Abstract
Objectives
Food reinforcement (modified relative reinforcement value (RRV)), self-control (the ability to delay gratification (ATDG)), and eating outside of homeostatic need (eating in the absence of hunger (EAH)) are associated with overweight/obesity. These constructs have typically been studied in isolation in children and little is known about how they interrelate and whether these associations differ by sex.
Methods
In a low-income sample of 230 seven- to ten-year-old children, we assessed RRV, ATDG, and EAH. We separately tested by sex the model that elevated RRV, lower ATDG and greater EAH are each independent direct predictors of overweight in middle childhood. We predicted that greater RRV and less ATDG would also have indirect effects on overweight through EAH. We investigated the association between RRV and ATDG.
Results
For girls, higher RRV was indirectly associated with overweight through EAH. For boys, no associations of RRV, ATDG, or EAH with overweight were significant. Finally, for girls RRV and ATDG were significantly positively associated.
Conclusions
In girls, higher food reinforcement appears to be an important contributor to overweight. During middle childhood, ATDG may be assessing food reinforcement rather than self-control. Future studies are needed to identify the mechanisms underlying childhood overweight in boys.
Keywords: Overweight, food reinforcement, ability to delay gratification, eating in the absence of hunger, sex differences
Introduction
Overweight and obesity impact 32% of children in the United States (1) and childhood overweight often continues into adulthood (2). Existing interventions have limited long-term effectiveness (3). Improved understanding of the behavioral mechanisms of overweight in children may inform the development of interventions.
Three key behavioral mechanisms theorized to underlie childhood overweight risk are food reinforcement (defined as the relative reinforcement value (RRV) of food), self-control (defined as the ability to delay gratification (ATDG)), and eating outside of homeostatic need (defined as eating in the absence of hunger (EAH)), The RRV task measures an individual's willingness to work to gain access to food when an alternative reinforcer is available (4, 5). In children, higher RRV for food is associated with greater caloric intake, higher likelihood of obesity (6) and excess future weight gain (7). ATDG measures an individual's ability to wait longer for a larger reward as opposed to receive a smaller reward immediately, and is typically measured with a food stimulus in children (8). Greater ATDG has been linked to a lower risk of childhood obesity (8, 9), but also to greater caloric intake (10), suggesting that ATDG may capture elements of food reinforcement. EAH measures the degree to which children consume palatable snacks following a satiating meal (11) and greater EAH has been linked to obesity (12).
The associations between RRV, ATDG, and EAH have rarely been examined. Specifically, it is unknown if greater RRV and poorer ATDG predict greater EAH (13)(14). The two prior studies testing links between ATDG and EAH have shown either a null (15) or unexpectedly positive association (10). In addition, potential sex differences in the associations of these behavioral constructs with overweight in children have rarely been examined (4, 6, 8), and results have been inconsistent.(16)(13)(17)(18, 19).
Therefore, the objective of this study was to test the direct and indirect associations of RRV for food, ATDG, and EAH with childhood overweight, as well as the interrelationships among these constructs, in boys and girls. We hypothesized that greater RRV for food, lower ATDG and greater EAH are independent direct predictors of overweight. We also hypothesized that greater RRV for food and less ATDG are indirectly associated with overweight through EAH. (see Figure 1 for Conceptual Model).
Figure 1. Conceptual Model.
