Abstract
Maintaining a pool of adult stem cells is essential for tissue homeostasis and wound repair. In mammalian tissues, notably hair follicles, blood, and muscle, stem cells acquire quiescence and infrequently divide for self-renewal. Mechanistic understanding of stem cell quiescence is critical for applying these multipotent cells in regenerative medicine and interrogating their roles in human diseases such as cancer. Quiescent and dividing epithelial stem cells located in hair follicle are conspicuously organized in a spatiotemporally specific manner, allowing them to be studied at a considerable depth. Recent advancements in mouse genetics, genomics and imaging have revealed unprecedented insights into establishment, maintenance and regulation of quiescent hair follicle stem cells. In this Concise Review, I summarize the progress with a focus on mechanisms mediated by signaling pathways and transcription factors and discuss their implications in the understanding of stem cell biology.
Graphical Abstract
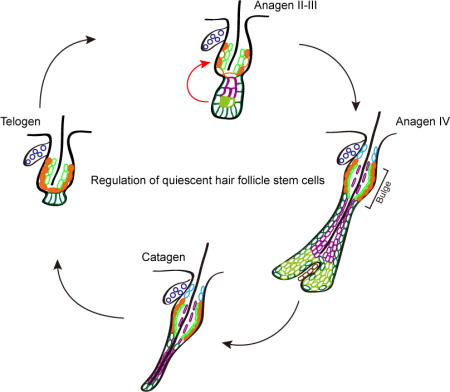
In mammalian skin, hair follicles periodically go through a cycle of growth, regression and rest. These macroscopic activities are driven by the activation and quiescence of hair follicle stem cells. This Concise Review discusses recent progresses in understanding the regulation of quiescent hair follicle stem cells through transcriptional mechanisms and signaling pathways.
A brief history of quiescent hair follicle stem cells
Skin and its appendages are hierarchically organized into multiple lineages such as epidermis, sebaceous gland, hair follicle and sweat gland with resident stem cells for their self-renewal and differentiation during homeostasis and injury repair. For more information about these different epithelial stem cells, I refer readers to several excellent reviews [1–3]. It should also be noted that hair follicle contains multiple stem cell and progenitor populations including epithelial and melanocyte stem cells. In this review, I will focus on the epithelial stem cells located in the bulge region of hair follicles and refer them as HF-SCs. The hair follicle is a fascinating mini-organ undergoing continuous regeneration throughout life. During embryonic development, hair follicles are specified from a population of epidermal and dermal progenitors under the control of Eda/Edar/NF-κB and Wnt signaling, among other pathways [4–7]. In the adult, hair follicles undergo periodic phases of growth (anagen), regression (catagen) and rest (telogen). Hair follicle characteristics associated with each phase are morphologically distinct, such that the different phases were readily distinguishable by early observers [8–10]. The anagen phase was correlated with the growth of the hair shaft and an elongated hair follicle, whereas the telogen phase was associated with the lack of hair growth and shortened hair follicle. Thus, early researchers associated anagen with active cell division and growth, and telogen with cellular quiescence. Although this quiescence of hair growth should not be confused with the quiescence of HF-SCs, the cyclic nature of hair follicle growth is a prominent feature of this mini-organ, and provided an initial hint for the periodic activities of HF-SCs.
One of the first insights into the quiescent nature of HF-SCs came from observations that DNA label-retaining cells (LRCs) resided in the bulge area [11], the lower region of the permanent portion of the hair follicle, below the sebaceous gland. Analyses of cell proliferative potential and transplantation assays using different hair follicle regions identified the bulge epithelial cells with the highest clonogenicity and the ability to form all hair follicle lineages [12]. To isolate these enigmatic LRCs at the bulge, Tumbar and colleagues from the Fuchs group, designed a genetically engineered Tet-off mouse model that enables universal labeling of cell nuclei with transgenic histone H2B-GFP expression. In the absence of doxycycline, H2B-GFP is robustly expressed in all cells regardless of their proliferation and differentiation states. Upon application of doxycycline to the Tet-off system, which in turn shuts off the expression of H2B-GFP, LRCs can be identified by the bright GFP signals retained in the nuclei of slow-cycling, long-lived cells after a prolonged “chase” period of usually >4 weeks. The bulge cells were conspicuously label-retaining among all skin populations [13]. Shortly after the successful isolation of the bulge cells, the Fuchs group further demonstrated the multipotency of these cells by culturing and expanding individual bulge cells in vitro and grafting these cells together with competent dermal cells to regenerate hair follicles [14]. Using a different approach, the Cotsarelis group identified a Krt15 promoter that is specifically activated in the bulge cells [15] and generated a Krt15-CrePR transgenic mouse model [16]. This model allows labeling and lineage analysis of the bulge cells directly in intact skin. By using genetic lineage tracing, the researchers demonstrated the stemness of the bulge cells in vivo [15–17]. Helped with the discovery that CD34 and α6 integrin mark the bulge stem cells [13,14,18], these groups further characterized the unique transcriptome of these cells. Transcriptome of the bulge cells was highlighted by low expression of cell proliferation markers and low expression of Wnt ligands [13,16], indicative of a relatively quiescent cellular state. Together, these pioneering studies formally established the bulge as the HF-SC compartment and paved the road to studying molecular mechanisms that govern the cellular state of stem cells under physiological and pathological conditions.
