Abstract
Objective
To evaluate whether wearing auditory assistive devices can improve gait and dynamic balance.
Patients
Three adult users of bilateral hearing assistive devices: one with cytomegalovirus exposure wearing cochlear implants, one with Meniere’s disease wearing hearing aids, and one with presbystasis wearing hearing aids.
Intervention
Rehabilitative intervention involved participants performing gait and dynamic posture tasks with and without their hearing assistive devices.
Main Outcome Measures
Gait velocity and Mini-BESTest score.
Results
The participant with Meniere’s disease showed a clinically significant improvement in gait in the aided versus the unaided condition (20.5 cm/sec higher velocity and 5 point better Mini-BESTest score). The other two participants also improved with augmented audition, but to a lesser degree.
Conclusions
Bilateral hearing augmentation may promote clinically significant improvements in gait, although the effects are not uniform among patients. Hearing aids or cochlear implants may be important interventions for improving stability during ambulation in some people with hearing loss.
Introduction
Vestibular, visual, and proprioceptive inputs are critical for maintaining balance. Spatial audition is an additional input that can affect stability during quiet standing (1-4). The ability of external auditory inputs to stabilize gait has not been examined and is particularly important as falls are particularly likely to occur during ambulation (5). Here, we compared gait performance in three people with hearing loss with and without the addition of their hearing assistive devices.
Methods
All participants were experienced users of hearing assistive devices. Participant M was a 38-year old woman with a history of Meniere’s disease. She had a flat symmetric sensorineural hearing loss of about 50 dB HL and was aided with bilateral hearing aids. No laboratory vestibular testing was completed, but she had normal bedside head impulse testing. Participant C was a 21-year old man with a history of congenital cytomegalovirus (CMV) infection who wore bilateral cochlear implants. He had bilateral vestibular areflexia confirmed with calorics, cervical vestibular evoked myogenic potentials (cVEMPs), and rotational chair testing. Participant W was an 82-year old woman with symmetric presbycusis declining to 80 dB at 8 kHz, aided with bilateral hearing aids. She had a distant history of benign positional vertigo but normal calorics. All participants achieved normal or near-normal hearing levels with their devices on.
Each participant completed an ambulation task on a 90 cm wide by 700 cm long strip instrumented with pressure sensors to measure gait speed and step length (GAITRite, CIR Systems, Sparta, NJ). People with gait speeds below 70 cm/sec have been shown to be at a 1.5 times higher risk of falling (6). The minimal detectable change (MDC) for gait speed has been measured as 10.8 cm/sec in community-dwelling older adults (7) and 10–17 cm/sec in adults with pathology (8). The minimal clinically important difference (MCID) has been calculated to be 16 cm/sec in stroke patients (9).
Each participant also completed the mini-BESTest, which evaluates gait and balance and is analogous to other tests such as the Berg Balance Scale, the Tinetti Balance Test, and the Timed Up and Go Test (10,11). The test includes sitting to standing, rising onto tiptoe, stepping to avoid a fall when leaning forward, backward, or laterally, changing gait speed, turning head while walking, reversing direction while walking, stepping over a box while walking, and a sequence of rising from a chair, walking 3 meters, turning, and returning to sit. The static components of the test include standing with eyes open on a firm flat surface, standing with eyes open on a firm inclined surface, standing on one leg, and standing with eyes closed on a foam pad. A Mini-BESTest score below 17 identifies older adults with high fall risks (12). The MDC and MCID for the Mini-BESTest have been reported to be 3.5 and 4 points respectively in patients with balance disorders (13).
We also measured other variables, although consider them secondarily here because their MDC and MCID are unknown. The Functional Ambulation Performance (FAP) is a comprehensive measure of gait including velocity, step length, dynamic base of support, and step symmetry ratio (14) with lower scores corresponding to higher fall risk (15). Romberg testing was performed on a foam block (Airex, Sins, Switzerland) with feet together and hands crossed on the chest.
