Abstract
Exposure to pollutants, such as ozone, exacerbates airway inflammation and hyperresponsiveness (AHR). TNF-stimulated gene 6 (TSG-6) is required to transfer inter-α-inhibitor heavy chains (HC) to hyaluronan (HA), facilitating HA receptor binding. TSG-6 is necessary for AHR in allergic asthma, because it facilitates the development of a pathological HA–HC matrix. However, the role of TSG-6 in acute airway inflammation is not well understood. Here, we hypothesized that TSG-6 is essential for the development of HA- and ozone-induced AHR. TSG-6−/− and TSG-6+/+ mice were exposed to ozone or short-fragment HA (sHA), and AHR was assayed via flexiVent. The AHR response to sHA was evaluated in the isolated tracheal ring assay in tracheal rings from TSG-6−/− or TSG-6+/+, with or without the addition of exogenous TSG-6, and with or without inhibitors of Rho-associated, coiled-coil–containing protein kinase (ROCK), ERK, or PI3K. Smooth-muscle cells from mouse tracheas were assayed in vitro for signaling pathways. We found that TSG-6 deficiency protects against AHR after ozone (in vivo) or sHA (in vitro and in vivo) exposure. Moreover, TSG-6−/− tracheal ring non-responsiveness to sHA was reversed by exogenous TSG-6 addition. sHA rapidly activated RhoA, ERK, and Akt in airway smooth-muscle cells, but only in the presence of TSG-6. Inhibition of ROCK, ERK, or PI3K/Akt blocked sHA/TSG-6–mediated AHR. In conclusion, TSG-6 is necessary for AHR in response to ozone or sHA, in part because it facilitates rapid formation of HA–HC complexes. The sHA/TSG-6 effect is mediated by RhoA, ERK, and PI3K/Akt signaling.
Keywords: asthma, cell signaling, extracellular matrix, hyaluronan, lung, TSG-6, inter-alpha-inhibitor
Introduction
The role of extracellular matrix (ECM)2 in airway inflammation is increasingly recognized. Hyaluronan (HA) is a major component of the ECM, and several studies have highlighted the involvement of HA in the pathobiology of airway inflammation. HA synthesis and metabolism are up-regulated through the course of the inflammatory process (1–4). Furthermore, HA undergoes a “size switch”, whereby lower-molecular weight, “short-fragment” HA (sHA; 100–350 kDa) is produced either de novo or through breakdown of existing structural high-molecular weight HA (HMW-HA; >1,000 kDa). HA in pathological conditions associates with the heavy chain (HC) component of the serum protein inter-α-inhibitor (IαI) when it exudes into the tissue ECM. This covalent attachment promotes optimal binding of HA to CD44 (5) and thus optimal HA signaling and is mediated by the protein encoded by TNF-stimulated gene 6 (TSG-6). TSG-6, a 35-kDa secreted ECM protein, is itself a hyaladherin that catalyzes the transfer of HC from IαI to HA. In asthmatic patients and animal models of allergic airway disease, TSG-6 mRNA expression, TSG-6–HC complexes, and HA–HC complexes are increased, suggesting that TSG-6 may play an important role in the pathogenesis of allergic airway disease (6).
Inflammation in chronic airway disease is exacerbated by exposure to environmental pollutants. For example, urban ozone pollution is common in developed countries and leads to increased morbidity in susceptible individuals, leading to 800 premature deaths, 4,500 hospital admissions, 900,000 school absences, and more than 1 million restricted activity days every year with an estimated $5 billion annual economic burden (7–11). Each 10-ppb increase in the 1-h daily maximum level of ozone is associated with an increase in mortality risk of individuals with cardio-respiratory disease of 0.39–0.87% (8, 10, 12, 13). Exposure to ozone causes acute exacerbation of airway disease and increased visits to health-care facilities within 24 h. Susceptible populations (e.g. children) are at increased risk. In 2005, 27,000 admissions and 19,000 emergency department visits for asthma were attributed to increased ozone levels (14). Despite the significance of their effects, the biological mechanisms that regulate the response to pollution exposure are not well understood. However, an important role for ECM is emerging. Exposure to ozone or cigarette smoke and asthma induce the release of sHA (4, 15, 16), which activates the TLR4 pathway, leading to airway inflammation (17–19) and hyperresponsiveness (AHR) (15, 17–20). Importantly, IαI is necessary for the development of ozone-induced AHR (15), as well as after other short-acting oxidative injury, like chlorine gas exposure (17). This suggests that formation of HA–HC complexes is involved in acute airway inflammation; however, this has not been conclusively proven.
Existing evidence suggests that TSG-6 mediates the development of allergic airway disease (1–3). However, TSG-6 also has prominent anti-inflammatory activity and ameliorates inflammation in endotoxin- and bleomycin-induced lung injury (21, 22) and extrapulmonary disease as diverse as osteoarthritis (23), peritonitis (24), myocardial injury (25), and corneal injury (26). Thus, the effects of TSG-6 in inflammation may well depend on the inflammatory mechanism, context, and association with sHA or HMW-HA. Furthermore, allergic asthma is a chronic condition, which supports a continued accumulation of a pathological HA–HC matrix over time. On the other hand, the relevance of TSG-6–HA interactions in acute, short-term inflammation is unknown. In this report, we investigated whether TSG-6 is necessary for the development of AHR after ozone exposure, a non-allergic acute inflammatory process. We demonstrate that absence of TSG-6 ameliorates ozone-induced or sHA-induced AHR in vivo. Importantly, we demonstrate for the first time that the TSG-6–mediated formation of HA–HC complexes transpires rapidly in biological tissues.
Results
TSG-6 is partly necessary for ozone induced inflammation
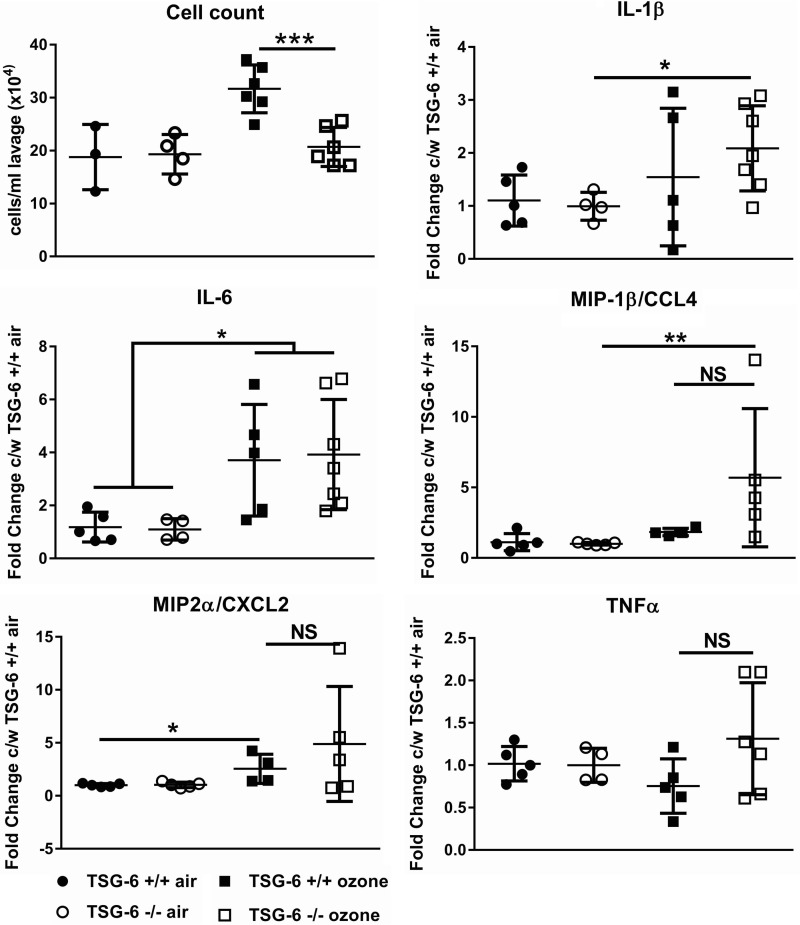
We first analyzed cellular and humoral inflammation 24 h after mouse exposure to ozone. Total cell counts were elevated only in TSG-6–sufficient mice (Fig. 1). Most cells at this time point were macrophages (not shown), in agreement with our previous observations (15). The TSG-6 dependence of ozone-induced cellular inflammation is similar to effects of CD44 and IαI deficiency (15) and supports a role for HA in the process of cell recruitment to the lung. We then evaluated cytokine expression by quantitative RT-PCR (Fig. 1). TSG-6–deficient mice were not significantly different from TSG-6–sufficient mice in their pro-inflammatory cytokine expression after ozone exposure. This result is similar to the previously observed failure of TSG-6 deficiency to alter cytokine expression in allergic airway inflammation (3). Post-ozone cytokines were not statistically different between TSG-6–deficient and –sufficient mice. In aggregate, these results suggest that TSG-6 is necessary for cell influx, but not for cellular activation after ozone exposure.
