Abstract
Hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) in premature infants. In this study, we tested the hypothesis that newborn transgenic mice carrying the human CYP1A1-Luc promoter will display transcriptional activation of the human CYP1A1 promoter in vivo upon exposure to hyperoxia, and that these mice will be less susceptible to hyperoxic lung injury and alveolar simpliFIcation than similarly exposed wild type (WT) mice. Newborn WT (CD-1) or transgenic mice carrying a 13.2 kb human CYP1A1 promoter and the luciferase (Luc) reporter gene (CYP1A1-luc) were maintained in room air or exposed to hyperoxia (85% O2) for 7–14 days. Hyperoxia exposure of CYP1A1-Luc mice for 7 and 14 days resulted in 4- and 30-fold increases, respectively, in hepatic Luc (CYP1A1) expression, compared to room air controls. In lung, hyperoxia caused a 2-fold induction of reporter Luc at 7 days, but the induction declined after 14 days. The newborn CYP1A1-Luc mice were less susceptible to lung injury and alveolar simplification than similarly exposed wild type (WT) CD-1 mice. Also, the CYP1A1-Luc mice showed increased levels of hepatic and pulmonary CYP1A1 expression and hepatic CYP1A2 activity after hyperoxia exposure. Hyperoxia also increased NADP(H) quinone reductase (NQO1) pulmonary gene expression in both CD-1 and CYP1A1-Luc mice at both time points, but this was more pronounced in the latter at 14 days. Our results support the hypothesis that hyperoxia activates the human CYP1A1 promoter in newborn mice, and that increased endogenous expression of CYP1A1 and NADP(H) quinone reductase (NQO1) contributes to the decreased susceptibilities to hyperoxic lung injury in the transgenic animals. This is the first report providing evidence of hyperoxia-mediated transcriptional activation of the human CYP1A1 promoter in newborn mice, and this in conjunction with decreased lung injury, suggests that these phenomena have important implications for BPD.
Keywords: Hyperoxia, Bronchopulmonary dysplasia (BPD), Cytochrome P450, Human CYP1A1-luciferase, Lung injury, Newborn
1. Introduction
Hyperoxia is commonly encountered in premature infants with pulmonary insufficiency [1,2]. Studies link hyperoxia exposure to the development of bronchopulmonary dysplasia (BPD), which is a major risk factor for mortality and morbidity in premature infants [1–6]. Hyperoxia causes lung injury in animal models [2,7,8], and reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, and hydrogen peroxide are likely candidates in eliciting lung injury [9–13]. The cytochrome P450 (CYP) enzymes belong to a superfamily of hemoproteins that participate in the metabolism of numerous endobiotics and xenobiotics [14]. The CYP1A family comprises of two proteins, i.e. CYP1A1 and 1A2, which are induced by polycyclic aromatic hydrocarbons (PAHs), such as benzo[a]pyrene or 3-methylcholanthrene [15]. Earlier work in rat and mouse models has shown that hyperoxia induces CYP1A enzymes [9,12,13,16–18], but the underlying mechanisms are not fully understood. We showed earlier that in adult mice, hyperoxia-mediated induction of CYP1A enzymes involves the arylhydrocarbon receptor (AHR) [18], which is the transcription factor that plays a pivotal role in the regulation of CYP1A genes [19–23].
Whereas induction of CYP has been implicated in hyperoxic lung injury [9,12,13,16,17,24], several groups, including ours have shown that CYP1A1 may play a protective role. Pretreatment of rats [25,26] with inducers of CYP1A enzymes attenuates hyperoxic lung injury. Also, adult mice lacking Cyp1a1 [27], or 1a2 [28], and newborn mice deficient in Cyp1a1 [29] are more susceptible to lung injury by hyperoxia, compared to similarly exposed wild type (WT) mice. The mechanisms of induction of human CYP1A1 by hyperoxia are not well understood. It is also not known if transgenic newborn mice expressing human CYP1A1 promoter will display altered susceptibility to hyperoxic lung injury. Therefore, in this study, we tested the hypothesis that newborn transgenic mice expressing the human CYP1A1-Luc promoter will display transcriptional activation of the human promoter in vivo upon exposure to hyperoxia, and that these mice will be less susceptible to hyperoxic lung injury and alveolar simplification than similarly exposed wild type (WT) mice.
