COLUMN ARTICLE
Fiber-reinforced composite (FRC) molding compounds with micromechanic requirements for fiber lengths longer than critical length (Lc) are now the most important breakthrough in Dentistry since the amalgam. The Lc is a measure of the minimum perfectly aligned fiber length dimension needed before maximum fiber stress transfer starts to occur within the cured resin [1–3]. Mechanical properties tested on flexural strength, yield strength, modulus, resilience, work of fracture (WOF), critical strain energy release (SIc), critical stress intensity factor (KIc) and Izod impact toughness for FRCs using pure quartz fibers have shown large statistically significant increases over the dental particulate-filled composites (PFCs) as 3M Corp. Z100® and Kerr Corp. Herculite® [4–10] and a PFC with microfibers that cannot satisfy Lc as Alert® from Jeneric Pentron [5–8,10]. In addition, FRCs have shown large statistically significant increases for all mechanical properties tested except modulus when compared to a widely used amalgam alloy Tytin® from Kerr Corp. [9,10]. Further, FRCs have shown other greatly improved properties for wear less than enamel [10,11], significantly increased condensing packability force with significant larger interproximal contacts [10,12] and ability to incorporate the antimicrobial triclosan without PFC sticky glueyness [10]. Industrially FRCs are accepted as high-performance molding compounds that can pack with control to form into intricate geometric cavities and used extensively in the electrical and automotive industries so that FRC development for Dentistry can proceed on firm dedicated principles.
Common problems with poor service longevity for dental PFCs when compared to amalgams [13–21] accentuate the importance for dental FRC molding compound use as an amalgam alternative [10]. Evidence-based randomized controlled clinical trials over 5 to 7 years have determined that the current PFCs used in dentistry fail at a rate 2 to 3 times greater than the amalgam [16,17,19,20]. Both the PFCs and amalgams generally fail due to secondary caries at the margins where PFC secondary caries failure rates have been shown to be 3.5 times greater than amalgams [17]. Recent accurate mechanical tests show that PFCs have an extremely low modulus compared to the amalgam modulus that can be compared much better to the modulus for enamel [9,10]. Subsequent lower dental PFC modulus filling material that deflects much greater should be more susceptible to increased interlaminar shear stress debonding at a higher modulus tooth adhesive interface that helps to account for more occlusal marginal leakage with related secondary decay [9,10]. On the other hand, FRC molding compounds can have much higher moduli that are closer to the modulus of amalgam [9,10]. Larger occlusal fillings and restorations with more than three surfaces then accentuate PFC failures [16,17]. Subsequent larger fillings with more margin exposure to occlusal loading would increase the probability that debonding occurs at a margin [9,10]. Also, the ADA has recommended that dental PFCs not be used in “stress-bearing” areas periodically since 1994 [22–24]. Although accurate mechanical tests comparing dental PFCs to amalgams show superior PFC properties for strengths and fracture toughness [9], moisture adsorption greatly reduces dental PFC strength [25] that could be accelerated by low modulus PFC strain-related microcracking to determine the eventual fracture failure in larger PFC fillings [9,10].
