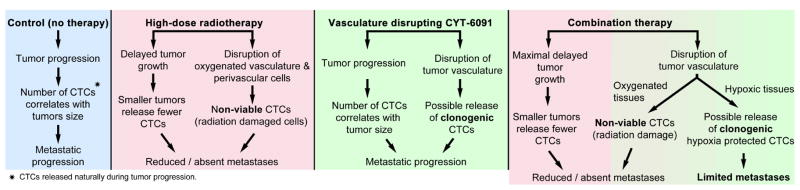
Abstract
Noninvasive biological readouts of tumor metastatic risk and therapeutic efficacy are needed as healthcare costs rise. Conventional imaging modalities are expensive; require significant periods of time to see appreciable change and repetitive use increases risk of hazardous side effects. Utilizing circulating tumor cells (CTCs) as a minimally invasive biological readout of tumor progression or therapeutic response is increasingly being studied. While focus has primarily been on the predictive value of naturally occurring CTCs, a more recent effort has emerged regarding the potential consequence of therapy induced CTCs. Here we report an investigation on the acute and long-term effect of vascular disrupting therapies (high-dose radiotherapy and tumor necrosis factor-alpha (TNF)) on CTCs monitored with a non-invasive real-time system. Acute mobilization of CTCs into the blood following both radiation and TNF treatment was observed. There was no increase in metastasis frequency or extent between control and TNF-treated mice; however, a significant reduction in lung metastasis was noted in the radiotherapy alone group. Mice treated with both TNF and radiotherapy had a slightly elevated metastatic profile between that of radiation alone and control (untreated) tumors. Possible mechanisms based on therapy specific vessel disruption and cell death are discussed. Overall, CTCs correlated with tumor progression and suggest CTC enumeration described herein may be useful in clinical management of solid tumor malignancies.
Keywords: radiation, TNF-α, vascular disrupting agents, circulating tumor cells, metastasis, in vivo fluorescent flow cytometry
Introduction
Therapy associated disruption of tumor vasculature allows immune cellular transit from blood into tumor 1,2 as well as creates a possibility for cells to exit from tumor into blood. The presence of breast tumor derived cells in blood have been reported to increase the risk of metastasis 3 leading to CTC-based tests becoming more accepted in oncological practice 4. However, little work was done to analyze the risks of tumor metastatic progression stemming from therapy induced vascular disruption 5,6. Fractionated radiation was recently reported to augment the release of viable CTCs into circulation potentially retaining metastatic potential 6,7 in patients with non-small cell lung cancer and squamous cell carcinoma of the head and neck. Routine biopsy and pressure also have been reported to temporarily increase the number of tumor cells in circulation as a result of tumor vessel damage 8–10. Thus, for the success of a multistage anti-cancer therapy based on disruption of tumor vasculature, it is crucial to understand both short- and long-term effects of vascular damage on release of CTCs and any possible influence on extent of metastatic disease.
The majority of patients with unresectable or metastatic solid tumors receive some type of radiotherapy during the course of treatment and, stereotactic body radiotherapy (high- dose radiotherapy) is increasingly being used in radiation oncology practice 11. The higher doses of radiation accompanying this treatment are being deposited within tumor tissues and have led to observations of associated tumor vessel damage 12, and release of viable circulating tumor cells (CTCs) 6. The vascular effects of high-dose radiotherapy are not completely understood or appreciated 4; moreover, a combination of vascular-disrupting and radiation-based therapies increasingly attracts attention 13 by providing a possibility of targeting both well oxygenated and hypoxic (low oxygen) tumor regions. We recently confirmed that a combination of vascular disrupting TNF-based nanoparticles, CYT-6091, with hypofractionated radiation induces synergistic tumor growth delay 14. The biological effects (vascular hemorrhaging) of TNF were concentrated in radioresistant, hypoxic regions acting complementary to radiation therapy which has lower efficacy in such regions.
