Abstract
Background
Reducing tuberculosis (TB) deaths among children requires a better understanding of the gaps in the care cascade from TB diagnosis to treatment completion. We sought to assess the child TB care cascade in 32 rural communities in Uganda and Kenya using programmatic data.
Methods
This is a retrospective cohort study of 160,851 children (ages < 15 years) living in 12 rural communities in Kenya and 22 in Uganda. We reviewed national TB registries from health centers in and adjacent to the 32 communities, and we included all child TB cases recorded from January 1, 2013 to June 30, 2016. To calculate the first step of the child TB care cascade, the number of children with active TB, we divided the number of reported child TB diagnoses by the 2015 World Health Organization (WHO) child TB case detection ratio for Africa of 27%. The remaining components of the Child TB Care Cascade were ascertained directly from the TB registries and included: diagnosed with TB, started on TB treatment, and completed TB treatment.
Results
In two and a half years, a total of 42 TB cases were reported among children living in 32 rural communities in Uganda and Kenya. 40% of the children were co-infected with HIV. Using the WHO child TB case detection ratio, we calculated that 155 children in this cohort had TB during the study period. Of those 155 children, 42 were diagnosed and linked to TB care, 42 were started on treatment, and 31 completed treatment. Among the 42 children who started TB treatment, reasons for treatment non-completion were loss to follow up (7%), death (5%), and un-recorded reasons (5%). Overall, 20% (31/155) of children completed the child TB care cascade.
Conclusion
In 32 rural communities in Uganda and Kenya, we estimate that 80% of children with TB fell off the care cascade. Reducing morbidity and mortality from child TB requires strengthening of the child TB care cascade from diagnosis through treatment completion.
Keywords: Child tuberculosis, Tuberculosis care cascade, HIV, HIV-exposed uninfected children
1. Introduction
The World Health Organization (WHO) estimates that among children worldwide there were one million incident cases of tuberculosis (TB) and at least 210,000 TB deaths in 2015 [1]. Despite the high burden of TB in children, it is estimated that at least two thirds of cases in sub-Saharan Africa go un-diagnosed or un-reported [2]. Even when a child is diagnosed with TB, treatment outcomes are often poor [3]. To reduce child TB deaths it is essential to strengthen the links in child TB care from diagnosis through treatment completion.
The “Cascades of Care” framework is used widely in the HIV/AIDS field to assess the quality of HIV care throughout the care continuum [4]. This framework can be adapted for Child TB Care to describe the sequential steps from TB diagnosis through linkage to TB care, treatment initiation, and treatment completion. The care cascades model defines one common outcome that all patients should achieve; for the TB care cascade, that goal is the completion of TB treatment and achieve a cure. To date there are no published studies that assess the child TB care cascade in its entirety. The few studies that report on components in the Child TB Care Cascade in sub Saharan Africa suggest stark gaps in components of the cascade including failure to diagnose TB [5], [6], [7], [8], [9] or complete treatment, [8], [9], [10], [11] especially in children under the age of five [5], [8], [9], [12], [13]. Applying the Childhood TB Care Cascade framework to local data in the public domain can help identify where children “fall off” the cascade and measure the efficacy of interventions to increase the number of children who are cured of TB.
Our primary objective was to describe the Child TB Care Cascade in 32 rural communities in Uganda and Kenya from 2013 to 2016 using local programmatic data from Uganda and Kenya national TB registers. We also sought to identify subpopulations of children that may be under diagnosed (i.e. children under five and children with smear negative disease) by applying the metrics of child TB diagnosis described by Seddon et al. [14] These metrics can be applied to data from local health centers, and deviations from the expected proportions can provide a snapshot of under- or over-diagnosis at the primary health care center level.
2. Methods
2.1. Study design and participants
This is a retrospective observational cohort study of children ages 0–15 years of age who were enumerated in a census from 2012 to 2013 from 32 rural communities in Kenya and Uganda (12 communities in Nyaanza province, Kenya, 10 in Eastern Uganda, 10 in Western Uganda). We reviewed the national TB registers in each of the 32 communities to assess the number of reported TB cases, the TB case characteristics, and to estimate the TB care cascade. We included cases diagnosed between January 1, 2013 to June 30, 2016 to allow for full documentation of 6 months of TB treatment by the time of database closure on May 5, 2017.
