Abstract
In the central nervous system, oligodendrocyte precursor cells are exclusive in their potential to differentiate into myelinating oligodendrocytes. Oligodendrocyte precursor cells migrate within the parenchyma and extend cell membrane protrusions that ultimately evolve into myelinating sheaths able to wrap neuronal axons and significantly increase their electrical conductivity. The subcellular force generating mechanisms driving morphological and functional transformations during oligodendrocyte differentiation and myelination remain elusive. In this review, we highlight the mechanical processes governing oligodendrocyte plasticity in a dynamic interaction with the extracellular matrix.
Keywords: cytoskeleton, myelination, oligodendrocyte-extracellular matrix feedback loops, oligodendrocyte mechanics
1 | INTRODUCTION
Myelination is a striking example of dynamic membrane specialization, cell-cell and cell-extracellular matrix (ECM) interactions. In the central nervous system (CNS), oligodendrocytes (OLs) produce myelin, a highly specialized protein and lipid-rich biological membrane that wraps around neuronal axons enabling fast saltatory nerve conduction and axon integrity protection (Nave & Werner, 2014). The importance of myelination is illustrated by the consequences of its absence or loss, in clinical conditions such as leukodystrophies, multiple sclerosis (MS), and contusion type spinal cord injury (Santos-Nogueira et al., 2015). However, it is becoming increasingly evident that neuro-psychiatric disorders and age-related cognitive decline are also related to deregulation of myelin homeostasis, and recent findings associate myelination with brain plasticity and learning (McKenzie et al., 2014; Yeung et al., 2014). OLs derive from oligodendrocyte precursor cells (OPCs), which persist in the adult brain and hold the capacity to proliferate, migrate, and differentiate into myelinating OLs (Kang, Fukaya, Yang, Rothstein, & Bergles, 2010). Endogenous OPC differentiation into myelinating OLs represents a potential regenerating strategy towards axonal remyelination and neuronal survival (Franklin & Goldman, 2015). The fundamental knowledge of OL physiology and the biophysical and biochemical properties ruling the processes of differentiation and myelination are crucial for designing specific remyelinating therapies in pathological conditions.
Morphologically, OLs are one of the most versatile and complex cells of the CNS. OPCs evolve into myelinating OLs by extending and ramifying multiple protrusive membrane structures that lead to myelin sheet formation. Mechanical transitions during OPC differentiation into myelinating OLs represent one of the most significant shifts in cell remodeling, tightly regulated in space and time by a dynamic interplay between actin, actomyosin and tubulin-based cytoskeleton and cell membrane adhesion complexes. Nevertheless, it remains elusive how these mechanisms are activated, cooperate and interexchange during OL differentiation and how they can be modulated via cell-ECM interactions. In this review, we will report the interdisciplinary advances in understanding cytoskeleton-based mechanosensors and mechano-transducers required for OL differentiation and how this knowledge can be integrated to help developing computational models able to predict OL behavior in the process of myelination.
2 | OLIGODENDROCYTE MECHANOSENSING FOR MYELINATION
2.1 | Sensing physical alterations in the CNS microenvironment
Adult OPCs are OL precursors with restricted lineage potential for the generation of myelinating OLs (Kang et al., 2010). They account for 5–8% of all cells in the CNS, are homogeneously distributed (Levine, Reynolds, & Fawcett, 2001), and participate in both normal myelin homeostasis and regeneration. The decision of an OPC to continue as a precursor cell or to differentiate into a myelinating OL depends on the nature of the surrounding CNS matrix (Jagielska et al., 2012; Lourenco et al., 2016; Urbanski et al., 2016). The healthy CNS is a mechanically heterogeneous tissue with stiffness values ranging from 0.03 kPa to 3 kPa dependent on the animal species, age, developmental stage and region considered (Arani et al., 2015; Chatelin, Constantinesco, & Willinger, 2010; Christ et al., 2010; Elkin, Ilankovan, & Morrison, 2010; Iwashita, Kataoka, Toida, & Kosodo, 2014; Weickenmeier et al., 2016).
OPCs as mechanosensitive cells (Rosenberg, Kelland, Tokar, De la Torre, & Chan, 2008) are able to probe altered stiffness of the surrounding tissue, and reprogram their fate and differentiation stage accordingly (Jagielska et al., 2012; Kippert, Fitzner, Helenius, & Simons, 2009; Lourenco et al., 2016; Urbanski et al., 2016). In vitro studies, using cell lines and primary OL cultures from mouse and rat, reported the optimal ECM stiffness for OL differentiation. Jagielska et al. compared glass coverslips (with elastic modulus of ~50 GPa) with poly-acrylamide gels with rigidities ranging between 0.1 and 70 kPa and observed that survival, proliferation, migration, and differentiation of primary OL cultures from rat are optimal at 0.4 to 1 kPa values, resembling the intermediate values of the physiological range of brain tissue stiffness (Jagielska et al., 2012). Kippert and colleagues showed that the OL mouse cell line Olineu when cultured on polyacrylamid gels of different rigidities (0.5 and 6 kPa) exhibits smaller cell surface area on softer matrices (Kippert et al., 2009). More recently, Lourenço and coworkers, using the rat CG-4 cell line, demonstrated that polyacrylamide substrates of 6.5 kPa promote OPC maturation more efficiently than stiff (~1 GPa) glass coverslips (Lourenco et al., 2016). Finally, Urbanski et al. compared soft matrix (1 kPa) with rigid MS chronic lesion-like matrix (30 kPa) and found that the softer matrix is more permissive to rat primary OPC branching and maturation into myelinating OLs while rigid lesion-like matrix is inhibitory (Urbanski et al., 2016). Altogether, these in vitro studies with both OL cell lines and primary cultures show that differentiation is more efficient in softer engineered ECMs. However, it should be noted that in most OL-related studies, the above mentioned types of in vitro OL cultures are grown on polystyrene cell culture plates or glass coverslips, which are substantially more rigid substrates (Jagielska et al., 2012), about six orders of magnitude higher than the rigidity of brain. This means that the need for a better understanding of both biochemical and mechanochemical pathways involved in OL differentiation would benefit from the use of substrates specifically developed to have a physiological rigidity, such as the polyacrylamide gels/hydrogels.