PFCs have many other problems that can account for increased secondary caries compared to amalgams. PFCs wear at a much greater rate than amalgams with much deeper related marginal ditching [26] that would tend to collect more bacteria in a pool next to margins on the occlusal surface [10,26]. Also, PFC wear rates increase with wider cavity sizes correlated with reduced “sheltering” by the enamel margins [27]. In addition, PFCs were shown to require 7 – 8 times more repairs than amalgams [16]. Dental PFCs are extremely technique sensitive [28,29] while the amalgam is far easier to fill a cavity preparation than the PFC [30–36]. Dental PFCs require about double the time to finish as a similar amalgam [31]. The dental PFC is a tacky paste as a difficult material to pack due to low viscosity or consistency where matrix resins further have a tendency to adhere to packing instruments resulting in noticeable voids [37]. Subsequent sticky tack and low consistency in dental PFCs are then known to produce problems related to class II fillings with voids in the proximal box [9,10,38–43], overhangs difficult to remove [9,10,30,36] and inadequate interproximal contacts [30,32,34–36,39,44–55] with food impaction [34,56–59]. Also, poor interproximal contacts are associated with a higher caries risk [34]. Consequential plaque is found 3.2X more frequently on interproximal margins with dental PFCs than amalgam [60] and interproximal secondary decay has been detected 5.4 times more frequently on the gingival margin in dental PFCs than amalgams [15]. As a related concern for voids in the dental PFC proximal box, the ADA Council on Dental Materials expressed alarm early in 1980 standard requirements that radiopacity be measurable for the detection of voids on x-rays [40]. Conversely, fibers greatly increase polymer consistency so that FRCs pack with positive controlled pressure into complex mold cavities to prevent void formation such as in the proximal box [9,10,12]. Further, FRCs have shown large significant statistically improved reductions in voids over dental PFC polymerization shrinkage test samples (p < 0.00001), increased interproximal contact areas over both high-viscosity Tytin® FC amalgam and Z100® PFC (p < 0.0001), and much higher packing forces than both high-viscosity Tytin® FC amalgam and Z100® PFC (p < 0.001) [10,12].
The FRC mechanical properties in a Z100® PFC matrix for different fiber lengths from Lc at approximately 0.5 mm for a 9 μm diameter quartz fiber and longer lengths up to 3.0 mm are compared with a Z100® PFC, Alert® PFC with microfibers well below Lc and Tytin® amalgam, Table 1 [9]. For the FRC with fiber lengths of 0.5 mm, at the Lc of 0.5 mm most of the fiber debonds from the polymer matrix that fails before the fiber breaks so that the full strength of the fiber can not be transferred through the composite. Consequently, small reductions in many mechanical properties occurred for the FRC at the 0.5 mm length when compared to the same polymer matrix composed of Z100® PFC. In fact, Alert® with microfibers well below Lc resulted in lower mechanical results for all properties when compared to the Z100® PFC probably due to a large extent from microfiber debonding that creates detrimental defects during the different loading conditions. But, as fiber lengths increase above Lc, increasing the fiber length to fiber diameter or aspect ratio increases strengths and modulus [5,10]. Further, increasing fiber length with aspect ratio above the Lc increases all fracture toughness properties for resilience, WOF, SIc, and KIc [5,10]. Subsequent lower mechanical properties for strength would then increase bulk fracture while lower fracture toughness properties would increase marginal chipping. Also, as fibers with some of the highest moduli known and above Lc bond well along the cavity walls at the occlusal margins interlaminar shear with the tooth and debonding related to secondary caries is expected to diminish greatly.
