Abstract
Pulmonary hypertension (PH) is a lung vascular disease with marked increases in pulmonary vascular resistance and pulmonary artery pressure (>25 mmHg at rest). In PH patients, increases in pulmonary vascular resistance lead to impaired cardiac output and reduced exercise tolerance. If untreated, PH progresses to right heart failure and premature lethality. The mechanisms that control the pathogenesis of PH are incompletely understood, but evidence from human and animal studies implicate nitrative stress in the development of PH. Increased levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) result in nitrative stress, which in turn induces posttranslational modification of key proteins important for maintaining pulmonary vascular homeostasis. This affects their functions and thereby contributes to the pathogenesis of PH. In this chapter, molecular mechanisms underlying nitrative stress-induced PH are reviewed, molecular sources of ROS and RNS are delineated, and evidence of nitrative stress in PH patients is described. A better understanding of such mechanisms could lead to the development of novel treatments for PH.
1. Introduction
Pulmonary hypertension (PH) is a progressive condition that was responsible for 5.5 deaths per 100,000 people in the United States in 2001, a number that increased to 6.5 deaths per 100,000 people in 2010 (National Vital Statistics System, Centers for Disease Control and Prevention, USA) (1). The pathogenesis of PH is characterized by progressive increases in pulmonary vascular resistance and pulmonary artery pressure (>25mmHg at rest). Features of PH include pulmonary vascular remodeling, endothelial dysfunction, impaired vasoconstriction, and intravascular thrombosis (2–4). Causes of PH have been classified in an expert consensus as class I to V (Table 1) (2). In severe cases of PH (e.g. idiopathic pulmonary arterial hypertension, IPAH), treatment options are limited, and lack of treatment can lead to right heart failure and premature lethality. Current pharmacological therapies targeting abnormalities in the prostacyclin, nitric oxide, and endothelin pathways can improve IPAH symptoms and lead to modest survival benefits, but do not reverse the disease pathogenesis (5, 6). Lung transplantation remains the best option for this devastating disease.
Table 1.
Clinical categories of pulmonary hypertension
| Class | Name |
|---|---|
| I | Pulmonary arterial hypertension |
| II | Pulmonary hypertension owing to left heart disease |
| III | Pulmonary hypertension associated with lung disease and/or hypoxemia |
| IV | Pulmonary hypertension due to chronic thrombotic and/or embolic disease |
| V | Pulmonary hypertension with unclear multifactorial mechanisms |
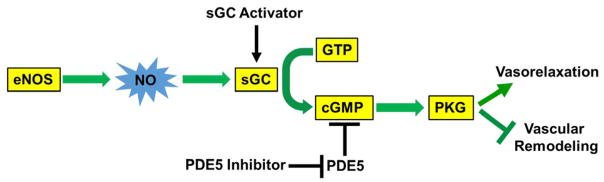
In healthy individuals, the prevention of pulmonary vascular remodeling and the preservation of a normal pulmonary tension appear to be controlled by cyclic guanosine monophosphate (cGMP)-dependent activation of protein kinase G (PKG); this occurs downstream of nitric oxide (NO) production and subsequent activation of soluble guanylate cyclase (sGC) (Figure 1). Genetic deletion of PKG-1α induces PH in mice (7), demonstrating the causal role of PKG dysfunction in the pathogenesis of PH. Therapeutic agents targeting this pathway by either inhibiting phosphodiesterase type 5 (PDE5)-dependent cGMP degradation or activating sGC-derived cGMP production have been shown to be effective in improving the symptoms of PH in humans. Early PH typically involves dysregulation of vasoactive pathways including reduced vasodilator pathway signaling (e.g. through impaired bioavailability of NO and downregulation of prostaglandin signaling), and enhanced vasoconstrictor pathway signaling (e.g. through increased production of endothelin-1 and reactive oxygen species, ROS) (8). Nevertheless, the precise molecular mechanisms that are responsible for aberrant pulmonary vascular remodeling and vasoconstriction in PH patients have not been fully defined.
Figure 1. Nitric oxide signaling maintains vascular homeostasis and inhibits the development of pulmonary hypertension.
eNOS generates basal levels of NO, which activate sGC; this in turn leads to cGMP production and subsequent activation of cGMP-dependent PKG. Activated PKG causes vasorelaxation and inhibits pulmonary vascular smooth muscle cell proliferation which underlies the mechanisms of pulmonary vascular remodeling, and thereby preserves normal pulmonary tension. Decreased NO bioavailability leading to impaired PKG activity is a common feature of PH. FDA-approved drugs by activating sGC (Riociguat) or inhibiting PDE5-mediated cGMP degradation (Sildenafil, Tadalafil) have been shown effectiveness in improving the symptom of PH and promoting modest survival, demonstrating the fundamental role of this signaling pathway in maintaining pulmonary vascular homeostasis. cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; GTP, guanosine triphosphate; NO, nitric oxide; PKG, protein kinase G; sGC, soluble guanylate cyclase.