Herewith, we hypothesized that vascular damaging high-dose radiation and/or CYT-6091 may lead to a measurable increase in CTCs detectable by our in vivo fluorescence detection platform. Furthermore, we sought to assess the possible use of CTC indices as markers of primary tumor treatment efficacy and whether or not certain vascular damaging therapies had distinguishable effects on CTC levels or correlated to metastatic progression. An in vivo flow cytometry method for real time CTC quantification developed by our group 10,15 provided an opportunity to reveal short term (within minutes) as well as enumerate at later dates a snapshot of CTCs after radiation or anti-vascular treatment. Prior to initiating the study described herein, an initial study was conducted to ascertain if CTCs are released following CYT-6091 therapy in a translational model. Syngeneic 4T1 murine breast tumors expressing GFP and grown in balb/c mice indicated a 3-fold increase in CTCs in a time window in-line with the known vascular damaging effects of CYT-6091 (data not shown).
Materials and Methods
Principles of in vivo fluorescence flow cytometry (FFC) detection
Real-time quantification of CTCs in mouse blood was performed by means of in vivo single color fluorescence flow cytometry described elsewhere 16. A 488-nm diode laser (total power of 7 mW in the sample) was used to excite fluorescent CTCs 17 circulating in arterial vessels. A 40x objective (NA 0.75, Plan Fluor, Nikon) was used to focus the laser beam into an arterial vessel and laser light and fluorescence were separated using dichroic mirrors. A cylindrical lens (focal length of 250 mm) shaped the laser beam in the sample into a line (50×5 μm). The fluorescence was filtered with a bandpass filter 520±15 nm (Semrock, Inc., Rochester, NY) and detected by a photomultiplier (PMT) tube (PMT, R928, Hamamatsu, Co., Bridgewater, NJ). The PMT signal was acquired using a PCI-5124 digitizer (National Instruments, Austin, TX) and analyzed using custom MatLab software (MathWorks, Natick, MA).
Cell lines and tumor model
Mouse mammary carcinoma, 4T1, cells transfected with green fluorescent protein (4T1-GFP) were cultured at 37°C and 5% CO2 in DMEM 4.5 g/L D-glucose (Mediatech Inc., Manassas, VA) supplemented with 10% FBS. For tumor inoculation, cells growing exponentially were harvested at 80% confluence with 0.125% trypsin, suspended in serum free media at 5 × 105 cells/0.05 mLs, and 0.05 mLs implanted subcutaneously in the left rear limb of female Nu/Nu mice (Harlan; weighing 20–25 g). Microchip transponders implanted in the flank were used for mouse identification (Bio Medic Data Systems, Inc., Seaford, DE). The 4T1 breast cancer model was chosen because it is a well-established model of metastatic cancer (e.g. sites of metastasis in lymph node and lung). Implantation in immune compromised, Nu/Nu mice, was inline with previously optimized conditions for monitoring CTCs 8–10,18–20. All animal procedures were performed with approval by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA).
Dorsal skinfold window chamber model and monitoring vascular permeability
The dorsal skinfold window chamber (DSWC) model was prepared as described previously 17. Briefly, custom aluminum frames with an approximately 12 mm diameter window opening were implanted on the dorsal skinfold. Tissue was removed from one side of the chamber leaving the subcutaneous tissue of the opposite side exposed for tumor spheroid implantation. 4T1-GFP cells grown as spheroids using the hanging drop method were implanted and a glass coverslip sealed the exposed tissue.
Animals were anesthetized with 1% isoflurane and placed in a custom holder for window chamber visualization on an Olypmus IX71 inverted microscope. Tumor tissue and neovascularization was confirmed by presence of GFP with characteristic tumor vessels observed throughout the fluorescent tissue. Mice were treated with sterile H2O (100 uL, n=3) or CYT-6091 (250 μg/kg, n = 3) IV via the lateral tail vein 1 hour prior to intracardiac injection of 0.8 mg 70kD texas red labeled dextrans (Invitrogen) for imaging of drug-induced vascular permeability. Time-lapse images were collected at 5, 20 and 35 minutes post dextran injection.
