Abstract
Background: Mucin1 (MUC1) is a highly glycosylated transmembrane protein that has gained attention because of its overexpression in various cancers. However, MUC1-targeted therapeutic antibodies have not yet been approved for cancer therapy. MUC1 is cleaved to two subunits, MUC1-N and MCU1-C. MUC1-N is released from the cell surface, making MUC1-C a more reasonable target for cancer therapy. Therefore, we produced a monoclonal antibody (anti-hMUC1) specific to the extracellular region of MUC1-C and evaluated its effects in vitro and in vivo.
Methods: We produced a monoclonal antibody (anti-hMUC1) using a purified recombinant human MUC1 polypeptide and our novel immunization protocol. The reactivity of anti-hMUC1 was characterized by ELISA, western blotting and immunoprecipitation analyses. The localization of the antibody in the breast cancer cells after binding was determined by confocal image analysis. The effects of the antibody on the growth of cells were also investigated. We injected anti-hMUC1 and performed in vivo tracing analysis in xenograft mouse models. In addition, expression of MUC1 in tissue sections from patients with breast cancer was assessed by immunohistochemistry with anti-hMUC1.
Results: The anti-hMUC1 antibody recognized recombinant MUC1 as well as native MUC1-C protein in breast cancer cells. Anti-hMUC1 binds to the membrane surface of cells that express MUC1 and is internalized in some cancer cell lines. Treatment with anti-hMUC1 significantly reduced proliferation of cells in which anti-hMUC1 antibody is internalized. Furthermore, the anti-hMUC1 antibody was specifically localized in the MUC1-expressing breast cancer cell-derived tumors in xenograft mouse models. Based on immunohistochemistry analysis, we detected significantly higher expression of MUC1 in cancer tissues than in normal control tissues. Conclusion: Our results reveal that the anti-hMUC1 antibody targets the extracellular region of MUC1-C subunit and may have utility in future applications as an anti-breast cancer agent.
Keywords: Mucin1, monoclonal antibody, breast cancer, antibody therapeutics, targeting
Introduction
Mucins are high-molecular-weight glycoproteins prominently expressed in the respiratory, gastrointestinal, and reproductive tracts. The human mucin (MUC) family contains mostly secreted and transmembrane mucins, both of which function as physical barriers 1-4. MUC1, a type I transmembrane mucin, is a heterodimer and is cleaved at the sea-urchin sperm protein, enterokinase, and agrin (SEA) domain to form two subunits. The MUC1 N-terminal subunit (MUC1-N) has an extracellular domain containing 20 to 125 tandem repeats of 20 amino acids that are O-glycosylated on serine and threonine residues. The MUC1 C-terminal subunit (MUC1-C) is composed of a 58-amino-acid extracellular domain, 28-amino-acid transmembrane domain, and 72-amino-acid cytoplasmic tail 4-7. MUC1 is expressed at the apical border of healthy epithelial cells and facilitates their protection. MUC1 is overexpressed in a wide range of human epithelial malignancies including breast, prostate, ovarian, pancreatic, and colon cancers and also in malignant plasma cells in multiple myeloma. In these conditions, the MUC1-overexpressing cells lose their polarity and MUC1 is aberrantly distributed throughout the entire surface of the cancerous cells 3, 4, 8. MUC1 modulates the metabolic program and is involved in survival and proliferation of tumor cells in hypoxic condition. MUC1 interacts with hypoxia-inducible factor-1α (HIF-1α) and p300, resulting in the aberrant regulation of glucose and amino acid metabolic pathways 9, 10.
Breast cancer is the leading cause of death in women worldwide and the second most common cancer after lung cancer 11. Breast cancer has a poor prognosis; therefore, even with the modern methods of treatment, breast cancer fatally claims almost half a million women per year worldwide 12. More than 90% of breast tumors have aberrant overexpression of MUC1 13. As a result, MUC1 has been studied as a potential candidate for tumor-specific therapies 14. The cytoplasmic tail of MUC1 is associated with its oncogenic effects, and this tail binds to various cancer-related proteins including c-Src 15, 16 and the epidermal growth factor receptor (EGFR) family proteins 17. MUC1-C interacts with EGFR and promotes EGFR-mediated signaling via recruitment of c-Src, phosphatidylinositol 3-kinase (PI3K), and GRB2 18. Interaction of the Src homology 2 (SH2) domain of PI3K with MUC1-C activates the PI3K-AKT pathway 19-21. Furthermore, the association of MUC1-C with the GRB2 SH2 domain and son of sevenless (SOS) leads to Ras activation, followed by stimulation of mitogen activated protein kinase (MAPK) signaling, which leads to the phosphorylation of extracellular signal-regulated kinases (ERK)1/2 17, 22, 23.
