kava extract (2 mg/ml) or the six kavalactones (100 μM) for 1 h, and then exposed to UVA at the light dose 0, 2 or 4 J/cm2. After UVA irradiation, the medium was replaced with DMEM containing 2% FBS and incubated for 24 h. Twenty μl of MTS were added to each well. After incubation at 37°C for 3 h, the absorbance was determined at wavelength 490 nm by a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT).
DCF Assay for Intracellular ROS Determination
Detection of ROS was performed with 2′,7′-dichlorofluorescin diacetate (DCFDA) as a probe and followed the procedure described previously (Venditti et al., 2011; Wen et al., 2011). The HaCaT cells were seeded in 24-well plate at a density of 105 cells/well. After 24 h incubation, the cells were treated with various concentrations of kava extract (0.5–10 mg/ml) or kavalactones (5,6-dehydrokawain or yangonin) at 37°C for 1 h, and irradiated with UVA at 2 J/cm2. Then the medium was replaced with DMEM containing 10 μM DCFDA and incubated at 37°C for 30 min. The fluorescence (emission 488 nm, excitation 520 nm) was measured using a Synergy 2 Multi-Mode Microplate Reader.
DNA Single Strand Cleavage
UVA light-induced DNA single strand cleavage by kava extract was carried out following the previously published procedure with modification (Dong et al., 2000). Supercoiled ΦX174 phage DNA (27 μM in base pairs) mixed with kava extract (13, 26, 40, 53 and 66 mg/ml) to make 60 μl of DNA solution and placed in a 96-well assay plate. The plate was exposed to UVA at the light dose of 4 J/cm2 (3.4 mW/cm2 for 20 min). After irradiation, each sample was mixed with 10 μl of a dye solution (bromophenol blue and xylene cyanole in 50% glycerol) and 12 μl of the mixture was loaded onto 1% agarose gel for electrophoresis at 70 V for 50 min. Two forms of DNA were formed and separated by electrophoresis.
Immunofluorescene Assay for Detection of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
Anti-8-OHdG monoclonal antibody (Trevigen) was used following the manufacturer’s instruction with modification. Briefly, cells seeded on a glass coverslip were treated with 10 mg/ml kava at 37°C for 1 h and exposed to UVA light 2 J/cm2 (7.8 mW/cm2, 4 min 20 s). The cells were fixed with 4% paraformaldehyde in PBS at 4°C overnight. After washing (with PBS) the cells were incubated with RNAse (100 μg/ml in 150 mM NaCl and 15 mM sodium citrate) for 1 h at 37°C. Non-specific binding was blocked with 5% BSA for 1 h at room temp. The cells were stained with anti-8-OHdG monoclonal antibody (1:250 dilution) at 4°C overnight, and FITC-conjugated goat anti-mouse IgG secondary antibody (Alexa Fluor 488; Molecular Probes) for 2 h in the dark at room temp, respectively. The cells were counter stained with DAPI, and the staining was viewed with an Olympus Confocal Microscope.
Statistical Analysis
Data are presented as mean ± S.D. Student’s t-test was used to determine the significance of difference in photoinduced hydroperoxidation between groups. The difference was considered statistically significant when the p value was less than 0.05.
Results
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of Kava and Six Kavalactones
Photoirradiation of 100 mM methyl linoleate alone and a mixture of 100 mM methyl linoleate and 1 or 2 mg/ml kava extract in water with 0, 14, 35, and 70 J/cm2 of UVA light was conducted in parallel. Comparison of the results indicated that photoirradiation of kava extract generated lipid peroxidation in a light dose-dependent manner, and at 35 and 70 J/cm2 of UVA light, was significantly higher than the control (p < 0.05) (Table 1) (Fig. 1).
Table 1.
Formation of UVA Light Induced Methyl Linoleate Lipid Peroxides by Kava Extract and Kava Lactones
| UVA (J/cm2) |
Methyl Linoleate |
Kava Extract 1 mg/ml |
Kava Extract 2 mg/ml |
7,8-Dihydro-Kawain 100 μM |
Kawain 100 μM |
Methysticin 100 μM |
7,8-Dihydro- methysticin 100 μM |
5,6-Dihydro-Kawain 100 μM |
Yangonin 100 μM |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 202 ± 21 | 200 ± 9 | 209 ± 18 | 199 ± 16 | 243 ± 21 | 231 ± 25 | 207 ± 25 | 203 ± 22 | 197 ± 24 |
| 14 | 385 ± 40 | 350 ± 26 | 426 ± 35 | 286 ± 16 | 300 ± 13 | 310 ± 11 | 305 ± 32 | 475 ± 57 | 483 ± 72 |
| 35 | 690 ± 62 | 953* ± 89 | 1056* ± 98 | 472 ± 50 | 524 ± 60 | 400 ± 47 | 542 ± 70 | 803 ± 96 | 673 ± 68 |
| 70 | 869 ± 69 | 1496* ± 117 | 3112* ± 325 | 712 ± 69 | 825 ± 58 | 689 ± 48 | 720 ± 55 | 1144* ± 132 | 1202* ± 149 |
Note: Data are expressed as means ± S.D. n = 3. Means with star are significantly higher than control values, p ≤ 0.05.
Figure 1.