The CNS tissue is organized in gray and white matter regions with distinct cellular compositions. While the gray matter regions contain essentially neuronal cell bodies and dendrites, the white matter region is highly enriched in myelinated neuronal axons. In these two different environments, the mechanisms that propel OPCs to migrate to axonal regions remain elusive. Can the different mechanical properties of white and gray matter in the CNS influence OL differentiation and myelin development? A recent study demonstrated that white matter is approximately two times stiffer than the gray matter (1.33 ±0.63 kPa vs. 0.68 ±0.20 kPa) due to the increased myelin content (Weickenmeier et al., 2016). This could explain why immature, incompletely myelinated brains are softer than mature, myelinated brains, and why aging brains are softer (Arani et al., 2015), probably due to progressive loss of myelin. Overall, these results suggest the existence of feedback loops between the mechanical properties of the CNS tissue and OPCs. OL differentiation and myelination may be influenced by the mechanical properties of the CNS tissue. In turn, the formation of compact myelin insulating neural axons and the presence of OLs alter the mechanical properties of axons.
Interesting studies have suggested that local biophysical interactions between OPCs in the adult CNS play an important role in their growth and this has large repercussions both in myelin homeostasis and in remyelination in disease conditions. For example, Hughes and co-workers investigated how the homeostatic cell density and distribution of adult OPCs are maintained in the CNS (Hughes et al., 2013). Using in vivo two-photon imaging in a line of transgenic mice that express a membrane-anchored form of EGFP under the control of the NG2 promoter to visualize specifically OPC in the adult brain, they found that these cells are extremely dynamic. OPCs survey the local environment by extension and retraction of their protrusions and continuous migration through the parenchyma, maintaining exclusive territories through self-repulsion. Moreover, Hughes and co-workers demonstrated that adult OPCs govern their cell density by local intercellular interactions with adjacent OPCs, as a positive correlation was found between OL differentiation, death and proliferation of neighboring OPCs (Hughes, Kang, Fukaya, & Bergles, 2013). This is in agreement with our previous findings where we demonstrated that in OPC-dorsal root ganglion (DRG) neuron co-cultures from rat, a critical density of OPCs along axons is required to trigger the initiation of OL differentiation in vitro (Rosenberg et al., 2008). This process of OPC differentiation occurs in the absence of dynamic axonal signaling and results from spatial and geometric constraints along an axon through lateral compression of OPCs. Moreover, using a neuron-free in vitro cell culture system of primary rat OPCs cultured with poly-L-lysine-coated polystyrene nanofibers that acted as pseudo-axonal scaffolds for myelination, we and others have also shown that OPCs can associate with the fibers and undergo differentiation and concentric membrane wrapping. We observed that the fiber diameter is permissive and sufficient as an axonal biophysical cue to initiate OL differentiation, with a threshold diameter value of 0.3 μm (Lee, Chong, Tuck, Corey, & Chan, 2013; Lee et al., 2012). Overall, these studies reinforce the notion that OLs are mechanosensitive cells and that the biophysical characteristics of the microenvironment play an important role in OPC fate decision.
It has long been considered that OLs are a functionally homogeneous population in the CNS. However, in agreement with the first observations of Pío del Río Hortega in 1928 (Del Rio Hortega, 1928), Marques et al. recently identified an unexpected OL transcriptional heterogeneity in the CNS (Marques et al., 2016). Surprisingly, they examined thousands of individual OLs of juvenile and adult mice by single-cell RNA sequencing and identified 12 individual populations, ranging from precursors to mature OLs. The authors suggest that this transcriptional heterogeneity in the OL lineage may be an intrinsic property of the cell or, possibly, the result of diverse local biophysical/biochemical interactions within the surrounding microenvironment. The observation of the enrichment of specific subsets of immature OLs in certain regions of the adult mouse brain (Marques et al., 2016) leads us to speculate that the different mechanical properties of brain regions may well influence the transcriptional and regional diversity of the OL population, possibly through epigenetic changes (Hernandez et al., 2016). This can also explain why only certain CNS regions are myelinated or why single axons can have distinct profiles of myelin distribution. This was elegantly demonstrated by Tomassy and colleagues when they traced individual axons of pyramidal neurons in the mouse neocortex by high-throughput electron microscopy reconstructions and observed at least three profiles of myelination (Tomassy et al., 2014).
2.2 | Mechanical regulation of OL differentiation: Actin and actomyosin-based force generating systems
In recent years, research has shown that OPCs receive and process information by mechanical signaling pathways, in addition to the well known biochemical pathways. Mechanical forces govern a host of cellular processes. However, at the molecular level, it remains elusive how OPCs can sense ECM parameters as elasticity and topology and transduce such information into morphological changes and lineage differentiation. Although much emphasis has been placed on the activation of OPC adhesion complexes as force sensors (e.g., integrin receptors (Olsen & Ffrench-Constant, 2005; Relvas et al., 2001)), the forces must nevertheless be transmitted through the cortical cytoskeleton. Yet, how the cytoskeleton senses, transmits and transduces forces into a biochemical response and downstream cellular processes is poorly understood.
