Abstract
Neuropathic pain is caused by a primary lesion or dysfunction of the nervous system and can occur in the cornea. However, neuropathic corneal pain (NCP) is currently an ill-defined disease. Patients with NCP are extremely challenging to manage and evidence-based clinical recommendations for the management of patients with NCP are scarce. The objectives of this review are to provide guidelines for diagnosis and treatment of patients with NCP and to summarize current evidence-based literature in this area. We performed a systematic literature search of all relevant publications between 1966 and 2017. Treatment recommendations are, in part, based on methodologically sound randomized controlled trials (RCTs), demonstrating superiority to placebo or relevant control treatments, and on the consistency of evidence, degree of efficacy, and safety. In addition, the recommendations include our own extensive experience in the management of these patients over the past decade. A comprehensive algorithm, based on clinical evaluation and complementary tests, is presented for diagnosis and subcategorization of patients with NCP. Recommended first-line topical treatments include neuro-regenerative and anti-inflammatory agents, whilst first-line systemic pharmacotherapy includes tricyclic antidepressants and the anticonvulsant. Second line oral treatments recommended include the opioid-antagonist and opiate analgesics. Complementary and alternative treatments, such as cardio-exercise, acupuncture, omega-3 fatty acid, and gluten-free diet, may have additional benefits, as do potential non-invasive and invasive procedures in recalcitrant cases. Medication selection should be tailored on an individual basis, considering side effects, comorbidities, and levels of peripheral and centralized pain. Nevertheless, there is an urgent need for long-term studies and RCTs assessing the efficacy of treatments for NCP.
Keywords: neuropathic pain, autologous serum tears, corneal pain, dry eye, in vivo confocal microscopy
INTRODUCTION
The International Association for the Study of Pain defines neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction of the nervous system”.1 The diagnosis of neuropathic pain requires confirmation of injury or disease affecting somatosensory pathways of peripheral and/or central nervous systems (CNS).2 Neuropathic pain can also occur in the cornea,3–7 the most richly innervated tissue in the body.8 Neuropathic corneal pain (NCP) remains an ill-defined entity (also termed corneal neuralgia, keratoneuralgia, corneal allodynia or corneal neuropathy),3–7 and can be perceived as pain,9 discomfort,10 aching,11 photoallodynia,6 burning,10 irritation10, dryness,11 and grittiness;11 symptoms that may overlap with diseases such as dry eye disease (DED).12 NCP can result from both peripheral nerve injury5–7, 9 or systemic etiologies,4,10, 9,13 and owes much of its understanding to advances in the pathophysiology and neurobiology of systemic neuropathic pain. While recent articles have attempted to elucidate the pathophysiology behind NCP, very limited literature exists on the management of NCP.6,13 This review article provides an evidence-based approach on management strategies for NCP, and further reflects our own extensive clinical experience on a large cohort of NCP patients. We have been treating NCP patients since late 2008. Our recent medical records review has shown that we are currently treating around 100 new patients NCP per year. We estimate that we have treated well over 700 patients over the past 9 years.
METHODS
We performed a systematic search of all relevant publications between 1966–2017, from Medline (National Library of Medicine), PubMed, PubMed Central, Embase, OVID and Cochrane Database. Search terms included: “pain, neuropathic pain, somatosensory pain, central pain, peripheral pain, dry eye, ocular discomfort, contact lens discomfort, ocular surface disease, corneal pain, confocal microscopy, trigeminal neuralgia and post-herpetic neuralgia”. We considered all systematic reviews, meta-analyses and randomized controlled trials (RCTs), retrospective studies, case series and case reports. Studies were evaluated according to the Oxford Centre for Evidence-based Medicine levels of evidence.14
PATHOPHYSIOLOGY OF NEUROPATHIC CORNEAL PAIN
The sensory nervous system consists of sensory neurons, neural pathways and the sensory cortex. Nociceptors are receptors necessary for pain perception,15 and may generate action potentials to thermal, mechanical, chemical or polymodal (more than one) stimuli.16 They are connected centrally to higher-order somatosensory pain pathways and the thalamus, where pain is perceived. During homeostasis, sensory neurons detect various stimuli to generate physiological pain responses, protecting tissues from acute injuries.17 However, tissue damage and inflammation of the ocular surface result in peripheral axonal injuries and the release of pro-inflammatory mediators,18,19 potentially resulting in increased sensitivity of peripheral nerves, thus intensifying peripheral pain signaling (peripheral sensitization). Over time this can result in central sensitization, with central neurons becoming highly responsive to similar magnitudes of pain and heightened pain awareness.20 The hallmark of central sensitization is pain that is disconnected from ongoing peripheral signs. Sensitization may results in allodynia7, photoallodynia (pain due to non-noxious stimuli or light),6 or hyperalgesia11 (enhanced pain response to infra-threshold noxious stimuli), causing unpleasant sensations. NCP may have a peripheral origin (e.g, ocular surgery9,21 or herpes zoster ophthalmicus22) or a systemic origin (e.g, small-fiber polyneuropathy or fibromyalgia).4,13 Additional underlying causes include DED, infectious keratitis, recurrent erosions, radiation keratopathy, contact lens wear, and many others as summarized in Table 1. Important co-morbid conditions include, anxiety,23,24 depression,23–25 and post-traumatic stress disorders.23,24
Table 1.
Etiology of Neuropathic Corneal Pain
|
MANAGEMENT OF PATIENTS WITH NEUROPATHIC CORNEAL PAIN
Diagnosing Neuropathic Corneal Pain
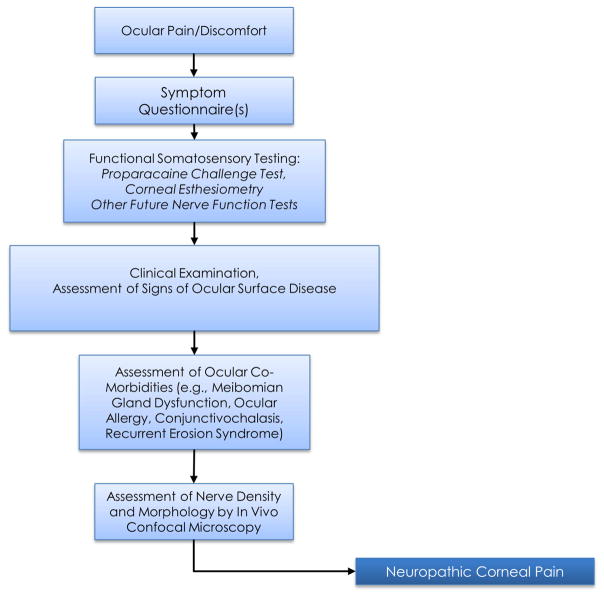
Diagnosing NCP has been challenging for vision care providers, partly due to the lack of understanding of this disease, as well as due to minimal or absent clinical signs, thus masking the underlying condition.5,6,11 NCP is typically diagnosed based on clinical history, symptoms, ophthalmological examination, and evidence of nerve injury (by in vivo confocal microscopy [IVCM]) and/or nerve dysfunction (nerve function tests) (Fig. 1). Patients typically complain of prolonged dry eye treatment, multiple treatment failures, note an inciting event (e.g., infection or surgery), and may complain about non-ocular pain, neurological, or psychiatric conditions upon questioning.
Figure 1. Steps for the Assessment of Patients with Neuropathic Corneal Pain.
Flow diagram illustrating initial approach to clinical assessment and diagnosis for patients with neuropathic corneal pain.
