Abstract
Background:
Testicular germ cell tumors (TGCT) were suggested to have a prenatal environmentally related origin. The potential endocrine disrupting properties of certain solvents may interfere with the male genital development in utero.
Objectives:
We aimed to assess the association between maternal and paternal occupational exposures to organic solvents during the prenatal period and TGCT risk in their offspring.
Methods:
This registry-based case control study included TGCT cases aged 14–49 y () diagnosed from 1978 to 2012 in Finland, Norway, and Sweden. Controls () were randomly selected from the central population registries and were individually matched to cases on year and country of birth. Occupational histories of parents prior to the child’s birth were extracted from the national censuses. Job codes were converted into solvent exposure using the Nordic job-Nordic Occupational Cancer Study Job-Exposure Matrix. Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI).
Results:
Overall, no association was found between prenatal maternal exposure to solvents and TGCT risk. In subset analyses using only mothers for whom occupational information was available in the year of or in the year prior to the child’s birth, there was an association with maternal exposure to aromatic hydrocarbon solvents (ARHC) (; CI: 1.08, 2.17), driven by exposure to toluene (; CI: 1.02, 2.73). No association was seen for any paternal occupational exposure to solvents with the exception of exposure to perchloroethylene in Finland (; CI: 1.32, 4.41).
Conclusions:
This study suggests a modest increase in TGCT risk associated with maternal prenatal exposure to ARHC. https://doi.org/10.1289/EHP864
Background
Testicular germ cell tumors (TGCT) are the most common malignancy in young men aged 15 to 39 years old (Ferlay et al. 2013). The age-standardized incidence rate of testicular cancer using the world standard population has increased drastically in the past 40 years, recording an 8-fold increase in Finland (from 1.8 to 15.0 per 100,000 person-years), 3-fold increase in Sweden (from 4.6 to 15.4) and Norway (from 7.8 to 22.9), and a 1.5-fold increase in Denmark (from 12.2 to 18.2) between 1970 and 2013 (Engholm et al. 2016). This steep increase, the large geographical variation in incidence, and the distinct patterns of incidence rates in migrants (Schmiedel et al. 2010) support the role of environmental factors in the etiology of TGCT. In Europe, prediction of the future burden of testicular cancer up to 2025 suggests continuing incidence increase (Le Cornet et al. 2014). Although causes and risk factors of TGCT remain poorly understood, the uniformly increasing incidence burden from testicular cancer in Europe suggests increasing risk in men in most populations, rather than ongoing changes in the national demographic profiles.
While many epidemiological studies have investigated risk factors for TGCT, findings have been inconclusive. The early age at TGCT diagnosis, together with established associations with congenital malformations such as cryptorchidism (Cook et al. 2010; Kratz et al. 2010) and with birth characteristics such as birth order, parity, and low birth weight (Cook et al. 2009, 2010), suggest a prenatal origin of TGCT. Also, at a recent international consensus meeting, it was concluded that the common precursor cells of TGCT (both seminomas and nonseminomas) have fetal characteristics, including embryonic markers (Moch et al. 2016). However, the majority of published epidemiological studies have focused on adulthood exposures (Béranger et al. 2013).
According to the testicular dysgenesis syndrome (TDS) hypothesis, TGCT, cryptorchidism, hypospadias, and reduced sperm count originate from a common fetal disorder hampering the normal male testicular development (Skakkebaek et al. 2016). Exposure to endocrine-disrupting chemicals (EDCs) has been suggested as a plausible factor for initiating TDS through interference with gonadal development by mimicking hormones or interfering with hormone production (Sharpe 2006). One epidemiological study found the concentration of persistent organic pollutants such as polychlorinated biphenyl (PCB), hexachlorobenzene (HCB), and polybrominated diphenyl ethers (PBDEs) to be significantly higher for mothers of testicular cancer cases compared to mothers of controls (Hardell et al. 2003, 2006).
Widely used solvents such as toluene, trichloroethylene, and perchloroethylene have possible endocrine disrupting properties (Brouwers et al. 2009), and might also interact with the masculinization process in utero (Sharpe 2006). Furthermore, benzene and trichloroethylene were classified as carcinogenic to humans (Group 1) (IARC 2012, 2014), and perchloroethylene (also called tetrachloroethylene) was classified as probably carcinogenic to humans (Group 2A) by the International Agency for Research on Cancer (IARC 2014). Organic solvents are used for extracting, dissolving, or suspending materials such as fats, waxes, and resins that are not soluble in water. Organic solvents can be found in various commonly used products such as paints, adhesives, cosmetics, glues, and plastics (Verma and Rana 2009), resulting in widespread exposures. Solvent exposure is particularly high in specific occupations and industries, such as plastic product workers, chemical and food industry, well drillers, laundry workers, and rubber product workers (Brouwers et al. 2009; Kauppinen et al. 2009).
Little is known about the role of fetal exposures to organic solvents in TGCT development (Béranger et al. 2013). Only one study, based on a small number of cases, investigated parental occupations that were potentially exposed to hydrocarbons (aircraft mechanics, engine repairers, machinists, light truck drivers, gas and service station attendants, painters, and printers) and found no association with TGCT in the offspring (Kardaun et al. 1991).
Given the TDS hypothesis and the endocrine properties of some solvents such as perchloroethylene (European Commission DG ENV 2000), we assessed parental occupational exposure to solvents during the prenatal period and the risk of developing TGCT in the offspring in a large-scale population-based case–control study linking the registries of the Nordic countries, the NORD-TEST study (Le Cornet et al. 2015).
Material and Methods
Study Population
The NORD-TEST study, which has been described previously in detail (Le Cornet et al. 2015), is a registry-based case–control study nested within the populations of Denmark, Finland, Norway, and Sweden. All residents of the Nordic countries are registered with a unique personal identity code, allowing linkage between registries for tracking personal medical information and occupational status for research purposes. The occupational information is based on job codes derived from censuses in Finland, Norway, and Sweden and on industry codes derived from the Supplementary Pension Fund’s database in Denmark. As we expected that the harmonization of data could result in uncertainty in solvent exposure estimates for Denmark, we decided to exclude data from Denmark from the present pooled analysis. All testicular cancer cases diagnosed between ages 14 and 49 y old from 1988–2012 in Finland, 1978–2010 in Norway, and 1979–2011 in Sweden were extracted from the respective nationwide cancer registry, using the following International Classification of Diseases (ICD) codes: ICD-7: 178 (WHO 1955); ICD-8 and ICD-9: 186 (WHO 1968, 1978); and ICD-O-3: C62 (WHO 2000). Four controls were randomly selected from the central population registries and were individually matched to cases by year and country of birth. Cases and their matched controls were eligible when they were free of any previous neoplasm (except nonmelanoma skin cancer) and, for controls, were alive on the date of testicular cancer diagnosis of their matched case. The parental occupational codes (Finland, Norway, and Sweden) prior to the cases’ or the controls’ birth had to be known for at least one of the parents to be included in the analysis. The final sample included 8,112 cases and 26,264 controls: 3,769 cases with four matched controls, 2,816 cases with three matched controls, 1,213 cases with two matched controls, and 314 cases with one matched control.