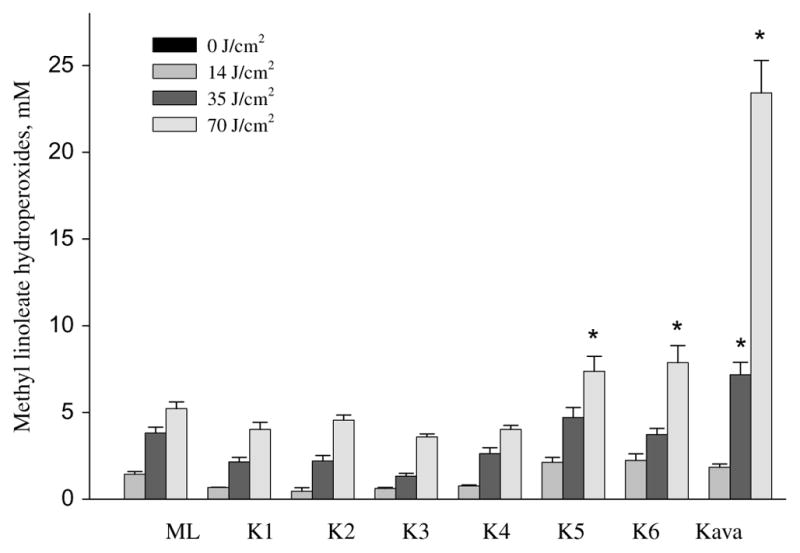
Formation of UVA light induced methyl linoleate hydroperoxides by kava extract and six kava lactones (K1,7,8-dihydrokawain; K2, kawain; K3, methysticin; K4, 7,8-dihydromethysticin; K5, 5,6-dehydrokawain; K6, yangonin). Data are expressed as means ± S.D. n = 3. Means with star are significantly higher than control values, p ≤ 0.05.
UVA photoirradiation of the six kavalactones (yangonin, 7,8-dihydrokawain, kawain, 7,8-dihydromethysticin, methysticin, and 5,6-dehydrokawain), each in 100 μM in methanol with 100 mM methyl linoleate, was conducted under similar experimental conditions (Table 1). Only at the highest UVA light dose (70 J/cm2), both 5,6-dehydrokawain and yangonin induced a low level of lipid peroxidation but significantly higher than the control (methyl linoleate) (p < 0.05) (Table 1); however, the other four kavalactones did not induce lipid peroxidation (Table 1 and Fig. 1).
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of Kava in the Presence of a Free Radical Scavenger or CH3OD
The involvement of free radical intermediates in the photoinduced peroxidation of methyl linoleate in the presence of kava extract was examined. NaN3 is a common free radical scavenger especially for singlet oxygen (1O2) (Basumodak and Tyrrell, 1993) and hydroxyl radical (•OH) (Sortino et al., 1998). Singlet oxygen has a longer half-life in deuterium solvents (Ogilby and Foote, 1983). Thus, methanol was replaced by CH3OD to determine whether or not lipid peroxidation would be enhanced.
The results shown in Fig. 2 clearly indicated that peroxidation of methyl linoleate by kava extract was significantly (p ≤ 0.05) inhibited by NaN3 as compared to the photoirradiation of kava extract. On the other hand, replacement of CH3OH by CH3OD significantly (p ≤ 0.05) enhanced peroxidation (Fig. 2) at a light dose of 70 J/cm2. The combined results suggested that singlet oxygen was involved in lipid peroxidation by UVA photoirradiation of kava extract.
Figure 2.
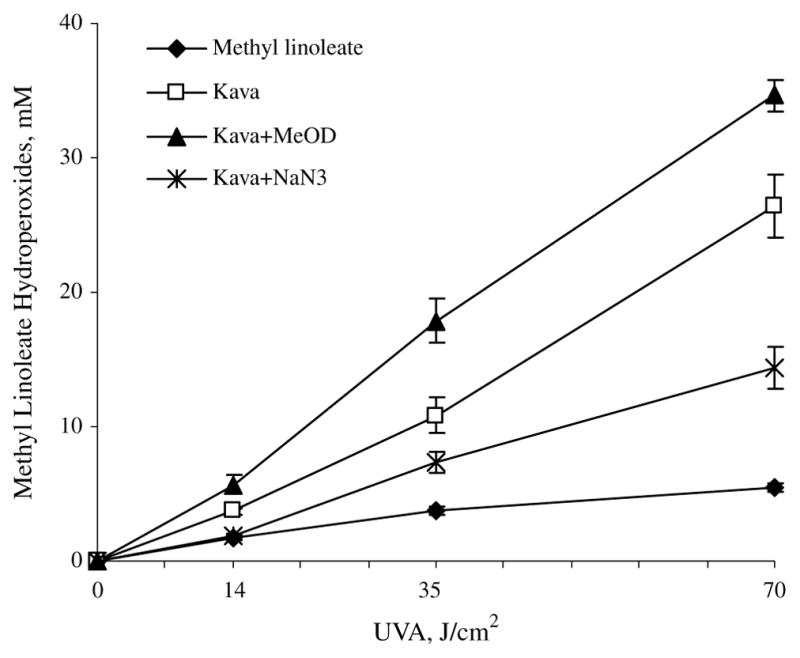
Effects of NaN3 and CH3OD on peroxidation of methyl linoleate (ML) initiated by 10 mg/ml kava extract in CH3OH under UVA irradiation. The levels of peroxidation were measured by HPLC analysis monitored at 235 nm. Data are expressed as means ± S.D. n = 3.
