Abstract
Conventional prostate cancer staging strategies have limited accuracy to define the location, grade, and burden of disease. Evaluations have historically relied upon prostate specific antigen levels, digital rectal examinations, random systematic biopsies, computed tomography, pelvic lymphadenectomy, and/or 99mTc-MDP bone scans. Today, risk-stratification tools incorporate these data in a weighted format to guide management. However, the limitations and potential consequences of their uncertainties are well-known. Inaccurate information may contribute to under-staging and under-treatment, or over-staging and over-treatment. Meanwhile, advances in multi-parametric MRI, whole body MRI, lymphotropic-nanoparticle MRI, and positron emission tomography are now available to improve the accuracy of risk stratification to facilitate more informed medical decisions. They also guide radiation oncologists to develop more accurate treatment plans. This review provides a primer to incorporate these advances into routine clinical workflow.
INTRODUCTION
A favorable outcome after prostate cancer radiotherapy depends on accurate staging and tumor delineation. Conventional strategies to date have relied upon the digital rectal exam (DRE), prostate specific antigen (PSA), random systematic biopsies, computed tomography (CT), pelvic lymphadenectomy, and 99mtechnetium-methylenediphosphonate (99mTc-MDP) bone scan. When combined with risk-stratification tools, these evaluations provide a best-practice approach to patient management. Unfortunately, this paradigm is known to underestimate the burden and location of disease in 20–30% of cases.1 This predicament contributes primarily to two separate dilemmas: (1) under-staging that can lead to under-treatment and relapse in patients initially considered to have a favorable prognosis and/or localized disease, and (2) insufficient confidence to consider active surveillance in a patient who is harboring a non-metastatic indolent prostate cancer.2 This review, therefore, highlights the utility of newer imaging technologies that can reduce some of the uncertainties of clinical staging and monitoring. It also provides practical guidance to incorporate these advanced images into radiotherapy treatment planning.
MULTI-PARAMETRIC MAGNETIC RESONANCE IMAGING
There are a number of dilemmas that radiation oncologists face when evaluating patients with newly diagnosed prostate cancer including: (1) uncertainties about the correct burden and grade of disease, and (2) uncertainties about occult extraprostatic extension (EPE), with or without seminal vesicle invasion (SVI). This is because a reliance on DREs and random systematic biopsies can easily miss occult high grade disease, or EPE when it is located in the lateral peripheral zone, anterior gland, superior base, or inferior apex. At the same time, CT images rarely provide further insights unless there is grossly visible EPE or SVI.
Today, multiparametric MRI (mpMRI) imaging of the prostate is available to overcome some of the limitations of random systematic biopsies and DRE. It includes three MRI sequences that are commonly referenced when evaluating the prostate: T2, diffusion weighted images (DWI), and dynamic contrast enhanced images (DCE). A key advantage of DWI and DCE imaging regards additional insights into the biological activity of intraprostatic lesions that are not appreciated with anatomic T2 sequences. When combining the three sequences, the radiographic characteristics from each are separately scored and commonly categorized with the PI-RADS system that relies upon a 5-point Likert scale to predict the Gleason’s score. For those unfamiliar with PI-RADS, a practical primer with pictorial essay is now available.3 It use represents a new best-practice, and one that has been endorsed by evidence-based guidelines. More specific advantages are summarized below.
Staging and Grading Newly Diagnosed Disease
Seminal Vesicles
SVI is often undetected with conventional biopsies, DRE, and CT images; this can carry significant clinical consequences given its impact on prognosis and treatment planning. For example, many radiation oncologists will eventually see patients with SV-only relapse after primary radiotherapy. These glandular structures are readily visible on T2-weighted MRI sequences and their evaluations for tumor invasion have close to 100% sensitivity and specificity. The number of patients needed to scan to identify SV invasion is inversely related to the clinical risk, and at present, the pre-test probability of SVI with PSA and GS typically determines the threshold for mpMRI utilization.4
According to PI-RADS v2, there are three types of SV invasion that can be readily visualized that includes extension along the ducts, extra-glandular extension into and around the SV, and metachranous tumor deposits (see TABLE 1).5 This can include invasion of the proximal portion of the seminal vesicle, which at times may extend in between the peripheral zone and transitional zones within the prostate. Invasion in this area might not be palpable by DRE, and arguably may not have the same ominous prognosis as a large tumor engulfing a majority of the seminal vesicle(s). However, the potential for this occurrence illustrates the challenges of assessing SVI without an MRI.
Table 1.
Guidance for evaluating tumor burden with mpMRI (adopted from PI-RADS v2)5
mpMRI - Evaluating SV Invasion
|
mpMRI - Evaluating Extra-Prostatic Extension
|
Extraprostatic Extension
Aggressive prostate cancers can extend beyond the gland in any direction. Evaluations for EPE are best reviewed on T2-weighted sequences and should consider axial, coronal, and sagittal reconstructions. The sensitivity and specificity to detect EPE has traditionally been about 50%. However, when broad tumor contact length is considered, the sensitivity can be as high as 75% to 90%.6 A summary of key evaluations that can guide the evaluation of EPE are provided in TABLE 2.
Table 2.
Strategies to optimize acquisition of mpMRI images
Pearls with Prostate mpMRI
|
Gleason Grade
A more recently recognized advantage of mpMRI images regards the ability of apparent diffusion coefficient (ADC) maps of diffusion weighted images (DWI) to identify occult high grade disease.7 This is because transrectal biopsy needles commonly avoid the bladder neck and/or apex, typically reach only the posterior 15 mm of the prostate, and collectively sample only 1% of the gland.8 Areas with high grade disease are typically dense, and thus accommodate less diffusion of water.7 When these areas of diffusion restriction are detected, biopsies of the lesions can be accomplished with targeted biopsies performed with trans-rectal,9 trans-perineal,10 or even trans-gluteal approaches.11 The ADC maps may be unable to detect insignificant foci of GS ≤3+4 disease, and can miss tumors ≤0.5 cc in 5% and ≤0.2 cc in 15% of cases.12 However, such small foci of disease are less likely to represent aggressive disease with metastatic potential, and at least one systematic review has summarized a negative predictive value up to 90% to rule out clinically significant disease.13 This latter information has been found to be incredibly valuable by the authors when discussing active surveillance in men with low-risk disease who commonly seek increased reassurances that they are not harboring occult higher grade disease.
