Abstract
Hemochorial placentation is orchestrated through highly regulated temporal and spatial decisions governing the fate of trophoblast stem/progenitor cells. Trophoblast cell acquisition of specializations facilitating invasion and uterine spiral artery remodeling is a labile process, sensitive to the environment, and represents a process that is vulnerable to dysmorphogenesis in pathologic states. Hypoxia is a signal guiding placental development, and molecular mechanisms directing cellular adaptations to low oxygen tension are integral to trophoblast cell differentiation and placentation. Hypoxia can also be used as an experimental tool to investigate regulatory processes controlling hemochorial placentation. These developmental processes are conserved in mouse, rat, and human placentation. Consequently, elements of these developmental events can be modeled and hypotheses tested in trophoblast stem cells and in genetically-manipulated rodents. Hypoxia is also a consequence of a failed placenta, yielding pathologies that can adversely affect maternal adjustments to pregnancy, fetal health, and susceptibility to adult disease. The capacity of the placenta for adaptation to environmental challenges highlights the importance of its plasticity in safeguarding a healthy pregnancy.
Keywords: hypoxia, placenta development, hypoxia inducible factor, trophoblast cell invasion
INTRODUCTION
Oxygen is an essential nutrient for all cells, including those within the fetus. Red blood cells deliver oxygen through the vasculature. Too little or too much oxygen can evoke responses directed to reestablishing an oxygen balance that is essential for optimal cellular function (Semenza, 2010). Insufficient adaptive responses lead to injury. Thus, oxygen homeostasis is vital to survival. Environmental oxygen has also served a broader purpose as a driver of the evolution of placental mammals (Falkowski et al., 2005). There is compelling evidence that intrauterine oxygen concentrations and mechanisms controlling oxygen homeostasis regulate placentation, affecting both placental structure and function (Fryer and Simon, 2006; Burton, 2009; Dunwoodie, 2009).
In this review, we focus on hypoxia-dependent adaptive responses associated with rodent placentation. For comparative purposes, aspects of hypoxia and uteroplacental biology in the human are discussed. Although species differences in placentation exist, structural and functional similarities are striking and represent the emphasis of the discussion. The reader is also directed to several excellent reviews examining relationships of oxygen, hypoxia, and placental development (Graham et al., 2000; Zamudio, 2003; Moore et al., 2004; Fryer and Simon, 2006; Cartwright et al., 2007; Burton, 2009; Pringle et al., 2010).
STRUCTURE OF THE MATERNAL-FETAL INTERFACE
The maternal-fetal interface is a dynamic site where uterine and placental structures cooperate to promote development of the fetus. These specialized extraembryonic tissues create a maternal environment conducive to efficient nutrient delivery to the developing fetus. The mouse, rat, and human each possess a hemochorial placenta (Enders and Welsh, 1993; Georgiades et al., 2002; Soares et al. 2012). Trophoblast cells are the parenchymal cells of the placenta and their appearance demarcates the first landmark differentiation event during embryogenesis. In this type of placentation, maternal uterine epithelium and vasculature are eroded by trophoblast cells, permitting their direct exposure to maternal blood (Kaufmann et al., 2003; Pijnenborg et al., 2006). Hemochorial placentation arises through interactions of trophoblast cells with two vascular beds: one arising within the uterus and the other associated with extraembryonic mesenchyme of the allantois. The latter provides a direct connection to the fetus. Trophoblast-vascular bed interactions in the uterus versus the allantois are distinct and involve the development of unique specialized trophoblast cell populations. Trophoblast cells associated with the uterine vasculature maximize the delivery of maternal blood to the placenta, whereas trophoblast cells developing in association with the allantoic vasculature efficiently extract nutrients from the maternal blood and transport them to the fetal vasculature. Trophoblast cell-directed modifications of the uterine vasculature represent adjustments critical to maximizing nutrient delivery without damaging the placenta (Burton et al., 2009). Although, this morphogenetic process exhibits species-specific features, there are conserved functions and regulatory events controlling hemochorial placentation (Pijnenborg et al., 1981; Soares et al., 2014). In the next few paragraphs, we discuss shared and unique features of placentation in the mouse, rat, and human, and address confusions arising from disparate nomenclature (Fig. 1).
Figure 1. Hemochorial placentation.
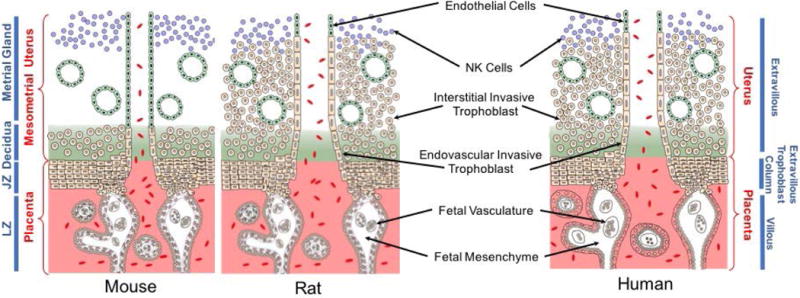
Schematic diagrams showing placentation sites of the mouse, rat, and human. Similarities and differences exist for each species. The mouse and rat share a trichorial labyrinth zone (LZ), while the human possesses a monochorial villous placenta structure. Labyrinth and villous compartments share functional properties. The mouse exhibits shallow intrauterine trophoblast invasion, whereas the rat and human possess deep intrauterine trophoblast invasion. Invasive and extravillous trophoblast cells are functional equivalents, and the junctional zone (JZ) of the rodent placentation site is homologous to the extravillous column of human placentation. Invasive/extravillous trophoblast cells and natural killer cells direct uterine spiral artery restructuring.
Mouse
Trophoblast cells arising from the trophectoderm layer of the blastocyst expand as trophoblast stem and progenitor cell populations into structures referred to as extraembryonic ectoderm and then the chorionic trophoblast and ectoplacental cone. Chorionic trophoblast cells, through their interactions with fetal mesenchyme and the basal portion of the ectoplacental cone, give rise to the labyrinth zone, which is composed of three trophoblast cellular constituents: trophoblast progenitors and two syncytial trophoblast cell layers (Walentin et al., 2016). Initially, trophoblast progenitor cells fuse to form the syncytial layers, and later during pregnancy undergo endoreduplication and differentiate into specialized trophoblast giant cells lining the maternal vascular space. Syncytial trophoblast represents the barrier to the movement of solutes between maternal and fetal compartments (Knipp et al., 1999; Watson and Cross, 2005). The ectoplacental cone is the source of trophoblast cells comprising the junctional zone, which is situated proximal to a specialized uterine stromal cell compartment referred to as decidua. Decidualization defines the process of uterine stromal cell differentiation and is associated with the infiltration and expansion of natural killer (NK) cells. These cells have a conserved role in regulating uterine spiral artery remodeling (Zhang et al., 2011). Junctional zone trophoblast progenitors can undergo endoreduplication forming trophoblast giant cells, a major endocrine cell type of the mouse placenta, and, also differentiate into spongiotrophoblast cells, glycogen trophoblast cells, and invasive trophoblast cells (Soares et al., 1996; Simmons and Cross, 2005; Soares et al., 2012). The latter migrate into mesometrial uterine spiral arterioles and, as gestation progresses, into the mesometrial uterine decidua, and are referred to as endovascular and interstitial invasive trophoblast, respectively. In the mouse, trophoblast invasion is restricted to the decidua (Adamson et al., 2002; Ain et al., 2003a). As invasive trophoblast cells advance, the presence of NK cells in the uterine mesometrial compartment is diminished (Ain et al., 2003a). The biology of spongiotrophoblast cells, glycogen trophoblast cells, and invasive trophoblast cells is yet to be fully elucidated.
Rat
Much of the organization of the rat placentation site is like the mouse. However, there are some key differences, which represent experimental opportunities. A fundamental and defining feature of placentation in the rat is the depth of trophoblast cell invasion (Ain et al., 2003a; Pijnenborg and Vercruysse, 2010; Soares et al., 2012). Interstitial and endovascular trophoblast cells migrate deep into the rat mesometrial uterus, through the decidua and completely infiltrating the myometrial compartment. Strain differences in the depth of trophoblast cell invasion (Konno et al., 2007, 2010, 2011) and responsiveness of endovascular trophoblast cell invasion to hypoxia represent additional characteristics of rat placentation that can be experimentally exploited (Rosario et al., 2008). This contrasts with shallow trophoblast invasion and superficial placentation of the mouse (Adamson et al., 2002; Ain et al., 2003a). Deep placentation is also a feature of human placentation. In the rat, junctional and labyrinth zones are structurally well-defined beginning at gestation day (gd) 13.5, and can be readily separated by dissection, permitting biochemical and molecular analyses of each compartment (Ain et al. 2006). The interdigitation of these placental zones in the mouse prohibits their separation. Collectively, these attributes elevate the rat as an animal model for placental investigation (Soares et al. 2012).
Human
The basic framework and functionality are shared by the human and rodent placentas. However, this is not always clear because of rodent- and human-specific nomenclature. The human placenta can be divided into two major functionally-distinct compartments: (i) villous and (ii) extravillous (Georgiades et al., 2002). A villus consists of an outer epithelial compartment and a core consisting of fetal mesenchyme and vasculature. The epithelial compartment is composed of an underlying cytotrophoblast (progenitor) layer, which declines and becomes discontinuous as gestation advances, and a superficial syncytial layer, which is bathed in maternal blood. The syncytial layer acts as a primary barrier controlling the trafficking of solutes between maternal and fetal compartments, and thus, is a functional equivalent of the rodent labyrinth zone. Extravillous trophoblast columns are operationally analogous to the junctional zone of the rodent placenta (Soares et al., 2014). The proximal portion of an extravillous column consists of progenitor cytotrophoblast cells that give rise to invasive trophoblast lineages (extravillous trophoblast cells), which are initially positioned at the distal end of the column. These invasive trophoblast cells move through the uterine decidua and myometrial compartments using interstitial and endovascular routes amidst NK cells and contribute to uterine spiral arteriole remodeling, tasks shared with rat invasive trophoblast cells. A hallmark of human placental disease is the failure of invasive trophoblast-directed uterine spiral artery remodeling (Brosens et al. 2011). Collectively, the essence of hemochorial placentation in the human exhibits remarkable conservation with the mouse and especially the rat (Fig. 1), justifying the utilization of animal models for investigation of fundamental properties of hemochorial placentation.
OPERATIONAL DEFINITIONS OF HYPOXIA AND NORMOXIA
It is essential that we make a few comments about the use of two terms, which describe the oxygen state: (i) hypoxia and (ii) normoxia. Use of these terms has not always been consistent. Most investigators would agree that hypoxia refers to a state of “low” oxygen, while normoxia refers to the “normal” condition of oxygen availability. Hypoxia can be effectively used to describe oxygen states relative to some normative value, or alternatively, it can be restricted to describing a state that evokes specific cellular responses (e.g., activation of a hypoxia signaling pathway, including hypoxia inducible genes, etc; Bruick, 2003; Semenza, 2010; see below). Normoxia is an especially problematic descriptor. For example, during the normal course of pregnancy, oxygen concentrations within the uterus change dramatically (Zamudio, 2003; Burton, 2009). Early pregnancy is characterized by low oxygen, whereas higher intrauterine oxygen levels are evident following establishment of the hemochorial placenta. Thus, normoxia could be appropriately used to describe various physiological oxygen states. The term normoxia is confusing when describing in vitro experimentation. Most cell culture is performed at ambient oxygen concentrations (sea level: 20.9%), which should not be denoted as normoxia. Such an oxygen concentration is convenient, but it is not physiologically relevant and certainly does not reflect a normoxic environment for any cell developing in utero.