Ocular Pain Questionnaires to Assess Symptoms
Validated pain questionnaires enable clinicians to evaluate patients’ symptoms and quality of life (QofL) changes. However, most validated questionnaires to date were designed to address DED symptoms, including Ocular Surface Disease Index (OSDI),26 McMonnies Dry eye Questionnaire,27 Standardized Patient Evaluation for Eye Dryness (SPEED),28 National Eye Institute Vision Function Questionnaire (NEI-VFQ),29,30 and Symptom Assessment in Dry Eye (SANDE).31,32 In contrast, the recently validated Ocular Pain Assessment Survey (OPAS)33 is a quantitative, multidimensional questionnaire, specifically designed for assessment of corneal and ocular surface pain and QofL changes. The OPAS assesses pain intensity, frequency of eye and non-eye pain, QofL changes, aggravating factors, associated factors, and symptomatic relief quantitative, allowing for monitoring of treatment responses.33 Other questionnaires, including the Neuropathic Pain Symptom Questionnaire (NPSI) modified for the eye have been used, although they have not been formally validated yet for the eye.34
Functional Somatosensory Testing
The Proparacaine Challenge Test
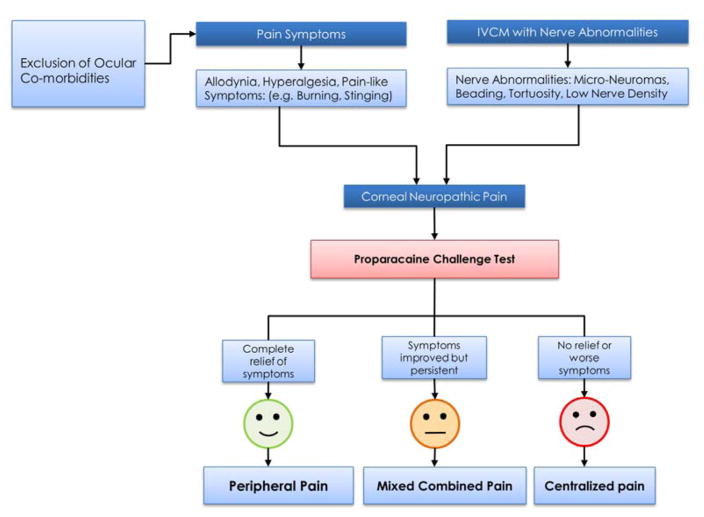
Establishing the origin of pain, whether central or peripheral is important for selecting appropriate treatment measures. Topical 0.5% proparacaine hydrochloride (Alcaine, Alcon, Fort Worth, TX) allows for differentiation of central from peripheral sources of pain.13 While proparacaine abolishes peripheral pain, it has no effect on pain from central sensitization. Patients experiencing complete or partial relief with proparacaine challenge, likely suffer from peripheral or mixed combined NCP. In contrast, patients not responding to proparacaine, suffer at least in part from central NCP (Fig. 2). It has been our clinical experience that many patients only achieve partial relief to topical proparacaine, suggesting that both peripheral and central sensitization are at play, albeit in different proportions depending on etiology and disease duration. Additional measures, including bandage contact lenses (CL) and moisture goggles may decrease evaporation-induced symptoms. It is important to highlight that this test cannot distinguish between patients with pathological dry eye symptoms and patients with peripheral symptoms from neuropathic origin. We believe that patients with signs of DED may also present with symptoms of NCP. However, no tests are currently available to distinguish between these entities.
Figure 2. Diagnosis of Neuropathic Corneal Pain.
Flow diagram illustrating clinical and diagnostic approach for differentiating peripheral and centralized neuropathic corneal pain.
Corneal Esthesiometry
Direct somatosensory measurement using esthesiometers, such as the Cochet-Bonnet contact esthesiometer, allows for evaluation of mechanical nociceptor responses,35 thus quantifying Aδ fibers function. Further, non-contact esthesiometry, such as with the Belmonte esthesiometer allows detection of polymodal function for both Aδ and C fibers.36 The new definition of dye eye disease includes the presence of somatosensory dysfunction.37 A recent study has demonstrated that patients with severe dry eye or neuropathic pain symptoms present with increased corneal sensitivity,34 while another study has shown decreased corneal sensitivity in patients with dry eye disease.38 Thus, measurement of corneal sensitivity could provide the first evidence for somatosensory abnormalities.
Clinical Examination
Ocular surface slit-lamp examination with vital dyes, such as fluorescein, lissamine green and rose Bengal, allow for the assessment of corneal and conjunctiva epithelial integrity and tear film stability. Other tests such as Schirmer’s and phenol red thread aid in evaluation of tear film volume. Further, tear osmolarity testing may be a useful screening method to diagnose tear film abnormalities.39 Monitoring patients with osmolarity remains controversial. While some studies demonstrate strong utility for longitudinal monitoring of patients through measurement of osmolarity,37 other studies have presented no correlation to changes in clinical signs and symptoms..40 Patients with NCP often present with symptoms out of proportion to signs,41 and may demonstrate minimal signs on slit-lamp examination.42 Ocular co-morbidities, as outlined below, need to be identified and treated.
In Vivo Confocal Microscopy to Confirm Corneal Nerve Damage
The cornea is innervated by branches of the nasociliary nerve, a branch of the ophthalmic division of the trigeminal nerve.43 Nerve bundles enter the peripheral cornea (limbus) in a radial fashion, migrate anteriorly, penetrate the Bowman’s layer, and form the subbasal plexus. However, given that corneal nerves cannot be quantitatively assessed by slit-lamp examination and that corneal biopsies cannot be readily performed, the objective assessment of the ocular surface neurobiology has remained challenging for clinicians. Laser IVCM (HRT3/RCM, Heidelberg Engineering, Heidelberg, Germany) is a non-invasive, high-resolution device that allows real-time visualization of corneal structures at the cellular level, providing optical biopsies at quasi-histological levels.8 IVCM studies in DED patients have demonstrated decreased nerve density, increased tortuosity, reflectivity and beading.44 Recently, a RCT demonstrated that patients with near-normal corneal nerve density showed improvement in both symptoms and signs of DED after therapy, while patients with low corneal nerve density showed no changes, providing a rationale for the notorious variability of responses observed with therapies.45 These findings could further be explained by potential neuropathic symptoms in patients with low nerve density. More recent studies in patients with NCP have demonstrated decreased corneal nerve density associated with allodynia,7 photoallodynia,6 and post-LASIK neuralgia.6,9 The presence of microneuromas by IVCM in these patients (Fig. 4) reflect sudden swelling of injured nerves at their terminal endings and have been shown to be specific for NCP,46 and thus potentially diagnostic. This parameter could particularly be helpful in patients with NCP who also present with signs of DED. Thus, in lieu of tissue biopsies, as performed in the skin, IVCM could allow for a definitive confirmation of nerve damage, allowing for diagnostic confirmation of NCP. In cases with systemic symptoms, referral to neurologists is suggested for further systemic somatosensory testing and skin biopsies.
Figure 4.
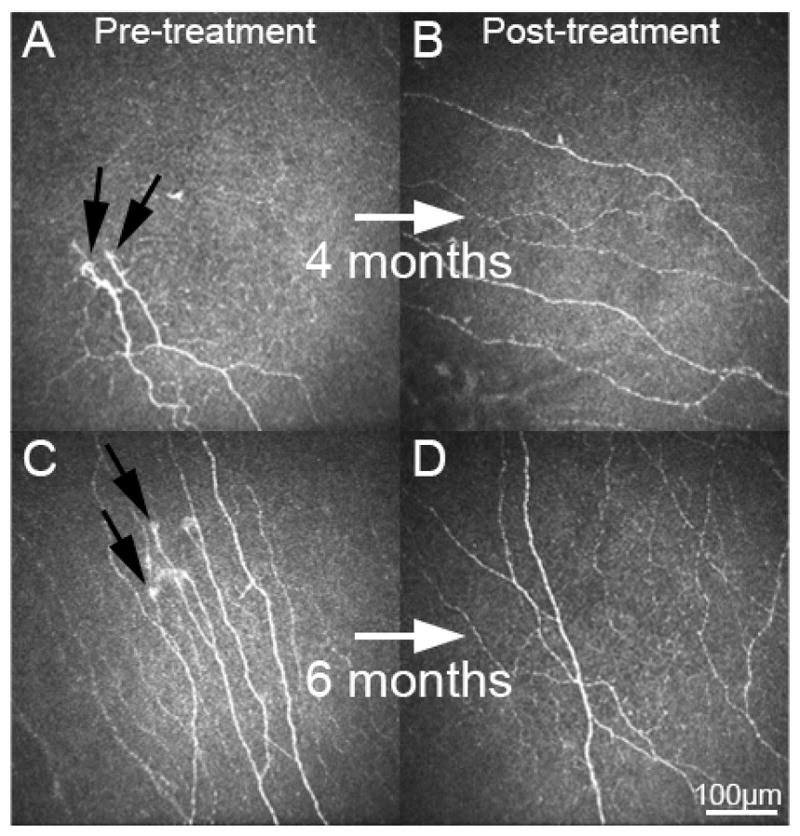
Corneal laser in vivo confocal microscopy images of patients with neuropathic corneal pain. Images pre-treatment (A and C) demonstrate presence of micro-neuromas (black arrows), decreased nerve density and increased tortuosity. Following 4 and 6 months of treatment with autologous serum tears 8x/day and low dose anti-inflammatory therapy, subbasal corneal nerve density is increased and micro-neuromas are not present (B and D). Size bar =100 μm.