The cytoskeleton is a network of subcellular filaments that form an extraordinary number of meshes and bundles within a fluid, the cytoplasm, and provides individual cells the ability to resist and react to applied external stress. Three cytoskeletal components are of specific interest to cell mechanical properties: actin filaments, myosin-II motors and microtubules. Filamentous actin (F-actin) is composed of actin monomers, which can polymerize into actin filaments or bundles and associate with myosin motors to form contractile microfilaments - the actomyosin network - that actively generate tension through an ATP-driven process. Actin filaments are mechanically different from microtubules in terms of mechanical stiffness, assembly dynamics, polarity and associated proteins. Actin filaments are more flexible than microtubules (Gittes, Mickey, Nettleton, & Howard, 1993) and have a higher turnover rate (Hill & Kirschner, 1982; Kirschner, 1980; Mogilner & Oster, 1999, 2003), rapidly reorganizing and enabling cells to change shape and migrate (Coles & Bradke, 2015; Etienne-Manneville, 2004, 2013; Fletcher & Mullins, 2010; Kueh & Mitchison, 2009).
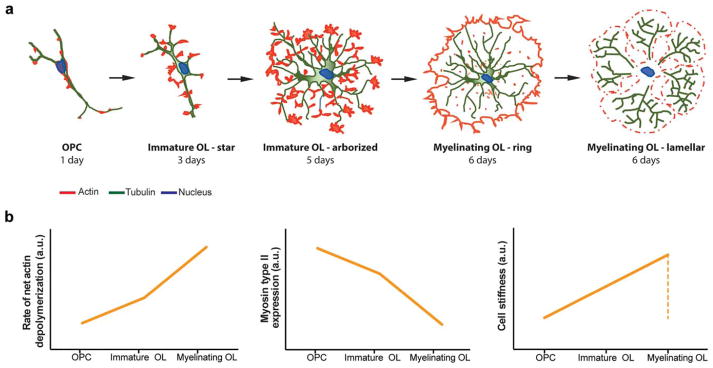
During in vitro differentiation of rat OLs, OPCs extend and ramify multiple cytoplasmic protrusions to enable proper myelination. OLs in their mature stage form a complex network of branched protrusions evolving in an extensive myelin-like membrane sheet. Importantly, morphological changes observed in in vitro cultures closely resemble those observed during in vivo developmental myelination (Kachar, Behar, & Dubois-Dalcq, 1986; Knapp, Bartlett, & Skoff, 1987). This process requires exquisite spatiotemporal reorganization and dynamics of the microtubules, actin and actomyosin-based cytoskeleton (Simpson & Armstrong, 1999; Song, Goetz, Baas, & Duncan, 2001) (Figure 1a, and 1b). In the first days of in vitro OL differentiation of mouse and rat cells, extension of membrane protrusions and arborization seem to be highly dependent on actin and tubulin polymerization. Actin filaments are mainly present at the protrusions leading edges while microtubules are also present in the cell body. In vitro OL maturation is usually reached between 5–7 days of culture, where more distal interbranch connections of actin are established with fewer invasions of microtubules. In this phase, an increased actin depolymerization rate allows the conversion of protrusions into sheets (Nawaz et al., 2015; Song et al., 2001) accompanied by significant changes in cell stiffness (Figure 1b) (Jagielska et al., 2012).
FIGURE 1.
Spatiotemporal distribution and dynamics of the cytoskeleton during in vitro OL differentiation and myelinization. (a) Organization of actin and microtubules in developing OL. In vitro differentiation of mouse/rat OPC into myelinating OL takes approximately 6 days and requires extensive remodeling of the actin and tubulin cytoskeleton. The first 3 days of culture are characterized by continuous extension of OPC membrane protrusions that originate star-shaped immature OL. Actin microfilaments are present mainly at the protrusions leading edge while microtubules are present both in the cell body and protrusions but do not extend to the most distal regions of the leading edges. By day 5, most cells form extensive branch networks, the actin microfilaments are present within newly formed connections between branches and microtubules invade them. Membrane protrusion and arborization seem to be highly dependent on actin and tubulin polymerization. Then, these highly arborized OL start the process of maturation by establishing more distal interbranch connections of actin with fewer invasions of microtubules. After day 6, the myelin sheet formation is accompanied by actin depolymerization (Song et al., 2001; Zuchero et al., 2015). (b) Dynamics of rate of net actin depolymerization, myosin-II expression and cell stiffness during in vitro OL differentiation as suggested by (Jagielska et al., 2012; Wang et al., 2008; Zuchero et al., 2015)
Recently, two studies proposed that spatiotemporal interplay between protrusive actin forces and actin depolymerization drives the formation, spreading and deformation of several myelin layers (Nawaz et al., 2015; Zuchero et al., 2015). In a zebrafish live imaging model, Nawaz et al., observed that actin filaments are initially present at the tips of exploratory growth cone-like protrusions due to actin polymerization but are later excluded from the mature compact myelin area, where actin depolymerization took place. Using in vitro OL cultures from rat, the same authors were able to analyze the leading edge of a myelin sheath, and observed that actin depolymerization increased membrane spreading by reducing the membrane surface tension. In contrast to OPCs, that form stable adhesions with the ECM, the myelinating OLs, at the leading edge, show low-adhesion to the axon, to allow the lateral movement of myelin layers and wrapping. Additionally, double mutant mice lacking two actin filament disassembly factors, cofilin-1 and ADF (also known as destrin), exhibited fewer myelin wraps, reinforcing the notion that actin depolymerization plays an important role in regulating myelin sheath spreading along the axon (Nawaz et al., 2015). Zuchero et al obtained complementary results using a pharmacological approach in in vitro rat co-cultures of OLs and neurons. They found that the initial extension of OL membrane protrusions and axonal binding requires a dynamic actin assembly, which is regulated by the Arp2/3 complex, a major actin nucleation and branching factor. However, upon initiation of the myelination process a rapid up-regulation of the actin disassembly factors gelsolin and cofilin-1 occurred and increased actin filament depolymerization rate, leading to OL membrane spreading and myelin wrapping (Zuchero et al., 2015). Aiming to identify the signal that induces actin disassembly and promotes myelin wrapping, the authors took advantage of structured illumination super-resolution microscopy to visualize the interaction between myelin basic protein (MBP) and actin in mature OLs. Interestingly, they found that regulation of the actin disassembly factors may be determined in part by the competition of the MBP protein for the binding to the phospholipid PI(4,5)P2 in the membrane, which induces the release of the actin disassembly factors, thus driving myelin wrapping and compaction.
