Abstract
Background and Objectives:
In addition to general advantages of laparoscopic over open surgery, such as better cosmesis and faster recovery, laparoscopic liver surgery offers specific advantages. Improved liver function and potentially earlier postoperative oncologic treatment are suggested by the literature as benefits of laparoscopic over open liver surgery. The purpose of this analysis was to analyze the outcomes of laparoscopic liver surgery in our department.
Methods:
All laparoscopic liver resections (LLRs) performed from January 2011 through July 2016 were identified from the institutional database and matched 1:2 to open liver resections (OLRs). Data were analyzed regarding perioperative outcome, and significance was set at P < .05.
Results:
Of 1525 liver resections, 120 patients were included in this analysis. Forty resections were performed laparoscopically. Patients in the LLR group more often had benign tumors. No patient died after LLR, but 2 required conversion to open surgery (5%) because of bleeding. Blood loss (200 vs 500 mL, P < .001) was less and hospital stay (6 vs. 7 days, P = .001) shorter after LLR. Iwate score, operating time, and the size of the resection margins did not differ between the groups. Iwate score correlated with operative time (P = .027).
Conclusions:
Laparoscopic liver surgery was safe, and several advantages over open surgery were confirmed in our series.
Keywords: Laparoscopic liver surgery, Match-pair analysis, Iwate score
INTRODUCTION
With the evolution of minimally invasive technologies, laparoscopic surgery has become the standard technique for many surgical procedures. It generally has the benefit of better cosmesis, and faster postoperative recovery, including less pain, earlier bowel movements, and many more advantages.1,2 Despite higher direct cost, laparoscopic surgery has become the standard procedure for colon and rectal cancer surgery in many centers.
Liver surgery is the standard of care for primary and many secondary liver tumors. It has specific aspects, for which laparoscopic liver surgery may have benefits in addition to the general advantages of laparoscopic surgery: Benign liver tumors such as adenoma (or focal nodular hyperplasia) are often diagnosed in young patients with high cosmetic needs. Surgery for hepatocellular carcinoma (HCC), which predominantly arises from cirrhotic liver, carries an increased risk of liver failure after liver resection. General anesthesia as well as the extent of surgery may worsen liver function in these patients, and minimally invasive surgery appears to be less harmful for liver function.3 Furthermore, surgery for metastases from colorectal (CRC) or other cancers is often embedded in multimodal treatment concepts, for which prolonged postoperative recovery is prohibitive. A recent analysis showed a shorter time to chemotherapy after laparoscopic liver surgery, in addition to a shorter hospital stay and a lower complication rate in 66 patients with colorectal liver metastases compared to 66 open resections.4 Moreover, laparoscopic surgery has become the standard for CRC in many centers, and additional laparoscopic liver resections (LLRs) appear attractive for both synchronous and metachronous liver metastases.
Because minimally invasive surgery theoretically serves these needs, many centers for liver surgery have been using this technique increasingly during recent years.5 The available literature suggests a shorter hospital stay, less blood loss, and fewer cirrhotic decompensations.3 Furthermore, a recent paper demonstrated a lower number of circulating tumor cells in the peripheral blood after laparoscopic compared to open surgery for hepatocellular carcinoma.6
All procedures performed in the following study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
MATERIALS AND METHODS
All liver resections performed at the Department for General, Visceral and Transplantation Surgery at the University Hospital of Mainz from January 2011 through July 2016 were identified from the prospective department database. Baseline information, intraoperative course, and postoperative outcome were analyzed. Intraoperative blood loss and application of blood products were identified from the anesthesiology records. The duration of surgery was defined from skin incision until closure. Surgical complications were scored according to the Clavien-Dindo classification.7 Information about completeness of resection (R0/R1), as well as the tumor type, was taken from the final histological reports.