Origin and basic property of quiescent hair follicle stem cells
During embryonic development, as soon as the hair germ is formed, HF-SCs are specified as Sox9-expressing progenitors [19]. Notably, Sox9+ cells are largely restricted away from the leading edge of the hair germ, which has high levels of Wnt activation and also produces Shh. Instead, Sox9+ cells reside one cell layer away from the leading edge of the hair germ [20] (Figure 1A). In contrast to adult HF-SCs, which also express high levels of Sox9 and are usually slow-cycling and quiescent, these embryonic Sox9+ cells are highly proliferative, and the robust cell division fueled by these cells is believed to be a main driving force for the initial hair morphogenesis [20] (Figure 1B). Therefore, there must be a transition during which at least a subset of these highly proliferative Sox9+ progenitors either give rise to a quiescent population of HF-SCs or stop cell division and become quiescent themselves. The precise molecular cascade underpinning this transition is not well understood, but the miRNA pathway has been implicated as serving a role. In the Dicer1 and Dgcr8 skin-conditional knockout (cKO) model, although HF-SCs can be specified, as judged by the appearance of Sox9+ cells in the hair germ and initially developed hair follicles at birth, these HF-SCs lose their identity 4~5 days after birth [21]. The requirement of miRNAs for maintaining HF-SCs was further supported by the complete loss of hair follicle lineages in grafted Dicer1 cKO skin [21], in conditional ablation of Dicer1 or Drosha in adult skin [22] and in Ago1/2 double KO skin [23]. The role of individual miRNAs in regulating the activity of HF-SCs has begun to emerge recently. Of note, miR-205 is highly expressed in the bulge HF-SCs and controlled by a super enhancer [21,24], and functions to promote pAkt levels by repressing multiple negative regulators of the PI(3)K pathway. Genetic deletion of miR-205 leads to significantly reduced pAkt levels and a premature exit of the cell cycle by the Sox9+ progenitors, leading to hypoproliferation of neonatal hair follicles [21]. However, the miR-205 null HF-SCs do not lose their SC identity. Thus, other miRNAs must contribute to the maintenance of HF-SCs as seen in the Dicer1 cKO. Of note, several negative regulators of the PI(3)K pathway, such as Inppl1 and Inpp4b are gradually upregulated in the Sox9+ hair follicle progenitor cells during hair morphogenesis, and their induction is generally correlated with cell cycle exit of these proliferative cells [21]. In addition, in Pten skin cKO animals, in which the PI(3)K pathway is abnormally activated, accelerated hair cycle and hyperproliferation was reported [25]. These data indicate a role of the PI(3)K pathway in promoting HF-SC proliferation and perhaps regulating the transition from proliferation to quiescence during the initial stage of hair development (Figure 1B). Future experiments that reveal the origin of quiescent HF-SCs and the underlying molecular cascades will be invaluable to understand how these cells exit the cell cycle while preserving the stemness.
Figure 1. Specification of hair follicle stem cells and the establishment of quiescent cellular state during embryonic skin development.
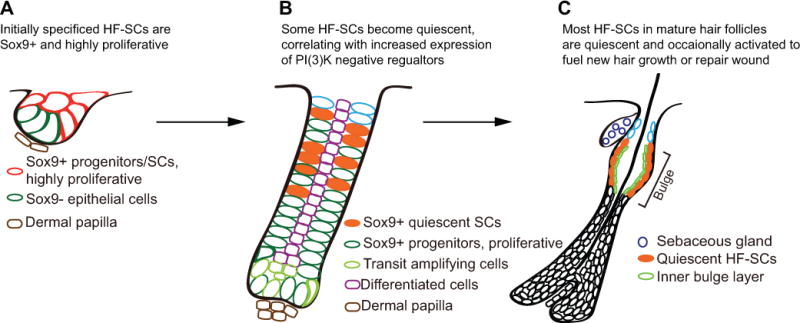
A. In newly formed hair germs, HF-SCs are specified as Sox9-expressing progenitors. At this stage, all Sox9-expressing cells are highly proliferative. B. When hair follicles continue to grow, some HF-SCs exit cell cycle and adapt the quiescent cellular state. Whereas the precise mechanism remains to be determined, the establishment of quiescence is generally correlated with increased expression of several negative regulators of the PI(3)K pathway. C. In fully developed hair follicles, HF-SCs are mostly quiescent and only occasionally activated to fuel hair growth or repair wound.
