Abstract
Objectives
We sought to evaluate predictors of stroke on LVAD from data available prior to implantation, and quantify stroke-related morbidity and mortality
Background
Stroke is a major complication after LVAD. Pre-implant factors that influence stroke are not well understood.
Methods
We evaluated all patients in the INTERMACS registry who were implanted with continuous-flow LVADs from May 1, 2012 to March 31, 2015. Preoperative risk factors for stroke, and stroke incidence, morbidity, and mortality were analyzed.
Results
During the study period, 7112 patients underwent CF LVAD placement. Median follow-up was 9.79 months (range 0.02–34.96 months). Of all patients, 752 (10.57%) had at least one stroke, with an incidence rate of 0.123 strokes per patient-year. 447 (51.38%) strokes were ischemic and 423 (48.62%) were hemorrhagic. Patients with hemorrhagic stroke had worse survival than those with ischemic strokes (30-day survival 45.3% vs. 80.7, p <0.001). Of patients with a first stroke, 13% had a second stroke. Pre-implant predictors of stroke were female gender (HR 1.51, 95% CI 1.25–1.82, p <0.001), pre-implant systolic blood pressure (HR 1.01, 95% CI 1.00–1.01, p = 0.002), heparin-induced thrombocytopenia (HIT) (HR 3.68, 95% CI 1.60–8.47, p = 0.002), intra-aortic balloon pump (IABP) (HR 1.21, 95% CI 1.01–1.46, p = 0.043), and primary cardiac diagnosis (ischemic/other/unknown) (p = 0.040).
Conclusion
Despite improvements in LVAD technology, stroke-related morbidity and mortality is substantial. Further investigation is necessary to decrease the risk of this devastating complication.
Keywords: left ventricular assist device, stroke
Introduction
Stroke in LVAD patients is associated with high mortality, significant morbidity, and impairment of quality of life in patients and caregivers. It frequently leads to ineligibility for transplant. Most importantly, despite advances in device longevity and patient survival, stroke rates have not improved significantly, and may even be increasing with some newer generation devices.(1)
The pathophysiology of strokes on LVAD is not well understood. Thromboembolism is an important contributor, but whether emboli originate from the ventricle, LVAD, aortic root, or aortic atheromas is difficult to discern in an individual case. Changes in cerebrovascular endothelial function and structure with CF-LVADs that may predispose to hemorrhagic strokes remain unknown. LVAD-related infections are associated with strokes but exact mechanisms and therapeutic targets remain elusive.
Recent investigations have focused on strict blood pressure and anticoagulation management as strategies to reduce strokes. While these approaches decrease stroke rates (3), stroke rates remain unacceptably high, even in patients with controlled BP and therapeutic INRs. It is difficult to predict the likelihood of achieving optimal BP and anticoagulation control post LVAD, avoiding LVAD infections, and adherence in an individual patient prior to LVAD implantation.
We sought to identify pre-implant variables that could predict stroke risk associated with device therapy. Identification of such factors prior to implantation may allow stratification of patients into stroke risk categories, enable formulation of management approaches to modify risk factors to reduce stroke in a more targeted manner, allow a more informed discussion of the risks and benefits with patients and families, dictate urgency of transplant listing for BTT candidates, and outline patient risk profiles and strategies for future studies. We also examine the impact of stroke on patient outcomes, particularly transplantation and mortality.
Methods
Study group
All patients in INTERMACS who received a durable CF-LVAD from May 1, 2012 to March 31, 2015 were included. The start date was chosen for consistency, to coincide with changes to INTERMACS data collection forms with more detailed, specific, and well-defined stroke data elements. For patients with multiple devices, only the events that occurred while patient had the first device were considered. Patients who had Total Artificial Hearts (TAH) or Biventricular Assist Devices (BIVAD) were excluded given small patient numbers and potentially different risk factors and pathophysiology compared to LVAD. TIA events were excluded because the diagnosis in LVAD patients is clinical and sometimes subjective, given the inability to perform MRIs to obtain the standard tissue-based diagnosis for TIA.(4)
Definitions
Ischemic stroke is defined in INTERMACS as a new acute neurologic deficit (or acute encephalopathy or seizures in children <6 months) of any duration associated with acute infarction on imaging corresponding anatomically to the clinical deficit. Acute symptomatic intracranial hemorrhage is defined as new acute neurologic deficit (or acute encephalopathy or seizures in children < 6 months) attributable to intracranial hemorrhage (ICH). Other definitions can be found on the INTERMACS website. (5)
Follow-up
Patients were followed until they died, were transplanted, explanted, or until the end of the data collection period. Patients were censored at transplant or explant.
