Abstract
Problem
Mental health conditions are prevalent in youth with type 1 diabetes (T1D). Anxiety symptoms and depressive symptoms are highly correlated and are two of the most prevalent mental health conditions in youth in the general population. The detrimental effect of depressive symptoms in youth with T1D has been well documented, but the effects of anxiety symptoms are not well understood.
Eligibility criteria
Studies were included if they were published between 1990 and 2015, and evaluated anxiety symptoms in a population of youth with T1D.
Sample
A total of 20 studies were identified from a sample of 338 papers.
Results
Anxiety symptoms were prevalent in youth with T1D. Anxiety symptoms were associated with higher glycosylated hemoglobin (HbA1c) levels, poorer self-management and coping behaviors, depressive symptoms, fear of hypoglycemia, and lower blood glucose monitoring frequency. State anxiety and trait anxiety symptoms affected health outcomes differently. Girls were at a higher risk of anxiety symptoms than boys.
Conclusions
Anxiety symptoms in youth with T1D have detrimental effects on health outcomes, including self-management, quality of life, and HbA1c.
Implications
Future research should aim to improve our current screening and treatment practices.
Keywords: Anxiety, Anxiety symptoms, Mental health conditions, Type 1 diabetes
Type 1 diabetes (T1D) affects more than 200,000 youth in the United States (American Diabetes Association [ADA], 2014) and the incidence continues to increase worldwide (ADA, 2014; Maahs, West, Lawrence, & Mayer-Davis, 2010). Youth with T1D are at an increased risk of mental health conditions, such as anxiety symptoms, eating and behavioral disorders, as well as depressive symptoms (Herzer & Hood, 2009; Kovacs, Goldston, Obrosky, & Bonar, 1997). Mental health conditions are prevalent in the general adolescent population, with approximately one in every five U.S. adolescents without T1D meeting criteria for a mental health condition (Merikangas et al., 2010). Mental health conditions occur in approximately 33% to 42% of youth with T1D, a rate two to three times higher than peers without T1D (Kovacs et al., 1997; Northam, Matthews, Anderson, Cameron, & Werther, 2005). Mental health conditions have been shown to increase the risk of both short and long-term disease-related complications, including episodes of severe hypoglycemia or hyperglycemia, weight gain, and microvascular disorders in youth with T1D (Grey, Boland, Davidson, Li, & Tamborlane, 2000; Herzer & Hood, 2009; McGrady, Laffel, Drotar, Repaske, & Hood, 2009; Rewers et al., 2002).
Depressive symptoms occur in youth with T1D at approximately twice the rate of youth in the general population without T1D, occurring in approximately 11.3% to 27.5% of youth with T1D (Grey, Whittemore, & Tamborlane, 2002; Herzer & Hood, 2009; Hood et al., 2006; Silverstein et al., 2005). Depressive symptoms have also been associated with poorer self-management and glycemic control in youth with T1D (Helgeson, Siminerio, Escobar, & Becker, 2008; Whittemore et al., 2002). Considerable research has been conducted on depressive symptoms in youth with T1D, and screening for depression is now part of the standard of care. However, little is understood about anxiety symptoms in this population.
Anxiety symptoms are also prevalent in youth with T1D, with approximately 13% to 21.3% of youth with T1D screening positive for anxiety symptoms at some point during their childhood or adolescence (Bernstein, Stockwell, Gallagher, Rosenthal, & Soren, 2013; Herzer & Hood, 2009; Kovacs et al., 1997). Anxiety symptoms have been associated with poorer quality of life, self-management, and glycemic control in youth with T1D (Garrison, Katon, & Richardson, 2005; Herzer & Hood, 2009; Herzer, Vesco, Ingerski, Dolan, & Hood, 2011; Naar-King, Idalski, et al., 2006; Naar-King, Podolski, et al., 2006). Approximately 18.4% of youth with T1D are diagnosed with an anxiety disorder at some point during childhood or adolescence (Silverstein et al., 2005).
Anxiety has been conceptualized as a state, a trait, a stimulus, a response, a future-oriented emotion, and an emotional state (Endler, Kocovski, & Macrodimitris, 2001). Spielberger proposed two types of anxiety symptoms, state and trait, which eventually led to the development of the State–Trait Anxiety Inventory. Trait anxiety was defined as an individual’s likelihood to respond anxiously to a stimulus, and state anxiety was defined as transient experience of the physiological arousal associated with feelings of dread and tension (Spielberger, 1966). Since this distinction was first proposed, state and trait anxiety symptoms have been widely accepted in the psychological literature (Endler et al., 2001). The differential effects of these types of anxiety symptoms on behavior and their underlying physiological differences are not fully understood (Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, 2010).
Anxiety symptoms in terms of hypervigilance and attentional biases have been explored (Eysenck, Derakshan, Santos, & Calvo, 2007). Some have theorized that the combination of hypervigilance and attentional biases together lead to greater sensitivity to negative stimuli (Bradley, Mogg, Falla, & Hamilton, 1998; Eysenck et al., 2007). One way to explain the distinction between state and trait anxiety symptoms may be related to the varying ways that individuals interpret threats. For example, state anxiety symptoms may increase the fear associated with a particular stimulus, while trait anxiety symptoms may lead an individual to constantly direct attention toward a threatening stimulus (Pacheco-Unguetti et al., 2010). In one study, young children with trait anxiety symptoms displayed faster detection of threatening faces (Hadwin et al., 2003).