Reactive nitrogen species (RNS) are usually unstable nitrogen-centered free radicals containing unpaired electrons (Table 2). RNS regulate many physiological processes including differentiation, metabolism, migration, and proliferation. These messenger molecules are also heavily involved in the nitrative modification of proteins that regulate PH pathogenesis. NO, for example, is formed by the NO synthase (NOS) enzymes and is stable under anoxic conditions. In the presence of excessive superoxide anion (O2·−) (i.e., oxidative stress), however, NO is converted to peroxynitrite (ONOO−) (i.e., nitrative stress) (Figure 2A). In general, when superoxide formation occurs at a threefold greater rate than NO synthesis, NO is being quantitatively converted to peroxynitrite, which leads to decreased NO bioavailability, and induces posttranslational modifications (e.g., nitrates tyrosine residues) of proteins and resultant dysregulation of protein functions (9). Nitrative stress-induced dysregulation of molecular signaling pathways have been implicated in the pathogenesis of PH in both animal models and patients (10–12).
Table 2.
Reactive nitrogen species
| Name | Formula |
|---|---|
| Dinitrogen trioxide | N2O3 |
| Nitric oxide | ·NO |
| Nitrite | NO2− |
| Nitrogen dioxide | ·NO2 |
| Nitronium ion | NO2+ |
| Nitrosothiols | RSNOs |
| Nitrosyl cation | NO+ |
| Nitrosyl chloride | NO2Cl |
| Nitrous acid | HNO2 |
| Nitrous oxide | N2O |
| Nitroxyl anion | NO− |
| Peroxynitrite | ONOO− |
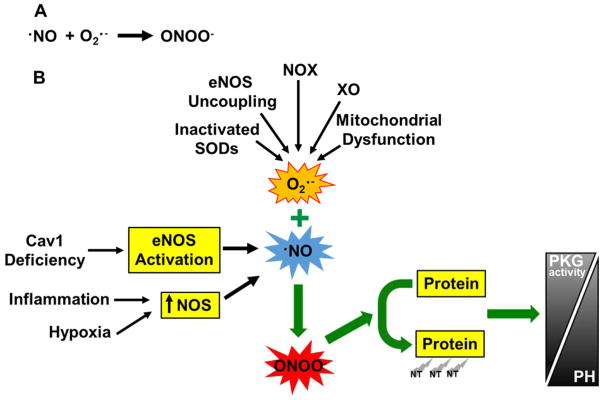
Figure 2. Molecular basis of nitrative stress in the pathogenesis of PH.
(A) Generation of peroxynitrite. In the presence of excessive superoxide anion (i.e., oxidative stress), nitric oxide and the reactive oxygen species, superoxide anion, react to form the potent, diffusible and damaging oxidant peroxynitrite. O2·−, superoxide anion; ONOO−, peroxynitrite. (B) Peroxynitrite induces tyrosine nitration of proteins leading to endothelial dysfunction, vasoconstriction and vascular remodeling and contributes to the pathogenesis of PH. Under various conditions, such as tissue inflammation, hypoxia, and Cav1 deficiency, excessive NO is generated because of increased expression of NOS or activation of eNOS (secondary to Cav1 deficiency). Under these conditions, excessive superoxide is also generated, which is attributable to increased activities of NOX (mainly NOX2 and NOX4) and xanthine oxidase (XO) whereas decreased anti-oxidant enzyme (SODs) activities, as well as eNOS uncoupling and mitochondrial dysfunction. Excessive NO and supeorxide anion leads to generation of peroxynitrite which induces tyrosine nitration of proteins and resultant dysfunction. For example, PKG nitration leads to impaired PKG activity which in turn induces vasoconstriction and vascular remodeling and thereby PH. The roles of nitration of other proteins in the pathogenesis of PH are listed in Table 3. NT, tyrosine nitration.
Given that the lung tissue of patients with severe PH demonstrate prominent levels of nitrative as well as oxidative stress (13, 14), delineation of the mechanistic role of oxidative/nitrative stress in the pathogenesis of PH and the signaling pathways regulating oxidative/nitrative stress in the pulmonary vasculature has become an actively-pursued area of research. The aim of this chapter is to review mechanisms that mediate nitrative stress-induced PH, delineate molecular sources of ROS and RNS in the context of PH, and describe evidence of nitrative stress in PH patients.
2. Molecular Mechanisms of Nitrative Stress in the Pathogenesis of Pulmonary Hypertension
2.1. Selectivity for tyrosine nitration by peroxynitrite
As mentioned above, excessive increases in NO and ROS levels give rise to peroxynitrite, a potent, diffusible, and damaging oxidant (15–17). It has been shown that high levels of peroxynitrite are cytotoxic and induce death of vascular endothelial cells and smooth muscle cells, which may contribute to the pathogenesis of PH (18, 19). Even at sublethal concentration, peroxynitrite reacts with amino acids leading to protein modifications such as tyrosine nitration. Nitration of tyrosine residues involves the addition of a nitro group (-NO2) to the hydroxyl group on the tyrosine residue. Peroxynitrite-induced tyrosine nitration is selective for certain tyrosine residues, which is not directed by specific tyrosine-containing signatures within the primary sequences, by the abundance of the proteins, or by the total amount of tyrosine. Instead, it is attributable to the local environment in which the tyrosine residue resides and the proximity of the protein to the nitrating agents (20). The presence of a proximal negatively charged Glu or Asp residue promotes the selective nitration of tyrosine, provided there is no Cys or Met near the tyrosine residue, as Cys or Met would otherwise preferentially react with nitrating agents (21). Additionally, the presence of enzymatic metal cofactors near the tyrosine residue is likely to confer specificity to nitration due to the metal-catalyzed formation of ·NO2 from ONOO−. Tyrosine nitration can be enhanced by the presence of heme-containing proteins (e.g. prostacyclin synthase) or in the presence of hydrogen peroxide through the generation of the nitrogen dioxide free radical by heme-peroxidases (e.g., myeloperoxidase) (22, 23). Specific molecules that can be modified by peroxynitrite and could be involved in the pathogenesis of PH are given in Table 3.