In adult mice, HF-SCs offer an ideal system to examine underlying mechanisms that regulate stem cell quiescence and activation (Figure 1C). During the early adult life, between postnatal day 18 (P18) and 70, body hair growth is largely synchronized [26,27], although the exact timeline for the synchronized hair cycle varies slightly in different genders and mouse strains. Generally based on studies of C57BL strain mice, between P18 and P23, hair follicles go through a short period of telogen phase before initiating a new anagen, which produces a new hair follicle between P23 and P35. By P37, the anagen hair follicle starts to regress and enters a transient catagen phase, during which the lower portion of hair follicle is degraded while the dermal papilla (DP) moves upwards with the remaining epithelial cells which eventually form a hair germ (HG) beneath the bulge stem cell compartment. By ~P44, with the completion of hair follicle regression and hair germ formation, the hair follicle enters a prolonged telogen phase. This telogen phase is considerably longer than the short one between P18 and P23, and typically lasts four weeks before the hair follicle enters the next anagen phase by ~P70. Therefore, the quiescent and activated HF-SCs can be isolated and examined by using FACS-purified HF-SCs in a temporally specific manner. HF-SCs isolated from the anagen phase are generally activated and undergo cell division, whereas HF-SCs isolated from the telogen phase are generally quiescent. Indeed, shortly after the discovery that CD34 and α6-integrin can specifically mark HF-SCs in the bulge region [13,14,18], many groups began to isolate telogen and anagen HF-SCs and perform transcriptome analyses to identify differentially expressed genes that regulate HF-SCs activities during these two phases. Not surprisingly, many cell cycle and cell division-related genes such as cyclin D1, Top2A, CenpE and signaling molecules such as Wnt, FGF and BMP components are found among highly differentially expressed genes [13,14,16,28,29]. To determine the dynamics of HF-SC division during the hair cycle, the Tumbar group showed that HF-SCs, on average, divide 2-3 times in a hair cycle and both cell migration and division contribute to the activation of HF-SCs, which fuels hair growth [29,30]. Furthermore, it was estimated that a single HF-SC divides less than 100 times for homeostasis during the typical lifespan of mice (~2-years) [30]. These findings provide initial clues for the different cellular states and the cell cycle dynamics of dividing and quiescent HF-SCs.
Regulatory mechanisms of quiescent hair follicle stem cells by signaling pathways
Studies of HF-SC quiescence and self-renewal with focus on transcription factors (TFs) and signaling pathways have provided mechanistic insights into the control of these two distinct cellular states. Like embryonic hair morphogenesis, hair growth in the adult mice is believed to be regulated by intimate interactions between hair follicle cells and the DP. Signaling pathways such as Wnt, BMP and FGF signaling have been shown to have profound roles in governing HF-SCs activities (Figure 2). When Wnt signaling is induced in the hair follicle, including in both the bulge compartment and HG progenitors, hair growth is precociously activated [31–33]. Interestingly, the activated hair growth caused by stabilization of β-catenin in the epithelial cells can be accomplished even when the DP cells are ablated, indicating the activated Wnt signaling in the epithelial cells is likely downstream of the signaling events from the DP [32]. Furthermore, stabilized β-catenin can further stimulate Wnt ligand production and, in turn, recruit neighboring cells into the action. Conversely, when β-catenin or Wntless (also known as Wls) genes, required for transcriptional activation of the Wnt pathway and for secretion of Wnt ligands respectively, are conditionally deleted in the skin, the telogen-to-anagen transition is compromised [32,34,35]. In the nuclei of Wnt responding cells, TCF3/4 switch from association with TLE4, which negatively regulates gene expression, to association with stabilized, nuclear β-catenin to activate a subset of Wnt-targeted genes. When TLE4 was overexpressed through genetic manipulation, the transition to Wnt activation and hair growth was delayed [36]. Under physiological conditions, Axin2 expressing HF-SCs are recently shown to produce low levels of Wnt ligands to maintain their stemness during quiescence [33]. The potent role of Wnts in regenerating hair was further highlighted by their ability to induce wound-induced hair neogenesis in mouse [37]. And in this case, the Wnts are induced by Fgf9, which is produced by γδ T cells when these cells enter the wound dermis [38]. Altogether, these data provide strong evidence for the key role of the Wnt signaling in orchestrating HF-SC activation.