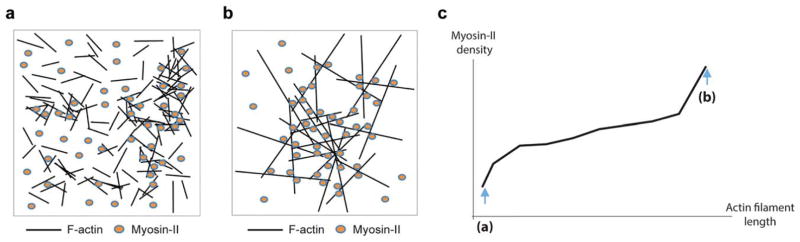
The first demonstrations that actin and myosin-II were required for force generation in OL physiology came from pharmacological studies where disruption of the actomyosin complex markedly impaired OPC migration and chemotactic response (Simpson & Armstrong, 1999), compromised normal process outgrowth and branching (Song et al., 2001) and maintenance of the myelin sheath (Wilson & Brophy, 1989). However, it remains elusive how these structures are physically and functionally linked in the different phases of OPC differentiation and myelination. In particular, few molecules of the actomyosin network are characterized in OL biology. The group of Melendez-Vasquez has contributed significantly to the knowledge of non-muscle myosin IIB in OL biology. Non-muscle myosin IIB is involved in OL contractility and was found to be a negative regulator of differentiation and myelination (Rusielewicz et al., 2014; Wang et al., 2012; Wang, Tewari, Einheber, Salzer, & Melendez-Vasquez, 2008). The expression of non-muscle myosin IIB decreases during in vitro maturation of primary rat OLs (Figure 1b) (Wang et al., 2008). Moreover, OPC-DRG neuron co-cultures from rat treated with blebbistatin to inhibit myosin II activity increased the complexity of OL branching and enhanced myelin production (Wang et al., 2008). In vivo, knockout mice for non-muscle myosin IIB displayed accelerated myelination (Wang et al., 2012). In opposition, overexpression of non-muscle myosin IIB prevented OPC branching and differentiation (Wang et al., 2012). In terms of localization, non-muscle myosin IIB is highly expressed in OPCs, where it co-localizes with actin filaments at the tips of growing protrusions and is also present in OPC protrusion branches and the cortex (Wang et al., 2012). In a model of lysolecithin-induced demyelination and remyelination, it was shown that depletion of non-muscle myosin IIB enhanced myelin repair by promoting OL maturation (Rusielewicz et al., 2014). Altogether, these data support a role for myosin IIB in OPC branching and differentiation. Kippert and colleagues identified the role of myosin IIB in the actomyosin contractility system in cell surface spreading. Reduction of myosin IIB activity by inhibition with blebbistatin promoted the spreading of membrane sheets and this was dependent on the physical properties of the supporting matrix (Kippert et al., 2009). This work establishes a direct connection between the mechanical properties of the matrix and the myosin IIB-mediated actomyosin cytoskeleton through a specific signaling pathway involving integrins and RhoA/ROCK. What is the specific role of myosin IIB in OL maturation and myelination? In light of recent findings from Nawaz et al., and Zuchero et al., described above (Nawaz et al., 2015; Zuchero et al., 2015) it is quite possible that non-muscle myosin IIB activity is dependent on (or affects) the state of actin polymerization in OLs. When bound to polymerized actin in OPCs, non-muscle myosin IIB provides the necessary cytoskeletal tension for protrusion outgrowth. However, as OLs mature, the myelin membrane is elaborated and the expression of non-muscle myosin IIB decreases. This is correlated with actin depolymerization behind the membrane leading edge, necessary for surface tension reduction that enables myelin wrapping around the axon. We believe that the role of actin depolymerization in the reduction of actomyosin contractility is two-fold. First, significant shrinking of F-actin decreases density of myosin generating contractile force as the number of filament intersections where myosins can bind to actin drops sharply (compare Figure 2a with 2b). Additionally, a single dense connected filament structure breaks into a number of smaller unconnected structures, which also contribute to the contractile force decrease (Figure 2c).
FIGURE 2.
Influence of F-actin depolymerization on stress generation in OL membrane. (a) 125 short filaments with mean length Lm = 1 and standard deviation STD =0.2. A single connected structure breaks down into several disconnected actin filaments highly reducing the stress in the membrane. (b) 25 long filaments having random angular orientation and centroid location with lengths distributed normally with Lm = 5 and STD = 0.8. The number of filament intersections is relatively large and there exist a contiguous connected filament structure. (c) A qualitative dependence of the maximal surface density of force-generating myosin on the average actin filament length
The structural organization and the spatial and subcellular distribution of the actin and actomyosin cytoskeletons during OL differentiation are poorly characterized. This could be achieved using different techniques, including super-resolution fluorescence microscopy. It also remains elusive how the co-existence and interplay of the actin and actomyosin cytoskeletons impact cell dynamics during OL differentiation. Most conceivably, other yet undescribed myosins are expressed in OLs and may play important roles in actomyosin-based contractility and transport-based processes. A systems-oriented study aiming to track or predict the behavior of myosins and correlate with the status of F-actin turnover will be important to understand the mechanisms of contractility and vesicle transport during OL extension of membrane protrusion, myelin membrane growth and leading edge protrusion in a spatial and temporal manner. Techniques such as 3D analysis of the actin and actomyosin cytoskeleton by cryo-electron microscopy, super resolution microscopy and computational modeling will be fundamental for the elucidation of these aspects.
