Abstract
Overview
Although the agreed‐upon standard is circumferential pathology analysis of the interface between the resected specimen and the patient, there is currently no consensus on the optimal methodology to achieve this in head and neck cancer specimens. This is most commonly conducted by either sampling the wound bed after resection or obtaining samples from the specimen. Regardless of the technique, only a fraction of the area of interest can be sampled due to the labor‐intensive nature of frozen sections.
Objective
This review will cover and define the possible role for optical mapping of the surgical specimen using fluorescence imaging in head and neck cancer.
Level of Evidence
NA
Keywords: Surgery, image‐guidance, fluorescence, probes, oncology
INTRODUCTION
Need for Imaging in Surgery
Despite the advent of targeted chemotherapeutic agents, almost 80% of early stage solid tumors undergo surgery at some point in their treatment course. In head and neck cancers, the majority of patients undergo surgery to remove their tumor.1, 2 An incremental gain in improving surgical outcomes and cost‐savings obtained by accurate and efficient identification of positive surgical margins in real‐time would have a significant impact on overall outcomes for cancer survival in the United States. However, the current gold standard in detecting tumor remains gross inspection followed by cryosection and Hematoxylin and Eosin (H&E) assessment by a pathologist.3 The biggest gap in quality of care is the high rate of positive margins in surgical resections, which correlates directly with poor survival and local‐regional relapse.4, 5 Furthermore, given the highly subjective nature of sampling, it is possible that positive margin status is under‐detected. Some have suggested that the optimal method for identifying positive margins requires detailed evaluation of the specimen itself through close cooperation with the pathologist rather than through sampling of the wound bed. Here, we review applications of this approach in combination with fluorescence optical imaging technique.
Surgeons and pathologists currently use subjective criteria such as palpation and visual cues to identify cancerous areas but tumor margins are positive in 30% of head and neck cancer (HNC) resections,1, 2, 6 indicating the insensitive nature of gross examination. Because sampling error limits the confidence of frozen section evaluation, routine use of a highly sensitive technique for “scanning” the specimen to identify suspicious areas to be evaluated by frozen section may improve diagnostic outcomes. Sampling error confounds most attempts to accurately determine margin status–if a tumor is not present on the slide, the pathologist cannot identify a positive margin. To improve this, whole specimen imaging of the surgical specimen can be of added value in the intraoperative management of a patient by identifying the highest yield foci to sample for frozen section analysis.
There are many types of techniques that use light to enhance the visual field of the surgeon
To address the need for real‐time detection of small foci of tumor in the operative setting, fluorescently labeled, tumor‐targeting agents that fluoresce or “glow” under near‐infrared light have been developed for intraoperative imaging. Initially proposed as intraoperative tools, recent clinical trials utilizing these agents have demonstrated a potential role in imaging of pathology specimens. Sampling error plagues the accuracy of frozen sections since only a limited surface area can be examined under the microscope for any given tumor. To this end, mapping of the tumor using optical imaging may allow the pathologist or surgeon to target frozen section sampling to areas that are fluorescent to identify close or positive margins. Mapping of the specimen using fluorescence with subsequent annotation of the specimen will facilitate precise communication between the surgeon and pathologist to identify suspicious regions on the specimen in real‐time. Failure to accurately detect close or positive margins remains a significant cause of poor patient outcome in head and neck cancer.
The two techniques that have gained the most ground are autofluorescence and contrast enhanced near‐infrared fluorescence (NIR).7, 8, 9, 10 Fluorescence imaging requires systemic administration of a cancer targeting agent coupled with a fluorophore and imaging time‐point with the best tumor‐to‐background ratio (TBR). Fluorophores that leverage the NIR range (700–900 nm) have attracted the most attention due to their improved depth‐penetration (up to 5–7 mm) as compared with fluorophores that emit below 600 nm and in the visible wavelengths as shown in Fig. 1A. Additionally, in vivo background fluorescence and scattering from water and chromophores are minimized in the NIR range as shown in Fig. 1B.