Exposure Assessment
Job codes for both parents were retrieved from the last census conducted prior to the child’s birth and the first census conducted after the child's birth. The Nordic Occupational Cancer Study Job-Exposure Matrix (NOCCA-JEM), constructed by a team of experts in 2009 (Kauppinen et al. 2009), was used to assign parental exposures. The NOCCA-JEM converts the occupational codes into country- and time-specific quantitative exposure estimates of solvents. The parental exposure estimates were calculated as the product of the proportion of exposed workers (P) within an occupation and the mean level of exposure to the agent among the exposed workers (L) (Kauppinen et al. 2014). The solvent exposure is expressed in parts per million (ppm) in the workroom air for the working day. Censuses were mandatory for the whole population in the Nordic countries and took place every 5 y in Sweden (1960–1990, except for the 1965 census) and in Finland (1970–1990), and every 10 y in Norway (1960–1990). The occupational exposure to solvents was estimated based on the occupational titles recorded in the census in the year of the child’s birth or the last census before the child’s birth.
Parental exposures available in NOCCA-JEM include the following six individual solvents: benzene, toluene, perchloroethylene, methylene chloride, trichloroethylene, and 1,1,1-trichloroethane. Additionally, three groups of solvents were created, which include the following: aromatic hydrocarbon solvents (ARHC, combining exposure to benzene and toluene), chlorinated hydrocarbon solvents (CHC, combining exposure to trichloroethanes, trichloroethylene, perchloroethylene, and methylene chloride), and “any solvent” exposure, combining exposure to the six individual solvents.
Additional data obtained are as follows: testicular cancer family history was retrieved for fathers and brothers of cases and controls from the cancer registries, and information on diagnosis and/or surgery of cryptorchidism, hypospadias, and inguinal hernia in cases and controls was extracted from the Medical Birth Registry and the Hospital Discharge Registry, as well as for Finland, from the registry of congenital malformations. Further details have been described previously (Le Cornet et al. 2015).
Statistical Methods
Pairwise correlations between binary solvents exposure variables were calculated using the phi coefficient. Bivariate analysis was used to assess associations of TGCT with the following factors: father’s history of testicular cancer; brother’s history of testicular cancer; personal history of inguinal hernia, hypospadias, and cryptorchidism; age of father at childbirth; and age of mother at childbirth. Conditional logistic regression was used to estimate the strength of the associations given by odds ratios (OR) and the corresponding 95% confidence intervals (CI).
Each parental occupational exposure was classified into two categories (exposed/unexposed) as well as, for the analyses of the individual solvents, into three categories (unexposed, low exposure, and high exposure). The cutoffs of exposure were specific to each solvents’ exposure, were based on their distribution among parents of controls, and were set at the 90th (paternal exposure) or 75th percentile (maternal exposure) to have enough subjects in the high exposure category (see Table S1). It should be noted that the estimation of exposure levels results in a semiquantitative variable rather than fully quantitative one, as illustrated with the example of methylene chloride exposure levels among mothers and 1,1,1-trichloroethane exposure levels among fathers in Supplementary Figure S1. This explains why in the categorization by percentile, the cutoff is not always at the exact 90th or 75th cutoff. It is also the reason why we decided in favor of categorical analyses rather than more complex modeling of dose–response trends. Models were built using the unexposed group of each solvent individually as the reference category. Age of father at birth, age of mother at birth, and family history of testicular cancer [father and/or brother(s)] were introduced as potential confounders in the models, but did not change the OR estimate with any solvent by 10% or greater.
Analyses were stratified, and the results were reported by TGCT subtype and by country. Country-specific analyses were not conducted for maternal exposures because the number of exposed cases was too small. The Wald test was used to assess the heterogeneity of effects across strata. An alternative analysis was performed where the reference group was redefined as those that were not exposed to any of the solvents evaluated. Furthermore, since there is no strong biological argument supporting a role of parental exposure before 1 y of pregnancy in the etiology of testicular cancer, a sensitivity analysis was performed to target the conception and pregnancy time, thereby reducing the potential misclassification due to job changes. Therefore, the population was restricted to both parents for whom the census was available the year of or the year prior to the child’s birth as well as to parents for whom the census before and after childbirth recorded the same occupation, assuming that the parents held this occupation also the year of and the year prior to the child’s birth.
Additionally, the Kappa coefficient was calculated to measure the degree of agreement between prenatal and adulthood occupational exposure to solvent in cases and controls.
All analyses were performed using SAS statistical package (version 9.3; SAS Institute, Inc.).
NORD-TEST has been approved by the relevant data protection and ethical committees in Finland, Norway, and Sweden, and by the IARC ethics committee.
Results
A total of 8,112 TGCT cases (seminoma and nonseminoma) and 26,264 controls were included in the study. Country-specific distributions of cases and controls have been described previously (Le Cornet et al. 2015). Table 1 shows the distributions of different characteristics for cases and controls. Overall, 94% of the cases were diagnosed between 1990 and 2012, and 81% were born between 1960 and 1980. The proportion of nonseminoma cases was 55% with the median age of 26.7 years, whereas that of seminoma was 45% with the median age of 32.2 years. About 87% of the mothers and 97% of the fathers had an occupational status known before the child’s birth, whereof 2.3% of the mothers and 13.1% of the fathers were estimated as having been exposed to solvents in their workplace. The prevalence of occupational exposure to solvents was relatively low in mothers, ranging from 0.6% for perchloroethylene to 1.1% for 1,1,1-trichloroethane. The prevalence was somewhat higher in fathers, ranging from 1.2% for perchloroethylene to 10.7% for 1,1,1-trichloroethane. Pairwise correlations between binary solvent exposures ranged from 0.00 to 0.73 for maternal exposures and from to 0.89 for paternal exposures (Table S2). The lowest correlation was found between toluene and perchloroethylene, whereas the highest was found for trichloroethylene and 1,1,1-trichloroethane in maternal exposures and for toluene and benzene in paternal exposures.