2.2. Setting
This study is set in 32 rural communities in Uganda and Kenya that were participating in the SEARCH HIV “test and treat” cluster-randomized trial (NCT 01864503). The SEARCH trial design and community selection have been previously described [15].
In these 32 rural communities, TB is primarily evaluated, diagnosed, and treated in government-sponsored primary health centers. The Kenyan and Ugandan national TB programs generally rely on passive case finding. Patients present to primary health centers for evaluation and, if diagnosed with TB, are referred to the National TB Program where they are recorded in the TB treatment registry and offered treatment. Primary health centers have access to sputum microscopy on site. None of the primary health centers have access to mycobacterium tuberculosis (MTB) culture or onsite radiographic service. Isoniazid preventive therapy and active case finding are not widely implemented in the 32 communities. Children with HIV receive HIV care at government-sponsored primary health centers and receive TB screening at each clinical visit per national Ministry of Health protocols.
2.3. Variables and Definitions
2.3.1. Reported cases of child TB
We retrospectively reviewed national TB treatment registries from health centers within and adjacent to the 32 study communities. We defined a child TB case as a case if it was (a) documented in the national TB registry as new, relapsed, or lost to follow up; (b) recorded in the registry when the child was less than 15 years of age; and (c) matched to a child enumerated in the SEARCH baseline census. TB cases from the national TB registry were linked to SEARCH census by matching name, sex, date of birth, and residence. We excluded cases from children classified as “lost to follow up” and “relapsed” if they were already included as a new case within our study period. The same methods were used to define adult TB cases.
2.3.2. TB contact
We defined a child as having an adult TB contact if the child's household identification number from the SEARCH study matched the household identification number of an adult TB case from the national TB registry.
2.3.3. Child TB case characteristics
National TB treatment registries include date, name, residence, sex, type of tuberculosis (pulmonary or extrapulmonary), initial and follow up smear results, treatment start date, and final treatment outcome. We classified the site of TB disease as pulmonary if the sputum acid-fast bacilli (AFB) smear was positive, even if concomitant extra-pulmonary disease was present. Standard definitions of the Ugandan and Kenyan National TB Program (NTP) were used to define TB treatment outcomes: completed treatment, incomplete treatment- not taking anti-TB drugs for two months or more after starting treatment, transferred out. If a treatment outcome was not documented in the registry, then we classified the outcome as treatment not completed.
2.3.4. HIV status
HIV status, CD4 count, and HIV viral load were obtained during the ongoing community-wide SEARCH trial, which at baseline achieved testing coverage in 89% adults [15] and 81% children [16] via a hybrid mobile and community based HIV testing strategy. For this analysis, we used the CD4 and viral load collected during the SEARCH trial's baseline year in 2013–2014. We used the HIV status documented in the TB registry for two children, as they were both missing a baseline HIV test from the SEARCH trial. A child was defined as likely HIV-exposed uninfected (HEU) if the child was HIV negative and the child's mother had a positive HIV antibody test result during the baseline year of the SEARCH study, 2013–2014. As part of the SEARCH study, we were able to link all children under the age of 12 to the de-identified identification number of their birth mothers. This identifier was then linked to the mother's HIV status. A mother's HIV status was missing if the mother was deceased at the time of the study, the mother did not live within the child's household, or the mother declined HIV testing.
2.4. Assessing the child TB care cascade
The components of the Childhood TB Care cascade were adapted from the HIV care cascade [4], and the measures of the cascade included:
-
(1)
Children with active TB: the estimated number of children with active TB disease was defined as the number of child TB cases reported in the study population divided by the case detection rate (CDR) for Africa. We used the 2015 WHO child TB case detection ratio (CDR) for Africa of 27%, as the CDR for child TB has not yet been established for rural areas of East Africa. [17]. The 2015 WHO Global TB Report reports that in Africa there were an estimated 330,000 cases of child TB, 90,523 of which were reported (CDR = 27%). The global child TB CDR was 36%.