Restaging Following Post-Prostatectomy Failure
When the PSA is detectable after prostatectomy, either immediately or at a later time point, clinicians rarely know with certainty if the source is confined to the pelvis. Regardless, salvage pelvic radiotherapy is often recommended as there is almost always a decline in the PSA with several retrospective series suggesting such a strategy prolongs survival.14,15 In fact, the American Urological Society and American Society of Radiation Oncology in 2013 published a joint guideline that recommends “offering” salvage radiotherapy so that patients are informed of its potential benefits.16 However, this management approach remains controversial as the exact location of residual or recurrent disease is often elusive. That is because historical reliance on restaging CT and 99mTc-MDP bone scintigraphy studies are rarely helpful. Next, there are currently no completed prospective randomized trials that evaluate early salvage radiotherapy compared to observation. Consequently, radiation oncologists have wide variability in their preferred strategies for selecting patients for salvage radiotherapy, whether to cover the pelvic lymph nodes, and/or consider ADT.17
An opportunity to improve patient selection and outcomes with salvage radiotherapy relies upon the development of more accurate restaging strategies. This includes imaging of the bones, described below, or pelvis with modalities such as MRI. For example, a multi-disciplinary group in Seoul recently demonstrated the value of restaging MRI scans in a report on 118 men with a median post-prostatectomy rPSA of 0.43 ng/mL (range 0.20–5.78).18 The location of gross residual disease that was identified offered not only an opportunity to ensure the radiotherapy treatment fields encompassed all of the visible disease, but provides a rationale target for investigations that assess the potential benefit of dose-escalation to the MRI visible lesion [NCT01411345].
Utility for Active Surveillance
Since the introduction of PSA screening, a majority of men with prostate cancer are diagnosed with low-grade, organ-confined disease that is suitable for careful monitoring rather than immediate radical treatment.2 However, despite the consequences of radical treatment, including urinary, sexual and bowel dysfunction, only 40% of men in the United States with low-risk prostate cancer choose active surveillance.19 A major reason relates to the concern that standard random systematic biopsies might under-classify the grade and burden of disease. Meanwhile, mpMRI staging studies offers an opportunity to provide more accurate prognostic information which is of particular interest for patients who wish to delay and/or avoid the risks of radical treatment.
Many active surveillance protocols today recommend a confirmatory prostate biopsy within one year of the initial pathological diagnosis.1 Meanwhile, recent reports suggests that ADC maps of DWI sequences, when not concerning, provide a high negative predictive value to rule out occult higher grade disease.20,21 This has led to enthusiasm to replace the confirmatory biopsy with a staging mpMRI. However, a recently published study from Memorial Sloan Kettering raises concerns with such an approach.22 Their study evaluated the value of mpMRI scans in 206 men with low risk prostate cancer, among whom 135 (66%) harbored a radiographically concerning lesion. Each patient underwent a confirmatory biopsy with a targeted biopsy of any concerning lesions. Overall, 35% were found to harbor higher grade disease. However, higher grade cancer was detected by random systematic biopsies outside the PIRADS 3 or 4 lesions in 17% and 12% of patients, respectively. Although the study was non-randomized, these data suggest that active surveillance might not be wise for patients who do not undergo a confirmatory random systematic biopsy within one year, even if they have a negative staging mpMRI. At least one randomized trial in the US is currently evaluating this further by comparing two annual confirmatory random systematic biopsies versus mpMRI staging studies [NCT02564549].
Today, many institutions have adopted serial mpMRI scans not only for diagnostic reclassification, but also into their longer-term active surveillance pathways. It’s believed such an approach might help reduce the need for multiple surveillance biopsies. However, such a strategy has yet to be validated for men who wish to avoid treatment long-term and numerous questions remain. These include the optimal frequency and interval between unremarkable surveillance mpMRI scans, how often to consider a surveillance random systematic biopsy, and whether the added costs lead to clinically relevant reductions in metastatic disease progression and/or prostate cancer mortality. It’s also unclear if surveillance mpMRI scans outperform clinical factors associated with a higher risk of occult high grade disease such as: interval since diagnosis, older age, higher PSA, higher PSA density, increased total tumor length on random biopsy cores, and a dominant lesion detected on any MRI sequence, regardless of the PIRADS score.23 Additional questions regard the potential value of genomic testing for active surveillance with at least one study already showing that both mpMRI and genomic tests may improve the accuracy of risk-stratification.24
Opportunities to Intensify Local Treatment
With improved imaging techniques to recognize the initial location and burden of disease, pre-treatment MRI images now provide clues to better understand the mechanisms that contribute to tumor progression following a course of definitive radiotherapy. While prostate cancers are commonly multifocal, they often have a dominant intraprostatic lesion (DIN).25 The DIN is considered an index lesion, and multiple reports have associated its size with tumor control after radiotherapy.26–28 Post-radiotherapy recurrences are often at the site of original DIN,29 a finding that has driven interest in dose-escalation to the DIN with either an EBRT or brachytherapy boost at the time of initial treatment (e.g. NCT01802242).30 While there is promise in the idea to intensify local treatment to the DIN, it remains unclear what role dose escalation to the DIN may play when ADT is used. There is also uncertainty as to what the optimal approach should be for patients without a DIN who still harbor diffuse high-volume disease as seen on random systematic biopsies, as such patients are also at a high risk of local progression after definitive radiotherapy.
MRI-Selection of Intermediate-Risk Patients for Brachytherapy Monotherapy
Brachytherapy for intermediate-risk disease has long been considered an appropriate treatment option.31,32 However, outcomes at times are suboptimal when compared to lower risk patients. Though the risk of occult nodal and/or distant disease may be higher in this cohort, relapse after brachytherapy might often be the result of unrecognized EPE. An increased risk of relapse may also be associated with unrecognized disease at the anterior base where dose coverage can be difficult.
Over the past several decades, efforts to improve outcomes inspired a significant number of investigations to evaluate the potential benefit of supplemental external beam radiotherapy (sEBRT).33,34 The premise that it may help rests on an opportunity to improve treatment of areas that are unrecognized by DRE and random systematic biopsies. Fortunately, the final results of phase III studies initiated more than 15 years ago are now available to clarify the role of routinely prescribing sEBRT. These include the randomized clinical trials performed at the Schiffler Cancer Institute (n=630),35,36 and the RTOG 0232 study (n=588).37 Each demonstrated that sEBRT did not improve progression-free or overall survival (REF: ASTRO 2016 PLENARY – Need IJROBP supplemental ref – CURRENTLY EMBARGOED).