2. Materials and methods
2.1. Animal studies and treatment protocol
Transgenic mice expressing the human 13.2 Kb CYP1A1 promoter to drive luciferase expression (CYP1A1-Luc) on CD-1 background were obtained from Xenogen Corporation (Alameda, CA) [30–32]. Newborn wild type (WT) (CD-1) or CYP1A1-Luc mice were placed in plexiglass chambers within 12 h of birth, and were either maintained in room air or were exposed to hyperoxia (85% O2) for 7 or 14 days [29,33]. Details of hyperoxia exposures were as reported previously [29]. The dams were rotated between room air and hyperoxia-exposed litters every 24 h to prevent oxygen toxicity in the dams. The mice were sacrificed on PND 8 or 15. The animals were anesthetized with sodium pentobarbital (200 mg/kg i.p.) and euthanized by exsanguination while under deep pentobarbital anesthesia. Six animals from each group were used for lung histopathology. Lung and liver tissues were harvested and stored at −80 °C for subsequent for mRNA and protein isolation and analyses. This study was approved and performed in accordance with federal guidelines for the humane care and use of laboratory animals by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
2.2. Analysis of lung morphometry
Lungs were inflated through an intratracheal catheter with buffered zinc formalin (10%) and fixed in with the same solution at a constant pressure of 25 cm H20 for at least 10 min. Lungs were sectioned at 4 μm thickness on a rotary microtome and were stained with hematoxylin and eosin (H&E). Alveolar development was evaluated at PND8 and 15 (n = 6/group) by radial alveolar counts (RAC) [34] and mean linear intercept (MLI) [35] as described before [36]. Fifteen randomly chosen areas were photographed (200x magnification). Fields containing large airways and vessels were not included. Analysis of each section was carried out in a blinded fashion.
2.3. Quantitative real time PCR assays
RNA extraction from liver and lung tissues was according to previously published protocols [29]. Real time quantitative PCR was conducted according our previous publication [29]. The cDNA (2 μl) and the real-time PCR primers for CYP1A1 (Forward 5′-GGTTAAC-CATGACCGGGAACT-3′ Reverse 5′-TGCCCAAACCAAAGAGAGTGA-3′) and NQO1 (Forward 5′-GGAAGCTGCAGACCTGGTGA-3′ Reverse 5′CCTTTCAGAATGGCTGGCA-3′) were used in final 20 μL qPCR reaction with a SYBR-green master mix (Qiagen-Cat#2014143). Real-time qPCR was performed in an ABI-Prism7700 sequence detection system. Data was analyzed by ΔΔ Ct method; the expression of the target genes such as CYP1A1 and NQO1 were normalized to β-Actin as an endogenous control.
2.4. Enzyme assays
Ethoxyresorufin-O-deethylase (EROD) (CYP1A1) and methoxyresorufin O-deethylase (MROD) activities in the liver and lung whole proteins were assayed essentially as described previously [9,17,18,29]. The luciferase assays in snap frozen lung and liver tissues were performed using a kit purchased from Promega (Cat# E1501), and the assay protocol was according to the manufacturer’s instructions. Tissue luciferase activity was measured with a Luminometer (Spectra Max M3, Molecular Devices, California, USA) and recorded as relative light units (RLU).
2.5. Electrophoresis and western blotting
Liver or lung tissue total proteins (~20 μg protein) were subjected to SDS-polyacrylamide gel electrophoresis in 10% acrylamide gel followed by Western blotting to detect CYP1A1, and NQO1 proteins, as reported in the recent articles from our laboratory [9,17,18,29]. The primary monoclonal antibody to CYP1A1 was a gift from Dr. P.E. Thomas (Rutgers University, Piscataway, NJ).
2.6. Statistical analyses
The data were analyzed by using two-way analyses of variance (ANOVA) followed by modified t-tests, which were used to assess significant differences arising from exposure to hyperoxia and room air in WT and CYP1A1-Luc mice. *, P-values < 0.05 were considered significant.