Figure 1.
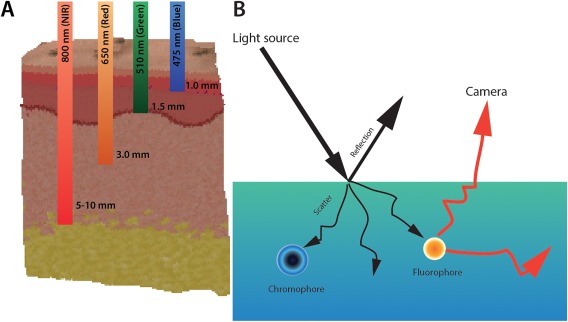
(A) Near infrared (NIR) light has the best penetration depth through soft‐tissue. (B) Light when entering a medium can be reflected, scattered and absorbed by molecules within the tissue (chromophores) or excite endogenous or exogenously administered molecules to emit light at a different wavelength.
Although widely evaluated in preclinical models, it has only been in the last several years that these agents have been successfully translated into human trials. By using the method of targeting specific receptors that are overexpressed on cancer cells such as vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), cancer‐specific regions can be identified. Example of such trials included VEGF targeting Bevacizumab‐IRDye800CW which is now undergoing trials for breast cancer (NCT01508572), premalignant esophageal lesions (NCT02129933), rectal cancer (NCT01972373), and familial adenomatous polyposis (NCT01691391).11, 12, 13 The first antibody‐based near‐infrared imaging trial was the use of Cetuximab‐IRDye800CW in head and neck cancer (NCT01987375)14 in the operating room and in post‐processing of tissues. Results from this trial clearly defined: (1) the diagnostic accuracy of Cetuximab‐IRDye800 for disease localization, (2) sensitivity and specificity between 85–95% depending on the imaging modality, and (3) the safety of fluorescently labeled antibodies for systemic administration. The development of these agents for intraoperative application has been widely considered, but their role in imaging of pathology specimens has not really been explored. To this end we propose to review the possible benefits of fluorescence imaging for surgical specimens.
Opportunities for Optical Imaging Throughout the Patient's Management
Recently, a phase I dose‐escalation study to determine the safety profile of fluorescently labeled anti‐EGFR antibody in subjects with squamous cell carcinoma (PI: E. Rosenthal, NCT01987375) was completed and a similar study evaluating panitumumab‐IRDye800 in head and neck cancer (NCT01998273) is being conducting. These studies clearly show the fluorescence of the therapeutic antibodies to EGFR are well correlated with the presence of subclinical non‐palpable, tumor fragments within patient derived specimens, suggesting successful EGFR expressing tumor targeting with high sensitivity and specificity as shown in Fig. 2.13
Figure 2.
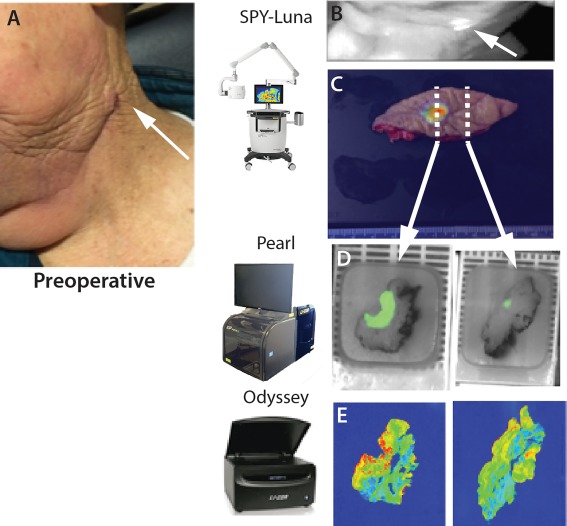
Cetuximab‐IRDye800 identifies cancer with high specificity. Patient data shown from clinical trial using systemically administered cetuximab‐IRDye800 at 25 mg/m2 3 days prior to surgical excision of head and neck cancer. Patient with oral cavity cancer enrolled for evaluation was noted to have a 5 mm lesion arising within the previous neck dissection scar. (A) Left neck dissection scar, arrow marks 5–6 mm suspicious lesion. (B, C) Intraoperative assessment demonstrated an isolated area of fluorescence within the scar (arrow). (C) The scar underwent wide local excision and repeat LUNA imaging. (D) Ex‐vivo imaging in surgical pathology: specimens were placed into the Pearl Triology system and imaged. (E) Tissue localization of the antibody‐dye bioconjugate in permanent histology sections may assist with disease identification.