While the above studies provide insights that a majority of patients with intermediate-risk disease do not benefit from sEBRT, the data are not entirely reassuring as men in this cohort have variability in their clinical presentation. Thus, caution needs to be preserved whenever considering the omission of sEBRT in patients with Gleason score 7 disease. This is because it is well known that patients with any pattern 4 disease have an increased likelihood for harboring occult EPE or SVI.38 They are also at risk for under-appreciated malignancy near the bladder neck where brachytherapy coverage might be intentionally compromised to reduce the risk of urinary side effects. The Schiffler Cancer Institute and RTOG 0232 phase III trials did not require pre-treatment MRI staging studies. Thus, it’s unclear how many men were included who harbored under-appreciated disease at the bladder neck, or who might have been found to have EPE and/or SVI. It has been argued that awareness of these occult characteristics, even when they exist, might not impact clinical outcomes.39 However, any radiation oncologist is aware that tumor coverage can be compromised whenever the burden of disease exceeds that which is predicted.
To date, at least one well-designed prospective clinical trial of intermediate-risk patients has evaluated the role of MRI to select men with intermediate risk disease for brachytherapy as a single modality.40 This includes the phase II trial at the MD Anderson Cancer Center where 300 patients with any volume GS 7 and a PSA ≤10 ng/mL, or GS 6 and PSA 10–15, were treated with permanent low dose rate brachytherapy after EPE and SVI was ruled out with a staging MRI. With a median follow-up of 4.1 years, the biochemical progression free and overall survival rates were 95.6% and 96.9%, respectively. These findings, when considered with the aforementioned randomized trial evidence, suggest that MRI staging studies provide assurance that intermediate-risk patients treated with brachytherapy as a single modality may fare as well as those who receive sEBRT, once EPE and/or SVI are ruled out with MRI.
Improving the Quality of Treatment Planning
Contouring the prostate with CT images is notoriously inaccurate. When there are uncertainties, a “better safe than sorry” approach risks over-contouring juxtaposed structures due to a fear of missing the target.41 This strategy is in direct conflict with today’s highly conformal techniques that afford the opportunity to maximally spare normal tissues. Consequently, preferences emerge to avoid critical structures such as the rectum and/or bladder neck, representing a paradigm found to contribute to under-contouring the prostate by as much as 15%.42 Taken together, the uncertainties that contribute to over-contouring or under-contouring often diminish some of the advantages gained by image guidance radiation therapy.
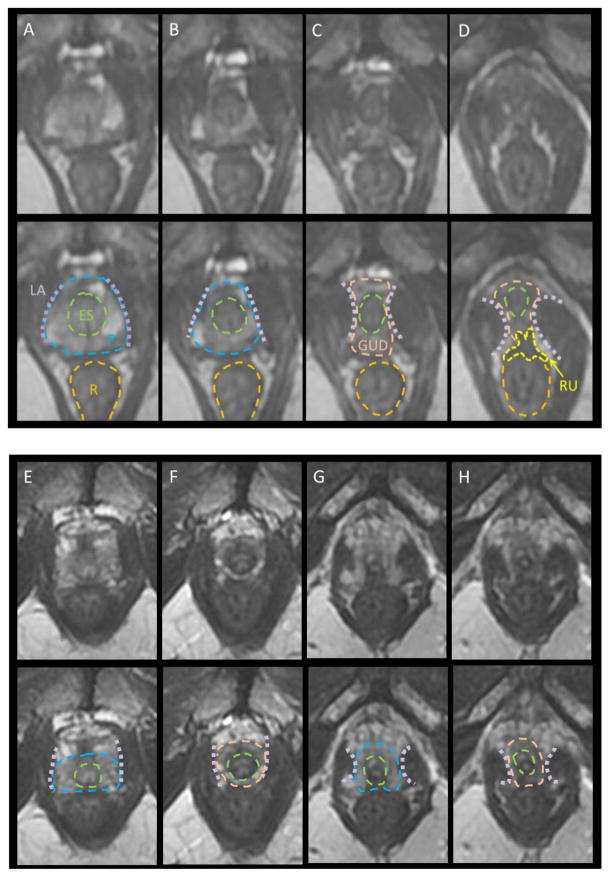
Many of the contouring errors are well defined and commonly occur at the inferior, superior, and anterior aspects of the prostate. At the apex, variations in the genitourinary diaphragm (GUD) are often seen, given it is one of the most variable anatomic regions in the male pelvis (Figure 2). When the GUD is short, the apex may be 0.5 cm from the penile bulb; when the GUD is long, the apex may be over 3 cm from the penile bulb. These examples illustrate the limitations of CT-based contouring “rules” that define the apex in relation to the penile bulb (the 1–1.5 cm rule). A reliance on this approach can lead to gross overestimations or underestimations of the apex. For example, if contouring stops 1.5 cm above the penile bulb, and the apex is actually 0.5 cm away, the resultant underestimation risks tumor progression after treatment since the non-contoured apex is frequently a site of tumor involvement. Meanwhile, contouring the prostate to 1.5 cm from the PB in a patient with an apex at 3 cm can result in unnecessary high dose exposure to the GUD, a sensitive structure associated with urethral strictures.
Figure 2.
MRI Anatomy of Apex (note that the blue hashed lines represent the prostatic boundaries). (A–D): classic configuration with concave levator ani becoming convex below the apex, pinching in on the external sphincter. Note the thickening of the anterior rectal wall; the rectourethralis (RU) muscle extends from the rectum to the GUD, maintaining the rectal angle toward the anus below the prostate. (E–F) concave levator ani extending down an elongate GUD. (F) is a full cm below the apex and the levator has not pinched in as in (C). In (G–H), the levator pinches in but there is still definite prostate. Recognition of light PZ usually clarifies the apex extent. The clearest apex definition is on coronal T2. Legend: LA=levator ani, P=prostate, R=rectum, ES=external sphincter, GUD=genitourinary diaphragm, RU=rectourethralis muscle.