Treatments and tumor growth response
Rear limb tumors were grown to an average size of 190 mm3 and randomized to various treatment groups: tumor control without treatment (n=6), CYT-6091 (250 μg/kg q2dx2; n=6), radiation (12 Gy q2dx3; n=6), and combined therapy (n=6). Ionizing radiation was administered using a Faxitron X-ray cabinet system (CP-160, Faxitron X-Ray Corp., Wheeling, IL) at a dose rate of 1.079 Gy/min (150 kVp and 6.6 mA) under ketamine/xylazine anesthesia. TNF was delivered via a pegylated gold nanoparticle carrier 21, administered IV on day 0 and day 2 immediately following the first two rounds of radiation for the combined treatment group. Tumor diameter was measured manually in the x- and y-axis using calipers. The tumor volume was calculated as: , and plotted as average tumor volume per group.
Circulating tumor cell monitoring
The number of CTCs in the blood circulation was enumerated using FFC under isoflurane anesthesia (inhalation, 0.5–1.2%). An anesthetized mouse was placed on a temperature-controlled microscope stage (37°C), with an ear spread flat over the glass window located on the microscope stage. A small artery (typically 30 – 50 μm in diameter) of the ear was used to monitor CTCs present in circulation. GFP expression acted as an inherent marker for CTCs in the blood circulation. The accuracy of the FFC method was additionally verified, spiking blood samples with GFP expressing CTCs and performing in vitro flow cytometry as described previously 8,9,16,18–20,22. The duration per FFC monitoring was 60 minutes following the schedule: 1) before treatment; 2) immediately post-treatment; 3) 8 hours post-treatment; 16 hours post-treatment; and 4) 21 days post-treatment.
Acute CTC release was quantified immediately following-0 hr, 8 hrs post- and 16 hrs post-administration of the initial treatment. The continuous-monitoring CTCs results from each mouse were binned into a single window. Six mice were inoculated with tumor cells, but did not receive therapy. This group served as the control group to which the treatment groups were compared for differences in the number of CTCs, CTC-release rates and number of metastases.
Metastatic tumor analysis
At the completion of the study, day 21, lung tissue was harvested, fixed in neutral buffered formalin and paraffin embedded. Five micrometer histological sections were prepared and stained with hematoxylin and eosin (H&E) and imaged on an Aperio Scancope (Leica Biosystems Imaging, Inc). A board certified pathologist identified and outlined metastatic lesions in the lungs using Aperio’s Imagescope software. The metastatic tumor burden was calculated in comparison to the total tissue present within the lung tissue section and is reported as percent lung metastasis. Groups and number analyzed are as follows: untreated control (n=6), CYT-6091 alone (n=6), radiation alone (n=6), and combination (n=6).
Statistical analysis
DSWC analysis – A two-way analysis of variance with post-hoc Sidak’s test for multiple comparisons was performed to analyze the fluorescent signal.
CTC analysis – A one-way analysis of variance with post-hoc Tukey test for multiple comparisons was performed to analyze day 21 differences between groups. A two-way analysis of variance with post-hoc Dunnet’s multiple comparison tests was performed to analyze differences within individual groups compared to pretreatment CTC numbers.
Metastatic analysis – Unpaired t-tests with post-hoc Welsh’s correction were performed. Probability of p<0.05 indicated a significant difference in all analyses.
