Abstract
Quercetin is proven to have anticancer effects for many cancers. However, the role of tumor suppressor p53 on quercetin’s radiosensitization and regulation of endoplasmic reticulum (ER) stress response in this process remains obscure. Here, quercetin exposure resulted in ER stress, prolonged DNA repair, and the expression of p53 protein; phosphorylation on serine 15 and 20 increased in combination with X-irradiation. Quercetin pretreatment could potentiate radiation-induced cell death. The combination of irradiation and quercetin treatment aggravated DNA damages and caused typical apoptotic cell death; as well the expression of Bax and p21 elevated and the expression of Bcl-2 decreased. Knocking down of p53 could reverse all the above effects under quercetin in combination with radiation. In addition, quercetin-induced radiosensitization was through stimulation of ATM phosphorylation. In human ovarian cancer xenograft model, combined treatment of quercetin and radiation significantly restrained the growth of tumors, accompanied with the activation of p53, CCAAT/enhancer-binding protein homologous protein, and γ-H2AX. Overall, these results indicated that quercetin acted as a promising radiosensitizer through p53-dependent ER stress signals.
Keywords: quercetin, p53, endoplasmic reticulum stress, DNA double-strand breaks, eIF-2α (eukaryotic initiation factor 2α), ATM kinase
Introduction
At least 50% of newly diagnosed cancer patients will receive some form of radiotherapy during treatment. Ionizing radiation (IR) produces a wide variety of DNA lesions including double-strand breaks (DSBs), which are considered to be the major reason for cell death.1 But most tumor patients get radioresistance and the risk of tumor recurrence increases after IR, regardless of the initial sensitivity of radiotherapy.2,3 Therefore, there is an urgent need to develop radiosensitizers to overcome the radioresistance.
Most of the current radiosensitization approaches promote cellular apoptosis by aiming to target DNA damage responses (DDR).4 ATM kinase, ATM phosphorylates p53 on serine 15, is one of the pivotal DDR elements.5 In a previous study, ATM has been demonstrated to directly phosphorylate serine 15 of p53 in response to IR.6 Therefore, activities of p53 are greatly increased in response to DNA damage.7 p53 can influence downstream genes including p21, GADD45, and Bax to elicit cell cycle arrest, apoptosis, and DNA damage.8 However, upon DNA damage, activated p53 can also be synthesized by serum deprivation, cell-cycle progression, or endoplasmic reticulum (ER) stress,9,10 which acts as a transcription factor for many DDR proteins including MLH1 and Rad51.11
Recent evidence has shown that ER stress activated by IR is one of the mechanisms of the induction of DNA damage.5,12 The ER stress plays an important role as a sensor for cellular stress to detect the alteration in cell homeostasis and triggers a coordinated adaptive program called unfolded protein response (UPR).13–15 Prolonged ER stress can also lead to apoptosis via activation of eIF2α or proapoptotic genes such as Bax via the p53 pathway.16 For example, tunicamycin, a classic ER stress inducer, is proven to be a radiosensitizer of tumor cells.17 Despite these findings, the mechanism by which radiosensitizers enhance the effectiveness of radiotherapy has not yet been elucidated.
Recently, more and more natural products have been investigated for their antitumor potential.18 Quercetin, a ubiquitous bioactive flavonoid, can inhibit the proliferation of cancer cells.19,20 Furthermore, we observed that irradiation-induced capillary dilation and congestion in hepatic lobules were reversed by quercetin, as described in a previous study.1 Due to the multifunctional nature of quercetin, we got a hypothesis that quercetin might increase sensitivity of tumor radiotherapy. To test this hypothesis, we conducted in vitro and in vivo studies. The results showed that quercetin can enhance tumor radiosensitivity by p53-mediated ER stress pathways.
Materials and methods
Cell culture
OV2008, A2780, and GM9607 cells were procured from China Center for Type Culture Collection (CCTCC, Wuhan, China), and cultured according to CCTCC guidelines. Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 2 mM glutamine (Sigma-Aldrich, St Louis, MO, USA), 100 units of penicillin/mL (Sigma-Aldrich), and 100 μg of streptomycin/mL (Sigma-Aldrich), incubated at 37°C in 5% CO2, and subcultured every 2−3 days. Quercetin and pifithrin-α were purchased from Sigma-Aldrich and used for 24 h before radiation was given.
Reagents and materials
Antibodies were purchased from Abcam (Cambridge, MA, USA), Cell Signaling Technology, Inc. (Beverley, MA, USA), StressGen Bioreagents (Ann Arbor, MI, USA), Calbiochem-Novabiochem Corporation (San Diego, CA, USA), and SantaCruz Biotechnology (Santa Cruz, CA, USA). DMEM and fetal bovine serum were obtained from Gibco-BRL (Grand Island, NY, USA). Polyvinylidene difluoride (PVDF, 0.22 mm) membrane was purchased from Bio-Rad Laboratories (Hercules, CA, USA). All the other reagents were obtained from Sigma-Aldrich.
Irradiation
After quercetin pretreatment, cells were irradiated with X-rays, which were generated with an X-ray machine (FAXITRON RX-650; Faxitron Bioptics, LLC, Tucson, AZ, USA) operated at 100 kVp energy. The dose rate was about 2 Gy/min. All irradiations were carried out at room temperature.