Liver Resection Technique
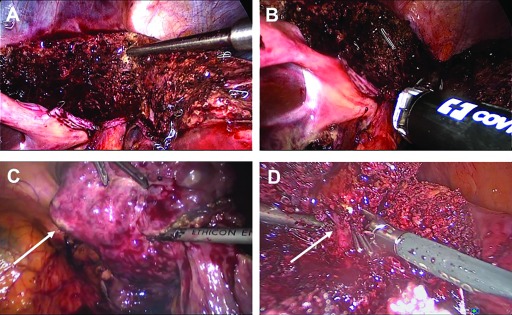
LLRs were performed either in the French position for tumors in segments 2 through 6 or in the left lateral position for tumors in the segments 6 and 7. A 10-mm camera (30°) was used in all cases, and 2 additional 10-mm trocars were used by the first surgeon. One to 2 more 5-mm trocars were placed if necessary for liver retraction or preparation of the Pringle maneuver. The resection margins and intrahepatic anatomy were confirmed by laparoscopic ultrasound. The parenchymal dissection was initially performed with an ultrasonic dissector (Ultracision; Ethicon, Norderstedt, Germany). With growing experience, the laparoscopic Cavitron ultrasonic surgical aspirator (CUSA; Söring, Quickborn, Germany) was used in addition for dissection, to minimize blood loss. Major vascular structures, such as the hilar plate or hepatic veins were closed with endo-GIA staplers (Figure 1). The fascia at all trocar sites ≥10 mm was closed with Vicryl (0) interrupted sutures.
Figure 1.
Intraoperative photographs obtained during laparoscopic liver surgery. Parenchymal transection along the falciform ligament with the CUSA device for a left lateral sectorectomy (A). The hilar plate is dissected by endo-GIA staplers (B). Resection of segment 6 in a cirrhotic patient with HCC (arrow, C). Preparation of a major venous branch (arrow) during resection of a liver metastasis in segment 5/8 (D).
For open surgery, the extent of resection was always confirmed by palpation and intraoperative ultrasound. Parenchymal dissection was performed with Kelly clamps or by scratching the parenchyma with the scissor tip, whereby small vessels were sealed with bipolar forceps and larger ones with clips and sutures. Hilar structures and hepatic veins were resected over vascular clamps, and the respective stump closed with Prolene 4-5/0 running sutures. The abdominal wall was closed by suturing the fascia with Vicryl 2 and polydioxanone (PDS II; Ethicon) loops.
Patient Matching
All LLRs were matched 1:2 to open liver resections (OLRs) of the same period, according to patient age, sex, tumor type, tumor size, and localization, American Society of Anesthesiologists (ASA) class, body mass index (BMI), extent of liver resection, and the presence of liver cirrhosis.
Statistical Analysis
Parameters with expected normal distribution are presented with mean values and confidence intervals and others with median and ranges. Categorical data were compared by the χ2 test and continuous data with Student's t test for parametric analyses and Mann-Whitney U-test for nonparametric analyses. Statistical significance was set at P < 0.05.
RESULTS
From January 2011 through July 2016, 1525 liver resections were performed in our department. Of those, 40 operations (2.6%) were laparoscopic, of which 37 (93%) were performed by a single surgeon (SH). Eighty OLRs served as controls in the matched-pair analysis. The patient cohorts did not differ regarding BMI, presence of liver cirrhosis, type and extent of surgery, or median tumor size (Table 1). In the beginning, most laparoscopic resections were performed for benign tumors, and over the time, the proportion of malignant tumors increased (Figure 2). Consequently, patients in the LLR group were younger in the beginning, but later, age did not differ (data not shown). Currently, tumor biology (benign or malignant) of the tumor does not affect patient selection for laparoscopic surgery.
Table 1.