UVA photoirradiation of kava extract in the presence of the superoxide inhibitor, superoxide dismutase (SOD), was conducted under similar experimental conditions. No inhibition of the formation of lipid peroxidation was observed (data not shown).
The Measurement of Kava Extract and Kavalactones Induced Cytotoxicity in Human Skin HaCaT Keratinocytes with and without UVA Light Irradiation
The induction of cytotoxicity by kava extract and the six kavalactones in human skin HaCaT keratinocytes with and without UVA light irradiation was studied. After 24 h exposure to kava extract dissolved in serum free DMEM, cell viabilities were examined by the MTS assay. Without light irradiation, kava extract did not induce cytotoxicity. Upon UVA irradiation, as compared to the control (methyl linoleate alone), kava induced cytotoxicity and at light dose-dependent manner group (p < 0.05) (Table 2).
Table 2.
The Cytotoxicity of Kava Extract and Kava Lactones with/without UVA Irradiationa
| UVA | 0 J/cm2 | Survival (%) | 2 J/cm2 | Survival (%) | 4 J/cm2 | Survival (%) | |
|---|---|---|---|---|---|---|---|
| Control medium | 1.42 ± 0.054 | 100 | 1.17 ± 0.054 | 100 | 1.11 ± 0.036 | 100 | |
| Kava extract | 1 mg/ml | 1.38 ± 0.102 | 97 | 1.17 ± 0.086 | 100 | 0.83 ± 0.085 | 75* |
| Kava extract | 2 mg/ml | 1.38 ± 0.056 | 98 | 0.67 ± 0.126 | 57* | 0.27 ± 0.016 | 25* |
| 7,8-Dihydrokawain | 100 μM | 1.43 ± 0.041 | 101 | 1.23 ± 0.076 | 105 | 1.17 ± 0.049 | 105 |
| Kawain | 100 μM | 1.41 ± 0.077 | 99 | 1.28 ± 0.090 | 110 | 1.23 ± 0.164 | 111 |
| Methysticin | 100 μM | 1.40 ± 0.086 | 99 | 1.30 ± 0.118 | 111 | 1.15 ± 0.091 | 104 |
| 7,8-Dihydromethysticin | 100 μM | 1.37 ± 0.048 | 96 | 1.27 ± 0.221 | 109 | 1.06 ± 0.094 | 96 |
| 5,6-Dehydrokawain | 100 μM | 1.35 ± 0.076 | 95 | 1.31 ± 0.181 | 112 | 1.08 ± 0.124 | 98 |
| Yangonin | 100 μM | 1.39 ± 0.090 | 98 | 1.24 ± 0.049 | 106 | 1.02 ± 0.152 | 93 |
| 7,8-Dihydrokawain | 200 μM | 1.46 ± 0.108 | 103 | 1.17 ± 0.106 | 100 | 1.10 ± 0.120 | 99 |
| Kawain | 200 μM | 1.41 ± 0.972 | 99 | 1.19 ± 0.089 | 102 | 1.14 ± 0.087 | 103 |
| Methysticin | 200 μM | 1.43 ± 0.110 | 101 | 1.16 ± 0.111 | 99 | 1.11 ± 0.095 | 100 |
| 7,8-Dihydromethysticin | 200 μM | 1.49 ± 0.075 | 105 | 1.18 ± 0.085 | 101 | 1.12 ± 0.063 | 101 |
| 5,6-Dehydrokawain | 200 μM | 1.38 ± 0.106 | 97 | 1.16 ± 0.181 | 99 | 1.08 ± 0.91 | 97 |
| Yangonin | 200 μM | 1.45 ± 0.123 | 102 | 1.15 ± 0.084 | 98 | 1.07 ± 0.102 | 96 |
| Berberineb | 10 μM | 1.67 ± 0.079 | 118 | 0.73 ± 0.097 | 62 | 0.34 ± 0.042 | 30 |
Note:
Data are expressed as treatment means ± SD, n = 3. Means with star are significantly lower than control values, p ≤ 0.05.
Berberine serves as a positive control.
All the six kavalactones did not exhibit cytotoxicity when tested either with or without light irradiation (Table 2).
DCF Assay for Intracellular Reactive Oxygen Species (ROS) Determination
The formation of ROS from incubation of kava extract (0.5–5 mg/ml) concomitantly exposed to UVA at 2 J/cm2 was examined with 2′,7′-dichlorofluorescin diacetate (DCFDA) as a probe in HaCaT cells. The results shown in Fig. 3 that kava extract induced a positive DCF response in a dose response manner. These results suggest that ROS was generated by kava extract concomitantly exposed to UVA light.
Figure 3.