Apoptosis analysis
Apoptosis was analyzed by fluorescence activated cell sorting (FACS) as described previously.20 After 5×105 cells/well were seeded to a 6-well plate for 24 h, the cells were treated with or without quercetin and/or 4 Gy of IR for 24 h. Then, the cells were harvested, washed with PBS, and stained using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). The apoptosis of cells was analyzed by flow cytometry using Cell Quest Software.
Immunofluorescence microscopy
Exponentially growing cultures of OV2008 or A2780 cells were seeded on 22 cm2 coverslips. Cells were treated with 4 Gy of irradiation (in the presence or absence of quercetin) and harvested at 12 h. The induction and maintenance of γ-H2AX and Rad51 foci were assessed by immunofluorescence microscopy. The number of foci per nucleus was then counted from a population of ≥25 cells and graphed as the mean and SDs.
Comet assay
Cells were either sham irradiated or exposed to 4 Gy IR 1 h after pretreatment with vehicle or 100 μM quercetin. Cells were collected at various times after irradiation and processed by neutral comet assay using a CometAssay® kit from Trevigen, Inc. (Gaithersburg, MD, USA) per the manufacturer’s protocol. Approximately, 100 nucleus images were captured for each slide and processed by a Zeiss Axio Observer.Z1 microscope. The tail moments from the cells were measured by the TriTek CometScore™ software (Version 1.5.2.6; TriTek Corporation, Summerduck, VA, USA).
Colony formation assay
Cell survival was measured with the standard colony-forming assay. Briefly, cells were pretreated with 100 μM quercetin for 24 h, followed by X-irradiation. Immediately after irradiation, the cells were collected by trypsinization, diluted, and seeded in 100-mm petri dishes. The dishes were cultured for 10 days in DMEM without quercetin, then fixed with alcohol, and stained with crystal violet. Colonies containing more than 50 cells were identified as survivors under a stereomicroscope. Each experiment was performed in triplicate.
Transient transfection
The p53 plasmid was transiently transfected into cells with Lipofectamine 2000 Reagent. The p53 siRNA was transiently transfected into cells with DharmaFECT 4 siRNA Transfection Reagent. After 48 h, the cells were treated with quercetin and then exposed to IR for 24 h.
Human tumor xenograft
This study was performed with approval from the Committee on the Ethics of Animal Experiments in the Hubei province. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of Tongji Hospital in Hubei. Animal studies were done as previously described.20 About 5×106 OV2008 cells were injected subcutaneously into both right and left flanks of mice. The mice (eight mice per group) were treated (oral gavage) with quercetin alone or quercetin (1 h before radiation) plus irradiation at 2 Gy fractions to a total dose of 20 Gy. The tumor volume and size were recorded thrice weekly. At the termination of the experiment, mice were euthanized 24 h after the last administration of compound. Tumor samples were fixed in paraformaldehyde.
Immunohistochemistry
Muscle tissues from the inoculation sites of the treated mice were surgically excised for immunohistochemistry analysis as previously described.20 The sections were fixed by acetone and incubated overnight at 4°C with rabbit anti-human p53 or eIF2α or γ-H2AX monoclonal antibody diluted at 1:100, followed by incubation with horseradish peroxidase-linked secondary antibody, and finally developed using diaminobenzidine kit (BD Biosciences) for optimal staining intensity.
Western blot analysis
Following incubation of cells in the presence or absence of quercetin and/or IR (2 Gy), cells were lysed or homogenized for Western blot analysis as previously described.20 Primary antibodies and horseradish peroxidase-conjugated secondary antibodies were purchased from StressGen Bioreagents, Abcam, SantaCruz Biotechnology, Calbiochem-Novabiochem Corporation, and Cell Signaling Technology, Inc.
Statistical analysis
The results are expressed as the mean value ± SD and are interpreted by analysis of variance repeated-measures test. Differences are considered statistically significant when p-value is <0.05.
Results
ER stress is essential for potentiation of quercetin affecting DSB
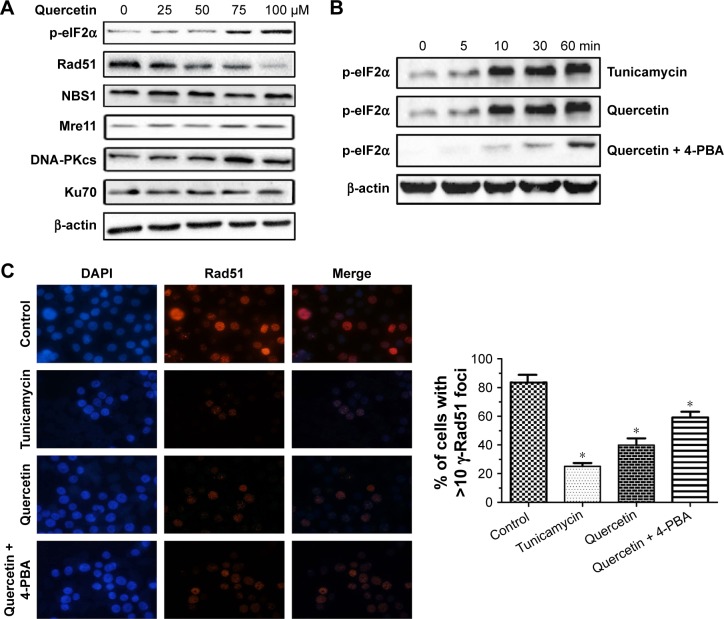
In an intratumoral environment, ER stress could be evoked when tumor cells are exposed to quercetin treatment.20 To examine whether quercetin-induced ER stress affected DSB repair, we investigated the relationship between them in human ovarian cancer OV2008 cells. Since ER stress is known to activate PERK, which leads to the phosphorylation of eIF2α,21,22 we used phospho-eIF2α as a marker of ER stress. As shown in Figure 1A, phospho-eIF2α was increased in a concentration-dependent manner after OV2008 cells were treated with quercetin for 12 h, which indicated that quercetin could induce ER stress. Moreover, Rad51, a key homologous recombination (HR) pathway protein, was decreased after quercetin treatment in a concentration-dependent manner. Meanwhile, quercetin did not alter expression levels of other HR-related proteins (Mre11 and NBS1) or NHEJ proteins (DNA-PKcs and Ku70; Figure 1A). Furthermore, tunicamycin, a classic ER stress inducer, could also induce eIF2α phosphorylation result in Rad51 decreased in a time-dependent (Figure 1B and C). In addition, 4-phenyl butyrate (4-PBA), an ER stress inhibitor, could partially abrogate the above phenomenon (Figure 1B and C). Overall, these results suggested that ER stress plays a critical role in the potentiation of quercetin affecting DSB repair.