-
(2)
Diagnosed with TB and linked to care: the number of children diagnosed with TB (clinical or via smear) and who were registered in the national TB Registry. We do not have information on people who were diagnosed with TB, but who were lost to follow up prior to linking to TB care.
-
(3)
Started on TB treatment: the number of children who were diagnosed and linked to care that started TB treatment.
-
(4)
Completed TB treatment: number of children who were started on TB treatment and were recorded as completing TB therapy in the government TB registers. Children who died, defaulted, or transferred out were defined as not completing TB treatment. We did not have data on TB cure, so we used TB treatment completion as a proxy for TB cure.
We combined data from Kenya and Uganda case into one cascade as we did not detect statistically significant differences in the four components of the cascade when stratified by country.
2.5. Measures of under-diagnosis or over-diagnosis of childhood TB
To assess over or under-diagnosis of childhood TB in subpopulations of children we used the following indicators described in Seddon et al. [14] and assumptions about child TB diagnosis: (1) expected proportion of overall burden found in children: 15–20% [18] (2) expected proportion of child TB cases that are smear positive: 10% (3) expected proportion of child TB cases under the age of 5: 50% [18] (4) expected proportion of cases of extra-pulmonary TB: 25% (<5 years) and 15% (<15 years). These metrics can be applied to data from local health centers and deviations from the expected proportions can provide a snapshot of under- or over-diagnosis at the primary health care center level.
2.6. Statistical analysis
Data was analyzed with STATA 12. We used the chi-squared and Fisher exact test, where appropriate, to compare proportions. Confidence intervals for point estimates were calculated using the Fisher exact method.
3. Ethics
The Makerere University School of Medicine Research and Ethics Committee (Uganda), the Ugandan National Council on Science and Technology (Uganda), the Kenya Medical Research Institute Ethical Review Committee (Kenya), and the University of California San Francisco Committee on Human Research (USA) approved the consent procedures and the SEARCH trial.
4. Results
160,851 children ages 0–15 years of age were enumerated in a census from 2012 to 2013; 103,409 children from the 22 communities in Uganda and 57,422 children from the 12 communities in Kenya. From January 1, 2013 to June 30, 2016, a total of 42 child TB cases (27 cases in Kenya and 15 in Uganda) were reported among the children enumerated in the census from the 32 communities.
4.1. Demographics of children diagnosed with TB
Demographic characteristics of the children in Kenya and Uganda diagnosed with TB are described in Table 1. Among the children diagnosed active TB, 40% (17/42) were between the ages of 10 to 14 years, 19% (7/42) lived with an adult who was diagnosed with TB from January 2013 to June 2016, and 40% (17/42) of the children were co-infected with HIV. Among the 8 children with a viral load measurement, 3 (38%) had a suppressed viral load (viral load ≤ 500 copies/ml) prior to being diagnosed with TB. Among the 37 children ≤ 12 years old, 57% (21/37) lived in a household with at least one HIV-infected adult, 46% (17/37) had a mother infected with HIV, and 23% (6/37) met our definition of a child who was likely HIV-exposed, but uninfected (HIV-infected mother, HIV-uninfected child).
Table 1.
Demographics of Children Diagnosed with Tuberculosis (TB) in 32 rural communities in Uganda (N = 15) and Kenya (N = 27), January 1, 2013 to June 30, 2016.