Figure 2. Signaling pathways control the quiescence and activation of hair follicle stem cells.
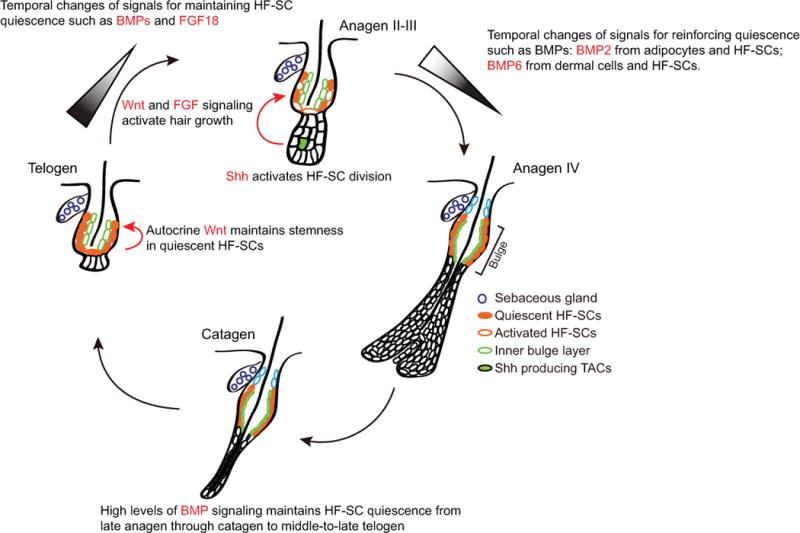
BMP signaling and FGF18 governs the quiescent cellular state whereas Wnt and FGF7/10 promotes hair growth. Shh produced by transit amplifying cells at the Matrix activates self-renewal of HF-SCs in early Anagen such as Anagen II and III.
BMP signaling, on the other hand, plays an essential role in governing HF-SC quiescence. One of the first pieces of evidence came from genetic deletion of Bmpr1a, a key receptor for the epithelial cells to interpret BMP ligands produced in various skin populations [39–41]. When Bmpr1a was specifically deleted from adult HF-SCs, HF-SCs lost quiescence and became hyperproliferative but did not lose their SC identity [42]. However, differentiation of the expanded HF-SCs and progenitors appears to be compromised, and the Bmpr1a cKO mice fail to regenerate hair shafts. Thus, BMP signaling has a dual role in maintaining HF-SC quiescence and promoting terminal differentiation of HF-SCs, likely through its action in distinct hair follicle cell populations. Bmpr1a studies provided evidence for cell intrinsic functions of BMP signaling. However, it was critical to determine how different cell populations in the skin produce BMP ligands and collectively regulate HF-SC activities. Multiple sources of BMP ligands have been identified in the skin. In the adipocytes located in the subcutaneous epidermis, BMP2 was found to be periodically expressed by these cells. Intriguingly, the oscillation of BMP2 expression in the adipocytes is correlated with the anagen-to-telogen transition, hair growth and HF-SC quiescence [43]. In late anagen to the middle of telogen, BMP2 is highly expressed in the adipocytes, and HF-SCs are rested in a refractory phase, generally resistant to activation signals. Beginning during late telogen to early anagen, BMP2 expression in the adipocytes is reduced considerably and correlates with the permissive phase for HF-SC activation. In addition to BMP2 produced by the adipocytes, BMP6 is found to be produced by the inner bulge layer, a potential population of the HF-SC niche [44]. BMP6 expression in these cells plays a role in restricting HF-SCs from activation. Of note, BMP6 is also produced from the DP [45]. Furthermore, in our recent study of Foxc1 in the HF-SCs, we also found that the transient expression of Foxc1 in the HF-SCs enhances expression of BMP2 and BMP6 cell autonomously [46], likely through direct transcriptional control. Therefore, many cell populations in the skin produce BMP ligands, which play a role in regulating HF-SC activities. HF-SCs are predicted to respond to the collective output of all BMP ligands from all of these cell populations. Although it remains an open question how these BMP producing cells coordinate to govern HF-SC quiescence and whether there is a hierarchical organization of BMP producing cells in their ability to regulate HF-SCs, it is clear that active BMP signaling is required to maintain HF-SC quiescence, and reduced or compromised BMP signaling is correlated with HF-SC activation.