3. Results
3.1. Effect of hyperoxia on transcriptional activation of human CYP1A1 and endogenous mouse CYP1A1 gene expression
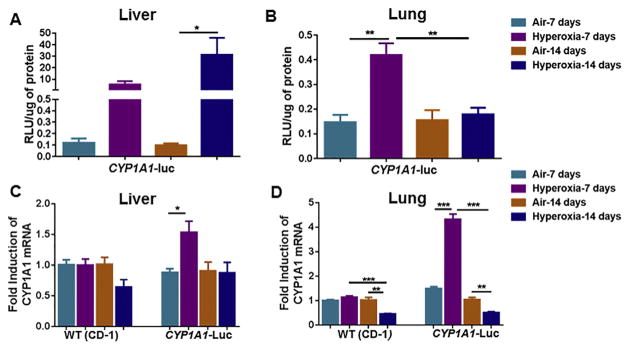
Hyperoxia elicited significant induction (~30-fold) of hepatic CYP1A1-Luc expression after 14 days of hyperoxia, compared to the room air group (Fig. 1A). In lung, hyperoxia caused a 2-fold increase in CYP1A1-Luc expression after 7 days, but the induction declined to control levels after 14 days (Fig. 1B). Hyperoxia did not alter the levels of endogenous hepatic CYP1A1 mRNA levels in WT, but it elicited about 60% induction in CYP1A1-Luc mice at the 7 but not the 14 day time point (Fig. 1C). In lung, similar to liver, hyperoxia did not alter mouse CYP1A1 levels in WT mice, but elicited a marked induction (~4-fold) of CYP1A1 mRNA in CYP1A1-Luc mice at 7 days (Fig. 1D).
Fig. 1.
Effect of hyperoxia on luciferase expression in liver (A) and lung (B) tissues of CYP1A1-Luc mice, and endogenous hepatic (C) and pulmonary (D) Cyp1a1 gene expression in WT as well as CYP1A1-Luc mice at 7 and 14 day time points. Exposure to hyperoxia and determination of luciferase and mRNA expression are described under Materials and Methods. Values represent means ± SE of 3–5 mice from each group. *,**, and ***, Statistically significant differences between air and hyperoxia exposed newborn mice at P < 0.05, 0.01, and 0.001, respectively, by two-way ANOVA.
3.2. Effect of hyperoxia on lung injury and alveolar simplification in newborn WT (CD-1) and human CYP1A1-Luc mice
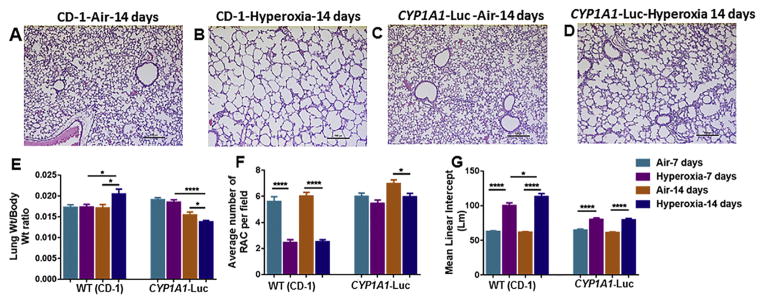
As shown in Fig. 2, WT mice in room air at postnatal day 15 (14 days of room air exposure) showed normal lung architecture (Fig. 2A), but after hyperoxia, these mice showed impaired alveolarization (Fig. 2B). In transgenic mice maintained in room air, lung architecture was normal (Fig. 2C), while mice exposed to hyperoxia showed alveolar simplification (Fig. 2D), but this was less pronounced than in WT (CD-1) mice (Fig. 2B).
Fig. 2.
Histological analysis of Hematoxylin and Eosin (H&E) stained lungs from WT (CD-1) and CYP1A1-Luc newborn mice exposed to room air and hyperoxia as described in methods. Zinc-formalin fixed, and paraffin-embedded sections were stained using H& E. Panels A and B are newborn WT (CD-1), and C and D are representative sections from CYP1A1-Luc exposed to air or hyperoxia for 14 days at 20X magnification. WT (CD1) group showed increased lung injury with perivascular edema and increased alveolar simplification after hyperoxia exposure (B). However, CYP1A1-Luc newborn mice even after hyperoxia exposure showed lesser lung injury and alveolar simplification (D). LW/BW ratio, which is an index of pulmonary edema, and RAC and MLI, which are quantitative indexes of alveolar simplification are depicted in panels E, F, and G, respectively. Values are means ± S.E.M. from 5 to 8 individual mice in air or hyperoxia groups exposed for 7 or 14 days. Significant differences in air and hyperoxia exposed newborn mice of different genotypes are indicated by *, **** at P < 0.05 or 0.0001, respectively.