Results
CYT-6091 induced tumor vascular permeability
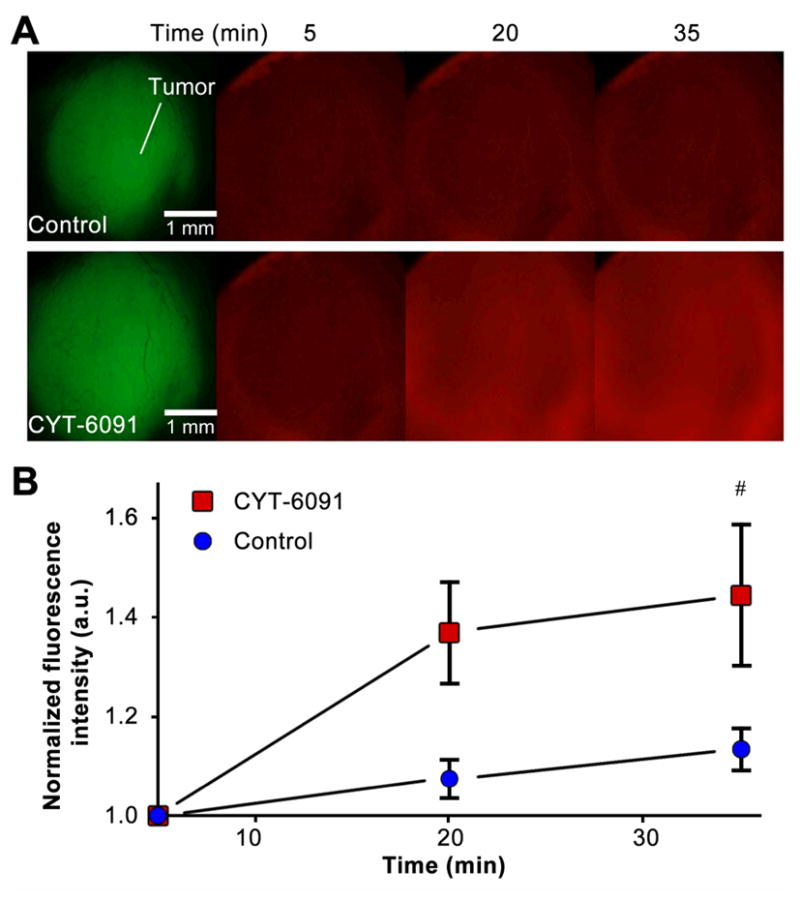
Tumor necrosis factor-α (TNF) is a cytokine possessing diverse biological effects upon ligand binding with one of its two known receptors. In the tumor microenvironment, TNF selectively induces vascular instability ultimately leading to tumor specific vascular hemorrhaging 23,24. We first evaluated the kinetics and acute vascular effects of TNF therapy (CYT-6091) using real-time imaging of fluorescent dextrans leaking out of damaged tumor vessels. Within 60 minutes post CYT-6091 administration, enhanced tumor vascular permeability was observed with fluorescently labeled dextrans accumulating in the tumor parenchyma (figure 1). The average pixel intensity increased 44.5% by 35 min in the CYT-6091 treated group compared to 13.5% in the control group, relative to baseline values (1.135 ± 0.04 v 1.445 ± 0.14, mean ± SEM, p<0.05). A significant increase in fluorescent intensity was also observed within the CYT-6091 group at 20 and 35 min compared to 5 min (p<0.05).
Figure 1. TNF induced vascular permeability in a breast tumor model.
(A) 4T1-GFP tumors growing in the DSFWC of mice were treated with sterile water (control) or TNF 1h prior to administration of fluorescent dextrans in the bloodstream. TNF induces vascular permeability, allowing dextrans to accumulate in the tumor over time. Images show tumors (green) and leaking of fluorescently tagged dextran (red) through tumor vessel walls.(B) Quantified data of fluorescent signal in tumor tissue represented as average ± SEM, n = 3, (#, p<0.05).