Figure 1.
TSG-6 effects on ozone-induced inflammation. Mice were exposed to 2 ppm ozone for 3 h and sacrificed 24 h later. Lung lavage cells were counted, and whole-lung tissue mRNA was assayed for gene expression. The absence of TSG-6 leads to a significant decrease in cellular influx but no significant difference in cytokine expression after ozone exposure. Results are represented as means ± S.E. (error bars) of measurements. The experiment was repeated at least twice. n = 4–8 mice. NS, nonsignificant. *, p < 0.05; **, p < 0.01; ***, p < 0.001, ANOVA with Tukey's multiple-comparison correction.
TSG-6 is necessary for the development of HA–HC complexes after ozone injury
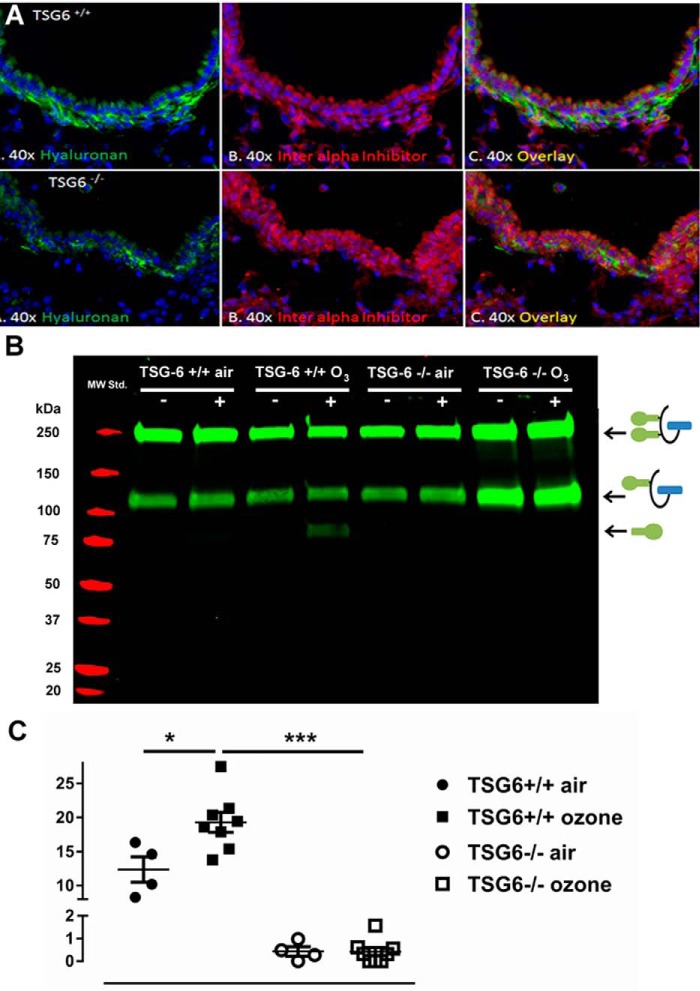
Previous work has highlighted the importance of TSG-6 for the formation of complexes between HA and HC of IαI in allergic inflammation (3), but this is a chronic process with ample time for the formation of these complexes. We wanted to determine whether TSG-6–mediated HA–HC formation can be detected within 24 h of ozone exposure (i.e. in acute lung injury). Immunohistochemistry for IαI and HA showed increased colocalization of HA and IαI after ozone exposure in TSG-6–sufficient mice only (Fig. 2A). IαI-HA colocalization was specific, as both IαI and HA staining was abolished after digestion of the histological sections with hyaluronidase (Fig. S1). After digestion of lung tissue to free HC from the HA–HC complex, we confirmed increased HC levels in TSG-6–sufficient mice (Fig. 2B). Quantification of the gels using ImageJ showed an absence of HC–HA deposition regardless of ozone or air exposure in TSG-6–deficient mice, whereas there was a significant increase in sufficient mice (Fig. 2C). These results suggest that TSG-6 mediates HA–HC complex formation within 24 h after ozone exposure.
Figure 2.
HA–HC deposition after ozone exposure. Mice were exposed to 2 ppm ozone for 3 h and sacrificed 24 h later. A, lung tissue was fixed in paraffin and stained for hyaluronan and IαIp. In TSG-6–sufficient mice, there is immunohistochemical evidence of HA–HC deposition in the subepithelial space, which is absent in TSG-6–deficient mice. B, tissue was digested with hyaluronidase, and released heavy chains were detected by Western blotting. Detection of HA-bound HC shows an increase of HA–HC complex formation only in TSG-6–sufficient mice after ozone exposure (note free HC band at ∼80 kDa, which appears only after hyaluronidase treatment). Schematics of the full IαI molecule (at 250 kDa), the pre-αI molecule (at 120 kDa), and the free HC (at 80 kDa) are provided to the right of the blot for clarification. +, hyaluronidase treatment; −, no hyaluronidase treatment. C, quantification of HA-bound HC shows a significant increase in TSG-6–sufficient mice after ozone exposure. TSG-6–deficient mice have virtually no HA-bound HC before or after ozone exposure. Results are represented as means ± S.E. (error bars). The experiment was repeated at least twice. n = 4 (air exposure) to 8 (ozone exposure). *, p < 0.05; ***, p < 0.001, ANOVA with Tukey's multiple-comparison correction.
TSG-6 is necessary for ozone-induced AHR
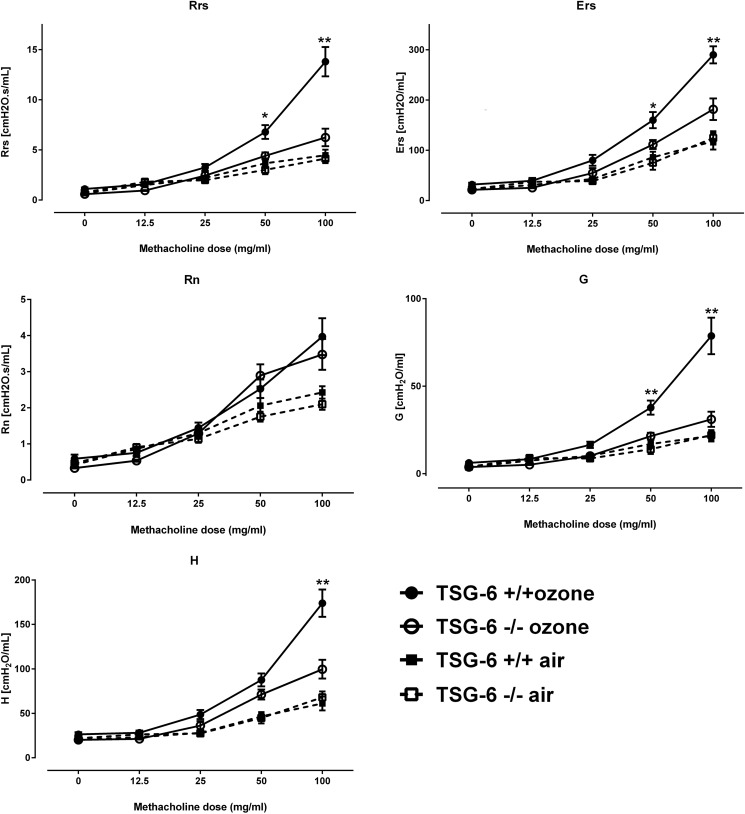
Previous work has established that HA–HC formation is necessary for the development of AHR in a chronic allergic asthma model (3). We therefore examined the effects of the presence of TSG-6 on ozone–induced AHR in vivo. We found that TSG-6 deficiency led to a significant decrease in ozone-induced airway resistance (respiratory system resistance; Rrs) and lung elastance (stiffness) (Ers) (Fig. 3). Analysis of the effects in different lung compartments suggested that the observed decrease in Rrs was not due to a decrease in large-airway (Newtonian) resistance (Rn), but rather to a dramatic decrease in peripheral tissue resistance (also called damping; G). Peripheral lung elastance (stiffness) H was also significantly decreased in the absence of TSG-6. In aggregate, these results suggest that TSG-6 mediates small airway narrowing after ozone exposure, which in turn leads to an increase of total airway resistance in response to methacholine (i.e. AHR).
Figure 3.