Figure 1.
Effect of quercetin on the expression levels of DSB repair related proteins.
Notes: (A) OV2008 cells were treated with quercetin at the indicated concentrations for 12 h. After incubation, cell extracts were analyzed by Western blotting. Actin was used as a loading control. (B) OV2008 cells were treated with vehicle, quercetin (100 μM), tunicamycin (5 μM), or quercetin (100 μM) in the presence of the ER stress inhibitor 4-PBA (10 μM) for the indicated times. After incubation, cell extracts were analyzed by Western blotting. Actin was used as a loading control. (C) OV2008 cells were treated with quercetin (100 μM) in the presence or absence of 4-PBA (10 μM), or the ER stress inducer tunicamycin (5 μM), for 12 h as indicated. After incubation, cell extracts were analyzed by immunofluorescence microscopy conducted using anti-Rad51 staining. Shown are mean foci per nucleus counted from three independent experiments. *p<0.05, when compared with control.
Abbreviations: 4-PBA, 4-phenyl butyrate; DAPI, dihydrochloride; DSB, double-strand break; ER, endoplasmic reticulum.
Quercetin enhances IR, induces DSB, and sensitizes cells to radiosensitivity
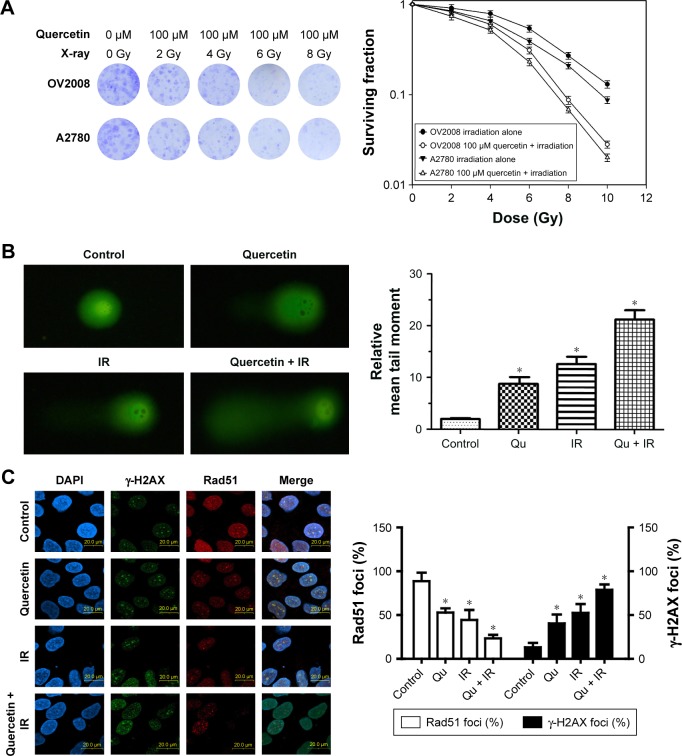
Because knocking out Rad51 sensitizes tumor cells to IR,23 we next analyzed whether quercetin treatment influenced cellular radiosensitivity by using a clonogenic survival assay. The survival curves of cells exposed to X-rays are shown in Figure 2A. Quercetin enhanced the radiosensitivity of both cell lines to radiation, with enhancement ratios of 1.43±0.08 for OV2008 and 1.36±0.13 for A2780 cells at a 10% cell survival (IC10). In addition, DNA damage was visualized in the form of a comet tail experiment. Our results showed that the combination of quercetin and IR caused dramatically increased DNA damage in OV2008 cells, compared with IR (23.07%±0.24% vs 12.47%±0.18%) or quercetin (23.07%±0.24% vs 9.35%±0.11%) alone (Figure 2B). These data suggested that quercetin might be a powerful radiosensitizer.
Figure 2.
Effect of quercetin on cellular sensitivity to IR.