Real-time monitoring of therapeutic effects on circulating tumor cells
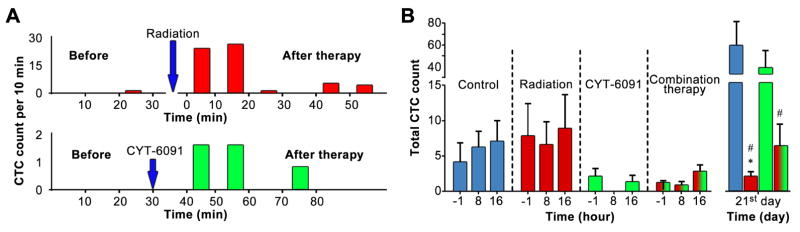
Rapid vascular disruption by CYT-6091 (20–60 min) followed by the collapse of damaged vessels 25 limits the time frame for successful release of CTCs from the damaged zones into blood circulation. In order to reliably assess the potential acute changes in the number of CTCs, we analyzed the effects of high-dose radiotherapy, CYT-6091 and the combined therapy using a real-time in vivo flow cytometry platform. Our real-time monitoring data indicated a statistically significant (compared to pre-therapy levels) increase in the number of CTCs starts soon (10–15 min) after therapy. The increase in CTC concentration lasts on average 20 min (figure 2A) before returning to pre-therapy level.
Figure 2. Therapeutic effect on CTCs.
A) Examples of an acute effect of radiation (top) and TNF (bottom) on CTCs. B) Acute and long-term effect of radiation, TNF or combined treatment on CTCs in immune deficient mice. Average CTCs detected prior to therapy (-1h) and within 24h after (8h, 16h) along with 21 days (21 d) post-therapy are plotted, n = 6 (# v. control, * v. CYT-6091 p<0.05).
No significant changes were observed in CTC numbers (p > 0.05) before, 8 and 16 h after therapy across all treatments tested (Fig. 3B). CTCs were detectable at all timepoints except the 8h timepoint in the CYT-6091 group. By day 21 following the initial treatment, radiation significantly reduced the number of tumor cells in circulation compared to control or the CYT-6091 treated group (1.99 ± 0.7 v. 60.2 ± 19.2, mean ± SEM, p<0.05), figure 2B. The combination therapy also significantly reduced the number of tumor cells in circulation compared to control (6.2 ± 3.056 v. 60.2 ± 19.2, mean ± SEM, p<0.05).
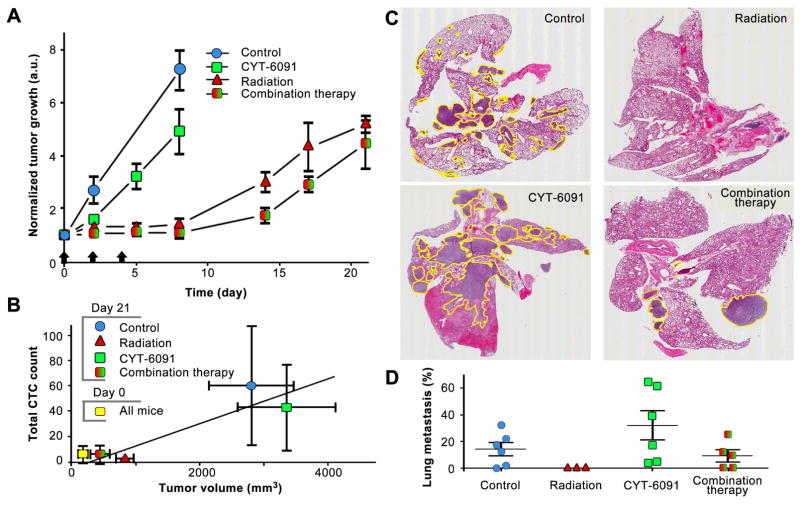
Figure 3. Metastatic tumor burden reduced with therapeutic intervention.
A) Tumor growth delay indicates an additive effect of combined TNF and radiation therapy in immune deficient mice. B) Correlation of CTCs with tumor volume. C) Representative images of whole lung tissue H&Es from tumor bearing mice treated with radiation, TNF or combination therapy. D) Quantified data on the percent of lung tissue harboring metastatic tumor cells, n = 6.
