Abstract
CT-guided percutaneous biopsy is a resourceful and widely used tool to evaluate pulmonary nodules that frequently avoids costly and unnecessary surgeries. Severe complications occur in less than 1% of cases and include gas embolism, which is rarely documented. We report a case of gas embolism after transthoracic biopsies and discuss the pathophysiology and the benefits of early diagnosis and proper management.
Keywords: Computed tomography, Lung tumor, CT guided lung biopsy, Air embolism, Gas embolism
CASE REPORT
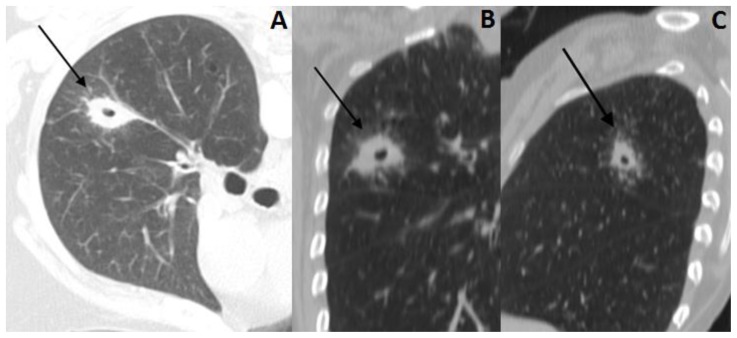
A 57-year-old female with history of locally advanced squamous cell carcinoma of the larynx and tracheostomy underwent a routine cervical CT scan, which revealed a cavitary lung nodule in the right upper lobe, measuring 2.5 × 1.4 cm, that was suspicious for malignancy (Figure 1).
Figure 1.
57 year old female with systemic air embolism after percutaneous lung biopsy.
FINDINGS: Axial CT scan (A) and reformatted images (coronal-B and sagittal-C) shows a cavitary lung nodule (black arrow) in the right upper pulmonary lobe, suspicious for malignancy.
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
A biopsy was performed in an interventional suite with CT fluoroscopy capabilities (Brilliance 40 CT Scanner, Phillips, Netherlands). The procedure was performed with local anesthesia and a 16/18-gauge semi-automatic coaxial core-biopsy needle (QCS-18-15.0-10T, Cook, USA). No sedation was employed and the patient was placed in the prone position.
Immediately after the needle was positioned inside the nodule (Figure 2), the patient developed cough, hemoptysis and agitation. Immediate imaging revealed gas inside the thoracic aorta (Figures 3, 4). The patient quickly developed cardiac arrest with pulseless electrical activity, and cardiopulmonary resuscitation procedures, including norepinephrine administration, were performed. Return to spontaneous circulation and blood pressure stabilization occurred within approximately five minutes. Decision was made to take the patient immediately to the intensive care unit for clinical management, and no CT of the brain was performed at that time. The clinical staff opted not to perform a CT of the brain later because the patient did not develop neurological deficits. A chest radiograph performed three hours after the procedure (Figure 5) showed no gas inside the thoracic aorta or coronary arteries. The patient remained hospitalized for thirteen days, and was discharged without any sequelae related to the gas embolism.
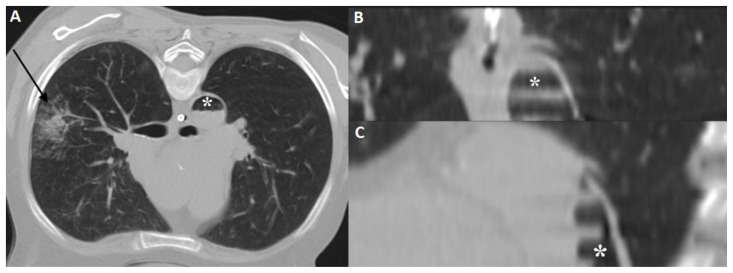
Figure 2.
57 year old female with systemic air embolism after percutaneous lung biopsy.