Table 1.
Averages and T-test (p value) Comparisons Between Composites and Amalgam
| Fiber Length (mm) 30wt% (28.2 Vf) | Flexural Strength (MPa) | Modulus (GPa) | Yield Strength (MPa) | Resilience (kJ/m2) | WOF (kJ/m2) | SIc (kJ/m2) | KIc (MPa*m1/2) | Strain at Peak Load |
|---|---|---|---|---|---|---|---|---|
| 0.0 mm (PFC) Z100® 3M | 117.6 (0.0012) | 19.5 (0.00102) | 95.4 (0.01337) | 3.03 (0.01882) | 4.48 (5.1×10−5) | 0.036 (0.16055) | 1.71 (0.06584) | 0.0079 (0.9740) |
| 0.5 mm (FRC) | 113.8 (0.1399) | 23.0 (0.0008) | 92.8 (0.0372) | 2.35 (0.0400) | 3.91 (0.0984) | 0.075 (0.1794) | 1.93 (0.07953) | 0.0062 (0.9189) |
| 1.0 mm (FRC) | 173.6 (0.00318) | 26.2 (0.001875) | 126.1 (0.00018) | 3.84 (0.00083) | 8.7 (0.01879) | 0.097 (0.0584) | 2.77 (0.00797) | 0.0084 (0.2993) |
| 2.0 mm (FRC) | 373.9 (5.2×10−5) | 34.0 (0.00116) | 329.8 (0.00168) | 19.7 (0.00287) | 28.2 (0.00046) | 1.882 (0.0338) | 11.01 (0.00579) | 0.0121 (0.1290) |
| 3.0 mm (FRC) | 374.9 (2.2×10−8) | 31.5 (0.01236) | 343.5 (0.00014) | 23.3 (0.00348) | 30.1 (4.2×10−5) | 2.4 (0.00296) | 12.01 (3.89×10−5) | 0.0131 (0.0677) |
| Alert® (PFC) with microfibers | 90.4 (0.7057) | 17.6 (0.00019) | 62.3 (0.9791) | 1.52 (0.0440) | 3.23 (0.0077) | 0.034 (0.2950) | 1.33 (0.7523) | 0.0069 (0.7130) |
| Amalgam Tytin® | 86.0 | 43.6 | 62.6 | 0.67 | 1.40 | 0.013 | 0.91 | 0.0078 |
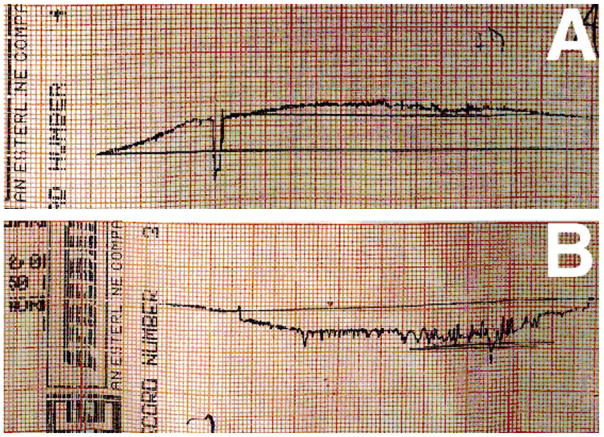
By related FRC strength improvements wear rates are reduced as fibers better support surface loading conditions and in particular as the fiber lengths become longer than the wearing plowing grooves [61,62]. FRCs with high modulus fibers reduce microcracking and water adsorption related to lower strain [63,64] that should further reduce wear. In fact, during a typical University of Alabama at Birmingham 3-body generalized wear simulator test at 400,000 cycles on a flat occlusal tooth sample corresponding to 3 clinical years of service FRC molding compound with fibers above Lc produced wear less than enamel. Accordingly, FRC wear produced a smooth filling material transition with the enamel margin. Conversely, the Alert® PFC with microfibers below Lc wears more than enamel to produce the characteristic material depression with ditch trenches at the enamel margins [10,11]. Profilometer tracings of the wear surfaces for an FRC with quartz fibers above Lc and the Alert® PFC with microfibers that were well below Lc are shown in Figure 1 [10,11]. Although amalgams wear much less than dental PFCs, amalgams still wear more than enamel to create small depressions at the margins [26] and microfills that wear slightly more than amalgams nevertheless fail from low fracture toughness properties resulting in marginal fracture [65,66]. While the polymer matrix is sheltered by nanoparticulate that fit closer together and do not debond by wear the larger particulate of microhybrid PFCs shear under loading conditions into the polymer matrix and debond to accelerate wear and produce a much deeper marginal ditching trench [27].
Figure 1.
Profilometer wear tracings. (A) FRC with fibers above Lc with less wear than enamel transition with a smooth cleaner surface at the margin (B) PFC Alert® with microfibers below Lc show margins ditched with greater wear than enamel.