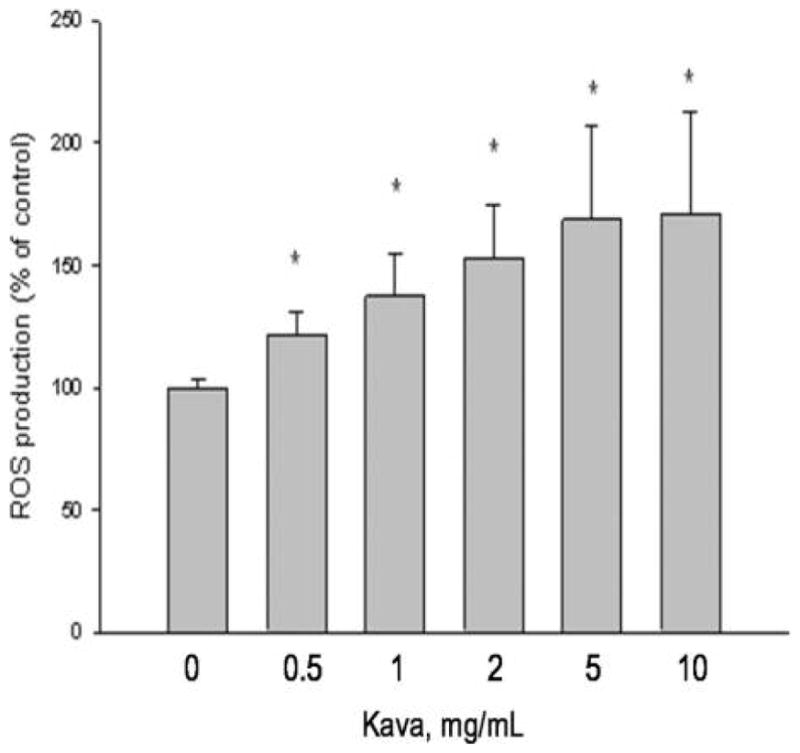
DCF assay of kava extract induced cellular ROS in human skin keratinocytes under UVA irradiation. Data are expressed as means ± SD. Means with star are significantly higher than control values, p ≤ 0.05.
DNA Single Strand Cleavage
UVA light-induced DNA single strand cleavage by kava extract was carried out with supercoiled ΦX174 phage DNA. As indicated in Fig. 4, kava extract mixed with DNA without UVA irradiation did not induce DNA damage. While irradiated with UVA at 4.3 J/cm2, kava induced ΦX-174 supercoiled plasmid DNA damage in a concentration (substrate) dependent manner.
Figure 4.
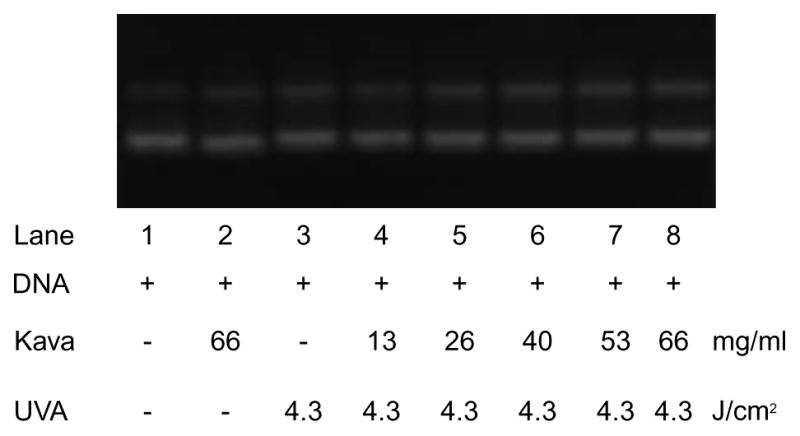
Concentration dependent of UVA irradiation (4.3 J/cm2) induced ΦX-174 supercoiled plasmid DNA damage by kava. Lanes 1–3 were controls: lane1, DNA only without UVA irradiation; lane 2, DNA and 66 mg/ml kava extract without UVA irradiation; and lane 3, only DNA only with UVA irradiation. Lanes 4–8 were UVA irradiated in the present of increasing concentrations of extract.
Immunohistochemistry Assay of 8-Hydroxy-2′-Deoxyguanosine Adducts (8-OHdG)
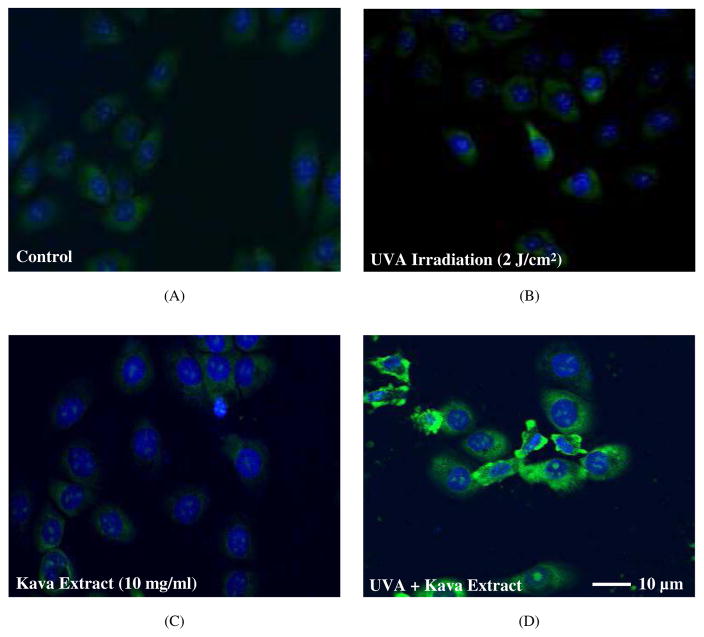
The immunohistochemistry staining assay of 8-OHdG was conducted in HaCaT cells. As shown in Figs. 5A and 5B, neither control group nor UVA irradiation (2 J/cm2) alone induced 8-OHdG. Kava extract (10 mg/ml) mixed with DNA induced marginal level of 8-OHdG adduct (Fig. 5C). Remarkable increase in expression levels of 8-OHdG immunostaining was observed in the cells exposed to kava extract (10 mg/ml) plus UVA (2 J/cm2) irradiation (Fig. 5D) compared with control (Fig. 5A), and UVA irradiation alone (Fig. 5B) or kava extract alone (Fig. 5C).