Table 3.
Proteins modified by peroxynitrite and involved with pulmonary hypertension
| Name | Action of Nitration | Pathological Function of Nitration | Refs |
|---|---|---|---|
| PKG | Inhibition | Pulmonary vasoconstriction and remodeling | (10, 11) |
| Prostacyclin synthase | Inhibition | Decreased production of vasodilator prostacyclin and increased production of vasoconstrictors | (25, 26) |
| eNOS | Inhibition | Induction of eNOS uncoupling generating superoxide and decreasing NO production | (11, 55, 59) |
| Mitochondrial SOD | Inhibition | Increased oxidative and nitrative stress | (30, 31) |
| ERK | Activation | Vascular cell proliferation: vascular remodeling | (33, 39) |
| PKC | Activation | Vascular cell proliferation: vascular remodeling | (36, 39) |
| p85PI3K | Inhibition (of PI3K) | Endothelial cell apoptosis and endothelial dysfunction | (40) |
| Src kinase | Activation | Vascular cell proliferation and migration: vascular remodeling | (41) |
Abbreviations: eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-related kinase; PI3K, phosphoinositide 3 kinase; PK, protein kinase; SOD, superoxide dismutase.
2.2. Nitration of protein kinase G impairs its activity and induces PH
In the lungs of Caveolin 1 (Cav1)-deficient mice that develop PH, there are enhanced levels of nitrotyrosine (a surrogate marker for peroxynitrite) (11). Tyrosine nitration of PKG is also enhanced in the lung of these mice versus wild types, while PKG activity is impaired at baseline or with its activator, cGMP (11). In the same study, authors demonstrated that alterations in PKG activity that are induced by Cav1 deficiency are eNOS-dependent and occur at least partly through nitration of PKG-1α tyrosine residues 345 and 549, which results in decreased kinase activity (Figure 2B). Genetic deletion of eNOS in Cav1 null mice results in normalization of the hypertensive pulmonary phenotype. Finally, this study also showed that PH phenotypes observed in Cav1 null mice could be reversed by treatment of these mice with either a superoxide dismutase mimetic (MnTMPyP, which scavenges superoxide) or the NOS inhibitor, L-NAME (11). Additionally, in Cav1 null mice, restoration of PKG activity through increased expression of PKG attenuates PH. Another genetic study also demonstrated the causal role of impaired PKG activity in the pathogenesis of PH by showing that Prkg1 (encoding PKG-1) knockout mice develop PH (7). Decreased PKG activity induces vasoconstriction and vascular remodeling partly through activation of Rho A/Rho kinase signaling (7). Together, these studies provide unequivocal evidence for the role of nitrative stress-induced tyrosine nitration of PKG and the resultant inhibition of its activity in the pathogenesis of PH.
In another study, it has been shown that nitration of tyrosine 247 in PKG-1α results in decreased cGMP binding and thereby decreased PKG activity in pulmonary artery smooth muscle cells (12). Tyrosine nitration of PKG has also been shown to occur in ovine fetal intrapulmonary veins in a hypoxia-dependent manner that is endothelial NOS (eNOS)-independent but through increased levels of nitrite and nitrate (10). As little as 30min exposure to hypoxia induces PKG tyrosine nitration and inhibition of its activity, which is attributable to hypoxia-induced impairment of pulmonary vessel vasorelaxation (10).
2.3. Nitration and inactivation of prostacyclin synthase induces vasoconstriction
Prostacyclin, generated primarily by the vascular endothelium, is a potent vasodilator through activation of adenylate cyclase in vascular smooth muscle cells, which increases synthesis of cyclic adenosine monophosphate (cAMP). It has been shown that prostacyclin synthase can be nitrated at residue 430 and that this results in impairment of its activity (24). Prostacyclin synthase nitration was also observed to be increased in pulmonary arterial endothelial cells from newborn lambs with persistent PH (25). Inactivation of prostacyclin synthase through tyrosine nitration impairs production of the potent vasodilator, prostacyclin, but also promotes the generation of the vasoconstrictors, prostaglandin H2 (PGH2) and thromboxane A2, and thereby contributes to vasoconstriction and development of PH (26, 27).
2.4. Nitration of endothelial nitric oxide synthase leads to endothelial nitric oxide synthase uncoupling and endothelial dysfunction
Studies show that eNOS can be modified by peroxynitrite through tyrosine nitration, which results in impairment of eNOS activity and diminished synthesis of the vasodilator, NO (27). Furthermore, peroxynitrite-mediated damage to eNOS induces eNOS uncoupling, which occurs when eNOS is not coupled with its cofactors or substrate, and the synthase activity is redirected away from generation of NO to superoxide (28, 29). In eNOS uncoupling, superoxide is generated by dissociation of the ferrous-dioxygen complex from the oxygenase domain (28, 29). eNOS uncoupling has been shown to be involved in eNOS-dependent tyrosine nitration of prostacyclin synthase. Thus, nitration of eNOS and its resultant uncoupling leads to decreased bioavailability of the vasodilators, NO and prostacyclin, and augmented oxidative/nitrative stress.