2.3 | The makeover: Myelin maturation and stabilization
The lipid-rich myelin sheath is an evolutionary acquisition of vertebrates and might have occurred simultaneously in the peripheral (PNS) and central nervous system (CNS). The lipid-rich constitution of myelin enables the formation of an electrically insulating layer around the axon. Because of its insulation properties, which allow fast, saltatory nerve impulse conduction, myelin is crucial for the proper functioning of the vertebrate nervous system (Zalc, 2016; Zalc, Goujet, & Colman, 2008). Understanding the underlying mechanisms of myelin membrane biogenesis and homeostasis is crucial to comprehend myelin disassembly in pathological demyelinating conditions.
Structurally, the myelin sheath is a tightly packed, multilayered membrane wrapped around selected nerve axons. In the CNS, axonal myelination by OL is one of the most remarkable biological examples of membrane remodeling and requires extensive cytoskeletal rearrangements that must be coordinated with myelin production. Some of the current key issues needed to be addressed are 1) how the different layers of myelin membrane are incorporated into the growing myelin sheath; 2) how is newly synthesized membrane delivered to the leading edge; 3) how is myelin membrane compacted; 4) which factors influence myelin thickness and internode length; and 5) how the myelin sheath is maintained (lipid and protein turnover). Some of the details of such complex processes during axon ensheathment and myelin wrapping have been elucidated in the recent years using techniques such atomic force microscopy (AFM), high-resolution cryo-electron microscopy (EM), three-dimensional EM reconstruction, interference reflection microscopy and other microscopy techniques, and in vivo zebrafish and mouse models. In vivo time-lapse imaging of active myelination in zebrafish has shown that individual oligodendrocytes generate new myelin sheaths within a 5-hour period (Czopka, Ffrench-Constant, & Lyons, 2013). Snaidero and colleagues proposed a new model of axonal myelination and showed that during myelin wrapping it is the plasma membrane’s innermost tongue that maintains contact with the axon segment while the outermost newly formed layers gradually spread out to the lateral sides to form the myelin internode. The authors also identified the presence of cytoplasmic channels in myelin sheaths that provide a connection from the outer to inner tongue of myelin, therefore enabling membrane trafficking to the leading edge (Snaidero et al., 2014). But, what is the driving force that propels the new myelin membrane around the axon? As discussed above, researches of Nawaz et al. and Zuchero et al. showed that actin filament disassembly may be a major driver that induces the formation of several myelin layers (Nawaz et al., 2015; Zuchero et al., 2015). However, as myelin is composed of up to 50 wraps of membrane, it is hard to imagine that actin disassembly alone is responsible for the concentric membrane wrapping.
For proper axonal insulation, myelin should have the necessary physical stability for membrane wrapping. This is achieved by compaction of the cytoplasmic leaflets of the myelin lamellae that includes adhesion of the different layers of myelin membranes and exclusion of cytoplasm. Usually, membrane-to-membrane interaction is assured by interaction between molecules with strong adhesion forces that overcome repulsing negative charges from oligosaccharides that usually cover the plasma membrane and have the role of protecting cells from non-specific adhesion. In the case of the myelin membranes, it was shown that during differentiation, OLs decrease the expression of the negatively charged sialic acids, contributing to myelin bilayers compaction and adhesiveness through the loss of electrostatic repulsion forces (Bakhti et al., 2013). Moreover, the authors identified PLP as an important myelin protein that increases myelin membrane adhesiveness and stability. MBP also regulates the morphology and stability of the complex myelin membrane architecture. MBP has a high content of positively charged residues and, therefore, binds to the negatively charged lipids of the cytoplasmic leaflets of the bilayer via electrostatic interaction. Moreover, by its association to the inner leaflet of the membrane bilayer, hydrophobic interactions take over and MBP undergoes a phase transition into a cohesive mesh-like protein network (Aggarwal et al., 2013; Raasakka et al., 2017). Using AFM, it was found that MBP adsorbs to myelin bilayer surfaces in a cooperative fashion, involving close protein–protein and protein–lipid/lipid domain interactions, suggesting that MBP contributes to myelin stability through strong adhesion forces (Lee et al., 2014). MBP and PLP are the main proteins found in the compact myelin and, therefore, may contribute to protein and cytoplasmic extrusion, and compact the membrane in a zippering fashion. On the other hand, Snaidero et al. showed that another myelin protein, CNP, delays myelin compaction, suggesting that equilibrium between CNP and MBP should exist to prevent premature or excessive myelin compaction at the leading edge (Snaidero et al., 2014). This work was recently complemented by the same authors who demonstrated that CNP antagonizes the activity of MBP in compacting myelin membrane layers by associating with and organizing the actin cytoskeleton within the cytoplasmic regions of the myelin sheath (Snaidero et al., 2017). Altogether, these data show that the bilayer-associated proteins MBP, PLP and CNP play an essential role in stabilizing and maintaining the myelin structure. This fundamental biophysical knowledge has strong implications in the understanding pathological demyelinating conditions such as MS, where alterations in myelin membrane occur, such as changes in adhesion forces and lipid composition and electric charge imbalance between lipid molecules and myelin proteins.