Increased airway resistance and stiffness in TSG-6–sufficient mice after ozone exposure, which is due to increases in peripheral tissue values. Mice were exposed to 2 ppm ozone for 3 h, and 24 h later, they were anesthetized, paralyzed, tracheotomized, intubated, and phenotyped for airway physiology measurements via flexiVent. Rrs, respiratory system (whole-lung) resistance; Ers, respiratory system (whole-lung) elastance; Rn, Newtonian (large-airway) resistance; G, damping (peripheral lung resistance); H, peripheral lung elastance. Results are represented as means ± S.E. (error bars) of measurements. The experiment was repeated twice. n = 8/group. *, p < 0.05; **, p < 0.01 t test with Holm–Sidak correction for multiple testing.
TSG-6 is necessary for HA-induced airway hyperresponsiveness
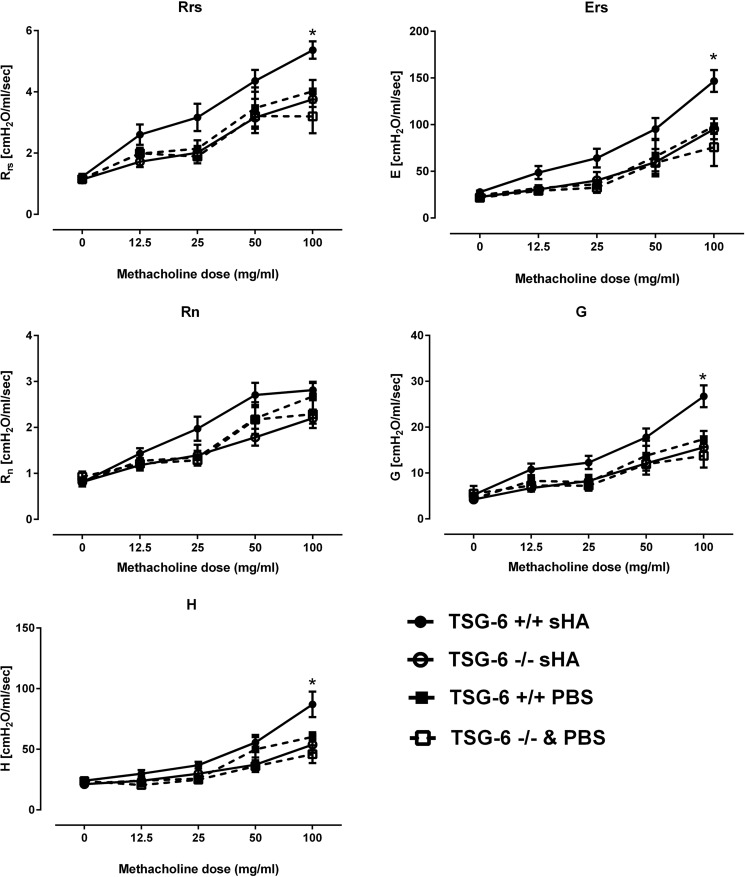
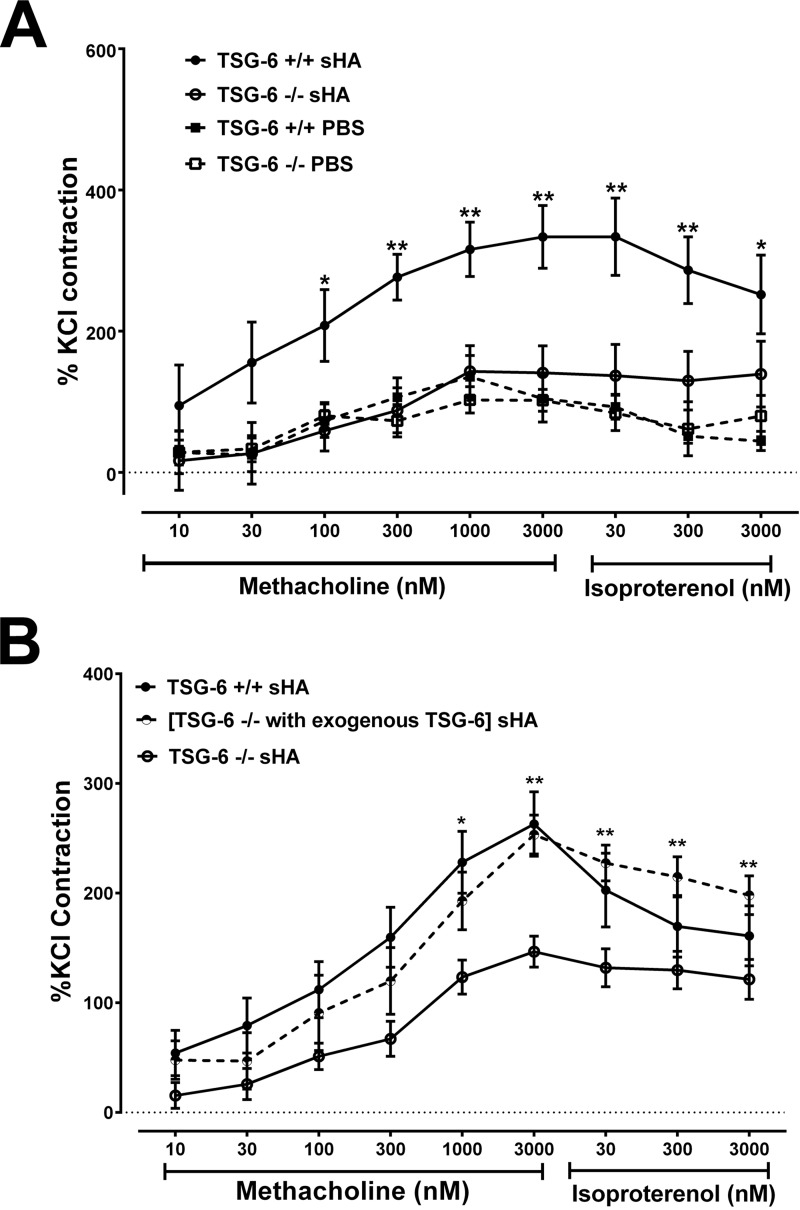
We have previously shown that HA mediates ozone-induced AHR (15, 18). Because we showed that TSG-6 was necessary for ozone-induced AHR, we next wanted to determine whether this role extends to HA-induced airway constriction. The results presented a striking similarity to the ozone phenotype (Fig. 4); TSG-6–sufficient mice exposed to sHA by oropharyngeal aspiration demonstrated increased Rrs and Ers, which were due to an increase in G and elastance H, but not Rn. The absence of TSG-6 completely abolished the sHA effect on the airways. Because we have previously shown that sHA effects after oxidative lung injury directly involve the airway smooth muscle (17), we further investigated whether the observed TSG-6 phenotype could be replicated in isolated tracheal rings, an in vitro system that does not contain inflammatory cells. Analysis using an isometric force transduction assay demonstrated absent contractility of tracheal rings exposed to sHA in TSG-6–deficient tracheal rings (Fig. 5A), whereas TSG-6–sufficient rings had a robust contractile response. Extracellular HA induces smooth-muscle depolarization and contractility via interaction with CD44 (17). We therefore hypothesized that exogenous, extracellular TSG-6 can rescue the contractile phenotype in response to sHA. Indeed, we observed that tracheal ring contractility of TSG-6–deficient rings, which were supplemented with pathological-range (27) TSG-6 in the bath, was indistinguishable from contractility of genetically TSG-6–sufficient mice (Fig. 5B). Exogenous TSG-6 did not significantly alter the contractile response of TSG-6–sufficient rings (not shown).
Figure 4.
Increased airway resistance and stiffness in TSG-6–sufficient mice after sHA exposure, due to increases in peripheral tissue values, parallels the ozone-induced changes. Mice received 50 μl of 3 mg/ml sHA by retropharyngeal aspiration, and 2 h later, they were anesthetized, paralyzed, tracheotomized, intubated, and phenotyped for airway physiology measurements via flexiVent. Rrs, respiratory system (whole-lung) resistance; Ers, respiratory system (whole-lung) elastance. Rn, Newtonian (large-airway) resistance. G, damping (peripheral lung resistance). H, peripheral lung elastance. PBS was used as vehicle. Results are represented as means ± S.E. (error bars). The experiment was repeated at least twice. n = 8/group. *, p < 0.05, t test with Holm–Sidak correction for multiple testing.
Figure 5.
Tracheal ring hyperresponsiveness to methacholine after 30-min sHA exposure. Isolated tracheal rings were bathed in Krebs solution with or without sHA for 30 min and then assayed for their contractile response to methacholine. A, TSG-6–sufficient tracheal rings demonstrate increased contractile response to methacholine after sHA exposure compared with PBS, whereas TSG-6–deficient tracheal rings do not increase their contractile response. B, in TSG-6–deficient tracheal rings, the addition of exogenous TSG-6 together with sHA for 30 min increases the contractile response to levels similar to TSG-6 genetically sufficient tracheal rings. PBS was used as vehicle. Results are represented as means ± S.E. (error bars). The experiment was repeated at least three times. n = 8/group. *, p < 0.05; **, p < 0.01, ANOVA with Tukey's multiple-comparison correction.