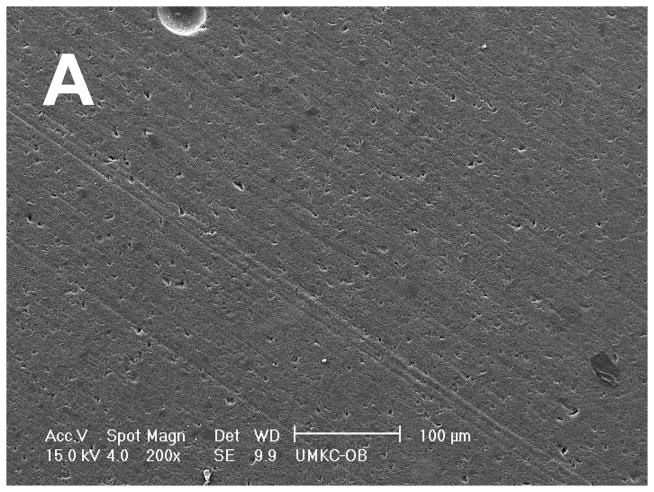
Scanning Electron Microscopy (SEM) of the same wear sample surfaces show the discontinuous chopped FRC molding compound with fibers greater than Lc to be vastly smoother and polished even at twice the magnification, Figure 2A, when compared to the rough surface for the PFC Alert® with short microfibers below Lc that lie in random planar fashion, Figure 2B [10,11]. Fibers above Lc will not be found on the wear surface where “sheltering” of the Z100® spherical nanohybrid particulate polymer matrix is accentuated by fibers that align parallel to the occlusal surface from packing forces. An SEM of the FRC compound at much higher 5000X magnification shows how a high-strength 9 μm diameter quartz fiber wears by thinning until sufficiently skeletal to break up into fine flat plate-like particulate with sizes from much less than 200 nm to about 3 μm that press fairly level back into the Z100® PFC polymer matrix, Figure 2C. Subsequent thin flat particulate would not be expected to shear debond from the composite matrix by wear loading but rather break down further into smaller particles. Finer nanoparticulates that debond from the polymer matrix might then tend to even polish the FRC across a dryer surface above the flat sample enamel plane. On the other hand, depressions that exist following PFC wear would pool fluids and bacteria that tend to dissolve the polymer for increased wear and marginal secondary decay. Also, the FRC polymer matrix may experience creep from wear pressure that results in the scission of some polymer molecules thus leaving a free radical on either side of the dissociated bond. Subsequent dangling free radicals may possibly then reinitiate free-radical crosslinking of methacrylate end groups with coupling to quartz particulate and better help explain the ideal smooth wear surface created in Figure 2A.
Figure 2.
SEMs (A) FRC polished smoothly with extremely low wear surface 200X magnification scale bar 100 μm (B) Rough PFC Alert® with debonding microfiber wear surface debris 100X magnification scale bar 200 μm. (C) FRC 5000X magnification reveals how a quartz fiber wears thin into flat plate-like particulate producing the smooth surface in figure 2A, scale bar 5 μm.
PFCs have developed 3.2 times more plaque than amalgam on class II margins [60]. Leachable monomers of dental PFCs [67,68] have been found capable of supporting bacterial growth [67,69]. Further, dental PFCs can support decay under restorations that do not occur below amalgams under identical conditions [67]. However, amalgam has silver antimicrobial properties [70–74]. Similarly, high-viscosity FRC consistency allows incorporation of broad-spectrum triclosan antimicrobial whereas lower viscosity PFCs are deprived of entire consistency and become gluey when triclosan is added by disrupting the resin and nanoparticulate weak secondary bonds [75–77]. The nonpolar or hydrophobic antimicrobial triclosan is a wetting agent to reduce viscosity during the mixing stage for resin and fiber incorporation, but and on the other hand is a toughening agent for the cured polymer to further increase flexural and adhesive bond strengths [75–77]. The hydrophobic or nonpolar principles for chemistry with triclosan should also reduce material breakdown by repelling polar molecules such as water and acid. An unusual odd alarmist triclosan controversy over bacterial resistance from unwarrantable extreme laboratory conditions that cannot be found in a normal microenvironment has been unjustifiable without any bacterial resistance to triclosan reported in over 40 years resulting in a government report recommendation for triclosan use wherever a health benefit is possible [76,77] as in dentistry. In some similar manner as triclosan disruption of secondary bonding needed for PFC consistency [75–77], incorporation of water-repelling hydrophobic low-viscosity resin that does not form secondary hydrogen bonds is not effective in providing PFCs with adequate consistency [10]. On the other hand, accentuated consistency with high-viscosity FRCs above Lc allows incorporation of more hydrophobic low-viscosity resin with a reduction in leachable monomer to suggest that much better polymer systems can be designed for future dental filling materials [10]. Also, higher FRC packing forces squeeze monomer, resin and particulate away from the molding compound fiber network and cavity margins to seal the adhesive bond with the insoluble high-modulus quartz fibers, nanofibers and particulate [4,9,10]. Subsequent elevated concentrations of insoluble fibers that align parallel to the cavity walls and occlusal plane with particulate along the margins should then better provide an enduring seal as a thin adhesive bond moisture barrier [9,10,63,78].