Differences in the experience of anxiety symptoms by gender have also been reported. Gender differentially affects types, rates, comorbidities, antecedents, and trajectories of anxiety symptoms (Crawford, Cohen, Midlarsky, & Brook, 2001; Zahn-Waxler, Shirtcliff, & Marceau, 2008). Anxiety symptoms are more prevalent in boys in preadolescence and in girls in adolescence (Zahn-Waxler et al., 2008). While the distinction in the experience of anxiety symptoms in males and females is not yet fully understood, it is likely due to a combination of biological factors and childhood adverse experiences.
Anxiety symptoms may affect self-management and quality of life in youth with T1D. There have been few studies on the prevalence and the effect of anxiety symptoms on health outcomes in this population. To better understand the role of anxiety symptoms in youth with T1D, we aimed to describe the prevalence of anxiety symptoms in youth with T1D, the effect of anxiety symptoms on health outcomes, and to identify differences in health outcomes related to state anxiety and trait anxiety.
Design
An integrative review of the literature was performed. Whittemore and Knafl’s (2005) method for data collection, analysis, and synthesis guided the analysis. The steps included: (1) a well-defined literature search strategy in consultation with a medical librarian was conducted; (2) eligible primary sources were obtained and organized into subgroups, from which data were extracted and categorized; (3) quality appraisal of the literature was performed using appropriate, validated quality measures; (4) data within subgroups were re-organized, grouped together appropriately, and displayed in tabular and chart formats; (5) conclusions were drawn capturing the breadth and depth of the research. The 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed.
Methods
Search Methods
The electronic databases searched during September 2015 were Medline, EMBASE, PsycINFO, and CINAHL. The search terms included “anxiety disorders,” “anxiety,” and “T1D,” and these were used consistently in each database when the search term matched a keyword. For example, the search strategy for Medline was as follows: (1) exp. anxiety disorders, (2) exp. anxiety, (3) exp. diabetes mellitus, type 1, (4) 1 or 2 and 3 with an age limitation. Keywords from all included articles were reviewed to ensure that all relevant keywords were included in the search. The reference lists of included papers were reviewed to assess for outliers. Detailed search strings for all databases are available upon request. Figure 1 shows the data collection process.
Figure 1.
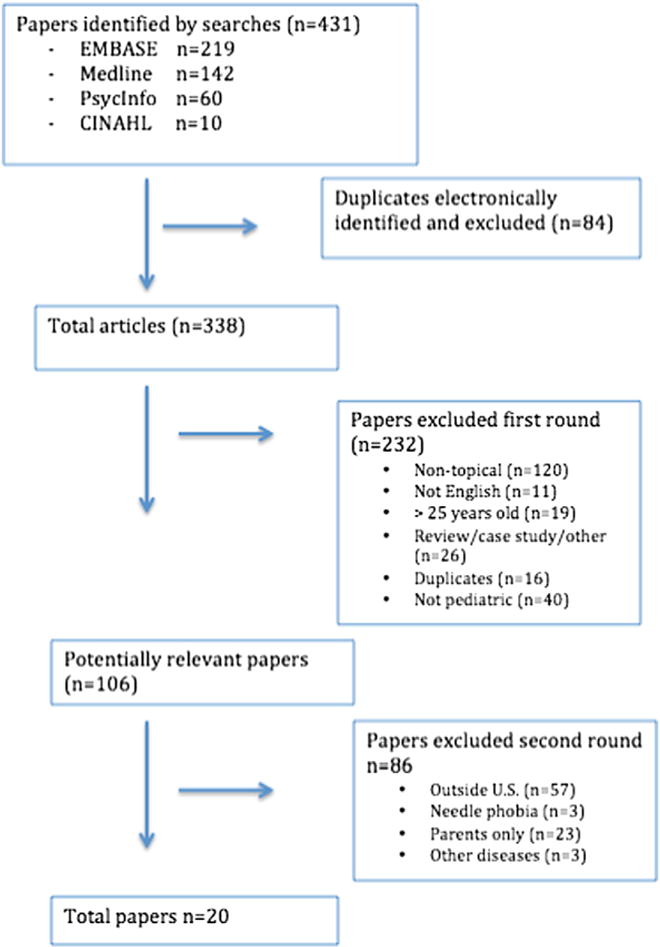
Data collection.
Studies were included if they were published between 1990 and 2015, evaluated a pediatric population, and the sample was youth. First order exclusion criteria were: (1) studies that were not topically appropriate (did not address anxiety in youth with T1D directly); (2) studies written in a language other than English; (3) review articles; (4) case studies; (5) practice guidelines; (6) duplicates; and (7) studies that did not include only pediatric participants. Second order exclusion criteria included: (1) studies conducted outside of the U.S.; (2) needle phobia or fear of hypoglycemia as the only measures of anxiety; (3) measurement of anxiety only in parents; (4) samples including participants with other chronic illnesses.
Review Process
The full citations, including bibliographic details, keywords, abstract, and Web addresses (when available), of all identified titles were imported into the online bibliographic management program RefWorks™ and combined into a database. Duplicate papers were removed. One author read the titles and abstracts of each article and ineligible papers were excluded; potentially relevant studies were retained for further review. This process was conducted twice to ensure that all eligible articles were included.
Quality Appraisal
The National Institutes of Health (NIH) “Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies” was used to determine relative quality of non-experimental studies (NIH, 2014). The “Cochrane Collaboration’s tool for assessing risk of bias” was used to determine relative quality of the experimental studies (Higgins et al., 2011).