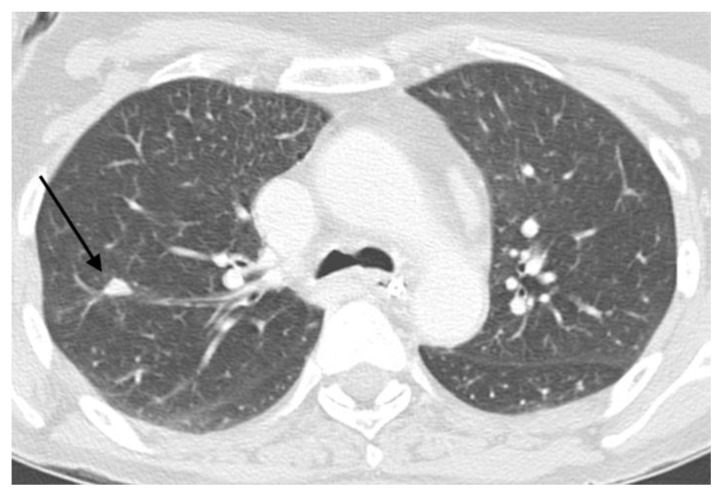
FINDINGS: Axial CT scan (A) and reformatted images (coronal-B and sagittal-C) shows the thoracic aorta (asterisk) in the beginning of the procedure, without abnormalities. Axial CT scan (A) shows the inferior portion of the lung nodule (black arrow).
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
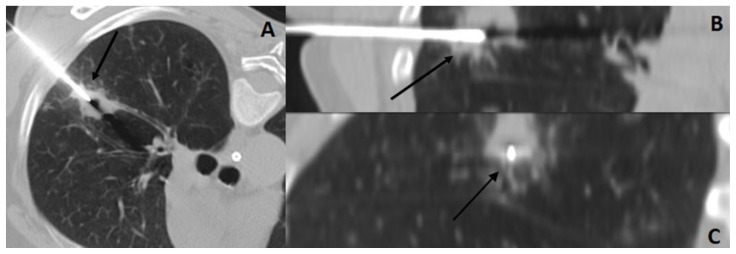
Figure 3.
57 year old female with systemic air embolism after percutaneous lung biopsy.
FINDINGS: Axial CT scan (A) and reformatted images (coronal-B and sagittal-C) shows the coaxial needle positioned inside the nodule (black arrow).
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
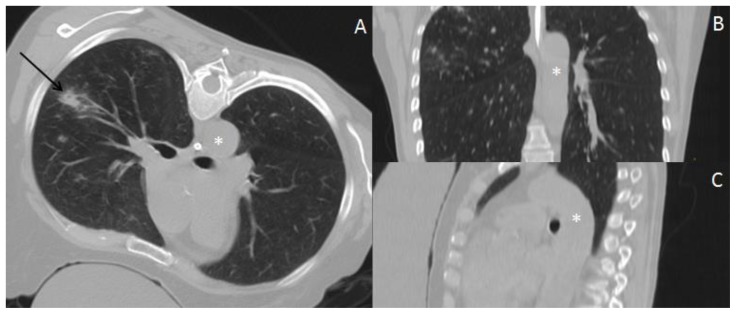
Figure 4.
57 year old female with systemic air embolism after percutaneous lung biopsy.
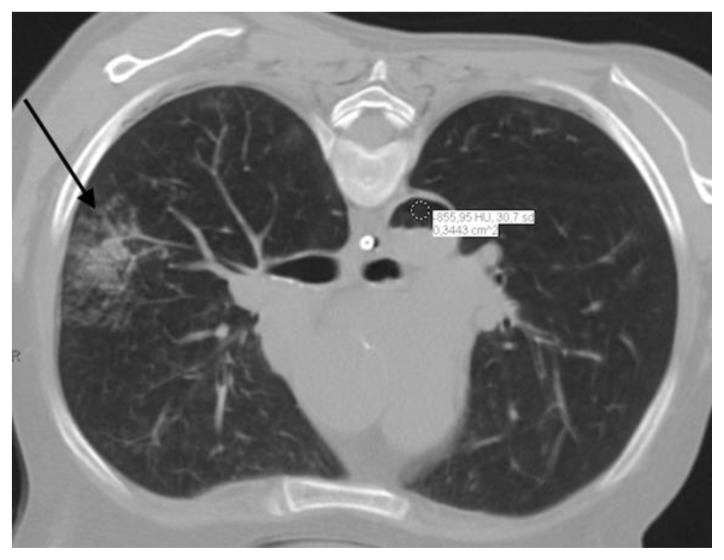
FINDINGS: Postprocedural axial CT scan (A) and reformatted images (coronal-B and sagittal-C) shows alveolar hemorrhage (black arrow) adjacent to the biopsied nodule and a significant amount of gas inside the thoracic aorta (asterisk), compatible with air embolism.