Acknowledgments
Support in part from funding through the National Institutes of Health grant numbers T32DE07042 and T32DE014300. SEMs Dr. Vladimir M. Dusevich Director Electron Microscopy Laboratory, University of Missouri-Kansas City.
BIBLIOGRAPHY
- 1.Cox HL. The elasticity and strength of paper and other fibrous materials. British Journal of Applied Physics. 1952;3(3):72–79. [Google Scholar]
- 2.Kelly A, Tyson WR. Tensile properties of fibre-reinforced metals: copper/tungsten and copper/molybdenum. Journal of the Mechanics and Physics of Solids. 1965;13(6):329–350. [Google Scholar]
- 3.Chawla KK. Composite Materials. 2. New York: Springer; 1998. Micromechanics of composites, monotonic strength and fracture, and fatigue and creep; pp. 303–346.pp. 377–410. [Google Scholar]
- 4.Petersen RC. Discontinuous fiber-reinforced composites above critical length. Journal of Dental Research. 2005;84(4):365–370. doi: 10.1177/154405910508400414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, et al. Fiber length micromechanics for fiber-reinforced composites with a photocure vinyl ester resin. Polymer Composites. 2006;27(2):153–169. doi: 10.1002/pc.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC, et al. Micromechanics for fiber volume percent with a photocure vinyl ester composite. Polymer Composites. 2007;28(3):294–310. doi: 10.1002/pc.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC, et al. Fracture toughness micromechanics by energy methods with a photocure fiber-reinforced composites. Polymer Composites. 2007;28(3):311–324. doi: 10.1002/pc.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen RC. Accurate critical stress intensity factor Griffith crack theory measurements by numerical techniques. Society for Advanced Materials and Process Engineering, SAMPE 2013, Symposium; Long Beach, CA. 2013. pp. 737–752. [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen RC, Liu P-R. Mechanical properties comparing composite fiber length to amalgam. Journal of Composites. 2016:3823952. doi: 10.1155/2016/3823952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC. Advancing discontinuous fiber-reinforced composites above critical length for replacing current dental composites and amalgam. Journal of Nature and Science (JNSCI) 2017;3(2):e321. [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen RC. Micromechanics/Electron Interactions for Advanced Biomedical Research. Saarbrücken, Germany: LAP LAMBERT Academic Publishing Gmbh and Co. KG; 2011. Chapter 11 Wear of fiber-reinforced composite; pp. 163–167. [Google Scholar]
- 12.Petersen RC. Micromechanics/Electron Interactions for Advanced Biomedical Research. Saarbrücken, Germany: LAP LAMBERT Academic Publishing Gmbh and Co. KG; 2011. Chapter 23 Fiber-reinforced composite molding compound for dental fillings; pp. 395–415. [Google Scholar]
- 13.Collins CJ, et al. A clinical evaluation of posterior composite resin restorations: 8-year findings. Journal of Dentistry. 1998;26(4):311–317. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwenhuysen JP, et al. Long-term evaluation of extensive restorations in permanent teeth. Journal of Dentistry. 2003;31(6):395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 15.Levin L, et al. Cross-sectional radiographic survey of amalgam and resin-based composite posterior restorations. Quintessence International. 2007;38(6):511–514. [PubMed] [Google Scholar]
- 16.Soncini JA, et al. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth Findings from the New England Children’s Amalgam Trial. Journal of the American Dental Association. 2007;138(6):763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo M, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. Journal of the American Dental Association. 2007;138(6):775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 18.Antony K, et al. Longevity of dental amalgam in comparison to composite materials. GMS Health Technology Assessment. 2008 [PMC free article] [PubMed] [Google Scholar]