Therapeutic Strategies for Patients with Neuropathic Corneal Pain
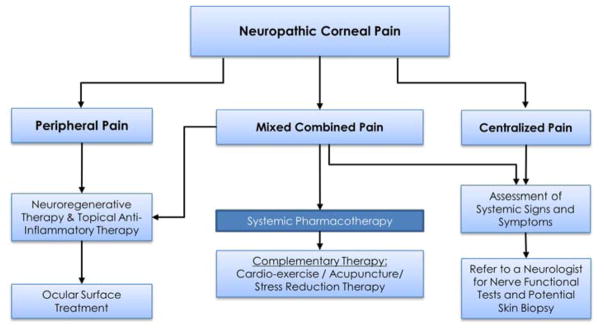
Devising management strategies for NCP patients requires differentiation of peripheral, mixed, and centralized sources of pain (Fig. 2 and Fig. 3). Many of the proposed therapies discussed for NCP are derived from evidence-based literature for systemic neuropathic pain and ocular post-herpetic neuralgia. In addition, we have been treating NCP patients since late 2008. Our recent medical records review has shown that we are currently treating around 100 new patients NCP per year. We estimate that we have treated well over 700 patients over the past 9 years.
Figure 3. Proposed Treatement Paradigm of Patients with Neuropathic Corneal Pain.
Proposed treatment strategy for neuropathic corneal pain.
Neuro-Regenerative Therapy
Peripheral sensitization in NCP is initiated by corneal nerve injury and subsequent inflammation. Recently, therapeutic strategies targeting neuronal regeneration have been shown to alleviate patient symptoms with autologous serum tears (AST).6 The rationale for this approach was based on previous reports from preclinical non-ocular neuropathic pain models on the use of neurotrophic factors, in particular nerve growth factor (NGF).47,48 NGF reduced allodynia and hyperalgesia through reduction of reactive astrocytosis and glial modulation. Recovery of corneal nerve topography has been demonstrated with the use of AST in patients with NCP,49 photoallodynia,6 DED,50 as well as with autologous plasma in patients with neurotrophic keratopathy.51 Traditional and more widespread therapeutic approaches, like tear substitutes, fail to show any trophic results and are unable to restore physiological innervation.45 Thus, neuro-regenerative approaches to treat NCP, such as with AST, plasma-rich in growth factors, platelet-rich plasma, autologous plasma, and more recently NGF, are promising.52–54
Autologous Serum Tears (AST)
AST contain neurotrophic factors, including NGF and insulin-like growth factor −1.55,56 The use of AS with an infusion pump was first described in 1975 for severe DED and chemical burns.57 Fox et al. then described the use of AST for the treatment of severe DED.58 The detailed protocol and guidelines for the preparation of AST, allowing its widespread use was presented by Tsubota et al.59 Although a concentration of 20% is most commonly used, no standardization and RCTs have been performed to determine the optimal concentration, frequency, and duration of therapy.60 Numerous studies have, however, reported the efficacy of AST in DED, demonstrating improvement in ocular discomfort and corneal staining61–67 Noble et al. demonstrated in a crossover RCT significant improvement in DED symptoms with 50% AST compared to conventional therapy.68 Further, Kojima et al. showed significant improvement in signs and symptoms with AST compared to preservative-free artificial tears in a RCT.69 In yet another RCT, Urzua et al. demonstrated a 50.95% improvement in OSDI scores with 20% AST, compared to 22.19% improvement with artificial tears.70 In NCP patients, we have demonstrated significant improvement of symptoms of allodynia9, 6,49 and photoallodynia6 with 20% AST within 3.6 months of treatment. In addition to improvement in patients’ symptoms, IVCM clearly demonstrates a significant increase in corneal subbasal nerve density, as well as decrease in nerve reflectivity and tortuosity. Taken together, the ability of AST to regenerate corneal nerves has been associated with the improvement of the symptoms of NCP.6,13,49 We recommend the use of 20% AST 8x/daily until significant relief/resolution of symptoms is achieved, followed by a very slow taper in order to prevent rebound. It has been our experience that initial symptom relief is observed within 3–4 months in NCP of peripheral origin and that taper can be successfully attempted within 9–12 months. Limitations of AST are the limited availability, cost due to lack of insurance coverage, and the storage requirements.
Anti-Inflammatory Therapy
The pathophysiology of NCP includes injury to peripheral nerves (e.g. due to direct trauma, inflammation, toxicity) resulting in release of pro-inflammatory neuropeptides from both injured nerves and cytokines from surrounding healthy nerves,71,72 leading peripheral sensitization.73,74 Chronic inflammation can decrease neurite outgrowth and increased calcium influx across cell membranes, causing axonal degeneration.75,76 Thus, the critical role of inflammation in the pathophysiology of NCP provides the rationale for the use of anti-inflammatory therapy. Our clinical experience has corroborated this rationale in that concurrent use of low dose topical corticosteroids with AST resulted in significant increase in cornea nerve regeneration as compared to AST alone.77
Topical corticosteroids have been used as a mainstay of anti-inflammatory therapy due to their inhibitory mechanism of action on cytokines, prostaglandins and leukotriene synthesis, as well as the inhibition of leukocyte migration.78 Among corticosteroids, loteprednol 0.5% suspension or gel have demonstrated lower rates of increased intraocular pressure and cataract formation due to decreased intraocular penetration.79, 80 Moreover loteprednol 0.5% gel has a much lower concentration of the neurotoxic81–83 preservative benzalkonium chloride (BAK; 0.003%), as compared to other corticosteroids (0.05–0.01%).84 In several recent RCTs in DED patients, significant symptom reduction was demonstrated with loteprednol 0.5% as compared to placebo.45,85 Thus, for NCP we recommend use of loteprednol 0.5% suspension or gel with a slow taper of four times daily for two weeks, followed by twice daily for two weeks and once daily over a 6- to 12-week period depending on individual patient response. Anti-inflammatory therapy is then attempted with steroid-sparing therapies, such as topical calcineurin inhibitors cyclosporine A 0.05% two to four times daily,86 and tacrolimus 0.03% three times daily,87 the interleukin-1 receptor antagonist Anakinra (Kineret) 2.5% three times daily,88 as well as topical testosterone 0.03% three times daily.89 Further, a new class of anti-inflammatory agents has become available with Lifitegrast 5%, which was recently approved by the FDA for the treatment of signs and symptoms of dry eye disease.90 Moreover, antibiotics such as topical and oral tetracycline and azithromycin have been used successfully for anti-inflammatory therapy.91,92 Nevertheless, topical loteprednol is the author’s first-line choice as an anti-inflammatory agent.
It is important to note that in patients with severe hyperalgesia, even low BAK concentrations are not tolerated, in which case preservative-free formulations are recommended, such as compounded methylprednisolone 1%. Depending on the level of inflammation, topical anti-inflammatory therapy may result in rapid decrease of pain. However, depending on the agent and preservatives used, they can result in significant discomfort.
Ocular Surface Rehabilitation and Managing Co-Morbidities
Palliative treatment with lubrication of the ocular surface can provide additional short-term relief for patients with NCP. Particularly, in cases with concurrent DED, lubrication may result in decreased tear osmolarity and dilution of pro-inflammatory mediators. Further, after initial anti-inflammatory therapy, additional of punctal plugs results in an increased tear lake.93 However, in cases with concurrent ocular allergies, placement of plugs may increase contact time with allergens and result in subsequent inflammatory responses.94 In patients with increased tear evaporation and decreased TBUT, approaches decreasing evaporation can be of benefit. These include, emulsion-based lubricants, treating concomitant meibomian gland dysfunction (MGD) with hot compresses and lid massage, use of moisture chamber goggles, or medical therapy with topical or oral antibiotics, including doxycycline or azithromycin. By improving tear film stability and reducing inflammation, these agents may act as an adjunct therapy in NCP. In refractory cases, procedures including intraductal MG probing, thermal pulsation devices, and intense pulse light therapy may be of benefit.95–96,97 In addition, treatment of blepharitis, including recognition and treatment of demodex blepharitis may remove inciting stimuli. Moreover, addressing other co-morbidities, such as ocular allergies, conjunctivochalasis, exposure keratopathy, and essential blepharospasm are important steps in mitigating patient symptoms.