Data Abstraction and Synthesis
The articles remaining in the sample were read and summarized systematically using a data extraction form. This technique allowed for iterative analyses across sources. Articles were organized initially into categories based on the type of study: descriptive, correlational, or experimental. Data were then analyzed within categories and further organized by state anxiety symptoms and trait anxiety symptoms.
Results
There were 431 potentially relevant papers initially identified, and after removal of duplicates, 338 papers remained. Applying the first order exclusion criteria, 232 papers were eliminated. The remaining 106 papers were subject to a full review and an additional 86 papers were excluded based on second order exclusion criteria. The remaining 20 papers met all inclusion/exclusion criteria (Figure 1). Quality assessments were completed for each article and results are displayed in Tables 1 and 2.
Table 1.
Quality assessment tool for observational cohort and cross sectional studies, NIH (2014).
|
Bernstein et al. (2013) |
Daviss et al. (1995) |
Di Battista, Hart, Greco, and Gloizer (2009) |
Garrison et al. (2005) |
Gelfand et al. (2004) |
Gonder-Frederick et al. (2006) |
Grey, Cameron, and Thurber (1991) |
Grey, Cameron, Lipman, and Thurber (1995) |
Herzer and Hood (2009) |
Herzer et al. (2011) |
Hilliard, Herzer, Dolan, and Hood (2011) |
Kovacs et al. (1990) |
Kovacs et al. (1997) |
La Greca, Swales, Klemp, Madigan, and Skyler (1995) |
Naar-King, Idalski, et al, 2006; Naar-King, Podolski, et al., 2006 |
Wiebe, Alderfer, Palmer, Lindsay, and Jarrett (1994) |
Weist, Finney, Barnard, Davis, and Ollendick (1993) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ql | G | F | G | F | G | G | G | G | G | F | G | G | F | F | F | F | F |
| Q2 | F | G | G | G | P | G | G | G | G | F | F | G | G | G | G | G | P |
| Q3 | U | F | U | NA | NA | U | U | U | G | G | G | G | G | P | G | G | P |
| Q4 | F | F | P | G | P | G | G | G | G | F | F | G | G | G | G | G | P |
| Q5 | G | P | P | P | P | P | P | P | F | F | P | P | P | G | G | P | P |
| Q6 | NA | NA | NA | NA | F | NA | NA | G | NA | NA | NA | G | G | NA | NA | G | F |
| Q7 | G | G | G | G | G | G | G | G | G | G | G | G | G | G | G | G | F |
| Q8 | F | F | G | F | P | G | G | F | NA | G | G | G | G | F | G | G | F |
| Q9 | G | G | G | P | P | G | G | G | G | G | G | G | F | F | G | F | G |
| Q10 | NA | P | NA | F | NA | NA | NA | G | NA | G | NA | G | G | NA | NA | NA | NA |
| Q11 | G | G | G | G | F | G | G | G | G | G | G | G | F | G | G | P | G |
| Ql2 | U | NA | NA | NA | NA | NA | NA | U | U | U | U | U | U | U | U | U | U |
| Ql3 | G | G | G | NA | NA | P | G | G | G | G | G | P | G | P | P | G | U |
| Ql4 | G | G | G | F | P | G | G | G | G | G | G | F | G | G | G | G | F |
Key: G = good; F = fair; P = poor; U = unknown; NA = not applicable.
Table 2.
Cochrane Collaboration’s Tool for Assessing Risk of Bias.
| Briery and Rabian (1999) | DirecNet (2006) | Hains, Davies, Parton, and Silverman (2001) | |
|---|---|---|---|
| Random sequence generation (selection bias) | P | G | P |
| Allocation concealment (selection bias) | P | G | P |
| Blinding of participants and personnel (performance bias) | P | P | P |
| Blinding of outcome assessment (detection bias) (patient-reported outcomes) | G | G | F |
| Blinding out outcome assessment (detection bias) (all-cause mortality) | G | G | F |
| Incomplete outcome data (attrition bias) (short term) | G | G | P |
| Incomplete outcome data (attrition bias) (long term) | P | G | P |
| Selective reporting (reporting bias) | F | F | P |
Key: G = good; F = fair; P = poor.
The final sample included 2 descriptive studies, 15 correlational studies, and 3 intervention studies. Data abstraction and synthesis are displayed in Table 3. Age of study participants ranged from 3 years of age to 25 years of age, and all studies except one limited the sample to youth up to 18 years of age, except one, that included participants 11 to 25 years of age.
Table 3.
Data abstraction summary.