2.5. Nitration of the anti-oxidant enzyme manganese superoxide dismutase contributes to oxidative stress and PH
Given that the reaction between NO and superoxide anion leads to generation of the potent oxidant peroxynitrite, dysregulation of anti-oxidant enzymes that scavenge and reduce levels of superoxide will enhance oxidative and nitrative stress and thereby contribute to the pathogenesis of PH. It has been shown that the mitochondrial manganese superoxide dismutase (MnSOD) is nitrated at residue 34, which results in inactivation of its activity (30). Tyrosine nitration of MnSOD has been demonstrated in vivo in fetal lambs with persistent PH (31). This study shows that decreased MnSOD activity contributes to oxidative stress and resultant endothelial dysfunction and thereby facilitates the development of persistent PH.
2.6. Nitration of key signaling molecules involved in pulmonary vascular remodeling
Extracellular signal-related kinase (ERK), p38 mitogen-activated protein (MAP) kinase, and protein kinase C (PKC) are important mediators of pulmonary vascular cell proliferation underlying pulmonary vascular remodeling. It has been shown that peroxynitrite can activate these key signaling molecules (32–36). Peroxynitrite also activates nuclear factor kappa B (NF-kB), which in turn induces transcription of inducible NOS (iNOS, see section 3.3). NF-kB is activated in the pulmonary vessels of end-stage IPAH patients (37), while NF-kB inhibition reduces experimental PH in mice (38). Importantly, another study demonstrates that peroxynitrite activates pulmonary artery smooth muscle cell and endothelial cell proliferation through activation of ERK and PKC (39). Additionally, it has been shown that peroxynitrite induces nitration of p85, the regulatory subunit of phosphatidylinositol 3-kinase (PI3K) (40), and Src kinase (41). Nitration of p85 inhibits its binding to the catalytic domain of PI3K and thereby attenuates PI3K activity, whereas nitration of Src kinase leads to Src activation. These signaling molecules play an important role in pulmonary vascular cell survival, migration and proliferation. However, tyrosine nitration of these molecules in pulmonary vascular cells or in lung tissue from animal models of PH or from patients with PH has not yet been reported. Future study is warranted to assess the role of tyrosine nitration of these molecules in the regulation of pulmonary vascular remodeling.
3. Molecular Sources of Reactive Nitrogen Species in Pulmonary Hypertension
3.1. Induced expression of endothelial nitric oxide synthase
While controversy exists regarding the expression levels of eNOS in the lungs of PH patients compared with controls, eNOS is robustly expressed in the plexiform lesions of IPAH lungs (42), which may contribute to nitrative stress in these lesions. Animal models of PH have also consistently demonstrated increases in the levels of eNOS mRNA, protein, and activity (43–46). Despite these inconsistent findings, which may be a product of the different stages of disease progression being assessed, it is widely believed that NO signaling is impaired in PH patients. It has been suggested that impaired NO bioavailability and activity during PH is a result of diminished NOS co-factor availability (47) or eNOS uncoupling (48), as opposed to reductions in NOS levels per se. Reducing intracellular tetrahydrobiopterin levels, for example, reduces NO synthesis and enhances superoxide generation in endothelial cells and isolated blood vessels (49), while administration of tetrahydrobiopterin inhibits superoxide production in a dose-dependent manner (50).
3.2. Activation of endothelial nitric oxide synthase secondary to Caveolin 1 deficiency
eNOS-derived NO is in general considered be beneficial and plays an important role in maintaining vascular homeostasis through the activation of PKG (Figure 1). Activity of eNOS is regulated by its interaction with effector molecules including Cav1. It has been shown that eNOS activity is negatively regulated by Cav1 binding to eNOS (51). Administration of the Cav1 scaffolding domain inhibits eNOS activity (52). In Cav1-deficient mice compared with wild type controls, eNOS is activated in blood vessels (53), NO levels in plasma and lung are increased (11, 54). Cav1 deficiency induces PH as shown by increased pulmonary vascular resistance, medial thickness, and muscularization, along with enhanced right ventricle: left ventricle plus septum weight ratio (11, 54). PH did not occur, however, when both Cav1 and eNOS were genetically deleted, or when NOS was inhibited pharmacologically by administration of L-NAME to Cav1 knockout mice (11, 55). These experimental studies together demonstrate the essential requirement for eNOS activation secondary to Cav1 deficiency in the pathogenesis of PH. Cav1 deficiency has been shown in several animal models of PH (56–58) and in the lungs of IPAH patients, which is associated with eNOS activation and tyrosine nitration of other proteins including PKG (11, 57, 59, 60).