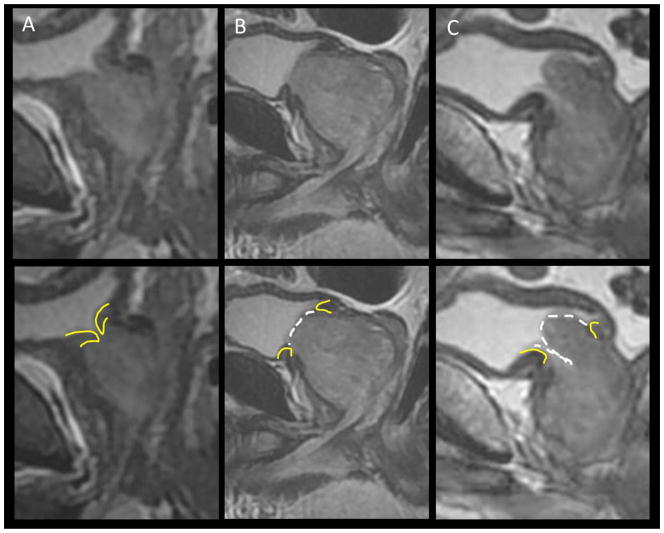
Another major challenge when contouring the prostate relates to the anterior base, a region that is very dynamic with gradual transition zone (TZ) enlargement over decades that can lead to effacement at the bladder neck (see Figure 3). On CT images, the prostate and bladder neck muscles merge, and delineating the boundary is frequently challenging. Meanwhile, on MRI the bladder muscle is distinct and dark relative to the prostate.41 Variants at the bladder neck include an intact and distinct bladder neck without prostate enlargement, a widened bladder neck with minimal TZ enlargement, a bladder neck that is obliterated by TZ projection, or actual bladder penetration by the median lobe. While the vast majority of acute radiotherapy-related symptoms are due to bladder neck swelling with radiation, the incidence of cancer is quite low in the TZ. Fortunately, DWI sequences can help guide treatment planning since it can now help rule out the likelihood of high grade disease within the TZ. This offers an attractive option to limit the amount of dose to the bladder neck as opposed to the historical strategy of uniformly sparing this region without MRI confirmation of tumor free TZ, an approach that potentially results in higher rates of treatment failure.
Figure 3.
Base variation. (A) reveals an intact bladder neck with narrow inlet. (B) reveals expansion and effacement of the bladder neck without intra-bladder extension. (C) reveals intra-bladder extension and overarching median lobe
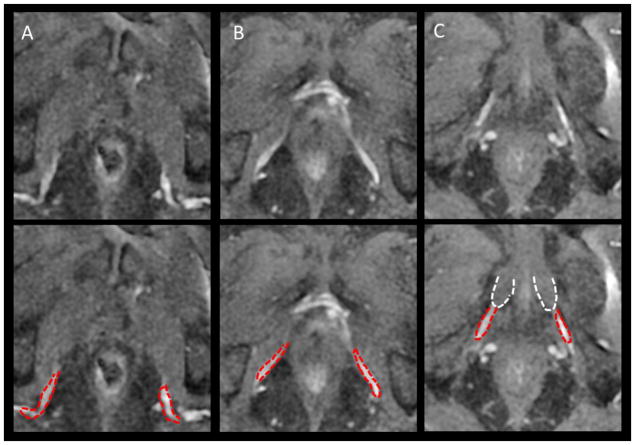
In addition to increased awareness of variations at the apex and bladder neck, an emerging area of attention relates to defining the critical structures that affect erectile function. This includes the internal pudenal artery, periprostatic nerve fibers, and penile bulb (see Figure 4). A recent review is available to learn more about the value and opportunities to avoid the internal pudenal artery and prescribe “vessel sparing radiation”.43 Nascent strategies to define periprostatic nerve fibers have also emerged, namely using diffusion tensor imaging magnetic resonance tractography (DTI-MRI).44 Ultimately, with ongoing efforts, there is promise that radiotherapy treatment planning will continue to shift the emphasis from severe complication avoidance (rectal fistulas, strictures), to toxicity limitation, with an eventual transition to emphasizing strategies that preserve baseline erectile function and quality of life.
Figure 4.
DCE improves definition of the full course of the internal pudendal artery (IPA, red) to its termination in the corpus cavernosa (A–C): upper, mid and terminal IPA to corpus cavernosa (white)
For now, there are two broad solutions to improve radiotherapy treatment planning. First is training in MRI anatomy to recognize the structures that are more clearly defined. This approach has been tested and validated in a group practice where greater agreement in CT contours was achieved after training in MRI anatomy.45 The other relates to accurate image registration of CT to MRI. The technical aspects of registering prostate CT to MRI is beyond the scope of this review, but is nonetheless critical to avoid errors during image fusion that contribute to incorrect target definitions. When this process is found to be challenging, it is often due to confounding by limited image resolution. Fortunately, awareness of prostate anatomy on MRI as well as CT, and ensuring a critical review of sagittal images to confirm anatomic landmarks such as the GUD, can be helpful to chaperone the registration and contouring process that may not be always achieved through an automated process.41
LYMPHOTROPIC NANOPARTICLE MRI
A potentially more meaningful development in MRI technology regards the opportunity to improve lymph node assessments with lymphotropic magnetic nanoparticles (LN-MRI). The potential impact on patient outcomes may be larger than that gained through improved mpMRI-guided assignments of T-stage and Gleason grade. This is because regional metastasis to pelvic lymph nodes occurs in up to 50% of patients with high risk disease, and is a primary reason for treatment failure.46
For now, the current approach for nodal characterization is limited to cross sectional imaging modalities such as CT or MRI, both of which primarily rely on size criteria and thus have a sensitivity of only 30%. Meanwhile, nodal staging with LN-MRI has demonstrated 94–96% sensitivity, with close to 100% specificity independent of their size.47 The current nanoparticle formulation that is being studied for nodal staging is a FDA-approved agent for iron replacement therapy (ferumoxytol, AMAG pharmaceuticals) that is not yet approved for imaging. Investigators at the National Cancer Institute (NCT01296139, NCT02141490) and Massachusetts General Hospital (NCT00087347) are leading the initial evaluations of ferumoxytol’s role as a LN-MRI imaging agent with promising results, though a timeline for FDA review is not currently known.