| Author (date) | Study design | Age (mean) | Scale utilized (alpha) | Key findings | Comments |
|---|---|---|---|---|---|
| Correlational Daviss et al. (1995) |
Cross-sectional |
10 to 16 (12.8) |
Revised Children’s Manifest Anxiety Scale (not reported) |
Duration of illness, family size, and adherence predicted HbA1c |
Anxiety was not a significant predictor of HbA1c |
| Di Battista et al. (2009) | Cross-sectional | 13 to 18 (15.9) | Social Anxiety Scale for Adolescents (alpha =0.76–0.91) | There were no differences in social anxiety between genders. In boys, social anxiety was associated with poorer diet and decreased insulin adherence. Social anxiety is associated with poorer quality of life in boys and girls | Social anxiety negatively impacts adherence and quality of life |
| Garrison et al. (2005) | Retrospective Cohort | 13 to 18 (14.87) | ICD-9 codes (NA) | Adolescents with internalizing disorders (anxiety or depression) were much more likely to be rehospitalized for diabetes | Anxiety is associated with increases in repeat hospitalizations related to diabetes |
| Gelfand et al. (2004) | Retrospective Cohort | 3 to 21 (12.86) | Chart abstraction (NA) | HbA1c improved by 0.8% in children who received a psychological referral and consultation | Psychological referral may improve glycemic control |
| Gonder-Frederick et al. (2006) | Cross-sectional | 12 to 17 (15.36) | State–Trait Personality Inventory, Anxiety Subscale (alpha =0.79 to 0.92) | Trait anxiety predicts fear of hypoglycemia. Fear of hypoglycemia was not related to HbA1c in this population | Trait anxiety is an important predictor of fear of hypoglycemia |
| Grey et al. (1995) | Longitudinal | 8 to 14 (11.3) | State–Trait Anxiety Inventory for Children (alpha =0.78–0.85) | Children with T1D had slightly higher reported rates of anxiety than controls, but the findings were not significant | 2 years after diagnosis, children with T1D had twice as much depression and adjustment problems as their peers |
| Grey et al. (1991) | Cross-sectional | 8 to 18 (12.9) | State–Trait Anxiety Inventory for Children (alpha =0.78–0.85) | Adolescents with T1D had more depression, anxiety, and negative coping than children with T1D | Adolescents with internalizing behaviors also exhibited more negative coping |
| Herzer and Hood (2009) | Cross-sectional | 13 to 18.5 | State–Trait Anxiety Inventory (alpha =0.87) | State anxiety was significantly associated with HbA1c, blood glucose monitoring frequency, and depressive symptoms. Trait anxiety was significantly associated with blood glucose monitoring frequency and depressive symptoms | Trait and state anxiety both affect disease outcomes |
| Herzer et al. (2011) | Longitudinal | 13 to 18 (15.63) | State–Trait Anxiety Inventory (alpha = 0.86) | Baseline family conflict was associated with baseline and 6-month state anxiety. Baseline state anxiety was associated with 9-month HbA1c. Anxiety mediated and account for 20% of the family conflict and glycemic control link | Anxiety is triggered and exacerbated by family conflict. Anxiety is associated with poorer glycemic control |
| Hilliard et al. (2011) | Prospective Cohort | 13 to 18 (15.5) | State–Trait Anxiety Inventory for Children (alpha = 0.87) | Higher state anxiety scores predicted higher HbA1c | State anxiety was directly related to glycemic control |
| Kovacs et al. (1990) | Longitudinal | 8 to 13 (11.1) | Revised Children’s Manifest Anxiety Scale (not reported) | Over time, anxiety increases in girls and decreases in boys. Children with higher levels of anxiety displayed poorer self-management | Girls are at a higher risk for anxiety symptoms than boys |
| Naar-King, Idalski, et al., 2006; Naar-King, Podolski, et al., 2006 | Cross-sectional | 10 to 16 (13.3) | The Behavior Assessment System for Children (not reported) | Boys had worse adherence than girls. Girls reported more internalizing symptoms than boys. Boys reported more externalizing symptoms than girls. Externalizing symptoms were associated with HbA1c. | Girls report more internalizing symptoms. Boys reported more externalizing symptoms, which were associated with poorer glycemic control. |
| Wiebe et al. (1994) | Prospective Cohort | (16.35) | State–Trait Anxiety Inventory for Children – Trait Screen (alpha = 0.84) | Youth with trait anxiety were more likely to misattribute non-diabetes related symptoms to blood glucose levels. Youth who were over-attentive to internal physical symptoms tended to have higher anxiety and poorer glycemic control | Trait anxiety was associated with incorrect interpretation of internal physical symptoms |
| Weist et al. (1993) | Cross-sectional | 8 to 19 (13.4) | State–Trait Anxiety Inventory (not reported) | There were no differences in anxiety in youth in good glycemic control and youth in poor glycemic control | Anxiety did not differ in youth in good control as compared to youth in poor control |
| Descriptive Bernstein et al. (2013) |
Cross-sectional |
11 to 25 (17.1) |
Screen for Child Anxiety Related Emotional Disorders (not reported) |
21% of the population screened positive for an anxiety disorder. Those with a positive mental health screening were twice as likely to be in poor glycemic control |
Anxiety is the most prevalent mental health condition in this population by almost double |
| Kovacs et al. (1997) | Longitudinal | 8 to 13 (11.0) | Interviews by a mental health professional (NA) | 47.6% developed a mental health condition by the 10th year of T1D diagnosis. 19.6% developed an anxiety disorder | Overall, there is elevated mental health morbidity in youth with T1D. Anxiety disorders were one of the three most prevalent conditions |
| Experimental Briery and Rabian (1999) |
Quasi-experimental |
6 to 16 (10.27) |
State–Trait Anxiety Inventory for Children (not reported) |
Participation in a summer camp for youth with T1D improved attitudes toward disease and decreased anxiety levels |
Peer-related interventions may help to alleviate anxiety |
| DirecNet Study Group (2006) | Randomized control trial | 7 to 18 (12.3) | Diabetes Worry Scale (alpha = 0.97) | Continuous glucose monitoring was not more beneficial than typical care with regard to | Continuous glucose monitoring did not benefit psychological outcomes |
| Hains et al. (2001) | Quasi-experimental | 13 to 18 (not reported) | Revised Children’s Manifest Anxiety Scale (not reported) | psychological outcomes Out of 6 youth that received a cognitive behavioral intervention, 4 displayed improvement in anxiety, anger, or stress | CBT may be a useful intervention in youth with T1D and anxiety symptoms |
Measures of anxiety were not consistent across the studies. Anxiety symptoms and anxiety disorders were both measured. Anxiety symptoms were measured using the State–Trait Anxiety Inventory (n = 11), the Revised Children’s Manifest Anxiety Scale (n = 3), the Behavior Assessment System for Children (n = 1), the Anxiety Scale for Adolescents (n = 1), and the Diabetes Worry Scale (n = 1). Alternatively, some studies examined anxiety disorders and used the Screen for Child Anxiety Related Emotional Disorders (n = 1) or psychological diagnoses by a mental health professional (n = 2).