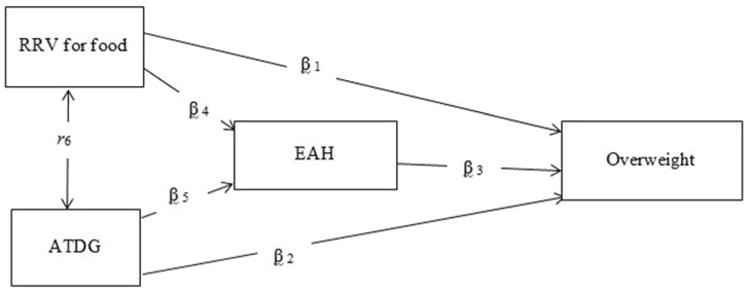
Note: The association between RRV and ATDG is represented by a correlation coefficient
Methods
Study Design and Participants
Participants were seven- to ten-year-old children recruited from an existing cohort participating in a multi-wave longitudinal study (20). The participation rate in the current study was 91%. The parent study into which children were recruited was designed to examine obesity risk in low-income preschoolers attending Head Start. The inclusion criteria at the time of parent study enrollment were: (1) Primary caregiver has < 4-year college degree; (2) Child aged 3 or 4 years; (3) Child born at 36+ weeks gestation, with no significant perinatal or neonatal complications as assessed by the study pediatrician (Dr. Lumeng). Exclusions were: (1) History of food allergies or serious medical problems affecting appetite/eating; (2) Non-fluency in English; (3) Foster child; (4) Significant developmental delay as assessed by Dr. Lumeng.
Of the 275 participants who enrolled in the current study, 230 had complete data for RRV, ATDG, EAH, and weight status. Due to scheduling difficulties, the 45 participants with missing data completed a partial protocol that did not include in-person behavioral tasks.
Study procedures were approved by the University of Michigan Medical School Institutional Review Board. Parents/guardian provided written informed consent and children provided verbal assent.
Measures
Laboratory visits took place across two days in a private conference room at a community center near the families' homes in the late afternoon. All of the measures in the current study were assessed on the first visit day, which typically lasted between 1.5-2 hours (the second day included an assessment of stress reactivity; data not reported here). To reduce variability in hunger at the time of arrival, the child was asked to have an after-school snack. If the parent reported that the child had not eaten a snack, a packet of crackers (Pepperidge Farm® Goldfish Crackers, 1.5 ounces, 210 kilocalories (kcals)) was offered (n=103). The tasks were administered in the following order: 1) ATDG, 2) RRV, 3) EAH. A 5 minute break was provided between each task to reduce carryover. Anthropometry was collected by trained study staff at the end of the protocol.
Relative Reinforcement Value (RRV) for Food
RRV for food is determined by measuring the number of responses on a computer task to obtain palatable food (4). The child was provided access to two identical computer stations that he/she could move freely between; one station where the child could work to access food (e.g., chocolate, gummy candy) and another station where the child could work to access small toys (e.g., bouncy balls, rings). The toy computer station was provided to prevent participants from working for food out of boredom. Each computer screen displayed three boxes containing different shapes. Each time either of the mouse keys on the laptop was pressed the shapes rotated and changed. When all shapes matched the participant received a point. In a standard RRV task, children consume the snack or play with the toy after they earn it (6). However, in the current study the RRV protocol was modified, so that for every time the child earned five points he/she was given a ticket to redeem a prize that corresponded to the station he/she was on (i.e., food or toy) at the end of the visit. This modification was made as variability in the amount of candy consumed during the RRV task could lead to differences in sensory specific satiety to sweet foods, which could impact the EAH task that followed. The schedule of reinforcement for food and toys began at 10 presses to earn one point and then doubled each time the child earned a ticket (Progressive Ratio (PR) 10, 20, PR 40, PR 80, PR 160, PR 320, PR 640, PR 1280, PR 2560, PR 5120, PR 10240). The child was instructed to move back and forth between the stations as many times as he/she would like and that the session would end when he/she no longer wanted to earn points. Food reinforcement was identified by the highest reinforcement schedule completed by the child to earn candy.