Once a reliable LN-MRI imaging agent becomes available, there are several implementation strategies that can be considered. Previous LN-MRI studies have already demonstrated the regional distribution and frequencies of lymph node involvement in the para-aortic region.48,49 This includes approximately 20% of patients with high-risk disease or a relapsed PSA <1.0 ng/mL following prostatectomy. With more accurate information, positive lymph nodes might be selectively boosted within a field of elective nodal irradiation.50 An alternative strategy might be to selectively target only the involved nodes, or deliver ablative doses of stereotactic radiotherapy when there are 3 or fewer lymph nodes involved. This latter approach has already demonstrated it can delay the need for additional therapies between 17–70 months in patients with nodal recurrence.51
WHOLE BODY MRI
Besides the utility of MRI images to detect and stage local disease, it can also aid in the evaluation of nodal involvement or distant bony metastases via whole body MRI scans.52 Whole body diffusion weighted MRI scans (WB-DWI) can visually allow detection of metastases based on the increased cellularity and resultant restricted diffusion of water molecules within either nodal or bony tissues. Recently, WB-DWI in conjunction with anatomic WB-MRI (T1W, fat suppressed T2W MRI) has been reported to accurately depict metastases with both higher sensitivity (100% vs. 85%) and specificity (100% vs. 88%), when compared to conventional imaging techniques.53 These authors’ WB-MRI protocol required a 56 minute image acquisition period. Furthermore, they described the utility of this single-step TNM staging method to aid treatment planning decisions. While WB-MRI (also known as WB-DWI) is not commonly used today, promising research is ongoing. A primary question that remains relates to the issue of magnetic field strengths for WB-DWI. Currently, 3T MRI scanners are found to provide better results for local staging compared to 1.5T MRI scanners, even though the experience with 1.5T is more extensive. At the same time, artifacts on 3T scanners can be more challenging to control due to factors such as greater B1 field inhomogeneity at 3 T field strengths. This can create difficulties in achieving uniform fat suppression across large fields of view with resultant chemical-shift and ghosting artifacts.54 Another challenge is the reliance on subjective interpretation of WB-DWI sequences that are typically performed by radiologists with more extensive experience in MRI interpretation, especially during the follow up of metastatic patients receiving systemic therapy.
MRI QUALITY ASSURANCE
While the above sections highlight the advantages with MRI images, a critical consideration relates to the importance of quality assurance. That is because many of these MRI capabilities are novel and cannot be readily implemented as a plug-and-play system. For example, a similar MRI scanner with the same coil designs can show different results at different centers due to variations within a wide range of factors. This can include variability with pulse sequence parameters and the dedication and experience of radiology technologists and radiologists. When mishandled, image acquisition, processing, and interpretation can lead to erroneous clinical decision making. Worse, it can lead to adverse patient outcomes. Fortunately, with awareness, support, and proper training, prostate mpMRI can be readily adopted into any medical institution.
To overcome some of these challenges, a MRI program is best started with a MRI physicist. Their expertise would include awareness of novel coil designs, sequence optimization, and novel pulse sequences to setup a high quality program. With expertise, sequences can be appropriately optimized and ensured to be internally consistent over time. This facilitates continuous improvement of mpMRI prostate imaging programs that will inevitably grow due to increasing demands for scans in patients with newly diagnosed disease, a rising PSA following radiotherapy or prostatectomy, or even those who prefer to be followed with image surveillance while on active surveillance.55
While many challenges remain when implementing a new prostate MRI imaging program, the American College of Radiology and European Society of Uroradiology have recently published guidelines for mpMRI image acquisition and interpretation (PI-RADSv2).56 This document serves as an introductory guide that relies primarily on expert opinion, but provides guidance for optimal image acquisition parameters, magnet strength and coil designs, along with key information about patient preparation. It is a document that will likely be continuously revised as more scientific data emerges.
POSITRON EMISSION TOMOGRAPHY
There are now multiple FDA-approved positron emission tomography (PET) tracers to non-invasively evaluate the location, burden, and molecular activity of prostate cancer. When fused with CT, the images provide significant advantages over conventional bone scans because of the opportunity to evaluate for sclerotic changes and better identify the anatomic location of biological activity; the latter helps distinguish activity that is more associated with inflammatory processes. Each of these radioactive tracers provides valuable information to guide decisions that regard the risks and benefits of local versus systemic therapy. This is particularly useful when managing patients who present with multiple high risk features or with relapse after primary treatment. Although not the focus of this review, several also have an increasing role for early response assessments to systemic therapy.
18F-FDG
Early studies with 18F-Fluorodeoxyglucose PET/CT scans found it to be a promising tool when compared to 99mTc-MDP bone scans because the former detects tumor directly by metabolic activity rather than by increased bone mineral turnover.57 While the specificity of 18F-FDG may be greater than bone scan for detecting metastases, it can be problematic with false positive results. That is because any metabolic activity resulting in increased glucose utilization (e.g. osteomyelitis, trauma) may result in a positive PET scan. Also, its urinary secretion into the bladder interferes with evaluations of soft tissues within the pelvis. At least one report has reported a detection rate of osseous metastases as low as 16% with 18F-FDG when compared with bone scintigraphy.58 It is likely that this limited sensitivity for staging motivated efforts to develop additional tracers for prostate cancer.
18F-Fluoride
There has been a recent growth of interest in 18F-Fluoride PET/CT, though the radioactive tracer was approved initially in 1999. Increased 18F-Fluoride uptake in malignant bone lesions reflects an increase in regional blood flow and bone turnover characterizing these lesions.59,60 Taking advantage of both favorable characteristics of 18F-Fluoride and the better performance of PET scanners, 18F-Fluoride has been found to be more sensitive for detection of bone metastases than 99mTc-MDP bone scanning.61–64 Even-Sapir and colleagues showed that 18F-Fluoride PET/CT is more sensitive and specific for detecting bone metastases in patients with high risk prostate cancer.65 Unfortunately, follow up duration for patients with negative 18F-Fluoride PET/CT scans was only 6 months, which may not have been sufficient to truly rule out false negative cases. Moreover the imaging findings were not correlated to serial PSA measurements, nor were they quantified.