From the studies, intravenous administration of fluorescently labeled anti‐EGFR antibody can image cutaneous and mucosal squamous cell carcinoma in vivo and ex vivo and throughout histology processing since the dye is not significantly degraded by formalin fixation and paraffin embedding.15 Using a range of devices as listed in T, the tissue can be imaged at each step–intraoperatively or in the clinic (Fig. 2B), ex‐vivo for tumor mapping (Fig. 2C), and then paraffin blocks (Fig. 2C) and histology sections can be imaged (Fig. 2E). Table 1 shows devices currently being evaluated for antibody‐based fluorescent imaging.
Table 1.
Potential Devices for Intraoperative Fluorescence Imaging
| Device Class | Examples | Primary Application | Secondary Application |
|---|---|---|---|
| Intraoperative (wide field) | Luna, PINPOINT (Novadaq), Exporer Air (SurgVision) | Tumor and wound intraoperative imaging | ‘Back‐table’ imaging of specimens immediately after resection |
| Pathology (closed system) | Pearl (Licor Biosciences) | Imaging of primary specimens | Imaging of any resected tissues |
| Post‐Processing | Odyssey (Licor Biosciences) | Imaging of histology sections | Correlation of fluorescence with histology |
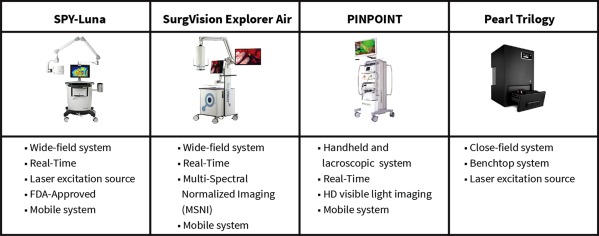
| |||
During this clinical trial, a closed field small animal imaging system (Pearl Imaging System, LI‐COR Biosciences, Lincoln, Nebraska), see Fig. 3D was utilized for imaging of the resected primary specimens.16 The consistency and accuracy of data obtained on this device throughout the trial suggested the value of a close field imaging system to the participating surgeons and pathologists.
Figure 3.
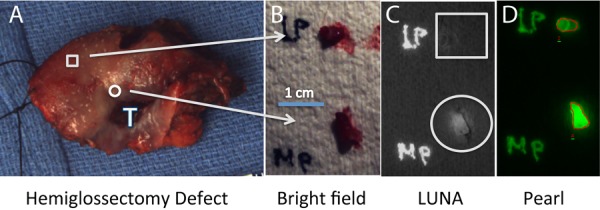
Primary tongue specimen from cetuximab‐IRDye800 clinical trial data using microdose. A patient with a lateral tongue squamous cell carcinoma underwent systemic injection of cetuximab‐IRDye800 3 days prior to hemiglossectomy and cervical lymph node dissection. Tongue specimen (A) underwent 4‐mm punch biopsies peripheral (square) and proximal (circle) to the tumor (T). Brightfield imaging (B) and LUNA imaging (C) was performed in the operating room and subsequently imaged in Pathology using the PEARL (D). Histology was used to determine the absence (peripheral biopsy, square) or presence (proximal biopsy, circle) of tumor.