Ability to Delay Gratification (ATDG)
The standard ATDG task used with preschool-aged children (12) was adjusted to be more developentally appropriate to the current age group (seven-to-ten year olds) by both increasing the number of trials (six trials in our modified task compared to one trial in the original task) and progressively increasing the waiting time to acquire food. These modifications were made to obtain sufficient variability, given that we anticiapted that all children in the cohort would be capable of waiting through the single trial in the standard task. We elected not to use the standard delay tasks employed in work with adults given the abstract nature and cognitive demands of these tasks, which we did not feel were appropriate for this age range. The modified task was piloted prior to use in this study to confirm that it captured variablity in child beahvior. The child was asked to choose the candy option he/she preferred (15-gram packet of M&M's® chocolate candy or 15-gram packet of Skittles® fruit candy). The child was then shown two piles of candy: one with a large quantity (two packets of candy) and the other with a small quantity (one packet of candy). The child was told that he/she would be allowed to eat the large quantity if he/she waited until the examiner returned, and that this would be repeated up to five times. If the child could not or did not wish to wait, the child could ring the bell to summon the examiner, at which time the child would receive the smaller quantity of candy. The examiner would leave the room and after one minute, return and ask the child if he/she would like the candy now, or if he/she would like to wait longer to get more candy. If the child decided to wait longer, the researcher would add one candy packet to both the large and small piles and told the child again that if he/she waits, he/she may have the larger pile. This procedure was repeated up to five times, with the waiting period extended by one minute each time. The number of candy packets increased from one v. two, two v. three, three v. four, four v. five, and five v. six. Each time, if the child waited the prescribed number of minutes until the examiner returned, he/she was scored as a ‘pass’ on that trial. The number of trials (zero to five) the child passed was used to indicate ATDG, which provides additional, progressively more challenging assessments relative to versions of this task used with younger children that score children as pass/fail from one trial.
Eating in the Absence of Hunger (EAH)
The parent and child (plus other family members present) were served a standardized meal consisting of a 12-inch deli meat sandwich, baked potato chips, apple sauce, fruit cups, condiments (mustard and mayonnaise) and water. When the child indicated he/she was finished, the researcher then invited the child (without the parent) to a separate room. Children were instructed, “You can have dessert. You can't take it with you, but you can eat as much as you like here for five minutes. If you are ready to be done before that, all you have to do is let me know. I'm going to do some work now.” For five minutes the child was given free access to pre-measured bowls of four Little Debbie Oatmeal Cream Pies® (152g, 680 kilocalories (kcals)), two Little Debbie Cosmic Brownies with Chocolate Chip Candy® (124g; 560 kcals), eight Nabisco/Chips Ahoy Chewy Chocolate Chip Cookies® (124g; 560 kcals), eight Keebler Fudge Stripe Cookies® (108g; 560 kcals), eight Little Debbie Mini Powdered Donuts® (100g, 440 kcals), and three Kellogg's Original Rice Krispy Treats® (66g, 270 kcals). The food that remained was weighed and this value was subtracted from the initial weight.
Body Mass Index (BMI)
Children were weighed using a Detecto Portable Scale Model #DR550C and measured using a Seca 214 portable stadiometer. BMI was calculated and weight status categorized as overweight (BMI ≥ 85th percentile) or not overweight (BMI < 85th percentile) based on the US Centers for Disease Control reference growth curves for age and sex (21).
Data Analysis
Data analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Univariate and bivariate statistics were used to describe the sample. The distribution of RRV schedule was skewed, therefore we log transformed this variable before doing any modeling. Path models were conducted (using MPLUS version 6.1 (Muthen & Muthen, Los Angeles, CA)) to test both the direct and indirect associations between RRV for food, ADTG, and EAH with overweight (Figure 1). To investigate for potential sex differences, path models were tested separately for boys and girls. Bayesian estimation technique in MPLUS was used to fit path models. Bayesian posterior predictive checks (PPC) using Chi-square statistics and the corresponding posterior predictive p-values were used to assess the goodness of fit for each model (22)
Results
Sample Characteristics
Sample characteristics are shown in Table 1. The sample was 45.6% Hispanic or not white, 48.7% female, and 50.0% of participants were classified as overweight/obese. The age range that was the focus of recruitment was seven- to eight-years-old and 95% of the sample was within this age range. Eleven children were slightly older (nine- to ten-years-old). The median food reinforcement schedule completed was PR 320 (range 0-5120). The average number of trials passed in the ATDG task was 2.08 (SD = 1.22; range 0-5). The mean total number of kcals consumed in the EAH task was (M =347.71; SD=155.49; Range 0.00-768.60).