Statistical analysis
Baseline characteristics and stroke incidence were analyzed using standard summary statistics. T-tests and chi-squared tests compared continuous and categorical variables respectively. Stroke rates were calculated as events-per-patient-year(EPPY). Kaplan-Meier curves evaluated patient survival and freedom from stroke. Univariate Cox proportional hazards models assessed the association between the risk of stroke and each of the demographic and pre-implant covariates. Any covariates statistically significant at p<0.05 were included in a multivariable Cox model. Covariates evaluated were ACE-Inhibitor/Angiotensin receptor blocker use, age group, albumin, total bilirubin, body mass index(BMI), blood urea nitrogen(BUN), Cardiac Index, chronic coagulopathy, currently smoking, HIT, gastrointestinal ulcers, history of atrial arrhythmias, limited social support, liver dysfunction, limited cognition, major stroke, other cerebrovascular disease(CVD), peripheral vascular disease(PVD), malnutrition, recent pulmonary embolus, repeated noncompliance, severe diabetes mellitus, thoracic aortic disease, total cholesterol, creatinine, c-reactive protein, diastolic blood pressure, dialysis, feeding tube, IABP, intubation, major MI, ventilator, gender, hemoglobin, heart rate, implant year, INR, LV ejection fraction, lymphocyte count, antiplatelet therapy, beta blocker use, warfarin use, NYHA class, INTERMACS profile, platelet count, previous cardiac operation, primary cardiac diagnosis(ischemic/other/unknown), sodium, systolic blood pressure(SBP), time since first cardiac diagnosis and device strategy. Competing risk analysis was used to estimate cumulative incidences for various patient outcomes.
SAS 9.4 was used for analysis.
Results
A total of 7112 patients met study criteria. The majority were male, INTERMACS profiles 2–3, and age 50–69. (Table 1) Follow-up ranged from 0.02 to 34.96 months (median 9.79 months).
Table 1.
Baseline Characteristics of patients enrolled (n=7,112)
| Age by group n(%) | |
| 19–29 | 267 (3.75%) |
| 30–39 | 518 (7.28%) |
| 40–49 | 971 (13.65%) |
| 50–59 | 1904 (26.77%) |
| 60–69 | 2389 (33.59%) |
| 70–79 | 1009 (14.19%) |
| 80+ | 54 (0.76%) |
|
| |
| Female sex | 1533 (21.56%) |
|
| |
| Device strategy n(%) | |
| Bridge to transplant | 1793 (25.2%) |
| Destination therapy | 3204 (45.1%) |
| Bridge to candidacy | 2077 (29.2%) |
| Other | 38 (0.53%) |
|
| |
| INTERMACS patient profiles* | |
| 1. Critical Cardiogenic Shock | 939 (13.2%) |
| 2. Progressive Decline | 2457 (34.55%) |
| 3. Stable but inotrope dependent | 2328 (32.73%) |
| 4. Resting symptoms | 1013 (14.24%) |
| 5. Exertion Intolerant | 182 (2.56%) |
| 6. Exertion limited | 49 (0.69%) |
| 7. Advanced NYHA III | 36 (0.51%) |
|
| |
| Creatinine (mg/dL) mean(SD) | 1.38 (0.66) |
|
| |
| Cholesterol (mg/dL) mean(SD) | 138.00 (203.91) |
|
| |
| Total bilirubin (mg/dL) mean(SD) | 1.37 (1.84) |
|
| |
| BMI mean(SD) | 28.63 (6.98) |
|
| |
| Atrial arrhythmias (%) | 86 (1.21%) |
|
| |
| Previous stroke (%) | 55 (0.77) |
|
| |
| Severe DM (%) | 244 (3.43%) |
|
| |
| Previous Cardiac Operation(%) | 2349 (33.03%) |
|
| |
| Median follow-up, months (range) | 9.79 (0.02–34.96) |
INTERMACS patient profiles was not reported for 108 (1.52%) of patients
The number of strokes per patient ranged from 0 to 4; 6360 (89.43%) had no strokes, 654 (9.20%) had one stroke, 82 (1.15%) had 2 strokes, 12 (0.17%) had 3 strokes, and 4 (0.06%) had 4 strokes. There were a total of 870 strokes reported in 7065.22 patient-years, yielding an estimated incidence rate of 0.123 strokes per patient-year. 447 (51.38%) of the reported strokes were ischemic and 423 (48.62%) were hemorrhagic.
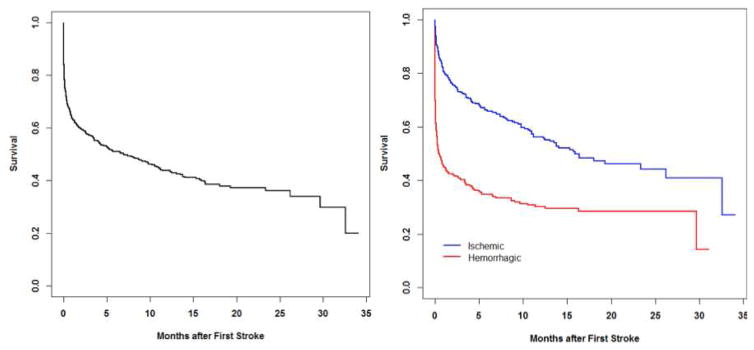
Of all subjects with a first stroke, 1-month survival was 63.6%, 6-month survival was 50.9%, and 12-month survival was 43.7%. Patients whose first stroke was a hemorrhagic stroke had significantly worse prognosis than those whose first stroke was an ischemic stroke. (P < 0.001)(Figure 1)
Figure 1.