| n/N (%) | |
|---|---|
| Gender | |
| Female | 27/42 (64%) |
| Age (years) | |
| 0–4 | 13/42 (31%) |
| 5–9 | 12/42 (29%) |
| 10–14 | 17/42 (40%) |
| Child living in a household with adult diagnosed with TBa | 7/42 (19%) |
| Child's HIV status | |
| HIV positive | 17/42 (40%) |
| HIV negative | 25/42 (60%) |
| Mother's HIV status | |
| HIV-infected | 17/37 (46%) |
| HIV-uninfected | 13/37 (35%) |
| HIV status unknown | 7/37 (19%) |
| HIV-exposed uninfected child | |
| (HIV-infected mother, HIV-uninfected child) | 6/37 (23%) |
| Child lives in household with HIV-infected adult | 21/37 (57%) |
| Clinical characteristics of HIV-infected childrenb | |
| On antiretroviral therapy prior to TB diagnosis | 9/12 (75%) |
| On antiretroviral therapy before or after TB diagnosis | 10/12 (83%) |
| HIV viral load < 500 copies/ml at baseline | 3/8 (38%) |
| CD4+ count < 200 cells/ul | 2/14 (14%) |
| Householdcharacteristics | |
| Median (IQR) number of household members | 6 (5–9) |
| Wealth Index (tertiles) | |
| 1 (lowest) | 6/37 (16%) |
| 2 | 12/37 (49%) |
| 3 (highest) | 19/37 (51%) |
A child was defined as living with an adult diagnosed with TB if an adult in their household was diagnosed with TB and entered in the registrar from January 1, 2013 through June 30, 2016.
Excludes children missing data on antiretroviral therapy start and viral load.
4.2. Child TB case characteristics
TB case characteristics and treatment completion are described in Table 2. Among children diagnosed with TB, 52% (22/42) of children had pulmonary TB and 48% (20/42) had extra-pulmonary TB. Children under 5 made up 31% of the total child TB cases. Treatment outcomes were as follows: 74% (31/42) of children completed TB treatment, 7% (3/42) were documented to have not completed treatment, 5% (2/42) died, 10% (4/42) were documented to have transferred out, and 5% (2/42) did not have a documented treatment outcome. The 2 children who died were both HIV-infected.
Table 2.
Case characteristics of children diagnosed with tuberculosis (TB) in 32 rural communities in Kenya and Uganda (N = 42), January 1, 2013–June 30, 2016.
| Case type | |
| New | 37/42 (88%) |
| Relapse | 4/32 (10%) |
| Default | 1/42 (2%) |
| TB type | |
| Pulmonary-smear positive | 8/42 (19%) |
| Pulmonary-smear negative | 10/42 (24%) |
| Pulmonary-smear not sent | 4/42 (10%) |
| Extra-pulmonary | 20/42 (48%) |
| TB type in children, age < 5year | |
| Pulmonary -smear positive | 0% |
| Pulmonary -smear negative | 3/13 (23%) |
| Pulmonary- smear not sent | 2/13 (15%) |
| Extra-pulmonary | 8/13 (62%) |
| 2 month follow up smear completed (of those with positive pre -treatment smear) | 6/8 (75%) |
| Recordedtreatmentoutcomes | |
| Completed Treatment | 31/42 (74%) |
| Did not complete treatment | 3/42 (7%) |
| Died | 2/42 (5%) |
| Transferred out | 4/42 (10%) |
| Outcome unknown | 2/42 (5%) |
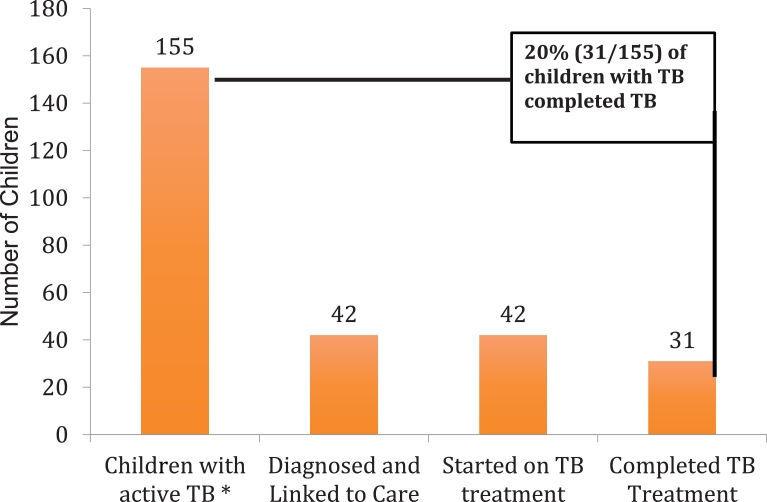
4.3. The childhood TB care cascade in 32 communities in Uganda and Kenya
The childhood TB care cascade for our study communities in Kenya and Uganda is presented in the Fig. 1. Using the WHO case detection rate for child TB of 27%, [17] we calculated that there were 155 children with TB disease in the 32 communities in Uganda and Kenya from January 1, 2013- June 30, 2016. This number was obtained by dividing the total number of reported child TB cases by the WHO child TB case detection ratio (42/0.27). Of the 155 children calculated to have TB, 42 children were diagnosed and linked to care, and 42 were started treatment. Of the 42 children started on treatment, 31 (70%) were documented to have completed treatment. Thus, 20% (31/155) of children with TB are estimated to have completed the childhood TB care cascade.
