Abstract
Objectives
Microsurgical techniques are essential for vessel anastomosis in free flap reconstructive surgery. However, teaching these skills intraoperatively is difficult. The chicken thigh microvascular model is a high‐fidelity model that has been previously validated to differentiate between skill levels of surgeons. This study aims to determine if this model objectively improves microsurgical skills.
Study Design
Validation study
Methods
Thirteen residents were given a tutorial on microvascular anastomosis and asked to perform anastomoses on the microvascular model. Anastomoses were video‐recorded and the time required for trainees to complete the first stitch of their first anastomosis was compared to the time required for the first stitch of their last anastomosis. Comparison of first and last stitch times was completed using a paired student t‐test. All participants completed a survey regarding their experience with the simulator.
Results
There was a statistically significant decrease between the time required for the first stitch (235 s, 95%CI 198–272 s) compared to last stitch (120 s, 95%CI 92–149 s), and an average 48.7% (115 s) decrease in time (p < 0.001). Junior (PGY 2/3) and senior (PGY 4/5) residents had similar decreases in time, 49.1% and 48.21%, respectively. One hundred percent of residents felt they improved during the session and 92% of residents agreed or strongly agreed that their final stitch was better than their last stitch. All residents agreed or strongly agreed that the simulation is realistic, effective in teaching the procedure, and would translate to improved intraoperative performance.
Conclusions
The chicken thigh model demonstrates objective improvements in resident microvascular surgical skills.
Level of Evidence
NA
Keywords: Microvascular reconstructive surgery, surgical simulators, head and neck surgery, facial plastics
INTRODUCTION
Microvascular surgery is an integral part of free flap reconstruction for oncologic procedures. Similarly, training in microvascular surgery has become an important component of Otolaryngology–Head and Neck Surgery (OHNS) residencies across the country.1 However, it represents one of the most technically challenging aspects of the operation and as a result is difficult to teach intraoperatively. The risk of surgical complications from poor anastomoses, especially in microvascular naïve trainees, makes ex vivo models ideal.
Many different surgical models have been proposed for surgical training in OHNS.2, 3, 4, 5, 6 These include training models for microvascular surgery that have even been used in the interview process for OHNS residency.4, 5, 7 Validated models using chicken thighs have been able to reliably differentiate between different levels of training using task‐specific Objective Structured Assessments of Technical Skills (OSATS).8, 9, 10 The chicken thigh model closely approximates arterial anastomosis in the head and neck in terms of both caliber of the anastomosis and required tissue handling. Despite the increasing use of this model for training purposes, only one published study has shown measured improvement of surgeon skill with use of the chicken thigh microvascular model, and this study was based off of OSAT scores, but did not incorporate surgical speed or other objective data into their assessment.11
The following study aims to determine whether or not surgical trainees show objective improvement in performing microvascular anastomosis after training on a chicken thigh model. Specifically, time to completion of an arterial microvascular suture was used as an objective measure. Subjective analysis was also performed by providing a comprehensive survey to each study participant.
METHODS AND MATERIALS
Experimental Design
After institutional review board approval was obtained, 13 residents were recruited from a single institution to perform the study. Participants spanned various levels of training–PGY‐2 to PGY‐5–and were all recruited from the OHNS department. Participants consisted of 4 female and 9 male residents. All participants were right‐hand dominant and age ranged from 28 to 36 years old.
A brief instructional video on the basics of microvascular surgery was shown to each participant. With the guidance of a resident preceptor, to start and stop video recordings, individuals were then allowed to practice arterial anastomosis on the chicken thigh model for 2 hours. Each participant was given a single chicken thigh. The anastomosis was resected after each attempt, allowing for reuse of the ischiatic artery. The first stitch of each attempted anastomosis was video recorded. Each study participant completed at least 3 anastomoses during the 2‐hour trial period. Timing to completion (TTC) of the first stitch of each anastomosis was recorded. Timing was recorded from when the needle first was attempted to be loaded until the suture was cut after throwing three square knots. The length of time to properly align the vessels using microvascular clamps was not incorporated into the timing. Times for the first stitch of the first anastomosis were compared to times for the first stitch of the last anastomosis. Participants completed a 16‐question survey after the completion of their anastomosis session. Each survey question was scored from 1 (strongly disagree) to 5 (strongly agree).