3.3. Induced expression of inducible nitric oxide synthase and neuronal nitric oxide synthase
Studies of hypoxia-induced PH have shown increased levels of iNOS mRNA and protein in the lungs of rats exposed to hypoxia compared with normoxia (61–64). Inhibition of iNOS attenuates hypoxia-induced PH in rats (64), indicating the pathogenic role of iNOS-derived NO in hypoxia-induced PH. Another study has shown similar increases in the levels of neuronal NOS (nNOS) gene and protein expression in the lungs of rats following exposure to hypoxia (47). Furthermore, nNOS-derived NO is the source of peroxynitrite that causes neuron damage in ischemic stroke (65).
3.4. Dietary nitrite
Along with the L-arginine-NO signaling pathway, the diet is a major source of nitrite in humans (8). The enterosalivary circulation of nitrate originates from intake of leafy greens and vegetables, along with food additives and preservatives. Salivary glands also concentrate nitrates from the plasma and secrete them into the mouth. Symbiotic bacteria in the mouth then reduces nitrate to nitrite, which enters the gastrointestinal tract. From here, nitrite can be absorbed into the circulation or reduced to form NO in the stomach. The enzymatic and non-enzymatic formation of NO from nitrite has been reviewed elsewhere (8). Non-enzymatic production of NO, for example, is enhanced by copper, ascorbate, and polyphenols, while enzymatic NO generation has been shown in metal-containing proteins such as carbonic anhydrase, hemoglobin, and myoglobin (8). Little is known, however, about the role of dietary nitrite-derived NO in nitrative stress-induced pathogenesis of PH. In fact, several studies show that inhalation of a low dose of nebulized sodium nitrite is protective from PH in animal models (66, 67).
4. Molecular Sources of Reactive Oxygen Species in Pulmonary Hypertension
4.1. Nicotinamide adenine dinucleotide phosphate oxidases
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) exist in numerous isoforms, namely NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase 1, and dual oxidase 2. NOX enzymes reduce oxygen to superoxide anion (O2·−). NOX5 is not present in rats or mice, and only NOX2 and NOX4 are expressed in the lungs (68). NOX enyzmes are the predominant source of ROS in endothelial cells (69), fibroblasts (70), and vascular smooth muscle cells (71). In pulmonary artery smooth muscle cells, for example, NOX4 expression and O2·− levels are increased by treatment with transforming growth factor β (72). In chronic hypoxia models of rodent PH, pulmonary vascular remodeling and PH are caused by NOX2- and NOX4-dependent generation of ROS (73, 74), while NOX2 and NOX4 deletion reverses PH through decreases in ROS levels (75). In a rat model of monocrotaline-induced PH, however, induction of PH was dependent on NOX1 but not NOX4 (76). Although the type of hypertensive insult may determine which NOX isoform is activated, their dysregulation is well established in the pathogenesis of PH (77).
4.2. Xanthine oxidase
Xanthine oxidase converts purine to uric acid under normoxia, but under hypoxic conditions that commonly occur in PH patients, hypoxanthine is formed from adenosine triphosphate, and oxygen is reduced to hydrogen peroxide and superoxide anion (78). Xanthine oxidase level is enhanced in the circulation of PAH patients, along with increased xanthine oxidase activity in the pulmonary arteries (79). Furthermore, xanthine oxidase activity is enhanced in a rodent model of PH and blood pressures can be restored by treatment of these animals with specific xanthine oxidase inhibitors (80, 81).
4.3. Endothelial nitric oxide synthase uncoupling
Paradoxical decreases in NO signaling that occur while eNOS expression is unaltered or even raised during PH may be a result of eNOS uncoupling. In this process, electrons being transferred to the NOS oxygenase domain from the reductase domain to form L-arginine are instead diverted to molecular oxygen, which results in the generation of superoxide anion instead of NO (for detailed review, see (48)). eNOS uncoupling is associated with changes in the quaternary structure of the enzyme, i.e. decreased assembly of the homodimer but increased assembly of the monomer. Uncoupling of eNOS is stimulated by depletion of cofactors L-arginine and tetrahydrobiopterin (BH4), or increased dihydrobiopterin (BH2) (82). Mice that have low levels of BH4 or decreased BH4:BH2 ratios exhibit PH (83). Administration of BH4 or a BH4 analogue attenuates PH in rat models challenged with monocrotaline or hypoxia (82, 84). These studies provide strong evidence for a pathogenic role of eNOS uncoupling-derived ROS in PH.
4.4. Mitochondrial electron transport chain dysfunction
Abnormal function of the mitochondrial electron transport chain leads to increased ROS generation in PH (85). Elevated levels of pyruvate dehydrogenase kinase leads to reduced levels of acetyl-CoA and a shift to aerobic glycolysis, which promotes pulmonary hyper-proliferation (86). It has also been suggested that removal of mitochondrial hydrogen peroxide leads to hypoxic pulmonary vasoconstriction, and nevertheless, increased levels of mitochondrial ROS contribute to hypoxic pulmonary vasoconstriction and hypoxic PH (87, 88).