- 19.Kovarik RE. Restoration of posterior teeth in clinical practice: Evidence base for choosing amalgam versus composite. Dental Clinics of North America. 2009;53(1):71–76. doi: 10.1016/j.cden.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Rasines Alcaraz MG, et al. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth (Review) The Cochran Collaboration. The Cochrane Database of Systematic Reviews. 2014;3:CD005620. doi: 10.1002/14651858.CD005620.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Rasines Alcaraz MG, et al. Amalgam or composite fillings-which materials lasts longer? Evidence Based Dentistry (EBD) 2014;15(2):50–51. doi: 10.1038/sj.ebd.6401026. [DOI] [PubMed] [Google Scholar]
- 22.Council on Dental Materials, Instruments and Equipment. Choosing intracoronal restorative materials. Journal of the American Dental Association. 1994;125(1):102–103. [PubMed] [Google Scholar]
- 23.Council on Scientific Affairs. Statement on posterior resin based composites. Journal of the American Dental Association. 1998;129(11):1627–1628. [PubMed] [Google Scholar]
- 24.Council on Scientific Affairs. Direct and indirect restorative materials. Journal of the American Dental Association. 2003;134(4):463–472. doi: 10.14219/jada.archive.2003.0196. [DOI] [PubMed] [Google Scholar]
- 25.Söderholm KJM, Roberts MJ. Influence of water exposure on the tensile strength of composites. Journal of Dental Research. 1990;69(12):1812–1816. doi: 10.1177/00220345900690120501. [DOI] [PubMed] [Google Scholar]
- 26.Lenifelder KF, Suzuki S. In vitro wear device for determining posterior composite wear. Journal of the American Dental Association. 1999;130(9):1347–1353. doi: 10.14219/jada.archive.1999.0406. [DOI] [PubMed] [Google Scholar]
- 27.Bayne SC, et al. Protection hypothesis for composite wear. Dental Materials. 1992;8(5):305–309. doi: 10.1016/0109-5641(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 28.Liebenberg WH. Assuring restorative integrity in extensive posterior resin composite restorations. Quintessence International. 2000;31(3):153–164. [PubMed] [Google Scholar]
- 29.Drummond JL. Degradation, fatigue and failure of resin dental composite materials. Journal of Dental Research. 2008;87(8):710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roulet JF. The problems associated with substituting composite resins for amalgam: a status report on posterior composites. Journal of Dentistry. 1988;16(3):101–113. doi: 10.1016/0300-5712(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Leinfelder KF. Posterior composite resins: the materials and their clinical performance. Journal of the American Dental Association. 1995;126(5):663–677. doi: 10.14219/jada.archive.1995.0247. [DOI] [PubMed] [Google Scholar]
- 32.Schriever A, et al. Tooth-colored restorations of posterior teeth in German dental education. Clinical Oral Investigations. 1999;3(1):30–34. doi: 10.1007/s007840050075. [DOI] [PubMed] [Google Scholar]
- 33.Cobb DS, et al. The physical properties of packable and conventional posterior resin-based composites: a comparison. Journal of the American Dental Association. 2000;131(11):1610–1615. doi: 10.14219/jada.archive.2000.0091. [DOI] [PubMed] [Google Scholar]
- 34.Keogh TP, Bertolotti RL. Creating tight anatomically correct interproximal contacts. Dental Clinics of North America. 2001;45(1):83–102. [PubMed] [Google Scholar]
- 35.El-Badrawy WA, et al. Evaluation of proximal contacts of posterior composite restorations with 4 placement techniques. Journal of the Canadian Dental Association. 2003;69(3):162–167. [PubMed] [Google Scholar]
- 36.Loomans BAC, et al. A randomized clinical trial on proximal contacts of posterior composites. Journal of Dentistry. 2005;34(4):292–297. doi: 10.1016/j.jdent.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Jordon RE, Suzuki M. The ideal composite material. Journal of the Canadian Dental Association. 1992;58(6):484–487. [PubMed] [Google Scholar]
- 38.Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Critical Reviews in Oral Biology and Medicine. 2000;11(4):467–480. doi: 10.1177/10454411000110040501. [DOI] [PubMed] [Google Scholar]