Quantitative analyses showed no changes in lung weight to body weight (LW/BW) ratios in WT mice exposed to hyperoxia for 7 days, compared to room air (Fig. 2E), but there was higher pulmonary edema after 14 days of hyperoxia compared to room air animals (Fig. 2E). In CYP1A1-Luc mice there were no changes in LW/BW ratios after 7 days of hyperoxia compared to room air (Fig. 2E). Interestingly, these mice showed lesser LW/BW ratios after 14 days of hyperoxia compared to air-breathing animals (Fig. 2E).
Lung morphometric studies showed significantly lower (~62%) radial alveolar counts (RAC) in WT mice after 7 as well as 14 days of hyperoxia, compared to the room air group (Fig. 2F). While RACs were not significantly different among room air and hyperoxic groups at 7 days in the CYP1A1-Luc mice (Fig. 2F), at the 14 day time point the air-breathing animals showed higher RACs than hyperoxic animals (Fig. 2F). The mean linear intercept (MLI) was significantly higher (~40–50%) after 7 and 15 days of hyperoxia in the WT mice compared to room air (Fig. 2G). On the other hand, the MLI was only slightly increased, albeit statistically significant, after hyperoxia in the CYP1A1-Luc mice (Fig. 2G).
3.3. Effect of hyperoxia on endogenous CYP1A1/1A2 activities and contents
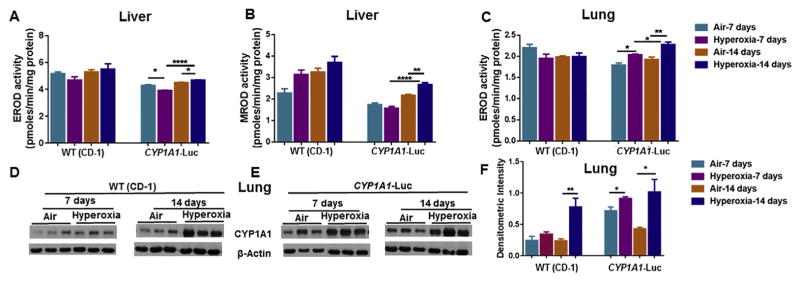
There was no significant effect of hyperoxia on liver EROD (CYP1A1) activities in WT mice (Fig. 3A), but there was a significant increase in the activity of this enzyme in CYP1A1-Luc mice at the 14 day time point (Fig. 3A). A similar effect was seen in MROD (CYP1A2) activities (Fig. 3B). In lung, hyperoxia did not alter EROD activities in WT mice, but it did elicit significant increase compared to room air controls at both the time points in CYP1A1-Luc mice (Fig. 3C). Western blotting analyses (Fig. 3D–F) showed that hyperoxia caused significant induction of pulmonary CYP1A1 protein expression in WT mice at 14 days and CYP1A1-Luc mice at both time points, compared to room air controls (Fig. 3E and F).
Fig. 3.
Effect of hyperoxia on hepatic EROD (A), MROD (B) and pulmonary EROD (C) activities, and apoprotein contents of endogenous pulmonary CYP1A1 (D,E,F) of WT (CD-1) and CYP1A1-Luc mice at 7 and 14 day time points. Values represent means ± SE of 3–5 mice from each group. *, **, and ****, Statistically significant differences between air and hyperoxia exposed newborn mice at P < 0.05, 0.01, and 0.0001, respectively, by two-way ANOVA.
3.4. Effect of hyperoxia on NQO1 mRNA and protein expression
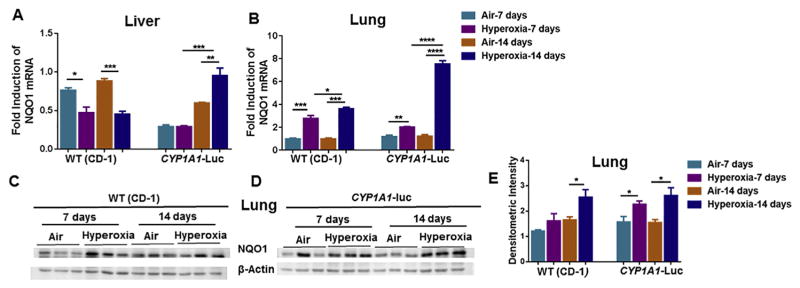
Hyperoxia exposure led to decrease in hepatic NQO1 mRNA in WT mice at both time points (Fig. 4A), but in CYP1A1-Luc mice, it elicited a significant induction of NQO1 gene expression at 14, but not 7, day time point (Fig. 4A). In lung, hyperoxia caused induction of NQO1 mRNA in WT and CYP1A1-Luc mice at both time points, but this was most pronounced in CYP1A1-Luc mice at 14 days (Fig. 4B). Western blotting analyses (Fig. 4C and D), followed by densitometric analyses (Fig. 4E), showed that hyperoxia induced NQO1 protein expression in WT at 14 days, and at both time points in the CYP1A1-Luc mice (Fig. 4E).