Table 1.
Demographic characteristics of the study population by case–control status and odds ratios (OR) for prenatal factors known or potentially related to testicular cancer risk.
| Demographic characteristic | Cases (%) | Controls (%) | OR (95% CI) |
|---|---|---|---|
| Total | 8,112 | 26,264 | |
| Country | |||
| Finland | 1,034 (12.7) | 4,030 (15.3) | |
| Norway | 3,163 (39.0) | 9,089 (34.6) | |
| Sweden | 3,915 (48.3) | 13,145 (50.0) | |
| Birth year | |||
| 1960–1964 | 1,651 (20.4) | 5,352 (20.4) | |
| 1965–1969 | 1,489 (18.4) | 4,490 (17.1) | |
| 1970–1974 | 1,926 (23.7) | 6,398 (24.4) | |
| 1975–1979 | 1,518 (18.7) | 4,848 (18.5) | |
| 1980–1995 | 1,528 (18.8) | 5,176 (19.7) | |
| Diagnostic period | |||
| 1978–1989 | 469 (5.7) | ||
| 1990–1994 | 825 (10.2) | ||
| 1995–1999 | 1,376 (17.0) | ||
| 2000–2004 | 1,965 (24.2) | ||
| 2005–2009 | 2,517 (31.0) | ||
| 2010–2012 | 960 (11.8) | ||
| Histological subtype | |||
| Seminoma | 3,687 (45.4) | ||
| Nonseminoma | 4,425 (54.6) | ||
| Age of mother at childbirth | |||
| 14–20 | 440 (5.4) | 1,500 (5.7) | 1.00 |
| 20–29 | 5,155 (63.6) | 16,625 (63.3) | 1.04 (0.93, 1.17) |
| 30–39 | 2,314 (28.5) | 7,484 (28.5) | 1.04 (0.92, 1.17) |
| 192 (2.4) | 647 (2.5) | 1.00 (0.82, 1.22) | |
| Missing | 11 (0.1) | 8 (0.0) | |
| Age of father at childbirth | |||
| 14–20 | 85 (1.1) | 250 (1.0) | 1.00 |
| 20–29 | 4,050 (49.9) | 13,205 (50.3) | 0.89 (0.69, 1.15) |
| 30–39 | 3,168 (39.1) | 10,353 (39.4) | 0.89 (0.69, 1.14) |
| 732 (9.0) | 2,285 (8.7) | 0.92 (0.71, 1.20) | |
| Missing | 77 (1.0) | 171 (0.7) | |
| Father’s history of testicular cancer | |||
| No | 8,043 (99.2) | 26,192 (99.7) | 1.00 |
| Yes | 69 (0.9) | 72 (0.3) | 2.95 (2.11, 4.12) |
| Brother’s history of testicular cancer | |||
| No | 8,055 (99.3) | 26,230 (99.9) | 1.00 |
| Yes | 57 (0.7) | 34 (0.1) | 5.30 (3.45, 8.12) |
| Personal history of inguinal hernia | |||
| No | 6,991 (86.2) | 22,820 (86.9) | 1.00 |
| Yes | 108 (1.3) | 414 (1.6) | 0.93 (0.75, 1.16) |
| Missing | 1,013 (12.5) | 3,030 (11.5) | |
| Personal history of hypospadias | |||
| No | 7,062 (87.1) | 23,187 (88.3) | 1.00 |
| Yes | 37 (0.5) | 47 (0.2) | 2.52 (1.62, 3.90) |
| Missing | 1,013 (12.5) | 3,030 (11.5) | |
| Personal history of cryptorchidism | |||
| No | 7,008 (86.4) | 23,115 (88.0) | 1.00 |
| Yes | 91 (1.1) | 119 (0.5) | 2.56 (1.94, 3.38) |
| Missing | 1,013 (12.5) | 3,030 (11.5) |
Note: CI, confidence interval; , number; OR, odds ratio.
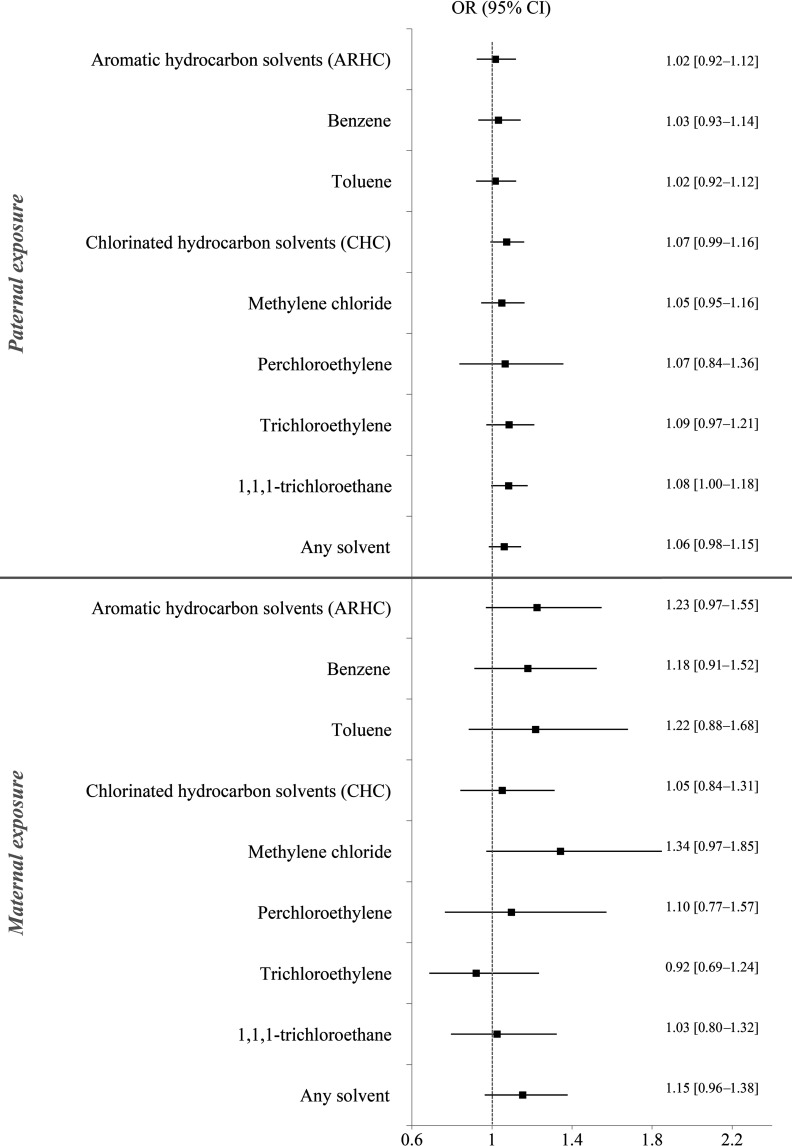
While personal history of inguinal hernia, age of father, and age of mother at childbirth were not associated with TGCT risk, cryptorchidism, hypospadias, and brother’s and father’s history of testicular cancer were associated with an increased TGCT risk (Table 1). When the covariates considered as potential confounders were introduced one by one in the models, the ORs corresponding to solvent exposures did not change by 10% or more. Adjusted ORs are therefore presented in the supplementary file (Table S3). Figure 1 shows the association between prenatal parental occupational exposures to solvents and the risk of TGCT in the offspring. Slightly elevated ORs were found among sons whose mothers were exposed to several individual solvents such as benzene, toluene, methylene chloride, and the grouped solvent ARHC, as illustrated in Figure 1, but none of the associations were statistically significant. When the analysis was repeated with a reference group of mothers occupationally not exposed to any solvents (Table 3), the associations remained not significant. Similarly, the analysis for low and high levels of ARHC exposure (Table 2) did not show an increased TGCT risk in the offspring. Only the low-exposure category to ARHC had a borderline significant association. However, the sensitivity analysis restricted to sons born in the year of or the year after the census, or sons of parents who held the same occupation at censuses before and after the child’s birth, showed a stronger statistically significant association between maternal prenatal exposure to ARHC and TGCT in offspring (; 95% CI: 1.08, 2.17, Table 4). Although ORs remained slightly elevated for several individual solvents, only toluene exposure was found significantly associated with TGCT risk (; 95% CI: 1.02, 2.73, Table 4) in the sensitivity analysis.