CELLULAR RESPONSES TO HYPOXIA
Low oxygen tension serves as a cellular trigger to alter metabolism and enhance mechanisms to promote the delivery of oxygen. Central to the cellular response to hypoxia is a transcription factor complex referred to as hypoxia inducible factor (HIF; Bruick, 2003; Semenza, 2010). HIF is composed of two subunits: an α regulatory subunit sensitive to oxygen and a constitutively expressed oxygen insensitive β-subunit (HIF1B) also referred to as aryl hydrocarbon receptor translocator (ARNT). The mammalian genome contains three α subunit genes: (i) HIF1A, (ii) HIF2A, also called endothelial PAS domain protein 1 (EPAS1), and (iii) HIF3A. HIF1A possesses the broadest tissue distribution and is the most thoroughly studied. Oxygen regulates the availability of HIF α subunits. Under oxygen replete conditions HIF α subunits become hydroxylated on prolyl residues by a family of oxygen sensitive prolyl hydroxylases (PHD1, PHD2, and PHD3, also referred to as the EGLN family) facilitating HIF α subunit association with the von-Hippel-Lindau (VHL) protein, leading to its ubiquitination and subsequent proteasome-mediated degradation (Kaelin and Ratcliffe, 2008). During conditions of low oxygen, the HIF α protein is stabilized allowing it to interact with ARNT, recruit the requisite coactivators [CREB binding protein (CBP), E1A binding protein p300, (EP300)], and bind to hypoxia regulatory elements (HREs) associated with target genes encoding proteins regulating adaptations to hypoxia. The transcriptional activity of HIF can also be modulated by other relevant regulatory proteins. An oxygen sensitive asparaginyl hydroxylase termed factor inhibiting HIF (FIH1 or HIF1AN) post-translationally modifies the C-terminal transactivation domain of HIFα subunits, especially HIF1A, disrupting their ability to interact with CBP/EP300 coactivators (Kaelin and Ratcliffe, 2008). CBP/EP300-interacting transactivator-2 (CITED2) serves as a negative feedback modulator of HIF transcriptional activity. CITED2 is hypoxia responsive and out competes HIF α for CBP/EP300 (Bhattacharya et al. 1999; Freedman et al. 2003; Berlow et al. 2017). Copper metabolism MURR1 domain 1 (COMMD1) interacts with HIF1A, leading to its instability and interfering with its dimerization to ARNT and its transcriptional activities (van de Sluis et al., 2007, 2009, 2010). FIH1, CITED2, and COMMD1 each act to negatively regulate HIF transcriptional activity. A simplified overview of cellular responses to hypoxia is presented in Fig. 2. It is also important to appreciate that several components of the HIF signaling pathway can be regulated independent of oxygen tension (Greer et al., 2012). Additional details of the cellular response to hypoxia can be found elsewhere (Bruick, 2003; Kaelin and Ratcliffe, 2008; Majmundar et al., 2010; Semenza, 2010).
Figure 2. Cellular responses to hypoxia.
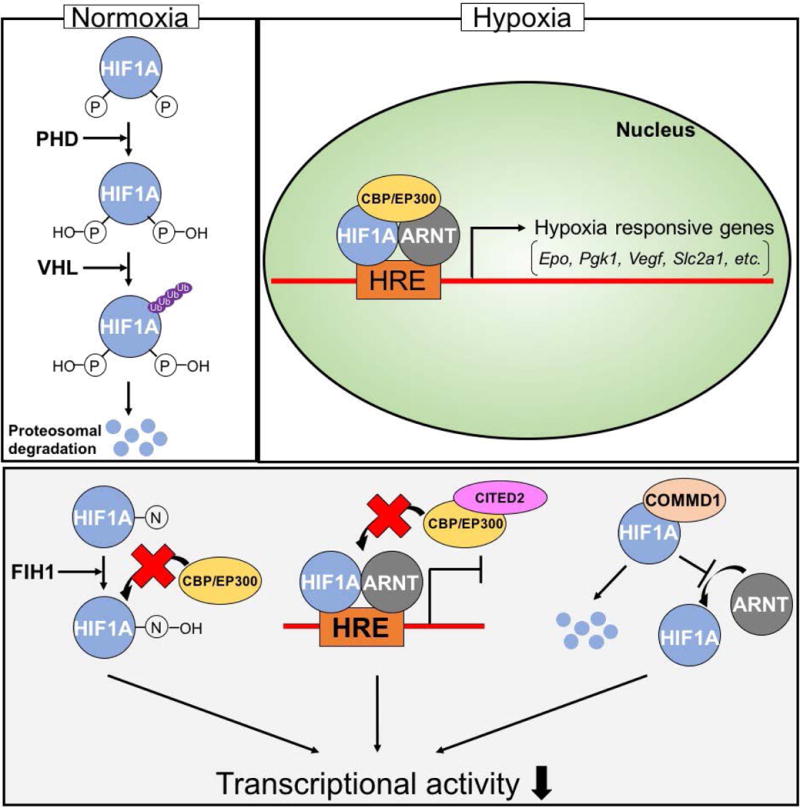
Homeostatic mechanisms guiding cellular adaptations are directed by the hypoxia inducible factor (HIF) signaling pathway. Upper panel) Under oxygen replete conditions PHDs hydroxylate HIF1A on prolyl residues, allowing VHL protein to ubiquitinate HIF1A resulting in proteosomal degradation of HIF1A. Under low oxygen conditions, HIF1A is stable and can dimerize with ARNT, recruit CBP/EP300, and bind to hypoxia/HIF response elements (HRE), inducing hypoxia responsive genes encoding proteins required for adaptations to hypoxia. Lower panel) Negative regulation of HIF transcriptional activity. FIH1 hydroxylates HIF1A on an asparagine residue, preventing HIF1A from interacting with CBP/EP300. CITED2 interacts with CBP/EP300, preventing CBP/EP300 from being recruited to the HIF1A/ARNT dimer complex. COMMD1 interacts with HIF1A, leading to proteolysis of HIF1A and disrupting dimerization of HIF1A with ARNT.
OXYGEN AND REGULATION OF PLACENTATION DURING NORMAL DEVELOPMENT
Oxygen as a signal for placentation
Oxygen concentrations at the site of embryo implantation and during the formation of the placenta are low (Rodesch et al., 1992; Fischer and Bavister, 1993; Jauniaux et al., 2001). Once hemochorial placentation is established then oxygenated blood flowing into the uterus elevates the oxygen tension within the placenta. Placental oxygen measurements during the first 10 weeks of human pregnancy are reported to be less than 20 mmHg (approximately 1–2% O2), but increase to about 60 mmHg (approximately 8% O2) during the second trimester of gestation (Rodesch et al., 1992; Jauniaux et al., 2001). Although comparable measurements have not been reported in rodents, pimonidazole-reactive placentation-associated structures and activation of a hypoxia/HIF reporter system have been detected and are indicative of exposures to low oxygen during critical early phases of normal gestation (Lee et al., 2001; Pringle et al., 2007; Leno-Duran et al., 2010; Chakraborty et al., 2011; Kenchegowda et al., 2017). We also gain insight from assessment of the stabilization of HIF1A and EPAS1 proteins, which are critically dependent upon oxygen tension. Both HIF α proteins are stabilized during early pregnancy, representing the formative stages of placentation (Caniggia et al., 2000; Rajakumar and Conrad, 2000; Letta et al., 2006; Pringle et al., 2007). Low oxygen tensions of the placentation site during early gestation may be facilitated by the presence of invasive trophoblast occluding the uterine spiral arterioles (Burton et al., 1999; Ain et al., 2003a) and can be viewed as a stimulus for placentation (Fryer and Simon, 2006), not unlike the role of tissue hypoxia in solid tumor growth (Harris, 2002; Gruber and Simon, 2006).
HIF signaling pathway and placentation
Insights about the role of oxygen as an intrinsic regulator of placentation have been derived from mutagenesis of the mouse genome. Phenotypes of mice with null mutations for several genes in the HIF signaling pathway are associated with failures in placentation (Fryer and Simon, 2006; Dunwoodie, 2009; see Table 1).
Table 1.
Placenta-related phenotypes associated with genetic disruption of components of the HIF signaling pathway.
| Gene symbol | Species | Mutationa | Placental phenotype | References |
|---|---|---|---|---|
| Arnt | Mouse | Global-LOF | Midgestation lethality (gd 9.5 to 10.5); failure of labyrinthine vascularization; decreased junctional zone trophoblast progenitor cells | Kozak et al., 1997; Maltepe et al., 1997; Abbott and Buckalew, 2000; Adelman et al., 2000 |
| Hif1a | Mouse | Global-LOF | Midgestation lethality (gd 10.5); impaired labyrinthine vascularization; decreased junctional zone trophoblast progenitor cells; not as severe as Arnt null placenta phenotype | Iyer et al., 1998; Ryan et al., 1998; Kotch et al., 1999; Cowden-Dahl et al., 2005a |
| Hif1a | Mouse | Maternal-LOF | Placental growth restriction; disrupted decidual natural killer cell expansion and interstitial trophoblast invasion | Kenchegowda et al., 2017 |
| Epas1 | Mouse | Global-LOF | Modest placental phenotype | Cowden-Dahl et al., 2005a |
| Hif1a/Epas1 | Mouse | Global-LOF | Phenocopies global Arnt null | Cowden-Dahl et al., 2005a |
| Egln1 | Mouse | Global-LOF | Lethality between gd 12.5 and 14.5; junctional zone expansion and compromised labyrinth zone development | Takeda et al., 2006; Ozolins et al., 2009 |
| Vhl | Mouse | Global-LOF | Midgestation lethality (gd 10.5 to 12.5); failure of labyrinthine vascularization | Gnarra et al., 1997 |
| Fih1 | Mouse | Global-LOF | None apparent | Zhang et al., 2010 |
| Cited2 | Mouse | Global-LOF | Placental growth restriction | Withington et al., 2006 |
| Cited2 | Rat | Global-LOF | Placental growth restriction | Dhakal et al., 2016 |
| Cited2 | Mouse | Trophoblast-LOF | Impaired labyrinthine vascularization | Moreau et al., 2014 |
| Commd1 | Mouse | Global-LOF | Midgestation lethality (gd 9.5 to 10.5); failure of labyrinthine vascularization | van de Sluis et al., 2007 |
| miR-210 | Mouse | Global-LOF | None apparent | Krawczynski et al., 2016 |
| miR-210 | Mouse | Trophoblast-GOF | None apparent | Krawczynski et al., 2016 |
| Kdm3a | Rat | Trophoblast-LOF | Disruption of hypoxia-activated placenta adaptations | Chakraborty et al., 2016 |
| Mmp12 | Rat | Global-LOF | Disruption of hypoxia-activated placenta adaptations | Chakraborty et al., 2016 |
LOF, loss of function; GOF, gain of function
HIF subunits
Placentation has been investigated in mice possessing null mutations of Arnt, Hif1a, and Epas1. Arnt deficient mice exhibit a defect in placentation that leads to midgestation (gd, 9.5 to 10.5) lethality (Kozak et al., 1997; Maltepe et al. 1997; Abbott and Buckalew, 2000; Adelman et al., 2000). Specifically, there is a failure in maturation of the fetal vasculature within the labyrinth zone. Junctional zone trophoblast progenitor cells are also adversely affected, especially those destined to spongiotrophoblast and invasive trophoblast lineage differentiation (Adelman et al. 2000). Hypoxia and HIF signaling promote rodent TS cell development into invasive trophoblast (Adelman et al. 2000; Cowden Dahl et al. 2005b; Chakraborty et al. 2011; Chakraborty et al., 2016). Similar observations of hypoxia-driven invasive trophoblast/extravillous trophoblast development from human progenitor trophoblast cells have also been reported (Robins et al., 2007; Chakraborty et al., 2016; Horii et al., 2016; Wakeland et al., 2017). Disruption of Hif1a leads to midgestation (gd 10.5) embryonic death (Iyer et al., 1998; Ryan et al., 1998; Kotch et al., 1999). Hif1a null placentas show deficits in labyrinthine vascularization and junctional zone growth, but are not as severe as the Arnt null placental phenotype. Modest placental disruptions are observed in placentation sites with a null mutation of Epas1; while, Hif1a/Epas1 double nulls exhibited placental phenotypes resembling Arnt nulls (Cowden Dahl et al., 2005a). Expansion of junctional zone trophoblast progenitors and their differentiation may be linked to hypoxia/HIF-dependent regulation of Achaete-scute family BHLH transcription factor 2 (ASCL2; Cowden Dahl et al., 2005a). ASCL2 is also regulated by hypoxia/HIF-signaling in human cytotrophoblast cell populations, supporting conservation in disparate species possessing hemochorial placentation (Jiang et al., 2000; Jiang and Mendelson, 2003). Hypoxia/HIF-driven development of the invasive trophoblast lineage is mediated in part by lim domain kinase 2 (Choi et al. 2013; Zhou et al. 2014) and the epigenetic regulator lysine demethylase 3A (KDM3A) and its downstream target, matrix metalloproteinase 12 (MMP12; Chakraborty et al. 2016). In the absence of HIF signaling, trophoblast differentiation favors labyrinthine trophoblast derivatives (Cowden Dahl et al., 2005a; Maltepe et al., 2005). Hypoxia antagonism of labyrinthine trophoblast development may be mediated via downregulation of peroxisome proliferator-activated receptor γ (PPARG, Tache et al., 2013).