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
Figure 5.
57 year old female with systemic air embolism after percutaneous lung biopsy.
FINDINGS: Postprocedural axial CT scan shows alveolar hemorrhage (black arrow) adjacent to the biopsied nodule and a significant amount of gas inside the thoracic aorta. The finding is confirmed with the ROI inside the aorta demonstrating the density of -856 Hounsfield Units, which is corresponds to air density (air embolism).
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
Follow-up chest CT (Figure 7) two months after the procedure demonstrated spontaneous decrease in size of the lung nodule, suggesting a benign etiology.
Figure 7.
57 year old female with systemic air embolism after percutaneous lung biopsy.
FINDINGS: axial CT scan performed two months and fourteen days after the procedure shows significant volumetric reduction of the lung nodule (black arrow). The patient was not under chemotherapy, suggesting a benign nature of the nodule.
TECHNIQUE: Axial non-enhanced CT, 300mAs,120kV, 3mm slice thickness.
DISCUSSION
Etiology & Demographics
Pulmonary nodules represent a challenge to daily medical practice [1]. Pulmonary nodule detection became common after the development of multidetector computed tomography (CT) scanners in the late 1990s [2], and cancer screening trials will likely result in the detection of an even greater number of pulmonary nodules in the near future [3].
Despite the importance of CT scans in the evaluation of pulmonary nodules (table 2), these nodules are often classified as indeterminate. An image-guided percutaneous biopsy is therefore an important resource for the evaluation of pulmonary nodules [2,4], with an overall accuracy of 95% and a positive predictive value for the diagnosis of malignancies of up to 99.3%, even for nodules smaller than 1.0 cm in size [5]. Thus, this method may help avoid costly and unnecessary surgeries [4].
Table 2.
Differential diagnosis table for solitary pulmonary nodules and main features on CT.
| Group | Diagnosis | CT Presentation |
|---|---|---|
| Malignant neoplasms | Adenocarcinoma | Usually peripheral solid nodule. May also present as subsolid or ground-glass nodule. |
| Squamous cell carcinoma | Most common carcinoma to cavitate. When central, often results in intraluminal obstruction. When peripheral, may be associated with mucocele and other obstructive changes. | |
| Bronchioloalveolar carcinoma | Variable appearance, ranging from consolidation to multifocal subsolid nodules or masses. | |
| Small cell carcinoma | Early metastases, particularly nodal. Most common primary lung malignancy to cause superior vena cava obstruction. Necrosis and hemorrhage are common. May appear similar to lymphoma. | |
| Primary pulmonary lymphoma | Many presentations, ranging from interstitial infiltrates to masses. | |
| Primary pulmonary carcinoid | Usually central lesions in association with bronchial obstruction. Often homogeneous contrast enhancement. Eccentric calcification can occur. When peripheral, usually an incidental finding, presenting as a small solitary nodule. | |
| Solitary metastasis | Typically a well circumscribed rounded lesion, more often in the periphery of the lung. | |
| Benign neoplasms | Hamartoma | Well-circumscribed pulmonary nodule without significant growth in control images. May contain intralesional fat or calcification (popcorn configuration). |
| Chondroma | Well-circumscribed mass with calcified components | |
| Arteriovenous malformation | Homogeneous, well-circumscribed nodule. May be associated with phleboliths. Contrast-enhanced CT demonstrates feeding and draining vessels | |
| Fibroma | Pleural based mass, usually with intense contrast enhancement | |
| Neural tumor (schwannoma, neurofibroma) | Well-circumscribed round or elliptic mass in the paravertebral region or along the nerves courses. May erode adjacent bone and calcify | |
| Pulmonary pneumocytoma (sclerosing hemangioma) | Well-defined juxta-pleural intraparenchymal nodule or mass, with inhomogeneous enhancement. May have areas of calcification. | |
| Infectious |
Granuloma
|
Calcified pulmonary nodules (calcification may be central or diffuse). |
| Bacterial (Nocardia, Actinomycosis, round pneumonia) | Several patterns, which include solitary lung mass (may cavitate). | |
| Abscess | Thick irregular walls, abruptly interrupt the bronchovascular structures. | |
| Septic embolus | Peripheral nodules with feeding vessels, associated with lung abscesses, or subpleural nodular lesions (may contain necrosis/cavitation). | |