Model
A previously validated chicken thigh model was used to simulate microvascular anastomosis. Individuals were provided with a single store‐bought chicken thigh to provide constant arterial caliber throughout each trial. The inner diameter of these arteries (2.5–3 mm) was similar to those seen in head and neck surgery and served as a close surrogate. After the chicken thigh ischiatic artery, shown in Figure 1, was skeletonized, participants were given a vessel dilator and microvascular forceps. Participants used 9‐0 monofilament suture and 8 interrupted stitches were performed in the usual fashion. Microscope settings were fixed at 4x magnification with a focal distance of 250 mm.
Figure 1.
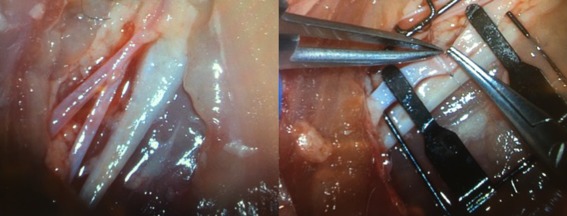
Photos showing the neurovascular structures of the chicken thigh model (left) and use of microvascular instrumentation to perform anastomosis (right).
Assessment and Statistical Analysis
For each of the 13 participants, the TTC of the first stitch of their first anastomosis was compared to the TTC of the final stitch of their final anastomosis. Paired t‐tests were used to compare the TTC from the first and final stitches with significance being attributed to a p‐value of less than 0.05. Results were also stratified between junior (PGY 2/3) and senior (PGY 4/5) residents. Survey responses were averaged over a qualitative 5‐point scale.
RESULTS
Video recordings of each participant were reviewed to determine the TTC of each stitch. For all participants, the average TTC of their first stich was 235 s (3 m 55 s) compared to an average TTC of 120 s (2 m 00 s) for their final stitch (p < 0.001). This relative difference is depicted in Figure 2 and represents a mean improvement of 48.7% across all participants. Junior and senior residents had similar improvements in TTC of 49.1% and 48.2%, respectively. Junior residents had an average TTC of 260 s (4 m 19 s) for their first stitch compared to 131 s (2 m 10 s) for their last stitch (p < 0.001). Senior residents had an average TTC of 206 s (3 m 25 s) compared to 108 s (1 m 48 s) for their last stitch (p < 0.001).
Figure 2.
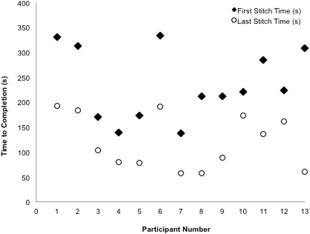
Graphical depiction of time to completion (TTC) of first stitch and final stitch for all microvascular simulator participants.
Average TTC of the first stitch for junior residents was 260 s (4 m 19 s) compared to 206 s (3 m 25 s) for senior residents (p > 0.05). Similarly, average TTC of the final stitch for junior residents was 131 s (2 m 10 s) compared to 108 s (1 m 48 s) for senior residents (p > 0.05). These results are summarized in Table 1.
Table 1.
Summary of Results for Time to Completion (TTC) of First and Final Stitch of Microvascular Simulator Participants, Stratified by Junior (PGY 2–3) and Senior (PGY 4–5) PGY Level.
| PGY Level | Time to Completion | p‐value | |
|---|---|---|---|
| First Stitch(s) | Last Stitch(s) | ||
| All residents | 235 | 120 | <0.001 |
| Junior | 260 | 131 | <0.001 |
| Senior | 206 | 108 | <0.001 |
Qualitative self‐assessment was performed using a 16‐question exit survey completed by all participants. One hundred percent (13/13) of participants agreed or strongly agreed that they improved at performing microvascular anastomosis throughout the simulation session. Ninety‐two percent (12/13) of participants agreed or strongly agreed that their last stitch was better than their first. One hundred percent (13/13) of participants agreed or strongly agreed that the simulation is effective in teaching surgical skills and that is was helpful in learning microsurgical technique. Similarly, 100% (13/13) of participants agreed or strongly agreed that the model tissue felt realistic, appeared life‐like, was easy to set up, and that the suturing felt realistic. The complete survey and average participant responses are shown in Figure 3.