Applications of Closed Field System
Successful use of the tabletop small animal system for specimen imaging was performed in the clinical trial to overcome some of the limitations of the intraoperative device–normalization, highly specific and rapid data acquisition. Given the value of this device first in human clinical trials, the large number of new agents becoming available for this technique, and the absence of any competing technology, it is worth reviewing the potential value of this device for detection of cancer during intraoperative specimen assessment. There are several properties of NIR light that have improved tissue penetration as compared to lower wavelength of light, leading to reliable tissue penetration of about 5 mm but not 10 mm. This means that tumor can be detected within 5 mm of the cut surface, which is a close margin for head and neck cancers. Furthermore, the technique will detect very small fragments of tumor in a field of view of the surgeon. The use of a closed system (as opposed to the open field operative systems) allows for consistent and clear fluorescence imaging since exogenous light can be carefully controlled.
Screening whole specimens for directed frozen sections
Whole specimen imaging, by placing the resected tissue within the closed field device and then imaging at multiple angles, can be used to identify suspicious areas where residual tumor may be present. This could be considered fluorescence surface mapping of the tumor to identify areas amendable to sampling for frozen section examination.
Detection of microscopic fragments of tumor
To determine if the tabletop closed field fluorescence imaging device could be successfully repurposed to image microscopic fragments of tumor, 3–100 mg of human tumor from patients treated with anti‐EGFR antibody‐IRDye800 was measured against the patient's matched normal tissue. We showed that 5 mg of tumor could be identified in the clinical setting as shown in Fig. 4; this exceeds current detection thresholds reported in the literature.15
Figure 4.
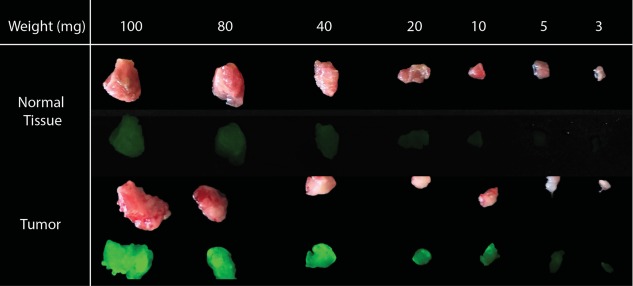
Serial section of tumor and normal muscle by weight from patient treated with anti‐EGFR antibody‐IRDye800. Tumor can be detected as low as 5 mg.
Most importantly we found that fluorescence (sensitivity: 85%) outperformed the surgeon (sensitivity: 54%) and pathologist (sensitivity: 49%) in identifying positive margins (Fig. 2: device sensitivity and specificity). Using fluorescence assessment, the clinician is able to identify the highest yield areas to assess by frozen section and may reduce the false negative margin results, especially in larger specimens.
Deeper analysis of samples
During our clinical trial, we found that the tissue could be tumor positive but was called negative on frozen section by pathology, in 8% of those cases we discovered the tissue of permanent sections contained cancer (unpublished data, Rosenthal). If sections of a tissue block from a highly fluorescent area are initially interpreted as “no tumor,” the pathologist could consider obtaining deeper sections or leveling completely through the block. This could conceivably turn a false negative margin into a true positive result. Fluorescence imaging can be especially helpful in identify suspicious areas in large specimens that can be targeted for more thorough histologic examination and improve overall accuracy in margin assessment.
Improving communication and workflow
Once the specimen is removed from the patient, the surgeon needs to relay to the pathologist the specimen orientation and areas that may have positive margins. To accomplish this, many surgeons physically carry the specimen to the frozen section room. Unfortunately, this only allows for limited communication (surgeon to pathologist performing frozen section), can lead to delays (urgent surgical issue), is time consuming, and requires the surgeon to leave the patient unattended in the operating room (Fig. 5). Although sutures and inking can be used, it is not feasible with many head and neck specimens. Furthermore, these orientation techniques are an approximation and represent a static mode of communication.
Figure 5.