PET-labeled acetate is transported across the cellular membrane through monocarboxylate transporter and participates in the synthesis of fatty acids from acetyl-CoA and malonyl-CoA through the action of fatty acid synthase, which is upregulated in prostate cancer. 11C-acetate is useful in the localization of tumor recurrence in men with biochemical failure, with a detection rate that tends to be positively associated with increasing serum PSA level.66
PET-labeled choline is preferentially taken up by prostate cancer cells harboring up-regulated choline kinase enzymes, leading to the incorporation of phosphatidylcholine in tumor cell membranes. Newer radiotracers that image choline include 11C-Choline and 18F-Flourocholine. 11C-Choline has less urinary excretion, but has a short half-life of only 20 minutes.67 In a study by Panebianco et al,68 the authors showed that when comparing contrast enhanced MR and spectroscopy with PET/CT with 18F-choline, the sensitivity of MR was greater than that of PET in local recurrences <10mm, with similar results in those >10mm in size in patients with an elevation in PSA after radical prostatectomy. PET/CT with 11C-choline has a positive predictive value of 86% (100% with PSA > 2 ng/ml) in the detection and localization of radical post- treatment lymph node recurrence in prostate cancer.69 Soyka et al showed that whole body PET/CT with 18F-choline also allows changes in the therapeutic management of 48% of these patients.70 Detection of biochemical relapse with 18F-choline might also allow for selective dose escalated planning of radiotherapy to areas of gross relapse without increasing toxicity.71
18F-FACBC (Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) is a synthetic non-metabolized amino acid analog that accumulates in prostate cancer via overexpression of the ASC (alanine, serine, and cysteine) transport system and other amino acid transport systems.72 Nanni et al showed that anti-18F-FACBC may be advantageous over 11C-choline for localization of disease in biochemical failure.73 Patients can be scanned within minutes after injection, and only a fraction is excreted in the urine. It was recently approved by the FDA on May 27, 2016,74 and has already been shown to improve the accuracy of localizing disease within the prostate compared to T2 weighted images alone.75
PSMA (Prostate Specific Membrane Antigen) is a transmembrane protein that is located in the epithelium that surrounds the prostatic ducts. It’s expression in prostate cancer cells is 100 –1,000 fold that of normal cells and increases with grade and stage of disease. It was initially introduced into prostate cancer imaging as an antibody conjugated to 111In-capromab-pendetide (Prostascint) that was imaged with single-photon emission computed tomography. This targeted the intracellular epitope and had limited imaging capabilities. An improvement was seen with the J591 antibody that targeted the extracellular epitope conjugated to 89Zr, though it too had limited utilities given slow tumor uptake and plasma clearance. A more promising strategy today for PSMA imaging includes small molecule inhibitors radiolabeled with 68Ga that bind with high affinity to the PSMA receptor.76 Multiple studies now suggest that 68Ga-PSMA provides superior diagnostic information compared to CT, MRI, and choline-based PET imaging and is also valuable in prostate cancers that exhibit low PSA values.77 Most of the available data has been in the recurrent and metastatic setting, though there is promise that it could be useful for upfront staging of patients with high risk disease. It has not yet received FDA approval, but may soon be available for routine clinical use.
CONCLUSION
Innovative imaging modalities that are available today, utilizing advanced MRI and PET technologies, provide opportunities to increase both diagnostic and prognostic accuracy and to improve treatment planning strategies. They can be considered for patients with any risk of disease. Their value in low-risk patients is improved selection of patients for active surveillance while detecting those who may be harboring more aggressive disease. For intermediate-risk patients interested in brachytherapy, it can help identify men unlikely to benefit from supplemental external beam radiotherapy. In high-risk patients, it can provide useful information to ensure proper tumor coverage. And finally, in men with recurrent or metastatic disease, it offers opportunities for more accurate assessment of tumor burden and treatment response.
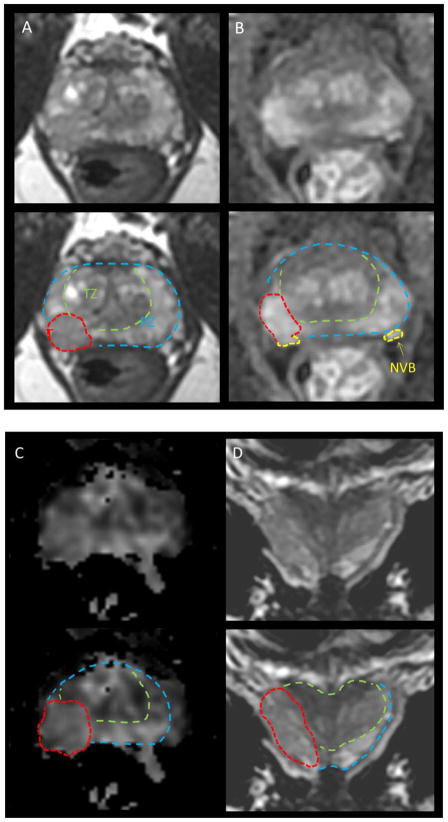
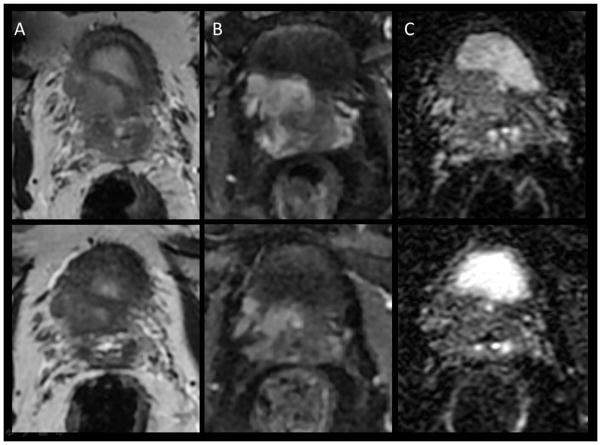
Figure 1.
mpMRI in a 63 yo with PSA 6.5, Gleason 3+4 in 3/6 biopsies on right, 3+3=6 in 2/6 on left. DRE stage = cT2b (right lobe). (A) T2 demonstrates a right sided lesion with probably ECE, (B) DCE demonstrates enhancement with probable ECE and probable extension to the NVB on right, (C) DWI demonstrates definite ECE, (D) coronal T2 view demonstrates extension throughout the lobe with effacement of the boundary consistent with ECE, though no SVI. Legend: T = tumor, PZ=peripheral zone TZ=transition zone, NVB= neurovascular bundle
Figure 5.
Tumor response to neoadjuvant androgen deprivation therapy (ADT). Upper panel pre ADT, lower panel post ADT. (A) T2, (B) DCE, (C) diffusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Drew Moghanaki, Hunter Holmes McGuire VA Medical Center, Virginia Commonwealth University.
Baris Turkbey, National Cancer Institute.
Neha Vapiwala, University of Pennsylvania.
Behfar Ehdaie, Memorial Sloan Kettering Hospital.
Steven J. Frank, MD Anderson Cancer Center.
Patrick W. McLaughlin, University of Michigan.
Mukesh Harisinghani, Massachusetts General Hospital.