- 39.Christiansen GJ. Overcoming challenges with resin in class II situations. Journal of the American Dental Association. 1997;128(11):1579–1580. doi: 10.14219/jada.archive.1997.0101. [DOI] [PubMed] [Google Scholar]
- 40.Council on Dental MaterialsInstruments and Equipment. The desirability of using radiopaque plastics in Dentistry: a status report. Journal of the American Dental Association. 1981;102(3):347–349. doi: 10.14219/jada.archive.1981.0058. [DOI] [PubMed] [Google Scholar]
- 41.Overton JD, Sullivan DJ. Early failure of class II resin composite versus class II amalgam restorations placed by dental students. Journal of Dental Education. 2012;76(3):338–340. [PubMed] [Google Scholar]
- 42.Martinim AP, et al. Influence of voids in the hybrid layer based on self-etching adhesive systems: a 3-D FE analysis. Journal of Applied Oral Science. 2009;17:19–26. doi: 10.1590/S1678-77572009000700005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purk JH, et al. Adhesive analysis of voids in class II composite resin restorations at the axial and gingival cavity walls restored under in vivo versus in vitro conditions. Dental Materials. 2007;23:871–877. doi: 10.1016/j.dental.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham J, et al. Clinical evaluation of three posterior composite and two amalgam restorative materials: 3-year results. British Dental Journal. 1990;169(10):319–323. doi: 10.1038/sj.bdj.4807369. [DOI] [PubMed] [Google Scholar]
- 45.Roulet JF, Noack MJ. Criteria for substituting amalgam with composite resins. International Dental Journal. 1991;41(4):195–205. [PubMed] [Google Scholar]
- 46.Wilkie R, et al. Class II glass ionomer cermet tunnel, resin sandwich and amalgam restorations over 2 years. American Journal of Dentistry. 1993;6(4):181–184. [PubMed] [Google Scholar]
- 47.Kaplowitz GJ. Achieving tight contacts in class II direct resin restorations. Journal of the American Dental Association. 1997;128(7):1012–1013. doi: 10.14219/jada.archive.1997.0308. [DOI] [PubMed] [Google Scholar]
- 48.Leinfelder KF. New developments inResin restorative systems. Journal of the American Dental Association. 1997;128(5):573–581. doi: 10.14219/jada.archive.1997.0256. [DOI] [PubMed] [Google Scholar]
- 49.Kraus S. Achieving optimal interproximal contacts in posterior direct composite restorations. Journal of the American Dental Association. 1998;129(10):1467. doi: 10.14219/jada.archive.1998.0052. [DOI] [PubMed] [Google Scholar]
- 50.Opdam NJM, et al. Five-year clinical performance of posterior resin composite restorations placed by dental students. Journal of Dentistry. 2004;32(5):379–383. doi: 10.1016/j.jdent.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Brackett MG, et al. Restoration of proximal contact in direct class II resin composites. Operative Dentistry. 2005;31(1):155–156. doi: 10.2341/04-198. [DOI] [PubMed] [Google Scholar]
- 52.Lee IB, et al. Rheological characterization of composites using a vertical oscillation rheometer. Dental Materials. 2007;23(4):425–432. doi: 10.1016/j.dental.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Kampouropoulos D, et al. The influence of matrix type on the proximal contact in class II resin composite restorations. Operative Dentistry. 2010;35(4):454–462. doi: 10.2341/09-272-L. [DOI] [PubMed] [Google Scholar]
- 54.Chuang S-F, et al. Morphological analysis of proximal contacts in class II direct restorations with 3D image reconstruction. Journal of Dentistry. 2011;39(6):448–456. doi: 10.1016/j.jdent.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Chhabra N, et al. Twist to matricing: restoration of adjacent proximal defects in a novel manner. International Journal of Applied and Basic Medical Research. 2016;6(1):71–72. doi: 10.4103/2229-516X.174022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gohil KS, et al. Proximal contacts in posterior teeth and factors influencing interproximal caries. The Journal of Prosthetic Dentistry. 1973;30(3):295–302. doi: 10.1016/0022-3913(73)90186-8. [DOI] [PubMed] [Google Scholar]