Prevalence of Anxiety
The prevalence of anxiety symptoms in youth with T1D was explored in two studies and a high prevalence rate was found. In a sample of 150 youth with T1D, 34.7% screened positive for a mental health condition, 14.7% screened positive for two or more mental health conditions, and 21.3% of the population screened positive for an anxiety disorder, almost double the rate for those who screened positive for depression (11.3%) (Bernstein et al., 2013). In a longitudinal sample of 92 youth with T1D, 47.6% of the sample developed a mental health condition within 9 years after diagnosis; of these, 19.6% developed an anxiety disorder and 26.1% developed major depressive or dysthymic disorders (Kovacs et al., 1990).
Association Between Anxiety and Diabetes Outcomes
Of the 15 correlational studies exploring anxiety in youth with T1D, an association between anxiety symptoms and poor health outcomes was reported in 75% of studies across a range of clinical and psychosocial outcomes using bivariate and multivariate analyses. Outcomes included glycemic control, fear of hypoglycemia, worry about hypoglycemia, family conflict, depressive symptoms, blood glucose monitoring, and quality of life. We examined the associations between state anxiety, trait anxiety, and general anxiety with outcomes separately.
In bivariate analyses, state anxiety was correlated with higher HbA1c (r = 0.30, p < 0.01), higher family conflict (r = 0.30, p < 0.01), higher depressive symptoms (r = 0.55, p < 0.01), lower blood glucose monitoring rates (r = −0.25, p < 0.01), poorer quality of life (r = −0.18, p < 0.05), and older age in adolescence (F = 6.10, p < 0.01) (Herzer & Hood, 2009; Herzer et al., 2011; Hilliard et al., 2011). In multivariate analyses, state anxiety was associated with higher HbA1c levels (r = 0.32, p < 0.01), higher family conflict (beta = 0.39, p < 0.01), and decreased blood glucose monitoring rates (r2 = 0.25, p < 0.01) in multivariate models (Herzer & Hood, 2009; Herzer et al., 2011).
In bivariate analyses, trait anxiety was correlated with higher fear of hypoglycemia (r = 0.48, p < 0.01), more worry about hypoglycemia (r = 0.53, p < 0.01), more episodes of hypoglycemia (r = 0.37, p < 0.05), higher depressive symptoms (r = 0.72, p < 0.01), lower ability to differentiate physical symptoms of anxiety from physical symptoms related to blood glucose (r = −0.49, p < 0.05), and decreased blood glucose monitoring rates (r = −0.17, p < 0.01) (Herzer & Hood, 2009; Gonder-Frederick et al., 2006; Wiebe et al., 1994. Parent and adolescent trait anxiety was correlated (r = 0.51, p < 0.01). In multivariate analyses, trait anxiety was associated with higher fear of hypoglycemia (r2 = 0.23, p < 0.01), more worry about episodes of hypoglycemia (r2 = 0.20, p < 0.01), and with a greater likelihood of misattributing non-diabetes related symptoms to blood glucose (r2 = 0.29, p < 0.05) in multivariate models (Gonder-Frederick et al., 2006; Wiebe et al., 1994).
General anxiety, rather than state or trait anxiety, was examined in four studies. Anxiety mediated the relationship between family conflict and HbA1c in youth with T1D, accounting for 20% of the variance (beta = 0.39, p < 0.01) (Herzer et al., 2011). General anxiety was also associated with a higher risk of diabetes-related rehospitalization (OR = 1.79, CI 1.27–2.52) (Garrison et al., 2005), and poorer coping (beta = 0.39, p < 0.01) (Grey et al., 1991). Treatment of anxiety symptoms was associated with improved glycemic control by an average of 0.8 percentage points (p < 0.01) (Gelfand et al., 2004).
Associations Among Gender, Anxiety, and Diabetes Outcomes
The associations among gender, anxiety, and glycemic control or quality of life were examined in four studies of youth with T1D. Girls exhibited higher anxiety (t = 2.03, p < 0.05) (Kovacs et al., 1997; Naar-King, Idalski, et al., 2006; Naar-King, Podolski, et al., 2006) and worse glycemic control than boys, with HbA1c an average of 1.58% higher in girls (p < 0.05) (La Greca et al., 1995). Girls were more likely to be worried about episodes of hypoglycemia than boys (t = −2.45, p < 0.02) (Gonder-Frederick et al., 2006). Social anxiety was associated with poorer quality of life in boys (r = 0.54, p < 0.01) and girls (r = 0.51, p < 0.01) (Di Battista et al., 2009). In addition, social anxiety was associated with decreased adherence (r = −0.39, p < 0.05) (Di Battista et al., 2009).
Interventions for Anxiety Symptoms
There were three intervention studies exploring anxiety in youth with T1D. These studies evaluated the effects of different types of interventions, including participation in a summer camp, cognitive behavioral therapy, and use of continuous glucose monitoring. These are the only intervention studies that have sought to improve anxiety symptoms in youth with T1D to date.