Patient Characteristics
| All liver Resections | Laparoscopic Resection | Open Resection | P* | |
|---|---|---|---|---|
| Number | 1525** | 40 | 80 | |
| Age, years (median, range) | 63 (16–93) | 60 (20–83) | 62,5 (24–82) | 0.08 |
| Sex, female/male, n | 656/829 | 19/21 | 29/51 | 0.24 |
| BMI, kg/m2, mean (SD) | 26.5 (4.9) | 28.3 (5.8) | 27.5 (5.6) | 0.62 |
| ASA score, n (%) | 0.05 | |||
| I | 22 (1.4) | 3 (7.5) | 0 | |
| II | 741 (48.6) | 23 (57.5) | 41 (51.3) | |
| III | 701 (46) | 14 (35) | 38 (47.5) | |
| IV | 21 (1.4) | 0 | 1 (1.3) | |
| Liver cirrhosis, n (%) | 147 (9.6) | 8 (20) | 16 (20) | 1.0 |
| Tumor size, cm (median, range) | 4.4 (0.5–10.5) | 4.35 (0.5–13) | 0.64 | |
| Biology (benign/malignant) | 14/26 | 7/73 | 0.001 | |
| Tumor type, n | 0.007 | |||
| Adenoma | 18 | 6 | 2 | |
| FNH | 33 | 8 | 4 | |
| HCC | 232 | 11 | 22 | |
| CCC | 179 | — | 6 | |
| CRC mets | 558 | 11 | 32 | |
| Endocrine mets | 33 | 4 | 13 | |
| Non-CRC/nonendocrine mets | 121 | |||
| Klatskin tumor | 107 | — | — | |
| Gallbladder cancer | 34 | — | — | |
| Others | 170 | — | — | |
| Type of resection, n (%) | 0.99 | |||
| Left lateral sectionectomy | 131 (8.8) | 16 (40%) | 32 (40%) | |
| Segmentectomy | 438 (29.5%) | 23 (57.5%) | 46 (57.5%) | |
| Atypical resection | 392 (26.4%) | 1 (2.5%) | 2 (2.5%) | |
| Hemihepatectomy (extended) | 524 (35.3%) | |||
| Iwate score, median (range) | — | 5 (4–9) | 5 (4–8) | 0.76 |
Laparoscopic versus open resection;
1485 fully evaluable. CCC, cholangiocellular carcinoma; mets, metastases.
Figure 2.
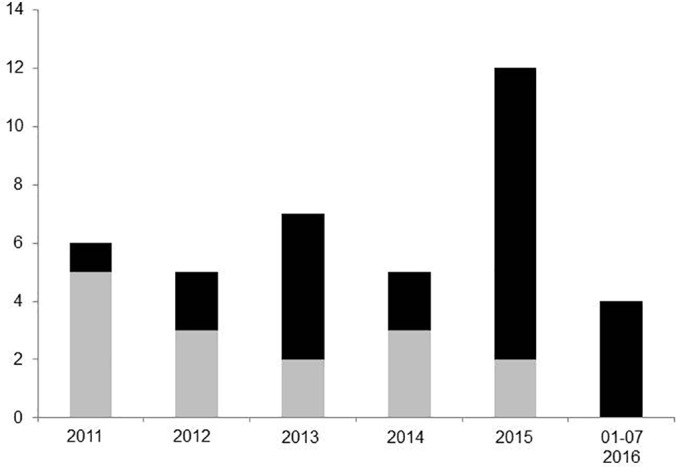
Changes in surgical indications over time. Laparoscopic liver resections for malignant tumors (e.g., HCC and CRC) are increasingly used (black bar: malignant tumor, gray bar: benign tumor).
Of the entire cohort of liver resections (1525), 40 patients (2.6%) had a liver resection for focal nodular hyperplasia (FNH) and 28 (1.8%) for adenoma. Of these 68 patients, 32 are included in this analysis. Twelve patients were symptomatic, 20 underwent surgery for an unclear diagnosis; in 2 of those malignancy was suspected.
Two laparoscopic resections required conversion to open surgery because of bleeding from the left hepatic vein (5%) in patients with cirrhosis. The results of these patients are included in the laparoscopic group as an intent-to-treat analysis (Table 1).