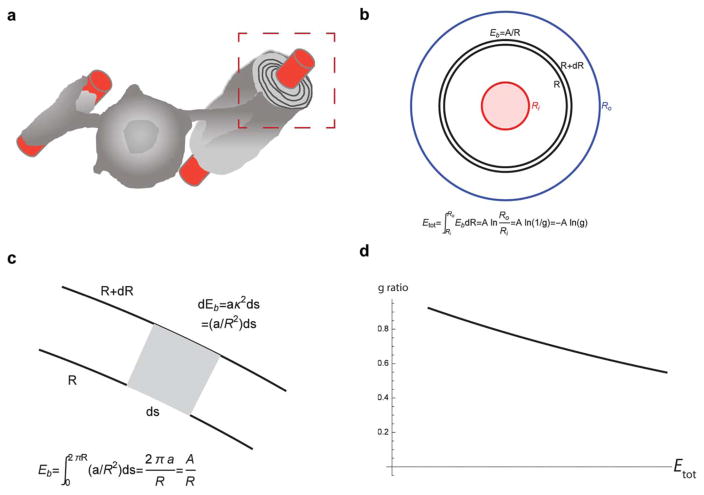
Finally, during myelin wrapping how does an OL sense the maximum number of wraps permitted for that axon? For efficient conduction, a myelinated axon should have an appropriate g-ratio (the diameter of the axon divided by the total diameter of the axon plus myelin) with thicker axons having a thicker myelin sheath. Yet, while there is a threshold of 0.3 μm in diameter, myelinated nanofibers do not have typical g-ratios (Lee et al., 2012). This suggests that axonal molecular clues may instruct OLs to make the correct number of wraps. Gibson and colleagues showed that, indeed, increasing neuronal activity by channelrhodopsin enhances myelin thickness (Gibson et al., 2014). Another possible and simple explanation is that the value of g-ratio is self-regulated by the composition, geometrical and mechanical properties (e.g., stored elastic energy) of the myelin sheath and there is no need for wrap number sensing. The elastic bending energy Eb of a thin membrane sheath of the radius R is proportional to its curvature Eb = A/R, where A is a constant factor. Considering a myelin sheath with an inner (axon) radius Ri and an outer radius Ro, the total bending energy E stored in this sheath is found by integration of the layer energy over the radius in the range Ri < R < Ro. This gives the value Etot = A ln(Ro/Ri) = A ln (1/g) = −A ln g. Assuming that the total bending energy is limited by a maximal value Emax beyond which the sheath loses its stability, we find g = exp(−Emax/A) independent of the axon radius Ri (Figure 3a–d).
FIGURE 3.
A mechanical model of an elastic myelinated layer around an axon. (A) Mature OL myelinating neuronal axons by extending and wrapping the myelin sheath. (B) A cross section of the axon (pink, red boundary of the radius Ri) and the myelinated layer (white, blue outer boundary of the radius Ro) normal to the axon axis. A thin circular slice of the radius R and width dR (bounded by black curves) stores a bending elastic energy Eb = A/R, where A is constant parameter (explanation in C). The total bending energy Etot stored in the layer is found by the integration of the slice energy Eb over radius in the range (Ri ≤ R ≤ Ro) and is proportional to the logarithm of g-factor. (C) A small curvilinear sector (gray) inside the thin slice (zoomed inset in B) has bending energy dE proportional to the segment length ds and the square of the local curvature k2 =1/R2. The energy Eb of the circular slice is found by the integration of dE along the slice, when s changes from 0 to 2πR. (D) The dependence of the g-factor on the maximal elastic energy stored in myelin sheath
2.4 | Biophysical systems analysis of OL differentiation and myelination
The progress of neurophotonics imaging techniques such as multiphoton microscopy has enabled vizualization of deep CNS tissue (Bovetti et al., 2017; Kong, Tang, & Cui, 2016; Park, Sun, & Cui, 2015) in mouse and zebrafish animal models where sparsely fluorescently labeled OL-lineage cells allow the real time analysis of individual cells during the process of axonal myelination (Almeida & Lyons, 2016; Chong et al., 2012). However, in vivo studies with sufficient resolution to understand quantitatively the complex cellular interactions and subcellular OL (e.g., cytoskeletal) dynamics are still lacking.
An emerging interface between engineering and molecular neuro-sciences has advanced our understanding of in vitro OL model systems. Engineered microstructures can position and connect cells in a specific and oriented manner in order to study specific intercellular interactions. Conventional myelinating co-cultures of neurons and glial cells fail to provide the means to locally manipulate the biophysical and biochemical environments in culture, making it difficult to investigate local axon-glia interactions. The lack of robust in vitro CNS myelination models has led to the development and optimization of microfluidic-based compartmentalized culture systems to achieve segregation of neuron and OL cell bodies while simultaneously allowing the formation of myelin sheaths (Kerman et al., 2015; Park, Koito, Li, & Han, 2009a, 2009b, 2012). Interestingly, some microfluidic systems have included electrical stimulation to achieve increased levels of myelination (Lee et al., 2016; Yang et al., 2012). Altogether, microfluidic cell culture systems have become an important platform for studying development, function and de(re)generation of the nervous system at the molecular and cellular level. However, the optimization of such technologies and their association with techniques such as live cell imaging and super resolution microscopy will, in the future, allow dissecting the biophysical mechanisms of axon-glia interactions. Moreover, a high throughput platform adapted to such microfluidic systems would allow to perform drug-screening assays to identify molecules able to promote myelin repair. We and others have described the fabrication of micropillar arrays for modeling myelination and as a high-throughput screening platform to identify potential remyelination therapeutics (Mei et al., 2014). OLs co-cultured with micropillars of conical shape interact with and ensheathed the pillars in a concentric fashion. We believe that the use of such micropillars as a scaffold for membrane wrapping could also be a useful tool to help elucidate the biophysical mechanisms underlying myelin wrapping by allying to other techniques such as live-cell imaging and super resolution microscopy.
3 | CONCLUSION
In in vitro culture, OLs are characterized by several membrane extensions and flat myelin-based membrane sheets that spirally wrap axons forming a compact insulating layer in vivo. By analogy with other cell types, maintenance of these protrusive structures, as well as the formation of the myelin sheath, rely on a pronounced cytoskeleton consisting of microtubules and actin filaments. While an extensive cytoskeletal network of microtubules provides cellular structural support, and is specifically important for cellular sorting and organelle trafficking, actomyosin networks operate as force sensors and force generators required for mechanical plasticity during OL differentiation and myelination. In recent years, actin depolymerization and actomyosin-based mechanisms have been shown to play a role in OL differentiation. However, it remains elusive their spatiotemporal regulation, stoichiometric contribution to force generation and modulation via brain matrix interactions. The ECM appears to be an important component of the feedback loop controlling the myelin membrane sheet spreading, which in turn affects the mechanical properties of ECM. Subsequently, the mechanical parameters of the membrane might determine an outcome of the myelination process (for example, the invariance of the g-ratio suggested in this manuscript). These considerations underline an important role of deeper quantitative analysis of the actomyosin/microtubule dynamics as well as usage of biophysical models in this field of research.