4.5. Anti-oxidant enzyme dysfunction
Given that the reaction between NO and superoxide anion leads to generation of the potent oxidant peroxynitrite, enzymes that scavenge and reduce levels of superoxide will reduce peroxynitrite-induced injury. While NO reacts with oxyhemoglobin and is rapidly converted to nitrate in erythrocytes, superoxide is removed by SOD enzymes (89). Mitochondrial SOD is rendered inactive, however, under conditions of nitrative stress when increases in peroxynitrite lead to nitration of tyrosine residue 34 in vitro (30). Tyrosine nitration of SOD has also been demonstrated in vivo in newborn lambs with persistent PH (31). Although mitochondrial SOD nitration is seen in human renal allografts (30), it is unknown whether mitochondrial SOD is nitrated and inactivated in lung tissues of patients with severe PH such as IPAH. However, one study has shown decreased activities of SOD isoforms (including CuZnSOD, ECSOD, and MnSOD) in airway epithelial cells and in bronchial tissues of IPAH patients (90), suggesting that levels of superoxide could be increased in IPAH lung tissue.
5. Nitrative Stress in Pulmonary Hypertension Patients
Human studies that provide evidence for a role of nitrative stress in the pathogenesis of PH can be observational studies of nitrative stress factors in PH patients or studies showing an impact of therapies that target nitrative stress signaling pathways on clinical outcome. For example, levels of peroxynitrite are elevated in the lungs of patients with severe PH, and these levels are thought to be raised in part by conditions of tissue hypoxia and inflammation (13, 80). Prominent tyrosine nitration, a hallmark of nitrative stress, is evident in lung tissues of severe PH patients including IPAH patients (13). Increased tyrosine nitration of PKG, for instance, is observed in lung tissues of IPAH patients (12). The activity of eNOS is markedly increased in the lungs of IPAH patients compared with healthy controls, without significant change in its protein levels (11). There is also evidence of high levels of eNOS in the plexiform lesions of lungs from IPAH patients (42), although eNOS levels have been reported to be upregulated (43), unaltered (91), or downregulated (92) in the lungs of PH patients versus control subjects. 8-hydroxyguanosine, the product of the reaction between superoxide and guanine, is markedly increased in the endothelium of plexiform and concentric lesions from IPAH patients but not in control subjects (13). In the lungs of the same IPAH patients, the amount and activity of MnSOD was lower compared with controls. Another study also shows marked decreases in the expression and activity of all 3 of the SOD isoforms in lungs of IPAH patients (90). In contrast, expression and activity of some of the ROS-generating enzymes are markedly increased in lung tissues of IPAH patients compared with controls; NOX4 expression was markedly increased in the pulmonary vasculature of IPAH patients (i.e. predominantly in the thickened medial layer of pulmonary arteries) (93). Xanthine oxidase level is also enhanced in the circulation of IPAH patients, along with increased xanthine oxidase activity in the pulmonary arteries (79). Together these studies demonstrate increased oxidative and nitrative stress in PH patients, especially in IPAH patients.
The presence of nitrative and oxidative stress in PH patients suggests that antioxidant therapy could prove beneficial in the clinic. Despite examples of antioxidant therapy suppressing PH in animal models, such treatments in human PH have proven to be predominantly ineffective (94). The antioxidant, coenzyme Q, for instance, gave rise to a modest improvement in right ventricle function, without lengthening 6-minute walking time (95). Large-scale randomized clinical studies of therapies that reduce nitrative stress will continue to improve our understanding of the importance of the nitrative stress pathways in the pathogenesis of PH in humans.
6. Conclusions
Increased oxidative/nitrative stress is a hallmark of severe PH in patients including those with IPAH. Peroxynitrite, nitrotyrosine, and nitration of specific proteins are prominent in lung tissues of IPAH patients. Recent studies have demonstrated that increased nitrative stress induces cytotoxicity in pulmonary vascular cells, posttranslational modification (tyrosine nitration) of proteins and resultant dysregulation of their functions (Table 3), and decreased bioavailability of vasodilators NO and prostacyclin. Nitrative stress-induced tyrosine nitration of PKG results in impairment of its function (via decreased kinase activity or cGMP binding), which causes vasoconstriction and vascular remodeling leading to PH. Nitration of prostacyclin synthase inhibits its ability to synthesize the vasodilator, prostacyclin, while eNOS nitration induces eNOS uncoupling leading to decreased NO production and increased superoxide generation. Nitration-mediated inhibition of anti-oxidant enzymes such as MnSOD enhances superoxide levels and oxidative/nitrative stress. Nitrative stress also leads to activation of key signaling molecules such as ERK and PKC, which promotes pulmonary vascular cell proliferation that underlies pulmonary vascular remodeling. Thus, nitrative stress plays an important role in the pathogenesis of PH by inducing vasoconstriction and pulmonary vascular remodeling (Figure 2B, and Table 3). Although current approaches that restore aberrant nitrative signaling and alleviate nitrative stress can reduce PH in animal models, the potential benefit of such treatments on patient survival is yet to be conclusively proven. Development of novel pathologically-relevant animal models of PH (91) and of therapies that inhibit nitrative stress (thereby normalizing the functions of key proteins such as PKG), could ultimately lead to improved clinical outcome in patients with PH.