| Others | Sarcoidosis | Many presentations. May present with small nodules or large opacities or consolidation. |
| Lipoid pneumonia | Fat-containing consolidations. May be associated with ossification | |
| Amyloid | Many presentations. May present with slowly growing nodule, with smooth or lobulated contours, and central or irregular calcification. | |
| Subpleural lymph nodule | Measures 3.0–8.5 mm, may show a thin tag extending to the pleura. | |
| Rheumatoid arthritis | Many presentations. May present as peripheral nodule. May cavitate. | |
| Granulomatosis with polyangiitis | Usually multiple nodules, may cavitate. | |
| Pulmonary infarction | Wedge-shaped (or rounded) juxtapleural opacification without bronchograms (“Hampton hump”) | |
| Congenital | Bronchogenic cyst | Well-circumscribed spherical or ovoid mass of variable attenuation. May present calcium oxalate (“milk of calcium”). Usually no enhancement after contrast injection |
| Bronchial atresia with mucoid impaction | “Finger in glove” appearance. Distal lung parenchyma can be hyperlucent. | |
| Sequestration | Lung parenchyma with systemic arterial supply. Usually not aerated. |
The most frequent complication resulting from percutaneous pulmonary biopsies is pneumothorax (~35% of procedures) [6]. Known risk factors for pneumothorax include underlying chronic obstructive pulmonary disease, underlying moderate-to-severe emphysema, longer needle path (>4 cm), subpleural lesions, smaller lesions and wider insertion angle of the needle, among others [7,8]. Severe complications such as malignant seeding, tension pneumothorax, severe alveolar hemorrhage, hemothorax and gas embolism occur only in less than 1% of cases [9,10].
From January 2009 to October 2014, approximately 2750 thoracic percutaneous biopsies were performed at the author’s institutions. To date, only two cases of arterial gas embolism have been diagnosed (0.07%).
The incidence of arterial air embolism reported in the literature ranges from 0.02 to 0.4%. The actual rate is estimated to be approximately 3.8% because it is often asymptomatic and not diagnosed [11–13]. No large case series have been published.
Air embolism is likely caused by a fistula between the pulmonary vein and the atmosphere or the airway (bronchi or alveoli) created by the biopsy needle. Factors that increase the pressure gradient between the airway and pulmonary vein, such as positive end-expiratory pressure ventilation, coughing during biopsy, obstructive pulmonary disease with air trapping, and the prone position, are thought to increase the risk of air embolism [11,12,14]. Lower lobe localization and larger caliber needles may also increase the risk of air embolism [15,16]. However, no controlled series has been published in the literature to establish definitive risk factors. The reported patient was in the prone position, with a relatively large-caliber coaxial needle, and coughed during the procedure. Given the association between alveolar hemorrhage and coughing, it is controverse to suggest cough as an independent risk factor. More importantly, cough is not actually preventable. In our institution, many lung biopsies are performed with patients in the prone position because it is usually less stressful for patients (who often become anxious when viewing the needles during the procedure). The prone position also facilitates promptly turning the patients biopsy side down after the procedure to reduce the risk of pneumothorax [17]. In addition, we believe that choosing one lesion as a target over another based on the risk of such a rare event, such as an arterial air embolism, instead of more practical technical issues (such as attempting to traverse fewer vessels and less parenchyma or choosing larger and easier targets or less necrotic lesions) might not be ideal. Given this, we believe that instead of being concerned with non-modifiable, patient-related risk factors or debatable target-related issues, interventional radiologists should be concerned with early recognition and the adequate treatment of air embolisms.
The most serious concern regarding gas entering the arterial system is the occlusion of functional end arteries within the cardiac and cerebral circulation, which may result in severe morbidity or death [18,19]. Air bubbles in the coronary and cerebral arteries may cause mechanical obstruction, vasospasm induced by the intra-arterial air or air-induced platelet activation and thrombogenesis, or an inflammatory response in the endothelium resulting in impaired blood flow [19–21]. Again, the most critical issue in managing this situation is an accurate and early diagnosis.