Effect of high-dose radiotherapy and TNF on tumor growth, CTC count and metastatic progression
The tumor growth delay was analyzed for high-dose radiation, vascular disrupting TNF (CYT-6091) and combination therapy (figure 3A). The CYT-6091 therapy delayed growth to reach 4-fold the starting tumor volume by 3 days (day 4 v. day 7, 1.75-fold inhibition) compared to control (figure 3A). Radiation alone delay growth by 12 days (day 4 v. day 16, 4-fold inhibition), while the combination group reached 4-fold the starting volume by day 19 of evaluation (day 4 v. day 19, 4.75-fold inhibition). The therapeutic effect on tumor volume correlated well with the total CTC count (figure 3B) at the end of the evaluation period.
Histological evidence of tumor metastasis was evident in all groups except those mice treated with radiation alone (figure 3C). Despite lacking histological evidence of metastasis, radiation metastatic burden was only significantly different from control and the CYT-6091 monotherapy (p<0.05). Tumor burden varied in these groups from 14.3% - 31.89% of total lung tissue and were not determined to be significantly different from each other (figure 3D).
Discussion
The real-time monitoring of CTCs provides a window into acute therapeutic events while traditional approaches can take months to detect therapy associated changes. In select cases, the current study revealed an acute increase in CTCs in a window of time consistent with changes in tumor vascular permeability following either radiation therapy or CYT-6091 (figure 1 and figure 2A). The short-lived increase in CTC concentration was followed by a notable absence of CTCs 8 h post CYT-6091 (figure 2B), consistent with physiological suppression of blood flow in tumor tissue reported earlier by our group 25. This is indirectly confirmed by the return of CTC count back to pre-therapy levels 16 h after CYT-6091 monotherapy, when vessel occlusion effects are minimized. Interestingly, the combination therapy also had a similar trend; however, these changes were not as appreciable. The very short circulation times of therapy-released CTCs may indicate a cluster of tumor cells were released or aggregates containing both tumor and healthy cells. Such aggregates would be rapidly cleared from circulation by lungs or spleen, while individual tumor cells usually remain in circulation for much longer periods of time (up to 30–90 min). Real-time in vivo flow cytometry data may provide a unique advantage over blood draw based tests; allowing monitoring of fast changes in the numbers of CTCs in the early hours to many days after therapy.
Long-term effects were analyzed using conventional tumor growth delay analysis and histopathology data in addition to a last CTC scan at day 21 after treatments commenced. Our data indicate CYT-6091 monotherapy had little effect delaying tumor growth, while radiation and combination therapies provided significant protection. Metastatic burden was clearly present in both control and CYT-6091 treated mice while the radiation alone and combined CYT-6091 and radiation groups had the lowest amount of CTCs detected 21 days post-treatment and the lowest metastatic tumor burden among groups. The extent of metastatic progression generally correlated with the tumor volume at day 21 however, the radiation alone group showed the lowest amount of metastasis even though the primary tumor volume was slightly greater than in the combined therapy group (Figure 3A). This is further evidence that CTC enumeration in blood provides a unique opportunity for non-invasive monitoring while utilizing a cost saving modality to evaluate tumor progression and therapeutic response.