Fig. 4.
Effect of hyperoxia on hepatic (A) and pulmonary (B) NQO1 mRNA and pulmonary NQO1 protein levels (C,D,E) in WT (CD-1) and CYP1A1-Luc mice. NQO1 mRNA levels were determined by real time-PCR as described in Materials and Methods. Western blot analysis was performed using NQO1 specific antibodies with β-actin as a loading control. Values represent means ± SE of 3–5 mice from each group. *, **, ***, and ****, Statistically significant differences between air and hyperoxia exposed newborn mice at P < 0.05, 0.01, 0.001, and 0.0001, respectively, by two-way ANOVA.
4. Discussion
In this study, we tested the hypothesis that hyperoxia leads to transcriptional activation of the human CYP1A1 promoter in vivo. Induction of luciferase expression in liver (Fig. 1A) and lung of newborn transgenic mice expressing CYP1A1-Luc (Fig. 1B) was probably due to transcriptional activation of the corresponding human promoter. These findings were consistent with our earlier study showing induction of luciferase in adult transgenic mice harboring the human CYP1A1-Luc or mouse CYP1A2-Luc [32]. Our observation that hyperoxia elicited induction of endogenous mouse Cyp1a1 gene expression in liver (Fig. 1C) and lung (Fig. 1D) of CYP1A1-Luc, but not WT mice, was intriguing, and could have been due to activation of mouse Cyp1a1 gene due to incorporation of the human CYP1A1-Luc promoter into the mouse genome.
Our results (Fig. 2A,B, E–G) showing increases in lung injury and alveolar simplification in WT (CD-1) mice following hyperoxia (85% O2) was consistent with our previous studies in newborn C57BL/6J mice [29,37] and Fisher 344 rats [38] that showed similar lung phenotype after prolonged hyperoxia. Our observations showing decreased susceptibility of newborn transgenic mice carrying the human CYP1A1-Luc promoter to lung injury and alveolar simplification supported the hypothesis that mice harboring the human CYP1A1 promoter display beneficial effects probably by modulating the endogenous expression of mouse CYP1A and phase II antioxidant enzymes such as NQO1.
The finding that hyperoxia caused significant increases of hepatic EROD (Fig. 3A) and MROD activities (Fig. 3B) at the 14 day time point of newborn CYP1A1-Luc mice indicated induction of CYP1A1 and 1A2 activities, respectively [9,17,18]. That the transgenic newborn mice displayed induction of pulmonary EROD after 7 or 14 days of hyperoxia (Fig. 3C) supported the hypothesis that hyperoxia induced functional endogenous CYP1A1 expression. The increased apoprotein contents of CYP1A1 in lungs of CYP1A1-Luc suggested that the increase in gene expression contributed to the augmented expression of the corresponding proteins (Fig. 3F).
The decrease in hepatic gene expression of NQO1 in WT mice by hyperoxia and the increase in the expression of this gene in CYP1A1-Luc mice (Fig. 4A) suggested that introduction of CYP1A1-Luc transgene altered the regulation of NQO1 via an as yet identified mechanism. This phenomenon may have in part contributed to the decreased susceptibility of CYP1A1-Luc mice to hyperoxia compared to WT mice. The observation in lung of hyperoxia-mediated induction of NQO1 to a greater extent in CYP1A1-Luc compared to that observed in WT mice (Fig. 4B) lends further credence to the hypothesis that increased NQO1 expression contributed to the protective effects of CYP1A1-Luc in the newborn transgenic mice. The increase in NQO1 gene expression in WT as well as CYP1A1-Luc mice by hyperoxia was accompanied by a concomitant increase in the corresponding protein levels (Fig. 4C–E).