HIF subunits are also prominently expressed in the mouse uterus during the establishment of pregnancy (Daikoku et al. 2003). Natale and Fisher and colleagues (Kenchegowda et al., 2017) conditionally disrupted Hif1a in maternal tissues at gd 8.5. Maternal Hif1a deficiency was associated with small placentas and disruptions in uterine NK cell accumulation and decidual interstitial trophoblast invasion.
Finally, the importance of HIF activation in the maternal liver on placentation has also been explored. Overexpression of adenoviral vector packaged cytomegalovirus promoter driven constitutively active HIF1A at gd 8 in the mouse results in prominent HIF1A expression in the liver, growth restriction of the placenta and fetus, without affecting the organization and morphology of the placenta (Tal et al., 2010).
Collectively, it is evident that HIF signaling within trophoblast cells at the uterine interface regulates hemochorial placentation.
PHD2
Of the three prolyl hydroxylase domain proteins (PHD1, PHD2, and PHD3), only mutation of the Egln1 gene, which encodes PHD2, affects placentation (Takeda et al. 2006). Egln1 deficiency interferes with mouse embryonic development, resulting in prenatal death between gd 12.5 and 14.5 and yields prominent effects on placental development. The junctional zone and its cellular constituents are expanded and labyrinthine development is compromised. Similar disruptive effects on placental development are also observed following transgenesis with Egln1-specific short hairpin RNA (Ozolins et al. 2009). Such a phenotype is consistent with removal of HIF inhibition and the above discussion of HIF signaling on trophoblast development.
VHL
Inactivation of the Vhl gene in the mouse leads to failures at the placental-fetal interface and embryonic lethality between gd 10.5 and 12.5 (Gnarra et al., 1997). The labyrinth zone fails to vascularize and syncytial trophoblast within the labyrinth zone does not develop properly. Disruption of VHL should lead to constitutive HIF signaling, which could foretell an expansion of the junctional zone at the expense of the labyrinth zone. Unfortunately, the integrity of the junctional zone and its trophoblast derivatives was not described in Vhl null placentas.
FIH1
The Fih1 gene has been globally disrupted (Zhang et al. 2010). Body weights are decreased at birth in Fih1 null mice, which could imply a defect in placental function. However, placental or pregnancy impairments were not reported. Adult Fih1 mutants possess significant disturbances in metabolic function (Zhang et al. 2010).
CITED2
CITED2 modulates interactions between transcription factors and CBP/EP300, which includes HIF (Bhattacharya et al. 1999; Freedman et al. 2003; Berlow et al. 2017). The outcomes of these interactions with CITED2 are transcription factor-specific. In some instances, CITED2 facilitates coupling of a transcription factor with CBP/EP300 (Bamforth et al. 2001), while in other instances CITED2 interferes with transcription factor-CBP/EP300 recruitment (Freedman et al. 2003; Lou et al. 2011; Berlow et al. 2017). As indicated above, CITED2 interferes with HIF-CBP/EP300 interactions and inhibits HIF-driven gene expression (Freedman et al. 2003; Berlow et al. 2017). CITED2 is prominently expressed in trophoblast cells of mouse and rat placentas (Withington et al. 2006; Moreau et al. 2014; Dhakal et al. 2016). Mutagenesis of the Cited2 gene in the mouse is associated with deficits in placental growth leading to in utero death, beginning at gd 14.5 (Withington et al. 2006; Moreau et al. 2014; Dhakal et al. 2016). All trophoblast lineages are negatively affected by CITED2 deficiency, as is vascularization of the labyrinth zone (Withington et al. 2006; Moreau et al. 2014). Cited2 gene deletion in the rat negatively affects placental growth, but does not compromise placental-fetal survival through pregnancy (Dhakal et al. 2016; P. Dhakal and M.J. Soares, unpublished findings). Cited2 mutant placental phenotypes are complex, due to the wide range of transcription factors utilizing CBP/EP300, and thus, likely extend beyond the influence of HIF signaling on placentation. Furthermore, observations in the mouse and rat reflect some similarities and differences for the role of CITED2 as a regulator of placentation.
COMMD1
Null mutation of Commd1 results in lethality between gd 9.5 and 10.5 and a placental phenotype resembling Vhl deficiency, e.g., failure of labyrinthine vascularization (van de Sluis et al., 2007).
miR-210
The hypoxia regulated micro RNA, miR-210, has been implicated as a downstream effector of HIF action (Ivan and Huang, 2014), and its expression is reported to be dysregulated in placental pathologies (Lee et al. 2011). However, genetic ablation of miR-210 or placental specific overexpression does not adversely affect placentation or placental adaptations to hypoxia (Krawczynski et al. 2016).
Overview
Key components of the hypoxia/HIF signaling pathway are essential contributors to the regulation of hemochorial placentation during the normal course of gestation. Their disruption curtails specific morphogenetic events critical to the formation of a healthy placenta. It is also necessary to acknowledge that other cellular signaling pathways (e.g., mTOR and endoplasmic reticulum stress pathways) can respond to low oxygen (Burton et al., 2010); however, their integration into the role of hypoxia in placental development is less well understood and will not be discussed further.
EXPERIMENTAL MANIPULATION OF OXYGEN
Analyses of the effects of hypoxia on hemochorial placentation have utilized both in vitro and in vivo model systems. Manipulating oxygen tensions in cultured trophoblast cells provides insights into “cellular potential” and when coupled with in vivo experimentation can be physiologically relevant.
In vitro model systems
There is a wide range of trophoblast cell culture systems that have been utilized to investigate responses to low oxygen tensions. These include an assortment of transformed and immortalized trophoblast cell lines, primary trophoblast cells, trophoblast stem (TS) cells, and TS-like cells (Kliman et al., 1986; Faria and Soares., 1991; Tanaka et al., 1998; Asanoma et al., 2011; Lee et al., 2016). Transformed and immortalized cell lines are easy to culture and manipulate, but possess limitations regarding their relevance to trophoblast cells of the placenta (Lee et al., 2016). Primary trophoblast cells isolated from the placenta can possess elements of authentic behavior; however, in most cases, have limited proliferative capacity and plasticity, and can show dysregulation reflecting displacement from their in situ residence. First and second trimester primary trophoblast cells exhibit some plasticity and a broader range of responsiveness to low oxygen than do term trophoblast cells, but can be a challenge to obtain. TS cell models are extraordinary in that they can be maintained in a stem state and expanded or induced to differentiate into specialized trophoblast lineages (Faria and Soares, 1991; Tanaka et al., 1998; Asanoma et al., 2011). Thus far, TS cells have been successfully derived from mouse and rat blastocysts, but not human blastocysts (Tanaka et al., 1998; Asanoma et al., 2011; Kunath et al., 2014). Human embryonic stem cells and induced pluripotent stem cells exhibit the capacity to differentiate along the trophoblast lineage (Xu et al., 2002; Renaud et al., 2015; Horii et al., 2016), and have been used to investigate potential mechanisms controlling human trophoblast cell differentiation (Ezashi et al., 2012). However, their relevance for elucidating regulatory mechanisms controlling trophoblast cell development has been debated (Roberts et al., 2014; Lee et al., 2016; Soares and Vivian, 2016). Below we focus on developmental events sensitive to oxygen tension, and describe salient findings from investigations using rodent TS cells and human primary trophoblast cells.
In vitro analyses
Oxygen tension and TS cell development
In vitro analyses with TS cells have been instructive and suggest that HIF signaling is critical for trophoblast cell lineage development. TS cells typically differentiate into trophoblast cell lineages of the junctional zone; however, TS cells lacking ARNT or those that are doubly deficient in HIF1A and EPAS1 preferentially differentiate into trophoblast cell lineages of the labyrinth zone (Cowden Dahl et al., 2005a; Maltepe et al., 2005).
Exposure of TS cells to low oxygen can disrupt differentiation towards a trophoblast giant cell phenotype and instead promote development of the invasive trophoblast cell lineage (Cowden Dahl et al., 2005a; Cowden Dahl et al., b; Gultice et al., 2006; Chakraborty et al., 2011, 2016). These responses are dependent upon HIF signaling, which redirects the epigenetic landscape and gene expression of trophoblast cells (Chakraborty et al., 2011, 2016). Hypoxia-dependent HIF activation drives pivotal events, including the downregulation of E-cadherin and upregulation of KDM3A, which promotes the expression of MMP12 and a pro-invasive phenotype (Chakraborty et al., 2011, 2016). TS cell responses are dependent upon oxygen tension (Gultice et al., 2009; Zhou et al., 2011). Low oxygen (~0.5%) slows cellular expansion and promotes differentiation, oxygen concentrations approximating 2% are most conducive to cell proliferation and antagonize differentiation, while ambient oxygen exhibits intermediate effects on TS cell proliferation and differentiation (Zhou et al., 2011; Xie et al., 2014; Yang et al., 2016). The in vitro response to hypoxia includes an upregulation in the expression of Ascl2 mRNA (Cowden Dahl et al., 2005a). ASCL2 is a transcription factor that is essential for the development of the junctional zone (Guillemot et al., 1994). A deficiency in ASCL2 leads to placental insufficiency and pregnancy failure. TS cell responses to low oxygen can also be modulated by other cellular regulators, including connexin 31 (Koch et al., 2012), extracellular matrix composition (Choi et al., 2013), and PPARG (Tache et al, 2013).
Oxygen tension and human trophoblast cells
Parallels between TS cell and human trophoblast cell responses to low oxygen have been observed. Low oxygen can promote human trophoblast cell proliferation and inhibit differentiation (Alsat et al., 1996; Genbacev et al., 1996; Genbacev et al., 1997; Nelson et al., 1999; Caniggia et al., 2000; Jiang et al., 2000) and have profound effects on the trophoblast cell epigenetic landscape (Yuen et al., 2013). Under appropriate conditions, low oxygen exposure can also facilitate development of the human invasive trophoblast lineage, as indicated by an increase in human leukocyte antigen-G expression (Robins et al., 2007; Horii et al., 2016; Wakeland et al., 2017). Hypoxia-dependent activation of the invasive trophoblast lineage is dependent upon HIF signaling (Wakeland et al., 2017) and HIF-dependent downregulation of E-cadherin (Arimoto-Ishida et al., 2009). ASCL2 is also upregulated by low oxygen in human trophoblast cells and is viewed as one of the mediators of the inhibitory effects of hypoxia on syncytial trophoblast development (Jiang et al., 2000; Jiang and Mendelson, 2003).