Self-Retained Cryopreserved Amniotic Membrane (CAM)
CAM has been shown to have anti-inflammatory, anti-fibrotic and neurotrophic effects on the ocular surface.98–102 CAM is generally well-tolerated, resulting in symptomatic relief and improved surface staining in patients with DED.98 The authors have had initial encouraging results with self-retained CAM (PROKERA®Slim and Clear, Bio-Tissue, Miami, FL, USA) in patients with NCP, resulting in rapid relief of symptoms.103 In cases with severe hyperalgesia, where patients may not tolerate the polycarbonate ring, we recommend removal of the ring and placement of the CAM into bandage contact lenses (BCLs). Our recent experience with 14 NCP patients that were not able tolerate the PROKERA® ring, but were treated with CAM into BCLs, has shown that 11/14 patients (78.57%) tolerated CAM/BCL well.
Protective Contact Lenses
Patients with peripheral source of pain refractory to topical therapies may benefit from temporary trials with extended wear soft bandage CLs or scleral lenses, such as the prosthetic replacement of the ocular surface ecosystem (PROSE, Boston Foundation for Sight, Needham, MA)104 for immediate symptom relief. While the exact mechanisms of symptom relief are not yet elucidated, they likely include shielding of corneal nociceptors from external environmental stimuli. Pain relief associated with corneal diseases has been reported in a recent series of 40 patients.105 Several studies have demonstrated symptom relief with therapeutic soft CLs in patients with ocular surface disease, such as ocular graft versus host disease (GVHD).106,107 Similarly, soft lenses improved OSDI scores as compared to AST in patients with Sjögren’s syndrome DED.108 However, potential risks of infections with prolonged wear make them less attractive long-term options.
Scleral lenses have been shown to result in decreased light sensitivity and discomfort in 92% of patients with ocular surface disease.109 Further, improved OSDI scores have been shown in several studies in patients with GVHD.110 Similarly, patients reported decreased pain with PROSE.111 A more recent study, however, indicated that long-term PROSE wear did not result in increased corneal nerve density by IVCM.112 Nevertheless, while some patients with NCP may experience immediate pain relief, using CLs may be challenging in NCP patients with severe underlying hyperalgesia, in whom lenses can provide strong noxious stimuli.
Systemic Pharmacotherapy
Symptoms of NCP can present due to central sensitization (Fig. 2). In these cases, systemic pharmacotherapy is required for pain relief.1,113–115 In addition, systemic therapies may aid in treatment of peripheral sensitization and result in accelerated relief. Although data on use of systemic pharmacotherapy for NCP is scarce, and no RCTs have been performed specifically in NCP, their use can be extrapolated from treatments of post-herpetic neuralgia (PHN) and neuropathic pain elsewhere.22,116,117 These approaches have been specifically utilized in NCP patients by the authors. While in many cases the use of a single drug may be sufficient, combination therapy may be necessary. We recommend that ophthalmologists not familiar with prescribing these drugs, initially co-manage patients requiring systemic pharmacotherapy in conjunction with neuropathic pain specialists. As they familiarize themselves with these treatments, they could subsequently manage these patients independently.
First-Line Agents
Tricyclic Antidepressants (TCAs)
TCAs have previously been used effectively in the treatment of neuropathic pain as first-line agents.118–120 TCAs exert their action by inhibiting pre-synaptic reuptake of serotonin and norepinephrine, as well as by blocking cholinergic, histaminergic, and sodium channels.118 The clinical efficacy of 25–150 mg amitriptyline daily in PHN has been demonstrated in a RCT117, which showed a significant reduction in pain in 66% of patients within 3 weeks. Another RCT, comparing nortriptyline directly to amitriptyline for PHN showed similar efficacy of both drugs, with nortriptyline demonstrating fewer side effects.121 In PHN, the time between onset of disease and start of TCAs has been shown to impact outcomes.122 Nortriptyline, preferred due to superior side effect profile, is started at a dose of 10–25 mg at bedtime and increased every 3–7 days to a final dose of 25–100 mg at bedtime as tolerated. Common side effects include dry mouth, constipation, and sedation. Our personal experience with nortriptyline in NCP has been very encouraging.
Anticonvulsant Carbamazepine (CBZ)
CBZ is a sodium channel-blocker commonly used for trigeminal neuralgia (TGN).123 A systematic review concluded that CBZ should be offered as a first-line agent for pain control in TGN (level A). The recommendations were based on the pooled results of four placebo-controlled studies including a total of 147 patients, treated with 300–2400/day.124 In 4 RCTs in TNG, comparing CBZ to placebo, superiority of CBZ was demonstrated, with 70% of CBZ-treated patients showing partial or complete pain relief125. The most common side effects include drowsiness, headache, and dizziness125. In patients with NCP, CBZ is started at 200 mg at night and gradually increased by 200 mg every 7 days to a final dose of 400–1200 mg, divided in 2–3 doses per day. Once response has been achieved and patients maintain pain relief, the dose can be tapered to a minimal effective dose.
Second Line Agents
Low-Dose Naltrexone (LDN)
LDN is an opioid antagonist for the μ-opioid and κ-opioid receptors.126,127 It has further been shown to be an antagonist to toll-like receptor 4 that has been linked to neuropathic pain,126,127 reducing the release of pro-inflammatory cytokines and modulating microglial activity. LDN (3–5 mg) has recently been used effectively as an off-label treatment in patients with chronic neuropathic pain, including fibromyalgia, complex regional pain syndrome, low-back pain, and painful diabetic neuropathy. In a RCT of 31 patients with fibromyalgia, use of LDN 4.5mg resulted in significant decrease of pain and improved satisfaction with life as compared to placebo.127 Another recent paper reported a single case of successful treatment with LDN in a patient with refractory painful diabetic neuropathic pain.128 Common side effects include headache, tachycardia and vivid dreams.127 LDN is recommended by us in NCP patients at 1.5 mg at bedtime with gradual bi-weekly increase of 1.5 mg to a final maximum dose of 4.5 mg taken at bedtime.
Tramadol
Tramadol is a weak μ-opioid agonist in addition to being a norepinephrine and serotonin reuptake inhibitor.129 Opioids in have been shown to be superior to placebo in reducing pain intensity, and in improving physical functioning.114 Common side effects include nausea, vomiting, constipation, and sedation. Further potential dependence limits it as a second-line agent, when the first-line medications fail to achieve a satisfactory response. Tramadol is suggested in NCP patients at 50 mg once or twice daily with gradual increase to a maximum dose of 400 mg daily. In cases where immediate and short-term relief for NCP is desired, its use as a short-term first-line agent may be justified.1,114,115
Third Line Agents
Calcium channel α 2-δ ligands
Gabapentin and pregabalin, both originally designed as anticonvulsants are widely used as single agents for the treatment of diabetic neuralgia, PHN, and central neuropathic pain.116,130,131 Gamma-aminobutyric acid (GABA) is the key inhibitory neuro-transmitter in the CNS. These drugs bind to the α 2-δ subunit voltage gated calcium channels and inhibit the release of glutamate, norepinephrine and substance P, and stabilize neurons from ectopic discharge.132 In patients with PHN, pregabalin improved short-term pain intensity.133 In a RCT of 563 patients with PHN, the efficacy of gabapentin (up to 3600 mg/day) has been reported, demonstrating significant decrease in pain compared to placebo.133 In another RCT 900 mg gabapentin resulted in 66% decreased pain and allodynia levels, compared to 33% decrease with placebo.134 Similarly, in a RCT of 776 patients with PHN, pregabalin (300–600 mg/day) resulted in significant decrease of pain compared to placebo.135 Both gabapentin and pregabalin are FDA approved for treatment of PHN. Common side effects include dizziness, somnolence, dry mouth, and constipation.136,137 Gabapentin is initiated as a single 600 mg dose on day 1 and increased every 3 days to a dose of 1800 mg, divided in 3 doses. The maximum analgesic dose of gabapentin recommended for adults is usually between 1,800 and 3,600 mg per day.112 Pregabalin is started at a dose of 75 mg at bedtime, with a gradual weekly increase to a maximum of 600 mg daily. While the authors have had similar success in patients with ocular PHN, the response rate in patients with NCP of other origins has been very limited.