Clinical & Imaging Findings
The clinical manifestations are mainly related to air embolism in the coronary or brain circulation, and include neurologic alterations (focal neurologic deficits, seizures, and altered levels of consciousness) and manifestations related to heart ischemia (chest pain, hypotension, dyspnea, dysrhythmias, and cardiopulmonary arrest). Importantly, these signs and symptoms may occur either immediately or several minutes after the biopsy. Moreover, an under-recognized proportion of patients submitted to lung biopsy likely develop air emboli and are asymptomatic [14,15,19]. Given the non-specific nature of these clinical alterations, we suggest that when these conditions are present, interventional radiologists should perform control CT scans that include the lung in the biopsied region, which is frequently performed, and include the heart and great vessels to search for intravascular and intracardiac air. This search might increase the detection of asymptomatic cases, and careful monitoring of these cases after biopsy may be important given the occurrence of delayed manifestations. However, performing control CT scanning should never delay emergency procedures, such as cardiopulmonary resuscitation, when indicated.
Some authors have even suggested the acquisition of systematic control images, including the entire aortocardiac region [22] or the entire chest [16], after every biopsy. However, it is unclear whether the benefits of performing this procedure for asymptomatic cases outweigh the resulting increase in radiation exposure for patients, given the extreme rarity of this complication. Moreover, we disagree with the suggestion by Rott and Boecker [16] of imaging the entire chest before repositioning the patient after biopsy to search for intravascular or intracardiac gas bubbles that could migrate when turning the patient; promptly turning the patient puncture side down significantly reduces the risk of pneumothorax [17], which is a much more common complication than gas embolism.
Treatment & Prognosis
If an air embolism is detected, the ideal management includes stopping the procedure, readying cardiopulmonary resuscitation procedures, warning the rapid response team, placing the patient in the right lateral decubitus position, supplying oxygen at 100%, and, if possible, arranging placement in a hyperbaric chamber within 4–6 hours. The right lateral decubitus position prevents air bubbles from entering the systemic circulation. Hyperbaric chambers may improve oxygenation in the ischemic tissue (Henry’s law) and reduce the dimensions of the gas bubbles (Boyle’s law). The chamber may also dissolve the bubbles (nitrogen reabsorption) and prevent cerebral edema [11]. It should be noted that most of these management steps, which have been published in the literature, are based on theories and personal experience. The good results observed in our patient and in others reported in the literature in which no hyperbaric chamber was available attest to the fact that the absence of this device should not be seen as a predictor of poor outcome.
In conclusion, arterial air embolism is a rare, non-predictable and non-avoidable potentially severe complication that is associated with percutaneous lung biopsies. Therefore, all interventional radiologists who perform this procedure must be prepared for early detection and proper initial treatment. While an arterial air embolism remains a potentially serious complication, the risk should not discourage interventional radiologists from performing well-indicated lung biopsies.
Differential Diagnoses
General imaging differential considerations for solitary pulmonary nodules include benign and malignant conditions. Among the benign etiologies, the most common are granulomas and hamartomas [6]. The most common malignant etiologies are divided between primary lesions, such as bronchioloalveolar carcinoma and squamous cell carcinoma [23], and solitary metastases (melanoma, osteosarcoma, testicle, colon, and others). Characteristics such as cavity wall thickness greater than 16mm, irregular speculated edge, size greater than 3 cm, history of malignancy, standardized uptake value higher than 2.5 on positron emission tomography (PET) and patient’s age over 70 years old, present likelihood ratios for malignancy above 4, whereas benign calcification pattern at CT, volume doubling time greater than 465 days and volume doubling time inferior than 7 days present likelihood ratios for malignancy below 0.01 [6].
Air embolism might be seen in association with surgery, trauma, intravascular catheters, barotrauma and rapid ascent in scuba divers [24]. Among those, notable causes are neurosurgical and otolaryngological procedures, penetrating or blunt trauma to the chest, intravenous injections of contrast media, insertion or removal of intravascular catheters, positive pressure mechanic ventilation and, as previously mentioned, rapid ascent without exhalation on scuba diving (table 3).