Patient survival
Kaplan-Meier curves of patient survival following stroke: overall (left) and by stroke type (right)
Device strategies used were BTT (n=1793), bridge to candidacy (BTC)(n=2077), destination therapy (DT) (n=3204), bridge to recovery (n=17), rescue therapy (n=16), and other (n=5). Stroke free survival did not differ by device strategy (p> 0.20)(Supplementary appendix). DT patients 60 years or older did not have a higher stroke risk than younger patients (p>0.20).
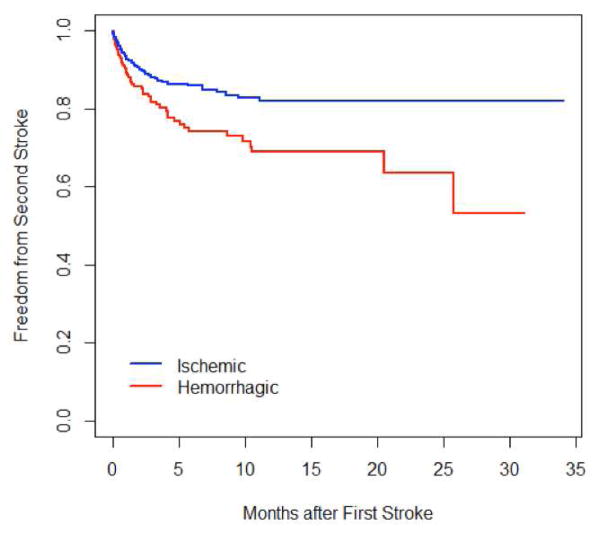
Of 752 patients who had a first stroke, 98 (13.03%) went on to have a second stroke. After a first stroke, 91.5%, 84.5%, and 77.1% of patients were free of a second stroke at 30 days, 6 months, and 1 year respectively. Patients who initially had a hemorrhagic stroke had a higher risk of a second stroke than patients who initially had an ischemic stroke (p=0.003)(Figure 2).
Figure 2.
Risk of second stroke
Kaplan-Meier curves of second of stroke after a first stroke, stratified by type of first stroke.
In univariate analysis, age group, albumin, heparin-induced thrombocytopenia (HIT), malnutrition/cachexia, severe DM, dialysis, feeding tube, IABP, gender, hemoglobin, antiplatelet therapy, warfarin use, primary cardiac diagnosis, and systolic blood pressure were statistically significant predictors of stroke. When entered in a multivariable Cox model, female gender (HR= 1.51, 95% CI:1.25–1.82, p<0.001), pre-implant systolic blood pressure (HR=1.01, 95% CI:1.00–1.01, p=0.002), HIT (HR=3.68, 95% CI:1.60–8.47, p=0.002), IABP (HR=1.21, 95% CI:1.01–1.46, p= 0.043), and primary cardiac diagnosis (p=0.040) remained statistically significant. Primary cardiac diagnosis is a three-level variable coded as ischemic/CAD, other, or unknown.
Early vs. Late strokes
Of 870 total strokes, 143 (16.4%) occurred within 2 weeks of implant and 727 (83.6%) occurred after 2 weeks. Univariate predictors of first early stroke (within 2 weeks) were HIT, intubation, ventilator, female gender, INR, previous cardiac operation, primary cardiac diagnosis, antiplatelet therapy, and history of warfarin use. When entered in a multivariable Cox model, HIT (HR=5.11, 95% CI:1.26–20.77 p=0.023), INR (HR=0.46, 95% CI: 0.22–0.96, p=0.038), previous cardiac operation (HR=0.39, 95% CI:0.26–0.58, p <0.001) remained significant. For first late stroke (after 2 weeks), univariate predictors were age group, albumin, HIT, malnutrition, severe DM, dialysis, feeding tube, IABP, gender, hemoglobin, and systolic blood pressure. When entered in a multivariable Cox model, malnutrition (HR=1.80, 95% CI:1.03–3.17, p=0.04), severe DM (HR=1.54, 95% CI:1.06–2.25, p=0.024), IABP (HR=1.23, 95% CI:1.01–1.50, p=0.043), and female gender (HR=1.52, 95% CI:1.25–1.84, p <0.0001), and systolic blood pressure (HR=1.008, 95% CI:1.002–1.013, p=0.004) were statistically significant.