Overview
In vitro investigations of oxygen tension manipulation and trophoblast cell development have been fraught with inconsistencies and confusion (James et al., 2006; Tuuli et al., 2011). Nonetheless, it is apparent that developmental state defines trophoblast cell responses to low oxygen. Trophoblast cells exhibiting maximal plasticity have the potential for a diversity of behaviors that are influenced by the concentration and duration of oxygen exposures. In general, oxygen tensions present in utero during early gestation are sufficient to activate HIF signaling and promote trophoblast cell expansion. During this time, there is an allocation of progenitors to specialized trophoblast cell lineages. Deficits in oxygen delivery favor differentiation of the invasive/extravillous trophoblast cell lineages. Increasing oxygen tensions may serve as a stimulus for the movement of trophoblast cells committed to the invasive/extravillous trophoblast cell lineage (Huppertz et al., 2009). Finally, it is important to acknowledge that in vivo physiological oxygen exposures cannot be readily achieved in a static tissue culture system. In vivo cellular responses to hypoxia are accompanied by changes in the intrauterine microenvironment and complex maternal systemic adaptations. Thus, in vitro analyses of the role of oxygen tension in trophoblast development are approximations of a reality that is best supported by in vivo investigations.
In vivo model systems
The mouse and rat have been extensively utilized to investigate the effects of hypoxia on placental development. Pregnant animals can be acutely or chronically exposed to hypoxic conditions using hypobaric or gas-regulated chambers. Some analyses in rodents and primates have been obtained from manipulations of the vasculature designed to limit uterine oxygen delivery. In general, the objectives of most experiments have been to elucidate responses to the potentially injurious effects of low oxygen leading to placental pathology and fetal growth restriction. Other research efforts have focused on disease states, or from individuals inhabiting high altitudes. Unlike in vitro experimentation, it is important to appreciate that in vivo conditions are dynamic and accompanied by efforts to adapt to the environmental challenge and attempts to achieve a favorable homeostatic balance.
In vivo analyses
Experimental oxygen manipulation in chambers
In vivo responses to maternal hypoxia are complex. Experimental exposure of pregnant rodents to hypoxic environments can impact placental and fetal development. The specific effects are dependent upon the magnitude, duration, and the gestational timing of the hypoxia (Huang et al., 2004; Higgins et al., 2016).
Some exposures to maternal hypoxia result in placental adaptations (Ain et al., 2004; Schäffer et al., 2006; Alam et al., 2007; Rosario et al., 2008; Chakraborty et al., 2011, 2016; Bu et al., 2016; Higgins et al., 2016; Matheson et al., 2016). Maternal hypoxia can lead to an increase in vascularity of the uterine mesometrial compartment, expansion of the junctional zone, and enhanced endovascular trophoblast invasion and endovascular trophoblast-guided uterine spiral artery remodeling (Rosario et al., 2008; Chakraborty et al., 2011, 2016; Bu et al., 2016; Fig. 3). Critical periods of placentation site sensitivity to hypoxia have been defined in the rat (Rosario et al., 2008). Hypoxia exposure between gd 8.5 and 9.5 is critical for activation of endovascular trophoblast invasion on gd 13.5, whereas, the window of sensitivity for junctional zone expansion is broader. Pregnant mice respond to hypoxia with a junctional zone enlargement, but not a stimulation of endovascular trophoblast invasion (Bu et al., 2016). Increased trophoblast proliferation is characteristic of hypoxia-exposed guinea pigs (Thompson et al., 2016). There is also evidence of sexually dimorphic placental responses to hypoxia exposures (Matheson et al., 2016).
Figure 3. Maternal hypoxia stimulates adaptations at the mouse and rat placentation site.
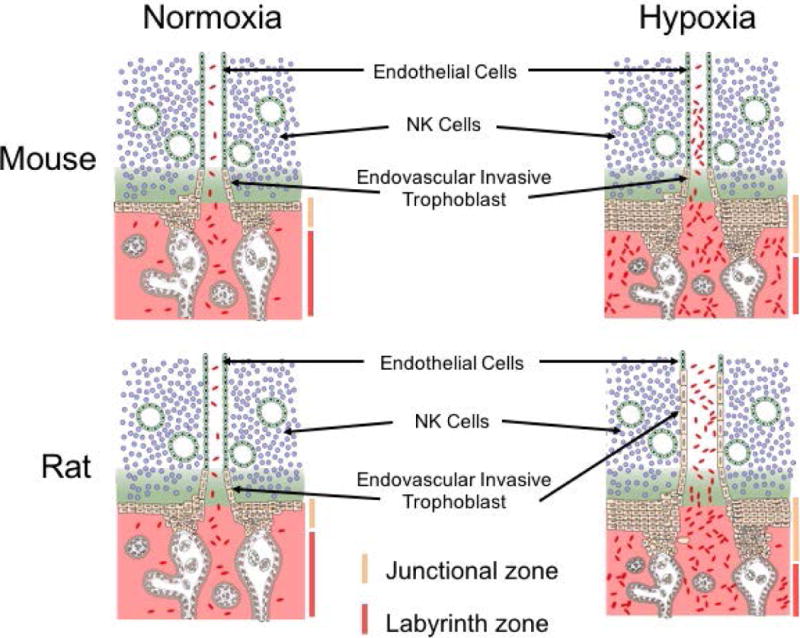
Exposure of pregnant mice and rats to hypoxia (10–11% oxygen) during a critical developmental phase of development (gd 7.5 to 9.5) results in striking changes in the organization of hemochorial placentation. In both species, there is an increased allocation of trophoblast progenitor cells to differentiation of junctional zone trophoblast cell lineages instead of labyrinth zone trophoblast cell lineages. In the rat but not the mouse, maternal hypoxia also activates development of endovascular trophoblast cell lineage, and accelerates endovascular trophoblast cell invasion, which restructure uterine spiral arterioles. The schematic presentation is based on previously reported findings (Rosario et al., 2008, Bu et al., 2016).
Severe, prolonged, or temporally-sensitive exposures to maternal hypoxia can lead to placental growth restriction and diminished trophoblast invasion (Kimball et al., 2015), narrowing and increased vascular resistance of fetoplacental arteries (Jakoubek et al., 2008; Hampl and Jakoubek, 2009; Hvizdosova-Klescova et al., 2013), alterations in the placental transcriptome (Huang et al., 2004; Gheorghe et al., 2007), placental injury (Tomlinson et al., 2010; Bobek et al., 2013), impaired fetal glucose utilization (Sakuragawa et al., 1988), and fetal growth restriction (Nelson et al., 1975; De Grauw et al., 1986; Faridy et al., 1988; Tapanainen et al., 1994; Lueder et al., 1995; Schwartz et al., 1998; Kimball et al., 2015; Higgins et al., 2016; Matheson et al., 2016). Hypoxia-induced placental injury can be monitored by magnetic resonance imaging (Tomlinson et al., 2010; Bobek et al., 2013). Chronic maternal hypoxia in the rat (10.5% O2 — gd 6 to 20) results in elevated blood pressure, impairments in trophoblast invasion and uterine spiral artery remodeling, and fetal growth restriction (Zhou et al., 2013). A similar phenotype has been reported in the pregnant guinea pig exposed to chronic hypoxia (10.5% O2 — gd 20 to 40 or gd 20 to 60–65; Thompson et al., 2016). In the rat, these pathologies can be moderated by treatment with an endothelin-1 type A receptor antagonist (Zhou et al., 2013).
It is important to appreciate that exposure to hypoxia can affect other aspects of maternal physiology, including food intake, which can also impact placentation (Higgins et al., 2016).
Restriction of uterine vascular perfusion
Experimental manipulations have been performed to mechanically restrict blood flow to the uterus and thus the movement of nutrients, including oxygen to the placenta. Such research models have been established to investigate maternal impacts on hemochorial placentation, and are referred to as reduced uterine perfusion pressure (RUPP) models (Li et al., 2012). In the rat, clips are applied to the aortic and uterine vessels on gd 14, decreasing uterine blood flow by approximately 40%. Maternal hypertension and kidney pathology ensues. The placenta exhibits hypoxia, stabilization of HIF1A, and shifts in gene expression (Granger et al., 2002; Gilbert et al., 2009; George et al., 2014). Many of the pathologies associated with the RUPP rat model can be ameliorated via treatment with a PPARG agonist (McCarthy et al., 2011). A similar restrictive manipulation of the abdominal aorta of the pregnant rhesus monkey results in an increase depth of intrauterine interstitial trophoblast cell invasion (Zhou et al., 1993).
Placental disease
Disruptions in oxygen homeostasis are associated with human placental disease. Preeclampsia is a disorder characterized by maternal hypertension, kidney glomerular injury, uteroplacental disruptions, fetal health concerns, and pre-term birth (Myatt and Roberts, 2015). Hallmarks of early onset preeclampsia are deficits in intrauterine trophoblast invasion and trophoblast-guided uterine spiral artery remodeling (Kaufmann et al., 2003; Pijnenborg et al., 2006; Brosens et al., 2011). Failures in uterine spiral artery remodeling result in poor placental perfusion, endothelial dysfunction, and hypoxia. A characteristic feature of a preeclamptic placenta is an upregulation of hypoxia/HIF signaling (Rajakumar et al., 2001, 2004; Caniggia and Winter, 2002; Vaiman et al., 2005; van Uitert et al., 2015; Korkes et al., 2017). There is some evidence for disruptions of oxygen sensing (Rolfo et al., 2010) and disruptions in proteasomes responsible for HIF α protein degradation in preeclamptic placentas (Rajakumar et al., 2008). Hypoxia associated with preeclampsia is a consequence of failed placentation.
Hypoxic states associated with disease can also drive trophoblast invasion and vascular remodeling. Huppertz and colleagues (Kadyrov et al., 2003) have demonstrated that maternal anemia, a systemic state of attenuated oxygen delivery, is associated with an increase in the depth of intrauterine trophoblast invasion. This finding is consistent with observations made with the primate RUPP model (Zhou et al., 1993) and with the hypoxia-exposed rat model (Rosario et al., 2008).
High altitude
Comparisons of pregnant humans exposed to sea level and higher altitudes possessing diminished oxygen concentrations have been investigated. Several reports indicate an impact on placental morphology at elevations of 2500 meters and above (Palmer et al., 1999; Zamudio 2003; Moore et al. 2004; Tissot van Patot et al., 2009). Such studies have focused on populations, which have recently migrated to cities located at higher altitudes (within three generations). The decreased oxygen tension at high altitude stimulates placental blood vessel development, resulting in increased vascularity (Burton et al. 1996; Mayhew 2003; Zhang et al. 2002; Tissot van Patot et al. 2003, 2004; Zamudio 2003). This represents an adaptive response to facilitate nutrient flow to the placenta and fetus. Blood vessel development at the placentation site is stimulated at high altitude, resulting in alterations to the vasculature, particularly, an increase in fetal capillary density and the number of uteroplacental arteries (Burton 2001; Tissot van Patot et al., 2003, 2004), a decrease in remodeling of the decidual ends of the uteroplacental arteries (Tissot van Patot et al., 2003), and an increase in uterine artery blood flow (Moore et al., 2011). HIF1A protein is stabilized at high altitude and HIF targets (e.g., vascular/endothelial growth factor, erythropoietin, etc) activated (Zamudio et al., 2007). Adenosine monophosphate-activated protein kinase-α1 has been implicated in increased blood flow associated with high altitude pregnancies (Skeffington et al., 2016). Fetal growth restriction is a common feature of pregnancies at high altitude (Moore et al., 2004). Preeclampsia is also more frequent at higher altitudes (Moore et al., 2004; Tissot van Patot et al., 2009).