Figure 5.
Immunohistochemical assay of 8-OHdG in HaCaT cells. 8-OHdG was examined by immunofluorescence staining and nuclei were counterstained with DAPI. (A) control, (B) UVA irradiation (2 J/cm2), (C) kava (10 mg/ml) and (D) kava extract (10 mg/ml) under UVA irradiation (2 J/cm2). Scale bar = 10 μm.
Electron Spin Resonance (ESR) Spectral Measurements-Detection of Singlet Oxygen, Superoxide, and Carbon-Centered Free Radicals
Detection of singlet oxygen formation
The specific ESR spin trapping agent of singlet oxygen, TEMP (Lion et al., 1976), was employed to investigate whether the UVA irradiation of kava extract generates singlet oxygen species. In the absence of kava extract, photoirradiation of TEMP with UVA light did not result in an ESR signal (data not shown). The photoirradiation of kava extract (1 mg/ml) and TEMP (40 mM) in water without UVA light irradiation at 350 nm for up to 30 sec did not produce an ESR signal (data not shown). With concomitant exposure to UVA light for 20 sec, singlet oxygen was generated, as evidenced by an ESR spectral profile which is typical of TEMPO (Fig. 6) (Rinalducci et al., 2004). The intensity of these ESR signals progressively enhanced when photoirradiation time increased from 0 to 20, 70, 120, 180, 240, and 300 sec, respectively. These results provide direct evidence that UVA irradiation of kava extract generates singlet oxygen and that the quantity of singlet oxygen produced is dependent on the light dose.
Figure 6.
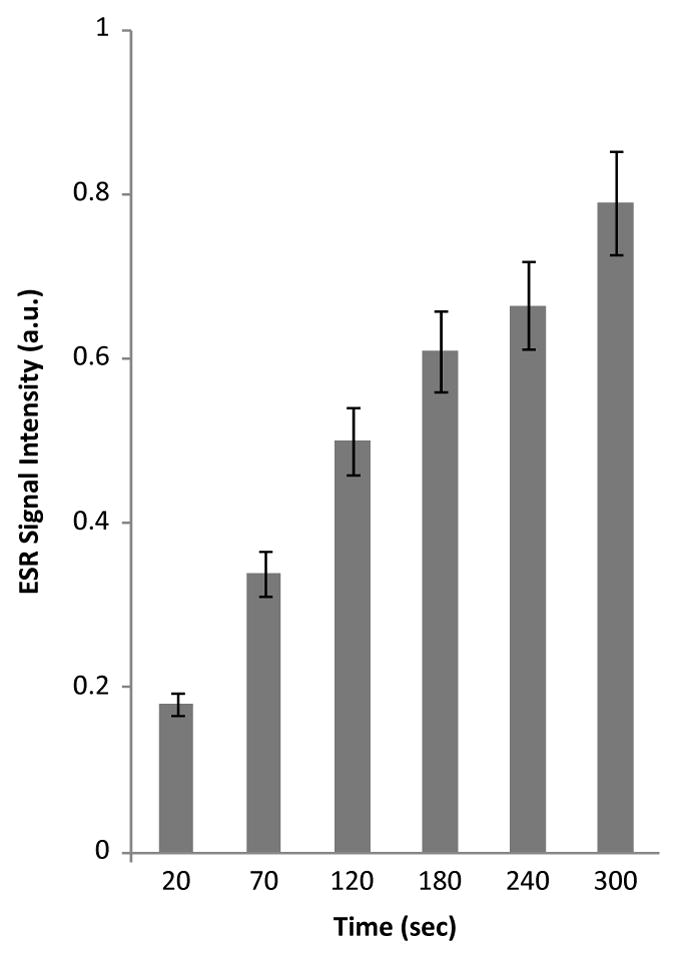
Time-dependent changes in the steady-state ESR spectra of TEMPO formed from reaction of 1 mg/ml kava extract and 40 mM TEMP in distilled water. Samples were irradiated at 350 nm for 0, 20, 70, 120, 180, 240, and 300 min, respectively. ESR instrument settings were as follows: 20 mW microwave power, 100 G sweep width, 1 G field modulation amplitude, and 100 kHz modulation frequency.