TSG-6 mediates activation of RhoA and Rho kinase after ASMC exposure to sHA
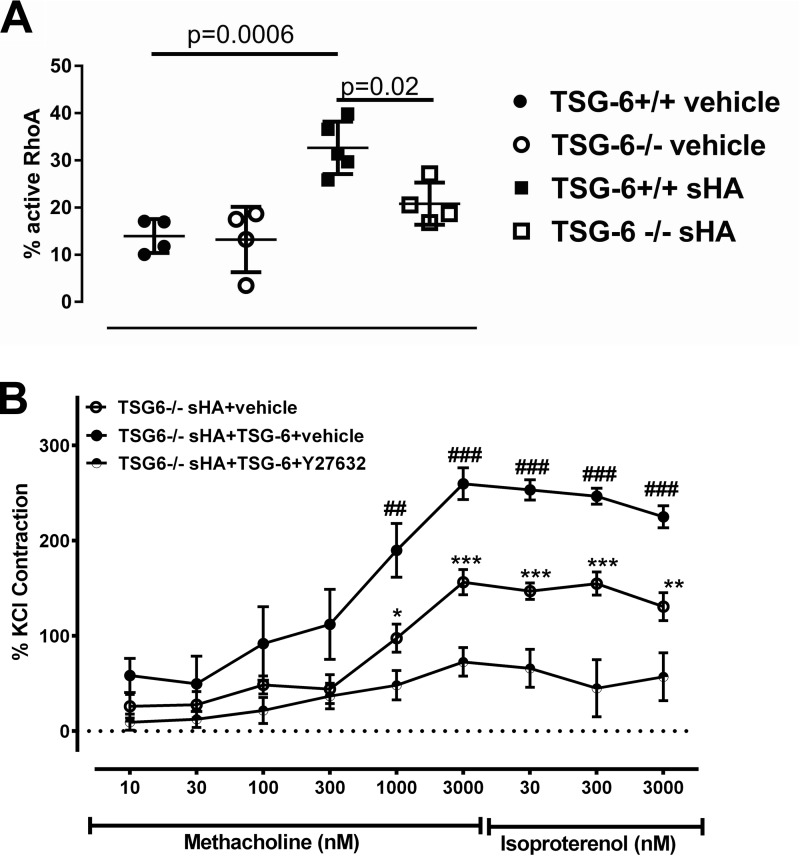
We have previously shown that sHA-induced airway hyperresponsiveness is mediated through RhoA and Rho kinase (ROCK) activation (17). We therefore tested whether TSG-6 activity in this context is also dependent on the RhoA pathway. We exposed isolated murine ASMC to sHA in vitro and found that RhoA is increased only in TSG-6–sufficient cells (Fig. 6A). In tracheal rings, inhibition of the RhoA pathway using ROCK inhibitor Y27632 ameliorated the TSG-6–induced contractility in sHA exposure (Fig. 6B). These results suggest that the TSG-6–mediated HA–HC complexes induce AHR through the RhoA pathway.
Figure 6.
TSG-6 effects on ASMC are mediated through the RhoA pathway. Primary mouse ASMC were cultured to 80% confluence and then washed repeatedly and serum-starved for 4 h and finally exposed to sHA or vehicle, as indicated. A, sHA induces activation of RhoA in murine ASMC, but only in TSG-6–sufficient cells. The experiment was repeated at least twice. n = 4–5/group. B, exogenous TSG-6 rescues sHA-induced hyperresponsiveness to methacholine, but this effect is inhibited by ROCK inhibitor Y27632. Isolated tracheal rings were bathed for 30 min in Krebs solution with sHA and added TSG-6 with Y27632 or respective vehicles, as indicated, and then assayed for their contractile response to methacholine. Results are represented as means ± S.E. (error bars). The experiment was repeated at least twice. n = 8/group. *, p < 0.05; **, p < 0.01; ***, p < 0.001, TSG-6 sHA + vehicle compared with TSG-6 sHA + TSG-6 + Y27632; ##, p < 0.001; ###, p < 0.001, TSG-6 sHA + TSG-6 + vehicle compared with TSG-6 sHA + vehicle and with TSG-6 sHA + TSG-6 + Y27632; ANOVA with Tukey's multiple-comparison correction.
TSG-6 effects on HA-induced contractility are dependent on ERK and PI3K/Akt
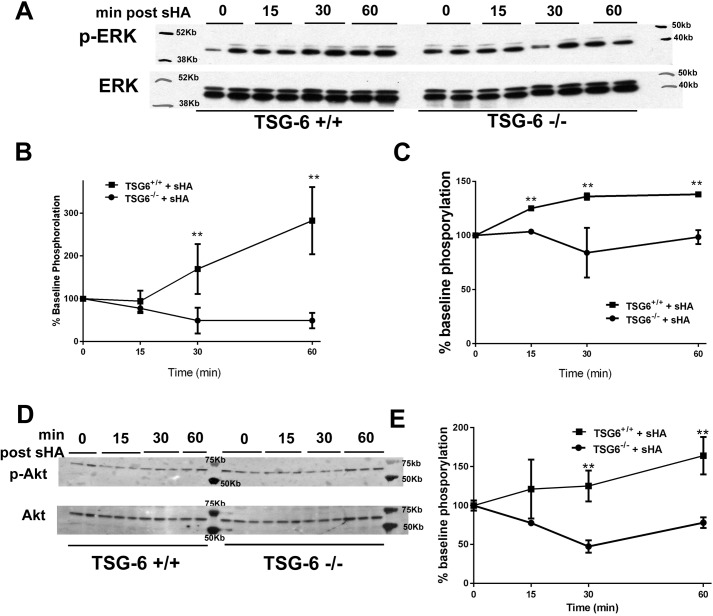
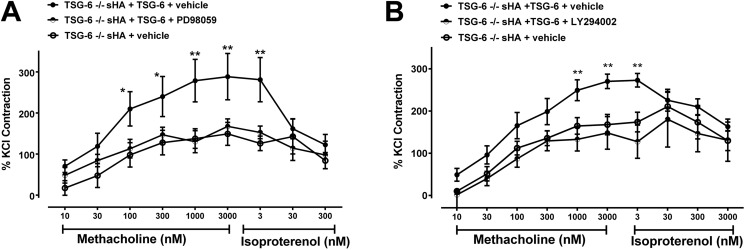
We further investigated the role of TSG-6 in the signaling pathway of sHA-induced contractility in vitro using isolated ASMC from TSG-6–deficient and –sufficient mice. We first performed a time course of kinase activation after sHA exposure. We found a significant increase in ERK phosphorylation of p42 and p44 within 15–30 min after sHA exposure, which was absent in TSG-6–deficient cells (Fig. 7, A–C). TSG-6 was also necessary for the phosphorylation of Akt threonine 308, which followed similar kinetics after sHA exposure in TSH-6–sufficient cells (Fig. 7, D and E). We then evaluated whether the observed activation of ERK and Akt had functional consequences. We again used the model of sHA-induced hyperresponsiveness in the tracheal ring assay and replicated the rescue of hyperresponsiveness by the addition of exogenous TSG-6 to deficient tracheal rings as our baseline observation. We found that both PD98059 (ERK inhibitor) and LY27632 (PI3K/Akt inhibitor), given for 30 min, ameliorated the TSG-6 rescue effects (Fig. 8). These results suggest that ERK and Akt are necessary for the HA–HC–TSG-6–induced airway hyperresponsiveness.
Figure 7.
ERK and Akt activation after sHA exposure is TSG-6–dependent. Primary mouse ASMC were cultured to 80% confluence and then washed repeatedly and serum-starved for 4 h and finally exposed to sHA or vehicle, as indicated. A, Western blotting for phospho-p42 and phospho-p44 ERK. B, quantification of phospho-p42 by densitometry shows a rapid increase of phosphorylated protein in TSG-6–sufficient cells, whereas deficient cells show no change. C, quantification of phospho-p44 by densitometry shows a rapid increase of phosphorylated protein in TSG-6–sufficient cells, whereas deficient cells show no change. D, Western blot for phospho-Akt at threonine 308 shows increased protein phosphorylation after sHA exposure, which is absent in TSG-6–deficient cells. E, quantification shows that the increase in phosphorylation is significant in TSG-6–sufficient cells. Results are represented as means ± S.E. (error bars). The experiment was repeated at least three times. n = 3–6/condition. **, p < 0.01, TSG-6–sufficient versus TSG-6–deficient, ANOVA with Tukey's multiple-comparison correction.
Figure 8.