Figure 1.
Forest plot of the risk to develop testicular germ cell tumor after paternal or maternal exposure to solvents prior to birth. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were derived based on logistic regression analysis conditional on year and country of child's birth, the reference category (unexposed) was the parents that were not occupationally exposed to the solvent of interest. Aromatic hydrocarbon solvents (ARHC) include benzene and/or toluene. Chlorinated hydrocarbon solvents (CHC) include methylene chloride, perchloroethylene, trichloroethylene, and/or 1,1,1-trichloroethane. Any solvent category includes at least one of the following: benzene, toluene, methylene chloride, perchloroethylene, trichloroethylene, 1,1,1-trichloroethane.
Table 3.
Parental occupational exposure to different solvents before childbirth and TGCT in the offspring with unexposed to any of the solvents as reference category.
| Exposure | Maternal exposure | Paternal exposure | ||||
|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | OR (95% CI)a | Cases (%) | Controls (%) | OR (95% CI)a | |
| Aromatic hydrocarbon solvents (ARHC) | ||||||
| Any of ARHC solventsb | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 104 (1.5) | 287 (1.2) | 1.23 (0.97, 1.55) | 612 (7.8) | 1,951 (7.7) | 1.03 (0.93, 1.13) |
| Exposed only to others | 89 (1.3) | 275 (1.2) | 1.09 (0.86, 1.40) | 545 (6.9) | 1,666 (6.5) | 1.10 (1.00, 1.22) |
| Benzene | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 86 (1.2) | 241 (1.0) | 1.18 (0.91, 1.53) | 540 (6.9) | 1,686 (6.6) | 1.04 (0.94, 1.15) |
| Exposed only to others | 107 (1.5) | 321 (1.4) | 1.15 (0.91, 1.44) | 617 (7.9) | 1,931 (7.6) | 1.08 (0.98, 1.19) |
| Toluene | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 55 (0.8) | 160 (0.7) | 1.22 (0.89, 1.69) | 581 (7.4) | 1,852 (7.3) | 1.02 (0.93, 1.13) |
| Exposed only to others | 138 (2.0) | 402 (1.7) | 1.14 (0.93, 1.39) | 576 (7.3) | 1,765 (6.9) | 1.10 (1.00, 1.22) |
| Chlorinated hydrocarbon solvents (CHC) | ||||||
| Any of CHC solventsc | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 108 (1.5) | 344 (1.5) | 1.06 (0.85, 1.32) | 967 (12.3) | 2,972 (11.7) | 1.07 (0.99, 1.16) |
| Exposed only to others | 85 (1.2) | 218 (0.9) | 1.33 (1.03, 1.73) | 190 (2.4) | 645 (2.5) | 1.00 (0.85, 1.18) |
| Methylene chloride | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 54 (0.8) | 142 (0.6) | 1.34 (0.97, 1.86) | 533 (6.8) | 1,648 (6.5) | 1.06 (0.95, 1.17) |
| Exposed only to others | 139 (2.0) | 420 (1.8) | 1.10 (0.90, 1.35) | 624 (7.9) | 1,969 (7.7) | 1.07 (0.97, 1.17) |
| Perchloroethylene | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 41 (0.6) | 125 (0.5) | 1.10 (0.77, 1.58) | 92 (1.2) | 276 (1.1) | 1.08 (0.84, 1.37) |
| Exposed only to others | 152 (2.2) | 437 (1.9) | 1.18 (0.97, 1.43) | 1,065 (13.6) | 3,341 (13.1) | 1.06 (0.98, 1.14) |
| Trichloroethylene | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 60 (0.9) | 215 (0.9) | 0.93 (0.69, 1.24) | 459 (5.8) | 1,423 (5.6) | 1.09 (0.98, 1.22) |
| Exposed only to others | 133 (1.9) | 347 (1.5) | 1.31 (1.06, 1.61) | 698 (8.9) | 2,194 (8.6) | 1.04 (0.95, 1.14) |
| 1,1,1,trichloroethane | ||||||
| Unexposed | 6,825 (97.2) | 22,519 (97.6) | 6,698 (85.3) | 21,879 (85.8) | ||
| Exposed | 81 (1.2) | 266 (1.2) | 1.03 (0.80, 1.33) | 838 (10.7) | 2,559 (10.0) | 1.08 (1.00, 1.18) |
| Exposed only to others | 112 (1.6) | 296 (1.3) | 1.28 (1.02, 1.61) | 319 (4.1) | 1,058 (4.1) | 1.01 (0.88, 1.14) |
Note: CI, confidence interval; , number; OR, odds ratio; TGCT, testicular germ cell tumor risk; Exposed, exposed to the solvents of interest but can also be exposed to other solvents; Exposed only to others, exposed to any other solvents but the solvent of interest; Unexposed, unexposed to any of the individual or groups of solvents.