Complexity of Liver Resection
To confirm comparability of laparoscopic and OLRs, the Iwate score was applied to all cases8: the localization and size of the tumor, the proximity to major blood vessels, the extent of surgery, the underlying liver function (Child score) and the use of a pure laparoscopic or hand-assisted surgery is assigned individual scores. The sum of these scores depicts an objective measure of the complexity of the procedure and defines the difficulty of surgery (score 0–3, low; 4–6, intermediate; 7–9, advanced; and 10–12, expert).
According to the Iwate score, laparoscopic and open resections were well matched with a median score of 5 each (P = .76). Only a very few cases were of a low-difficulty level, and some were classified as advanced (Figure 3). These cases were scored with 7 points because of tumor size (4–4.6 cm) and localization in segments 4b, 5, and 7. Two lesions with a maximum diameter of 3.5 cm (segment 4a) and 4.5 cm (segment 7) were scored with 8 and 9 points, respectively.
Figure 3.
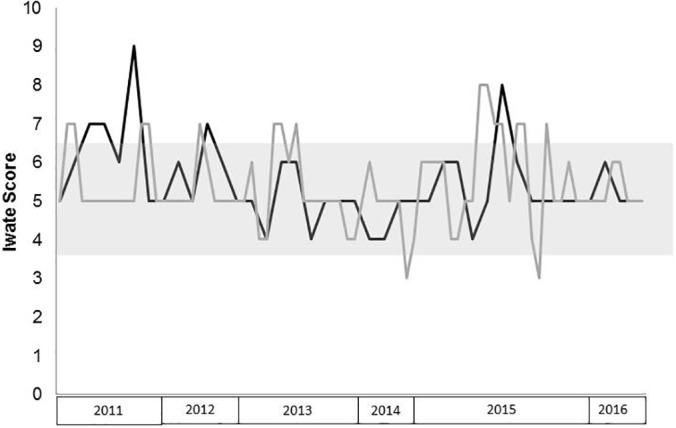
The Iwate score was used to describe the difficulty of laparoscopic (black) and open (gray) liver resections over time. The gray box indicates intermediate difficulty of the procedure according to this score (4–6 points); scores of 7 to 9 refer to advanced and 10 to 12 to expert difficulty levels.
Moreover, the Iwate score as a measure of complexity of LLRs correlated highly with the operative time for laparoscopic and open procedures (P = .027).
Surgical Access or Technique
For open surgery, 69/80 (86.3%) resections were performed through a Makuuchi incision (upper median laparotomy with extension to the right costal arch). Four patients had a bilateral subcostal incision and 2 patients required a median extension in addition to the bilateral incision. In 5 patients, only an upper median laparotomy was used.
The standard trocar positions for laparoscopic resections have been described above. Most resections were performed using 3 (22/40) or 4 (17/40) ports. Only one patient with synchronous rectal resection required 5 trocars for liver and rectal resection. The resected specimen was removed from the abdominal cavity through an extension of a trocar site in 15 of 40 (37.5%) patients. For larger specimens, a suprapubic transverse incision was used in 9 (22.5%) patients, and preexisting incisions were reopened in 14 patients (35%). One patient had a synchronous ileostomy closure, and 1 an umbilical hernia repair, through which the resected specimen were removed at the end of surgery.
We used the Ultracision device for parenchymal transection during the first 17 resections. Of these, 2 required conversion for injury of the left hepatic vein, both in patients with liver cirrhosis. Because we have been using the CUSA device for parenchymal transection, none of the 23 resections required conversion.
Additional Surgical Procedures
Two patients with liver metastases from CRC had simultaneous rectal resections. Four patients in the laparoscopic group had additional cholecystectomy, and one patient each had closure of an ileostomy and incisional hernia repair. Finally, a patient with a small liver metastasis from rectal cancer had laparoscopic cholecystectomy, placement of a diverting ileostomy, repair of a fixed umbilical hernia, and port implantation, together with the LLR. During open liver surgery, 1 patient had simultaneous umbilical hernia repair, and 1 patient each had ileostomy closure, small bowel resection, partial resection of the diaphragm, or right hemicolectomy. The median duration of surgery was comparable between the 2 groups.