TSG-6 rescue of sHA-induced AHR is abolished by ERK or PI3K/Akt inhibition. Isolated tracheal rings were bathed for 30 min in Krebs solution with sHA and added TSG-6 with ERK inhibitor PD98059, PI3K/Akt inhibitor LY294002, or respective vehicles, as indicated, and then assayed for their contractile response to methacholine. A, ERK inhibitor PD98059 abolished the exogenous TSG-6 effect in restoring sHA-induced contractility to methacholine. B, PI3K/Akt inhibitor LY294002 has similar effects. Results are represented as means ± S.E. (error bars). The experiment was repeated at least three times. n = 6–8 tracheal rings/group. *, p < 0.05; **, p < 0.01, TSG-6–deficient with sHA, exogenous TSG-6, and respective inhibitor, compared with TSG-6–deficient with sHA, exogenous TSG-6, and respective vehicle; ANOVA with Tukey's multiple-comparison correction.
Discussion
In this work, we provide proof for a role of TSG-6 in the development of AHR in acute airway inflammation. We show that TSG-6 mediates the formation of HA–HC complexes within minutes ex vivo and within hours in vivo and that TSG-6 is necessary for HA-induced AHR. Furthermore, we provide novel proof that the TSG-6/HA–mediated AHR pathway involves activation of kinases ERK and Akt.
HA signaling plays a central role in the response to airway injury. sHA is released in response to oxidative stress, either by fragmentation of existing structural HA or by de novo synthesis, and promotes inflammation and AHR. Increased airway sHA levels (e.g. in bronchoalveolar lavage fluid) are a hallmark of virtually every lung disease studied to date, notably found in asthma, COPD, fibrotic lung disease, and lung allograft rejection (28). However, sHA can only mediate inflammation and AHR after further interaction with the ECM. Indeed, airway injury promotes the development of a pathological HA matrix (i.e. linkage of sHA to IαI HC) (6), which is mediated by TSG-6, supports sHA binding to CD44 (5) and unfolds its downstream effects. sHA signaling is therefore subject to an intricate regulatory process that includes TSG-6 and IαI as well as a complex array of receptors. This offers intriguing targets for potential therapeutic applications in airway disease. Indeed, HMW-HA or IαI-blocking antibodies can inhibit AHR in oxidative lung injury (15, 17). Furthermore, the absence of TSG-6 protects from the development of a pathological HA matrix as well as AHR in an allergic murine airway inflammation model (3). Finally, CD44 blockade ameliorates inflammation and AHR in allergic asthma (29). Collectively, these studies highlight the central role of HA in the development of airway disease and the need to fully elucidate HA airway pathobiology to predict the best targets for therapeutic or prophylactic interventions.
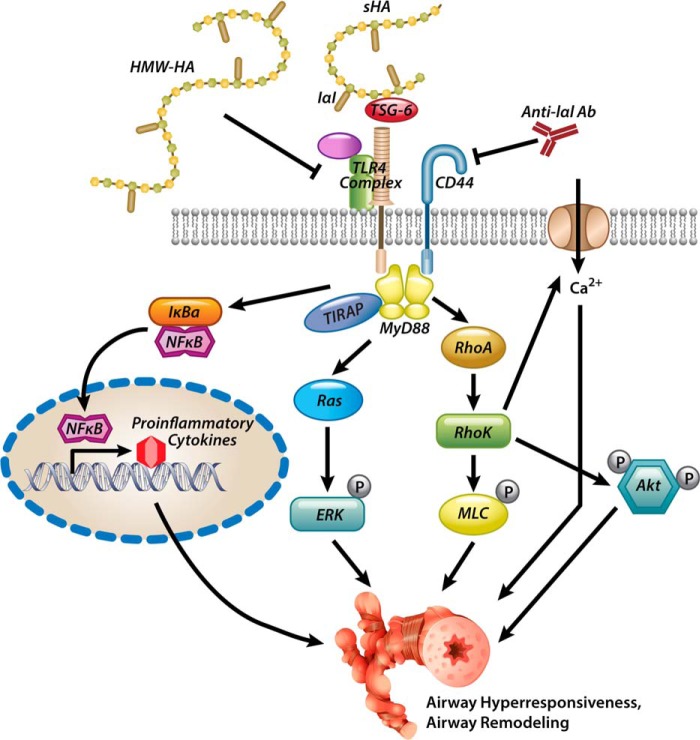
We have shown previously that sHA is released in the airway compartment and is necessary and sufficient for the development of AHR after ozone (15) and chlorine gas (17) exposure. These findings suggest that oxidative stress in the airways induces HA-innate immune interactions, which ultimately mediate AHR. The signaling pathway involves IαI (6), CD44 (6), TLR4 (7), and its downstream adaptors MyD88 and TIRAP as well as RhoA and activation of calcium channels. We are now expanding on these findings to demonstrate that TSG-6 is necessary for the development of ozone-induced AHR and that sHA/TSG-6 signaling is mediated by intracellular kinases Rho kinase, ERK, and PI3K/Akt (Fig. 9). These results elucidate the HA pathway in inflammatory AHR.
Figure 9.
Proposed model of HA-induced AHR. Oxidative lung injury leads to the release of sHA (15, 17), which forms complexes with IαI HC via TSG-6 (*). HA–HC complexes activate CD44 and TLR4 signaling (15, 18), leading to AHR. High-molecular weight HA and anti-IαI antibodies (15, 17) or genetic absence of IαI (15) or TSG-6 (*) can inhibit the development of AHR. MyD88 (19), RhoA (*), ERK (*), and Akt (*), and calcium influx (17) are intracellular mediators of the sHA-induced AHR. The asterisks in parentheses denote novel contributions of the present paper to the proposed model.
TSG-6 is crucial for the development of a pathological HA matrix and airway remodeling in allergic airway disease, which is a chronic inflammatory process leading to the deposition of a pathological HA matrix (6). We now show that extracellular TSG-6 is also necessary for sHA signaling and the development of AHR in acute airway inflammation without significant airway remodeling. TSG-6–deficient mice had decreased formation of HA–HC complexes by Western blotting and decreased colocalization of HA with IαI by immunohistochemistry. Although we used a polyclonal antibody against IαI, this antibody has been reported previously to have low affinity to murine bikunin (30). The immunohistochemical findings do not lend themselves easily to quantification; however, the staining conveyed the impression of decreased hyaluronan deposition as well. In conjunction with the Western blotting results showing increased free HC after hyaluronidase digestion in ozone-exposed lungs (Fig. 2, B and C), our results thus suggest that the formation of pathological HA–HC complexes takes place in acute inflammation and is necessary for the development of AHR within hours after exposure. Indeed, our data suggest that the development of pathological HA–HC complexes, mediated by TSG-6, must take place in minutes, because TSG-6–mediated up-regulation of RhoA was evident as early as 5 min after sHA exposure, and other kinases were elevated 15 min after sHA exposure; furthermore, instillation of sHA in vivo led to a TSG-6–dependent AHR within 2 h. This is a significantly accelerated process, compared with previously described in vitro kinetics (31). This may suggest that conditions in tissue significantly shift the equilibrium toward formation of HA–HC complexes in the presence of TSG-6. Further research will be needed to determine whether this is due to the presence of additional ECM factors or the specific conditions (temperature, pH, ionic strength) in tissues as opposed to those in the test tube.
An interesting observation derived from Fig. 2 (B and C) is also that there is more anti-IαI immunoreactivity in TSG-6–deficient lungs than in their wild-type counterparts (bands at 250 and 125 kDa). Because most IαI in the airways after ozone exposure is derived from inflammatory extravasation, this may suggest that TSG-6 deficiency leads to increased epithelial leakage. Indeed, TSG-6–deficient mice have increased total protein in their lavage fluid compared with wild-type mice after ozone exposure (Fig. S2), which is an indicator of epithelial leakage. Others have shown that epithelial high-molecular weight HA promotes epithelial resilience after injury (32). Thus, it is possible that TSG-6 not only contributes to sHA signaling toward increased AHR, but also high-molecular weight HA signaling supporting epithelial resiliency to ozone-induced leakage. Increased IαI (through extravasation or de novo local expression) may also contribute to the development of AHR after ozone exposure, because its availability would contribute to the development of pathological HA matrix, when TSG-6 is present to facilitate HA–HC binding.