Table 1. Characteristics of the sample (n=230)*.
| Variable | N (%) or Mean (Standard Deviation) | ||
|---|---|---|---|
|
| |||
| All (n = 230) | Male (n = 118) | Female (n = 112) | |
|
| |||
| Age (years) | 7.84 (0.66) | 7.83 (0.61) | 7.85 (0.70) |
| Race/Ethnicity | |||
| Non-Hispanic white | 125 (54.35%) | 61 (51.69%) | 64 (57.14%) |
| Black, non-Hispanic | 35 (15.22%) | 17 (14.41%) | 18 (16.07%) |
| Other, non-Hispanic | 3 (1.30%) | 3 (2.54%) | 0 (0.00%) |
| Biracial, non-Hispanic | 44 (19.1%) | 28 (23.73%) | 16 (14.29%) |
| Hispanic, any race | 23 (10.00%) | 9 (7.63%) | 14 (12.50%) |
| Weight status | |||
| Non-Overweight | 115 (50.00%) | 67 (56.78%) | 48 (42.86%) |
| Overweight | 54 (23.48%) | 24 (20.34%) | 30 (26.79%) |
| Obese | 61 (26.52%) | 27 (22.88%) | 34 (30.36%) |
| RRV, median (IQR) | 320 (80-640) | 320 (80-640) | 320 (80-640) |
| ATDG | 2.08 (1.22) | 2.02 (1.17) | 2.15 (1.28) |
| EAH | 347.71 (155.49) | 370.71 (167.32) | 323.48 (138.63) |
The 230 participants included in this analysis did not differ from the excluded participants (n=45) with regard to child sex, race/ethnicity, and overweight status. Child who were not included in the analysis were older, on average, than children who were included in the analysis (8.4 years (SD 0.9) vs. 7.8 years (SD 0.7), p=0.001).
Model Fit
All of the models showed good fit, with posterior predictive p-values ranging from 0.583 to 0.667, well within the 0.05 – 0.95 range. Model fit was optimized by separating boys and girls into two distinct models. Two sample t-tests were used to confirm that the beta estimates from the girl and boy models were statistically significantly different (all models p values < .0001).
Path Estimates for Girls
Path estimates for girls are shown in Table 2. There was a trend-level direct effect of RRV (p=0.06) and a direct effect of higher EAH (p<0.0001) on overweight, but no direct effect ATDG (p=0.46) There was an indirect effect of higher RRV for food through higher EAH on overweight (p<0.0001), but no indirect effect of ATDG (p=0.18). RRV for food and ATDG were positively correlated (p< 0.0001).
Table 2. Path Estimates.
| Girls | Boys | ||||||
|---|---|---|---|---|---|---|---|
| Pathway code | Pathway description | Estimate (posterior SD) | 95% CI | p-value | Estimate (posterior SD) | 95% CI | p-value |
| B1 | RRV for food → overweight | 0.15 (0.10) | -0.01 -0.36 | 0.06 | -0.17 (0.12) | -0.43 -0.07 | 0.12 |
| B2 | ATDG→ overweight | -0.10 (0.11) | -0.27 – 0.11 | 0.46 | 0.004 (0.13) | -0.23 – 0.29 | 0.98 |
| B3 | EAH →overweight | 0.54 (0.10) | 0.33 – 0.70 | < 0.0001 | 0.12 (0.11) | -0.09 – 0.36 | 0.24 |
| B4 | RRV for food → EAH | 0.21 (0.09) | 0.02 – 0.41 | < 0.0001 | -0.09 (0.09) | -0.25 – 0.08 | 0.24 |
| B5 | ATDG→ EAH | 0.14 (0.10) | -0.06 – 0.34 | 0.18 | 0.04 (0.09) | -0.12 – 0.25 | 0.68 |
| R1 | Correlation RRV for food with ATDG | 0.26 (0.08) | 0.09 – 0.42 | < 0.0001 | 0.20 (0.09) | 0.00 – 0.34 | 0.06 |
Note: RRV = Relative Reinforcing Value; ATDG = Ability to Delay Gratification; EAH = Eating in the Absence of Hunger
Path Estimates for Boys
Path estimates for boys are shown in Table 2. There were no direct effects of RRV, ATDG or EAH on overweight (all ps >0.12). There were no indirect effects of RRV for food or ATDG through EAH on overweight (all ps >0.24). There was a trend-level positive association between RRV for food and ATDG (p=0.06).