Postoperative Outcome
The intra- and postoperative outcomes are summarized in Table 2: the median blood loss was significantly lower after laparoscopic surgery (P < .001). This effect was only true in patients without liver cirrhosis (500 vs 175; P < .001), whereas blood loss was not different in patients with cirrhosis (P = .55). Although the median intensive care unit stay was not different between the two groups, patients stayed significantly longer in the hospital after OLR.
Table 2.
Intra- and Postoperative Outcomes
| Laparoscopic Resection | Open Resection | P | |
|---|---|---|---|
| Duration of surgery, minutes (median, range) | 149:37 (40:33–369)* | 150:40 (60–540)* | 0.54 |
| ICU stay (median, range) | 0 (0–6) | 0 (0–5) | 0.69 |
| Hospital stay, days (median, range) | 6 (4–34) | 7 (4–39) | 0.002 |
| Surgical complications, n/total group (%) | 3/40 (7.5%) | 9/80 (11.3%) | 0.384 |
| Grade IIIa, % | — | 6 | |
| Grade IIIb, % | 1 | 3 | |
| Grade Iva, % | 2 | 1 | |
| Grade IVb | — | — | |
| Grade V | — | — | |
| Blood loss, mL (median, range) | 200 (50–3000) | 500 (100–3000) | <0.001 |
| R0/R1 resection | 33/7 | 74/67/0 | 0.097 |
| Benign tumor (R0/R1) | 9/5 | 67/6 | 0.07 |
| Malignant tumor (R0/R1) | 24/2 | 0.93 | |
| Resection margin, cm (median, range) | 0.3 (0–4) | 0.4 (0–3.6) | 0.776 |
Operative time related to extrahepatic procedures, such as port implantation, synchronous rectal resection, and hernia repair, among others.
According to the final histology, neither the minimal resection margin nor the R1 resection rate differed between the groups. (Table 2).
DISCUSSION
Minimally invasive techniques are increasingly used for liver surgery. General advantages of laparoscopic over open surgery are better cosmesis and shorter hospital stay. In the absence of randomized trials, the current evidence for LLRs derives mainly from retrospective case series. Most of these series report an equal or even longer duration of the operation, less blood loss, and a shorter hospital stay for laparoscopic compared to OLR. Also, decompensation of liver function in patients with cirrhosis appears less frequent in several series, and laparoscopic surgery appears therefore attractive for HCC in liver cirrhosis.3,9 Although any liver resection can be performed laparoscopically, in the literature, standard indications for laparoscopic surgery are resections of the left lateral section and anterior segments 4 to 6.5
Because the proportion of extensive and complicated (often repeat) liver surgery at our department is high and such cases were excluded from laparoscopic surgery, the proportion of laparoscopic surgery (3%) is very low in our series. Therefore, we performed a matched-pair analysis to minimize the bias of retrospective analyses and used standard risk factors for liver surgery as matching items: ASA classification and underlying liver diseases, of which liver cirrhosis is the most severe. Because the extent of liver resection may vary according to the size of the resected tumor in nonanatomic resections and segment resections, we also matched for tumor size to achieve better comparability of the technical risk. The Iwate score confirms an equal complexity of open and laparoscopic resections, and the correlation of Iwate score and operative time further underlines the validity of this score. Furthermore, we increased the power of our analysis by matching (1:2): 1 laparoscopic with 2 open resections.