ORs and the corresponding 95% CIs were derived based on logistic regression analysis conditional on year and country of child’s birth, the reference category (unexposed) being the parents that were occupationally unexposed to any of the individual or groups of solvents.
Benzene and/or toluene.
Methylene chloride, perchloroethylene, trichloroethylene, and/or 1,1,1-trichloroethane.
Table 2.
Parental occupational exposure to solvents before the child’s birth categorized into low/high exposure and the risk of testicular germ cell tumor in the offspring.
| Agents | Level of exposurea | Maternal exposure | Paternal exposure | ||||
|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | OR (95% CI)b | Cases (%) | Controls (%) | OR (95% CI)b | ||
| Aromatic hydrocarbon solvents (ARHC) | |||||||
| Benzene | Unexposed | 6,932 (98.8) | 22,840 (99.0) | 7,315 (93.1) | 23,810 (93.4) | ||
| Low | 55 (0.8) | 155 (0.7) | 1.22 (0.89, 1.68) | 267 (3.4) | 862 (3.4) | 1.01 (0.88, 1.16) | |
| High | 31 (0.4) | 86 (0.4) | 1.10 (0.71, 1.71) | 273 (3.5) | 824 (3.2) | 1.06 (0.92, 1.22) | |
| Toluene | Unexposed | 6,963 (99.2) | 22,921 (99.3) | 7,274 (92.6) | 23,644 (92.7) | ||
| Low | 40 (0.6) | 110 (0.5) | 1.32 (0.90, 1.93) | 505 (6.4) | 1,615 (6.3) | 1.02 (0.92, 1.13) | |
| High | 15 (0.2) | 50 (0.2) | 1.01 (0.55, 1.84) | 76 (1.0) | 237 (0.9) | 1.01 (0.77, 1.31) | |
| Chlorinated hydrocarbon solvents (CHC) | |||||||
| Methylene chloride | Unexposed | 6,964 (99.2) | 22,939 (99.4) | 7,322 (93.2) | 23,848 (93.5) | ||
| Low | 40 (0.6) | 102 (0.4) | 1.36 (0.94, 1.99) | 423 (5.4) | 1,311 (5.1) | 1.06 (0.95, 1.19) | |
| High | 14 (0.2) | 40 (0.2) | 1.28 (0.69, 2.40) | 110 (1.4) | 337 (1.3) | 1.01 (0.81, 1.26) | |
| Perchloroethylene | Unexposed | 6,977 (99.4) | 22,956 (99.5) | 7,763 (98.8) | 25,220 (98.9) | ||
| Low | 28 (0.4) | 92 (0.4) | 1.03 (0.67, 1.58) | 86 (1.1) | 248 (1.0) | 1.11 (0.87, 1.43) | |
| High | 13 (0.2) | 33 (0.1) | 1.28 (0.67, 2.47) | 6 (0.1) | 28 (0.1) | 0.67 (0.28, 1.63) | |
| Trichloroethylene | Unexposed | 6,958 (99.1) | 22,866 (99.1) | 7,396 (94.2) | 24,073 (94.4) | ||
| Low | 38 (0.5) | 148 (0.6) | 0.85 (0.59, 1.23) | 213 (2.7) | 639 (2.5) | 1.12 (0.96, 1.32) | |
| High | 22 (0.3) | 67 (0.3) | 1.06 (0.65, 1.72) | 246 (3.1) | 784 (3.1) | 1.05 (0.91, 1.22) | |
| 1,1,1,trichloroethane | Unexposed | 6,937 (98.8) | 22,815 (98.8) | 7,017 (89.3) | 22,937 (90.0) | ||
| Low | 45 (0.6) | 162 (0.7) | 0.95 (0.68, 1.33) | 380 (4.8) | 1,147 (4.5) | 1.10 (0.98, 1.25) | |
| High | 36 (0.5) | 104 (0.5) | 1.14 (0.77, 1.67) | 458 (5.8) | 1,412 (5.5) | 1.07 (0.95, 1.19) | |
Note: CI, confidence interval; , number; OR, odds ratio.
The exposed group was divided into two groups, based on the 75th percentile of mothers who were exposed and on the 90th percentile of fathers who were exposed.
ORs and the corresponding 95% CIs were derived based on logistic regression analysis conditional on year and country of child’s birth. The reference category (unexposed) consists of the parents who were not occupationally exposed to the solvent of interest.
Table 4.
Sensitivity analysis: testicular germ cell tumor risk when parents have been exposed to solvents within the year before childbirth.