The lack of metastatic tumor burden following release of CTCs after high dose radiation therapy indicates low CTC viability, clonogenicity or both. We and others have recently demonstrated in a variety of studies and endpoints that at doses of 10 Gy or above, radiation disrupts the tumor vasculature leading to irreparable damage of nearby well oxygenated tumor cells. In hypoxic (low oxygen) tumor regions where cells are capable of surviving radiation, the vessels may remain intact and not contribute to CTC load in the circulation. However, TNF may preferentially damage tumor vasculature in hypoxic, perinecrotic regions of solid tumor, while radiation damages oxygenated vasculature 14,26,27. Hypoxic tumor tissues are known to be radio- and chemo-resistant, harbor and expand cancer stem cell populations and promote prometastatic phenotypes 28,29. Thus, the disruption of hypoxic tumor vessels by CYT-6091 alone may produce CTCs capable of surviving radiation therapy, while retaining the potential to form distant metastases. This could be a reason that a slight, but statistically insignificant rise in the CTCs and metastasis was detected in the combination treatment group at 21 days after treatment. However, this highlights the importance and relevance of adjuvant chemotherapy to control systemic disease with the use of vascular disrupting drugs in combination with radiation or not. Moreover, the comparison between our current data (figure 2A) from immune deficient mice with similar data from immune competent mice14 suggests that the combination of high-dose radiation and CYT-6091 is even more effective in the presence of an active immune response. Thus, further optimization of TNF based therapies and radiotherapy 30,31 should consider the role of immune response in assessing the actual danger of CTCs and optimizing therapy sequences and combinations, especially if combined with immunotherapy 32.
To our knowledge, these preclinical results are one of the first to monitor the increased release of CTCs during high-dose radiotherapy and vascular-disrupting therapy and a correlation to CTC viability/metastatic progression. Current clinical trials are being led by portions of our group investigating the release of CTCs in rectal carcinoma patients before and after radiochemotherapy and surgery. Additional clinical trials in melanoma patients where the technology for CTC enumeration was described previously22 are being conducted. Melanin overexpression in melanoma cells is detectable photoacoustically and would eliminate the need for fluorescent detection method described here. We also acknowledge the value of further testing in pre-clinical models using orthotopically implanted tumors, patient-derived xenograft (PDX) models or transgenic tumor models. The real-time quantification of CTCs by in-vivo flow cytometry observed in the current study demonstrates the potential of predicting and controlling acute and long-term effects of high-dose radiotherapy and vascular disrupting agents to personalize and tailor multi-modality therapy in each patient.
Supplementary Material
Figure 4.
Therapy induced release of CTCs and effect on tumor progression
Acknowledgments
Financial support: Supported by a grant from The Brown Foundation, NIH/NCI grant CA44114, NIH EB017217, and Deutsche Forschungsgemeinschaft (German Research Foundation Grant, JU 2814/1-1).
This work was supported by NIH CA44114 to RJG and NIH EB017217 for VZ. MAJ was partly supported by the Deutsche Forschungsgemeinschaft (German Research Foundation Grant, JU 2814/1-1). We thank S. Ferguson for assisting with laser measurements and Azemat Jamshidi-Parsian for helping with the cell culture.
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
Contribution
N.A.K, M.A.J. and R.J.G. designed the research. N.A.K, M.A.J., and C.C performed the experiments and analyzed the data. Y.A.M. and D.A.N. built the instruments and helped with experiments. M.S. developed the data-processing algorithms and also assisted with data processing. M.C.Q. analyzed histological samples N.A.K., M.A.J., R.P.M.D., D.A.N., V.P.Z., and R.J.G. wrote the manuscript. V.P.Z. and R.J.G. directed the project. All authors discussed the results and commented on the manuscript.