Serotonin-Norepinephrine Inhibitors (SSNRIs)
Duloxetine and venlafaxine are SSNRIs with both antidepressant and central analgesic properties.138 Their dual mechanism of action has been studied in several clinical trials on painful polyneuropathies. Duloxetine is FDA approved for the treatment of painful diabetic polyneuropathy.139 A recent meta-analysis on pharmacological treatment for neuropathic pain included nine studies with duloxetine, seven of which showed positive results with doses of 20–120 mg/day and final conclusion of “strong recommendations for use”. Common side effects include nausea, dry mouth, headache, decreased libido, dizziness, somnolence or insomnia.140 Duloxetine is contraindicated in patients with severe hepatic and renal impairment.138
Sodium Channel Blocker (Mexiletine)
Mexiletine, a sodium channel blocker that is an orally active local anesthetic agent, is structurally related to lidocaine, and prescribed as a second- or third-line treatment for neuropathic pain.140 It is an orally active local anesthetic and anti-arryhythmic agent that can be used at doses of 225–675 mg/day. 140 The most common side effects are nausea, headache, sleep disturbances, and tiredness. Due to its poor side effect profile, we only recommend in NCP patients refractory to other treatments.
Lifestyle Changes
Preclinical and clinical evidence suggests efficacy of cardio-exercise in pain relief through inhibition of pain pathways that alter pain perception, resulting in improvement of allodynia and hyperalgesia.141 In addition, studies have shown the anti-inflammatory effect of exercise by diminishing neuro-immunologic signaling after nerve injury,142 as well as increase in neurotrophic factors in the CNS contributing to neuroplasticity and neuro-restoration.143,144 Hence, we recommend trials of cardio-exercise for at least 30 minutes twice weekly.
Nutritional intervention strategies, such as the increase in the ratio of omega-3 to omega-6 fatty acids may regulate inflammation and optimize health in NCP patients.145 Further, omega-3 fatty acids supplementation has been reported in clinical trials to improve TBUT and Schirmer’s test results and may decrease symptoms of hyperalgesia.146 Doses of 1000 mg bid to tid daily are recommended. Moreover, gluten sensitivity may be etiologically linked to several idiopathic neuropathies through induction of neuronal inflammation.147 A study 101 patients with idiopathic peripheral neuropathies showed a 40% prevalence of gluten sensitivity.148 In the authors experience with NCP patients, trials of gluten-free diet have resulted in variable decrease in pain.
Meditation and mindfulness, may contribute positively to pain management through activation of multiple brain regions that contain a high expression of opioid receptors. They may have a positive impact on overlapping co-morbidities, such as depression and anxiety in patients with NCP.
Alternative Therapy
Given the many underlying etiologies, some cases can be refractory to the proposed therapies above. The authors have found that use of adjunctive therapies, such as acupuncture may provide additional pain relief. Acupuncture has been shown to stimulate endogenous opioid mechanisms, and may stimulate secretion of neuropeptides.149 Patients with NCP have reported pain relief up to several days from a single session, and if resulting in pain relief, twice weekly sessions are recommended.
In extreme refractory cases, several experimental procedures are available, including non-invasive transcranial magnetic stimulation (TMS) and scrambler therapy (ST), as well as more invasive neuromodulation, and intrathecal drug delivery systems. The recommendation for these therapies is based on the published literature for non-ocular neuropathic pain, as well as our own personal experience.
TMS utilizes non-invasive MRI-strength magnetic pulses to stimulate cortical neurons.150 While it is FDA-approved for depression, recent evidence suggests its use in pain management (in particular for central neuropathic pain).150–152 A recent Japanese RCT in patients with refractory neuropathic pain showed significant short-term effects with TMS,92 while other RCTs have shown good response in patient with TGN.153 Further, ST that uses electric pulses to stimulate C fiber receptors, interferes with pain signal transmission, by confusing the nervous system ability to sense pain.154 A non-controlled case series found a significant reduction in pain scores among 201 chronic pain patients treated with an average of 10 sessions.154 Further, a RCT in patients with chronic neuropathic pain showed significantly reduced pain scores compared to the control group.155
Finally, neuromodulation is an invasive procedure that involves neuronal stimulation through device implantation or administration of medications into the nervous system.156 Invasive procedures, such as deep brain stimulation and implantable spinal cord stimulators have been studied for relief of neuropathic pain in rare cases of intractable neuropathic pain.156,157 Several case reports have recently been published on their use in post-surgical NCP.158,159
SUMMARY
The management of patients with NCP remains extremely challenging in clinical practice, although much progress has been made, resulting increased awareness, diagnostics, and treatments of these patients. No single therapy will likely be successful and combination therapy will remain the mainstay of treatment, addressing the various and complex underlying factors in this disease. There is an urgent need for well-conducted RCTs in this area. Meanwhile, interdisciplinary treatment with both conventional (e.g. neurology, psychiatry, and rheumatology) and alternative medicine is suggested.
Table 2.
Topical Treatments for Neuropathic Corneal Pain
| Topical Agent | Mechanism of Action | Pathology | Efficacy, Level of Evidence |
|---|---|---|---|
| Autologous Serum Tears 20% | Neurotrophic factors: NGF, substance P, insulin-like growth fator-1 | Injured nerves and epithelial cells | MLE, Level 3 and 46,44,50–65 |
| Corticosteroids (e.g., Loteprednol 0.5%) | -Anti-inflammatory -Inhibit leukocyte migration - Inhibit cytokines, prostaglandin and leukotriene synthesis |
Ocular surface inflammation | HLE, Level 175–81 |
| Cryopreserved Amniotic Membrane | -Anti-inflammatory -Neurotrophic |
Ocular surface inflammation | MLE, Level 394–99 |
| Bandage contact lens, Scleral Lens | -Protective effect against the environment triggers | Ocular surface injury | MLE, Level 2103–108 |
| Artificial tears (Preservative free, Emulsion-based) | Decrease tear osmolality – dilution Protective mechanism in evaporative dry eye | Ocular surface disease | HLE, Level 189 |
HLE- High Level of Evidence
MLE- Medium Level of Evidence
MGD – Meibomian Gland Dysfunction
NGF- Nerve Growth Factor
Table 3.
Systemic Pharmacotherapy for Neuropathic Corneal Pain
| Medication (class) | Mechanism of action | Starting dosage | Maximum dosage | Side Effects | Precaution and contraindications |
|---|---|---|---|---|---|
| First-Line Agents | |||||
| (Tricyclic Antidepressants) Nortriptyline, Desipramine Use a tertiary amine TCAs only if a secondary are not available Ref. 112–119 Nortriptyline/Desipramine are FDA-approved for treatment of symptoms of depression |
Monoamine reuptake inhibition, sodium channel blockade and anticholinergic effects | 10–25 mg at bedtime | 100 mg at bedtime | Dry mouth, constipation, somnolence, anticholinergic effects, weight gain | Cardiac disease, prostatic adenoma and seizure disorder High doses should be avoid in adults>65 years of age |
| Carbamazepine (Anticonvulsant) Ref. 120,121 FDA-approved for epilepsy, trigeminal neuralgia, and manic and mixed episodes of bipolar disorder |
Sodium channel– blocker | 200mg daily | 400–800mg/day, divided in 2–3 doses | Hyponatremia, Drowsiness, headache, Dizziness, rash and nausea | Concomitant use of MAO inhibitors Cardiac or hepatic disease Renal failure Prostatic hyperplasia |
| Second-Line Agents | |||||
| Low-Dose Naltrexone (Opioid Antagonist) Ref. 122–124 FDA-approved at higher doses (50 mg to 300 mg) for treatment of drug and alcohol addiction |
At low doses has an antiinflammatory effect, reducing the proinflammatory cytokines Modulating microglial activity Opioid antagonist - μ-opioid and κ-opioid receptors |
1.5 mg at bed time | 4.5 mg/bedtime | Headache, vivid dreams, nightmares, tachycardia and anxiety | Past organ transplant and use of immunosuppressive drugs |
| Tramadol (Opioid Agonist) Ref. 110,111,125 FDA-approved for treatment of moderate to moderately severe pain |
μ-receptor agonist and monoamine reuptake inhibitor | 50mg/day | 100 mg/day in divided doses every 3–7 days as tolerated | Nausea, vomiting, constipation, dizziness, and somnolence | History of substance abuse, suicide risk and antidepressant in elderly patients |
| Third-Line Agents | |||||
| Calcium channel α 2-δ ligands - Gabapentin (Anticonvulsants) FDA-approved for treatment of post-herpetic neuralgia Pregabalin (Anticonvulsant) Ref. 113, 126–136 FDA-approved for treatment of neuropathic pain and fibromyalgia |
Act on the α2δ subunit of voltage-gated calcium channels, which decrease central sensitization | 100–300 mg three times/day 50mg three times/day or 75mg twice a daily |
2400 mg/day 300 mg/day |
Sedation, dizziness, peripheral edema | Reduced dose in renal insufficiency |
| (Serotonin-noradrenaline reuptake inhibitors) Duloxetine Ref. 137–139 FDA-approved for treatment of diabetic peripheral neuropathic pain and fibromyalgia |
Serotoninnoradrenaline reuptake inhibitors | 30 mg/day | 60 mg twice/day | Nausea, abdominal pain, constipation | Hepatic disorders Use of tramadol hypertension |
| Mexiletine (Sodium Channel Blocker) Ref. 140 FDA approved for treatment of cardiac arrhythmia |
Voltage-gated sodium channel blocker Lidocaine analogue Class IB anti-arrhythmic |
225–675 mg/day | 675 mg/day | Nausea, headache, sleep disturbances, tiredness, Gastritis is the most common side effect | Hepatic impairment Severe heart failure Sinus node dysfunction or intraventricular conduction defect |
Acknowledgments
Financial Support: NIH R01-EY022695 (PH), NIH R01-EY026963 (PH), NIH R21-EY025393 (PH)
The funding organizations had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: None
Financial interest: None
Conflict of Interest: No conflicting relationship exists for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dworkin RH, O’Connor AB, Kent J, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154(11):2249–2261. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10(1):2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–134. doi: 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal P, Borsook D, Moulton EA. Oculofacial Pain: Corneal Nerve Damage Leading to Pain Beyond the Eye. Invest Ophthalmol Vis Sci. 2016;57(13):5285–5287. doi: 10.1167/iovs.16-20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamrah P, Qazi Y, Shahatit B, et al. Corneal Nerve and Epithelial Cell Alterations in Corneal Allodynia: An In Vivo Confocal Microscopy Case Series. Ocul Surf. 2017;15(1):139–151. doi: 10.1016/j.jtos.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15(1):15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optom Vis Sci. 2015;92(9):e233–240. doi: 10.1097/OPX.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 10.Galor A, Covington D, Levitt AE, et al. Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. J Pain. 2016;17(3):310–318. doi: 10.1016/j.jpain.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29(3):301–312. doi: 10.1038/eye.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galor A, Levitt RC, Felix ER, Sarantopoulos CD. Understanding the true burden of dry eye disease. Expert Rev Ophthalmol. 2015;10(5):403–405. doi: 10.1586/17469899.2015.1061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1–2):59–70. doi: 10.3109/08820538.2015.1114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxford Centre for Evidence-based Medicine. Level of evidence and grades of recommendation. www.cebm.net/levels_of_evidence.asp.