Ischemic strokes
The number of ischemic strokes per patient ranged from 0 to 4; 6710 (94.35%) had no ischemic strokes, 361 (5.08%) had one ischemic stroke, 38 (0.53%) had 2 ischemic strokes, 2 (0.03%) had 3 ischemic strokes, and 1 (0.01%) had 4 ischemic strokes. Patient survival after the first ischemic stroke was 80.7%, 65.8%, and 56.2% at 1 month, 6 months, and 1 year respectively. In univariate analysis, age group, BMI, gender, INR, LVEF, previous cardiac operation, primary cardiac diagnosis, sodium, and systolic blood pressure were all statistically significant predictors of ischemic stroke. When entered in a multivariable Cox model, only female gender (HR=1.46, 95% CI:1.14–1.88, p=0.003) and previous cardiac operation (HR=0.78, 95% CI:0.61–0.99, p=0.038) remained statistically significant.
Hemorrhagic strokes
The number of hemorrhagic strokes per patient ranged from 0 to 4; 6717 (94.45%) had no ischemic strokes, 370 (5.20%) had one hemorrhagic stroke, 23 (0.32%) had 2 hemorrhagic strokes, 1 (0.01%) had 3 hemorrhagic strokes, and 1 (0.01%) had 4 hemorrhagic strokes. Patient survival after the first hemorrhagic stroke was 45.3%, 34.8%, and 30.3% at 1 month, 6 months, and 1 year respectively. In univariate analysis, albumin, HIT, IABP, gender, hemoglobin, use of beta-blockers, and primary cardiac diagnosis were all statistically significant predictors of hemorrhagic stroke. When entered in a multivariable Cox model, HIT (HR:5.04, 95% CI:1.85–13.70, p=0.002), IABP (HR:1.38, 95% CI:1.08–1.77, p=0.011), female gender (HR: 1.65, 95% CI: 1.28–2.11, p<0.001), and primary cardiac diagnosis (p=0.004) remained significant.
Stroke and transplantation
Of 1793 BTT patients, 167 (9.31%) had at least one stroke. Of these patients with a first stroke, 65 (38.92%) died before receiving a transplant or having a second stroke, 23 (13.77%) received transplants before having a second stroke, 61 (36.53%) were alive and second stroke-free at last follow-up, and 18 (10.78%) had at least one more stroke. Three patients with a second stroke were later transplanted.
Of 1,626 BTT patients who did not have a stroke, 542 (33.33%) were transplanted, 145 (8.92%) died before receiving a transplant, and 939 (57.75%) were alive and stroke-free at last follow-up.
Overall, the rate of transplant was significantly lower for patients who had a stroke (15.57%) than for those who did not have a stroke (33.33%).(p < 0.001).
The cumulative incidence functions for three competing outcomes (death, first stroke, and transplant) for BTT patients are displayed in Figure 3. For this analysis, the first stroke was treated as a terminating event; that is, any events occurring after the first stroke were ignored. At the end of the follow-up period, the estimated cumulative incidence of death is 18.2% (95% CI:7.7%–32.3%); the estimated cumulative incidence of stroke is 13.0% (95% CI:10.7%–15.6%); the estimated cumulative incidence of transplant is 49.7% (95% CI:45.4%–53.9%).
Figure 3.
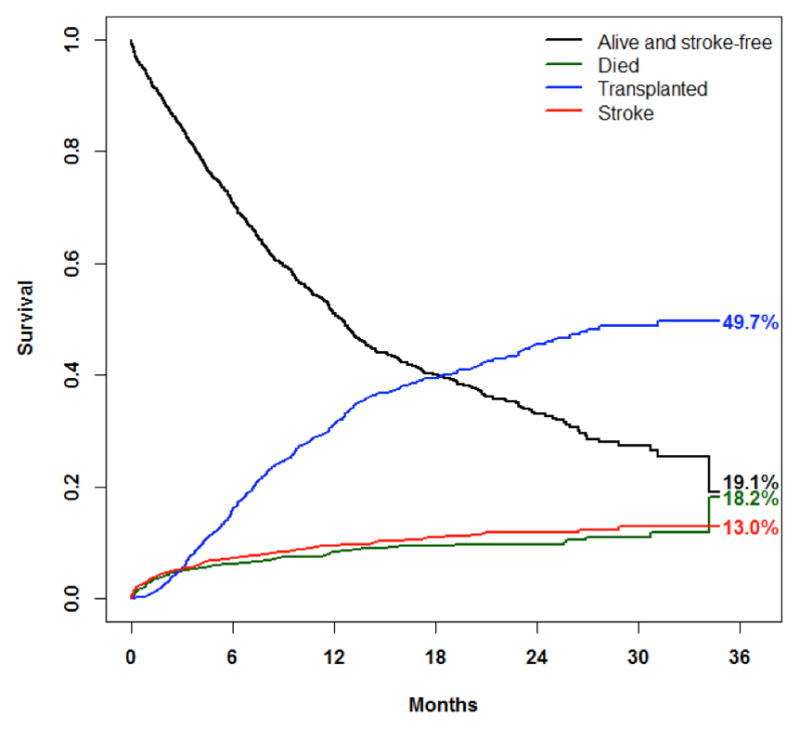
Competing outcomes
Competing risks for bridge-to-transplant patients.