There were two sound conditions: aided by assistive devices and unaided. Auditory conditions were pseudorandomized. Background noise (56 dB A-weighted) for the GAITRite strip experiment was provided by a line of three treadmill machines oriented parallel to the strip at a distance of about 3 meters, and for the mini-BESTest was produced by an interstate highway approximately 50 meters distant (≈59-62 dB A-weighted). Each sound source provided a diffuse, but directional, auditory cue. Participants walked the length of the strip five times in each sound condition with their eyes closed and the results were averaged. All testing was performed in accordance with the Institutional Review Board at Washington University in St. Louis and the Helsinki Declaration.
Results
Gait velocity improved for each participant when wearing assistive devices. For the participant M with Meniere’s, gait velocity went from 51.0 to 71.5 cm/s with hearing assistive devices. For participants W and C, gait velocity increased from 46.0 to 49.9 cm/s and 85.1 to 92.5 cm/s, respectively. Results on the mini-BESTest for participants M, W, and C went from 21 to 26, 17 to 20, and 26 to 27, points respectively (Figure 1).
Figure 1.
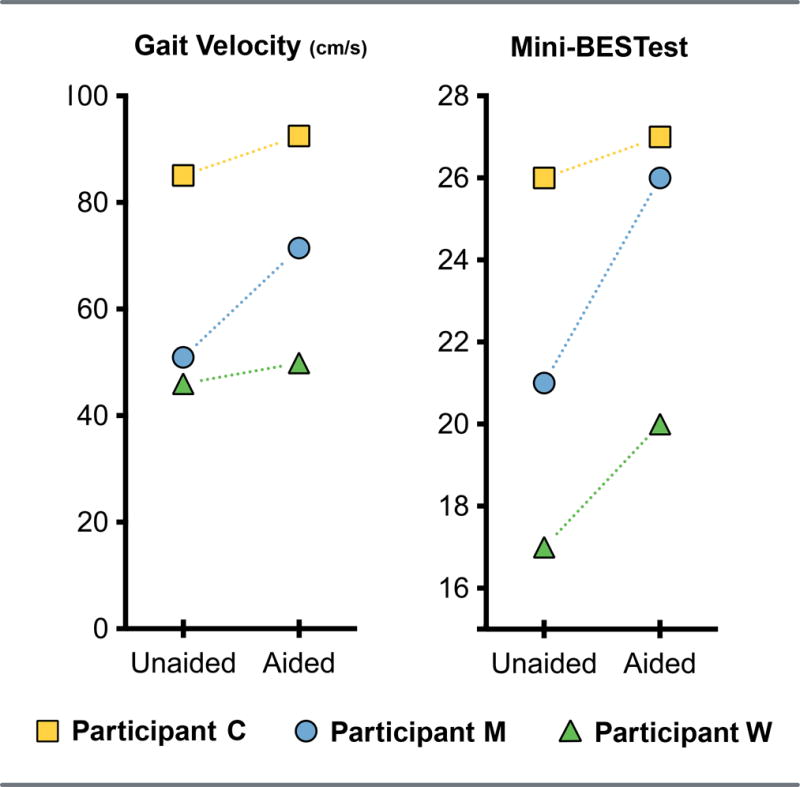
Primary outcome variables for three participants with and without their hearing assistive devices. Left: Gait speed (cm/sec). Right: Mini-BESTest Overall Score (maximum score = 28 points). Participant C had cytomegalovirus exposure, participant M had Meniere’s disease, and participant W had presbystasis.
Secondary outcome variables are shown in Figure 2. The FAP score improved from the unaided to aided auditory condition in all participants (participant M: 75-87; participant W: 60-69; participant C: 88 to 95). Step length, defined as the distance from heel strike of one foot to subsequent heel strike of the other foot and measured in centimeters, showed similar changes (participant M: 34.5 to 44.2; participant W: 27.1 to 29.2; participant C: 48.7 to 53.7) as did the Romberg test results, measured in seconds (participant M: 0 to 11; participant W: 0 to 3; participant C: 11 to 30).
Figure 2.