- 15.Lee Y, Lee CH, Oh U. Painful channels in sensory neurons. Mol Cells. 2005;20(3):315–324. [PubMed] [Google Scholar]
- 16.Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207(1):19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. 2015;3(2):111–121. doi: 10.1007/s40135-015-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20(16):6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CR, Amaya F, Barrett L, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319(3):1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 20.Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci. 2004;24(20):4832–4839. doi: 10.1523/JNEUROSCI.0300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. 2010;8(3):135–145. doi: 10.1016/s1542-0124(12)70224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavan-Langston D. Herpes zoster antivirals and pain management. Ophthalmology. 2008;115(2 Suppl):S13–20. doi: 10.1016/j.ophtha.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2017;101(2):227–231. doi: 10.1136/bjophthalmol-2015-308214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–384. e372. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012;154(2):340–346. e342. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 27.Gothwal VK, Pesudovs K, Wright TA, McMonnies CW. McMonnies questionnaire: enhancing screening for dry eye syndromes with Rasch analysis. Invest Ophthalmol Vis Sci. 2010;51(3):1401–1407. doi: 10.1167/iovs.09-4180. [DOI] [PubMed] [Google Scholar]
- 28.Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 29.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 30.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21(6):578–583. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Amparo F, Schaumberg DA, Dana R. Comparison of Two Questionnaires for Dry Eye Symptom Assessment: The Ocular Surface Disease Index and the Symptom Assessment in Dry Eye. Ophthalmology. 2015;122(7):1498–1503. doi: 10.1016/j.ophtha.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–57. doi: 10.1016/s1542-0124(12)70053-8. [DOI] [PubMed] [Google Scholar]
- 33.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology. 2016;123(7):1458–1468. doi: 10.1016/j.ophtha.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spierer O, Felix ER, McClellan AL, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Invest Ophthalmol Vis Sci. 2016;57(2):617–625. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochet P, Bonnet R. L’esthesie corneenne. Clin Ophthalmol. 1960;4:3–27. [Google Scholar]
- 36.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40(2):513–519. [PubMed] [Google Scholar]
- 37.Sullivan BD, Nelson JD, Craig JP, et al. Conclusion and recommendation from the TFOS dry eye Workshop II. ARVO; Baltimore. USA: 2017. [Google Scholar]
- 38.Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000–1008. doi: 10.1097/ICO.0b013e318242fd60. [DOI] [PubMed] [Google Scholar]
- 40.Amparo F, Hamrah P, Schaumberg DA, Dana R. The value of tear osmolarity as a metric in evaluating the response to dry eye therapy in the clinic and in clinical trials. Am J Ophthalmol. 2014;157(4):915–916. doi: 10.1016/j.ajo.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161–166. doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7(1):28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 43.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5–6):138–148. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kheirkhah A, Dohlman TH, Amparo F, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122(4):662–668. doi: 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moein HR, Dieckmann G, Abbouda A, et al. In vivo confocal microscopy demonstrates the presence of microneuromas and may allow differentiation of patients with corneal neuropathic pain from dry eye disease. Invest Ophthalmol Vis Sci. 2017;58 ARVO E-Abstract 2656. [Google Scholar]
- 47.Cirillo G, Cavaliere C, Bianco MR, et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 2010;30(1):51–62. doi: 10.1007/s10571-009-9430-2. [DOI] [PubMed] [Google Scholar]
- 48.Colangelo AM, Bianco MR, Vitagliano L, et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci. 2008;28(11):2698–2709. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of severe corneal pain in patients with corneal neuropathy: An In Vivo Confocal Microscopy Study. Invest Ophthalmol Vis Sci. 2014;55 ARVO E-Abstract 1468. [Google Scholar]
- 50.Cruzat A, Cavalcanti B, Williams C, Trinidad M, Dana R, Hamrah P. Corneal nerve regeneration with autologous serum eye drops in treatament of severe dry eye syndrome. The Cornea Society/Fall educational symposium; 2012; Chicago. [Google Scholar]
- 51.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94(5):584–591. doi: 10.1136/bjo.2009.164780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu P, Song W, Niu Z, et al. Platelet-derived growth factor promotes the proliferation of human umbilical cord-derived mesenchymal stem cells. Cell Biochem Funct. 2013;31(2):159–165. doi: 10.1002/cbf.2870. [DOI] [PubMed] [Google Scholar]
- 53.Bonini S, Aloe L, Bonini S, Rama P, Lamagna A, Lambiase A. Nerve growth factor (NGF): an important molecule for trophism and healing of the ocular surface. Adv Exp Med Biol. 2002;506(Pt A):531–537. doi: 10.1007/978-1-4615-0717-8_75. [DOI] [PubMed] [Google Scholar]
- 54.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23(4):296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- 55.Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85(10):1188–1197. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–1120. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Ralph RA, Doane MG, Dohlman CH. Clinical experience with a mobile ocular perfusion pump. Arch Ophthalmol. 1975;93(10):1039–1043. doi: 10.1001/archopht.1975.01010020815015. [DOI] [PubMed] [Google Scholar]
- 58.Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27(4):459–461. doi: 10.1002/art.1780270415. [DOI] [PubMed] [Google Scholar]
- 59.Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol. 1999;83(4):390–395. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Q, Angelina A, Zambrano A, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2013;(8):CD009327. doi: 10.1002/14651858.CD009327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hussain M, Shtein RM, Sugar A, et al. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. Cornea. 2014;33(12):1245–1251. doi: 10.1097/ICO.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 62.Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int. 2014;2014:826970. doi: 10.1155/2014/826970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang J, Chung SH, Jeon S, Kwok SK, Park SH, Kim MS. Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjogren syndrome. Cornea. 2014;33(7):663–667. doi: 10.1097/ICO.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 64.Celebi AR, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):619–626. doi: 10.1007/s00417-014-2599-1. [DOI] [PubMed] [Google Scholar]
- 65.Spaniol K, Koerschgen L, Sander O, Koegler G, Geerling G. Comparison of application systems for autologous serum eye drops. Curr Eye Res. 2014;39(6):571–579. doi: 10.3109/02713683.2013.855237. [DOI] [PubMed] [Google Scholar]
- 66.Jirsova K, Brejchova K, Krabcova I, et al. The application of autologous serum eye drops in severe dry eye patients; subjective and objective parameters before and after treatment. Curr Eye Res. 2014;39(1):21–30. doi: 10.3109/02713683.2013.824987. [DOI] [PubMed] [Google Scholar]
- 67.Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016;100(1):22–27. doi: 10.1136/bjophthalmol-2015-306842. [DOI] [PubMed] [Google Scholar]
- 68.Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88(5):647–652. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139(2):242–246. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 70.Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37(8):684–688. doi: 10.3109/02713683.2012.674609. [DOI] [PubMed] [Google Scholar]