References
- 1.George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest. 2014;146(2):476–95. doi: 10.1378/chest.14-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med. 2013;34(4):639–50. doi: 10.1016/j.ccm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Morrell NW, Archer SL, Defelice A, Evans S, Fiszman M, Martin T, et al. Anticipated classes of new medications and molecular targets for pulmonary arterial hypertension. Pulm Circ. 2013;3(1):226–44. doi: 10.4103/2045-8932.109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2015;65(18):1976–97. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 7.Zhao YD, Cai L, Mirza MK, Huang X, Geenen DL, Hofmann F, et al. Protein kinase G-I deficiency induces pulmonary hypertension through Rho A/Rho kinase activation. Am J Pathol. 2012;180(6):2268–75. doi: 10.1016/j.ajpath.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno M, Wang J, Mora AL, Gladwin MT. Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxid Redox Signal. 2013;18(14):1797–809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox biology. 2013;1:244–57. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L1012–20. doi: 10.1152/ajplung.00061.2007. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119(7):2009–18. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal S, Gross CM, Rafikov R, Kumar S, Fineman JR, Ludewig B, et al. Nitration of tyrosine 247 inhibits protein kinase G-1alpha activity by attenuating cyclic guanosine monophosphate binding. J Biol Chem. 2014;289(11):7948–61. doi: 10.1074/jbc.M113.534313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169(6):764–9. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 14.Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, et al. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2001;164(6):1038–42. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Li W, Su J, Liu W, Altura BT, Altura BM. Peroxynitrite induces apoptosis in rat aortic smooth muscle cells: possible relation to vascular diseases. Experimental biology and medicine. 2004;229(3):264–9. doi: 10.1177/153537020422900307. [DOI] [PubMed] [Google Scholar]
- 19.Gow AJ, Thom SR, Ischiropoulos H. Nitric oxide and peroxynitrite-mediated pulmonary cell death. Am J Physiol. 1998;274(1 Pt 1):L112–8. doi: 10.1152/ajplung.1998.274.1.L112. [DOI] [PubMed] [Google Scholar]
- 20.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305(3):776–83. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274(2):842–8. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 22.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272(12):7617–25. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 23.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101(12):4003–8. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt P, Youhnovski N, Daiber A, Balan A, Arsic M, Bachschmid M, et al. Specific nitration at tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J Biol Chem. 2003;278(15):12813–9. doi: 10.1074/jbc.M208080200. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan CN, Afolayan AJ, Eis A, Teng RJ, Konduri GG. Altered prostanoid metabolism contributes to impaired angiogenesis in persistent pulmonary hypertension in a fetal lamb model. Pediatr Res. 2015;77(3):455–62. doi: 10.1038/pr.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins & other lipid mediators. 2007;82(1–4):119–27. doi: 10.1016/j.prostaglandins.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium: journal of endothelial cell research. 2004;11(2):89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 28.Luo S, Lei H, Qin H, Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr Pharm Des. 2014;20(22):3548–53. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama M, Hirata K. Endothelial nitric oxide synthase uncoupling: Is it a physiological mechanism of endothelium-dependent relaxation in cerebral artery? Cardiovasc Res. 2007;73(1):8–9. doi: 10.1016/j.cardiores.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366(1):82–8. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 31.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, et al. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;303(10):L870–9. doi: 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bapat S, Verkleij A, Post JA. Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS Lett. 2001;499(1–2):21–6. doi: 10.1016/s0014-5793(01)02511-x. [DOI] [PubMed] [Google Scholar]
- 33.Pesse B, Levrand S, Feihl F, Waeber B, Gavillet B, Pacher P, et al. Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol. 2005;38(5):765–75. doi: 10.1016/j.yjmcc.2005.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Wang YZ, Kagan E, Bonner JC. Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J Biol Chem. 2000;275(29):22479–86. doi: 10.1074/jbc.M910425199. [DOI] [PubMed] [Google Scholar]
- 35.Guner YS, Ochoa CJ, Wang J, Zhang X, Steinhauser S, Stephenson L, et al. Peroxynitrite-induced p38 MAPK pro-apoptotic signaling in enterocytes. Biochem Biophys Res Commun. 2009;384(2):221–5. doi: 10.1016/j.bbrc.2009.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281(10):6366–75. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 37.Price LC, Caramori G, Perros F, Meng C, Gambaryan N, Dorfmuller P, et al. Nuclear factor kappa-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS One. 2013;8(10):e75415. doi: 10.1371/journal.pone.0075415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Wei C, Kim IK, Janssen-Heininger Y, Gupta S. Inhibition of nuclear factor-kappaB in the lungs prevents monocrotaline-induced pulmonary hypertension in mice. Hypertension. 2014;63(6):1260–9. doi: 10.1161/HYPERTENSIONAHA.114.03220. [DOI] [PubMed] [Google Scholar]
- 39.Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulmonary pharmacology & therapeutics. 2011;24(1):100–9. doi: 10.1016/j.pupt.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Hellberg CB, Boggs SE, Lapetina EG. Phosphatidylinositol 3-kinase is a target for protein tyrosine nitration. Biochem Biophys Res Commun. 1998;252(2):313–7. doi: 10.1006/bbrc.1998.9581. [DOI] [PubMed] [Google Scholar]
- 41.Minetti M, Mallozzi C, Di Stasi AM. Peroxynitrite activates kinases of the src family and upregulates tyrosine phosphorylation signaling. Free Radic Biol Med. 2002;33(6):744–54. doi: 10.1016/s0891-5849(02)00891-2. [DOI] [PubMed] [Google Scholar]
- 42.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, et al. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol. 1998;185(3):313–8. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Xue C, Johns RA. Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(24):1642–4. doi: 10.1056/NEJM199512143332416. [DOI] [PubMed] [Google Scholar]
- 44.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107(5):2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L46–56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, et al. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. Journal of applied physiology. 2008;104(1):110–8. doi: 10.1152/japplphysiol.00698.2005. [DOI] [PubMed] [Google Scholar]