In contrast to the Wnt and BMP signaling pathways, whose downstream effects in HF-SC activities are consistent with HF-SC activation or quiescence distinctively, FGF signaling pathways have shown diverse regulatory outcomes. FGF7 and FGF10 are produced by the DP, and their presence stimulates hair growth initiated in the HG [28]. In contrast, FGF18 is largely produced by the inner bulge layer and the bulge HF-SCs [47]. Genetic deletion of Fgf18 significantly shortens the duration of the quiescent telogen phase, leading to activation of hair growth and HF-SC division. It has been suggested that Fgfr2 IIIb is responsible to transduce the FGF7/10 signaling, whereas Fgfr3c is responsible for mediating the effect of FGF18 [47,48]. Given the diversity and complexity of FGF signaling [49], it will be interesting to further dissect the downstream mediator of FGF7/10-Fgfr2b and FGF18-Fgfr3c axis and understand how different FGFs produce different HF-SC responses. Further analyses of potential synergy or antagonism of these pathways can provide new insights into how HF-SCs and HG cells interpret complex input from the environment and orchestrate quiescence and activation of HF-SCs.
In addition to these signaling pathways that regulate interaction between SCs and their niche, there is increasing evidence documenting interactions between SCs and their progenies. In this regard, the Shh signaling pathway has been shown to play a critical role in promoting HF-SC cell division shortly after the activation of hair growth. It is notable that Shh is prominently produced by a subset of transit amplifying cells located in the hair bulb. These cells form only when the new growing hair progresses through the initial anagen phases (anagen I and II). In turn, Shh produced by the TACs stimulates HF-SCs cell division in a cell non-autonomous manner [50]. This mode of regulation for HF-SC division after hair growth is initiated represents an elegant and possibly adaptive mechanism to control the number of HF-SCs.
Regulatory mechanisms of quiescent hair follicle stem cells by transcription factors
Given the complexity of signaling pathways, which have many regulatory branches such as ligands, receptors, downstream effectors as well as potential crosstalk, it is not surprising that studies focusing on individual TFs have provided perhaps more clear pictures for underlying mechanisms that govern HF-SC activities. Lef1, a key TF of the Wnt signaling pathway, was one of the first TFs shown to be required for hair development [51,52]. Sox9, on the other hand, is required to maintain the HF lineage but not the initial morphogenesis [53]. Subsequent studies have further defined the spatiotemporally specific pattern of Sox9 induction in HF-SC and progenitors as early as when embryonic HGs are formed [19]. ChIP-seq data and global enhancer studies have further determined direct targets of Sox9 and revealed a dominant role of Sox9 in defining the HF fate [24,54]. Because Sox9 is highly expressed in the HF-SCs in both quiescent and active phases, it is generally believed that Sox9 is required to define the HF-SC cellular state. However, it remains an open question whether Sox9 functions differently by targeting a distinct set of targets in quiescent and activated HF-SC populations, respectively.
Several TFs such as Runx1, Lhx2, Nfatc1, Foxc1, Tcf3/4, also known as Tcf7l1/2 respectively, have been shown to regulate HF-SC quiescence (Figure 3). Runx1 negatively regulates HF-SC quiescence and is implicated to have an oncogenic role in skin cancer [55,56]. In contrast, Lhx2 KO HF-SCs show compromised quiescence and disrupted polarity [57,58]. Interestingly, loss of Lhx2 also reduces differentiation toward the hair follicle fate and promotes the differentiation toward the sebaceous gland, another skin appendage. In this regard, Lhx2 governs not only quiescence, but also the differentiation of HF-SCs. However, it is unclear whether these two functions are linked by a common set of Lhx2 targets or are mediated by different targets. In contrast, Nfatc1, Tcf3/4 and Foxc1 all regulate HF-SC quiescence but not the fate specification [46,59–63]. However, the mode of regulation is different among these factors. Nfatc1 was one of the first TFs shown to regulate HF-SC quiescence. By repressing CDK6, Nfatc1 was shown to repress HF-SC activation during the telogen-anagen transition in early adult mice [60]. A longitudinal study further identified Nfatc1 as a factor functioning in HF-SC aging [59]. Namely, the elevated Nfatc1 levels in aged HF-SCs render the HF-SCs more quiescent and more difficult to activate, leading to reduced hair regeneration. Deletion of Nfatc1 ameliorates the compromised HF-SC activation in aged mice and promotes hair regeneration.
Figure 3. Transcription factors regulate the quiescence and activation of hair follicle stem cells.