Fig. 1.
The Childhood TB Care Cascade for 32 rural communities in Kenya and Uganda.
*The number of children with TB disease was calculated by dividing the number of recorded child TB cases (42) by the WHO case detection rate for Africa of 0.27 [17]. The number of children diagnosed and linked to care, started on TB treatment, and completed TB treatment was extracted directly from the National TB Registries from the 32 communities in Kenya and Uganda.
4.4. Metrics of over and under diagnosis of child TB
The metrics of over- and under-diagnosis of childhood TB in the 32 communities in Kenya and Uganda are presented in Table 3. Childhood TB accounted for 7% (95% CI: 5%−10%) of the TB burden in our 32 study communities: 5% (95% CI: 3%−8%) and 9% (95% CI: 6%−13%) in Uganda and Kenya, respectively. In both Kenya and Uganda Indicator 1, the measured proportion of overall TB burden found in children, is lower in both than the expected value of 15–20% [18] Indicator 2, the measured proportion of smear positive cases (45% in Uganda and 27% in Kenya), also exceeds the expected number of 10%. This suggests clinicians may rely too heavily on smear microscopy and not enough on clinical algorithms to make childhood TB diagnoses. In Kenya and Uganda, indicators 2 and 4, the measured proportion of pediatric smear positive TB and extra pulmonary diseases, exceed expected values, which suggests under-diagnosis of smear negative disease and pulmonary TB cases are missed.
Table 3.
Programmatic indicators of how well childhood TB is diagnosed in 32 communities in rural Uganda and Kenya, indicators adapted from Seddon et al. [14].
| Measured |
Expected | |||||
|---|---|---|---|---|---|---|
| Uganda |
Kenya |
Approximate expected valuea | Interpretation of the Comparison of Reported Value to Expected Value | |||
| Indicator | n/N | % (95% CI) | n/N | % (95% CI) | % | |
| 1. Proportion of overall burden found in children | 15/292 | 5% | 27/297 | 9% | 15–20%b | Measured values under expected value. Suggests under diagnosis of childhood TB. |
| (3%−8%) | (6%−13%) | |||||
| 2. Proportion of pediatric pulmonary TB cases that are smear positive | 5/11 | 45% | 3/11 | 27% | 10% | Measured values over expected value. Suggests not enough children treated for TB on clinical grounds in Kenya |
| (17%−78%) | (6%−61%) | |||||
| 3. Proportion of pediatric cases aged < 5 years | 4/15 | 27% | 9/27 | 33% | 50% | Measured value under expected value. Suggests possible under diagnosis of childhood TB among young children |
| (8%−55%) | (17%−54%) | |||||
| 4.Proportion of pediatric cases that are EPTB | 4/15 | 27% | 16/27 | 59% | 10% in children < 15 years, 25% in children < 5 years | Measured value over expected value. Suggests possibility of missed diagnoses of pulmonary TB and over diagnosis of extra-pulmonary TB |
| (8%−55%) | (39%−78%) | |||||
5. Discussion
In this study of 32 rural communities in Kenya and Uganda, we used programmatic data to assess the child TB care cascade. We found that a fifth of children completed the TB care cascade. Our analysis of the Seddon et al. [14] metrics of child TB diagnosis suggest that failure to diagnose TB is a critical gap in the cascade. Even if children are diagnosed, the probability that they complete treatment is suboptimal. Only 70% of children who were diagnosed and linked to TB treatment completed treatment, which falls short of the WHO's End TB goal of 90%. [19]. TB is a curable disease and these data highlight the need for ongoing interventions to ensure that children do not fall off the TB care cascade.