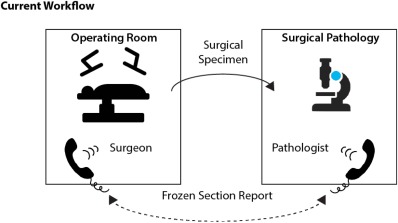
Current workflow. Surgeon sends specimen to pathologist (or leaves the OR to hand carry) with sutures for orientation. A phone conversation required to communicate complex 3D anatomy and areas of suspicion.
In contrast, images obtained during fluorescence mapping of the specimen could be transmitted to the pathologist and used to facilitate communication regarding fluorescence‐positive areas for frozen section evaluation. Images can be viewed concurrently on touch displays in different locations. Annotation of the suspicious foci by marking, tagging, pinning, and/or highlighting together with real‐time verbal communication will enhance efficient interaction between the surgeon in the operating room and the pathologist in the frozen section room (Fig. 6). Moreover, the communication can take place while the samples are being transported or processed, which can save time and allow the pathologist and surgeon to address specific questions.
Figure 6.

Proposed new workflow. Annotation of the suspicious area can be identified and improved overall communication between surgeon and pathologist.
SUMMARY
We propose the use of closed field imaging systems located in Pathology or in the “back‐table” of the operating room for fluorescence mapping of tumors to increase the accuracy of frozen section evaluation for margin status. This technique requires an overlay of the fluorescence image onto a color image of the specimen in order to appropriately orient the pathologist to the location of the closest margins. Data from early clinical studies suggests that this will be effective in reducing false negative results due to sampling error.
Conflict of interests
N.T., C.S.K., JMW, and E.L.R. declare no conflicts of interests
BIBLIOGRAPHY
- 1. McMahon J, O'Brien CJ, Pathak I, et al. Influence of condition of surgical margins on local recurrence and disease‐specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg 2003;41:224–231. [DOI] [PubMed] [Google Scholar]
- 2. Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol 2009;45:361–385. [DOI] [PubMed] [Google Scholar]
- 3. Jaafar H. Intra‐operative frozen section consultation: concepts, applications and limitations. Malays J Med Sci MJMS 2006;13:4–12. [PMC free article] [PubMed] [Google Scholar]
- 4. Eldeeb H, Macmillan C, Elwell C, Hammod A. The effect of the surgical margins on the outcome of patients with head and neck squamous cell carcinoma: single institution experience. Cancer Biol Med 2012;9:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravasz LA, Slootweg PJ, Hordijk GJ, Smit F, van der Tweel I. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J Craniomaxillofac Surg 1991;19:314–318. [DOI] [PubMed] [Google Scholar]
- 7. Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res 2006;12:6716–6722. [DOI] [PubMed] [Google Scholar]
- 8. Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high‐risk oral premalignant disease using a simple hand‐held device. Head Neck 2007;29:71–76. [DOI] [PubMed] [Google Scholar]
- 9. Poh CF, MacAulay CE, Zhang L, Rosin MP. Tracing the “at‐risk” oral mucosa field with autofluorescence: steps toward clinical impact. Cancer Prev Res (Phila) 2009;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poh CF. Improving public awareness and outcomes for oral cancer . Future Sci OA 2016;2:FSO103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Scheltinga Terwisscha AG, van Dam GM, Nagengast WB, et al. Intraoperative near‐infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med 2011;52:1778–1785. [DOI] [PubMed] [Google Scholar]
- 12. Arjaans M, Oude Munnink TH, Oosting SF, et al. Bevacizumab‐induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res 2013;73:3347–3355. [DOI] [PubMed] [Google Scholar]
- 13. Rosenthal EL, Warram JM, de Boer E, et al. Safety and tumor specificity of Cetuximab‐IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 2015;21:3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenthal EL, Warram JM, de Boer E, et al. Successful translation of fluorescence navigation during oncologic surgery: a consensus report. J Nucl Med 2016;57:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warram JM, de Boer E, van Dam GM, et al. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J Pathol Clin Res 2016;2:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warram JM, de Boer E, Moore LS, et al. A ratiometric threshold for determining presence of cancer during fluorescence‐guided surgery. J Surg Oncol 2015;112:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