In our model (Fig. 9), the absence of TSG-6 inhibits ozone-induced AHR and cell infiltration but does not significantly affect cytokine gene expression. Furthermore, sHA and TSG-6 mediate AHR in naive ASMC, without the presence of inflammatory cells. These results suggest that pathological HA matrix can promote AHR in the absence of immune cell–mediated inflammation. Notably, in a model of allergic airway inflammation, TSG-6 deficiency ameliorated AHR without changing Th2 cytokine expression (3). In aggregate, these data suggest that AHR, although primed and induced by the inflammatory process, needs the interaction of ASMC with their pericellular matrix in order to fully unfold. In our proposed model, the ASMC is an independent effector unit, which will only develop AHR by interaction with a pathological HA matrix (sHA bound to IαI HC via TSG-6). This offers the intriguing prospect of HA signaling modification as a broad-spectrum treatment for AHR, regardless of the underlying inflammatory type. Indeed, inhibition of HA signaling through the absence of TSG-6 ameliorates AHR after ozone exposure (a Th1-mediated process) and after ovalbumin exposure (a fundamentally different, Th2-mediated process). In this process, cell influx may be an important component of HA generation. Neutrophils infiltrate the airways early after ozone exposure, and neutrophil depletion inhibits ozone-induced AHR (33). Neutrophils are an important source of reactive oxygen species, which are known to fragment high-molecular weight HA in the ECM into pro-inflammatory sHA, which attracts more neutrophils (34). This feed-forward loop may be ameliorated by inhibition of sHA binding, as occurs in the absence of IαI (15) and TSG-6 (current work).
We have shown previously that RhoA activation is induced by sHA and leads to ASMC membrane depolarization and AHR in oxidative lung injury (17). We are now expanding the knowledge about intracellular kinases that are activated by sHA/TSG-6 and mediate AHR. Our results suggest that ERK and PI3K/Akt are involved in mediating sHA-induced AHR and are necessary for its development. ERK has been shown to mediate inflammatory AHR (35, 36) as well as calcium/calmodulin signaling (37) in previous studies. We are currently investigating whether RhoA and ERK are acting in the same pathway (38, 39) or whether they represent parallel activated pathways that are independently necessary for the development of AHR. Akt is also downstream of the RhoA pathway (40) and has also been implicated in the pathogenesis of AHR (41–43). In aggregate, our results suggest that sHA is a crucial mediator of the activation of RhoA, ERK, and Akt in airway disease, thus offering an attractive treatment target for AHR.
It should be noted that TSG-6 has been shown to have prominent anti-inflammatory activity in a range of diseases and environmental exposures, including lung inflammation after endotoxin (21) and bleomycin exposures (22). In fact, TSG-6 has been postulated to be the crucial factor determining beneficial effects of therapeutically applied stem cells in diverse conditions, such as myocardial infarction (25), peritonitis (24), or myelosuppression (44). We believe that the apparent discrepancy between these reports and our study, as well as other studies supporting an adverse role for TSG-6 in chronic allergic lung inflammation (3), is due to the inflammatory context, in particular the dominant mechanism behind the observed phenotype. In conditions where pathological HA matrix formation is a necessary pathomechanism, such as the development of AHR after ozone or in asthma (1–3, 15, 18), TSG-6 may have adverse effects by mediating the formation of pathological HA matrix and airway smooth-muscle contraction. However, when HA deposition is not the major pathomechanism of disease, such as in endotoxin lung inflammation or myocardial infarction (21, 25), TSG-6 non-HA-related anti-inflammatory effects predominate. An interesting parallel has been demonstrated with another HA binding partner (i.e. IαI), which is necessary for ozone– and chlorine gas–induced AHR (15, 17) but protects from endotoxin-induced sepsis (45), complement-induced lung injury (46), and naphthalene-induced lung injury (47) not by hyaluronan-mediated effects, but by inhibiting disseminated intravascular coagulation, inhibiting complement activation, or promoting vitronectin binding, respectively.
It should be noted that the relative abundance of HA–HC complexes in our whole-lung analysis appears relatively low, compared with the amount of IαI and pre-αI. As discussed above, IαI extravasates into inflamed tissues (such as ozone-exposed lungs) very readily and is expected to reach high concentrations at the inflamed site, given its high concentrations in mammalian blood. However, TSG-6 may contribute to ozone-induced AHR independently of IαI as well. For example, TSG-6 can induce HA cross-linking independently of IαI (48). Because IαI inhibits TSG-6-HA complex formation (49), this mechanism is unlikely to play a significant role in late stages of HA matrix formation; however, it is entirely possible that this “preliminary” TSG-6–HA matrix mediates HA-dependent effects in the very early stages of response.
In conclusion, we have demonstrated that TSG-6 mediates the formation of HA complexes after in vivo exposure to the environmental pollutant ozone. These complexes are evident in vivo at 24 h after exposure. Ex vivo, TSG-6 is necessary for the activation of RhoA, ERK, and PI3K/Akt after sHA exposure, and this activation occurs within minutes, suggesting that the formation of HA–HC complexes is a very rapid process in tissues. TSG-6 is also necessary for the development of sHA-induced AHR, which is also a rapidly induced event, occurring 30 min after exposure. TSG-6–mediated formation of HA complexes is thus a rapidly occurring and necessary step in the development of environmentally induced AHR. These results suggest a novel therapeutic target in airway disease.
Experimental procedures
Mice
Experimental groups consisted of TSG-6−/− or TSG-6+/+ mice, on a BALB/cByJ background, purchased from Jackson laboratories (stock number 001026) and bred in our vivarium. All experimental procedures were approved by the NIEHS, National Institutes of Health, Animal Care and Use Committee.
Chemicals and enzymes
The following chemicals and enzymes were used: Pronase (catalog no. 10-165-921-001, Roche Applied Science); collagenase type IV (catalog no. 4188) and elastase (catalog no. 2292) (Worthington); penicillin/streptomycin/fungizone (catalog no. 15240112, Invitrogen); pancuronium bromide (P-1918), methacholine (A-2251), isoproterenol (I6504), urethane (U-2500), potassium chloride (P5405), Y27632 (Y0503), and PD98059 (P215) (Sigma-Aldrich); sHA (sonicated Healon) (Abbott); and recombinant TSG-6 (donated by H. Wisniewski). HA and TSG-6 were tested for LPS contamination by the Limulus amebocyte lysate assay; LPS was below the detection limit for both reagents.
Ozone exposure
Mice were exposed to 2 ppm ozone for 3 h, and underwent the flexiVent procedure for the measurement of AHR or necropsy 24 h later. Mouse lungs were lavaged for inflammatory cell counts. The right lung tissue was snap-frozen for later gene expression analysis by quantitative RT-PCR using Sigma Gensys prequalified SYBER Green primers or HA–HC extraction and quantification. The left lung was embedded in OCT for immunohistochemistry for HA and IαI.
Immunohistochemistry
The staining method for IαI and hyaluronan has been described (3). Briefly, formalin-fixed sections were briefly incubated with blocking buffer (1% BSA in PBS) and then incubated with 5 μg/ml biotinylated HABP (catalog no. 385911, EMD/Millipore, Billerica, MA) and a rabbit polyclonal antibody against IαI (1:100 dilution, A0301, Dako North America, Inc., Carpentaria, CA) for 1 h at room temperature or overnight at 4 °C. After washing, secondary reagents were added and incubated for 1 h at room temperature in the dark. Alexa Fluor 488–conjugated (for HABP) and Alexa Fluor 594–conjugated (for anti-IαI) donkey anti-goat streptavidin was used as the secondary reagent. For the hyaluronidase-digested sections, after the blocking step, sections were digested with Streptomyces hyaluronidase diluted 1:100 in blocking solution (catalog no. 389561, EMD Millipore/Calbiochem, Billerica, MA) for 30 min at 37 °C, after which step sections were washed with PBS and probed with antibodies as described above. Each staining included a control of only the secondary reagent to demonstrate the specificity of staining. Sections were examined with a Leica DMIRB upright microscope equipped with a Retiga 2000R camera and QCapture Pro version 6 software.
Detection of HA–HC complexes
The protocol has been described (3). Briefly, lung tissues of mice were homogenized on wet ice and transferred to prechilled Eppendorf tubes. Prechilled PBS was added to the tubes at 100 μl of cold PBS for every 33 mg of tissue. A 50-μl aliquot of the minced tissue suspension was transferred to two new prechilled 1.5-ml tubes, and Streptomyces hyaluronidase (5 μl of a 0.5-turbidity unit/ml stock; catalog no. 389561, EMD/Millipore) was added to one of these tubes, whereas PBS (5 μl) was added to the other. The tubes were incubated on ice for 30 min and then centrifuged at 13,200 rpm for 5 min at 4 °C. The supernatants were transferred to new prechilled 1.5-ml tubes and incubated for another 30 min at 37 °C. Then 25 μl of the digests was added per lane on 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and blotted using a Bio-Rad nitrocellulose and Trans-Blot Turbo system. The blots were blocked for 1 h with blocking buffer (catalog no. 927-40000, LI-COR (Lincoln, NE)) and probed with a rabbit polyclonal antibody against IαI (1:8,000 dilution; A0301, Dako North America). This antibody can specifically recognize IαI and HC but not the isolated light chain bikunin in the mouse (30). The secondary antibody was IRDye 800CW anti-rabbit IgG (1:15,000 dilution; catalogue no. 926-32211, LI-COR). The blots were washed and imaged using an Odyssey infrared imaging system (LI-COR).