Discussion
The hypothesized conceptual model was partially supported, particularly in girls. In girls, higher RRV was indirectly associated with overweight through EAH, and EAH was directly associated with overweight. Additionally, for girls, there was a trend-level direct association of RRV with overweight. Thus, for girls, this pattern is consistent with EAH potentially mediating the association between RRV and overweight. In contrast, for boys, there were no associations of RRV, ATDG, or EAH with overweight. Finally, for girls RRV and ATDG were positively associated and for boys there was a trend- level positive association.
The current study suggests that higher food reinforcement may be an important pathway to overweight in girls through greater EAH during middle childhood. Prior research has found that higher RRV is associated with elevated BMI in preschoolers, children, and adults (14, 23). Differences in RRV for food may be related to differences in the functioning of the mesolimbic dopamine system (24), which is implicated in motivational processes (25). RRV for food can even be assessed in the first year of life (23) and there is evidence that RRV for food may be malleable. In infants, repeated exposure to an alternative reinforcer (i.e., a music enrichment program) resulted in reduced RRV for food (26). Future research is needed to investigate to what degree RRV for food may be modified in middle childhood and whether this may be protective against future weight gain.
There was limited support in the current study for the association of ATDG with overweight in middle childhood. ATDG is typically considered a marker of self-control (27) and in many studies with preschool-aged children lower ATDG was associated with a greater risk for future weight gain (8, 9, 16). However, Hughes and colleagues (10) found that greater ATDG (interpreted as more self-control) was associated with greater caloric intake and was unrelated to other measures of self-control in Hispanic preschoolers. In the current study, ATDG was positively associated with RRV for girls and there was a trend-level positive association for boys, suggesting that ATDG may be capturing some aspect of reinforcement for food in middle childhood. There are similarities between the assessment of RRV for food (willingness to work for larger quantities of food) and ATDG (willingness to wait for larger quantities of food). As self-control increases with age, older children who find food particularly reinforcing may be more capable of waiting for food rewards. With older children other paradigms may be needed to more precisely assess self-control related to food, such as the cued go/no-go task (i.e., inhibiting one's response to unhealthy food cues).
Current findings are consistent with prior research that highlights EAH as an important factor associated with overweight (12, 13). Little is known about factors that may contribute to greater EAH. It is possible that children who are more prone to EAH are less sensitive to signals of hunger and satiety, however interventions designed to increase awareness of hunger and satiety were not effective in reducing EAH (28). The current study highlights the role of food reinforcement as an important factor in EAH, as girls with higher RRV for food have higher EAH. Prior research has found that children with higher EAH are also more likely to have alleles (AA and AT) of the FTO gene that have been implicated in risk for overweight (29). The same FTO alleles associated with EAH are also implicated in greater activation of neural regions related to food motivation in response to food cues (30). Future research should investigate whether reducing food reinforcement may also diminish EAH and risk for overweight.