| Exposure | Maternal exposure | Paternal exposure | ||||
|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | OR (95% CI)a | Cases (%) | Controls (%) | OR (95% CI)a | |
| Aromatic hydrocarbon solvents (ARHC) | ||||||
| Any of ARHC solventsb | ||||||
| Unexposed | 4,007 (98.6) | 13,567 (99.0) | 4,034 (91.9) | 13,482 (91.8) | ||
| Exposed | 56 (1.4) | 138 (1.0) | 1.53 (1.08, 2.17) | 354 (8.1) | 1,198 (8.2) | 0.98 (0.85, 1.13) |
| Benzene | ||||||
| Unexposed | 4,022 (99.0) | 13,589 (99.2) | 4,074 (92.8) | 13,638 (92.9) | ||
| Exposed | 41 (1.0) | 116 (0.8) | 1.24 (0.83, 1.85) | 314 (7.2) | 1,042 (7.1) | 0.99 (0.86, 1.15) |
| Toluene | ||||||
| Unexposed | 4,037 (99.4) | 13,642 (99.5) | 4,047 (92.2) | 13,542 (92.2) | ||
| Exposed | 26 (0.6) | 63 (0.5) | 1.67 (1.02, 2.73) | 341 (7.8) | 1,138 (7.8) | 0.99 (0.86, 1.14) |
| Chlorinated hydrocarbon solvents (CHC) | ||||||
| Any of CHC solventsc | ||||||
| Unexposed | 4,016 (98.8) | 13,568 (99.0) | 3818 (87.0) | 12,904 (87.9) | ||
| Exposed | 47 (1.2) | 137 (1.0) | 1.17 (0.83, 1.66) | 570 (13.0) | 1,776 (12.1) | 1.07 (0.95, 1.20) |
| Methylene chloride | ||||||
| Unexposed | 4,037 (99.4) | 13,646 (99.6) | 4,064 (92.6) | 13,636 (92.9) | ||
| Exposed | 26 (0.6) | 59 (0.4) | 1.54 (0.94, 2.51) | 324 (7.4) | 1,044 (7.1) | 1.03 (0.89, 1.19) |
| Perchloroethylene | ||||||
| Unexposed | 4,043 (99.5) | 13,658 (99.7) | 4,338 (98.9) | 14,532 (99.0) | ||
| Exposed | 20 (0.5) | 47 (0.3) | 1.43 (0.83, 2.45) | 50 (1.1) | 148 (1.0) | 1.02 (0.71, 1.46) |
| Trichloroethylene | ||||||
| Unexposed | 4,038 (99.4) | 13,618 (99.4) | 4,124 (94.0) | 13,873 (94.5) | ||
| Exposed | 25 (0.6) | 87 (0.6) | 0.98 (0.62, 1.54) | 264 (6.0) | 807 (5.5) | 1.10 (0.94, 1.28) |
| 1,1,1,trichloroethane | ||||||
| Unexposed | 4,022 (99.0) | 13,590 (99.2) | 3,886 (88.6) | 13,145 (89.5) | ||
| Exposed | 41 (1.0) | 115 (0.8) | 1.22 (0.84, 1.78) | 502 (11.4) | 1,535 (10.5) | 1.09 (0.96, 1.22) |
| Any solventd | ||||||
| Unexposed | 5,076 (97.6) | 16,899 (97.8) | 4,732 (86.0) | 15,975 (87.1) | ||
| Exposed | 127 (2.4) | 378 (2.2) | 1.15 (0.94, 1.42) | 773 (14.0) | 2,376 (12.9) | 1.09 (0.99, 1.19) |
Note: CI, confidence interval; , number; OR, odds ratio.
ORs and the corresponding 95% CIs were derived based on logistic regression analysis conditional on year and country of child’s birth, the reference category (unexposed) was the parents that were not occupationally exposed to the solvent of interest.
Benzene and/or toluene.
Methylene chloride, perchloroethylene, trichloroethylene, and/or 1,1,1-trichloroethane.
At least one of the following solvents: benzene, toluene, methylene chloride, perchloroethylene, trichloroethylene, 1,1,1-trichloroethane.
Most ORs associated with paternal exposures were close to 1.0. When exposure was subdivided into categories of low and high exposures, none of the exposures in fathers were found to be associated with TGCT risk (Table 2). In the sensitivity analyses assessing the paternal occupational exposure to solvents in the year of or the year prior to the childbirth, no associations with TGCT risks in offspring were found, which is consistent with the results of the primary analysis. Stratification by country (see Table S4) indicated an increased TGCT risk in Finland with prenatal paternal exposure to perchloroethylene (; 95% CI: 1.32, 4.41), and the test for heterogeneity between countries was statistically significant ().
No major differences in associations were observed between seminomas and nonseminomas for any of the maternal or paternal solvent exposures (Table S5).
Discussion
In this large-scale nested case–control study of more than 8,000 TGCT cases in Finland, Norway, and Sweden, we found little evidence of an association between parental occupational exposure to solvents and the risk of TGCT in their offspring. The exception was a statistically significant moderately increased risk with maternal exposure to ARHC and toluene when restricting the analysis to subjects whose maternal exposure information near the time of pregnancy/childbirth was available, possibly showing a stronger association than the overall analyses due to reduced exposure misclassification. Country-specific results showed no additional associations besides the association with paternal exposure to perchloroethylene, which was observed exclusively in Finland.
The finding of a role of ARHC and toluene exposure has to be interpreted with caution, as the number of mothers exposed was limited in the sensitivity analysis, and the strongest association was found in the low exposure category. Job-exposure matrices (JEMs) allocate the same level and probability of exposure to all individuals within one occupational code. The individual exposure estimates are thus prone to nondifferential misclassification, particularly when the probability of exposure within one occupational code is low. The exposure categorization (low, high) of the continuous distribution of exposure underlying random measurement error might induce a systematic nondifferential over- or underestimation (Brenner and Loomis 1994). The borderline significant OR observed for the low category of exposure of ARHC might be due to systematic nondifferential exposure misclassification. However, it might be noted that nonmonotonic dose–response curves have previously been described for exposures to EDCs in relation to various endpoints (Vandenberg et al. 2016). Also, the positive association found between maternal exposure to ARHC and TGCT in offspring might be driven by toluene exposure, since ARHC is the combination of benzene and toluene.
Only one other case–control study has investigated occupations of fathers and mothers potentially exposed to hydrocarbons, but no excess risk of TGCT was observed in the offspring (Kardaun et al. 1991). However, exposure to individual chemical agents were not assessed, and the study, including 223 cases, has low statistical power. Several agents belonging to the group of ARHC solvents, such as styrene or agents deriving from benzene and toluene (pentachlorobenzene, trichlorobenzene, HCB, nitrotoluene), have been classified in the priority list of EDCs by the European Commission (European Commission DG ENV 2000). Exposure to EDCs has been suggested to contribute to the development of TDS through interference with hormone synthesis, secretion, and signaling (Cook et al. 2011; Giordano et al. 2010; Sharpe 2006). In two studies, fetal exposure to toluene and styrene was reported to be associated with a reduced synthesis and secretion of testosterone in the fetal testes and decreased weight of male reproductive organs (Ohyama et al. 2007; Tsukahara et al. 2009).
One study reported a positive association between fathers working as painters and occupationally exposed to organic solvents within the 3 months before pregnancy and birth defect risk in children (Hooiveld et al. 2006). Our results on overall exposure to solvents do not support the association observed in this study, but the two studies are difficult to compare, since in the study by Hooiveld et al. (2006), the number of children with birth defects was very small () and involved a wide range of congenital malformations and disorders. The mechanisms underlying a potential effect of paternal exposure to solvents on TGCT in offspring is unclear, but a plausible pathway has been hypothesized; notably an effect on sperm DNA, producing mutations or chromosomal abnormalities (Olshan et al. 1991). Although experimental studies have supported a potential effect of solvent exposure on the testes, none have investigated the corresponding effect of paternal exposure on the testes in the offspring (Lamb and Hentz 2006; NTP 2002; Verma and Rana 2009; Xu et al. 2004). Therefore, the association found for paternal exposure to perchloroethylene in our study requires further support from mechanistic studies. However, it should be noted that neither the prevalence of exposure nor the exposure levels among the exposed to perchloroethylene were substantially higher in Finland compared to Norway or Sweden. While the differences in ORs observed across the countries might be explained by some difference in occupational practices, we cannot exclude the possibility of an observation by chance due to multiple comparisons.