Figure 3.
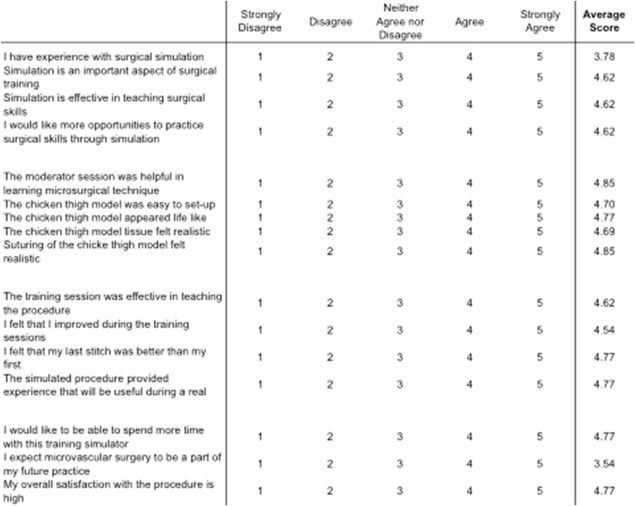
Microvascular simulator survey results for all resident participants with average response scores listed in the right column.
DISCUSSION
Previous microvascular simulation studies have validated ex vivo models and shown the ability to differentiate between different levels of training. Nimmons et al. showed this difference by grading OSATS for each participant and stratifying scores based on level of training. The significant difference in scores allowed for the differentiation of microvascular ability. However, this study–along with all other microvascular simulation studies–has not demonstrated objective improvement in user performance after use of the chicken thigh microvascular model.
The results herein demonstrate both an objective and subjective improvement in resident performance after use of a microvascular simulation model. All residents experienced a significant improvement in TTC after using the chicken thigh model. This study is the first to show improvement of an objective measure for resident microvascular surgical skill after using a microvascular anastomosis model.
Greater efforts have been made to develop and incorporate surgical simulation models into OHNS training curricula.12, 13, 14, 15 However, the tangible benefits associated with these models has remained uninvestigated. Existing models for microvascular surgery represent cost‐effective and validated simulation environments, but have not shown an objective improvement in resident performance for the designated task. The significant improvement in TTC for microvascular suturing after a single practice session with the chicken thigh model highlights the need and utility of microvascular simulation as a component of OHNS training.
Additionally, improvement in microvascular skills was not limited to a single subset of residents. Both junior‐ and senior‐level residents experienced a significant objective benefit from completing the surgical simulator. TTC of the last microvascular stitch was significantly reduced for both junior and senior residents, suggesting that practice on the microvascular simulator is beneficial across all levels of training. Early incorporation of this surgical simulator may yield even greater benefits to senior residents.
Participants also described a self‐reported improvement in performance after the simulation session. All residents noted a desire to continue practicing with the microvascular simulator to improve their surgical skills along with their perceived improvement. Continued longitudinal use would provide further benefit to resident training through increased operator confidence.16, 17 This may in turn lead to increased competence when first encountering microvascular tasks in the operating room.
Although our study investigated the TTC of microvascular suturing, several other objective metrics exist to evaluate individual performance. Other objective measures that may be investigated in future studies include the quality of individual ties or assessment of overall anastomosis for watertight seal. Other limitations include the small sample size of residents used for the analysis. Further longitudinal studies are necessary to show continued resident improvement over time in addition in a larger sample size and correlation with improved resident performance during in vivo settings.
CONCLUSION
Our current study is the first to demonstrate an objective improvement in resident microvascular surgical skills after use of a chicken thigh model. Resident response indicated that the simulator was useful in teaching the procedure. Individuals also perceived a subjective improvement in microvascular skills after using the chicken thigh model. Additional longitudinal testing with different objective measures is necessary to further define the utility of microvascular simulation models.