Notes: Cells were pretreated with or without quercetin (100 μM) for 12 h, followed by X-irradiation. (A) The cellular sensitivity to IR was assessed by clonogenic survival assay. (B) Cells were either sham irradiated or exposed to 4 Gy IR 1 h after pretreatment with vehicle or 100 μM quercetin. DNA damage was seen in the form of a comet tail under a fluorescence microscope (left) and it was quantified (right). For each comet image, PI intensities of the head and tail portions were obtained, and the percentage intensity of the tail portion was multiplied by the size of the tail (DNA migration) to yield a tail moment. *p<0.05, when compared with control. (C) Cells were treated with 4 Gy of irradiation (in the presence or absence of quercetin) and harvested at 12 h. Immunofluorescence microscopy was conducted using anti γ-H2AX or Rad51 staining. Shown are mean foci per nucleus counted from three independent experiments. *p<0.05, when compared with control.
Abbreviations: DAPI, dihydrochloride; IR, ionizing radiation; PI, propidium iodide; Qu, quercetin.
To elucidate the mechanism of quercetin-induced radio-sensitization, we evaluated the γ-H2AX foci formation in irradiated cells because γ-H2AX is phosphorylated immediately after DSB induction and forms nuclear foci.24 The γ-H2AX foci were significantly increased in the IR group, quercetin group, and quercetin in combination with IR group, and the highest increase was in the combined treatment group (Figure 2C). We also investigated the Rad51 focus formation. In accordance with the result of γ-H2AX foci, Rad51 foci presented the lowest level in cells treated with quercetin addition (Figure 2C). Together, these data suggested that quercetin sensitized cells to IR by aggravating γ-H2AX foci and reducing Rad51 foci.
p53 expression and phosphorylation were involved in quercetin sensitization to IR-mediated DSB in ovarian cancer cells
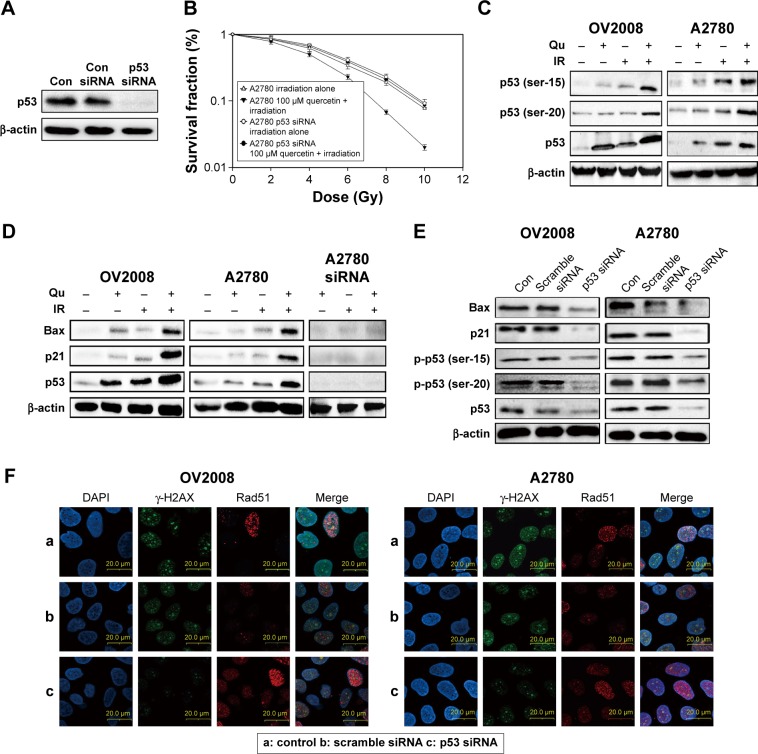
The transcriptional activity of p53 is induced by many forms of cellular stress, including IR, oncogene activation, hypoxia, and nutrient deprivation.25 As shown in Figure 3A and B, A2780 cells knocked down p53 with the specific siRNA were more resistant to IR in combination with quercetin, which suggested that the radiosensitivity effect of quercetin was dependent on p53. Also, phosphorylation at serine 15 and serine 20 are very important for p53 mediating DNA damage.26 As shown in Figure 3C, quercetin substantially elevated IR-induced p53 protein levels and increased phosphorylation of p53 on serine 15 and serine 20. In addition, p21 and BAX were significantly raised in OV2008 and A2780 under quercetin treatment in combination with IR, but not in A2780 cells knocked down p53 under the quercetin treatment in combination with IR (Figure 3D). Furthermore, the expressions of p53, p21, BAX, and γ-H2AX were markedly decreased and that of Rad51 was increased in OV2008 and A2780 cells transfected with the p53-specific siRNA (Figure 3E and F). The results suggested that p53 was involved in quercetin-sensitizing human ovarian cancer cells to IR.
Figure 3.
Quercetin increases levels of p53, phospho-p53 (serine 15, 20), and sensitizes ovarian cancer cells to DSB in response to irradiation.
Notes: (A and B) Scrambled siRNA and p53 siRNA were transfected into the cells. Then, cells were pretreated with or without quercetin (100 μM) for 12 h, followed by X-irradiation. The cellular sensitivity to IR was assessed by clonogenic survival assay. (C and D) Cells were treated with 4 Gy of IR in the presence or the absence of quercetin (100 μM, 1 h before IR and maintained for 24 h). After incubation for 48 h, cell extracts were analyzed by Western blotting. Actin was used as a loading control. (E and F) Scrambled siRNA and p53 siRNA were transfected into the cells. After incubation, cell extracts were analyzed by Western blotting. Actin was used as a loading control. (F) Immunofluorescence microscopy was conducted using anti γ-H2AX (left) or Rad51 (right) staining. Shown are mean foci per nucleus counted from three independent experiments. Errors bars represent standard deviation.