Taken together, our results suggest that the beneficial effects observed in CYP1A1-Luc mice against hyperoxic lung injury were due to a combination of mechanisms entailing induction of endogenous CYP1A enzyme as well as NQO1 by hyperoxia, and the elevated CYP1A and NQO1 expression protect against lung injury by catalyzing the detoxification of ROS-mediated metabolites (e.g., lipid hydroperoxides, quinones). Our recent studies in newborn [29] as well as adult [27,28] showing that mice lacking the genes for CYP1A1 [27] or 1A2 [28] are more susceptible to hyperoxic lung injury than WT mice lend credence to this hypothesis. Interestingly, adult mice carrying the human CYP1A1-Luc or mouse Cyp1a2-Luc were more susceptible to lung injury than WT (CD-1) mice [32], but the fact that the adult hCYP1A1-and and mouse Cyp1a2-Luc displayed decreased expression of CYP1A enzymes may have contributed to the increased lung injury in these mice. It is also possible that differences in the mechanisms of lung injury in adult and newborn by hyperoxia may have in part contributed to the differential phenotype in adult and newborn mice [39]. Further studies on the molecular regulation of CYP1A and NQO1 genes by hyperoxia could lead to much needed novel strategies [40] for the prevention and/or treatment of BPD in premature infants.
Acknowledgments
We thank Dr. Bhagavatula Moorthy for the critical reading of this manuscript and for offering us the human CYP1A1-Luc mouse model. This work was in part supported by R01 grant HL088343 (NIH) to XC and K08-HL-127103 (NIH) to KL.
Footnotes
Transparency document
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrc.2017.10.166
References
- 1.Northway WH, Jr, Rosan RC. Radiographic features of pulmonary oxygen toxicity in the newborn: bronchopulmonary dysplasia. Radiology. 1968;91:49–58. doi: 10.1148/91.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet. 2017 Apr 22;389(10079):1649–1659. doi: 10.1016/S0140-6736(17)30312-4. [DOI] [PubMed] [Google Scholar]
- 3.Bland RD, Coalson JJ. Chronic Lung Disease in Early Infancy. M. Dekker; New York: 2000. [Google Scholar]
- 4.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 5.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 8.Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, Chiarella SE, Radigan KA, Eisenbart J, Agrawal H, Berkelhamer S, Hekimi S, Zhang J, Perlman H, Schumacker PT, Jain M, Chandel NS. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am J Respir Crit Care Med. 2011;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- 10.Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J Biol Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Coalson JJ, Bobb HH, Carter JD, Banu J, Ghio AJ. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am J Physiol. 1999;277:L1214–L1223. doi: 10.1152/ajplung.1999.277.6.L1214. [DOI] [PubMed] [Google Scholar]
- 12.Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- 13.Moorthy B. The CYP1A subfamily. In: Ioannides E, editor. Cytochromes P450. Role in Drug Metabolism and Toxicity of Drugs and Other Xenobiotics. RSC Publishing; Cambridge,UK: 2008. pp. 97–135. [Google Scholar]
- 14.Guengerich FP. Cytochrome P450: what have we learned, what are the future issues? Drug Metab Rev. 2004;36:159–197. doi: 10.1081/dmr-120033996. [DOI] [PubMed] [Google Scholar]
- 15.Whitlock JP, Jr, Okino ST, Dong L, Ko HP, Clarke-Katzenberg R, Ma Q, Li H. Cytochromes P450: induction of cytochrome P4501A1: a model for analyzing mammalian gene transcription. Faseb J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun. 1993;197:878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- 17.Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 19.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor, [Ah] gene battery in the oxidative stress response, cell cycle control and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 21.Nukaya M, Moran S, Bradfield CA. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci U S A. 2009;106:4923–4928. doi: 10.1073/pnas.0809613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 2007;72:1369–1379. doi: 10.1124/mol.107.038968. [DOI] [PubMed] [Google Scholar]
- 23.Sato W, Suzuki H, Sasaki T, Kumagai T, Sakaguchi S, Mizugaki M, Miyairi S, Yamazoe Y, Nagata K. Construction of a system that simultaneously evaluates CYP1A1 and CYP1A2 induction in a stable human-derived cell line using a dual reporter plasmid. Drug Metab Pharmacokinet. 2010;25:180–189. doi: 10.2133/dmpk.25.180. [DOI] [PubMed] [Google Scholar]