Detection of superoxide formation
Photoirradiation of BMPO alone with UVA light did not generate ESR signals (data not shown). Similarly, no ESR signal was observed when kava extract was mixed with BMPO. Photoirradiation of kava extract with UVA light in the presence BMPO for 5 min also did not produce ESR spectral signals (data not shown). These results indicated that superoxide radical anion was not generated from photoirradiation of kava extract with UVA light.
Detection of carbon-centered free radical formation
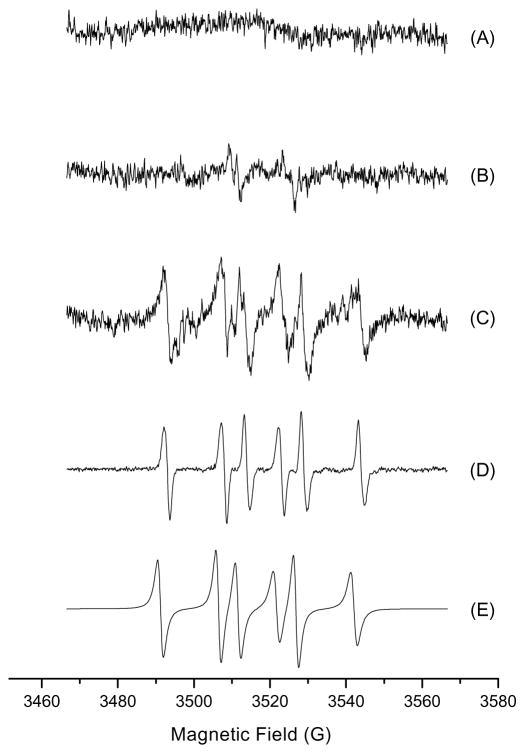
The ESR spin trapping agent POBN was first used for detection of carbon-centered radicals. A sample containing 0.36 g/ml kava extract and 50 mM POBN in 70% ethanol in water was irradiated at 275 nm and 350 nm for 2, 10, and 20 min, respectively. However, no ESR signals were detected (data not shown). When experiments were conducted with the sample dissolved in distilled water. Again, no ESR signals were detected (data not shown). The ESR spin trapping agent BMPO was then used for this study. No signal was found when kava extract mixed with BMPO without photoirradiation (Fig. 7A). Two extremely weak lines were generated from photoirradiation without kava extract (Fig. 7B). Photoirradiation of kava extract at 340 nm in 2 min generated an ESR pattern of six strong signals, which is typical of carbon-centered free radicals (Fig. 7C). For confirmation, Fenton reaction in the presence of ethanol was conducted, from which the generated hydroxyl radical rapidly reacted with ethanol to produce carbon-centered free radicals. The resulting ESR profile of the carbon-centered free radicals is identical to the previous reported data (Fig. 7D) (Reinke et al., 1994; Zhu et al., 2002; Cheng et al., 2007) and is also identical to that shown in Fig. 7C. Furthermore, a simulated carbon-centered ESR profile (Fig. 7E) was generated and the ESR profile is identical to that formed from photoirradiation of kava extract (Fig. 7C). Therefore, these overall results clearly confirm that UVA photoirradiation of kava extract generated carbon-centered free radicals.
Figure 7.
Kava generated carbon-centered free radicals. (A) 0.36 g/ml kava extract without photoexcitation; (B) control did not contain any sample; (C) 0.36 g/ml kava; (D) Fenton reaction performed in PBS buffer (20 mM, pH 7.4) containing 0.25 mM Fe2+, 0.25 mM H2 O2, and 20% (v/v) ethanol without photoexcitation; (E) simulation of (C) using the following parameters: BMPO/C• (aN = 15.2 G, aH = 20.4 G). Samples were photoexcitated at 340 nm, and ESR signal recorded after 2 min exposure. ESR instrument settings were as follows: 20 mW microwave power, 100 G sweep width, 1 G field modulation amplitude, and 100 kHz modulation frequency.
Discussion
In the present study, we determined that under our experimental conditions, the UVA photoirradiation of kava extract in the presence of a lipid, methyl linoleate, induced lipid peroxidation leading to the formation of methyl linoleate hydroperoxides. Among the six kavalactones tested under similar conditions, only 5,6-dehydrokawain and yangonin induced a low level of lipid peroxidation (significantly higher than the control methyl linoleate). The levels of lipid peroxidation (hydroperoxides) formed by the UVA irradiation of kava extract were higher than those formed by the UVA irradiation of any of the kavalactones, which suggests that the kava extract-induced lipid peroxidation is not attributed to its kavalactone components. At this time we don’t know which constituents in the kava extract exhibit phototoxicity.
We subsequently examined the role of free radicals in the generation of lipid hydroperoxides from the photoirradiation of kava extract. In the present study, we showed that the formation of lipid peroxides by photosensitized kava extract was inhibited by NaN3 and enhanced in the presence of CH3OD. These results suggest that singlet oxygen may be involved in the generation of lipid peroxides by photosensitized kava extract. Then the ESR spin trap method was employed to further confirm the free-radical mechanism so as to explain the enhanced production of lipid peroxides from the UVA irradiation of kava extract. When TEMP, the specific ESR spin trapping agent for singlet oxygen, was employed, a very strong signal was generated. The ESR study using BMPO and the comparison of the resulting ESR profile with the carbon-centered free radicals generated by Fenton reaction in the presence of methanol confirmed that carbon-centered free radicals were also generated. When considered together, these results suggest a free radical mechanism for the formation of lipid peroxides generated by the UVA irradiation of kava extract.