Abbreviations: Con, control; DAPI, dihydrochloride; DSB, double-strand break; IR, ionizing radiation; Qu, quercetin.
p53 is crucial for ER stress induction and enhanced IR-mediated apoptosis effect by quercetin
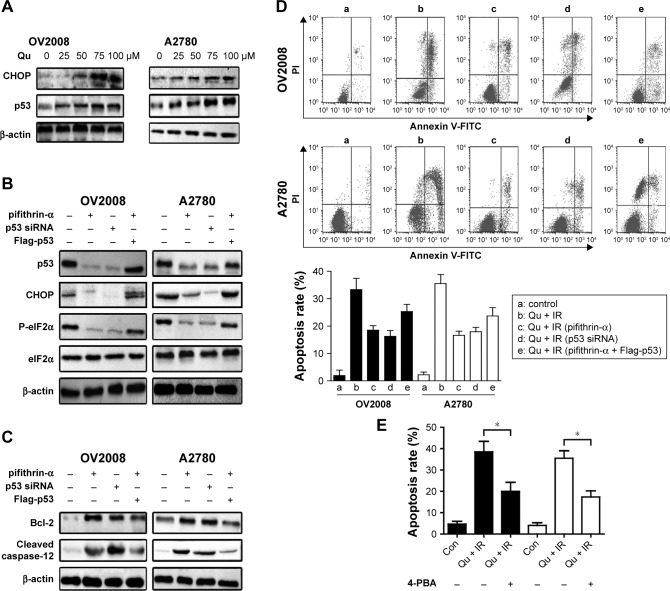
To find out the relationship between p53 and ER stress in the process of quercetin-sensitizing ovarian cancer cells to IR, we detected the expression of the ER stress biomarker CCAAT/enhancer-binding protein homologous protein (CHOP) and p53 at the same concentration of quercetin. The result showed that quercetin could induce protein expression of p53 in a dose-dependent manner parallel with the CHOP increase (Figure 4A). In previous reports, CHOP has been closely linked with p53 in regulation of cell cycle, and may be mediated by quercetin.24,25 To confirm this mechanistic link, we used a specific p53 inhibitor pifithrin-α or specific siRNA for p53 to examine the role of p53 in quercetin-induced ER stress. Knocking down p53 could abrogate CHOP expression and the proapoptosis effect of the combination treatment of quercetin and IR (Figure 4B and D). Meanwhile, overexpression of p53 in OV2008 and A2780 could partially abrogate the above phenomenon (Figure 4B and D). Moreover, p53 knockdown markedly increased Bcl-2 and cleaved caspase-12 in tumor cells (Figure 4C). Bcl-2 and cleaved caspase-12 have been demonstrated to have association with ER stress-mediated apoptosis.14,25 In addition, 4-PBA could reverse quercetin in combination with IR-induced cell apoptosis (Figure 4E). All together, quercetin-induced p53-dependent ER stress might be one of the mechanisms for quercetin-induced radiosensitization in ovarian cancer cells.26,27
Figure 4.
p53 plays a crucial role in enhancing the apoptotic effect of quercetin in ovarian cancer cells treated with IR.
Notes: (A) Cells were treated with indicated concentrations of quercetin for 24 h. The protein expressions of CHOP and p53 were analyzed by Western blot. (B and C) Cells were treated with IR (4 Gy) combined with quercetin (100 μM, 1 h before IR) followed by the presence or absence of the p53 inhibitor pifithrin-α (20 μM) for 24 h; Flag-p53 was transfected into the cells treated with IR (4 Gy) combined with quercetin (100 μM, 1 h before IR) followed by the presence of the p53 inhibitor pifithrin-α (20 μM) for 24 h; p53 siRNA was transfected into the cells followed by IR (4 Gy) combined with quercetin (100 μM, 1 h before IR) and maintained for 24 h. (B) Total cell lysates were subjected to Western blot analysis with specific antibodies to p53, CHOP, p-eIF2α, and eIF2α. (C) Western blot analysis for the detection of expression of Bcl-2 and cleaved caspase-12. (D) Flow cytometric analysis. Cells treated as in (B) were stained for the determination of annexin V/PI-positive populations using flow cytometry. (E) Flow cytometric analysis. Cells treated IR (4Gy) combined with quercetin (100 μM, 1 h before IR) followed by in the presence or absence of 4-PBA for 24 h, which were stained for the determination of annexin V/PI positive populations using flow cytometry. *p<0.05, when compared with control.
Abbreviations: CHOP, CCAAT/enhancer-binding protein homologous protein; Con, control; FITC, fluorescein isothiocyanate; IR, ionizing radiation; PI, propidium iodide; QU, quercetin.