- 71.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 72.Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, Dartt D, Gallar J, Belmonte C, Hamrah P, Willcox M TFOS International Workshop on Contact Lens Discomfort. The TFOS international workshop on contact lens discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS71-97. doi: 10.1167/iovs.13-13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMahon S, Koltzenburg M. The changing role of primary afferent neurones in pain. Pain. 1990;43(3):269–272. doi: 10.1016/0304-3959(90)90024-8. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez GG, Garcia de la Rubia P, Gallar J, Belmonte C. Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Invest Ophthalmol Vis Sci. 1993;34(12):3329–3335. [PubMed] [Google Scholar]
- 75.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Persson AK, Liu S, Faber CG, Merkies IS, Black JA, Waxman SG. Neuropathy-associated Nav1.7 variant I228M impairs integrity of dorsal root ganglion neuron axons. Ann Neurol. 2013;73(1):140–145. doi: 10.1002/ana.23725. [DOI] [PubMed] [Google Scholar]
- 77.Kheirkhah A, Cruzat A, Aggarwal S, et al. Outcome of autologous serum tears with concurrent anti-inflammatory treatment in ocular surface disease. American Academy of Ophthalmology Annual Meeting; 2013; p. PO 314. [Google Scholar]
- 78.de Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71(6 Suppl):89–95. doi: 10.1590/s0004-27492008000700017. [DOI] [PubMed] [Google Scholar]
- 79.Coffey MJ, Decory HH, Lane SS. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin Ophthalmol. 2013;7:299–312. doi: 10.2147/OPTH.S40588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holzer MP, Solomon KD, Sandoval HP, Vroman DT. Comparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification: prospective randomized double-masked study. J Cataract Refract Surg. 2002;28(1):93–99. doi: 10.1016/s0886-3350(01)01185-3. [DOI] [PubMed] [Google Scholar]
- 81.Sarkar J, Chaudhary S, Namavari A, et al. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012;53(4):1792–1802. doi: 10.1167/iovs.11-8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Namavari A, Chaudhary S, Chang JH, et al. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Invest Ophthalmol Vis Sci. 2012;53(2):732–740. doi: 10.1167/iovs.11-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan S, Li L, Xu Z, Zhao J. Effect of leukemia inhibitory factor on corneal nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett. 2011;499(2):99–103. doi: 10.1016/j.neulet.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 84.Tu EY. Balancing antimicrobial efficacy and toxicity of currently available topical ophthalmic preservatives. Saudi J Ophthalmol. 2014;28(3):182–187. doi: 10.1016/j.sjopt.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 86.Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28(10):1091–1096. doi: 10.1097/ICO.0b013e3181a16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moscovici BK, Holzchuh R, Chiacchio BB, Santo RM, Shimazaki J, Hida RY. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012;31(8):945–949. doi: 10.1097/ICO.0b013e31823f8c9b. [DOI] [PubMed] [Google Scholar]
- 88.Amparo F, Dastjerdi MH, Okanobo A, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013;131(6):715–723. doi: 10.1001/jamaophthalmol.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiffman RM, Bradford R, Bunnell B, Lai F, Bernstein P, Whitcup SW. A Multi–Center, Double–Masked, Randomized, Vehicle–Controlled, Parallel Group Study to Evaluate the Safety and Efficacy of Testosterone Ophthalmic Solution in Patients With Meibomian Gland Dysfunction. ARVO Annual Meeting Abstract; 2006. [Google Scholar]
- 90.Abidi A, Shukla P, Ahmad A. Lifitegrast: A novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198. doi: 10.4103/0976-500X.195920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(2):12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 92.Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29(7):781–788. doi: 10.1097/ICO.0b013e3181cda38f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ervin AM, Wojciechowski R, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2010;(9):CD006775. doi: 10.1002/14651858.CD006775.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tost FH, Geerling G. Plugs for occlusion of the lacrimal drainage system. Dev Ophthalmol. 2008;41:193–212. doi: 10.1159/000131090. [DOI] [PubMed] [Google Scholar]
- 95.Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. doi: 10.2147/OPTH.S33182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. 2015;33(1):41–46. doi: 10.1089/pho.2014.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thode AR, Latkany RA. Current and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD) Drugs. 2015;75(11):1177–1185. doi: 10.1007/s40265-015-0432-8. [DOI] [PubMed] [Google Scholar]
- 98.Cheng AM, Zhao D, Chen R, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14(1):56–63. doi: 10.1016/j.jtos.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stelnicki EJ, Doolabh V, Lee S, et al. Nerve dependency in scarless fetal wound healing. Plast Reconstr Surg. 2000;105(1):140–147. doi: 10.1097/00006534-200001000-00024. [DOI] [PubMed] [Google Scholar]
- 100.Sakuragawa N, Elwan MA, Uchida S, Fujii T, Kawashima K. Non-neuronal neurotransmitters and neurotrophic factors in amniotic epithelial cells: expression and function in humans and monkey. Jpn J Pharmacol. 2001;85(1):20–23. doi: 10.1254/jjp.85.20. [DOI] [PubMed] [Google Scholar]
- 101.Elwan MA, Thangavel R, Ono F, Sakuragawa N. Synthesis and release of catecholamines by cultured monkey amniotic epithelial cells. J Neurosci Res. 1998;53(1):107–113. doi: 10.1002/(SICI)1097-4547(19980701)53:1<107::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 102.Elwan MA, Ishii T, Sakuragawa N. Detection of dopamine D2 receptor mRNA and binding sites in monkey amniotic epithelial cells. J Neurosci Res. 1999;56(3):316–322. doi: 10.1002/(SICI)1097-4547(19990501)56:3<316::AID-JNR11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 103.Morkin M, Hamrah P. Pain control with amniotic membrane implant in patients with corneal neuralgia. 19th Annual International Ocular Surface Society Meeting; April, 30, 2016; Seattle, Washington. [Google Scholar]
- 104.Dimit R, Gire A, Pflugfelder SC, Bergmanson JP. Patient ocular conditions and clinical outcomes using a PROSE scleral device. Cont Lens Anterior Eye. 2013;36(4):159–163. doi: 10.1016/j.clae.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Smiddy WE, Hamburg TR, Kracher GP, Gottsch JD, Stark WJ. Therapeutic contact lenses. Ophthalmology. 1990;97(3):291–295. doi: 10.1016/s0161-6420(90)32589-7. [DOI] [PubMed] [Google Scholar]
- 106.Russo PA, Bouchard CS, Galasso JM. Extended-wear silicone hydrogel soft contact lenses in the management of moderate to severe dry eye signs and symptoms secondary to graft-versus-host disease. Eye Contact Lens. 2007;33(3):144–147. doi: 10.1097/01.icl.0000244154.76214.2d. [DOI] [PubMed] [Google Scholar]
- 107.Inamoto Y, Sun YC, Flowers ME, et al. Bandage soft contact lenses for ocular graft-versus-host Disease. Biol Blood Marrow Transplant. 2015;21(11):2002–2007. doi: 10.1016/j.bbmt.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li J, Zhang X, Zheng Q, et al. Comparative evaluation of silicone hydrogel contact lenses and autologous serum for management of sjogren syndrome-associated dry eye. Cornea. 2015;34(9):1072–1078. doi: 10.1097/ICO.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 109.Romero-Rangel T, Stavrou P, Cotter J, Rosenthal P, Baltatzis S, Foster CS. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130(1):25–32. doi: 10.1016/s0002-9394(00)00378-0. [DOI] [PubMed] [Google Scholar]
- 110.Schornack MM, Baratz KH, Patel SV, Maguire LJ. Jupiter scleral lenses in the management of chronic graft versus host disease. Eye Contact Lens. 2008;34(6):302–305. doi: 10.1097/ICL.0b013e318188e205. [DOI] [PubMed] [Google Scholar]