References
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn GL, Jr, Hahn PF, Tabatabaei S, Harisinghani M. A practical primer on PI-RADS version 2: a pictorial essay. Abdom Radiol (NY) 2016;41(5):899–906. doi: 10.1007/s00261-016-0705-z. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen PL, Whittington R, Koo S, et al. Quantifying the impact of seminal vesicle invasion identified using endorectal magnetic resonance imaging on PSA outcome after radiation therapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59(2):400–405. doi: 10.1016/j.ijrobp.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Vargas HA, Hotker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2015 doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkrantz AB, Shanbhogue AK, Wang A, Kong MX, Babb JS, Taneja SS. Length of capsular contact for diagnosing extraprostatic extension on prostate MRI: Assessment at an optimal threshold. J Magn Reson Imaging. 2016;43(4):990–997. doi: 10.1002/jmri.25040. [DOI] [PubMed] [Google Scholar]
- 7.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258(2):488–495. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142(1):71–74. doi: 10.1016/s0022-5347(17)38664-0. discussion 74–75. [DOI] [PubMed] [Google Scholar]
- 9.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186(4):1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189(3):860–866. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Zangos S, Eichler K, Engelmann K, et al. MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: technique and preliminary results. Eur Radiol. 2005;15(1):174–182. doi: 10.1007/s00330-004-2458-2. [DOI] [PubMed] [Google Scholar]
- 12.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176(6 Pt 1):2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Futterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol. 2015;68(6):1045–1053. doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. Jama. 2008;299(23):2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotter SE, Chen MH, Moul JW, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011;117(17):3925–3932. doi: 10.1002/cncr.25993. [DOI] [PubMed] [Google Scholar]
- 16.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190(2):441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Moghanaki D, Urdaneta AI, Karlin JD, Koontz BF, Anscher MS. Management of Postprostatectomy Biochemical Relapse With Salvage Radiotherapy: Results of an International Survey. Am J Clin Oncol. 2016;39(1):64–68. doi: 10.1097/COC.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, Park W, Pyo HR, et al. Suggestion for the prostatic fossa clinical target volume in adjuvant or salvage radiotherapy after a radical prostatectomy. Radiother Oncol. 2014;110(2):240–244. doi: 10.1016/j.radonc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman KE, Niu J, Shen Y, et al. Physician Variation in Management of Low-Risk Prostate Cancer: A Population-Based Cohort Study. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore CM, Petrides N, Emberton M. Can MRI replace serial biopsies in men on active surveillance for prostate cancer? Curr Opin Urol. 2014;24(3):280–287. doi: 10.1097/MOU.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 21.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188(5):1732–1738. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recabal P, Assel M, Sjoberg DD, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol. 2016 doi: 10.1016/j.juro.2016.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satasivam P, Poon BY, Ehdaie B, Vickers AJ, Eastham JA. Can Confirmatory Biopsy be Omitted in Patients with Prostate Cancer Favorable Diagnostic Features on Active Surveillance? J Urol. 2016;195(1):74–79. doi: 10.1016/j.juro.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porpiglia F, Cantiello F, De Luca S, et al. In-parallel comparative evaluation between multiparametric magnetic resonance imaging, prostate cancer antigen 3 and the prostate health index in predicting pathologically confirmed significant prostate cancer in men eligible for active surveillance. BJU Int. 2015 doi: 10.1111/bju.13318. [DOI] [PubMed] [Google Scholar]
- 25.Epstein JI, Lecksell K, Carter HB. Prostate cancer sampled on sextant needle biopsy: significance of cancer on multiple cores from different areas of the prostate. Urology. 1999;54(2):291–294. doi: 10.1016/s0090-4295(99)00105-3. [DOI] [PubMed] [Google Scholar]
- 26.Pucar D, Hricak H, Shukla-Dave A, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(1):62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 27.Mouraviev V, Villers A, Bostwick DG, Wheeler TM, Montironi R, Polascik TJ. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: active surveillance and focal targeted therapy. BJU Int. 2011;108(7):1074–1085. doi: 10.1111/j.1464-410X.2010.10039.x. [DOI] [PubMed] [Google Scholar]
- 28.Karavitakis M, Ahmed HU, Abel PD, Hazell S, Winkler MH. Tumor focality in prostate cancer: implications for focal therapy. Nat Rev Clin Oncol. 2011;8(1):48–55. doi: 10.1038/nrclinonc.2010.190. [DOI] [PubMed] [Google Scholar]
- 29.Arrayeh E, Westphalen AC, Kurhanewicz J, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012;82(5):e787–793. doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Iturriaga A, Casquero F, Urresola A, et al. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion High-Dose-Rate prostate brachytherapy. Prospective phase II trial. Radiother Oncol. 2016;119(1):91–96. doi: 10.1016/j.radonc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11(1):6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Buyyounouski MK, Davis BJ, Prestidge BR, et al. A survey of current clinical practice in permanent and temporary prostate brachytherapy: 2010 update. Brachytherapy. 2012;11(4):299–305. doi: 10.1016/j.brachy.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Lee WR, DeSilvio M, Lawton C, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys. 2006;64(3):804–809. doi: 10.1016/j.ijrobp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Lawton CA, Yan Y, Lee WR, et al. Long-term results of an RTOG Phase II trial (00–19) of external-beam radiation therapy combined with permanent source brachytherapy for intermediate-risk clinically localized adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2012;82(5):e795–801. doi: 10.1016/j.ijrobp.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Merrick GS, Wallner KE, Galbreath RW, Butler WM, Adamovich E. Is supplemental external beam radiation therapy essential to maximize brachytherapy outcomes in patients with unfavorable intermediate-risk disease? Brachytherapy. 2016;15(1):79–84. doi: 10.1016/j.brachy.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Merrick GS, Wallner KE, Galbreath RW, et al. Is supplemental external beam radiation therapy necessary for patients with higher risk prostate cancer treated with 103Pd? Results of two prospective randomized trials. Brachytherapy. 2015;14(5):677–685. doi: 10.1016/j.brachy.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Prestidge BR, Winter K, Sand MG, et al. Initial Report of NRG Oncology/RTOG 0232: A Phase III Study Comparing Combined External Beam Radiation and Transperineal Interstitial Permanent Brachytherapy with Brachytherapy Alone for Selected Patients with Intermediate Risk Prostatic Carcinoma. ASTRO Annual Meeting, Plenary; 2016. [Google Scholar]