Quercetin sensitizes human ovarian cancer cells to IRby activating ATM-mediated signaling pathways
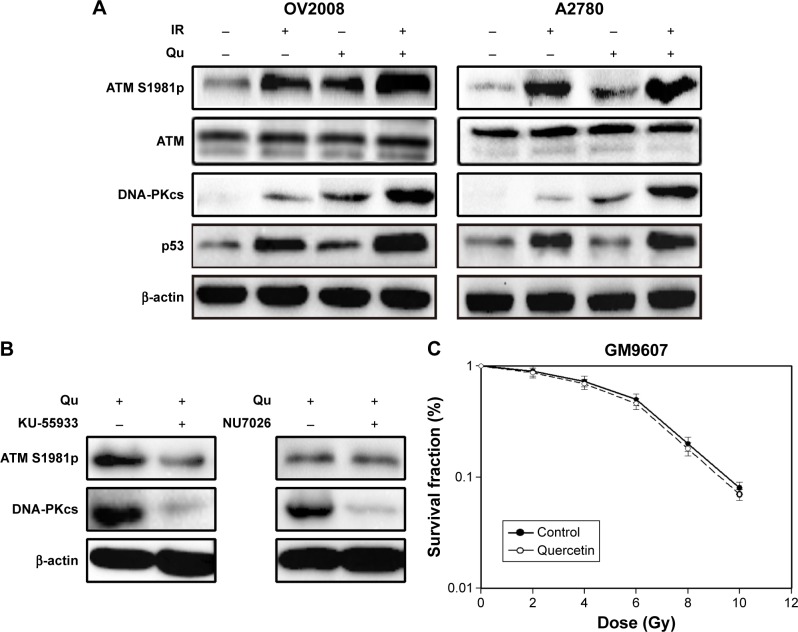
To further explore the mechanism of quercetin-induced radiosensitization, we focused on ATM activation, which is one of the most important factors of cellular radiosensitivity. As shown in Figure 5A, quercetin stimulated IR-induced Ser1981 phosphorylation in OV2008 cells, a marker of ATM activation in response to radiation.22 We also got the same result in A2780 cells (Figure 5A). In a previous study, IR stimulated transphosphorylation of DNA-PKcs at T2609 in ATM kinase dependent.18 As shown in Figure 5A, quercetin increased ATM kinase, and DNA-PKcs T2609 phosphorylation activity is stimulated by IR treatment. Therefore, cells were pretreated with quercetin for 12 h, followed by exposure to specific inhibitors of ATM (KU55933) or DNA-PKcs (NU7026) for 1 h. KU55933 could decrease the quercetin-induced ATM Ser1981 phosphorylation and reduce DNA-PKcs T2609 phosphorylation. In contrast, addition of the DNA-PKcs inhibitor led to a strong reduction of DNA-PKcs T2609 phosphorylation, and this was only slightly influenced by ATM Ser1981 phosphorylation (Figure 5B). These results demonstrated that quercetin could stimulate ATM activation and mediate downstream targets of ATM to result in radiosensitization. An SV-40-transformed human fibroblast cell line (GM9607) with deficient ATM was used to further confirm the specificity of quercetin on ATM activation. We found that IR sensitivity in this hypersensitive cell line was only slightly influenced by the presence of quercetin (Figure 5C), with a sensitizing enhancement ratio (SER) of 1.07, indicating that quercetin could specifically target ATM to induce radiosensitization.
Figure 5.
Quercetin targets ATM specifically in DDRs.
Notes: (A) Cells treated with mock or IR (4 Gy) in the presence or absence of quercetin (100 μM, 1 h before IR) were harvested 2 h after IR and subjected to Western blotting with indicated antibodies. (B) Cells treated with IR (4 Gy) combined with quercetin (100 μM, 1 h before IR) or with the ATM inhibitor KU-55933 (20 μM, 2 h before IR) or with the DNA-PKcs inhibitor NU7026 (20 μM, 2 h before IR) were harvested 2 h after IR and subjected to Western blotting with indicated antibodies. (C) The ATM-deficient fibroblast cell line GM9607 was treated with indicated doses of radiation in the presence or absence of quercetin (100 μM) followed by the colony formation assay.
Abbreviations: DDR, DNA damage response; IR, ionizing radiation; QU, quercetin.
Quercetin sensitizes cells to radiosensitivity in ovarian cancer cell xenografts
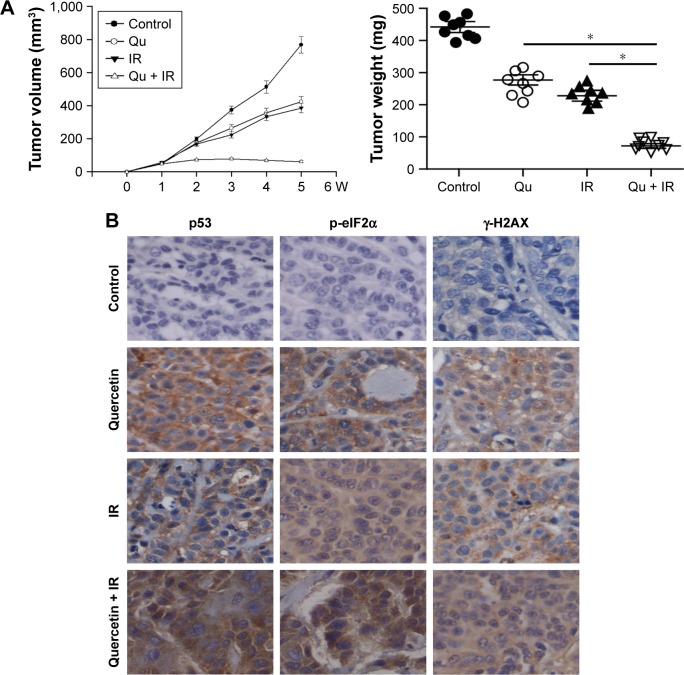
On the basis of the above results, we next investigated whether quercetin enhances radiosensitivity by inducing DSB in vivo. A2780 cells, 5×106, were injected subcutaneously on both right and left flanks of female athymic nude mice. As shown in Figure 6A, tumor growth was only significantly suppressed in the quercetin in combination with X-rays group. p53, CHOP, and γ-H2AX levels were detected in the tumor sections with immunohistochemistry, and were significantly increased in the quercetin in combination with X-ray-treated tumors (Figure 6B). Overall, these findings demonstrated that quercetin could increase ovarian cancer cells apoptosis by radiation in vivo associated with the p53-induced ER stress pathway.