There are other human populations indigenous to the Andes, Ethiopia, and Tibet, which represent altitude-adaptive natives (Bigham, 2016). These individuals have acquired uteroplacental adaptations that enhance pregnancy performance, fetal growth, and facilitate offspring survival (Hochachka and Rupert 2003; Beall et al. 2004; Zamudio et al., 2007). Most interestingly, these populations possess polymorphisms in genes that are directly linked to hypoxia-dependent adaptations (Bigham, 2016). Among the targeted loci are genes encoding key components of the HIF signaling pathway, including HIF1A, EPAS1, EGLN1, etc., which have also been linked to placentation (see above). Connections between polymorphisms in the HIF signaling pathway, adaptations to high altitude, and preeclampsia have recently been discussed (Ahmed et al., 2017). Evolutionary selection drives optimization of reproductive performance for a given set of environmental conditions, e.g., oxygen tension. Mobility of populations creates mismatches in the efficiencies of hypoxia-dependent adaptive mechanisms and environmental challenges, and the potential for pathologies (Petousi and Robbins, 2014).
Overview
Local oxygen concentrations during critical phases of placentation dictate the extent of trophoblast-directed uterine vascular remodeling and the development of the trophoblast-fetal vascular nutrient delivery system. Hypoxia exposures can elicit adaptations or they can overwhelm adaptive measures, resulting in structural and functional deficits in the placenta.
VASOACTIVE REGULATORS CONTROLLING OXYGEN DELIVERY TO THE PLACENTATION SITE
Regulatory processes controlling uteroplacental vascular connectivity can affect oxygen delivery and placental development.
NOS3
Endothelial nitric oxide synthase (ENOS, also called NOS3) is expressed in endothelial cells, is responsible for the biosynthesis of nitric oxide (a potent vasodilator), and has been linked to vascular homeostasis (Li and Forstermann, 2000) and adaptations at the placentation site (Boeldt et al., 2011). Pregnant mice with Nos3 null mutations are hypertensive, resulting in placental hypoxia, disrupted placental transport, and fetal growth restriction (Kulandavelu et al., 2012; Kusinski et al., 2012). Maternal Nos3 deficiency adversely affects uteroplacental vascular adaptations, whereas fetal Nos3 deficiency compromises the fetoplacental vasculature and fetal growth (Kulandavelu et al. 2013; Rennie et al. 2015).
ACE2
Angiotensin converting enzyme-2 (ACE2) degrades angiotensin II (Ang-II, a potent vasoconstrictor) to Ang1–7 (a vasodilator), and possesses a pivotal role in controlling vasomotor tone in uterine spiral arteries (Yamaleyeva et al., 2015). A null mutation at the mouse Ace2 locus results in disruptions in the maternal vasculature, resulting in placental hypoxia, and fetal growth restriction.
INFLAMMATION AND OXYGEN DELIVERY TO THE PLACENTATION SITE
Inflammatory states can regulate oxygen delivery to the placentation site and can be experimentally-induced via lipopolysaccharide (LPS) treatment. LPS-induced inflammation during rat pregnancy results in disruptions of intrauterine trophoblast invasion and the uteroplacental vasculature, and leads to fetal growth restriction (Renaud et al., 2011; Cotechini et al., 2014). These placental inflammatory events are associated with hypoxia and the stabilization of HIF1A (Robb et al., 2017). Treatments with interleukin 10 (IL-10), a nitric oxide mimetic, or a tumor necrosis factor α antagonist moderate inflammation-induced placental hypoxia and fetal growth restriction (Robb et al., 2017).
NK CELLS AND OXYGEN DELIVERY TO THE PLACENTATION SITE
NK cells are prominent contributors to remodeling uterine spiral arteries (Zhang et al., 2011; Chakraborty et al., 2012). They accumulate in the decidualized uterus and target uterine spiral arteries, where they facilitate arterial vessel migration toward the developing placenta and stimulate degenerative changes in the tunica media of uterine arteries/arterioles and their conversion to flaccid low resistance vessels. Through these actions, NK cells affect oxygen delivery to trophoblast progenitors within the developing placenta (Chakraborty et al., 2011). In the rat, NK cell deficiency results in local hypoxia at the gd 9.5 placentation site and the activation of endovascular trophoblast invasion and extensive trophoblast-guided uterine spiral arteriole restructuring (Chakraborty et al., 2011; Renaud et al., 2017). Thus, during pregnancy, uterine NK cells promote oxygen delivery to the placentation site and effectively attenuate and delay delivery of hypoxic signals capable of activating endovascular trophoblast development.
PLACENTAL HEMATOPOIESIS
The placenta plays a role in red blood cell development with the potential to directly affect oxygen homeostasis in the fetus (Dzierzak and Robin, 2010; Gekas et al., 2010). Stem cells with the capacity for multilineage hematopoiesis are found in the mouse placenta (Gekas et al., 2005; Ottersback and Dzierzak 2005). This stem cell population resides in the labyrinth zone and originates from chorionic and allantoic mesoderm (Rhodes et al., 2008) in a niche supported by endothelial-trophoblast platelet derived growth factor-B signaling (Chhabra et al., 2012). Hypoxia and HIF1A contribute to the regulation of hematopoietic stem and progenitor cell development in the placenta (Imanirad and Dzierzak, 2013; Imanirad et al., 2013). The capacity for blood cell development is conserved in the human placenta (Barcena et al., 2009; Robin et al., 2009; Van Handel et al., 2010).
REGULATORS OF HYPOXIA-DEPENDENT PLACENTAL ADAPTATIONS
Several regulators independent of the hypoxia/HIF signaling pathway have been identified, with roles in modulating placental adaptations to hypoxia (see Table 2).
Table 2.
Genetic control of hypoxia-dependent placental adaptations.
| Gene symbol | Species | Mutationa | Placental phenotype in response to hypoxia exposure | References |
|---|---|---|---|---|
| Prl family genes | ||||
| Prl4a1 | Mouse | Global-LOF | Disruption of trophoblast-vascular interactions leading to placental insufficiency and pregnancy failure | Ain et al., 2004 |
| Prl8a2 | Mouse | Global-LOF | Mesometrial vascular lesions, decreased endovascular trophoblast invasion, and pregnancy failure | Alam et al., 2007 |
| Prl7b1 | Mouse | Global-LOF | Failure of hypoxia-dependent expansion of the junctional zone | Bu et al., 2016 |
| Other genes | ||||
| Ndrg1 | Mouse | Global-LOF | Fetal growth restriction and compromised pregnancies | Larkin et al., 2014 |
| Isg15 | Mouse | Global-LOF | Pregnancy failure | Henkes et al., 2015 |
| Il10 | Mouse | Global-LOF | Hypertension, kidney pathologies, and increased pregnancy failure | Lai et al., 2011 |
| Nos3 | Mouse | Global-LOF | Pregnancy failure | Rueda-Clausen et al., 2014 |
| Comt | Mouse | Global-LOF | Fetal growth restriction | Rueda-Clausen et al., 2014 |
| Kdm3a | Rat | Trophoblast-LOF | Disruption of hypoxia-activated endovascular trophoblast invasion | Chakraborty et al., 2016 |
| Mmp12 | Rat | Global-LOF | Disruption of hypoxia-activated endovascular trophoblast invasion | Chakraborty et al., 2016 |
Prolactin (PRL) family
In mammals, PRL is a hormone/cytokine with strong links to pregnancy and lactation (Soares, 2004). The ancestral PRL gene has undergone species-specific expansions, resulting in a family of nearly two dozen related genes in the mouse and rat (Wiemers et al., 2003b; Mallon et al., 2004; Alam et al., 2006). PRL family paralogs are primarily hormones/cytokines of pregnancy, and are expressed predominantly in the anterior pituitary, uterus, and placenta (Soares, 2004). Some members of the PRL family regulate cellular function through interactions with the classical PRL receptor, while other PRL family ligands utilize non-canonical modes of action that are not yet well defined (Soares, 2004). Targeted mouse mutagenesis has led to insights regarding roles for some PRL family hormones as regulators of adaptations to maternal hypoxia (Soares et al., 2006, 2007). Roles for three non-canonical mouse PRL family ligands in pregnancy-dependent adaptations to hypoxia have been investigated: PRL-like protein A (PLP-A), decidual prolactin related protein (DPRP), and PLP-N.
PLP-A
PRL family 4, subfamily a, member 1 (Prl4a1) encodes PLP-A, which is expressed in spongiotrophoblast cells and trophoblast giant cells from midgestation to term (Campbell et al., 1989; Lin et al., 1997a; Müller et al., 1998). A deficit in the PLP-A protein does not adversely affect the reproductive performance of mice maintained under standard animal husbandry conditions. However, when pregnant Prl4a1 null mice are challenged by exposure to hypoxia, they are not able to successfully adapt and their pregnancies fail due to placental insufficiency (Ain et al., 2004). Under hypoxic conditions, the absence of PLP-A disrupts requisite trophoblast-vascular interactions, compromising nutrient delivery, and leading to placental growth restriction (Ain et al., 2004). This contrasts with pregnant mice expressing PLP-A, which successfully adapt to low oxygen and maintain their pregnancies. At least some of the actions of PLP-A are mediated through modulation of uterine NK cells (Müller et al., 1999; Ain et al., 2003b).
DPRP
PRL family 8, subfamily a, member 2 (Prl8a2) encodes DPRP, a heparin binding cytokine (Roby et al., 1993; Rasmussen et al., 1996; Wang et al., 2000; Alam et al., 2008). DPRP is expressed in decidual cells, representing a prominent marker of decidualization, and an early post-embryo implantation event (Gu et al., 1994; Rasmussen et al., 1997; Lin et al., 1997a; Orwig et al., 1997a; Orwig et al., 1997b, 1999). Reproductive performance under standard housing conditions does not differ significantly between Prl8a2 null and wild type mice (Alam et al., 2007). Unlike wild type pregnant female mice, DPRP deficient pregnant females are unable to adapt to a hypoxic environment and pregnancies fail by midgestation. Pregnancy failures in hypoxia-exposed Prl8a2 nulls are associated with prominent vascular lesions, enlarged mesometrial blood spaces, distorted chorioallantoic placentas, and decreased endovascular trophoblast invasion (Alam et al., 2007). Although the mechanism of DPRP action in promoting pregnancy-dependent adaptations is unknown, the ability of DPRP to suppress intrauterine endoplasmic reticulum stress responses may provide some insights (Alam et al., 2015).
PLP-N
PRL family 7, subfamily b, member 1 (Prl7b1) encodes PLP-N, which is uniquely expressed by invasive trophoblast cell lineages from midgestation to term (Ain et al., 2003a; Wiemers et al., 2003a; Wiemers et al., 2003b; Bu et al. 2016). Both endovascular and interstitial invasive trophoblast cells express PLP-N (Ain et al., 2003a; Bu et al. 2016). Disruption of the Prl7b1 gene does not adversely affect reproductive performance when mice are maintained under standard animal husbandry conditions (Bu et al. 2016). Wild type mice adapt to low oxygen with a robust expansion of the junctional zone. This placental adaptation to hypoxia is not observed in Prl7b1 deficient mice (Bu et al. 2016). Interestingly, failure to adapt in the mutant mice is not associated with pregnancy loss. In contrast, wild type mice exposed to 9.5% oxygen exhibit maladaptive responses, which lead to placental insufficiency and fetal death. In this instance, it may be advantageous to cull litter sizes when exposed to environmentally adverse conditions. These observations reflect a level of complexity not previously appreciated.
Other members of the PRL family
Biological activities of a subset of other members of the PRL family suggest that they may also contribute to hypoxia-dependent placental adaptations. Proliferin (PLF; PRL family 2, subfamily c, member 1; PRL2C1) and proliferin-related protein (PLF-RP; PRL family 7, subfamily d, member 1; PRL7D1) are expressed by trophoblast giant cells and spongiotrophoblast cells, respectively (Colosi et al., 1988; Lee et al., 1988), and act to reciprocally modulate angiogenesis (Jackson et al., 1994). Mouse PLP-E (PRL family 7, subfamily a, member 1; PRL7A1) and its rat ortholog, PLP-Fα (PRL family 7, subfamily a, member 3; PRL7A3), are produced by trophoblast giant cells under the control of oxygen tension (Lin et al. 1997b; Ho-Chen et al., 2007), and are regulators of blood cell development, including erythropoiesis (Lin and Linzer, 1999; Bittorf et al., 2000). Thus, through these ligands, the placenta can regulate oxygen delivery by modulating blood vessel formation and the kinetics of red blood cell expansion. The consequences of mutagenesis of these PRL family genes on placentation or placental adaptions to hypoxia have not been determined.