- 38.Pugh TJ, Frank SJ, Achim M, et al. Endorectal magnetic resonance imaging for predicting pathologic T3 disease in Gleason score 7 prostate cancer: implications for prostate brachytherapy. Brachytherapy. 2013;12(3):204–209. doi: 10.1016/j.brachy.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallner KE. Commentary on “Endorectal magnetic resonance imaging for predicting pathologic T3 disease in Gleason score 7 prostate cancer: Implications for prostate brachytherapy”. Brachytherapy. 2013;12(3):202. doi: 10.1016/j.brachy.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Frank SJ, Pugh TJ, Blanchard P, et al. Permanent Seed Implantation Prostate Brachytherapy for Intermediate Risk Prostate Cancer: Efficacy and Toxicity Outcomes from a Prospective Cohort of 300 Patients. Brachytherapy. 2016;15(Supplement 1):S201. [Google Scholar]
- 41.McLaughlin PW, Evans C, Feng M, Narayana V. Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate contouring accuracy. Int J Radiat Oncol Biol Phys. 2010;76(2):369–378. doi: 10.1016/j.ijrobp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Gao Z, Wilkins D, Eapen L, Morash C, Wassef Y, Gerig L. A study of prostate delineation referenced against a gold standard created from the visible human data. Radiother Oncol. 2007;85(2):239–246. doi: 10.1016/j.radonc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Spratt DE, Liss AL, McLaughlin PW. Vessel-sparing radiation and functional anatomy-based preservation for erectile function after prostate radiotherapy. Lancet Oncol. 2016;17(5):e198–208. doi: 10.1016/S1470-2045(16)00063-2. [DOI] [PubMed] [Google Scholar]
- 44.Finley DS, Ellingson BM, Natarajan S, et al. Diffusion tensor magnetic resonance tractography of the prostate: feasibility for mapping periprostatic fibers. Urology. 2012;80(1):219–223. doi: 10.1016/j.urology.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Khoo EL, Schick K, Plank AW, et al. Prostate contouring variation: can it be fixed? Int J Radiat Oncol Biol Phys. 2012;82(5):1923–1929. doi: 10.1016/j.ijrobp.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 46.Swanson GP, Thompson IM, Basler J. Current status of lymph node-positive prostate cancer: Incidence and predictors of outcome. Cancer. 2006;107(3):439–450. doi: 10.1002/cncr.22034. [DOI] [PubMed] [Google Scholar]
- 47.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 48.Meijer HJ, Fortuin AS, van Lin EN, et al. Geographical distribution of lymph node metastases on MR lymphography in prostate cancer patients. Radiother Oncol. 2013;106(1):59–63. doi: 10.1016/j.radonc.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Meijer HJ, van Lin EN, Debats OA, et al. High occurrence of aberrant lymph node spread on magnetic resonance lymphography in prostate cancer patients with a biochemical recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82(4):1405–1410. doi: 10.1016/j.ijrobp.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 50.Meijer HJ, Debats OA, Kunze-Busch M, et al. Magnetic resonance lymphography-guided selective high-dose lymph node irradiation in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):175–183. doi: 10.1016/j.ijrobp.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Ost P, Jereczek-Fossa BA, Van As N, et al. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin Oncol (R Coll Radiol) 2016 doi: 10.1016/j.clon.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 52.Blackledge MD, Tunariu N, Orton MR, et al. Inter- and Intra-Observer Repeatability of Quantitative Whole-Body, Diffusion-Weighted Imaging (WBDWI) in Metastatic Bone Disease. PLoS One. 2016;11(4):e0153840. doi: 10.1371/journal.pone.0153840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasoglou V, Larbi A, Collette L, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? Prostate. 2014;74(5):469–477. doi: 10.1002/pros.22764. [DOI] [PubMed] [Google Scholar]
- 54.Koh DM, Blackledge M, Padhani AR, et al. Whole-body diffusion-weighted MRI: tips, tricks, and pitfalls. AJR Am J Roentgenol. 2012;199(2):252–262. doi: 10.2214/AJR.11.7866. [DOI] [PubMed] [Google Scholar]
- 55.Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging. 2014;39(6):1443–1448. doi: 10.1002/jmri.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson JJ, Kransdorf MJ, O’Connor MI. Diagnosis of occult bone metastases: positron emission tomography. Clin Orthop Relat Res. 2003;415(Suppl):S120–128. doi: 10.1097/01.blo.0000093051.96273.7c. [DOI] [PubMed] [Google Scholar]
- 58.Yeh SD, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693–697. doi: 10.1016/0969-8051(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 59.Hawkins RA, Choi Y, Huang SC, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33(5):633–642. [PubMed] [Google Scholar]
- 60.Schiepers C, Nuyts J, Bormans G, et al. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med. 1997;38(12):1970–1976. [PubMed] [Google Scholar]
- 61.Oyen WJ, Witjes JA, Corstens FH. Nuclear medicine techniques for the diagnosis and therapy of prostate carcinoma. Eur Urol. 2001;40(3):294–299. doi: 10.1159/000049789. [DOI] [PubMed] [Google Scholar]
- 62.Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17(8):2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 63.Hoegerle S, Juengling F, Otte A, Altehoefer C, Moser EA, Nitzsche EU. Combined FDG and [F-18]fluoride whole-body PET: a feasible two-in-one approach to cancer imaging? Radiology. 1998;209(1):253–258. doi: 10.1148/radiology.209.1.9769840. [DOI] [PubMed] [Google Scholar]
- 64.Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40(10):1623–1629. [PubMed] [Google Scholar]
- 65.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47(2):287–297. [PubMed] [Google Scholar]
- 66.Mohsen B, Giorgio T, Rasoul ZS, et al. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 2013;112(8):1062–1072. doi: 10.1111/bju.12279. [DOI] [PubMed] [Google Scholar]
- 67.Vali R, Loidl W, Pirich C, Langesteger W, Beheshti M. Imaging of prostate cancer with PET/CT using (18)F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5(2):96–108. [PMC free article] [PubMed] [Google Scholar]
- 68.Panebianco V, Sciarra A, Lisi D, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP) Eur J Radiol. 2012;81(4):700–708. doi: 10.1016/j.ejrad.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 69.Scattoni V, Picchio M, Suardi N, et al. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol. 2007;52(2):423–429. doi: 10.1016/j.eururo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 70.Soyka JD, Muster MA, Schmid DT, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39(6):936–943. doi: 10.1007/s00259-012-2083-2. [DOI] [PubMed] [Google Scholar]
- 71.Pinkawa M, Piroth MD, Holy R, et al. Dose-escalation using intensity-modulated radiotherapy for prostate cancer - evaluation of quality of life with and without (18)F-choline PET-CT detected simultaneous integrated boost. Radiat Oncol. 2012;7:14. doi: 10.1186/1748-717X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okudaira H, Nakanishi T, Oka S, et al. Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl Med Biol. 2013;40(5):670–675. doi: 10.1016/j.nucmedbio.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Nanni C, Schiavina R, Boschi S, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S11–17. doi: 10.1007/s00259-013-2373-3. [DOI] [PubMed] [Google Scholar]
- 74.FDA approves new diagnostic imaging agent to detect recurrent prostate cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm503920.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery.
- 75.Turkbey B, Mena E, Shih J, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270(3):849–856. doi: 10.1148/radiol.13130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]