Despite matching characteristics, patients in the laparoscopic group had benign tumors more often than those in the open resection group. Furthermore, patients were younger in the first 30 LLRs (data not shown), but age does not differ in the current analysis. This imbalance in (age and) tumor histology is related to patient selection for laparoscopic surgery: we initially performed laparoscopic surgery in young patients with suspected benign histology, only, because of the greater demand for satisfactory cosmesis in younger patients with unclear and presumed benign lesions. Because our primary interest was safety of laparoscopic surgery rather than long-term outcome, we accepted larger differences in this matching pair. Also, we mainly performed laparoscopic surgery for superficial tumors or tumors in the anterior or left lateral segments. With increasing experience, we extended the indications for the laparoscopic technique to malignant tumors, which develop more often in older patients, and to tumors located in the posterior segments.
The indications for and types of surgery are in accordance with the literature.5 In general, only patients with symptomatic FNH should undergo liver surgery, because this entity is not associated with complications or malignancy. In contrast, liver adenoma may harbor the risk of bleeding and malignancy above a tumor diameter of 5 cm.10 Accordingly, liver resection is usually offered to patients with adenoma more than 3 to 5 cm in diameter, to prevent tumor-related complications (danger of malignancy >5 cm, danger of bleeding starts >3 cm).11 However, the differential diagnosis is often difficult, and patients with FNH may undergo liver resection to exclude a malignant tumor or an adenoma. Consequently, the incidence of adenoma and FNH in our cohort of patients was below 5%, and most of them underwent surgery for an unclear diagnosis.
As was true in most reports in the literature, the duration of surgery did not differ between the groups in our series, although several laparoscopic resections included additional extrahepatic procedures. Also, blood loss was lower, and the hospital stay was shorter after laparoscopic liver surgery in our series (Table 3).3,4,12–21 The reason for the lack of significance in blood loss in cirrhotic livers is probably the massive blood loss in the 2 patients who required conversion to open surgery because of bleeding from the left hepatic vein.
Table 3.
Most Recent Match-Pair Analyses of Laparoscopic Versus OLRs for HCC and CRC Mets
| Cancer | Study | n | Surgery | Cirrhosis (%) | P | Conversion Rate (%) | Blood Loss (mL) | P | R0 resection (%) | Hospital Stay (days) | P | 5-y Survival | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | Han et al3 | 88 | LLR | 62.5 | NS | 9.1 | 500 | NS | 98.9 | NS | 8 | =0.001 | 76.4 mo | NS |
| 88 | OLR | 59.1 | 525 | 94.3 | 10 | 73.2 mo | ||||||||
| Kim et al12 | 29 | LLR | 100 | NS | 23 | 485 | NS | 89.7 | NS | 7.7 | <0.001 | 47.9 mo | NS | |
| 29 | OLR | 100 | 262 | 96.6 | 13.4 | 59.5 mo | ||||||||
| Cheung et al13 | 32 | LLR | 87.5 | NS | — | 150 | =0.001 | 97 | NS | 4 | <0.001 | 76.6 | NS | |
| 64 | OLR | 79.7 | 300 | 94 | 7 | 57 | ||||||||
| Truant et al14 | 36 | LLR | 1001 | 19.4 | 452 | NS | — | 6.5 | =0.003 | 70 | NS | |||
| 53 | OLR | 1001 | 447 | — | 9.5 | 46 | ||||||||
| Hu et al15 | 30 | LLR | 83 | 0 | 520 | NS | — | 13 | <0.01 | 53.3 | NS | |||
| 30 | OLR | — | 480 | — | 20 | 50 | ||||||||
| Lee et al16 | 33 | LLR | 84.8 | =0.038 | 18.2 | 150 | NS | 97 | NS | 5 | <0.001 | 76 | NS | |
| 50 | OLR | 64 | 240 | 98 | 7 | 76,1 | ||||||||
| CRC metastases | Tohme et al4 | 66 | LLR | 4 | 150 | NS | 88 | NS | 4 | <0.001 | 51.3 | NS | ||
| 66 | OLR | 250 | 89 | 5 | 38.6 | |||||||||
| Guerron et al17 | 40 | LLR | 5 | 376 | =0.04 | 1 cm | NS | 3.7 | <0.001 | 892 | NS | |||
| 40 | OLR | 753 | 1 cm | 6.5 | 812 | |||||||||