Other modulators of hypoxia-dependent placental adaptations
NDRG1
N-myc downstream regulated gene 1 (NDRG1) is a known hypoxia responsive transcript in human trophoblast with protective roles in preventing hypoxia-induced injury (Roh et al., 2005). Disruption of the mouse Ndrg1 locus leads to fetal growth restriction and compromised placental adaptations to hypoxia (Larkin et al., 2014).
ISG15
Interferon-stimulated gene 15 (Isg15) is an interferon-responsive gene expressed in rodent and primate placentas (Hansen and Pru, 2014) and through covalent interactions modifies the activities of intracellular proteins (Zhao et al., 2005). A null mutation at the Isg15 locus compromises fetal survival, which is exacerbated in pregnancies maintained under hypoxic conditions (Henkes et al., 2015).
IL-10
Anti-inflammatory cytokines, such as IL-10 (Mosser and Zhang, 2008), have been linked to pregnancy and pregnancy-related diseases (Thaxton and Sharma, 2010). Genetic disruption of the Il10 gene compromises pregnant mice exposed to hypoxia, resulting in hypertension, kidney pathologies, and increased pregnancy loss (Lai et al., 2011). These adverse effects in hypoxia-exposed pregnant Il10 null mice can be reversed by treatment with IL-10 (Lai et al., 2011).
NOS3
A deficiency in NOS3 undermines hypoxia-dependent adaptations in pregnant mice (Rueda-Clausen et al., 2014). While wild type pregnant mice can adapt to hypoxia exposures, NOS3 deficient mice do not sufficiently adapt and pregnancies fail. Specifically, NOS3 appears to contribute to hypoxia-dependent adaptations in the labyrinth zone of the mouse placenta (Schäffer et al., 2006).
COMT
Catechol-O-methyltransferase (COMT) is an enzyme involved in the catabolism of catecholamines and other molecules possessing a catechol structure, such as estrogens (Shenoy et al., 2010; Thomas and Potter, 2013). Comt null mice can exhibit pregnancy complications (Kanasaki et al., 2008), and show failures in adaptations to hypoxia resulting in fetal growth restriction (Rueda-Clausen et al., 2014). In the guinea pig, chronic hypoxia results in the downregulation of placental COMT transcript expression (Thompson et al., 2016).
Overview
In summary, several regulatory mechanisms are utilized in placentation during exposure to hypoxia. Some of these regulators are not required for placenta development during the normal course of gestation but become essential contributors as the placenta adapts to physiological stressors. Members of the PRL family may facilitate placental and pregnancy-associated adaptations in some species, including the mouse, while in other species adaptations may be accomplished by other ligands or mechanisms. The placenta is the source of numerous species-specific gene family expansions whose biology is yet to be fully resolved but may contribute to pregnancy-dependent adaptations (Soares et al., 2006; Rawn and Cross, 2008). At least one of the drivers for the evolution of these gene families may have been oxygen deficits, with the goal of optimizing reproductive performance in a diversity of challenging environments.
FINAL THOUGHTS
Signaling pathways controlling oxygen homeostasis are integral regulators of hemochorial placental development. This is best illustrated by mouse mutagenesis experiments, showing components of the HIF signaling pathway contribute in fundamental ways to development of the placenta. Hemochorial placentation can exhibit exceptional plasticity that is demonstrably responsive to hypoxia. Oxygen tension can guide trophoblast lineage decisions, impacting how the placenta forms a functional interface with the maternal environment and its effectiveness in supporting fetal growth. The robust sensitivity of the invasive/extravillous trophoblast cell lineage to oxygen tension is illustrative of this concept. Placental plasticity is not a constant during gestation, but instead is most evident during early stages of pregnancy when trophoblast stem and progenitor cell populations are prominent and environmental challenges are capable of molding placental development to maximize performance. This represents an effective strategy when the environmental challenge present during the critical sensitive period persists through gestation. Investigations of high altitude-adapted native populations highlight how natural selection has directed optimization of placental adaptations through targeting genes in the HIF signaling pathway. The mobility of present day human populations creates mismatches and the potential for discordant responses to environmental challenges. Plasticity should be viewed as an intrinsic measure of a healthy placenta and placental disease a consequence when trophoblast cell adaptive responses are overwhelmed or dysregulated. Thus, experimental interrogation of mechanisms controlling plasticity can provide insights into the etiology of placental disease. Molecular dissection of TS cell models, which exhibit maximal plasticity, is an effective strategy for determining trophoblast cell potential and an efficient approach for generating hypotheses of mechanisms controlling placental adaptations. These hypotheses are best tested in relevant animal models of hemochorial placentation.
Acknowledgments
Research from the authors’ laboratory was supported by an American Heart Association postdoctoral fellowship to K.K. and grants from the National Institutes of Health (HD020676, HD079363, HD082535).
References
- Abbott BD, Buckalew AR. Placental defects in ARNT-knockout conceptus correlate with localized decreases in VEGF-R2, Ang-1, and Tie-2. Dev Dyn. 2000;219:526–538. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1080>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Adelman DM, Gertsenstein M, Nagy A, et al. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SIY, Ibrahim ME, Khalil EAG. High altitude and pre-eclampsia: Adaptation or protection. Med Hypotheses. 2017;104:128–132. doi: 10.1016/j.mehy.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003a;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Ain R, Dai G, Dunmore JH, et al. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA. 2004;101:16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003b;204:65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Alam SM, Ain R, Konno T, Ho-Chen JK, Soares MJ. The rat prolactin gene family locus: species-specific gene family expansion. Mamm Genome. 2006;17:858–877. doi: 10.1007/s00335-006-0010-1. [DOI] [PubMed] [Google Scholar]
- Alam SMK, Konno T, Dai G, et al. A uterine decidual-cell cytokine ensures pregnancy-dependent adaptations to hypoxia. Development. 2007;134:407–415. doi: 10.1242/dev.02743. [DOI] [PubMed] [Google Scholar]
- Alam SM, Konno T, Sahgal N, et al. Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions. J Biol Chem. 2008;283:18957–18968. doi: 10.1074/jbc.M801826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Konno T, Soares MJ. Identification of target genes for a prolactin family paralog in mouse decidua. Reproduction. 2015;149:625–632. doi: 10.1530/REP-15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsat E, Wyplosz P, Malassine A, et al. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168:346–353. doi: 10.1002/(SICI)1097-4652(199608)168:2<346::AID-JCP13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Arimoto-Ishida E, Sakata M, Sawada K, et al. Up-regulation of α5-integrin by E-cadherin loss in hypoxia and its key role in the migration of extravillous trophoblast cells during early implantation. Endocrinology. 2009;150:4306–4315. doi: 10.1210/en.2008-1662. [DOI] [PubMed] [Google Scholar]
- Asanoma K, Rumi MA, Kent LN, et al. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Bragança J, Eloranta JJ, et al. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Barcena A, Muench MO, Kapidzic M, Fisher SJ. A new role for the human placenta as a hematopoietic site throughout gestation. Reprod Sci. 2009;16:178–187. doi: 10.1177/1933719108327621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC, Goldstein MC. Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci USA. 2004;101:14300–14304. doi: 10.1073/pnas.0405949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlow RB, Dyson HJ, Wright PE. Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature. 2017;543:447–451. doi: 10.1038/nature21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, et al. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW. Genetics of human origin and evolution: high-altitude adaptations. Curr Opin Genet Dev. 2016;41:8–13. doi: 10.1016/j.gde.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittorf T, Jaster R, Soares MJ, et al. Induction of erythroid proliferation and differentiation by a trophoblast-specific cytokine involves activation of the JAK/STAT pathway. J Mol Endocrinol. 2000;25:253–262. doi: 10.1677/jme.0.0250253. [DOI] [PubMed] [Google Scholar]
- Bobek G, Stait-Gardner T, Surmon L, et al. Magnetic resonance imaging detects placental hypoxia and acidosis in mouse models of perturbed pregnancies. PLOS One. 2013;8:e59971. doi: 10.1371/journal.pone.0059971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeldt DS, Yi FX, Bird IM. eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium—new insights into eNOS regulation through adaptive cell signaling. J Endocrinol. 2011;210:243–258. doi: 10.1530/JOE-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- Bu P, Alam SMK, Dhakal P, et al. A prolactin family paralog regulates placental adaptations to a physiological stressor in the mouse. Biol Reprod. 2016;94:107. doi: 10.1095/biolreprod.115.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ. Oxygen, the Janus gas: its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jaunaiux E. Maternal emodeling ion of the human placenta: does the embryo develop in a hypoxic environment? Gynecol Obstet Fertil. 2001;29:503–508. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Reshetnikova OS, Milovanov AP, Teleshova OV. Stereological evaluation of vascular adaptations in human placental villi to differing forms of hypoxic stress. Placenta. 1996;17:49–55. doi: 10.1016/s0143-4004(05)80643-5. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Deb S, Kwok SC, Joslin JA, Soares MJ. Differential expression of placental emodelin-II and prolactin-like protein-A in the rat chorioallantoic placenta. Endocrinology. 1989;125:1565–1574. doi: 10.1210/endo-125-3-1565. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Winter JL. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies—a review. Placenta. 2002;23(Suppl A):S47–S57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Keogh RJ, Tissot van Patot MC. Hypoxia and placental remodeling. In: Roach RC, Wagner PD, Hackett PH, editors. Hypoxia and the Circulation. Springer; New York: 2007. pp. 117–130. [Google Scholar]
- Chakraborty D, Cui W, Rosario GX, et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci USA. 2016;113:E7212–E7221. doi: 10.1073/pnas.1612626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Rumi MAK, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating HIF-dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Rumi MA, Soares MJ. NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle. 2012;11:2427–2430. doi: 10.4161/cc.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A, Lechner AJ, Ueno M, et al. Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Dev Cell. 2012;22:651–659. doi: 10.1016/j.devcel.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Sanders TA, Tormos KV, et al. ECM-dependent HIF induction directs trophoblast stem cell fate via LIMK1-mediated cytoskeletal rearrangement. PLOS One. 2013;8:e56949. doi: 10.1371/journal.pone.0056949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosi P, Swiergiel JJ, Wilder EL, et al. Characterization of proliferin-related protein. Mol Endocrinol. 1988;2:579–586. doi: 10.1210/mend-2-6-579. [DOI] [PubMed] [Google Scholar]
- Cotechini T, Komisarenko M, Sperou A, et al. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211:165–179. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden Dahl KD, Fryer BH, Mack FA, et al. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol Cell Biol. 2005a;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden Dahl KD, Robertson SE, et al. Hypoxia-inducible factor regulates αvβ3 integrin cell surface expression. Mol Biol Cell. 2005b;16:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta RA, et al. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278:7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- De Grauw TJ, Myers RE, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate. 1986;49:85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- Dhakal P, Iqbal K, Soares MJ. Exploring roles for Cited2 in hemochorial placentation; 49th Annual Meeting of the Society for the Study of Reproduction; San Diego, CA. 2016. [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Robin C. Placenta as a source of hematopoietic stem cells. Trends Mol Med. 2010;16:361–367. doi: 10.1016/j.molmed.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool. 1993;266:578–587. doi: 10.1002/jez.1402660608. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Telugu BP, Roberts RM. Model systems for studying trophoblast differentiation from human pluripotent stem cells. Cell Tissue Res. 2012;349:809–824. doi: 10.1007/s00441-012-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Katz ME, Milligan AJ, et al. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–2204. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- Faridy EE, Sanii MR, Thliveris JA. Fetal lung growth: influence of maternal hypoxia and hyperoxia in rats. Respir Physiol. 1988;73:225–241. doi: 10.1016/0034-5687(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters, and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Kung AL, et al. Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol. 2003;10:504–512. doi: 10.1038/nsb936. [DOI] [PubMed] [Google Scholar]
- Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- Gekas C, Dieterien-Lievre F, Orkin SH, Mikkola HKA. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gekas C, Rhodes KE, Van Handel B, et al. Hematopoietic stem cell development in the placenta. Int J Dev Biol. 2010;54:1089–1098. doi: 10.1387/ijdb.103070cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, et al. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- George EM, Garrett MR, Granger JP. Placental ischemia induces changes in gene expression in chorionic tissue. Mamm Genome. 2014;25:253–261. doi: 10.1007/s00335-014-9505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Gheorghe CP, Mohan S, Oberg KC, Longo LD. Gene expression patterns in the hypoxic murine placenta: a role in epigenesis? Reprod Sci. 2007;14:223–233. doi: 10.1177/1933719107302860. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Postovit LM, Park H, et al. Role of oxygen in the regulation of trophoblast gene expression and invasion. Placenta. 2000;21:443–450. doi: 10.1053/plac.2000.0543. [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, et al. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M, Simon MC. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol. 2006;13:169–174. doi: 10.1097/01.moh.0000219663.88409.35. [DOI] [PubMed] [Google Scholar]
- Gu Y, Soares MJ, Srivastava RK, Gibori G. Expression of decidual prolactin-related protein in the rat decidua. Endocrinology. 1994;135:1422–1427. doi: 10.1210/endo.135.4.7925104. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, et al. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Gultice AD, Kulkarni-Datar K, Brown TL. Hypoxia-inducible factor 1alpha (HIF1A) mediates distinct steps of rat trophoblast differentiation in gradient oxygen. Biol Reprod. 2009;80:184–193. doi: 10.1095/biolreprod.107.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultice AD, Selesniemi KL, Brown TL. Hypoxia inhibits differentiation of lineage-specific Rcho-1 trophoblast giant cells. Biol Reprod. 2006;74:1041–1050. doi: 10.1095/biolreprod.105.047845. [DOI] [PubMed] [Google Scholar]
- Hampl V, Jakoubek V. Regulation of fetoplacental vascular bed by hypoxia. Physiol Res. 2009;58(Suppl 2):S87–S93. doi: 10.33549/physiolres.931922. [DOI] [PubMed] [Google Scholar]
- Hansen TR, Pru JK. ISGylation: a conserved pathway in mammalian pregnancy. Adv Exp Med Biol. 2014;759:13–31. doi: 10.1007/978-1-4939-0817-2_2. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Henkes LE, Pru JK, Ashley RL, et al. Embryo mortality in Isg15−/− mice is exacerbated by environmental stress. Biol Reprod. 2015;92:36. doi: 10.1095/biolreprod.114.122002. [DOI] [PubMed] [Google Scholar]
- Higgins JS, Vaughan OR, de Liger EF, et al. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594:1341–1356. doi: 10.1113/JP271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Rupert JL. Fine tuning the HIF-1 ‘global’ O2 sensor for hypobaric hypoxia in Andean high-altitude natives. Bioessays. 2003;25:515–519. doi: 10.1002/bies.10261. [DOI] [PubMed] [Google Scholar]
- Ho-Chen JK, Bustamante JJ, Soares MJ. Prolactin-like protein-F subfamily of placental hormones/cytokines: responsiveness to maternal hypoxia. Endocrinology. 2007;148:559–565. doi: 10.1210/en.2006-1146. [DOI] [PubMed] [Google Scholar]
- Horii M, Li Y, Wakeland AK, Pizzo DP, et al. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc Natl Acad Sci USA. 2016;113:E3882–3891. doi: 10.1073/pnas.1604747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ST, Vo KC, Lyell DJ, et al. Developmental response to hypoxia. FASEB J. 2004;18:1348–1365. doi: 10.1096/fj.03-1377com. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Gauster M, Orendi K, et al. Oxygen as modulator of trophoblast invasion. J Anat. 2009;215:14–20. doi: 10.1111/j.1469-7580.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvizdosova-Klescova A, Uhlik J, Malina M, et al. Remodeling of fetoplacental arteries in rats due to chronic hypoxia. Exp Toxicol Pathol. 2013;65:97–103. doi: 10.1016/j.etp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Ietta F, Wu Y, Winter J, et al. Dynamic HIF1A regulation during human placental development. Biol Reprod. 2006;75:112–121. doi: 10.1095/biolreprod.106.051557. [DOI] [PubMed] [Google Scholar]
- Imanirad P, Dzierzak E. Hypoxia and HIFs in regulating the development of the hematopoietic system. Blood Cells Mol Dis. 2013;51:256–263. doi: 10.1016/j.bcmd.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanirad P, Kartalaei PS, Crisan M, et al. HIF1α is a regulator of hematopoietic progenitor and stem cell development in hypoxic sites of the mouse embryo. Stem Cell Res. 2013;12:24–35. doi: 10.1016/j.scr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Huang X. miR-210: fine-tuning the hypoxic response. Adv Exp Med Biol. 2014;772:205–227. doi: 10.1007/978-1-4614-5915-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Volpert OV, Bouck N, Linzer DIH. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- Jakoubek V, Bíbová J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol. 2008;294:H1638–H1644. doi: 10.1152/ajpheart.01120.2007. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (Mammalian achaete-scute homologous protein-2) Mol Endocrinol. 2000;14:1661–1673. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- Jiang B, Mendelson CR. USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia. Mol Cell Biol. 2003;23:6117–128. doi: 10.1128/MCB.23.17.6117-6128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrov M, Schmitz C, Black S, et al. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- Kenchegowda D, Natale B, Lemus MA, et al. Inactivation of maternal Hif-1α at mid-pregnancy causes placental defects and deficits in oxygen delivery to the fetal organs under hypoxic stress. Dev Biol. 2017;422:171–185. doi: 10.1016/j.ydbio.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R, Wayment M, Merrill D, et al. Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR) Physiol Rep. 2015;3:e12651. doi: 10.14814/phy2.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, et al. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Knipp GT, Audus KL, Soares MJ. Nutrient transport across the placenta. Adv Drug Deliv Rev. 1999;38:41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Koch Y, van Fürden B, Kaiser S, et al. Connexin 31 (GJB3) deficiency in mouse trophoblast stem cells alters giant cell differentiation and leads to loss of oxygen sensing. Biol Reprod. 2012;87:37. doi: 10.1095/biolreprod.111.098079. [DOI] [PubMed] [Google Scholar]
- Konno T, Graham AR, Rempel LA, et al. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology. 2010;151:4537–4550. doi: 10.1210/en.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- Konno T, Rempel LA, Rumi MA, et al. Chromosome-substituted rat strains provide insights into the genetics of placentation. Physiol Genomics. 2011;43:930–941. doi: 10.1152/physiolgenomics.00069.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkes HA, De Oliveira L, Sass N, et al. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens Pregnancy. 2017;36:145–150. doi: 10.1080/10641955.2016.1259627. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- Krawczynski K, Mishima T, Huang X, Sadovsky Y. Intact feto-placental growth in microRNA-210 deficient mice. Placenta. 2016;47:113–115. doi: 10.1016/j.placenta.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulandavelu S, Whiteley KJ, Bainbridge SA, et al. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61:259–266. doi: 10.1161/HYPERTENSIONAHA.112.201996. [DOI] [PubMed] [Google Scholar]
- Kulandavelu S, Whiteley KJ, Qu D, et al. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension. 2012;60:231–238. doi: 10.1161/HYPERTENSIONAHA.111.187559. [DOI] [PubMed] [Google Scholar]
- Kunath T, Yamanaka Y, Detmar J, et al. Developmental differences in the expression of FGF receptors between human and mouse embryos. Placenta. 2014;35:1079–1088. doi: 10.1016/j.placenta.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Kusinski LC, Stanley JL, Dilworth MR, et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303:R86–R93. doi: 10.1152/ajpregu.00600.2011. [DOI] [PubMed] [Google Scholar]
- Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chen B, Shi XH, et al. NDRG1 deficiency attenuates fetal growth and the intrauterine response to hypoxic injury. Endocrinology. 2014;155:1099–1106. doi: 10.1210/en.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CQ, Gardner L, Turco M, et al. What Is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Reports. 2016;6:257–272. doi: 10.1016/j.stemcr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Romero R, Kim JS, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011;179:590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Talamantes F, Wilder E, et al. Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology. 1988;122:1761–1768. doi: 10.1210/endo-122-5-1761. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jeong CH, Koo SY, et al. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Leno-Duran E, Hatta K, Bianco J, et al. Fetal-placental hypoxia does not result from failure of spiral arterial modification in mice. Placenta. 2010;31:731–737. doi: 10.1016/j.placenta.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303:H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Linzer DI. Induction of megakaryocyte differentiation by a novel pregnancy-specific hormone. J Biol Chem. 1999;274:21485–21489. doi: 10.1074/jbc.274.30.21485. [DOI] [PubMed] [Google Scholar]
- Lin J, Poole J, Linzer DIH. Three new members of the mouse prolactin/growth hormone family are homologous to proteins expressed in the rat. Endocrinology. 1997a;138:5541–5549. doi: 10.1210/endo.138.12.5626. [DOI] [PubMed] [Google Scholar]
- Lin J, Poole J, Linzer DIH. Two novel members of the prolactin/growth hormone family are expressed in the mouse placenta. Endocrinology. 1997b;138:5535–5540. doi: 10.1210/endo.138.12.5636. [DOI] [PubMed] [Google Scholar]
- Lou X, Sun S, Chen W, et al. Negative feedback regulation of NF-κB action by CITED2 in the nucleus. J Immunol. 2011;186:539–548. doi: 10.4049/jimmunol.1001650. [DOI] [PubMed] [Google Scholar]
- Lueder FL, Kim S-B, Buroker CA, et al. Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism. 1995;44:532–537. doi: 10.1016/0026-0495(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon AM, Wilming L, Weekes J, et al. Organization and evolution of a gene-rich region of the mouse genome: a 12.7-Mb region deleted in the Del(13)Svea36H mouse. Genome Res. 2004;14:1888–1901. doi: 10.1101/gr.2478604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Krampitz GW, Okazaki KM, et al. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, et al. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Matheson H, Veerbeek JH, Charnock-Jones DS, et al. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594:1371–1388. doi: 10.1113/JP271073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM. Changes in fetal capillaries during preplacental hypoxia: growth, shape remodeling and villous capillarization in placentae from high-altitude pregnancies. Placenta. 2003;24:191–198. doi: 10.1053/plac.2002.0895. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, Kingdom J, et al. Peroxisome proliferator-activated receptor-γ as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58:280–286. doi: 10.1161/HYPERTENSIONAHA.111.172627. [DOI] [PubMed] [Google Scholar]
- Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol. 2011;178:181–190. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, et al. Maternal adaptation to high-altitude pregnancy: an experiment of nature—a review. Placenta. 2004;25:S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Artap ST, Shi H, et al. Cited2 is required in trophoblasts for correct placental capillary patterning. Dev Biol. 2014;392:62–79. doi: 10.1016/j.ydbio.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Ishimura R, Orwig KE, et al. Homologues for prolactin-like proteins A and B are present in the mouse. Biol Reprod. 1998;58:45–51. doi: 10.1095/biolreprod58.1.45. [DOI] [PubMed] [Google Scholar]
- Müller H, Liu B, Croy BA, et al. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein-A. Endocrinology. 1999;140:2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17:83. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Johnson RD, Smith S, et al. Hypoxia limits differentiation and upregulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol. 1999;180:896–902. doi: 10.1016/s0002-9378(99)70661-7. [DOI] [PubMed] [Google Scholar]
- Nelson ML, Cons JM, Hodgdon GE. Effects of simulated high altitude (3800 m) on reproductive function in the pregnant rat. Environ Physiol Biochem. 1975;5:65–72. [PubMed] [Google Scholar]
- Orwig KE, Dai G, Rasmussen CA, Soares MJ. Decidual/trophoblast prolactin-related protein: characterization of gene structure and cell-specific expression. Endocrinology. 1997a;138:2491–2500. doi: 10.1210/endo.138.6.5155. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Ishimura R, Müller H, et al. Identification and characterization of a mouse homolog for decidual/trophoblast prolactin-related protein. Endocrinology. 1997b;138:5511–5517. doi: 10.1210/endo.138.12.5628. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Soares MJ. Transcriptional activation of the decidual/trophoblast prolactin-related protein gene. Endocrinology. 1999;140:4032–4039. doi: 10.1210/endo.140.9.6954. [DOI] [PubMed] [Google Scholar]