The studies of kava extract and its kavalactones in human skin keratinocytes revealed that kava extract, 5,6-dehydrokawain, and yangonin are cytotoxic. The DCF assay confirmed that ROS were generated from concomitant exposure to kava extract and UVA light. UVA irradiation of kava extract in human skin keratinocytes also led to DNA strand cleavage and generation of 8-OHdG.
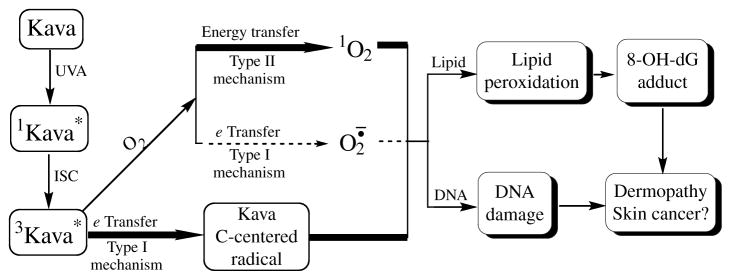
As seen from the proposed mechanism diagrammed in Fig. 8, the irradiation of kava extract with UVA light results in the formation of excited (sensitized) kava extract initially at the singlet state which turns to triplet state via an inter system crossing (ISC). The photoexcited kava extract serves as the initiating species to transfer energy to molecular oxygen and generate singlet oxygen (1O2) by a type II mechanism or to transfer an electron to molecular oxygen to generate the carbon-centered radicals by a type I mechanism (Foote, 1991; Kagan, 1993). In the presence of a lipid, the generated ROS (singlet oxygen) and carbon-centered radicals can initiate lipid peroxidation (Xia et al., 2007), which upon lipid degradation, can generate endogenous DNA adducts, including 8-OHdG.
Figure 8.
Proposed mechanism of kava extract-induced free radicals, lipid peroxidation, DNA strand cleavage, and 8-OHdG, leading to dermopathy and skin cancer.
It is well established that in addition to ROS, alkoxy radicals can initiate lipid peroxidation (Comporti, 1985; Aust et al., 1993; Harman, 1993). In our case, alkoxy radicals can be generated by reaction of the kava extract-derived carbon-centered free radicals with molecular oxygen through a free radical chain reaction. Consequently, it is apparent that the alkoxy radicals derived from the photoirradiation of kava extractcan initiate lipid peroxidation as well. It is known that ROS, carbon-centered free radicals, lipid peroxidation, and 8-OHdG are all well-known cancer causing agents (Comporti, 1985; Harman, 1993; Xia et al., 2006, 2007; Valavanidis et al., 2009). Since all these end-points are associated with aging-related diseases, including cancer, the results of this study suggest that human exposure to products that contain kava extract may enhance their sensitivity to skin dermopathy and skin cancer caused by ultraviolet A light or sunlight.
Acknowledgments
We thank Dr. Frederick A. Beland for critical review of this manuscript. We also thank a gift of kava from Dr. Po-Chuen Chan. This article is not an official guidance or policy statement of U.S. Food and Drug Administration (FDA). No official support or endorsement by the U.S. FDA is intended or should be inferred.
References
- Aust SD, Chignell CF, Bray TM, Kalyanaraman B, Mason RP. Free-radicals in toxicology. Toxicol Appl Pharm. 1993;120:168–178. doi: 10.1006/taap.1993.1100. [DOI] [PubMed] [Google Scholar]
- Balasaraswathy P, Kumar U, Srinivas CR, Nair S. UVA and UVB in sunlight, optimal utilization of UV rays in sunlight for phototherapy. Indian J Dermatol Venereol Leprol. 2002;68:198–201. [PubMed] [Google Scholar]
- Basu-Modak S, Tyrrell RM. Singlet oxygen — a primary effector in the ultraviolet-a/near-visible light induction of the human hemeoxygenase gene. Cancer Res. 1993;53:4505–4510. [PubMed] [Google Scholar]
- Cheng ZH, Zhou HP, Yin JJ, Yu LL. Electron spin resonance estimation of hydroxyl radical scavenging capacity for lipophilic antioxidants. J Agric Food Chem. 2007;55:3325–3333. doi: 10.1021/jf0634808. [DOI] [PubMed] [Google Scholar]
- Comporti M. Lipid-peroxidation and cellular-damage in toxic liver-injury. Lab Invest. 1985;53:599–623. [PubMed] [Google Scholar]
- Dong SM, Hwang HM, Shi XC, Holloway L, Yu H. UVA-induced DNA single-strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem Res Toxicol. 2000;13:585–593. doi: 10.1021/tx990199x. [DOI] [PubMed] [Google Scholar]
- Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- Foote CS. Definition of type I and type II photosensitized oxidation. J Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Guo L, Yu H, Chan P-C. Toxicity of kava. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:89–112. doi: 10.1080/10590500801907407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu PP, Chiang HM, Xia Q, Chen T, Chen BH, Yin JJ, Wen KC, Lin G, Yu H. Quality assurance and safety of herbal dietary supplements. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:91–119. doi: 10.1080/10590500902885676. [DOI] [PubMed] [Google Scholar]