| Qiu et al18 | 30 | LLR | 6.7 | NS | 6.7 | 215 | <0.001 | 9.9 cm | NS | 7.5 | <0.001 | — | ||
| 30 | OLR | 10 | 385 | 1 cm | 11.5 | — | ||||||||
| Cannon et al19 | 35 | LLR | — | 202 | <0.001 | 97 | p=0.02 | 4.8 | <0.001 | 36 | NS | |||
| 138 | OLR | 392 | 81 | 7.8 | 37 | |||||||||
| Cheung et al20 | 20 | LLR | 0 | 200 | =0.043 | 0.6 cm | NS | 4.5 | =0.021 | 69.4 mo | NS | |||
| 40 | OLR | 300 | 0.5 cm | 7 | 42.1 mo | |||||||||
| Schiffman et al21 | 242 | LLR | — | 262 | =0.049 | 94.5 | NS | 6.5 | =0.007 | 51.4 | NS | |||
| 386 | OLR | 385 | 87.4 | 8.8 | 45.9 | |||||||||
| Fretland et al23 | 133 | LLR | 2 | 300 | NS | 94 | NS | 53h | <0.001 | — | ||||
| 147 | OLR | 200 | 93 | 96h | — | |||||||||
All F3 and F4 fibrosis;
2-year survival; 3references 14–17 included in this meta-analysis. [-] information not provided; mo, months; NS, not significant.
The shorter hospital stay after laparoscopic surgery in our series is consistent with the literature (Table 3). To avoid financial disadvantage by discharging patients earlier than the diagnosis-related group (DRG) relevant minimum hospital stay for liver resections, only 1 patient was discharged before day 5. Despite this, the difference in hospital stay was highly significant in our series. To maximize the advantages of this technology, reimbursement of laparoscopic surgery should be adapted, respecting the higher direct cost and enabling an earlier discharge from the hospital.
One limitation of laparoscopic surgery is the retrieval of the resected specimen: whereas most surgical steps can be performed laparoscopically, the trocar sizes do not allow the retrieval of larger specimens. However, preexisting incisions or a transverse suprapubic incision can be used, which is appreciated by most (female) patients with cosmetic concerns, and most specimens were retrieved accordingly in this series. Only small specimens from segmental or atypical resections were retrieved through extensions of a trocar site.
During our initial experience, only the Ultracision device was used for parenchymal transection. This device allows a precise preparation and sufficient sealing of small intrahepatic vessels, but has limited hemostatic effect in the liver parenchyma. Furthermore, an apparently incomplete sealing of larger vascular structures becomes overt during the sealing phase, only. This limitation was the reason for 2 conversions in cirrhotic livers, in which the identification of vessels is more difficult. The CUSA device has no sealing capacity, but vascular structures are preserved, and vessels can be selectively stapled after complete transection of the surrounding parenchyma. Sufficient hemostasis can be achieved by additional bipolar coagulation.
Recent publications suggest oncological benefits of laparoscopic surgery: recovery from liver surgery requires an interruption or delay of oncologic treatments that might impair long-term survival. Tohme et al4 recently reported an earlier start of chemotherapy in patients with CRC liver metastases after laparoscopic surgery, and a longer time to adjuvant chemotherapy was associated with shorter survival among patients with resected CRC in a meta-analysis.22 Recently, the randomized OSLO-COMET trial has demonstrated a lower complication rate and a shorter hospital stay after laparoscopic liver surgery in patients with colorectal liver metastases.23 Moreover, laparoscopic surgery is associated with a lower number of circulating tumor cells in patients with HCC compared with open surgery.6 These effects are major advantages of laparoscopic surgery for patients with malignant diseases, but they must be confirmed.
In addition to less hepatic decompensation, Han et al3 found larger resection margins in patients with HCC who underwent LLR compared to OLR. As for most series in the literature, the resection margins did not differ between the groups in our series (Table 3).