- Ottersback K, Dzierak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ozolins TRS, Fisher TS, Nadeau DM, et al. Defects in embryonic development EGLN1/PHD2 knockdown transgenic mice are associated with induction of Igfbp in the placenta. Biochem Biophys Res Commun. 2009;390:372–376. doi: 10.1016/j.bbrc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Palmer SK, Moore LG, Young D, et al. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- Petousi N, Robbins PA. Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol. 2014;116:875–884. doi: 10.1152/japplphysiol.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Robertson WB, Brosens I, Dixon G. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L. Animal models of deep trophoblast invasion. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders: basic science and its translation to obstetrics. Cambridge University Press; Cambridge: 2010. pp. 127–139. [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Sferruzzi-Perri AN, et al. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010;16:415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Thompson JG, Roberts CT. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta. 2007;28:1147–1157. doi: 10.1016/j.placenta.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Brandon HM, Daftary A, et al. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000;63:559–569. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Michael HM, Daftary A, et al. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: implications for expression of HIF-alpha proteins. Placenta. 2008;29:290–299. doi: 10.1016/j.placenta.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Whitelock KA, Weissfeld LA, et al. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2α, in placentas from women with preeclampsia. Biol Reprod. 2001;64:499–506. doi: 10.1093/biolreprod/64.2.499. [DOI] [PubMed] [Google Scholar]
- Rasmussen CA, Hashizume K, Orwig KE, et al. Decidual prolactin-related protein: heterologous expression and characterization. Endocrinology. 1996;137:5558–5566. doi: 10.1210/endo.137.12.8940384. [DOI] [PubMed] [Google Scholar]
- Rasmussen CA, Orwig KE, Vellucci S, Soares MJ. Dual expression of prolactin-related protein in decidua and trophoblast tissues during pregnancy in rats. Biol Reprod. 1997;56:647–654. doi: 10.1095/biolreprod56.3.647. [DOI] [PubMed] [Google Scholar]
- Rawn SM, Cross JC. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol. 2008;24:159–181. doi: 10.1146/annurev.cellbio.24.110707.175418. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Chakraborty D, Mason CW, et al. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc Natl Acad Sci USA. 2015;112:E6175–6184. doi: 10.1073/pnas.1507397112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud SJ, Cotechini T, Quirt JS, et al. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186:1799–1808. doi: 10.4049/jimmunol.1002679. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Scott RL, Chakraborty D, et al. Natural killer cell deficiency alters placental development in rats. Biol Reprod. 2017;96:145–158. doi: 10.1095/biolreprod.116.142752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MY, Rahman A, Whiteley KJ, et al. Site-specific increases in utero- and fetoplacental arterial vascular resistance in eNOS-deficient mice due to impaired arterial enlargement. Biol Reprod. 2015;92:48. doi: 10.1095/biolreprod.114.123968. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb KP, Cotechnini T, Allaire C, et al. Inflammation-induced fetal growth restriction in rats is associated with increased placental HIF-1α accumulation. PLOS One. 2017;12:e0175805. doi: 10.1371/journal.pone.0175805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RM, Loh KM, Amita M, et al. Differentiation of trophoblast cells from human embryonic stem cells: to be or not to be? Reproduction. 2014;147:D1–D12. doi: 10.1530/REP-14-0080. [DOI] [PubMed] [Google Scholar]
- Robin C, Bollerot K, Mendes S, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JC, Heizer A, Hardiman A, et al. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta. 2007;28:1141–1146. doi: 10.1016/j.placenta.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Roby KF, Deb S, Gibori G, et al. Decidual prolactin-related protein. Identification, molecular cloning, and characterization. J Biol Chem. 1993;268:3136–3142. [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Roh C-R, Budhraja V, Kim H-S, et al. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Rolfo A, Many A, Racano A, et al. Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. PloS One. 2010;5:e13288. doi: 10.1371/journal.pone.0013288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Clause CF, Stanley JL, Thambiraj DF, et al. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod Sci. 2014;21:492–502. doi: 10.1177/1933719113503401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragawa N, Matsui A, Matsuzaka T, et al. Enhanced glucose metabolism and impaired placental function in hypoxic pregnant rats. Nucl Med Biol. 1988;15:645–650. doi: 10.1016/0883-2897(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Schäffer L, Vogel J, Breymann C, et al. Preserved placental oxygenation and development during severe systemic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2006;290:R844–R851. doi: 10.1152/ajpregu.00237.2005. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Kovach A, Meyer J, et al. Brief, intermittent hypoxia restricts fetal growth in Sprague-Dawley rats. Biol Neonate. 1998;73:313–319. doi: 10.1159/000013990. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metabol. 2010;21:529–536. doi: 10.1016/j.tem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Skeffington KL, Higgins JS, Mahmoud AD, et al. Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol. 2016;594:1357–1369. doi: 10.1113/JP270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Alam SMK, Konno T, et al. The prolactin gene family and pregnancy-dependent adaptations. Anim Sci J. 2006;77:1–9. [Google Scholar]
- Soares MJ, Chakraborty D, Kubota K, et al. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Intl J Dev Biol. 2014;58:247–259. doi: 10.1387/ijdb.140083ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Chakraborty D, Rumi MAK, et al. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Chapman BM, Rasmussen CA, et al. Differentiation of trophoblast endocrine cells. Placenta. 1996;17:277–289. doi: 10.1016/s0143-4004(96)90051-x. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Konno T, Alam SMK. The prolactin family: effectors of pregnancy-specific adaptations. Trends Endocrinol Metabol. 2007;18:114–121. doi: 10.1016/j.tem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Vivian JL. Tipping the balance toward trophoblast development. Proc Natl Acad Sci USA. 2016;113:5144–5146. doi: 10.1073/pnas.1604914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache V, Ciric A, Moretto-Zita M, et al. Hypoxia and trophoblast differentiation: a key role for PPARγ. Stem Cells Dev. 2013;22:2815–2824. doi: 10.1089/scd.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ho VC, Takeda H, et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Shaish A, Barshack I, et al. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol. 2010;177:2950–2962. doi: 10.2353/ajpath.2010.090800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, et al. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tapanainen PJ, Bang P, Wilson K, et al. Maternal hypoxia as a model for intrauterine growth retardation: effects on insulin-like growth factors and their binding proteins. Pediatric Res. 1994;36:152–158. doi: 10.1203/00006450-199408000-00004. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MP, Potter BVL. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol. 2013;137:27–49. doi: 10.1016/j.jsbmb.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP, Pence L, Pinkas G, et al. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95:128. doi: 10.1095/biolreprod.116.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot van Patot MC, Bendrick-Peart J, Beckey VE, et al. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L525–L532. doi: 10.1152/ajplung.00203.2003. [DOI] [PubMed] [Google Scholar]
- Tissot van Patot M, Grilli PA, Chapman P, et al. Remodeling of uteroplacental arteries is decreased in high altitude placentae. Placenta. 2003;24:326–335. doi: 10.1053/plac.2002.0899. [DOI] [PubMed] [Google Scholar]
- Tissot van Patot MC, Valdez M, Becky V, et al. Impact of pregnancy at high altitude on placental morphology in non-native women with and without preeclampsia. Placenta. 2009;30:523–528. doi: 10.1016/j.placenta.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Tomlinson TM, Garbow JR, Anderson JR, et al. Magnetic resonance imaging of hypoxic injury to the murine placenta. Am J Physiol Regul Integr Comp Physiol. 2010;298:R312–R319. doi: 10.1152/ajpregu.00425.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuuli MG, Longtine MS, Nelson DM. Oxygen and trophoblast biology – a source of controversy. Placenta. 2011;32:S109–S118. doi: 10.1016/j.placenta.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiman D, Mondon F, Garces-Duran A, et al. Hypoxia-activated genes from early placenta are elevated in preeclampsia, but not in intra-uterine growth retardation. BMC Genomics. 2005;6:111. doi: 10.1186/1471-2164-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sluis B, Groot AJ, Vermeulen J, et al. COMMD1 promotes pVHL and O2-independent proteolysis of HIF-1alpha via HSP90/70. PloS One. 2009;4:e7332. doi: 10.1371/journal.pone.0007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sluis B, Mao X, Zhai Y, et al. COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. J Clin Invest. 2010;120:2119–2130. doi: 10.1172/JCI40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sluis B, Muller P, Duran K, et al. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol. 2007;27:4142–4156. doi: 10.1128/MCB.01932-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel B, Rashad SL, Hassanzadeh-Kiabi N, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–3330. doi: 10.1182/blood-2010-04-279489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uitert M, Moerland PD, Enquobahrie DA, et al. Meta-analysis of placental transcriptome data identifies a novel molecular pathway related to preeclampsia. PloS One. 2015;10:e0132468. doi: 10.1371/journal.pone.0132468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeland AK, Soncin F, Moretto-Zita M, et al. Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor-dependent manner. Am J Pathol. 2017;187:767–780. doi: 10.1016/j.ajpath.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentin K, Hinze C, Schmidt-Ott KM. The basal chorionic trophoblast cell layer: An emerging coordinator of placenta development. Bioessays. 2016;38:254–265. doi: 10.1002/bies.201500087. [DOI] [PubMed] [Google Scholar]
- Wang D, Ishimura R, Walia DS, et al. Eosinophils are cellular targets of the novel uteroplacental heparin-binding cytokine decidual/trophoblast prolactin-related protein. J Endocrinol. 2000;167:15–28. doi: 10.1677/joe.0.1670015. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003a;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao LJ, Ain R, et al. The mouse prolactin gene family locus. Endocrinology. 2003b;144:313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- Withington SL, Scott AN, Saunders DN, et al. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhou S, Jiang Z, et al. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res. 2014;13:478–491. doi: 10.1016/j.scr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Yamaleyeva LM, Pulgar VM, Lindsey SH, et al. Uterine artery dysfunction in pregnant ACE2 knockout mice is associated with placental hypoxia and reduced umbilical blood flow velocity. Am J Physiol Endocrinol Metab. 2015;309:E84–E94. doi: 10.1152/ajpendo.00596.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Arenas-Hernandez M, Gomez-Lopez N, et al. Hypoxic stress forces irreversible differentiation of a majority of mouse trophoblast stem cells despite FGF4. Biol Reprod. 2016;95:110. doi: 10.1095/biolreprod.116.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen RK, Chen B, Blair JD, et al. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics. 2013;8:192–202. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Wu Y, Ietta F, et al. Human placental hypoxia-inducible factor-1α expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EG, Burton GJ, Smith SK, Charnock-Jones DS. Placental vessel adaptation during gestation and to high altitude: changes in diameter and perivascular cell coverage. Placenta. 2002;223:751–762. doi: 10.1016/s0143-4004(02)90856-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth. Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Fu Z, Linke S, et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010;11:364–378. doi: 10.1016/j.cmet.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Denison C, Huibregtse JM, et al. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci USA. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Xiao D, Hu Y, et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62:599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32:475–481. doi: 10.1016/j.placenta.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chiu K, Brescia RJ, et al. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yuge A, Rajah AM, et al. LIMK1 regulates human trophoblast invasion/differentiation and is down-regulated in preeclampsia. Am J Pathol. 2014;184:3321–3331. doi: 10.1016/j.ajpath.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