- 24.Bhakta KY, Jiang W, Couroucli XI, Fazili IS, Muthiah K, Moorthy B. Regulation of cytochrome P4501A1 expression by hyperoxia in human lung cell lines: implications for hyperoxic lung injury. Toxicol Appl Pharmacol. 2008;233:169–178. doi: 10.1016/j.taap.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour H, Brun-Pascaud M, Marquetty C, Gougerot-Pocidalo MA, Hakim J, Pocidalo JJ. Protection of rat from oxygen toxicity by inducers of cytochrome P-450 system. Am Rev Respir Dis. 1988a;137:688–694. doi: 10.1164/ajrccm/137.3.688. [DOI] [PubMed] [Google Scholar]
- 26.Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- 27.Lingappan K, Jiang W, Wang L, Wang G, Couroucli XI, Shivanna B, Welty SE, Barrios R, Khan MF, Nebert DW, Roberts LJ, Moorthy B. Mice deficient in the gene for cytochrome P450 (CYP)1A1 are more susceptible than wild-type to hyperoxic lung injury: evidence for protective role of CYP1A1 against oxidative stress. Toxicol Sci. 2014 Sep;141(1):68–77. doi: 10.1093/toxsci/kfu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Firoze Khan M, Gonzalez FJ, Jackson Roberts L, Moorthy B. Disruption of cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury. Free Radic Biol Med. 2015 May;82:147–159. doi: 10.1016/j.freeradbiomed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maturu Y, Wei-Liang W, Jiang L, Wang K, Lingappan R, Barrios Y, Liang B, Moorthy XI. Couroucli, Newborn mice lacking the gene for Cyp1a1 are more susceptible to oxygen-mediated lung injury and are rescued by postnatal beta-napthoflavone (BNF) administration: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol Sci. 2017;157:260–271. doi: 10.1093/toxsci/kfx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Moorthy B, Chen M, Muthiah K, Coffee R, Purchio AF, West DB. A Cyp1a2-luciferase transgenic CD-1 mouse model: responses to aryl hydrocarbons similar to the humanized AhR mice. Toxicol Sci. 2004;82:297–307. doi: 10.1093/toxsci/kfh260. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Wang L, Zhang W, Coffee R, Fazili IS, Moorthy B. Persistent induction of cytochrome P450 (CYP)1A enzymes by 3-methylcholanthrene in vivo in mice is mediated by sustained transcriptional activation of the corresponding promoters. Biochem Biophys Res Commun. 2009;390:1419–1424. doi: 10.1016/j.bbrc.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang W, Couroucli X, Wang L, Barrios R, Moorthy Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo. Biochem Biophys Res Commun. 2011;407:79–85. doi: 10.1016/j.bbrc.2011.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MS, Rieger-Fackeldey E, Schanbacher BL, Cook AC, Bauer JA, Rogers LK, Hansen TN, Welty SE, Smith CV. Altered expressions of fibro-blast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res. 2007;62:652–657. doi: 10.1203/PDR.0b013e318159af61. [DOI] [PubMed] [Google Scholar]
- 34.Cooney TP. Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax. 37:572–579. doi: 10.1136/thx.37.8.572. 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambo P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- 36.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen Y-F, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L738–L750. doi: 10.1152/ajplung.90603.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome P4501A inducer, B-naphtho-flavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol Appl Pharmacol. 2011;256:83–94. doi: 10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakur VS, Liang YW, Lingappan K, Jiang W, Wang L, Barrios R, Zhou G, Guntupalli B, Shivanna B, Maturu P, Welty SE, Moorthy B, Couroucli XI. Increased susceptibility to hyperoxic lung injury and alveolar simplification in newborn rats by prenatal administration of benzo[a]pyrene. Toxicol Lett. 2014;230:322–332. doi: 10.1016/j.toxlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med. 2006 Jul 1;41(1):4–18. doi: 10.1016/j.freeradbiomed.2006.01.027. Epub 2006 Feb 17 Review. [DOI] [PubMed] [Google Scholar]
- 40.Abman SH, Bancalari E, Jobe A. The evolution of bronchopulmonary dysplasia after 50 years. Am J Respir Crit Care Med. 2017;195(4):421–424. doi: 10.1164/rccm.201611-2386ED. [DOI] [PubMed] [Google Scholar]