Competing Risk Analysis
The cumulative incidence functions for four competing outcomes (death, transplant, ischemic stroke, hemorrhagic stroke) for the overall cohort are displayed in Figure 4. At the end of the follow-up period, the estimated cumulative incidence of death is 23.8% (95% CI:19.1%–28.9%); the estimated cumulative incidence of transplantation is 28.6% (95% CI:26.5%–30.7%); the estimated cumulative incidence of ischemic stroke is 7.2% (95% CI:6.4%–8.1%); the estimated cumulative incidence of hemorrhagic stroke is 8.1% (95% CI:6.9%–9.4%).
Figure 4.
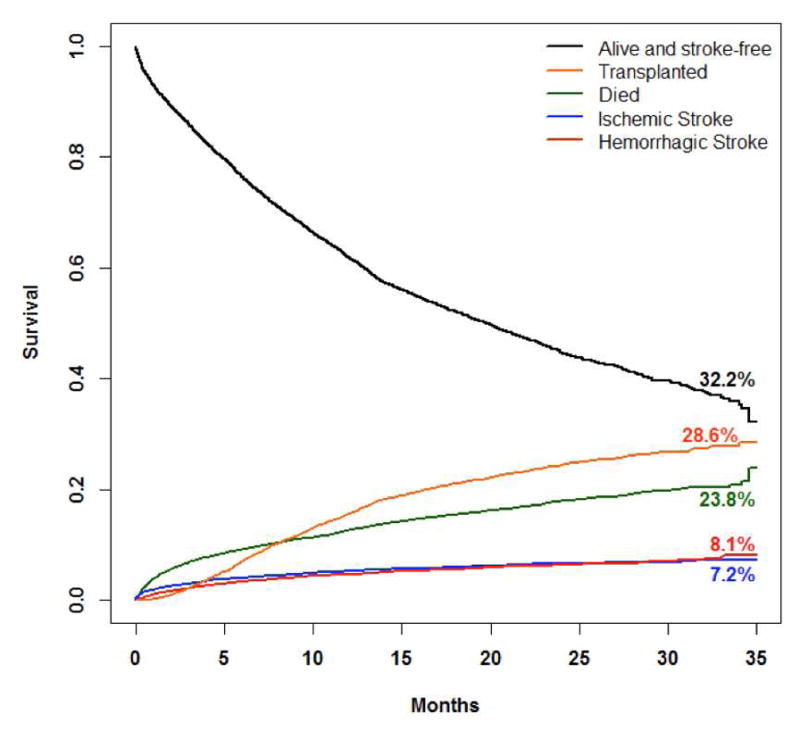
Competing outcomes
Competing risks for the overall cohort
Stroke and Gender
Women comprised 21.56% of study population (1533/7112). Women had 251 strokes in 1,482.22 patient-years, with an estimated incidence rate of 0.169 strokes per patient-year. Men had 619 strokes reported in 5,582.67 patient-years, with an estimated incidence rate of 0.111 per patient-year. The stroke incidence rate was significantly higher for women than for men (IRR=1.53, 95% CI:1.32–1.77, p<0.001). Of reported strokes in women, 116 (46.22%) were ischemic and 135 (53.78%) were hemorrhagic. Of reported strokes in men, 331 (53.47%) were ischemic and 288 (46.53%) were hemorrhagic. Men and women did not differ in their distribution of ischemic and hemorrhagic strokes (p=0.062).
Prior Stroke
Fifty-five patients (0.77%) had a history of stroke prior to LVAD placement, 6901 (97.03%) had no history of major stroke, and stroke history was unspecified for 156 (2.19%). Compared to patients without a prior stroke, patients with a history of stroke did not have a different risk of stroke on LVAD (p=0.169).
Risk Score
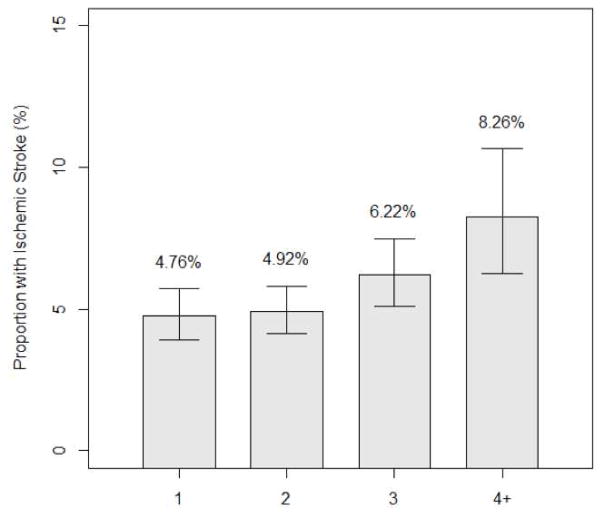
An ischemic stroke risk score in LVAD recipients was derived based on pre-implant data. Variables were initially based on those CHA2DS2VASC sore, but thresholds and some criteria had to be modified to match specific data elements and formats as collected by INTERMACS (Table 2). The score was significantly associated with risk of ischemic stroke (p<0.001). The risk of ischemic stroke increases by an estimated 19% for each 1-point increase in the score. (95% CI for increase:8%–30%) (Figure 5, Supplementary appendix.).