Acknowledgments
Funding information
Fundação para a Ciência e a Tecnologia (FCT), Grant/Award Number: SFRH/BPD/90268/2012; European Union’s Seventh Framework Programme (EU-FP7), Grant/Award Number: 600375; European Regional Development Fund (ERDF), Grant/Award Numbers: Norte-01-0145-FEDER-000008 and Norte-01-0145-FEDER-000019; National Institute of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Grant/Award Numbers: R01NS062796, R01NS097428, R01NS095889; United States National Multiple Sclerosis Society (US NMSS), Grant/Award Number: RG5203A4
Helena S. Domingues acknowledges the financial support by Fundaçãopara a Ciência e Tecnologia (FCT) through the fellowship with the reference SFRH/BPD/90268/2012. Inês M. Pinto and Andrea Cruz acknowledge the financial support from the Marie Curie COFUND Programme “NanoTRAINforGrowth”, from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 600375. Jonah R. Chan acknowledges grants from the US NMSS (RG5203A4) and NIH/NINDS (R01NS062796, R01NS097428, R01NS095889). João B. Relvas acknowledges funding from Norte-01-0145-FEDER-000008 - Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). This article is a result of the project Nanotechnology based functional solutions (NORTE-01–0145-FEDER-000019), co-financed by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- Aggarwal S, Snaidero N, Pahler G, Frey S, Sanchez P, Zweckstetter M, … Simons M. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biology. 2013;11:e1001577. doi: 10.1371/journal.pbio.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R, Lyons D. Oligodendrocyte development in the absence of their target axons in vivo. PLoS One. 2016;11:e0164432. doi: 10.1371/journal.pone.0164432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, Kruse SA, … Huston J., 3rd Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti M, Snaidero N, Schneider D, Aggarwal S, Mobius W, Janshoff A, … Simons M. Loss of electrostatic cell-surface repulsion mediates myelin membrane adhesion and compaction in the central nervous system. Proceedings of the National Academy of Sciences United States of America. 2013;110:3143–3148. doi: 10.1073/pnas.1220104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Moretti C, Zucca S, Dal Maschio M, Bonifazi P, Fellin T. Simultaneous high-speed imaging and optogenetic inhibition in the intact mouse brain. Scientific Reports. 2017;7:40041. doi: 10.1038/srep40041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelin S, Constantinesco A, Willinger R. Fifty years of brain tissue mechanical testing: from in vitro to in vivo investigations. Biorheology. 2010;47:255–276. doi: 10.3233/BIR-2010-0576. [DOI] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, … Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proceedings of the National Academy of Sciences United States of America. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ AF, Franze K, Gautier H, Moshayedi P, Fawcett J, Franklin RJ, … Guck J. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. Journal of Biomechanics. 2010;43:2986–2992. doi: 10.1016/j.jbiomech.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Coles CH, Bradke F. Coordinating neuronal actin-microtubule dynamics. Current Biology. 2015;25:R677–R691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Czopka T, Ffrench-Constant C, Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Developmental Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río Hortega P. Tercera aportación al conocimiento morfológico e interpretación funcional de la oligodendroglía. Memorias de la Real Sociedad Espanola de Historia Natural. 1928;14:5–122. [Google Scholar]
- Elkin BS, Ilankovan A, Morrison B., 3rd Age-dependent regional mechanical properties of the rat hippocampus and cortex. Journal of Biomechanical Engineering. 2010;132:011010. doi: 10.1115/1.4000164. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Microtubules in cell migration. Annual Review of Cell and Developmental Biology. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Goldman SA. Glia disease and repair-remyelination. Cold Spring Harbor Perspectives in Biology. 2015;7:a020594. doi: 10.1101/cshperspect.a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, … Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. Journal of Cell Biology. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Patzig J, Mayoral SR, Costa KD, Chan JR, Casaccia P. Mechanostimulation promotes nuclear and epigenetic changes in oligodendrocytes. Journal of Neuroscience. 2016;36:806–813. doi: 10.1523/JNEUROSCI.2873-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TL, Kirschner MW. Subunit treadmilling of microtubules or actin in the presence of cellular barriers: possible conversion of chemical free energy into mechanical work. Proceedings of the National Academy of Sciences United States of America. 1982;79:490–494. doi: 10.1073/pnas.79.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Kataoka N, Toida K, Kosodo Y. Systematic profiling of spatiotemporal tissue and cellular stiffness in the developing brain. Development. 2014;141:3793–3798. doi: 10.1242/dev.109637. [DOI] [PubMed] [Google Scholar]
- Jagielska A, Norman AL, Whyte G, Vliet KJ, Guck J, Franklin RJ. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells and Development. 2012;21:2905–2914. doi: 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Behar T, Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell and Tissue Research. 1986;244:27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman BE, Kim HJ, Padmanabhan K, Mei A, Georges S, Joens MS, … Gage FH. In vitro myelin formation using embryonic stem cells. Development. 2015;142:2213–2225. doi: 10.1242/dev.116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippert A, Fitzner D, Helenius J, Simons M. Actomyosin contractility controls cell surface area of oligodendrocytes. BMC Cell Biology. 2009;10:71. doi: 10.1186/1471-2121-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MW. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. Journal of Cell Biology. 1980;86:330–334. doi: 10.1083/jcb.86.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Bartlett WP, Skoff RP. Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens, and membrane production. Developmental Biology. 1987;120:356–365. doi: 10.1016/0012-1606(87)90238-7. [DOI] [PubMed] [Google Scholar]