This study was presented at the Triological Society meeting at the Combined Otolaryngology Sections Meeting in Chicago, IL May 20–21 2016
Work for this study was completed entirely at the Massachusetts Eye and Ear Infirmary, Boston, Massachusetts.
Conflicts of Interest: none
Funding: none
BIBLIOGRAPHY
- 1. Spiegel JH, Polat JK. Microvascular flap reconstruction by otolaryngologists: prevalence, postoperative care, and monitoring techniques. Laryngoscope 2007;117:485–490. [DOI] [PubMed] [Google Scholar]
- 2. Dedmon MM, Kozin ED, Lee DJ. Development of a temporal bone model for transcanal endoscopic ear surgery. Otolaryngol Head Neck Surg 2015;153:613–615. [DOI] [PubMed] [Google Scholar]
- 3. Dedmon MM, Paddle PM, Phillips J, et al. Development and validation of a high‐fidelity porcine laryngeal surgical simulator. Otolaryngol Head Neck Surg 2015;153:420–426. [DOI] [PubMed] [Google Scholar]
- 4. Sucur D, Konstantinovic P, Potparic Z. Fresh chicken leg: an experimental model for the microsurgical beginner. Br J Plast Surg 1981;34:488–489. [DOI] [PubMed] [Google Scholar]
- 5. Govila A. A simple model on which to practise microsurgical technique: a fresh chicken. Br J Plast Surg 1981;34:486–487. [DOI] [PubMed] [Google Scholar]
- 6. Erman AB, Deschler DG. The chicken thigh model for head and neck microvascular training. Triological Society Combined Sections Meeting Program 2011 Jan 27–29; Scottsdale, AZ.
- 7. Moore EJ, Price DL, Van Abel KM, et al. Still under the microscope: can a surgical aptitude test predict otolaryngology resident performance? Laryngoscope 2015;125:E57–E61. [DOI] [PubMed] [Google Scholar]
- 8. Nimmons GL, Chang KE, Funk GF, et al. Validation of a task‐specific scoring system for a microvascular surgery simulation model. Laryngoscope 2012;122:2164–168. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan DJ, Vaz‐Guimaraes F, Fernandez‐Miranda JC, et al. Validation of a chicken wing training model for endoscopic microsurgical dissection. Laryngoscope 2015;125:571–576. [DOI] [PubMed] [Google Scholar]
- 10. Douglas HE, Mackay IR. Microvascular surgical training models J Plast Reconstr Aesthet Surg 2011;64:e210–e202. [DOI] [PubMed] [Google Scholar]
- 11. Schoeff S, Hernandez B, Robinson DJ, Jameson MJ, Shonka DC Jr. Microvascular anastomosis simulation using a chicken thigh model: interval versus massed training. Laryngoscope 2017. doi: 10.1002/lary.26586. [DOI] [PubMed] [Google Scholar]
- 12. Wiet GJ, Stredney D, Wan D. Training and simulation in otolaryngology. Otolaryngol Clin North Am 2011;44:1333–1350, viii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arora A, Lau LY, Awad Z, et al. Virtual reality simulation training in Otolaryngology. Int J Surg 2014;12:87–94. [DOI] [PubMed] [Google Scholar]
- 14. Wiet GJ, Rastatter JC, Bapna S, et al. Training otologic surgical skills through simulation–moving toward validation: a pilot study and lessons learned. J Grad Med Educ 2009;1:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allak A, Liu YE, Oliynyk MS, et al. Development and evaluation of a rigid esophagoscopy simulator for residency training. Laryngoscope 2016;126:616–619. [DOI] [PubMed] [Google Scholar]
- 16. Leopold SS, Morgan HD, Kadel NJ, et al. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am 2005;87:1031–1037. [DOI] [PubMed] [Google Scholar]
- 17. Nishisaki A, Keren R, Nadkarni V. Does simulation improve patient safety? Self‐efficacy, competence, operational performance, and patient safety. Anesthesiol Clin 2007;25:225–236. [DOI] [PubMed] [Google Scholar]