- Harman D. Free-radical involvement in aging — pathophysiology and therapeutic implications. Drug Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- Jeanmougin M, Civatte J. Dosimetry of solar ultraviolet radiation. Daily and monthly changes in Paris. Ann Dermatol Venereol. 1987;114:671–676. [PubMed] [Google Scholar]
- Johnson TE, Hermanson D, Wang L, Kassie F, Upadhyaya P, O’Sullivan MG, Hecht SS, Lu J, Xing C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am J Chin Med. 2011;39:727–742. doi: 10.1142/S0192415X11009202. [DOI] [PubMed] [Google Scholar]
- Kagan J. Organic Photochemistry. Academic Press; San Diego: 1993. [Google Scholar]
- Lion Y, Delmelle M, Van De Vorst A. New method of detecting singlet oxygen production. Nature. 1976;263:442–443. doi: 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- Norton SA, Ruze P. Kava dermopathy. J Am Acad Dermatol. 1994;31:89–97. doi: 10.1016/s0190-9622(94)70142-3. [DOI] [PubMed] [Google Scholar]
- Norton SA. Herbal medicines in Hawaii from tradition to convention. Hawaii Med J. 1998;57:383–386. [PubMed] [Google Scholar]
- NTP. NTP TR 571, NIH Publication No 11-5913, National Toxicology Program. 2011. NTP technical report on toxicology and carcinogenesis studies of kava extract in F344/N rats and B6C3F1 mice. [Google Scholar]
- Ogilby PR, Foote CS. Chemistry of singlet oxygen. Effect of solvent, solvent isotopic substitution, and temperature on the lifetime of singlet molecular oxygen. J Am Chem Soc. 1983;105:3423–3430. [Google Scholar]
- Reinke LA, Rau JM, Mccay PB. Characteristics of an oxidant formed during iron(II) autoxidation. Free Radic Biol Med. 1994;16:485–492. doi: 10.1016/0891-5849(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Rinalducci S, Pedersen JZ, Zolla L. Formation of radical from signet oxygen produced during photoinhibition of isolated light-harvesting protein of photosystem II. Biochim Biophys Acta. 2004;1608:63–73. doi: 10.1016/j.bbabio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ruze P. Kava-induced dermopathy: a niacin deficiency? Lancet. 1990;335:1442–1445. doi: 10.1016/0140-6736(90)91458-m. [DOI] [PubMed] [Google Scholar]
- Sortino S, Condorelli G, De Guidi G, Giuffrida S. Molecular mechanism of photosensitization XI Membrane damage and DNA cleavage photoinduced by enoxacin. Photochem Photobiol. 1998;68:652–659. [PubMed] [Google Scholar]
- Tokita M, Morita M. Identification of new geometric isomers of methyl linoleate hydroperoxide and their chromatographic behavior. Biosci Biotechnol Biochem. 2000;64:1044–1056. doi: 10.1271/bbb.64.1044. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Venditti E, Bruge F, Astolfi P, Kochevar I, Damiani E. Nitroxides and a nitroxide-based UV filter have the potential to photoprotect UVA-irradiated human skin fibroblasts against oxidative damage. J Dermatol Sci. 2011;63:55–61. doi: 10.1016/j.jdermsci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Wen KC, Chiu HH, Fan PC, Chen CW, Wu SM, Chang JH, Chiang HM. Antioxidant activity of Ixora parviflora in a cell/cell-free system and in UV-exposed human fibroblasts. Molecules. 2011;16:5735–5752. doi: 10.3390/molecules16075735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton E. Herbal kava: reports of liver toxicity. Can Med Assoc J. 2002;166:777. [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Yin JJ, Cherng SH, Wamer WG, Boudreau M, Howard PC, Fu PP. UVA Photodecomposition of retinylpalmitate — formation of singlet oxygen and superoxide, and their role in induction of lipid peroxidation. Toxicol Lett. 2006;163:30–43. doi: 10.1016/j.toxlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Xia Q, Yin JJ, Fu PP, Boudreau MD. Photoirradiation of Aloe vera by UVA — formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett. 2007;168:165–175. doi: 10.1016/j.toxlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Miyahara E, Hihara J. Efficacy and safety of orally administered Lentinulaedodes mycelia extract for patients undergoing cancer chemotherapy: a pilot study. Am J Chin Med. 2011;39:451–459. doi: 10.1142/S0192415X11008956. [DOI] [PubMed] [Google Scholar]
- Yu HB, Jin W, Ho HL, Chan KC, Chan CC, Demokan MS, Stewart G, Culshaw B, Liao YB. Multiplexing of optical fiber gas sensors with a frequency modulated continuous-wave technique. Appl Opt. 2001;40:1011–1020. doi: 10.1364/ao.40.001011. [DOI] [PubMed] [Google Scholar]
- Zhu BZ, Zhao HT, Kalyanaraman B, Frei B. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: an ESR spin trapping study. Free Radic Biol Med. 2002;32:465–473. doi: 10.1016/s0891-5849(01)00824-3. [DOI] [PubMed] [Google Scholar]
- Zurer P, Hanson D. Chemistry puts herbal supplements to the test. Chem Eng News. 2004;82:16. [Google Scholar]