- Kong L, Tang J, Cui M. In vivo volumetric imaging of biological dynamics in deep tissue via wavefront engineering. Optics Express. 2016;24:1214–1221. doi: 10.1364/OE.24.001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science. 2009;325:960–963. doi: 10.1126/science.1168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Banquy X, Kristiansen K, Kaufman Y, Boggs JM, Israelachvili JN. Lipid domains control myelin basic protein adsorption and membrane interactions between model myelin lipid bilayers. Proceedings of the National Academy of Sciences United States of America. 2014;111:E768–775. doi: 10.1073/pnas.1401165111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HU, Nag S, Blasiak A, Jin Y, Thakor N, Yang IH. Subcellular optogenetic stimulation for activity-dependent myelination of axons in a novel microfluidic compartmentalized platform. ACS Chemical Neuroscience. 2016;7:1317–1324. doi: 10.1021/acschemneuro.6b00157. [DOI] [PubMed] [Google Scholar]
- Lee S, Chong SY, Tuck SJ, Corey JM, Chan JR. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature Protocols. 2013;8:771–782. doi: 10.1038/nprot.2013.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, … Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends in Neuroscience. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Lourenco T, Paes de Faria J, Bippes CA, Maia J, Lopes-da-Silva JA, Relvas JB, Graos M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Scientific Reports. 2016;6:21563. doi: 10.1038/srep21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, … Castelo-Branco G. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, Paes de Faria J, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SP, Shen YA, Niu J, Zhao C, Presley B, … Chan JR. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A, Oster G. The polymerization ratchet model explains the force-velocity relation for growing microtubules. European Biophysics Journal. 1999;28:235–242. [Google Scholar]
- Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophysical Journal. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annual Review of Cell and Development Biology. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nawaz S, Sanchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, … Simons M. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Developmental Cell. 2015;34:139–151. doi: 10.1016/j.devcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen IM, Ffrench-Constant C. Dynamic regulation of integrin activation by intracellular and extracellular signals controls oligodendrocyte morphology. BMC Biology. 2005;3:25. doi: 10.1186/1741-7007-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomedical Microdevices. 2009a;11:1145–1153. doi: 10.1007/s10544-009-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. A multi-compartment CNS neuronglia Co-culture microfluidic platform. Journal of Visualized Experiments. 2009b:31. doi: 10.3791/1399. https://doi.org/10.3791/1399. [DOI] [PMC free article] [PubMed]
- Park J, Koito H, Li J, Han A. Multi-compartment neuron-glia co-culture platform for localized CNS axon-glia interaction study. Lab on a Chip. 2012;12:3296–3304. doi: 10.1039/c2lc40303j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Sun W, Cui M. High-resolution in vivo imaging of mouse brain through the intact skull. Proceedings of the National Academy of Sciences United States of America. 2015;112:9236–9241. doi: 10.1073/pnas.1505939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasakka A, Ruskamo S, Kowal J, Barker R, Baumann A, Martel A, … Kursula P. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Scientific Reports. 2017;7:4974. doi: 10.1038/s41598-017-05364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, Ffrench-Constant C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Current Biology. 2001;11:1039–1043. doi: 10.1016/s0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proceedings of the National Academy of Sciences United States of America. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusielewicz T, Nam J, Damanakis E, John GR, Raine CS, Melendez-Vasquez CV. Accelerated repair of demyelinated CNS lesions in the absence of non-muscle myosin IIB. Glia. 2014;62:580–591. doi: 10.1002/glia.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Nogueira E, Lopez-Serrano C, Hernandez J, Lago N, Astudillo AM, Balsinde J, … Lopez-Vales R. Activation of lysophosphatidic acid receptor type 1 contributes to pathophysiology of spinal cord injury. Journal of Neuroscience. 2015;35:10224–10235. doi: 10.1523/JNEUROSCI.4703-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Armstrong RC. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia. 1999;26:22–35. [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, … Simons M. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, … Simons M. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Reports. 2017;18:314–323. doi: 10.1016/j.celrep.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Goetz BD, Baas PW, Duncan ID. Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Molecular and Cellular Neuroscience. 2001;17:624–636. doi: 10.1006/mcne.2001.0974. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, … Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski MM, Kingsbury L, Moussouros D, Kassim I, Mehjabeen S, Paknejad N, Melendez-Vasquez CV. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Scientific Reports. 2016;6:33751. doi: 10.1038/srep33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rusielewicz T, Tewari A, Leitman EM, Einheber S, Melendez-Vasquez CV. Myosin II is a negative regulator of oligodendrocyte morphological differentiation. Journal of Neuroscience Research. 2012;90:1547–1556. doi: 10.1002/jnr.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. Journal of Cell Biology. 2008;182:1171–1184. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickenmeier J, de Rooij R, Budday S, Steinmann P, Ovaert TC, Kuhl E. Brain stiffness increases with myelin content. Acta Biomateriala. 2016;42:265–272. doi: 10.1016/j.actbio.2016.07.040. [DOI] [PubMed] [Google Scholar]
- Wilson R, Brophy PJ. Role for the oligodendrocyte cytoskeleton in myelination. Journal of Neuroscience Research. 1989;22:439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]
- Yang IH, Gary D, Malone M, Dria S, Houdayer T, Belegu V, … Thakor N. Axon myelination and electrical stimulation in a microfluidic, compartmentalized cell culture platform. Neuromolecular Medicine. 2012;14:112–118. doi: 10.1007/s12017-012-8170-5. [DOI] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, … Frisen J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Zalc B. The acquisition of myelin: An evolutionary perspective. Brain Research. 2016;1641:4–10. doi: 10.1016/j.brainres.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Currrent Biology. 2008;18:R511–512. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Fu MM, Sloan SA, Ibrahim A, Olson A, Zaremba A, … Barres BA. CNS myelin wrapping is driven by actin disassembly. Developmental Cell. 2015;34:152–167. doi: 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]