The main advantage of the NORD-TEST study is the linkage between the population-based registries from the Nordic countries representing an exceptional setting to design a retrospective study, with access to prospectively registered exposure information. The extraction of the cases diagnosed during the last 30 years in three countries provides a large sample size to analyze parental occupational exposure to organic solvents, even though the prevalence of exposure remained limited for certain solvents and exposure categories. Another strength is the use of a country-specific JEM created for Nordic countries (NOCCA-JEM) that encompasses the exposure estimates of single substances that are likely to have their own mechanisms of action in contrast to groups of substances (Pukkala et al. 2009). One of the advantages of using a JEM is that it assigns occupational exposures in a systematic and objective way, therefore avoiding differential misclassification between cases and controls; this is particularly true for the NORD-TEST study collecting occupational data via censuses, which were carried out before the diagnosis of TGCT.
One of the limitations of the NORD-TEST study is the lack of information on the time and duration of each occupation and therefore the inability to precisely target the critical time window of exposure and calculate preconception cumulative exposures. Because of the study design, we had to assume that the job at the time of census reflected the exposure at the time of conception and during intrauterine development, even though the census might be several years before the child’s birth. Nevertheless, the sensitivity analyses, including parents for whom the census was available within the year before or the year of the child’s birth or parents who held the same occupation in census prior and after the child’s birth, yielded stronger associations. In addition, NOCCA-JEM assumes homogeneity of exposure within jobs. The true variance of solvent exposure within jobs represented by equal levels of exposure might have diluted the estimated strength of a possible association. Nevertheless, our approach allowed us to compare the parents with a higher risk of solvent exposure with those with a lower risk of solvent exposure.
A recent hypothesis suggests that TGCT could arise from a combined effect of prenatal and early- and later-life exposures (McGlynn and Trabert 2012). The germ cell neoplasia in situ (Berney et al. 2016) assumed to be generated in utero by primary prenatal exposure does not become invasive until puberty following activation of the pituitary–gonadal axis (Rajpert-De 2006; Skakkebaek et al. 1987, 2001; Sonne et al. 2009). The design of NORD-TEST study did not allow collection of individual lifelong exposures. However, some of the subjects had a known occupational history. With this information, we checked whether subjects exposed to solvents during the prenatal period were likely to be exposed later in life in their occupations, but found this was not the case (as measured by the kappa statistics of agreement of categorical variables; ).
Conclusions
This is the first large nested case–control study investigating associations between maternal and paternal exposure to several individual organic solvents and types of them, and the risk of TGCT in their offspring. We found no evidence of any association with paternal occupational exposure to solvents, except an elevated risk observed with perchloroethylene in Finland only. For maternal exposure, a moderately elevated risk was observed among sons of mothers occupationally exposed to ARHC, particularly toluene, when analyses were restricted to mothers for whom the occupation was known in the year of or the year before childbirth. Further studies are needed, including investigations of molecular mechanisms, to better understand the observed association between maternal exposures to toluene and TGCT risk in their offspring.
Supplemental Material
Acknowledgments
This work was supported by public funding from the Lyric Grant INCa-DGOS-4664 (Institute of Cancer Research, France), the International Agency for Research on Cancer (IARC), and the Cancéropôle Lyon Auvergne Rhône-Alpes (CLARA). We would like to acknowledge M. Steding-Jessen and A. Meersohn from the Danish Cancer Society Research Center, K. Fremling from the Institute of Environmental Medicine, and Karolinska Institutet and V. Luzon from IARC for their help with the data management. The Family Cancer Database was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry. Data from the Finnish Cancer Registry was extracted by the permission for the research No.THL/1123/5.05.00/2012. We would also like to thank the NOCCA team for approving our use of the NOCCA-JEM and their expertise on the occupational exposure in the Nordic countries.
References
- Béranger R, Le Cornet C, Schüz J, Fervers B. 2013. Occupational and environmental exposures associated with testicular germ cell tumours: systematic review of prenatal and life-long exposures. PLoS One 8(10):e77130, PMID: 24155923, 10.1371/journal.pone.0077130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney DM, Looijenga LH, Idrees M, Oosterhuis JW, Rajpert-De Meyts E, Ulbright TM, et al. 2016. Germ cell neoplasia in situ (GCNIS): evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 69(1):7–10, PMID: 26918959, 10.1111/his.12958. [DOI] [PubMed] [Google Scholar]
- Brenner H, Loomis D. 1994. Varied forms of bias due to nondifferential error in measuring exposure. Epidemiology 5(5):510–517, PMID: 7986865. [PubMed] [Google Scholar]
- Brouwers MM, van Tongeren M, Hirst AA, Bretveld RW, Roeleveld N. 2009. Occupational exposure to potential endocrine disruptors: further development of a job exposure matrix. Occup Environ Med 66(9):607–614, PMID: 19286684, 10.1136/oem.2008.042184. [DOI] [PubMed] [Google Scholar]
- Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, McGlynn KA. 2009. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer–experiences of the mother. Int J Epidemiol 38(6):1532–1542, 10.1093/ije/dyp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, McGlynn KA. 2010. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer–experiences of the son. Int J Epidemiol 39(6):1605–1618, PMID: 20660640, 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, Trabert B, McGlynn KA. 2011. Organochlorine compounds and testicular dysgenesis syndrome: human data. Int J Androl 34(4 Pt 2):e68–e84; discussion: e84–e85, PMID: 21668838, 10.1111/j.1365-2605.2011.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engholm G, Ferlay J, Christensen N, Kejs A, Johannesen T, Khan S, et al. 2016. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015). Copenhagen, Denmark:Association of the Nordic Cancer Registries, Danish Cancer Society. [Google Scholar]
- European Commission DG ENV. 2000. Towards the Establishment of a Priority List of Substances for Further Evaluation of their Role in Endocrine Disruption-Preparation of a Candidate List of Substances as a Basis for Priority Setting-BKH Report. Annex 13. http://ec.europa.eu/environment/archives/docum/01262_en.htm#European Workshop on Endocrine Disruptors [accessed 15 June 2015].