Of these, two used a one-group pretest–posttest design and one was a randomized controlled trial. Positive results were found only in the one-group pretest–posttest studies. Of the one-group pretest–posttest studies, participation in a one-week pediatric summer camp specifically for youth with T1D led to improved attitudes toward diabetes and lower levels of trait anxiety (n = 90, p < 0.001) (Briery & Rabian, 1999). In the other study, a cognitive behavioral intervention that included cognitive restructuring and problem solving skill building improved anxiety symptoms in four of six youth with T1D (Hains et al., 2001).
In a randomized control trial that measured the possible psychological benefit of continuous glucose monitoring (CGM) versus usual care was conducted in 200 youth with T1D. The ability to constantly monitor glucose levels had no effect on diabetes-related anxiety (p = 0.16) (DirecNet Study Group, 2006).
Differential Effects of State and Trait Anxiety on Health Outcomes
Based on this review, we propose a conceptual model in which we distinguish the differential relationships of state and trait anxiety on health outcomes in youth with T1D (Figure 2). State and trait anxiety influenced health outcomes directly in unique ways. State anxiety was associated with higher family conflict (beta = 0.39, p < 0.01), poorer glycemic control (r = 0.32, p < 0.01), and decreased blood glucose monitoring rates (r = 0.25, p < 0.01) (Herzer & Hood, 2009; Herzer et al., 2011). Trait anxiety was associated with fear of hypoglycemia (r2 = 0.23, p < 0.01), worry about hypoglycemia (r2 = 0.02, p < 0.01), and with a higher likelihood of misattributing physical symptoms to blood glucose (r2 = 0.29, p < 0.05) (Gonder-Frederick et al., 2006; Wiebe et al., 1994).
Figure 2.
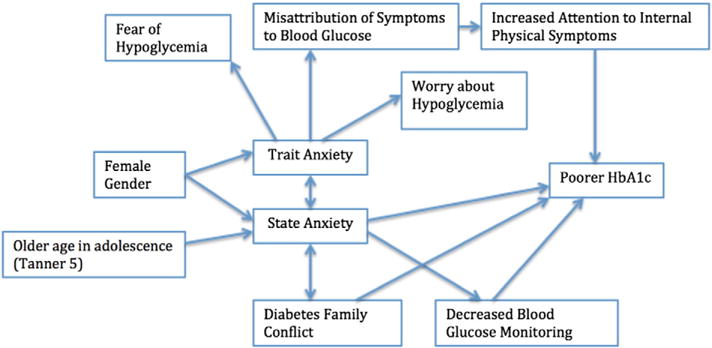
Conceptual model of anxiety in youth with T1D
Discussion
Approximately 20% of youth with T1D screen positive for clinically significant anxiety symptoms. This rate is concerning not only due to the symptom burden and the challenges it represents to youth in daily life, but also due to the potential detrimental effects on diabetes outcomes. Anxiety symptoms appear to increase the risk of poor self-management, poor quality of life, higher depressive symptoms, poorer HbA1C, and re-hospitalization. The prevalence of anxiety symptoms is similar to that of depressive symptoms in youth with T1D, and higher than that of the general adolescent population. Associations between anxiety and poorer health outcomes (e.g. poorer glycemic control, lower quality of life, poorer self-management, higher family conflict) were demonstrated in the majority of studies. More efficient and effective strategies to screen and treat anxiety in youth with T1D are needed.
We found a distinction between the behavioral effects of state anxiety and trait anxiety in youth with T1D, indicating that it is important to differentiate anxiety type when evaluating anxiety and designing interventions. Distinct pathways that demonstrate differential effects of anxiety type on self-management behaviors and health outcomes have been identified. Differences in magnitude of correlations between state and trait anxiety are also important. For example, we found that trait anxiety was more highly correlated with depressive symptoms than state anxiety.
Our framework captures the unique pathways that contribute to state and trait anxiety, and the unique pathways through which state and trait anxiety affect behavioral and physiological outcomes. Research beyond the scope of this review supports additional relationships in our proposed model distinguishing state and trait anxiety. For example, trait anxiety has been associated with fear of hypoglycemia. Fear of hypoglycemia has been linked to poorer HbA1c levels and poorer self-management in other studies in youth with T1D (Fidler, Elmelund Christensen, & Gillard, 2011; Wild et al., 2007). As a result, trait anxiety may contribute to poorer HbA1c levels and self-management behaviors through fear of hypoglycemia.
In addition to categorizing anxiety as state or trait, another way to categorize anxiety is by diagnosis (i.e. generalized anxiety disorder, panic disorder, agoraphobia, separation anxiety disorder, social anxiety disorder, and others). The State–Trait Anxiety Inventory is a commonly used and reliable measure of anxiety symptoms and allows researchers to consider the distinction between these types of anxiety. It is a measure that has also been commonly used in youth with T1D. Future research may be enhanced by the use of alternative measures to distinguish different types of anxiety. For example, the SCARED (the Screen for Child Anxiety Related Disorders) provides categorization by diagnosis: panic disorder, generalized anxiety disorder, separation anxiety disorder, social anxiety disorder, and significant school avoidance. Understanding how different anxiety diagnoses affect health outcomes would allow for the development of specialized interventions and would lead to a more robust understanding of how anxiety types impact behaviors.