- 111.Stason WB, Razavi M, Jacobs DS, et al. Clinical benefits of the boston ocular surface prosthesis. Am J Ophthalmol. 2010;149(1):54–61. doi: 10.1016/j.ajo.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Kornberg DL, St Clair RM, et al. Corneal nerve structure and function after long-term wear of fluid-filled scleral lens. Cornea. 2015;34(4):427–432. doi: 10.1097/ICO.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 114.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 115.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 116.Panlilio LM, Christo PJ, Raja SN. Current management of postherpetic neuralgia. Neurologist. 2002;8(6):339–350. doi: 10.1097/00127893-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 117.Pappagallo M, Haldey EJ. Pharmacological management of postherpetic neuralgia. CNS Drugs. 2003;17(11):771–780. doi: 10.2165/00023210-200317110-00001. [DOI] [PubMed] [Google Scholar]
- 118.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209. doi: 10.1002/14651858.CD011209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 120.Gilron I, Tu D, Holden RR, Jackson AC, DuMerton-Shore D. Combination of morphine with nortriptyline for neuropathic pain. Pain. 2015;156(8):1440–1448. doi: 10.1097/j.pain.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 121.Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology. 1998;51(4):1166–1171. doi: 10.1212/wnl.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 122.Bowsher D. Factors influencing the features of postherpetic neuralgia and outcome when treated with tricyclics. Eur J Pain. 2003;7(1):1–7. doi: 10.1016/s1090-3801(02)00060-5. [DOI] [PubMed] [Google Scholar]
- 123.Sidhu HS, Sadhotra A. Current Status of the new antiepileptic drugs in chronic pain. Front Pharmacol. 2016;7:276. doi: 10.3389/fphar.2016.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saeed T, Nasrullah M, Ghafoor A, et al. Efficacy and tolerability of carbamazepine for the treatment of painful diabetic neuropathy in adults: a 12-week, open-label, multicenter study. Int J Gen Med. 2014;7:339–343. doi: 10.2147/IJGM.S64419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jorns TP, Zakrzewska JM. Evidence-based approach to the medical management of trigeminal neuralgia. Br J Neurosurg. 2007;21(3):253–261. doi: 10.1080/02688690701219175. [DOI] [PubMed] [Google Scholar]
- 126.Noon K, Sturgeon J, Kao M, Darnall B, Mackey S. (418) A novel glial cell inhibitor, low dose naltrexone, reduces pain and depression, and improves function in chronic pain: A CHOIR study. J Pain. 2016;17(4S):S79. [Google Scholar]
- 127.Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–459. doi: 10.1007/s10067-014-2517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hota D, Srinivasan A, Dutta P, Bhansali A, Chakrabarti A. Off-label, low-dose naltrexone for refractory painful diabetic neuropathy. Pain Med. 2016;17(4):790–791. doi: 10.1093/pm/pnv009. [DOI] [PubMed] [Google Scholar]
- 129.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berger A, Dukes E, McCarberg B, Liss M, Oster G. Change in opioid use after the initiation of gabapentin therapy in patients with postherpetic neuralgia. Clin Ther. 2003;25(11):2809–2821. doi: 10.1016/s0149-2918(03)80335-1. [DOI] [PubMed] [Google Scholar]
- 131.Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003;36(7):877–882. doi: 10.1086/368196. [DOI] [PubMed] [Google Scholar]
- 132.Taylor CP. The biology and pharmacology of calcium channel alpha2-delta proteins pfizer satellite symposium to the 2003 society for neuroscience meeting. Sheraton New Orleans Hotel, New Orleans, LA November 10, 2003. CNS Drug Rev. 2004;10(2):183–188. doi: 10.1111/j.1527-3458.2004.tb00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salah S, Thomas L, Ram S, Clark GT, Enciso R. Systematic review and meta-analysis of the efficacy of oral medications compared with placebo treatment in the management of postherpetic neuralgia. J Oral Facial Pain Headache. 30(3):255–266. doi: 10.11607/ofph.1629. [DOI] [PubMed] [Google Scholar]
- 134.Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65(3):444–447. doi: 10.1212/01.wnl.0000168259.94991.8a. [DOI] [PubMed] [Google Scholar]
- 135.Frampton JE, Foster RH. Pregabalin: in the treatment of postherpetic neuralgia. Drugs. 2005;65(1):111–118. doi: 10.2165/00003495-200565010-00011. discussion 119–120. [DOI] [PubMed] [Google Scholar]
- 136.Lopez-Trigo J, Sancho Rieger J. Pregabalin. A new treatment for neuropathic pain. Neurologia. 2006;21(2):96–103. [PubMed] [Google Scholar]
- 137.Otsuki T, Higuchi T, Yamazaki T, Okawa E, Okada K, Abe M. Efficacy and Safety of Pregabalin for the Treatment of Neuropathic Pain in Patients Undergoing Hemodialysis. Clin Drug Investig. 2017;37(1):95–102. doi: 10.1007/s40261-016-0464-1. [DOI] [PubMed] [Google Scholar]
- 138.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 139.Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016;338:183–206. doi: 10.1016/j.neuroscience.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 140.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kami K, Tajima F, Senba E. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain. Anat Sci Int. 2017;92(1):79–90. doi: 10.1007/s12565-016-0360-z. [DOI] [PubMed] [Google Scholar]
- 142.Grace PM, Fabisiak TJ, Green-Fulgham SM, et al. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allen NE, Moloney N, van Vliet V, Canning CG. The Rationale for exercise in the management of pain in parkinson’s disease. J Parkinsons Dis. 2015;5(2):229–239. doi: 10.3233/JPD-140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Almeida C, DeMaman A, Kusuda R, et al. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156(3):504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 145.Raphael W, Sordillo LM. Dietary polyunsaturated fatty acids and inflammation: the role of phospholipid biosynthesis. Int J Mol Sci. 2013;14(10):21167–21188. doi: 10.3390/ijms141021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu A, Ji J. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med Sci Monit. 2014;20:1583–1589. doi: 10.12659/MSM.891364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hernandez-Lahoz C, Mauri-Capdevila G, Vega-Villar J, Rodrigo L. Neurological disorders associated with gluten sensitivity. Rev Neurol. 2011;53(5):287–300. [PubMed] [Google Scholar]
- 148.Hadjivassiliou M, Grunewald RA, Davies-Jones GA. Gluten sensitivity as a neurological illness. J Neurol Neurosurg Psychiatry. 2002;72(5):560–563. doi: 10.1136/jnnp.72.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 150.Yates E, Balu G. Deep Transcranial magnetic stimulation: a promising drug-free treatment modality in the treatment of chronic low back pain. Del Med J. 2016;88(3):90–92. [PubMed] [Google Scholar]
- 151.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;(4):CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 152.Shimizu T, Hosomi K, Maruo T, et al. Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an h-coil and figure-8 coil. J Neurosurg. 2017:1–9. doi: 10.3171/2016.9.JNS16815. [DOI] [PubMed] [Google Scholar]
- 153.Leung A, Donohue M, Xu R, et al. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain. 2009;10(12):1205–1216. doi: 10.1016/j.jpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Compagnone C, Tagliaferri F, Scrambler Therapy G. Chronic pain treatment and scrambler therapy: a multicenter retrospective analysis. Acta Biomed. 2015;86(2):149–156. [PubMed] [Google Scholar]
- 155.Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage. 2012;43(1):87–95. doi: 10.1016/j.jpainsymman.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 156.Deer TR, Lamer TJ, Pope JE, et al. The neurostimulation appropriateness consensus committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodulation. 2017;20(1):15–30. doi: 10.1111/ner.12564. [DOI] [PubMed] [Google Scholar]
- 157.Argoff CE. Review of current guidelines on the care of postherpetic neuralgia. Postgrad Med. 2011;123(5):134–142. doi: 10.3810/pgm.2011.09.2469. [DOI] [PubMed] [Google Scholar]
- 158.Sayegh RR, Sweet JA, Miller JP, Hayek SM. Electrical stimulation of the trigeminal ganglion and intrathecal drug delivery systems for the management of corneal neuropathic pain. Cornea. 2016;35(4):576–577. doi: 10.1097/ICO.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 159.Hayek SM, Sweet JA, Miller JP, Sayegh RR. Successful management of corneal neuropathic pain with intrathecal targeted drug delivery. Pain Med. 2016;17(7):1302–1307. doi: 10.1093/pm/pnv058. [DOI] [PubMed] [Google Scholar]