A critical failure in the child TB care cascade is the apparent under-diagnoses of TB. In East Africa children are expected to comprise 15–20% of the total TB burden; [14], [18] however, we found that children made up only 5% and 9% of the total burden Uganda and Kenya respectively. Other studies from community settings in sub-Saharan Africa have also found a lower than expected proportion of TB cases in children ranging from 6.3% in Nigeria [9], 7.5% in urban Uganda, [11] and 13% in Southern Ethiopia [5]. The gap is particularly stark among children under five. It is estimated that nearly 50% of child TB cases should be among children under the age of five, as children under the age of five have the highest likelihood of progressing to active TB once exposed [14], [20]. However, as in other studies in East Africa, [5], [7] we found that the number of child TB cases among children under the age of five (27% in Uganda and 33% in Kenya) fell short of the expected number of 50%. Screening children who are in contact with TB is one way to improve child TB case detection, [21] though this is rarely implemented.
Improving a front-line clinician's ability to make TB diagnoses, including clinical diagnoses, could help improve the child TB diagnosis gap. In this study, over a quarter of pulmonary TB cases were smear positive (45% in Uganda and 27% in Kenya), which is higher than the expected value of 10%, [14], [22], [23] and suggests that not enough cases are diagnosed clinically and an overreliance on smear to make a diagnosis. Our proportion of smear positive pulmonary disease is higher than in a study from urban clinics in Uganda, where 15% of TB cases were smear positive, [11] and a national study in Zambia where 6% of child TB cases were smear positive [6]. Scaling up training on the use of algorithmic diagnostic aids for Child TB, such as the Union's Pediatric TB Desk [24] could improve knowledge gaps in child TB diagnosis. Performance feedback on quality indicators has improved the quality of adult TB care in rural health centers in Uganda [25] may also be helpful for pediatric populations.
A central goal of the WHO End TB strategy is for 90% of children to complete TB treatment [19]. In this study, 69% of children were documented to have completed TB treatment. Data on child TB treatment completion in rural areas East Africa is sparse, making comparisons challenging. However, our treatment completion rate is similar to a study in Nyaanza province [13] that reported that 74% of children with TB completed treatment, and a study from a rural district in Ethiopia that reported that 82% of children completed TB treatment [10]. TB is a curable disease and we should aim for 100% treatment completion. Implementation of strategies for child friendly formulations and innovative community and family-based care delivery models might improve treatment completion rates.
We found a high burden of active TB among HIV-infected children. This may be because HIV-infected children are more likely to be screened for TB and because HIV-infected children are more vulnerable to developing active disease once exposed to TB. ART decreases the incidence of TB among HIV-infected children; [26], [27] however, among the children with a recorded viral load from 2013 to 2014, 55% (5/9) had a detectable viral load. Preventing TB in this vulnerable group of children will depend on improvements in ART adherence and implementation of isoniazid preventive therapy.
HIV-exposed uninfected (HEU) children, a population growing at a rate of 1 million annually, [28] may also be at high risk for TB disease. This growing group of children have an increased risk of morbidity and mortality, [29] and HIV-exposure has been associated with an increased risk of latent TB infection in children under the age of 5 years [30]. In this study, among children whose maternal HIV status was known, we found that a fifth of the children with TB were likely HIV-exposed uninfected children. Our data highlight the need to further quantify the risk of TB infection among HEU children, a vulnerable group of children who may need intensified TB screening and prevention efforts.
We also found that regardless of HIV status, the majority of children with TB were connected to the HIV care system. Over half of children lived with an HIV-infected adult and 45% had an HIV-infected mother. Leveraging HIV-care delivery systems to improve TB diagnosis and TB prevention among child TB contacts may be one way to strengthen the child TB care cascade.