Table 3.
Notable conditions associated with air embolism.
| Surgical procedures |
|
| Intravenous catheterization |
|
| Radiologic procedures |
|
| Trauma |
|
| Other |
|
More rarely, macroscopic fat emboli can also be seen on CT [25,26]. In those cases, the filling defect shows a negative attenuation coefficient between -120 and -60 HU [27].
TEACHING POINT
Arterial air embolism is a rare, non-predictable and non-avoidable potentially severe complication of percutaneous lung biopsies. It is exceedingly rare but might be associated with high morbidity and mortality and therefore all interventional radiologists who perform this procedure must be prepared for detection on procedural images and adequate management.
Figure 6.
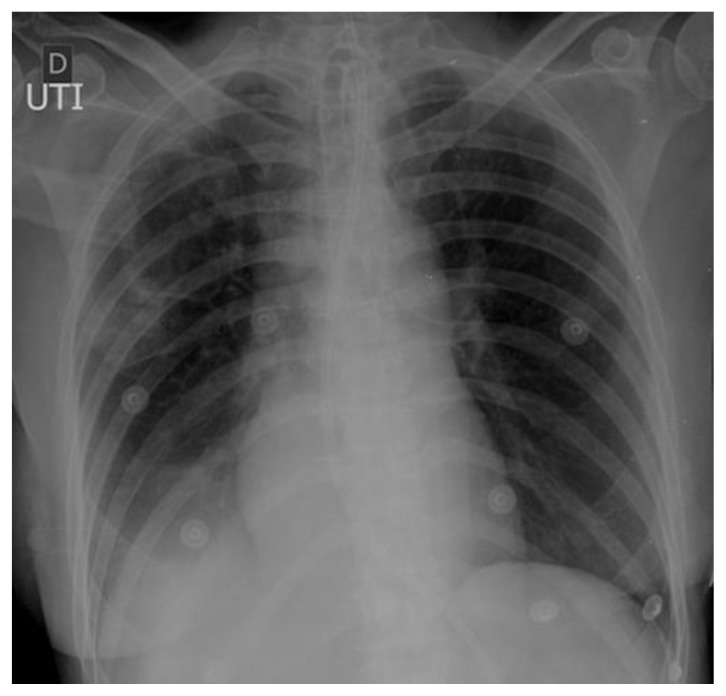
57 year old female with systemic air embolism after percutaneous lung biopsy.
FINDINGS: Chest radiograph performed 3h after the procedure in the ICU shows no gas detectable inside the thoracic aorta or coronary arteries.
TECHNIQUE: Radiography, 3.6 mAs, 85 kV, 200 mA.
Table 1.
Summary table for Systemic Air Embolism after Percutaneous Lung Biopsy.
| Etiology | Systemic air embolism after percutaneous lung biopsy. |
| Incidence | 0,02 to 0,4%. |
| Presumed Risk factors |
|
| Treatment |
|
| Prognosis | Majority of cases reported in literature are non-fatal, with full recoveries. |
| Findings on imaging | Intravascular and intracardiac air. |
ACKNOWLEDGEMENTS
I would like to express my deep gratitude to Dr. Marcello Silveira Rovella and Dr. Mauricio Ruettimann Liberato de Moura for their contributions in this paper.