Results also revealed marked sex differences. For girls only, higher RRV was indirectly associated with overweight through higher EAH. Thus, for girls, higher food reinforcement may be an important target for intervention. In contrast, in boys there was not a significant association of RRV with EAH or overweight. Prior research has found limited sex differences regarding the association of RRV with risk for overweight in children (4, 6, 8). The current study also found an association of EAH with overweight only for girls. This is consistent with prior research that EAH is associated with elevated BMI in girls (but not boys) (17), but inconsistent with other research that this association is present for boys only (18, 19). Prior research that failed to detect an association between EAH and overweight for girls was conducted in settings where the participant's food consumption could be observed by others (i.e., in school; at home), which may have altered eating behavior (18, 19). However, the lack of EAH-overweight associations for boys in the current study highlights the importance of identifying factors (e.g., physical inactivity, satiety responsiveness) that may underlie risk for overweight in boys and developing more individualized treatments that address potential sex differences.
There are limitations to consider. First, the current study is cross-sectional and therefore causality cannot be inferred. Second, the current study sample was low-income and it is possible that these results may not generalize to more well-resourced populations. Environmental drivers of obesity (e.g., less access to healthy foods, fewer safe spaces for physical activity) may account for a larger portion of the variance in BMI among low-income samples, which may reduce the effect size of individual differences. Third, the RRV task was modified to have participants work to earn tickets for food/toys (rather than consuming candy or playing with toys during the task). This was done to prevent performance on the RRV task from impacting the EAH task. Altering the RRV task in this manner requires the children to wait to receive the reinforcer, which adds an element of delay of gratification to the standard RRV task. This may have increased the similarities between ATDG and RRV. Future research is needed that utilizes the standard RRV task that provides food reinforcers contingent upon responding to remove ambiguity between reinforcement value and self-control. Finally, the ATDG, RRV and EAH protocols were always administered in the same order, thus the potential contribution of order effects cannot be ruled out. Future research would benefit from counterbalancing the order of tasks or conducting assessments on separate days to rule out potential carryover effects.
In summary, the current study has several important implications. First, for girls, RRV was indirectly through EAH associated with overweight. This suggests that girls with higher reinforcement for food are more prone to eat palatable food even when not calorically deprived. Second, ATDG was associated with reinforcement for food rather than self-control in middle childhood for girls, which suggests that other approaches are needed to assess self-control related to food in this developmental stage. Third, for girls, EAH was associated with overweight, which suggests that approaches to reducing food reinforcement through attention modification training or food cue extinction (28, 31) may be important for reducing EAH and overweight. Finally, the current study highlights the need to identify the mechanisms underlying overweight in boys and the potential need to consider sex differences in developing obesity interventions for children.
What is already known about this subject?
Food reinforcement, self-control, and eating outside of homeostatic need have been implicated in childhood overweight/obesity.
However, these constructs have been investigated in isolation and little is known about how they may interrelate or be differentially associated with overweight.
There has also been little investigation of potential sex differences.
What does your study add?
For girls, higher food reinforcement was indirectly associated with overweight through eating outside of homeostatic need, which suggests this is an important target for intervention for girls.
The ability to delay gratification was associated with reinforcement for food in middle childhood for girls, which suggests that other approaches may be needed to assess self-control related to food in this developmental stage.
None of the proposed constructs were related to overweight in boys, which highlights the need to identify the mechanisms underlying overweight in boys.
Acknowledgments
All phases of this study were supported by NIH grant # R01DK098983.