This study has important strengths and limitations. Our calculation of the total number of child TB cases likely underestimates the true number of child TB cases, as the WHO CDR for the African region is likely higher than that in rural areas of East Africa where access to TB diagnostics is limited. However, this is one of the first studies to adapt the “care cascades” framework to child TB and provides an example of how data in the public domain can be easily adapted to identify gaps in a child TB care cascade. There are also limitations to applying the Seddon et al. metrics of child TB diagnosis to our study. Firstly, the expected values for metrics are not specific to our study population, as these data are not available for rural areas of East Africa. Secondly, the number of TB cases are small, which limits our power to detect differences between expected and measured values. However, these metrics are intended to be provide a general snapshot of over and under diagnosis and we have shown that they can be easily applied to local data to identify potential areas for improvement.
In summary, to reveal local practice gaps in child TB care we applied the ‘cascades of care’ framework and metrics of child TB diagnosis to programmatic data from 32 rural communities in Kenya and Uganda. Less than a fifth of children made it through the child TB care cascade and the biggest gap in the cascade was child TB diagnosis. Our analysis of the metrics of child TB diagnosis suggests that strengthening the capacity of front-line clinicians to make clinical child TB diagnoses, especially in children under that age of five, is one way to increase child TB case detection in rural communities in East Africa. There is also room for improvement in child TB treatment outcomes. Strategies to improve diagnosis and child TB care delivery in rural areas of East Africa are urgently needed to ensure that all children make it through the TB care Cascade.
Conflicts of interest
DVH has received non-financial support (donation of the drug Truvada [emtricitabine-tenofovir]) for the SEARCH study from Gilead Sciences. The other authors do not have conflicts of interests to declare.
Acknowledgments
Acknowledgments
The authors would like to thank the Ministries of Health of Uganda and Kenya, our research team, collaborators and advisory boards, and all of the communities and all the individuals who generously participated in the SEARCH study. We also would like to thank Ronald Kakuru, Paul Malagala, and Samuel Ngome for their contributions to data collection.
Funding
Research reported in this manuscript was supported by the Division of AIDS, National Institute of Allergy and Infectious Diseases of the National Institutes of Health award numbers 5K23AI118592, U01AI099959 and in part by the President's Emergency Plan for AIDS Relief, the Bill and Melinda Gates Foundation, and Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, PEPFAR, or Gilead.
Footnotes
Conference presentation: These data were presented in part at the AIDS 2016 Conference, Durban, South Africa, Abstract WPEB401.
References
- 1.World Health Organization . WHO; 2016. Global tuberculosis report.http://www.who.int/tb/publications/global_report/en/ Accessed November 14, 2016. [Google Scholar]
- 2.Jenkins H.E. Global burden of childhood tuberculosis. Pneumonia Nathan Qld. 2016;8 doi: 10.1186/s41479-016-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins H.E., Yuen C.M., Rodriguez C.A. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(3):285–295. doi: 10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner E.M., McLees M.P., Steiner J.F., Del Rio C., Burman W.J. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dangisso M.H., Datiko D.G., Lindtjørn B. Low case notification rates of childhood tuberculosis in southern Ethiopia. BMC Pediatr. 2015;15:142. doi: 10.1186/s12887-015-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapata N., Chanda-Kapata P., O'Grady J. Trends in childhood tuberculosis in Zambia: a situation analysis. J Trop Pediatr. 2013;59(2):134–139. doi: 10.1093/tropej/fms065. [DOI] [PubMed] [Google Scholar]
- 7.Marquez C., Davis J.L., Katamba A. Assessing the quality of tuberculosis evaluation for children with prolonged cough presenting to routine community health care settings in rural Uganda. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries A.D., Hargreaves N.J., Graham S.M. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6(5):424–431. [PubMed] [Google Scholar]