Table 2.
Variables and points for the ischemic stroke risk score
| Variable | Point |
|---|---|
| CHF | 1 point |
| Sex | Female=1 point |
| Male=0 points | |
| Age | >70 years =2 points |
| 60–70=1 point | |
| <60=0 point | |
| Vascular | disease 1 point if any history of peripheral vascular disease, myocardial infarction, thoracic aortic disease, or other cerebrovascular disease. (If multiple factors, 1point) |
| Major stroke | 2 points |
| Severe diabetes | 1 point |
| Pre-implant systolic BP >120 | 1 point |
Figure 5.
Risk score
Risk of ischemic stroke according to number of risk factors.
Postoperative factors
We evaluated the influence of infection, gastrointestinal bleed, and pump thrombosis individually on stroke. For each adverse event, only events that occurred before the first stroke for patients with strokes, or before censoring (at transplant, explant, or pump exchange) for patients without stroke were considered, and the adverse events were treated as time-varying covariates. Infection significantly increased risk of stroke (HR=1.94, 95% CI:1.65–2.28, p < 0.001). Gastrointestinal bleed also significantly increased risk of stroke (HR=1.40, 95% CI: 1.15–1.71, p=0.001). Confirmed pump thrombosis did not significantly increase risk of stroke (p=0.164).
Discussion
Despite tremendous advances in LVAD technology, stroke rates and morbidity remain high. Stroke incidence was not statistically different in Heartmate II compared to Heartmate XVE in DT patients.(6) In the Heartmate II BTT and CAP, stroke rate was 0.14 EPPY whereas in the Heartmate II DT trial stroke rate was 0.13 EPPY.(7) Patients in the Heartmate II DT CAP and the Heartmate II BTT post-approval study had stroke rate of 0.08 EPPY.(8,9) With Heartware, stroke rates were 0.18 EPPY in the BTT and CAP and 0.27 EPPY in the DT population.(10) Prior studies have identified patient and device-specific stroke risk factors, including female gender, infection, atrial fibrillation, hypertension, anticoagulant levels, duration of LVAD support, and LVAD type.(1,3,11,12) However, given the small number of events, these studies had limited power to test a large number of contributing factors in a multivariable analysis.
We found almost equivalent rates of ischemic and hemorrhagic strokes in CF-LVAD patients, similar to the Heartware ADVANCE and Heartmate II BTT trials.(6,13) Hemorrhagic stroke rates were much higher than the 0.3–0.6% annual rate in other populations on warfarin. (14) Strict blood pressure management and anticoagulation monitoring have been shown to decrease the risk of hemorrhagic stroke in LVAD patients (3); data on the additional influence of speed modulation, induction of intermittent pulsatility, antiplatelet therapy adjustment or elimination, and the role of intensive blood pressure lowering in the acute setting of ICH is lacking. Cerebral amyloid angiopathy may predispose older LVAD patients to stroke, but autopsy/histopathologic studies in CF-LVAD patients are not available. AV malformations have gained significant attention in LVAD-associated GI bleeds, but their role in ICH has not been defined. These large knowledge gaps warrant further investigation to establish an evidence-based framework for prevention and management of this major LVAD complication.
Approximately 45% of LVAD patients with ischemic strokes and over 70% of patients with hemorrhagic strokes did not remain alive on device support at 1 year. These rates are higher than those of non-LVAD patients with strokes(15), likely related to multiple factors, including the persistent risk of stroke related to the device itself, unintended fluctuations in anticoagulation, the requirement after a hemorrhagic stroke to alter anticoagulation in a manner suboptimal for the LVAD, the association of stroke with sepsis, limited enthusiasm for neurosurgical interventions in LVAD patients, and comorbidities. LVAD patients with ischemic stroke may not be candidates for thrombolysis because of ongoing anticoagulation and a higher risk of hemorrhagic transformation with large cardioembolic strokes. The AHA/ASA guidelines now provide a Class IIa recommendation for endovascular therapy with stent retrievers for selected patients with anterior circulation occlusion who have contraindications to systemic rtPA.(16) Case reports in LVAD patients have shown favorable outcomes, and systematic evaluation of endovascular therapy for acute ischemic strokes in LVAD patients is warranted.(17)
Several investigators have demonstrated gender differences in CVA with CF LVADs, particularly with ICH. (12,18,19) In this report, female gender was an independent predictor of both ischemic and hemorrhagic strokes. In the non-LVAD population, premenopausal women have lower rates of stroke than men of comparable age, whereas postmenopausal women have increasing rates of stroke compared with men of similar age. (20) Endogenous estrogen is hypothesized to be the important biological factor that explains these differences. However, in contrast to the protective effect of endogenous estrogen, the use of oral contraceptives (OCs) modestly (1.4–2 times) increases the risk of stroke, and OC users who are smokers, obese, and hypertensive have higher risk of ischemic stroke.(21) Endothelial dysfunction as defined by lower ADAMTS13 and high Von Willebrand Factor increases the likelihood of ischemic stroke, and OCs further increase this risk. (22) Specific single nucleotide polymorphisms can increase susceptibility to hemorrhagic strokes in association with OCs.(23) In post-menopausal women, aggregate data suggests that hormone replacement may slightly increase the risk of ischemic but not hemorrhagic stroke. (21) Sex hormone-independent mechanisms are also implicated, and there are differences in X-chromosome gene expression in men compared with women with ischemic stroke.(24) In a study of 51 non-LVAD patients with ischemic stroke, there were differences in gene expression of pathways that modulated inflammation, immunity, and cell death between men and women. (25) These factors have not been specifically examined in the LVAD population, but could modulate gender differences in stroke incidence and outcomes.