Disclosure of Conflict of Interests
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science (New York, NY) 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 2.Welford AF, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. The Journal of clinical investigation. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidard F-C, et al. Time-Dependent Prognostic Impact of Circulating Tumor Cells Detection in Non-Metastatic Breast Cancer: 70-Month Analysis of the REMAGUS02 Study. International Journal of Breast Cancer. 2013;2013:130470. doi: 10.1155/2013/130470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkinson DR, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorsey JF, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: Pilot study results. Cancer. 2015;121:139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin OA, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. International journal of radiation oncology, biology, physics. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Tinhofer I, Hristozova T, Stromberger C, Keilhoiz U, Budach V. Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2012;83:e685–690. doi: 10.1016/j.ijrobp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Juratli MA, et al. Real-time monitoring of circulating tumor cell release during tumor manipulation using in vivo photoacoustic and fluorescent flow cytometry. Head & neck. 2014;36:1207–1215. doi: 10.1002/hed.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juratli MA, et al. Dynamic Fluctuation of Circulating Tumor Cells during Cancer Progression. Cancers (Basel) 2014;6:128–142. doi: 10.3390/cancers6010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juratli MA, et al. In Vivo Long-Term Monitoring of Circulating Tumor Cells Fluctuation during Medical Interventions. PLoS One. 2015;10:e0137613. doi: 10.1371/journal.pone.0137613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A Survey of Stereotactic Body Radiation Therapy Use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song CW, et al. Is Indirect Cell Death Involved in Response of Tumors to Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy? International journal of radiation oncology, biology, physics. 2014;89:924–925. doi: 10.1016/j.ijrobp.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Wachsberger P, Burd R, Dicker AP. Tumor Response to Ionizing Radiation Combined with Antiangiogenesis or Vascular Targeting Agents: Exploring Mechanisms of Interaction. Clinical Cancer Research. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 14.Koonce NA, et al. Combination of Gold Nanoparticle-Conjugated Tumor Necrosis Factor-alpha and Radiation Therapy Results in a Synergistic Antitumor Response in Murine Carcinoma Models. International journal of radiation oncology, biology, physics. 2015;93:588–596. doi: 10.1016/j.ijrobp.2015.07.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuchin VV, Tarnok A, Zharov VP. Towards in vivo flow cytometry. J Biophotonics. 2009;2:457–458. doi: 10.1002/jbio.200910546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedosekin DA, et al. Synergy of photoacoustic and fluorescence flow cytometry of circulating cells with negative and positive contrasts. J Biophotonics. 2013;6:425–434. doi: 10.1002/jbio.201200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upreti M, et al. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl Oncol. 2011;4:365–376. doi: 10.1593/tlo.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedosekin DA, Verkhusha VV, Melerzanov AV, Zharov VP, Galanzha EI. In vivo photoswitchable flow cytometry for direct tracking of single circulating tumor cells. Chemistry & biology. 2014;21:792–801. doi: 10.1016/j.chembiol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galanzha EI, Zharov VP. Photoacoustic flow cytometry. Methods. 2012;57:280–296. doi: 10.1016/j.ymeth.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer research. 2009;69:7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-α-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedosekin DA, Sarimollaoglu M, Ye JH, Galanzha EI, Zharov VP. In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers. Cytometry. Part A: the journal of the International Society for Analytical Cytology. 2011;79:825–833. doi: 10.1002/cyto.a.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farma JM, et al. Direct evidence for rapid and selective induction of tumor neovascular permeability by tumor necrosis factor and a novel derivative, colloidal gold bound tumor necrosis factor. International journal of cancer. Journal international du cancer. 2007;120:2474–2480. doi: 10.1002/ijc.22270. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe N, et al. Toxic Effect of Tumor Necrosis Factor on Tumor Vasculature in Mice. Cancer research. 1988;48:2179–2183. [PubMed] [Google Scholar]
- 25.Visaria RK, et al. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Molecular cancer therapeutics. 2006;5:1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 26.Koonce NA, et al. Targeting Artificial Tumor Stromal Targets for Molecular Imaging of Tumor Vascular Hypoxia. PLoS One. 2015;10:e0135607. doi: 10.1371/journal.pone.0135607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song CW, et al. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. International journal of radiation oncology, biology, physics. 2015;93:166–172. doi: 10.1016/j.ijrobp.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. http://www.nature.com/nrc/journal/v11/n6/suppinfo/nrc3064_S1.html. [DOI] [PubMed] [Google Scholar]
- 29.Conley SJ, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proceedings of the National Academy of Sciences. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Frontiers in oncology. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing Tumor Immunity With Fractionated Radiation. International Journal of Radiation Oncology*Biology*Physics. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. http://dx.doi.org/10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:5379–5388. doi: 10.1158/1078-0432.ccr-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.