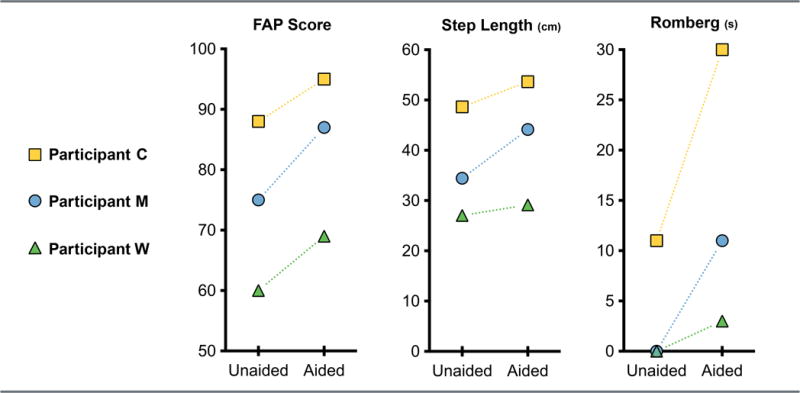
Secondary outcome variables for three participants with and without their hearing assistive devices. From left to right: Functional Ambulation Performance (FAP) score (maximum score = 100 points), step length (cm), and Romberg test on foam score (maximum time = 30 seconds). Participant key is the same as in Figure 1.
Discussion
Our results show for the first time that hearing assistive devices may improve gait performance. This extends results of previous reports suggesting that spatial auditory cues can improve static balance (1,16), and that hearing instruments may function as balance aids (3,4). Participant M demonstrated consistent, clinically significant improvement while aided, increasing gait velocity by 20.5cm/sec and mini-BESTest by 5 points. These changes in primary outcome cross thresholds for MDC (gait velocity: 10.8 cm/sec; mini-BESTest: 3.5 points) and MCID (gait velocity: 16 cm/sec as seen in stroke patients and 10-17 cm/sec in various pathologic populations; mini-BESTest: 4 points). Participants W and C showed consistent improvement but did not cross MDC and MCID thresholds. All participants demonstrated improvements on all secondary variables, but the significance of this is difficult to assess as MDC and MCID are not known for these variables.
Fall risk is indirectly correlated with gait speed among the elderly (6). All of our participants sped up with better audition, with participant W improving so much that she was no longer in the range of gait speeds considered indicative of increased fall risk (6,17). With hearing aids, participant W also crossed a mini-BESTest threshold score of 19 points, a value that has been used to differentiate between fallers and nonfallers in Parkinson patients (18). All of our participants improved their FAP scores when wearing their hearing assistive devices. The FAP scores and stride lengths also demonstrated improvement, although no numerical thresholds have been reported for fall risk (14). Improvements in Romberg score in participant C correspond to an improvement in odds ratio for falling of 3.4 (19). The change of less than 10 seconds seen in other participants fell within the same fall risk range reported by Agrawal et al. making change in odds ratio impossible to quantify.
The youngest participant performed best in all conditions, and the older participant with presbystasis performed the worst. This might be an age-related effect due to either peripheral causes such as poor proprioception or central changes such as diminished ability to combine balance-related information efficiently, but this is impossible to conclude with certainty from the limited data presented here.
The importance of auditory input likely increases when visual, proprioceptive, or vestibular inputs are not available due to environmental conditions (such as walking in the dark), and patient-related conditions (such as visual and proprioceptive loss in people with diabetes). We conducted our experiments in the dark to eliminate vision and accentuate the effect of audition, but principles of sensory integration suggest that even when vision is available the brain will use all accessible sensory cues, including audition, to maintain balance (20).
Given that all three participants were regular users of their hearing aids or cochlear implants, an alternative explanation for the performance differences seen between the aided and unaided conditions is that the participants were simply not used to perceiving their world without hearing assistive devices. While this explanation cannot be entirely dismissed, a recent study examining static balance with normal hearing listeners included non-spatial sound via headphones and found no improvement in balance (21), suggesting that the effects here are likely a result of spatial hearing.
Our results suggest that optimizing auditory inputs may be an effective tool to improve gait, as previously shown in static conditions (3,4), but may be of clinical utility only in some people. Optimization of benefit could include wearing assistive devices such as hearing aids or cochlear implants, or the thoughtful design of interior spaces to maximize the effectiveness of the acoustic environment.