Airway physiology measurements
Mice were treated with either ozone/free air or sHA/PBS before airway physiology assessment via flexiVent (Scireq, Montreal, Canada). Ozone (O3) or free air exposure was done 24 h before flexiVent. sHA (0.15 mg) or PBS was instilled 2 h before flexiVent via oropharyngeal aspiration. Mice were anesthetized with 2 g/kg of urethane and intubated with an 18-g cannula secured to the trachea with a suture. Mouse cannula was attached to flexiVent Legacy system module 1 with a 1.8-ml stroke on the default ventilation pattern. Mice were paralyzed with 0.8 mg/kg pancuronium bromide 5 min before starting experiment. Mice were assessed using an alternating script of prime waves and snapshot for 12 readings for each dose of methacholine. Between doses, two deep inflations were done to fully inflate lungs. Nebulized methacholine doses assayed were 0, 12.5, 25, 50, and 100 mg/ml. Scireq software provided results for Rrs, Ers, G, and H.
Isometric force transduction
Isolated and cleaned mouse tracheas from TSG-6−/− or TSG-6+/+ mice were cut into ∼3-mm pieces and suspended in a 5 ml of oxygenated Krebs solution bath and attached to an isometric transducer. After equilibration to 1 × g of force contractility, maximum contractility was measured by dosing with 80 mm KCl solution, and then the tissue was washed and the KCl dose was repeated. After re-establishing baseline, tracheal rings were then dosed with combinations of sHA (0.5 mg/ml), TSG-6 (0.25 μg/ml), an inhibitor (either 10 μm Y27632, 30 μm LY26042, or 125 μm PD98059), or appropriate vehicle and incubated for 30 min before generating a contraction curve using methacholine (1–3,000 nm), followed by a relaxation curve using isoproterenol (30–3,000 nm), a β2 agonist.
RhoA assay
Smooth-muscle cells were isolated from TSG-6+/+ and TSG-6−/− mice. Each mouse was euthanized by pentobarbital injections, and tracheas were harvested as close to weaning as possible. Trachea were cleaned, cut lengthwise, and incubated overnight in Ham's F-12 (Invitrogen) with 0.15% Pronase and 2× penicillin/streptomycin/fungizone overnight at 4 °C. Tracheas were washed two times with 20 ml of DMEM/F-12 and transferred to a 100-mm Petri dish containing 10 ml of DMEM/F-12 with 2× penicillin/streptomycin/fungizone, 0.05% collagenase, and 0.025% elastase. Tracheas were cut into small pieces and incubated for 3–5 h at 37 °C. Digestion was centrifuged at 1500 rpm for 5 min, and pellets were resuspended in DMEM/F-12 with 10% FBS and 2× penicillin/streptomycin/fungizone and incubated at 37 °C with 5% CO2. Media were changed every 2 days while cells reached desired confluence. Cells were plated at 80% confluence in 100-mm dishes and were exposed to sHA, TSG-6, or both. Cells were harvested in lysis buffer supplied by Cytoskeleton Inc. (Denver, CO). Protein lysates were assayed using Cytoskeleton's Glisa and Total RhoA colorimetric ELISA kits as per the manufacturer's instructions.
Pathway analysis
Primary isolated mouse airway smooth-muscle cells are cultured from TSG-6+/+ and TSG-6−/− mice as stated above. Cells were exposed to media or sHA for 5, 15, or 30 min. Cells were harvested in lysis buffer supplied with the Cell Signaling PathScan intracellular signaling array kit and analyzed as per the manufacturer's protocol. This was validated by a standard Western blot assay using Novex NuPAGE 1.5-mm 4–12% bis-tris polyacrylamide gels loaded as per the manufacturer's specification for reducing conditions in 1× MOPS buffer. Gels were blotted onto PVDF membranes using program P3 in the I-Blot2 system. Membranes were blocked for 1 h with 4% BSA in TBST (0.1% Tween 20) and incubated overnight at 4 °C with antibodies against the following kinases: p44/42 MAPK (ERK1/2), phospho-p44/42 MAPK (ERK1/2 (Thr-202/Tyr-204)), Akt, or phospho-Akt (Ser-473), all from Cell Signaling Technologies. Blots were washed with 0.1% TBST four times for 5 min with agitation. Blots were then incubated with secondary anti-rabbit HRP (1:20,000) in 0.1% TBST for 1 h and washed four times with 0.1% TBST for 5 min with agitation. Signal detection used Thermo Scientific's Supersignal West Pico chemiluminescent substrate, which was detected using Kodak BioMax film. Film was scanned, and the image file was quantified using ImageJ.
Quantitative RT-PCR
RNA was isolated from snap-frozen tissue using the Qiagen RNeasy minikit as per the manufacturer's spin protocol. RNA was quantified with Nanodrop 1000. cDNA was generated using the Applied Biosystems high-capacity cDNA reverse transcription kit as per the manufacturer's protocol. A SYBER Green assay was done using Sigma predesigned KiCqStart primers for IRG-1 and MIP-2. Mouse Irg-1 primer sequences are GATGATCCTGGATTCTCTGG (forward) and AAAATCCATGGAGTGAACAG (reverse). Mouse Mip-2/CXCL2 primer sequences are GGGTTGACTTCAAGAACATC (forward) and CCTTGCCTTTGTTCAGTATC (reverse).
Statistical analysis
GraphPad Prism version 6 (La Jolla, CA) was used for analysis. Values were analyzed using ANOVA and Tukey's post hoc analysis or Holm–Sidak repeated measures correction, as appropriate.
Author contributions
V. P. S. performed most experiments for this project and wrote part of the paper. C. G. J., A. M., and V. C. performed experiments and assays for this project. R. J. M., M. E. L., H.-G. W., and M. A. A. contributed reagents and provided feedback and edits on the concept of the project. S. G. conceived the idea, planned and coordinated all experiments, and wrote the paper.
Supplementary Material
This work was supported partly through funds from the Division of Intramural Research, NIEHS, National Institutes of Health, by Grants P01HL107147 (to V. C. and R. M.) and HL103453 and HL081064 (to M. A. A.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work is dedicated to the memory of Mark E. Lauer, who passed away unexpectedly before completion of this work.
This article contains Figs. S1 and S2.
- ECM
- extracellular matrix
- HA
- hyaluronan
- sHA
- short-fragment HA
- HMW-HA
- high-molecular weight HA
- ASMC
- airway smooth-muscle cell(s)
- HC
- heavy chain(s)
- IαI
- inter-α-inhibitor
- AHR
- airway hyperresponsiveness
- ROCK
- Rho-associated, coiled-coil–containing protein kinase
- bis-tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ANOVA
- analysis of variance.