- 9.Adejumo O.A., Daniel O.J., Adebayo B.I. Treatment outcomes of childhood TB in Lagos, Nigeria. J Trop Pediatr. December 2015 doi: 10.1093/tropej/fmv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dangisso M.H., Datiko D.G., Lindtjørn B. Trends of tuberculosis case notification and treatment outcomes in the Sidama Zone, southern Ethiopia: ten-year retrospective trend analysis in urban-rural settings. PloS One. 2014;9(12) doi: 10.1371/journal.pone.0114225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wobudeya E., Lukoye D., Lubega I.R., Mugabe F., Sekadde M., Musoke P. Epidemiology of tuberculosis in children in Kampala district, Uganda, 2009-2010; a retrospective cross-sectional study. BMC Public Health. 2015;15(1):967. doi: 10.1186/s12889-015-2312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hailu D., Abegaz W.E., Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr. 2014;14:61. doi: 10.1186/1471-2431-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavanaugh J., Genga K., Marigu I., Laserson K., Ackers M., Cain K. Tuberculosis among children in Kenya: epidemiology and impact of HIV in two provinces. J Trop Pediatr. 2012;58(4):292–296. doi: 10.1093/tropej/fmr098. [DOI] [PubMed] [Google Scholar]
- 14.Seddon J.A., Jenkins H.E., Liu L. Counting children with tuberculosis: why numbers matter. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2015;1(12 (19 suppl.)):9–16. doi: 10.5588/ijtld.15.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamie G., Clark T.D., Kabami J. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3(3):e111–e119. doi: 10.1016/S2352-3018(15)00251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzer L.B., Sang N., Plenty A., et al. Baseline population HIV Cascade and 2-yr outcome of HIV+ children in the SEARCH trial. Conference on Retroviruses and Opportunistic Infections, Seattle, February 2017, Abstract #832.
- 17.WHO . 2015. Global Tuberculosis Report. [Google Scholar]
- 18.Dodd P.J., Gardiner E., Coghlan R., Seddon J.A. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2(8):e453–e459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 19.WHO. WHO | End TB Strategy. http://www.who.int/tb/post2015_strategy/en/. Accessed November 14, 2016.
- 20.Marais B.J., Gie R.P., Schaaf H.S., Hesseling A.C., Enarson D.A., Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2006;10(7):732–738. [PubMed] [Google Scholar]
- 21.Jaganath D., Zalwango S., Okware B. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57(12):1685–1692. doi: 10.1093/cid/cit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marais B.J., Gie R.P., Schaaf H.S. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 23.Marais B.J., Graham S.M., Maeurer M., Zumla A. Progress and challenges in childhood tuberculosis. Lancet Infect Dis. 2013;13(4):287–289. doi: 10.1016/S1473-3099(13)70031-8. [DOI] [PubMed] [Google Scholar]
- 24.Graham S. third ed. International Union Against Tuberuclosis and Lung Diseases; Paris, France: 2016. The union's deskguide for diagnosis and management of TB in children.http://www.theunion.org/what-we-do/publications/english/2016_Desk-guide_Africa_Web.pdf?utm_source=The+Union+E-newsletter&utm_campaign=43e9b2d8bb-E_news_September_20169_29_2016&utm_medium=email&utm_term=0_3de3c63f53-43e9b2d8bb-246912857 [Google Scholar]
- 25.Davis J., Katamba A., Vasquez J. Evaluating tuberculosis case detection via real-time monitoring of tuberculosis diagnostic services. Am J Respir Crit Care Med. 2011;184(3):362–367. doi: 10.1164/rccm.201012-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braitstein P., Nyandiko W., Vreeman R. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28(7):626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 27.Abuogi L.L., Mwachari C., Leslie H.H. Impact of expanded antiretroviral use on incidence and prevalence of tuberculosis in children with HIV in Kenya. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2013;17(10):1291–1297. doi: 10.5588/ijtld.12.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powis K.M., Slogrove A.L., Mofenson L. Protecting the health of our AIDS-free generation: beyond prevention of mother-to-child HIV transmission. AIDS Lond Engl. 2017;31(2):315–316. doi: 10.1097/QAD.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arikawa S., Rollins N., Newell M.-L., Becquet R. Mortality risk and associated factors in HIV-exposed, uninfected children. Trop Med Int Health TM IH. 2016;21(6):720–734. doi: 10.1111/tmi.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquez C., Chamie G., Achan J. Tuberculosis Infection in Early Childhood and the Association with HIV-exposure in HIV-uninfected Children in Rural Uganda. Pediatr Infect Dis J. 2016;35(5):524–529. doi: 10.1097/INF.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]