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- Giordano F, Abballe A, De Felip E, di Domenico A, Ferro F, Grammatico P, et al. 2010. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Part A Clin Mol Teratol 88(4):241–250, PMID: 20196143, 10.1002/bdra.20657. [DOI] [PubMed] [Google Scholar]
- Hardell L, Bavel B, Lindström G, Eriksson M, Carlberg M. 2006. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl 29(1):228–234, PMID: 16371110, 10.1111/j.1365-2605.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- Hardell L, van Bavel B, Lindström G, Carlberg M, Dreifaldt AC, Wijkström H, et al. 2003. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect 111(7):930–934, 10.1289/ehp.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooiveld M, Haveman W, Roskes K, Bretveld R, Burstyn I, Roeleveld N. 2006. Adverse reproductive outcomes among male painters with occupational exposure to organic solvents. Occup Environ Med 63(8):538–544, PMID: 16757511, 10.1136/oem.2005.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC monographs. 2012. Chemical Agents and Related Occupations. Lyon, France: IARC, 9–562. [PMC free article] [PubMed] [Google Scholar]
- IARC monographs. 2014. Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. Lyon, France:IARC, 1–512. [PMC free article] [PubMed] [Google Scholar]
- Kardaun JW, Hayes RB, Pottern LM, Brown LM, Hoover RN. 1991. Testicular cancer in young men and parental occupational exposure. Am J Ind Med 20(2):219–227, PMID: 1951369, 10.1002/ajim.4700200208. [DOI] [PubMed] [Google Scholar]
- Kauppinen T, Heikkilä P, Plato N, Woldbaek T, Lenvik K, Hansen J, et al. 2009. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA). Acta Oncol 48(5):791–800, PMID: 19225948, 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- Kauppinen T, Uuksulainen S, Saalo A, Mäkinen I, Pukkala E. 2014. Use of the Finnish Information System on Occupational Exposure (FINJEM) in epidemiologic, surveillance, and other applications. Ann Occup Hyg 58(3):380–396, PMID: 24401793, 10.1093/annhyg/met074. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Mai PL, Greene MH. 2010. Familial testicular germ cell tumours. Best Pract Res Clin Endocrinol Metab 24(3):503–513, PMID: 20833340, 10.1016/j.beem.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JC, Hentz KL. 2006. Toxicological review of male reproductive effects and trichloroethylene exposure: assessing the relevance to human male reproductive health. Reprod Toxicol 22(4):557–563, PMID: 16938429, 10.1016/j.reprotox.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Le Cornet C, Fervers B, Dalton SO, Feychting M, Pukkala E, Tynes T, et al. 2015. Testicular germ cell tumours and parental occupational exposure to pesticides: a register-based case-control study in the Nordic countries (NORD-TEST study). Occup. Environ. Med 72(11):805–811, 10.1136/oemed-2015-102860. [DOI] [PubMed] [Google Scholar]
- Le Cornet C, Lortet-Tieulent J, Forman D, Béranger R, Flechon A, Fervers B, et al. 2014. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer 50(4):831–839, PMID: 24369860, 10.1016/j.ejca.2013.11.035. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Trabert B. 2012. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol 9(6):339–349, PMID: 22508459, 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch H, Humphrey PA, Ulbright TM, Reuter VE. 2016. WHO Classification of Tumours Volume 8, Lyons, France:IARC, 189. [Google Scholar]
- NTP (National Toxicology Program). 2002. Toxicology and carcinogenesis studies of p-nitrotoluene (CAS no. 99-99-0) in F344/N rats and B6C3F(1) mice (feed studies). Natl Toxicol Program Tech Rep Ser 498:1–277, 10.1038/nrurol.2012.61. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Satoh K, Sakamoto Y, Ogata A, Nagai F. 2007. Effects of prenatal exposure to styrene trimers on genital organs and hormones in male rats. Exp Biol Med (Maywood) 232(2):301–308, PMID: 17259338. [PubMed] [Google Scholar]
- Olshan AF, Teschke K, Baird PA. 1991. Paternal occupation and congenital anomalies in offspring. Am J Ind Med 20(4):447–475, PMID: 1785611. [DOI] [PubMed] [Google Scholar]
- Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparén P, Tryggvadottir L, et al. 2009. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol 48(5):646–790. [DOI] [PubMed] [Google Scholar]
- Rajpert-De ME. 2006. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod. Update 12(3):303–323. [DOI] [PubMed] [Google Scholar]
- Schmiedel S, Schüz J, Skakkebaek NE, Johansen C. 2010. Testicular germ cell cancer incidence in an immigration perspective, Denmark, 1978 to 2003. J Urol 183(4):1378–1382, PMID: 20171682, 10.1016/j.juro.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. 2006. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab 20(1):91–110, PMID: 16522522, 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. 2016. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 96(1):55–97, PMID: 26582516, 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978, 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. 1987. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 10(1):19–28, PMID: 3034791. [DOI] [PubMed] [Google Scholar]
- Sonne SB, Almstrup K, Dalgaard M, Juncker AS, Edsgard D, Ruban L, et al. 2009. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res 69(12):5241–5250, PMID: 19491264, 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Nakajima D, Kuroda Y, Hojo R, Kageyama S, Fujimaki H. 2009. Effects of maternal toluene exposure on testosterone levels in fetal rats. Toxicol Lett 185(2):79–84, PMID: 19110042, 10.1016/j.toxlet.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. 2016. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma Y, Rana SV. 2009. Endocrinal toxicity of industrial solvents–a mini review. Indian J Exp Biol 47(7):537–549, PMID: 19761037. [PubMed] [Google Scholar]
- WHO (World Health Organization). 1955. International Classification of Diseases, 7th Edition. http://www.wolfbane.com/icd/icd7h.htm [accessed 16 June 2017]. [Google Scholar]
- WHO. 1968. International Classification of Diseases, 8th Edition. http://www.wolfbane.com/icd/icd8h.htm [accessed 16 June 2017]. [Google Scholar]
- WHO. 1978. International Classification of Diseases, 9th Edition. http://www.wolfbane.com/icd/icd9h.htm [accessed 16 June 2017]. [Google Scholar]
- WHO. 2000. International Classification of Diseases for Oncology, 3rd Edition. http://www.who.int/classifications/icd/adaptations/oncology/en/ [accessed 16 June 2017]. [Google Scholar]
- Xu H, Tanphaichitr N, Forkert PG, Anupriwan A, Weerachatyanukul W, Vincent R, et al. 2004. Exposure to trichloroethylene and its metabolites causes impairment of sperm fertilizing ability in mice. Toxicol Sci 82(2):590–597, PMID: 15375293, 10.1093/toxsci/kfh277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.