Areas for further research include: (1) understanding the prevalence of anxiety symptoms in youth with T1D, especially in relation to and comorbid with depressive disorders; (2) determining what, if any, factors predispose youth with T1D to develop anxiety symptoms; (3) identifying whether the association between anxiety symptoms with poorer glycemic control and poorer quality of life is causal; if so, (4) proposing validated screening tools specific to the T1D pediatric population and (5) developing accessible, feasible interventions. Promising interventions include cognitive behavioral therapy and interventions that emphasize connectedness among youth with T1D (Briery & Rabian, 1999; Hains et al., 2001). Studies that focus on improving anxiety symptoms in girls will also be important.
Limitations
This review had several limitations. There were a limited number of studies on anxiety in youth with T1D, some of which had methodological flaws. The quality of studies varied widely and the majority of studies were cross-sectional, which does not allow us to extrapolate causality.
These studies are limited by the wide age span of participants; each study used a different model; some included all pediatric patients; some included just adolescents; and all used different definitions of “youth” or “adolescent.” The articles were published between 1990 and 2015, which enhanced our yield. Given the limited literature available on this topic, we elected to use a wider date range in order to capture as much evidence as possible. The relationships found in the early studies may not be as relevant in the current clinical and social context. Despite this, all included studies utilized objective measures of anxiety that continue to be utilized in current anxiety research. These measure different forms of anxiety from levels of anxiety symptoms to types of anxiety disorders. Diabetes management and mental health screening practices have changed considerably since 1990. Nonetheless, there are still no standards of care for assessment, management, or prevention of anxiety in youth with T1D.
Several international studies were excluded from this review because concepts of anxiety and mental health differ across cultural contexts, as do screening methods and treatments. While we are unable to identify how these results may have changed our findings, it is important to note that these studies were excluded. Lastly, anxiety was not a primary variable of interest in some of the studies, but was measured incidentally and included in analyses. As a result, information about anxiety derived from some studies was less central to the main objective of the study.
Conclusion
Key gaps in the research concerning the prevalence and impact of anxiety symptoms in youth with T1D were identified in this review. Differential effects of state and trait anxiety on health outcomes in youth with T1D were identified. Future research in this area should be aimed at enhancing our understanding of the prevalence of anxiety symptoms in this population and to improve our current screening and treatment practices.
References*
* Indicates included in the sample.
- American Diabetes Association. Statistics about diabetes. 2014 Retrieved from http://www.diabetes.org/diabetes-basics/statistics/
- *.Bernstein CM, Stockwell MS, Gallagher MP, Rosenthal SL, Soren K. Mental health issues in adolescents and young adults with T1D prevalence and impact on glycemic control. Clinical Pediatrics. 2013;52:10–15. doi: 10.1177/0009922812459950. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition & Emotion. 1998;12:737–753. [Google Scholar]
- *.Briery BG, Rabian B. Psychosocial changes associated with participation in a pediatric summer camp. Journal of Pediatric Psychology. 1999;24:183–190. doi: 10.1093/jpepsy/24.2.183. [DOI] [PubMed] [Google Scholar]
- Crawford TN, Cohen P, Midlarsky E, Brook JS. Internalizing symptoms in adolescents: Gender differences in vulnerability to parental distress and discord. Journal of Research on Adolescence. 2001;11:95–118. [Google Scholar]
- *.Daviss WB, Coon H, Whitehead P, Ryan K, Burkley M, McMahon W. Predicting diabetic control from competence, adherence, adjustment, and psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:1629–1636. doi: 10.1097/00004583-199512000-00013. [DOI] [PubMed] [Google Scholar]
- *.Di Battista AM, Hart TA, Greco L, Gloizer J. T1D among adolescents reduced diabetes self-care caused by social fear and fear of hypoglycemia. The Diabetes Educator. 2009;35:465–475. doi: 10.1177/0145721709333492. [DOI] [PubMed] [Google Scholar]
- Diabetes Research in Children Network (DirecNet) Study Group. Psychological aspects of continuous glucose monitoring in pediatric T1D. Pediatric Diabetes. 2006p;7:32. doi: 10.1111/j.1399-543X.2006.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler NS, Kocovski NL, Macrodimitris SD. Coping, efficacy, and perceived control in acute vs chronic illnesses. Personality and Individual Differences. 2001;30:617–625. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: An overview of fear of hypoglycemia, quality-of-life, and impact on costs. Journal of Medical Economics. 2011;14:646–655. doi: 10.3111/13696998.2011.610852. [DOI] [PubMed] [Google Scholar]
- Garrison MM, Katon WJ, Richardson LP. The impact of psychiatric comorbidities on readmissions for diabetes in youth. Diabetes Care. 2005;28:2150–2154. doi: 10.2337/diacare.28.9.2150. [DOI] [PubMed] [Google Scholar]
- *.Gelfand K, Geffken G, Lewin A, Heidgerken A, Grove MJ, Malasanos T, Silverstein J. An initial evaluation of the design of pediatric psychology consultation service with children with diabetes. Journal of Child Health Care. 2004;8:113–123. doi: 10.1177/1367493504041870. [DOI] [PubMed] [Google Scholar]