Figure 6.
In vivo antitumor effects of quercetin treated with IR in OV2008 xenografts.
Notes: (A and B) OV2008 tumor cells were implanted into athymic nude mice. Mice received (2 mg/day, once daily for 14 doses) quercetin by oral gavage with/without radiotherapy (quercetin was given 1 h before IR) at 2 Gy fractions for a total dose of 20 Gy. (A) Effect of IR, and quercetin combined with or without IR on ovarian cancer growth, measurements of tumor volumes (left) or weights (right); *p<0.05, when compared with control. (B) Expression of p53, p-eIF2α, and γ-H2AX in tumor tissues of the OV2008 xenograft mouse model.
Abbreviations: IR, ionizing radiation; Qu, quercetin; W, week.
Discussion
IR is one of the most widely used therapeutics for cancer therapy. Unfortunately, some tumor patients are inherently more resistant to IR or acquire radioresistance after radiotherapy. In previous studies, quercetin is proven to have antioxidant and pro-oxidant ability.19,27 Therefore, it was reasonable to hypothesize that quercetin could be a radiosensitizer. In our study, both in vitro and in vivo evidence was in agreement with this hypothesis. Also, the SERs reached at least 1.36 in several cultured cell lines after quercetin treatment which compares favorably with other known radiosensitizers. Quercetin had recently been reported to enhance chemotherapeutic drug-induced apoptosis.28,29 Our data were consistent with these investigations.
ER stress-induced UPR is an important mechanism by which tumor cells determining their malignancy and resulting therapy resistance.30 In the present study, we found that quercetin-induced ER stress resulted in the proteasomal degradation of Rad51 and reduction of DSB repair. We considered this as one mechanism of quercetin-induced radio-sensitization because cells with lacking functional of Rad51are defective in HR repair and more susceptible to IR.31 Moreover, we found that quercetin enhanced IR-induced apoptosis by inducing CHOP through PERK/ATF4/eIF2α pathway.20 CHOP is a transcriptional factor and can promote apoptosis through mediating the ratio of the prosurvival Bcl-2 and the proapoptotic Bax.32 Our results showed that the combination treatment led to upregulation of Bax level and downregulation of Bcl-2 level in OV2008 and SKOV3 cell lines compared with that in the cells exposed to quercetin or X-rays alone.
DNA DSBs are precursors to micronucleus formation and lead to phosphorylation of histone protein H2AX and degradation of Rad51.5,24 These two markers were considered as a measure for DNA damage in this study. We found that quercetin could increase the expression of H2AX phosphorylation proteins and inhibit the expression of Rad51 in a p53-dependent manner. Furthermore, although p53 is highly expressed in cells following DNA damage,11,25,26 it is also possible that the released phosphorylated p53 enhanced the DNA damage checkpoints and transcriptional activation of genes to be involved in quercetin-enhancing IR-induced DSB in ovarian cancer cells. In addition, P21 is a key downstream target gene of p53.32 Also, phosphorylation of serine 15 was a key target site of p53 for its activation and stabilization.5,11,16 We found that quercetin increased the levels of total p53, phospho-p53 (serine 15), and p21 proteins in ovarian cancer cells. Knocking down of p53 by specific siRNA was significantly reduced on increase of DSBs, concomitant with decrease of p21 protein expression in OV2008 and A2780 cells treated by quercetin, which demonstrated that quercetin induced radiosensitization through p53 pathway. How p53 triggers ER stress is still unclear. Our study demonstrated that the eIF2a kinase was also a p53 target gene and contributed to quercetin-induced radiosensitivity. In the present study, we also showed that quercetin specifically stimulated ATM activation in DDR pathways. The detailed mechanism of quercetin activation of the ATM kinase is still unknown. Previous reports have shown that quercetin is an inhibitor of CYP2C9.33,34 CYP2C9 is a member of the cytochrome P450 mixed function oxidase system.
The current study provided basic information about the effects of a ubiquitous bioactive flavonoid as a candidate chemical for DNA damage pathways in human cancer cells. We present strong in vitro and in vivo evidence for quercetin as a radiosensitizer in tumor, which provides important data to fully understand this new pathway for radiotherapy.