References
- 1.Kanegaonkar RG, Amin K, Clarke M. The contribution of hearing to normal balance. J Laryngol Otol. 2012;126:984–8. doi: 10.1017/S002221511200179X. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X, Yost WA. Relationship between postural stability and spatial hearing. J Am Acad Audiol. 2013;24:782–8. doi: 10.3766/jaaa.24.9.3. [DOI] [PubMed] [Google Scholar]
- 3.Rumalla K, Karim AM, Hullar TE. The effect of hearing aids on postural stability. Laryngoscope. 2015;125:720–3. doi: 10.1002/lary.24974. [DOI] [PubMed] [Google Scholar]
- 4.Vitkovic J, Le C, Lee SL, Clark RA. The contribution of hearing and hearing loss to balance control. Audiol Neurotol. 2016:195–202. doi: 10.1159/000445100. [DOI] [PubMed] [Google Scholar]
- 5.Boyé ND, Mattace-Raso FU, Van Der Velde N, et al. Circumstances leading to injurious falls in older men and women in the Netherlands. Injury. 2014;45:1224–30. doi: 10.1016/j.injury.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg A, Schepens S. Measurement error and minimum detectable change in 4-meter gait speed in older adults. Aging Clin Exp Res. 2011;23(5-6):406–412. doi: 10.1007/BF03325236. [DOI] [PubMed] [Google Scholar]
- 8.Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J Eval Clin Pract. 2014;20(4):295–300. doi: 10.1111/jep.12158. [DOI] [PubMed] [Google Scholar]
- 9.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful Gait Speed Improvement During the First 60 Days Poststroke: Minimal Clinically Important Difference. Phys Ther. 2010;90(2):196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitney SL, Poole JL, Cass SP. A review of balance instruments for older adults. Am J Occup Ther. 1998;52:666–71. doi: 10.5014/ajot.52.8.666. [DOI] [PubMed] [Google Scholar]
- 11.King LA, Horak FB. On the Mini-BESTest: scoring and the reporting of total scores. Phys Ther. 2013;93:571–5. doi: 10.2522/ptj.2013.93.4.571. [DOI] [PubMed] [Google Scholar]
- 12.Yingyongyudha A, Saengsirisuwan V, Panichaporn W, Boonsinsukh R. The Mini-Balance Evaluation Systems Test (Mini-BESTest) demonstrates higher accuracy in identifying older adult participants with history of falls than do the BESTest, Berg Balance Scale, or Timed Up and Go Test. J Geriatr Phys Ther. 2016;39:64–70. doi: 10.1519/JPT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 13.Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of Reliability, Validity, and Responsiveness of the Mini-BESTest and Berg Balance Scale in Patients With Balance Disorders. Phys Ther. 2013;93(2):158–167. doi: 10.2522/ptj.20120171. [DOI] [PubMed] [Google Scholar]
- 14.Gouelle A. Use of Functional Ambulation Performance score as measurement of gait ability: Review. J Rehabil Res Dev. 2014;51:665–74. doi: 10.1682/JRRD.2013.09.0198. [DOI] [PubMed] [Google Scholar]
- 15.Nelson AJ, Certo LJ, Lembo LS, et al. The functional ambulation performance of elderly fallers and non-fallers walking at their preferred velocity. NeuroRehabilitation. 1999;13:141–6. [Google Scholar]
- 16.Easton RD, Greene AJ, DiZio P, Lackner JR. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp Brain Res. 1998;118:541–50. doi: 10.1007/s002210050310. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt MC. Risk Factors for Recurrent Nonsyncopal Falls. JAMA. 1989;261(18):2663. [PubMed] [Google Scholar]
- 18.Mak MKY, Auyeung MM. The mini-bestest can predict parkinsonian recurrent fallers: A 6-month prospective study. J Rehabil Med. 2013;45(6):565–571. doi: 10.2340/16501977-1144. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test: normative data in U.S. adults. Otol Neurotol. 2011;32:1309–11. doi: 10.1097/MAO.0b013e31822e5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 2014;15:520–35. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens MN, Barbour DL, Gronski MP, Hullar TE. Auditory Contributions to Maintaining Balance. J Vestib Res. doi: 10.3233/VES-160599. Accepted for publication. [DOI] [PubMed] [Google Scholar]