We did not find a history stroke pre-LVAD to be a risk factor for stroke on LVAD. However, caution must be advised in interpretation, since patients with prior strokes who were considered for LVAD were likely highly selected, with little residual deficits and favorable neurologic prognosis. Nevertheless, the data suggests that prior stroke by itself should not be an absolute contraindication to LVAD placement.
Previous studies have reported conflicting results on the influence of preoperative atrial fibrillation on thromboembolic events after LVAD. (11,26) We did not find a history of atrial arrhythmias to be a predictor for stroke. Therefore, data don’t support routine intensification of anticoagulation or adjunctive procedures such as left atrial appendage ligation or Maze procedure during LVAD implant in patients with atrial fibrillation.
This study provides some predictive capability for strokes, and can guide individual patient management. For example, a woman who is on IABP prior to LVAD has 2.3 times the risk of stroke as someone without these risk factors. Similarly, a 70-year old diabetic woman with prior stroke has a substantially higher ischemic stroke risk on device than a young man. Therefore, transplant strategies should therefore be carefully thought out prior to LVAD implantation, and if destination therapy is the anticipated strategy, the higher risk of stroke should be part of the informed consent.
The majority of identified preoperative risk factors are not modifiable. Pre-implant BP was a risk factor, but may not be modifiable by the time patients present for LVAD. Similarly, IABP use may reflect patient clinical acuity rather than a direct effect on stroke. However, those risk factors that are modifiable, such as malnutrition for late strokes, may present appropriate targets for interventions and further investigation.
It is important to note that traditional stroke risk factors such as diabetes, hyperlipidemia, age, and smoking were not independently predictive of stroke in multivariable analysis although several of the risk factors (age, sex, diabetes, stroke, vascular disease) were predictive of ischemic stroke in the composite score. Incremental increases in pre-implant systolic blood pressure had a small effect size. DT patients did not have a higher risk of stroke than BTT patients, and stroke rates were similar in older and younger DT patients. These findings, as well as differences in strokes between devices seen in other studies, support the notion that a significant proportion of the stroke risk on LVAD is related to the pump itself rather than the patient-related factors, and advancements in pump technology may have as much influence as the patients the pumps are implanted into. Postoperative LVAD complications such as infections and bleeds may lead to alteration in coagulation and increase subsequent risk of stroke. Improved understanding of other LVAD complications and development of strategies to prevent and better manage them may also have a significant impact on stroke.
Other factors not routinely measured may be relevant. Sleep apnea, common after heart failure, is a known risk factor for stroke. The few case reports of sleep apnea after LVAD reported varied results, including persistence, resolution, and interference by central sleep apnea on LVAD filling.(27,28). Sleep apnea can lead to nighttime spikes in blood pressure, which may go undetected in routine LVAD clinic visits yet predispose to hemorrhagic stroke.
As expected, stroke on LVAD was a major impediment to transplantation. Nevertheless, selected BTT candidates who have a stroke on LVAD could still be transplanted. The 13% recurrent stroke rate and anticoagulation challenges make it imperative to aggressively rehabilitate and relist transplantable patients before another potentially disabling and disqualifying event.
This study had several limitations. INTERMACS policy does not allow breakdown of pumps by manufacturers, so we could not evaluate differences in stroke risk factors and outcomes by device brand. Second, only data collected per registry protocol is available. Stroke diagnosis was not centrally adjudicated and management was not standardized. Data collection forms for the study period did not have a specific designation for hemorrhagic transformation of ischemic strokes, and some strokes listed as hemorrhagic may have initially been ischemic. Finally, evaluation of preoperative risk factors alone provides an incomplete picture of risk factors for stroke. However, the intent of this project was to assess predictive factors that could influence management strategies early in the process, including decisions regarding LVAD implantation, perioperative management and transplant listing. A complete analysis using preoperative and postoperative variables was not possible because institutional differences in surgical approach, anticoagulation, hypertension management, events immediately preceding the strokes on LVAD, and INR and BP at the time of stroke or in the last clinic visit prior to the stroke were not captured in INTERMACS. A conditional analysis using 1 month post-implant as time 0 and incorporating preoperative and 1-month post-implant variables is in the supplementary appendix.