Acknowledgments
This work was supported by the Educational Commission of Hubei Province of China (No B2016078).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Okayasu R. Repair of DNA damage induced by accelerated heavy ions–a mini review. Int J Cancer. 2012;130(5):991–1000. doi: 10.1002/ijc.26445. [DOI] [PubMed] [Google Scholar]
- 2.Buckel L, Savariar EN, Crisp JL, et al. Tumor radiosensitization by monomethyl auristatin E: mechanism of action and targeted delivery. Cancer Res. 2015;75(7):1376–1387. doi: 10.1158/0008-5472.CAN-14-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocard E, Oizel K, Lalier L, et al. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget. 2015;6(9):6840–6849. doi: 10.18632/oncotarget.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano MA, Li Z, Dangeti M, et al. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013;32(19):2452–2462. doi: 10.1038/onc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye R, Goodarzi AA, Kurz EU, et al. The isoflavonoids genistein and quercetin activate different stress signaling pathways as shown by analysis of site-specific phosphorylation of ATM, p53 and histone H2AX. DNA Rep. 2004;3(3):235–244. doi: 10.1016/j.dnarep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem. 2008;283(10):6572–6583. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- 7.Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 8.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 9.Bourougaa K, Naski N, Boularan C, et al. Endoplasmic reticulum stress induces G2 cell-cycle arrest via mRNA translation of the p53 isoform p53/47. Mol Cell. 2010;38(1):78–88. doi: 10.1016/j.molcel.2010.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SE, Malzer E, Ordonez A, et al. p53 and translation attenuation regulate distinct cell cycle checkpoints during endoplasmic reticulum (ER) stress. J Biol Chem. 2013;288(11):7606–7617. doi: 10.1074/jbc.M112.424655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias-Lopez C, Lazaro-Trueba I, Kerr P, et al. p53 modulates homologous recombination by transcriptional regulation of the RAD51 gene. EMBO Rep. 2006;7(2):219–224. doi: 10.1038/sj.embor.7400587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene. 2010;29(22):3241–3251. doi: 10.1038/onc.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 14.Badiola N, Penas C, Minano-Molina A, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2(4):e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai YC, Weissman AM. The unfolded protein response, degradation from endoplasmic reticulum and cancer. Genes Cancer. 2010;1(7):764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda N, Saitoh M, Kobayashi N, Miyazono K. Execution of BMP-4-induced apoptosis by p53-dependent ER dysfunction in myeloma and B-cell hybridoma cells. Oncogene. 2006;25(25):3509–3517. doi: 10.1038/sj.onc.1209393. [DOI] [PubMed] [Google Scholar]
- 17.Yamamori T, Meike S, Nagane M, Yasui H, Inanami O. ER stress suppresses DNA double-strand break repair and sensitizes tumor cells to ionizing radiation by stimulating proteasomal degradation of Rad51. FEBS Lett. 2013;587(20):3348–3353. doi: 10.1016/j.febslet.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun B, Ross SM, Trask OJ, et al. Assessing dose-dependent differences in DNA-damage, p53 response and genotoxicity for quercetin and curcumin. Toxicol In Vitro. 2013;27(6):1877–1887. doi: 10.1016/j.tiv.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Yi L, Zongyuan Y, Cheng G, Lingyun Z, Guilian Y, Wei G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014;105(5):520–527. doi: 10.1111/cas.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;4(1):8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 23.Du LQ, Wang Y, Wang H, Cao J, Liu Q, Fan FY. Knockdown of Rad51 expression induces radiation- and chemo-sensitivity in osteosarcoma cells. Med Oncol. 2011;28(4):1481–1487. doi: 10.1007/s12032-010-9605-1. [DOI] [PubMed] [Google Scholar]
- 24.Sak A, Stueben G, Groneberg M, Bocker W, Stuschke M. Targeting of Rad51-dependent homologous recombination: implications for the radiation sensitivity of human lung cancer cell lines. Br J Cancer. 2005;92(6):1089–1097. doi: 10.1038/sj.bjc.6602457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim MJ, Ahn JY, Han Y, et al. Acriflavine enhances radiosensitivity of colon cancer cells through endoplasmic reticulum stress-mediated apoptosis. Int J Biochem Cell Biol. 2012;44(8):1214–1222. doi: 10.1016/j.biocel.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Unger T, Sionov RV, Moallem E, et al. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene. 1999;18(21):3205–3212. doi: 10.1038/sj.onc.1202656. [DOI] [PubMed] [Google Scholar]
- 27.Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Natsume Y, Ito S, Satsu H, Shimizu M. Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology. 2009;258(2):164–175. doi: 10.1016/j.tox.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. 2011;68(5):1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 30.Henning W, Sturzbecher HW. Homologous recombination and cell cycle checkpoints: Rad51 in tumour progression and therapy resistance. Toxicology. 2003;193(1−2):91–109. doi: 10.1016/s0300-483x(03)00291-9. [DOI] [PubMed] [Google Scholar]
- 31.Toulany M, Mihatsch J, Holler M, Chaachouay H, Rodemann HP. Cisplatin-mediated radiosensitization of non-small cell lung cancer cells is stimulated by ATM inhibition. Radiother Oncol. 2014;111(2):228–236. doi: 10.1016/j.radonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kuo PC, Liu HF, Chao JI. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem. 2004;279(53):55875–55885. doi: 10.1074/jbc.M407985200. [DOI] [PubMed] [Google Scholar]
- 33.Sousa RL, Marletta MA. Inhibition of cytochrome P-450 activity in rat liver microsomes by the naturally occurring flavonoid, quercetin. Arch Biochem Biophys. 1985;240(1):345–357. doi: 10.1016/0003-9861(85)90040-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Xiao P, Ou-Yang DS, et al. Simultaneous action of the flavonoid quercetin on cytochrome P450 (CYP) 1A2, CYP2A6, N-acetyltransferase and xanthine oxidase activity in healthy volunteers. Clin Exp Pharmacol Physiol. 2009;36(8):828–833. doi: 10.1111/j.1440-1681.2009.05158.x. [DOI] [PubMed] [Google Scholar]