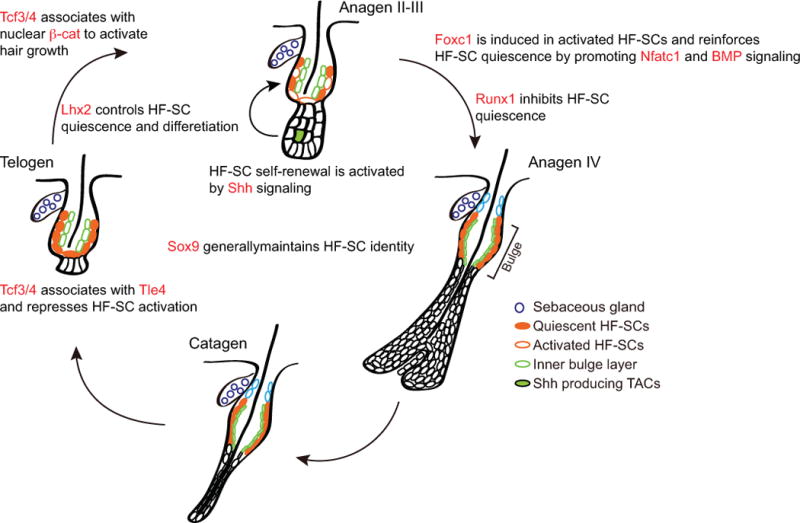
Both positive and negative transcription regulators are identified to control the quiescence of hair follicle stem cells at distinct stages of hair cycle.
Foxc1 was recently found to be induced in the HF-SCs during the self-renewal phase but not highly expressed in quiescent HF-SCs [46], in contrast to Sox9, Nfatc1 and Tcf3/4, which appear to be expressed constitutively in both quiescent and dividing HF-SCs. Of note, Foxc1 is induced in both the HF-SCs and the inner bulge layer. The function of Foxc1 in each layer seems to be different. Specific deletion of Foxc1 in anagen HF-SCs leads to premature HF-SC activation in the subsequent telogen, whereas deletion of Foxc1 in the inner bulge layer leads to the loss of club hair, which is the old hair follicle generated from the previous hair cycle, but no premature HF-SC activation. Genomic analyses using genome-wide open chromatin profiling (ATAC-seq) [64] and RNA-seq suggest that Foxc1 activates Nfatc1 and BMP signaling, two well-established quiescent control mechanisms, to reinforce the quiescent cellular state in self-renewing HF-SCs. Thus, Foxc1 represents a new class of adaptive regulators that govern HF-SC activity in a spatiotemporally specific manner. In a long-term study of Foxc1-deleted HF-SCs, it was shown that the loss of Foxc1 can compromise hair regeneration under a stressed condition, in which hair follicles are repeatedly plucked and HF-SCs are under increased demand to self-renew and regenerate hair [63]. Foxp1, another Fox family TF, was also shown to control quiescence of HF-SCs at least in part by governing FGF18 [65].
In addition to the TF-controlled mechanisms, epigenetic regulators such as Ezh1/2 and Dnmt1 have also been shown to play a role in maintaining HF-SCs, although their specific impact on the quiescence and activation of these cells is less clear. Mouse models with deletion of Ezh1/2 and subsequent depletion of the histone H3 lys27 trimethylation mark can still specify HF-SCs but show proliferation and differentiation defects, which lead to the rapid loss of the entire hair follicle lineage [66]. In contrast to this rapid decline, genetic deletion of Dnmt1 leads to progressive loss of hair regeneration over a year [67]. To determine the roles of Ezh1/2 and Dnmt1 in HF-SC activation and quiescence, specific deletion of these genes in HF-SCs using Krt15-CrePR or Lgr5-CreER is required. Recently, the rate of protein synthesis in skin and its appendages has been determined. It was shown to be vastly different in quiescent and activated HF-SCs and their differentiating progenies [68]. These initial observations have brought translation control mechanisms into the spotlight. Other mechanisms such as the ones mediated by the voltage-gated calcium channel Cav1.2 and atypical Protein Kinase C were also shown to play a role in regulating quiescence [69–71]. Further studies are needed to dissect these globally relevant mechanisms into specific interactions among individual regulators and their targets.
Concluding remarks and future perspective
Now it is well appreciated that the quiescent and activated HF-SCs have distinct transcriptome. Genetic deletion of TFs required for quiescence, such as Nfatc1, Foxc1, Lhx2 and Foxp1, usually leads to premature activation of hair growth, but the long-term effect of compromised quiescence in intact skin remains unclear. Of note, deleting Nfatc1 from aging skin lowers the threshold for stem cell activation and leads to more robust hair growth [59]. In contrast, deletion of Foxc1, combined with repeated hair plucking to stimulate stem cell division, leads to compromised stem cell function over a long term e.g., ~2 years [63]. These observations suggest that limited stem cell division could have long-term benefit for maintaining the stem cell population, at least when tissues are under constant pressure to regenerate. However, some fundamental questions remain, which are how the quiescent cellular state is directly linked to stem cell functions and whether quiescence is required for long-term HF-SC maintenance. Why are HF-SCs normally maintained as a largely quiescent cell population? Is it simply a consequence of the cyclic nature of hair growth or a means to control the number of stem cells, or does quiescence have a more direct link to stem cell maintenance? A key challenge is to identify molecular mechanisms that link quiescence and stemness functionally and directly. Given the mild compromise of quiescence in existing studies, perhaps a more profound break of the quiescent cellular state can help to unearth such links between quiescence and HF-SC functions. Furthermore, recent studies have shown that bulge HF-SCs can be regenerated from neighboring cells when specifically ablated by laser [72] or genetic manipulation [73,74]. These results hint a considerable degree of plasticity in the epithelial cells of hair follicles despite their distinct transcriptome measured at the single-cell level [75].