- 47.Shaul PW, North AJ, Brannon TS, Ujiie K, Wells LB, Nisen PA, et al. Prolonged in vivo hypoxia enhances nitric oxide synthase type I and type III gene expression in adult rat lung. Am J Respir Cell Mol Biol. 1995;13(2):167–74. doi: 10.1165/ajrcmb.13.2.7542896. [DOI] [PubMed] [Google Scholar]
- 48.Gielis JF, Lin JY, Wingler K, Van Schil PE, Schmidt HH, Moens AL. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med. 2011;50(7):765–76. doi: 10.1016/j.freeradbiomed.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Cosentino F, Katusic ZS. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91(1):139–44. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- 50.Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. 1997;237(2):340–4. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- 51.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272(30):18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 52.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6(12):1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 53.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 54.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99(17):11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulmonary pharmacology & therapeutics. 2008;21(3):507–15. doi: 10.1016/j.pupt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110(11):1499–506. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 57.Achcar RO, Demura Y, Rai PR, Taraseviciene-Stewart L, Kasper M, Voelkel NF, et al. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129(3):696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 58.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114(9):912–20. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 59.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88(6):555–62. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 60.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21(7):1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 61.Le Cras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol. 1996;270(1 Pt 1):L164–70. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- 62.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274(2 Pt 1):L212–9. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 63.Resta TC, O’Donaughy TL, Earley S, Chicoine LG, Walker BR. Unaltered vasoconstrictor responsiveness after iNOS inhibition in lungs from chronically hypoxic rats. Am J Physiol. 1999;276(1 Pt 1):L122–30. doi: 10.1152/ajplung.1999.276.1.L122. [DOI] [PubMed] [Google Scholar]
- 64.Hampl V, Bibova J, Banasova A, Uhlik J, Mikova D, Hnilickova O, et al. Pulmonary vascular iNOS induction participates in the onset of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L11–20. doi: 10.1152/ajplung.00023.2005. [DOI] [PubMed] [Google Scholar]
- 65.Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19(14):5910–8. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10(10):1122–7. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 67.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 68.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal. 2009;11(10):2505–16. doi: 10.1089/ars.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49(4–6):134–40. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94(26):14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L490–8. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- 72.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L661–L73. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 73.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, et al. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10(10):1687–98. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 74.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, et al. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein- 3. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L489–99. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 76.Veit F, Pak O, Egemnazarov B, Roth M, Kosanovic D, Seimetz M, et al. Function of NADPH oxidase 1 in pulmonary arterial smooth muscle cells after monocrotaline-induced pulmonary vascular remodeling. Antioxid Redox Signal. 2013;19(18):2213–31. doi: 10.1089/ars.2012.4904. [DOI] [PubMed] [Google Scholar]
- 77.Hansen T, Galougahi KK, Celermajer D, Rasko N, Tang O, Bubb KJ, et al. Oxidative and nitrosative signalling in pulmonary arterial hypertension - Implications for development of novel therapies. Pharmacol Ther. 2016;165:50–62. doi: 10.1016/j.pharmthera.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996;270(6 Pt 1):L941–6. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- 79.Spiekermann S, Schenk K, Hoeper MM. Increased xanthine oxidase activity in idiopathic pulmonary arterial hypertension. The European respiratory journal. 2009;34(1):276. doi: 10.1183/09031936.00013309. [DOI] [PubMed] [Google Scholar]
- 80.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. Journal of applied physiology. 2001;90(4):1299–306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 81.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L233–45. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 82.Kunuthur SP, Milliken PH, Gibson CL, Suckling CJ, Wadsworth RM. Tetrahydrobiopterin analogues with NO-dependent pulmonary vasodilator properties. Eur J Pharmacol. 2011;650(1):371–7. doi: 10.1016/j.ejphar.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 83.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, et al. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111(16):2126–33. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 84.Francis BN, Wilkins MR, Zhao L. Tetrahydrobiopterin and the regulation of hypoxic pulmonary vasoconstriction. The European respiratory journal. 2010;36(2):323–30. doi: 10.1183/09031936.00188809. [DOI] [PubMed] [Google Scholar]
- 85.Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Comprehensive Physiology. 2013;3(3):1011–34. doi: 10.1002/cphy.c120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115(1):148–64. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 87.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536(Pt 1):211–24. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Current opinion in pharmacology. 2009;9(3):287–96. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masri FA, Comhair SA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, et al. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clinical and translational science. 2008;1(2):99–106. doi: 10.1111/j.1752-8062.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–32. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 92.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 93.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101(3):258–67. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 94.Wong CM, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal. 2013;18(14):1789–96. doi: 10.1089/ars.2012.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharp J, Farha S, Park MM, Comhair SA, Lundgrin EL, Tang WH, et al. Coenzyme Q supplementation in pulmonary arterial hypertension. Redox biology. 2014;2:884–91. doi: 10.1016/j.redox.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]