- 57.Dörfer CE, et al. Factors influencing proximal dental contact strengths. European Journal of Oral Sciences. 2000;108(5):368–377. doi: 10.1034/j.1600-0722.2000.108005368.x. [DOI] [PubMed] [Google Scholar]
- 58.Jernberg GR, et al. Relationship between proximal tooth open contacts and periodontal disease. Journal of Periodontology. 1983;54(9):529–533. doi: 10.1902/jop.1983.54.9.529. [DOI] [PubMed] [Google Scholar]
- 59.Hancock EB, et al. Influence of interdental contacts on periodontal status. Journal of Periodontology. 1980;51(8):445–449. doi: 10.1902/jop.1980.51.8.445. [DOI] [PubMed] [Google Scholar]
- 60.Svanberg M, et al. Mutans streptococci in plaque from margins of amalgam, composite and glass-ionomer restorations. Journal of Dental Research. 1990;69(3):861–864. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 61.Giltrow JP. Friction and wear of self-lubricating composite materials. Composites. 1973:55–64. [Google Scholar]
- 62.Friedrich K. Advances in Composite Technology. Amsterdam: Elsevier; 1993. Wear models for multiphase materials and synergistic effects in polymeric hybrid composites; pp. 209–269. [Google Scholar]
- 63.Peters ST. Handbook of Composites. Chapman and Hall; London: 1998. pp. 630–631.pp. 797–801.pp. 1007–1012. [Google Scholar]
- 64.Robinson P, et al. Failure Mechanisms in Polymer Matrix Composites. Oxford: WP Woodhead Publishing; 2012. pp. 395–401. [Google Scholar]
- 65.Anusavice KJ. Science of Dental Materials. 11. St. Louis: Saunders; 2003. Bonding; pp. 381–398. [Google Scholar]
- 66.Leinfelder KF, et al. An in vitro device for predicting clinical wear. Quintessence International. 1989;20(10):755–761. [PubMed] [Google Scholar]
- 67.Geurtsen W. Biocompatibility of resin-modified filling materials. Critical Reviews in Oral Biology and Medicine. 2000;11(3):333–355. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 68.Spahl W, et al. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. Journal of Dentistry. 1998;26(2):137–145. doi: 10.1016/s0300-5712(96)00086-3. [DOI] [PubMed] [Google Scholar]
- 69.Hansel C, et al. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. Journal of Dental Research. 1998;77(1):60–67. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 70.Edwards-Jones V. The benefits of silver in hygiene, personal care and healthcare. Letters in Applied Microbiology. 2009;49(2):147–152. doi: 10.1111/j.1472-765X.2009.02648.x. [DOI] [PubMed] [Google Scholar]
- 71.Lara HH, et al. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. Journal of Nanobiotechnology. 2011;9:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrêa JM, et al. Silver nanoparticles in dental biomaterials. International Journal of Biomaterials. 2015 doi: 10.1155/2015/485275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franci G, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20(5):8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudramurthy GR, et al. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules. 2016;21(7):E836. doi: 10.3390/molecules21070836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen RC. Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer Biomaterials in Materials Science and Engineering. International Journal of Computational Materials Science and Engineering. 2014;3(1):1450003. doi: 10.1142/S2047684114500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petersen RC. Triclosan computational conformational chemistry analysis for antimicrobial properties in polymers. Journal of Nature and Science. 2015;1(3):e54. [PMC free article] [PubMed] [Google Scholar]
- 77.Petersen RC. Triclosan antimicrobial polymers. AIMS Molecular Science. 2016;3(1):88–103. doi: 10.3934/molsci.2016.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sideridou I, et al. Water sorption characteristics of light-cured dental resins and composites based on BisEMA/PCDMA. Biomaterials. 2004;25(2):367–376. doi: 10.1016/s0142-9612(03)00529-5. [DOI] [PubMed] [Google Scholar]