References
- 1. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., and Aronica M. A. (2011) Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 30, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., and Aronica M. A. (2013) Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology 23, 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swaidani S., Cheng G., Lauer M. E., Sharma M., Mikecz K., Hascall V. C., and Aronica M. A. (2013) TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J. Biol. Chem. 288, 412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang J., Jiang D., Jung Y., Xie T., Ingram J., Church T., Degan S., Leonard M., Kraft M., and Noble P. W. (2011) Role of hyaluronan and hyaluronan-binding proteins in human asthma. J. Allergy Clin. Immunol. 128, 403–411.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhuo L., Kanamori A., Kannagi R., Itano N., Wu J., Hamaguchi M., Ishiguro N., and Kimata K. (2006) SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 281, 20303–20314 [DOI] [PubMed] [Google Scholar]
- 6. Lauer M. E., Majors A. K., Comhair S., Ruple L. M., Matuska B., Subramanian A., Farver C., Dworski R., Grandon D., Laskowski D., Dweik R. A., Erzurum S. C., Hascall V. C., and Aronica M. A. (2015) Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J. Biol. Chem. 290, 23124–23134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dockery D. W., Pope C. A 3rd, Xu X., Spengler J. D., Ware J. H., Fay M. E., Ferris B. G. Jr., and Speizer F. E. (1993) An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 329, 1753–1759 [DOI] [PubMed] [Google Scholar]
- 8. Bell M. L., McDermott A., Zeger S. L., Samet J. M., and Dominici F. (2004) Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292, 2372–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gryparis A., Forsberg B., Katsouyanni K., Analitis A., Touloumi G., Schwartz J., Samoli E., Medina S., Anderson H. R., Niciu E. M., Wichmann H. E., Kriz B., Kosnik M., Skorkovsky J., Vonk J. M., and Dörtbudak Z. (2004) Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am. J. Respir. Crit. Care Med. 170, 1080–1087 [DOI] [PubMed] [Google Scholar]
- 10. Katsouyanni K., Zmirou D., Spix C., Sunyer J., Schouten J. P., Pönkä A., Anderson H. R., Le Moullec Y., Wojtyniak B., Vigotti M. A., et al. (1995) Short-term effects of air pollution on health: a European approach using epidemiological time-series data. The APHEA project: background, objectives, design. Eur. Respir. J. 8, 1030–1038 [PubMed] [Google Scholar]
- 11. Hubbell B. J., Hallberg A., McCubbin D. R., and Post E. (2005) Health-related benefits of attaining the 8-hr ozone standard. Environ. Health Perspect. 113, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ito K., De Leon S. F., and Lippmann M. (2005) Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology 16, 446–457 [DOI] [PubMed] [Google Scholar]
- 13. Levy J. I., Chemerynski S. M., and Sarnat J. A. (2005) Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology 16, 458–468 [DOI] [PubMed] [Google Scholar]
- 14. Fann N., Lamson A. D., Anenberg S. C., Wesson K., Risley D., and Hubbell B. J. (2012) Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. 32, 81–95 [DOI] [PubMed] [Google Scholar]
- 15. Garantziotis S., Li Z., Potts E. N., Kimata K., Zhuo L., Morgan D. L., Savani R. C., Noble P. W., Foster W. M., Schwartz D. A., and Hollingsworth J. W. (2009) Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J. Biol. Chem. 284, 11309–11317 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16. Bracke K. R., Dentener M. A., Papakonstantinou E., Vernooy J. H., Demoor T., Pauwels N. S., Cleutjens J., van Suylen R. J., Joos G. F., Brusselle G. G., and Wouters E. F. (2010) Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am. J. Respir. Cell Mol. Biol. 42, 753–761 [DOI] [PubMed] [Google Scholar]
- 17. Lazrak A., Creighton J., Yu Z., Komarova S., Doran S. F., Aggarwal S., Emala C. W. Sr., Stober V. P., Trempus C. S., Garantziotis S., and Matalon S. (2015) Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L891–L903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garantziotis S., Li Z., Potts E. N., Lindsey J. Y., Stober V. P., Polosukhin V. V., Blackwell T. S., Schwartz D. A., Foster W. M., and Hollingsworth J. W. (2010) TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am. J. Respir. Crit. Care Med. 181, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19. Li Z., Potts-Kant E. N., Garantziotis S., Foster W. M., and Hollingsworth J. W. (2011) Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6, e27137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20. Song W., Yu Z., Doran S. F., Ambalavanan N., Steele C., Garantziotis S., and Matalon S. (2015) Respiratory syncytial virus infection increases chlorine induced airway hyper-responsiveness. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L205–L210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danchuk S., Ylostalo J. H., Hossain F., Sorge R., Ramsey A., Bonvillain R. W., Lasky J. A., Bunnell B. A., Welsh D. A., Prockop D. J., and Sullivan D. E. (2011) Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res. Ther. 2, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foskett A. M., Bazhanov N., Ti X., Tiblow A., Bartosh T. J., and Prockop D. J. (2014) Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L120–L131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahoney D. J., Swales C., Athanasou N. A., Bombardieri M., Pitzalis C., Kliskey K., Sharif M., Day A. J., Milner C. M., and Sabokbar A. (2011) TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum. 63, 1034–1043 [DOI] [PubMed] [Google Scholar]
- 24. Choi H., Lee R. H., Bazhanov N., Oh J. Y., and Prockop D. J. (2011) Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 118, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee R. H., Pulin A. A., Seo M. J., Kota D. J., Ylostalo J., Larson B. L., Semprun-Prieto L., Delafontaine P., and Prockop D. J. (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oh J. Y., Roddy G. W., Choi H., Lee R. H., Ylöstalo J. H., Rosa R. H. Jr, and Prockop D. J. (2010) Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. U.S.A. 107, 16875–16880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forteza R., Casalino-Matsuda S. M., Monzon M. E., Fries E., Rugg M. S., Milner C. M., and Day A. J. (2007) TSG-6 potentiates the antitissue kallikrein activity of inter-α-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 36, 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lauer M. E., Dweik R. A., Garantziotis S., and Aronica M. A. (2015) The rise and fall of hyaluronan in respiratory diseases. Int. J. Cell Biol. 2015, 712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katoh S., Matsumoto N., Kawakita K., Tominaga A., Kincade P. W., and Matsukura S. (2003) A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J. Clin. Invest. 111, 1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lauer M. E., Fulop C., Mukhopadhyay D., Comhair S., Erzurum S. C., and Hascall V. C. (2009) Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-α-inhibitor heavy chain attachment. J. Biol. Chem. 284, 5313–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauer M. E., Glant T. T., Mikecz K., DeAngelis P. L., Haller F. M., Husni M. E., Hascall V. C., and Calabro A. (2013) Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J. Biol. Chem. 288, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., and Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 33. O'Byrne P. M., Walters E. H., Gold B. D., Aizawa H. A., Fabbri L. M., Alpert S. E., Nadel J. A., and Holtzman M. J. (1984) Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am. Rev. Respir. Dis. 130, 214–219 [DOI] [PubMed] [Google Scholar]
- 34. Gao F., Koenitzer J. R., Tobolewski J. M., Jiang D., Liang J., Noble P. W., and Oury T. D. (2008) Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem. 283, 6058–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rahman M. S., Yamasaki A., Yang J., Shan L., Halayko A. J., and Gounni A. S. (2006) IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J. Immunol. 177, 4064–4071 [DOI] [PubMed] [Google Scholar]
- 36. Sakai H., Nishizawa Y., Nishimura A., Chiba Y., Goto K., Hanazaki M., and Misawa M. (2010) Angiotensin II induces hyperresponsiveness of bronchial smooth muscle via an activation of p42/44 ERK in rats. Pflugers Arch. 460, 645–655 [DOI] [PubMed] [Google Scholar]
- 37. Midgley A. C., Rogers M., Hallett M. B., Clayton A., Bowen T., Phillips A. O., and Steadman R. (2013) Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 288, 14824–14838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu S., Korzh V., Gong Z., and Low B. C. (2008) RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene 27, 1580–1589 [DOI] [PubMed] [Google Scholar]
- 39. Zhao M., Discipio R. G., Wimmer A. G., and Schraufstatter I. U. (2006) Regulation of CXCR4-mediated nuclear translocation of extracellular signal-related kinases 1 and 2. Mol. Pharmacol. 69, 66–75 [DOI] [PubMed] [Google Scholar]
- 40. Li Z., Dong X., Wang Z., Liu W., Deng N., Ding Y., Tang L., Hla T., Zeng R., Li L., and Wu D. (2005) Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7, 399–404 [DOI] [PubMed] [Google Scholar]
- 41. Lee K. S., Lee H. K., Hayflick J. S., Lee Y. C., and Puri K. D. (2006) Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 20, 455–465 [DOI] [PubMed] [Google Scholar]
- 42. Choi Y. H., Jin G. Y., Li L. C., and Yan G. H. (2013) Inhibition of protein kinase C δ attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1α/VEGF pathway. PLoS One 8, e81773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng C., Ho W. E., Goh F. Y., Guan S. P., Kong L. R., Lai W. Q., Leung B. P., and Wong W. S. (2011) Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One 6, e20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie J., Broxmeyer H. E., Feng D., Schweitzer K. S., Yi R., Cook T. G., Chitteti B. R., Barwinska D., Traktuev D. O., Van Demark M. J., Justice M. J., Ou X., Srour E. F., Prockop D. J., Petrache I., and March K. L. (2015) Human adipose-derived stem cells ameliorate cigarette smoke-induced murine myelosuppression via secretion of TSG-6. Stem Cells 33, 468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jourdain M., Carrette O., Tournoys A., Fourrier F., Mizon C., Mangalaboyi J., Goudemand J., Mizon J., and Chopin C. (1997) Effects of inter-α-inhibitor in experimental endotoxic shock and disseminated intravascular coagulation. Am. J. Respir. Crit. Care Med. 156, 1825–1833 [DOI] [PubMed] [Google Scholar]
- 46. Garantziotis S., Hollingsworth J. W., Ghanayem R. B., Timberlake S., Zhuo L., Kimata K., and Schwartz D. A. (2007) Inter-α-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J. Immunol. 179, 4187–4192 [DOI] [PubMed] [Google Scholar]
- 47. Adair J. E., Stober V., Sobhany M., Zhuo L., Roberts J. D., Negishi M., Kimata K., and Garantziotis S. (2009) Inter-α-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J. Biol. Chem. 284, 16922–16930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baranova N. S., Nileback E., Haller F. M., Briggs D. C., Svedhem S., Day A. J., and Richter R. P. (2011) The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 286, 25675–25686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baranova N. S., Foulcer S. J., Briggs D. C., Tilakaratna V., Enghild J. J., Milner C. M., Day A. J., and Richter R. P. (2013) Inter-α-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J. Biol. Chem. 288, 29642–29653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.