ABBREVIATIONS
- CT
Computed tomography
REFERENCES
- 1.Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535–2542. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 2.Macmahon H, Austin JHM, Gamsu G, et al. Radiology guidelines for management of small pulmonary nodules detected on ct scans?: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 3.Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winer-muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34–49. doi: 10.1148/radiol.2391050343. [DOI] [PubMed] [Google Scholar]
- 5.Choi SH, Chae EJ, Kim J-E, et al. Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: analysis of outcomes of 305 procedures from a tertiary referral center. AJR Am J Roentgenol. 2013;201:964–970. doi: 10.2214/AJR.12.10156. [DOI] [PubMed] [Google Scholar]
- 6.Tomiyama N, Yasuhara Y, Nakajima Y, et al. 2006 CT-guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Wu CC, Maher MM, Shepard JA. Complications of CT-Guided Percutaneous Needle Biopsy of the Chest: Prevention and Management. AJR. 2011;196(6):678–82. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 8.Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;(Suppl 1):S99–S107. doi: 10.3978/j.issn.2072-1439.2013.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 10.Kothary N, Bartos JA, Hwang GL, et al. Computed tomography-guided percutaneous needle biopsy of indeterminate pulmonary pathology: efficacy of obtaining a diagnostic sample in immunocompetent and immunocompromised patients. Clin Lung Cancer. 2010;11:251–256. doi: 10.3816/CLC.2010.n.032. [DOI] [PubMed] [Google Scholar]
- 11.Hare SS, Gupta a, Goncalves aTC, Souza Ca, Matzinger F, Seely JM. Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol. 2011;66:589–596. doi: 10.1016/j.crad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Hiraki T, Fujiwara H, Sakurai J. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest. 2007;132:684–690. doi: 10.1378/chest.06-3030. [DOI] [PubMed] [Google Scholar]
- 13.Freund MC, Petersen J, Goder KC, et al. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med. 2012;12:2. doi: 10.1186/1471-2466-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke M, Reinhardt HC, von Bergwelt-Baildon M, et al. Massive air embolism after lung biopsy. Circulation. 2014;129:1046–1047. doi: 10.1161/CIRCULATIONAHA.113.004241. [DOI] [PubMed] [Google Scholar]
- 15.Ishii H, Hiraki T, Gobara H, et al. Risk factors for systemic air embolism as a complication of percutaneous CT-guided lung biopsy: multicenter case-control study. Cardiovasc Intervent Radiol. 2014;37:1312–1320. doi: 10.1007/s00270-013-0808-7. [DOI] [PubMed] [Google Scholar]
- 16.Rott G, Boecker F. Influenceable and avoidable risk factors for systemic air embolism due to percutaneous CT-guided lung biopsy: patient positioning and coaxial biopsy technique-case report, systematic literature review, and a technical note. Radiol Res Pract. 2014;2014:349062. doi: 10.1155/2014/349062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore EH, LeBlanc J, Montesi SA, et al. Effect of patient positioning after needle aspiration lung biopsy. Radiology. 1991;181:385–387. doi: 10.1148/radiology.181.2.1924776. [DOI] [PubMed] [Google Scholar]
- 18.Moon RE. Handbook on Hyperbaric Medicine. Milano: Springer Verlag Milan; 1996. pp. 229–248. [Google Scholar]
- 19.Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000;342:476–482. doi: 10.1056/NEJM200002173420706. [DOI] [PubMed] [Google Scholar]
- 20.Evans DE, Kobrine AI, Weathersby PK, et al. Cardiovascular effects of cerebral air embolism. Stroke. 1981;12:338–44A. [PubMed] [Google Scholar]
- 21.Dutka AJ. A review of the pathophysiology and potential application of experimental therapies for cerebral ischemia to the treatment of cerebral arterial gas embolism. Undersea Biomed Res. 1985;12:403–421. [PubMed] [Google Scholar]
- 22.Kuo H, Cheng L, Chung T. Systemic air embolism detected during percutaneous transthoracic needle biopsy: report of two cases and a proposal for a routine postprocedure computed tomography scan of the aorto-cardiac region. Clin Imaging. 2010;34:53–56. doi: 10.1016/j.clinimag.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Swensen SJ, Jett JR, Hartman TE, et al. Lung Cancer Screening with CT: Mayo Clinic Experience. Radiology. 2003;226:756–761. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 24.Dudney TM, Elliot CG. Pulmonary embolism from amniotic fluid, fat, and air. Prog Cardiovasc Dis. 1994;36:447. doi: 10.1016/s0033-0620(94)80053-7. [DOI] [PubMed] [Google Scholar]
- 25.Yeo SH, Chang HW, Sohn SI, et al. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5:239–242. doi: 10.4021/jocmr1251w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravenel JG, Heyneman LE, McAdams HP. J Thorac Imaging. 2002;17:154–156. doi: 10.1097/00005382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Ananthakrishnan L, Rajiah P, Ahn R, et al. Spectral detector CT-derived bib-contrast images: comparison of attenuation values with unenhanced CT. Abdom Radiol (NY) 2017;42:702–709. doi: 10.1007/s00261-016-1036-9. [DOI] [PubMed] [Google Scholar]