CONCLUSION
Laparoscopic liver surgery confers several general advantages over open surgery, which are confirmed by our analysis. Moreover, recent analyses suggest superiority of minimally invasive liver surgery compared to open surgery, in oncologic outcome. These observations, however, require confirmation by larger and prospective trials in the future. Because no disadvantage has been reported in the literature yet and the oncological outcome was the same as for OLR in our series, we will further extend the indications to more complicated laparoscopic resections.
Contributor Information
Stefan Heinrich, Department of General, Visceral, and Transplantation Surgery, University Hospital of Mainz, Germany..
Verena Tripke, Department of General, Visceral, and Transplantation Surgery, University Hospital of Mainz, Germany..
Tobias Huber, Department of General, Visceral, and Transplantation Surgery, University Hospital of Mainz, Germany..
Jens Mittler, Department of General, Visceral, and Transplantation Surgery, University Hospital of Mainz, Germany..
Hauke Lang, Department of General, Visceral, and Transplantation Surgery, University Hospital of Mainz, Germany..
References:
- 1. Park SK, Olweny EO, Best SL, Tracy ER, Mir SA, Cadeddu JA. Patient-reported body image and cosmesis outcomes following kidney surgery: comparison of laparoendoscopic single-site, laparoscopic, and open surgery. Eur Urol. 2011;60:1097–1104. [DOI] [PubMed] [Google Scholar]
- 2. Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010;CD001546. [DOI] [PubMed] [Google Scholar]
- 3. Han HS, Sheta A, Ahn S, Yoon Y-S, Cho JY, Choi Y-R. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–650. [DOI] [PubMed] [Google Scholar]
- 4. Tohme S, Goswami G, Han K, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015;19;2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection: 2,804 patients. Ann Surg. 2009;250:831–841. [DOI] [PubMed] [Google Scholar]
- 6. Li W, Zhou X, Huang Z-J, et al. Laparoscopic surgery minimizes the release of circulating tumor cells compared to open surgery for hepatocellular carcinoma. Surg Endosc. 2015;29:3146–3153. [DOI] [PubMed] [Google Scholar]
- 7. Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tranchart H, Di Giuro G, Lianas P, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. [DOI] [PubMed] [Google Scholar]
- 10. Belghiti J, Cauchy F, Paradia F, et al. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014;11:737–749. [DOI] [PubMed] [Google Scholar]
- 11. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386–398. [DOI] [PubMed] [Google Scholar]
- 12. Kim H, Suh K-S, Lee K.-W, et al. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28:950–960. [DOI] [PubMed] [Google Scholar]
- 13. Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–511. [DOI] [PubMed] [Google Scholar]
- 14. Truant S, Bouras AF, Hebbar M, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668–3677. [DOI] [PubMed] [Google Scholar]
- 15. Hu BS, et al. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4725–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg. 2011;35:2268–2274. [DOI] [PubMed] [Google Scholar]
- 17. Guerron AD, Aliyev S, Agcaoglu O, et al. Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc. 2013;27:1138–1143. [DOI] [PubMed] [Google Scholar]
- 18. Qiu J, Chen S, Pankaj P, Wu H. Laparoscopic hepatectomy for hepatic colorectal metastases: a retrospective comparative cohort analysis and literature review. PLoS One. 2013:8:e60153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon RM, Scoggins CR, Callender GG, McMasters KM, Martin RC., 2nd Laparoscopic versus open resection of hepatic colorectal metastases. Surgery 2012;152:567–573; discussion 573–574. [DOI] [PubMed] [Google Scholar]
- 20. Cheung TT, Poon RT, Yuen WK, et al. Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ J Surg. 2013;83:847–852. [DOI] [PubMed] [Google Scholar]
- 21. Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015:157:211–222. [DOI] [PubMed] [Google Scholar]
- 22. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 23. Fretland AA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2017;266, in press. [DOI] [PubMed] [Google Scholar]