In summary, despite improvements in LVAD technology, stroke-related morbidity and mortality is substantial. Preoperative factors associated with stroke risk should be managed or modified as possible. Further investigation is necessary to decrease the risk of this sudden, often catastrophic, and frequently disabling complication.
Supplementary Material
Clinical Perspectives.
Patients with multiple patient-related stroke risk factors may benefit from individualized strategies, such as more aggressive blood pressure and coagulation management, and early listing for transplantation.
Translational Outlook.
Improved understanding of genetic and molecular polymorphisms in coagulability as well as improved LVAD biocompatibility may lead to decreased LVAD stroke rates.
Acknowledgments
Funding: Division of Cardiovascular Diseases, UAB; NIH #HHSN268201100025C
Abbreviations
- BP
Blood pressure
- BTT
Bridge-to-transplant
- CF-LVAD
Continuous flow left ventricular assist device
- DT
Destination therapy
- INR
International Normalized Ratio
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LVAD
Left ventricular assist device
- NYHA
New York Heart Association
- TIA
Transient ischemic attack
- MI
Myocardial infarction
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stulak JM, Davis ME, Haglund N, et al. Adverse events in contemporary continuous-flow left ventricular assist devices:A multi-institutional comparison shows significant differences. J Thorac Cardiovasc Surg. 2016;151:177–89. doi: 10.1016/j.jtcvs.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Failure. 2015;21:119–25. doi: 10.1016/j.cardfail.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Teuteberg JJ, Slaughter MS, Rogers JG, et al. The HVAD Left Ventricular Assist Device:Risk Factors for Neurological Events and Risk Mitigation Strategies. JACC Heart failure. 2015;3:818–28. doi: 10.1016/j.jchf.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack. Stroke. 2009;40:2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 5.INTERMACS. INTERMACS User's guide. 2014 http://www.uab.edu/medicine/intermacs/appendices/app-a-5-0.
- 6.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Eng J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 7.Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–21. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart failure. 2012;5:241–8. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 9.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U. S Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS. J Am Coll Cardiol. 2011;57:1890–8. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter MS, Pagani FD, McGee EC, et al. Heart Ware ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–83. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thoracic Surg. 2013;96:2161–7. doi: 10.1016/j.athoracsur.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Bogaev RC, Pamboukian SV, Moore SA, et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–22. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy:recent data and ideas. Stroke. 2005;36:1588–93. doi: 10.1161/01.STR.0000170642.39876.f2. [DOI] [PubMed] [Google Scholar]
- 15.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurologic clinics. 2008;26:871–95. vii. doi: 10.1016/j.ncl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Derdeyn CP, Biller J, et al. 2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke. 2015;46:3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mufti F, Bauerschmidt A, Claassen J, Meyers PM, Colombo PC, Willey JZ. Neuroendovascular Interventions for Acute Ischemic Strokes in Patients Supported with Left Ventricular Assist Devices:A Single-Center Case Series and Review of the Literature. World neurosurgery. 2015 doi: 10.1016/j.wneu.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 18.Morris AA, Pekarek A, Wittersheim K, et al. Gender differences in the risk of stroke during support with continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015;34:1570–7. doi: 10.1016/j.healun.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Boyle AJ, Jorde UP, Sun B, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support:an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–8. doi: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 20.Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32:2100–7. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the AHA/ASA. Stroke. 2014;45:1545–88. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson HM, Siegerink B, Luken BM, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–60. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Li Y, Li H, et al. Increased risk of stroke in oral contraceptive users carried replicated genetic variants: a population-based case-control study in China. Human genetics. 2012;131:1337–44. doi: 10.1007/s00439-012-1161-7. [DOI] [PubMed] [Google Scholar]
- 24.Stamova B, Tian Y, Jickling G, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43:326–34. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Stamova B, Jickling GC, et al. Effects of gender on gene expression in the blood of ischemic stroke patients. J Cereb Blood Flow Metab. 2012;32:780–91. doi: 10.1038/jcbfm.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xuereb L, Go PH, Kaur B, et al. Impact of Preoperative Atrial Fibrillation on Postoperative Thromboembolic Events After Left Ventricular Assist Device Implantation. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Vermes E, Fonkoua H, Kirsch M, et al. Resolution of sleep-disordered breathing with a biventricular assist device and recurrence after heart transplantation. J Clin Sleep Med. 2009;5:248–50. [PMC free article] [PubMed] [Google Scholar]
- 28.Schaffer SA, Bercovitch RS, Ross HJ, Rao V. Central sleep apnea interfering with adequate left ventricular filling in a patient with left ventricular assist device. J Clin Sleep Med. 2013;9:161–2. doi: 10.5664/jcsm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.