- *.Gonder-Frederick LA, Fisher CD, Ritterband LM, Cox DJ, Hou L, DasGupta AA, Clarke WL. Predictors of fear of hypoglycemia in adolescents with T1D and their parents. Pediatric Diabetes. 2006;7:215–222. doi: 10.1111/j.1399-5448.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. The Journal of Pediatrics. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- *.Grey M, Cameron ME, Lipman TH, Thurber FW. Psychosocial status of children with diabetes in the first 2 years after diagnosis. Diabetes Care. 1995;18:1330–1336. doi: 10.2337/diacare.18.10.1330. [DOI] [PubMed] [Google Scholar]
- *.Grey M, Cameron ME, Thurber FW. Coping and adaptation in children with diabetes. Nursing Research. 1991;40:144–148. [PubMed] [Google Scholar]
- Grey M, Whittemore R, Tamborlane W. Depression in T1D in children: Natural history and correlates. Journal of Psychosomatic Research. 2002;53:907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Hadwin JA, Donnelly N, French CC, Richards A, Watts A, Daley D. The influence of children’s self-report trait anxiety and depression on visual search for emotional faces. Journal of Child Psychology and Psychiatry. 2003;44:432–444. doi: 10.1111/1469-7610.00133. [DOI] [PubMed] [Google Scholar]
- *.Hains AA, Davies WH, Parton E, Silverman AH. Brief report: A cognitive behavioral intervention for distressed adolescents with type I diabetes. Journal of Pediatric Psychology. 2001;26:61–66. doi: 10.1093/jpepsy/26.1.61. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology. 2008;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Herzer M, Hood KK. Anxiety symptoms in adolescents with T1D: association with blood glucose monitoring and glycemic control. Journal of Pediatric Psychology. 2009:jsp063. doi: 10.1093/jpepsy/jsp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Herzer M, Vesco A, Ingerski LM, Dolan LM, Hood KK. Explaining the family conflict-glycemic control link through psychological variables in adolescents with T1D. Journal of Behavioral Medicine. 2011;34:268–274. doi: 10.1007/s10865-010-9307-3. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hilliard ME, Herzer M, Dolan LM, Hood KK. Psychological screening in adolescents with T1D predicts outcomes one year later. Diabetes Research and Clinical Practice. 2011;94:39–44. doi: 10.1016/j.diabres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LMB. Depressive symptoms in children and adolescents with T1D: Association with diabetes-specific characteristics. Diabetes Care. 2006;29:1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- *.Kovacs M, Goldston D, Obrosky DS, Bonar LK. Psychiatric disorders in youths with IDDM: Rates and risk factors. Diabetes Care. 1997;20:36–44. doi: 10.2337/diacare.20.1.36. [DOI] [PubMed] [Google Scholar]
- *.Kovacs M, Iyengar S, Goldston D, Stewart J, Obrosky DS, Marsh J. Psychological functioning of children with insulin-dependent diabetes mellitus: A longitudinal study. Journal of Pediatric Psychology. 1990;15:619–632. doi: 10.1093/jpepsy/15.5.619. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Swales T, Klemp S, Madigan S, Skyler J. Adolescents with diabetes: Gender differences in psychosocial functioning and glycemic control. Children’s Health Care. 1995;24:61–78. [Google Scholar]
- Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of T1D. Endocrinology and Metabolism Clinics of North America. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with T1D mediational role of blood glucose monitoring. Diabetes Care. 2009;32:804–806. doi: 10.2337/dc08-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in US adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Naar-King S, Idalski A, Ellis D, Frey M, Templin T, Cunningham PB, Cakan N. Gender differences in adherence and metabolic control in urban youth with poorly controlled T1D: The mediating role of mental health symptoms. Journal of Pediatric Psychology. 2006a;31:793–802. doi: 10.1093/jpepsy/jsj090. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Podolski CL, Ellis DA, Frey MA, Templin T. Social ecological model of illness management in high-risk youths with T1D. Journal of Consulting and Clinical Psychology. 2006b;74:785. doi: 10.1037/0022-006X.74.4.785. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies. 2014 Accessed, 21, 14. [Google Scholar]
- Northam EA, Matthews LK, Anderson PJ, Cameron FJ, Werther GA. Psychiatric morbidity and health outcome in T1D–Perspectives from a prospective longitudinal study. Diabetic Medicine. 2005;22:152–157. doi: 10.1111/j.1464-5491.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety different attentional functioning under state and trait anxiety. Psychological Science. 2010;21:298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, Klingensmith G. Predictors of acute complications in children with T1D. JAMA. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Clark N. Care of children and adolescents with type 1 diabetes a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Theory and research on anxiety. Anxiety and Behavior. 1966;1 [Google Scholar]
- Weist MD, Finney JW, Barnard MU, Davis CD, Ollendick TH. Empirical selection of psychosocial treatment targets for children and adolescents with diabetes. Journal of Pediatric Psychology. 1993;18:11–28. doi: 10.1093/jpepsy/18.1.11. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Knafl K. The integrative review: Updated methodology. Journal of Advanced Nursing. 2005;52:546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Kanner S, Singleton S, Hamrin V, Chiu J, Grey M. Correlates of depressive symptoms in adolescents with T1D. Pediatric Diabetes. 2002;3:135–143. doi: 10.1034/j.1399-5448.2002.30303.x. [DOI] [PubMed] [Google Scholar]
- *.Wiebe DJ, Alderfer MA, Palmer SC, Lindsay R, Jarrett L. Behavioral self-regulation in adolescents with type I diabetes: Negative affectivity and blood glucose symptom perception. Journal of Consulting and Clinical Psychology. 1994;62:1204. doi: 10.1037//0022-006x.62.6.1204. [DOI] [PubMed] [Google Scholar]
- Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Education and Counseling. 2007;68:10–15. doi: 10.1016/j.pec.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]