Another question is how the quiescent cellular state and its associated molecular mechanisms may play a role in skin diseases, notably skin cancer. It has been proposed that quiescent cancer cells can escape from the cytotoxic effects imposed by many drugs, which target highly proliferative cells. Further understanding of normal mechanisms governing HF-SC quiescence, and potentially tumor-specific mechanisms conferring quiescence to a subset of tumor cells, may provide a basis to develop new cancer drugs to specifically target and eradicate these quiescent cancer cells.
In conclusion, HF-SCs have been an excellent model system to study quiescent and activated stem cells in adult tissue. As we combine the traditionally established tools of genetics, molecular, cellular, and developmental biology with innovative genomic tools such as transcriptomics, metabolomics, single-cell analyses and genome editing, our ability to unravel new biological and mechanistic insights has never been more promising. This new knowledge will certainly open new frontiers for harnessing the power of stem cells for regenerative medicine and developing new treatments for human diseases.
Acknowledgments
I thank all my colleagues for their seminal contributions to the field of skin and hair follicle biology. I apologize to those whose papers are not cited owing to space limitation. I also thank P. Muhlrad for comments and all members of the Yi laboratory for discussion. This work was supported by grants from the National Institute of Health (AR059697, AR066703 and AR071435) and American Cancer Society Research Scholar Award (124718-RSG-13-197-01-DDC).
References
- 1.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plikus MV, Gay DL, Treffeisen E, et al. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kretzschmar K, Watt FM. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar SE, Willert K, Salinas PC, et al. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- 5.Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurikkala J, Pispa J, Jung H-S, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Dev Camb Engl. 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- 8.Dry FW. The coat of the mouse (Mus musculus) J Genet. 1926;16:287–340. [Google Scholar]
- 9.Chase HB. Growth of the hair. Physiol Rev. 1954;34:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- 10.Straile WE, Chase HB, Arsenault C. Growth and differentiation of hair follicles between periods of activity and quiescence. J Exp Zool. 1961;148:205–221. doi: 10.1002/jez.1401480304. [DOI] [PubMed] [Google Scholar]
- 11.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 12.Oshima H, Rochat A, Kedzia C, et al. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 13.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Lyle S, Yang Z, et al. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 18.Morris RJ, Bortner CD, Cotsarelis G, et al. Enrichment for Living Murine Keratinocytes from the Hair Follicle Bulge with the Cell Surface Marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 19.Nowak JA, Polak L, Pasolli HA, et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouspenskaia T, Matos I, Mertz AF, et al. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Zhang Z, O’Loughlin E, et al. MicroRNA-205 controls neonatal expansion of skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol. 2013;15:1153–1163. doi: 10.1038/ncb2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teta M, Choi YS, Okegbe T, et al. Inducible deletion of epidermal Dicer and Drosha reveals multiple functions for miRNAs in postnatal skin. Development. 2012;139:1405–1416. doi: 10.1242/dev.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Zhang Z, O’Loughlin E, et al. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012;26:693–704. doi: 10.1101/gad.182758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam RC, Yang H, Rockowitz S, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521:366–370. doi: 10.1038/nature14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki A, Itami S, Ohishi M, et al. Keratinocyte-specific Pten Deficiency Results in Epidermal Hyperplasia, Accelerated Hair Follicle Morphogenesis and Tumor Formation. Cancer Res. 2003;63:674–681. [PubMed] [Google Scholar]
- 26.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol CB. 2009;19:R132–142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YV, Cheong J, Ciapurin N, et al. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waghmare SK, Bansal R, Lee J, et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowry WE, Blanpain C, Nowak JA, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschene ER, Myung P, Rompolas P, et al. β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science. 2014;343:1353–1356. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim X, Tan SH, Yu KL, et al. Axin2 marks quiescent hair follicle bulge stem cells that are maintained by autocrine Wnt/β-catenin signaling. Proc Natl Acad Sci U S A. 2016;113:E1498–1505. doi: 10.1073/pnas.1601599113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myung PS, Takeo M, Ito M, et al. Epithelial Wnt Ligand Secretion Is Required for Adult Hair Follicle Growth and Regeneration. J Invest Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YS, Zhang Y, Xu M, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lien W-H, Polak L, Lin M, et al. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol. 2014;16:179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 38.Gay D, Kwon O, Zhang Z, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andl T, Ahn K, Kairo A, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Dev Camb Engl. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 40.Yuhki M, Yamada M, Kawano M, et al. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Dev Camb Engl. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- 41.Ming Kwan K, Li AG, Wang X-J, et al. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genes N Y N 2000. 2004;39:10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- 42.Kobielak K, Stokes N, de la Cruz J, et al. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu Y-C, Pasolli HA, Fuchs E. Dynamics Between Stem Cells, Niche and Progeny in the Hair Follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Siegenthaler JA, Dowell RD, et al. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science. 2016;351:613–617. doi: 10.1126/science.aad5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura-Ueki M, Oda Y, Oki J, et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J Invest Dermatol. 2012;132:1338–1345. doi: 10.1038/jid.2011.490. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brewer JR, Mazot P, Soriano P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 2016;30:751–771. doi: 10.1101/gad.277137.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu Y-C, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kratochwil K, Dull M, Farinas I, et al. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 52.van Genderen C, Okamura RM, Fariñas I, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 53.Vidal VP, Chaboissier MC, Lutzkendorf S, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 54.Kadaja M, Keyes BE, Lin M, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28:328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osorio KM, Lee SE, McDermitt DJ, et al. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Dev Camb Engl. 2008;135:1059–1068. doi: 10.1242/dev.012799. [DOI] [PubMed] [Google Scholar]
- 56.Scheitz CJF, Lee TS, McDermitt DJ, et al. Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J. 2012;31:4124–4139. doi: 10.1038/emboj.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mardaryev AN, Meier N, Poterlowicz K, et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development. 2011;138:4843–4852. doi: 10.1242/dev.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomann P, Paus R, Millar SE, et al. Lhx2 is a direct NF-κB target gene that promotes primary hair follicle placode down-growth. Dev Camb Engl. 2016;143:1512–1522. doi: 10.1242/dev.130898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keyes BE, Segal JP, Heller E, et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci U S A. 2013;110:E4950–4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horsley V, Aliprantis AO, Polak L, et al. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldstein J, Fletcher S, Roth E, et al. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:983–994. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen H, Merrill BJ, Polak L, et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lay K, Kume T, Fuchs E. FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc Natl Acad Sci U S A. 2016;113:E1506–1515. doi: 10.1073/pnas.1601569113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buenrostro JD, Giresi PG, Zaba LC, et al. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leishman E, Howard JM, Garcia GE, et al. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Dev Camb Engl. 2013;140:3809–3818. doi: 10.1242/dev.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ezhkova E, Lien W-H, Stokes N, et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Jiang T-X, Hughes MW, et al. Progressive Alopecia Reveals Decreasing Stem Cell Activation Probability during Aging of Mice with Epidermal Deletion of DNA Methyltransferase 1. J Invest Dermatol. 2012;132:2681–2690. doi: 10.1038/jid.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blanco S, Bandiera R, Popis M, et al. Stem cell function and stress response are controlled by protein synthesis. Nature. 2016;534:335–340. doi: 10.1038/nature18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yucel G, Altindag B, Gomez-Ospina N, et al. State-dependent signaling by Cav1.2 regulates hair follicle stem cell function. Genes Dev. 2013;27:1217–1222. doi: 10.1101/gad.216556.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osada S-I, Minematsu N, Oda F, et al. Atypical Protein Kinase C Isoform, aPKCλ, Is Essential for Maintaining Hair Follicle Stem Cell Quiescence. J Invest Dermatol. 2015;135:2584–2592. doi: 10.1038/jid.2015.222. [DOI] [PubMed] [Google Scholar]
- 71.Niessen MT, Scott J, Zielinski JG, et al. aPKCλ controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol. 2013;202:887–900. doi: 10.1083/jcb.201307001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Driskell I, Oeztuerk-Winder F, Humphreys P, et al. Genetically induced cell death in bulge stem cells reveals their redundancy for hair and epidermal regeneration. Stem Cells Dayt Ohio. 2015;33:988–998. doi: 10.1002/stem.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcin CL, Ansell DM, Headon DJ, et al. Hair Follicle Bulge Stem Cells Appear Dispensable for the Acute Phase of Wound Re-epithelialization. STEM CELLS. 2016;34:1377–1385. doi: 10.1002/stem.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joost S, Zeisel A, Jacob T, et al. Single-Cell Transcriptomics Reveals that Differentiation and Spatial Signatures Shape Epidermal and Hair Follicle Heterogeneity. Cell Syst. 2016;3:221–237.e9. doi: 10.1016/j.cels.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