Footnotes
The authors have no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA: the journal of the American Medical Association. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108(3):712–8. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 3.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychological bulletin. 2006;132(5):667. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein LH, Leddy JL, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychological bulletin. 2007;133(5):884. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27(1):41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 6.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. The American journal of clinical nutrition. 2008;87(5):1121–7. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. The American journal of clinical nutrition. 2009;90(2):276–81. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 8.Caleza C, Yañez-Vico RM, Mendoza A, Iglesias-Linares A. Childhood Obesity and Delayed Gratification Behavior: A Systematic Review of Experimental Studies. The Journal of pediatrics. 2016;169:201–7.e1. doi: 10.1016/j.jpeds.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 9.French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite. 2012;59(2):541–9. doi: 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes SO, Power TG, O'Connor TM, Fisher JO. Executive functioning, emotion regulation, eating self-regulation, and weight status in low-income preschool children: How do they relate? Appetite. 2015;89:1–9. doi: 10.1016/j.appet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(Supplement 2):539–49. [PubMed] [Google Scholar]
- 12.Asta K, Miller AL, Retzloff L, Rosenblum K, Kaciroti NA, Lumeng JC. Eating in the absence of hunger and weight gain in low-income toddlers. Pediatrics. 2016:e20153786. doi: 10.1542/peds.2015-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lansigan RK, Emond JA, Gilbert-Diamond D. Understanding eating in the absence of hunger among young children: A systematic review of existing studies. Appetite. 2015;85:36–47. doi: 10.1016/j.appet.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & Behavior. 2010;100(5):438–45. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieper JR, Laugero KD. Preschool children with lower executive function may be more vulnerable to emotional-based eating in the absence of hunger. Appetite. 2013;62:103–9. doi: 10.1016/j.appet.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Connell LE, Francis LA. Positive parenting mitigates the effects of poor self-regulation on body mass index trajectories from ages 4–15 years. Health Psychology. 2014;33(8):757. doi: 10.1037/hea0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutting TM, Fisher JO, Grimm-Thomas K, Birch LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers' dietary disinhibition. The American journal of clinical nutrition. 1999;69(4):608–13. doi: 10.1093/ajcn/69.4.608. [DOI] [PubMed] [Google Scholar]
- 18.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behaviour research and therapy. 2007;45(6):1357–68. doi: 10.1016/j.brat.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Hill C, Llewellyn C, Saxton J, Webber L, Semmler C, Carnell S, et al. Adiposity and ‘eating in the absence of hunger’ in children. International Journal of Obesity. 2008;32(10):1499–505. doi: 10.1038/ijo.2008.113. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng JC, Miller A, Peterson KE, Kaciroti N, Sturza J, Rosenblum K, et al. Diurnal cortisol pattern, eating behaviors and overweight in low-income preschool-aged children. Appetite. 2014;73:65–72. doi: 10.1016/j.appet.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R. CDC Growth Charts. Adv Data. 2000;314:1–28. [PubMed] [Google Scholar]
- 22.Gelman A, Carlin J, Stern H, Dunson D, Vehtari A, Rubin D. Bayesian data analysis. Boca Raton, Fla: Chapman & Hall'CRC; 2004. [Google Scholar]
- 23.Kong KL, Anzman-Frasca S, Feda DM, Eiden RD, Sharma NN, Stier CL, et al. Infant Temperament Is Associated with Relative Food Reinforcement. Childhood Obesity. 2016;12(6):411–7. doi: 10.1089/chi.2016.0001. [DOI] [PubMed] [Google Scholar]
- 24.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121(17907820):877–86. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong KL, Eiden RD, Feda DM, Stier CL, Fletcher KD, Woodworth EM, et al. Reducing relative food reinforcement in infants by an enriched music experience. Obesity. 2016;24(4):917–23. doi: 10.1002/oby.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigal JJ, Adler L. Motivational effects of hunger on time estimation and delay of gratification in obese and nonobese boys. J Genet Psychol. 1976;128(1st Half):7–16. doi: 10.1080/00221325.1976.10533966. [DOI] [PubMed] [Google Scholar]
- 28.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: a randomized controlled trial. Journal of consulting and clinical psychology. 2011;79(6):759. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. International journal of obesity. 2009;33(1):42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 30.Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences. 2017;114(1):160–5. doi: 10.1073/pnas.1605548113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutelle KN, Knatz S, Carlson J, Bergmann K, Peterson CB. An Open Trial Targeting Food Cue Reactivity and Satiety Sensitivity in Overweight and Obese Binge Eaters. Cognitive and Behavioral Practice. 2016 doi: 10.1016/j.cbpra.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