Monoclonal antibodies have strong potential as anti-cancer therapeutic agents because of their ability to specifically target cancer cells, unlike the non-specific effects of chemotherapy and radiotherapy 24. Most of the therapeutic MUC1 antibodies produced so far target tandem repeats in MUC1-N 25, 26. However, MUC1-N is shed from the cellular surface and exists freely in the extracellular matrix and peripheral circulation 3, 27. Therefore, free MUC1-N domains can sequester most of the antibodies, which limits the amount of antibodies reaching the MUC1 proteins at the cell surface. To overcome this problem, antibodies targeting the extracellular region of MUC1-C are emerging as a promising line of therapeutics for the treatment of various cancers.
In this study, we developed a monoclonal antibody specific to the extracellular region of MUC1-C using a novel immunization protocol that we established 28. We found that the anti-hMUC1 monoclonal antibody (anti-hMUC1) could target overexpressed MUC1 in breast cancer cells in vitro and in vivo.
Materials and Methods
Purification of recombinant human MUC1 C-terminal protein
The human cDNA encoding a peptide sequence of 192 amino acids of MUC1 (from alanine 961 to serine 1152; GenBank Accession No. P15941) was obtained from MCF7 cells and amplified by RT-PCR using the following primer sets: sense 5ʹ-CC ATG GCC TCA GGC TCT GCA TC-3ʹ and anti-sense 5ʹ-CTC GAG AGA CTG GGC AGA GAA AGG AAA T-3ʹ. Using the Nco I and Xho I restriction enzyme sites, the amplified cDNA fragments were cloned into the expression vector pET-22b (Novagen, Darmstadt, Germany) containing a C-terminal His-tag. The plasmids were transformed into Escherichia coli RosettaTM (Invitrogen, Carlsbad, CA, USA) competent cells and protein expression was induced with 1 mM isopropyl β-D-1-thioglactopyranoside (IPTG, Sigma-Aldrich, Saint Louis, MO, USA) for 8 h at 37°C. Cells were lysed by sonication in lysis buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM EDTA, 0.5% Triton X100, 1 μg/mL lysozyme, and proteinase inhibitor cocktail) on ice. After centrifugation, the inclusion body fraction was mixed with buffer B (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 8.0), and purified using a Ni-NTA agarose (Qiagen, Valencia, CA, USA) system. The mixture was loaded onto the Ni-NTA column and washed with wash buffer C (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 6.3). The bound proteins were eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 4.5) and analyzed by SDS-PAGE. The resultant recombinant human (rh) protein including extracellular region of MUC1-C was named rhMUC1-EC192.
Mice and immunization
Female BALB/c and BALB/c nu/nu mice (four-week-old) were purchased from Nara Biotech, Inc. (Seoul, Korea). Mice were maintained under specific-pathogen free conditions in the Experimental Animal Center of Hallym University. All animal use and relevant experimental procedures were approved by the Institutional Animal Care and Use Committee of Hallym University (Permit Number: Hallym2015-81). BALB/c mice were immunized intraperitoneally (i.p.) with liposome complex comprised of 50 μg rhMUC1-EC192 protein and 50 μg CpG-DNA 4531(O) co-encapsulated with phosphatidyl-β-oleoyl-γ-palmitoyl ethanolamine: cholesterol hemisuccinate (DOPE: CHEMS) complex [called Lipoplex (O)], three times at 10-day intervals, as previously described 28, 29.
Antigen-specific Ig enzyme-linked immunosorbent assay (ELISA)
The rhMUC1-EC192-specific total IgG amount was measured by ELISA as previously described 28, 29. Immunoplates (96-well) were coated with 1 μg/mL rhMUC1-EC192 protein and blocked with 0.5% bovine serum albumin (BSA) in 0.2% Tween-20 in phosphate-buffered saline (PBS-T). The mouse sera, hybridoma cell culture supernatants, or purified antibody were diluted with PBS-T and incubated for 2 h at room temperature. The plates were washed three times with PBS-T and incubated with goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. To measure the IgG isotype, 96-well immunoplates were coated with rhMUC1-EC192, incubated with purified antibody, followed by incubation with HRP-conjugated anti-mouse IgG (each isotype) antibody.
Production of the mouse anti-human MUC1 monoclonal antibody
The splenocytes were prepared from the immunized mice producing high titers of anti- rhMUC1-EC192 antibody and used for fusion with SP2/0 myeloma cells using polyethylene glycol (Sigma-Aldrich). The fused cells were cultured and selected with hypoxanthine-aminopterin-thymidine (Sigma-Aldrich) medium. The obtained hybridoma clone was cultured in hypoxanthine-thymidine medium. For production of monoclonal antibody, mice were i.p. injected with pristine and inoculated with hybridoma cells 10 days later. The ascitic fluid was collected after 10 days and centrifuged at 1,000 × g for 30 min. The supernatant was purified using IgG-bound Protein-A chromatography (GE Healthcare Life Sciences, Buckinghamshire, UK). Hereafter, we refer to this antibody as the anti-hMUC1 monoclonal antibody.
Surface plasmon resonance (SPR) analysis
Binding affinity of the anti-hMUC1 monoclonal antibody (hMUC1-1H7 clone) to rhMUC1-EC192 protein was measured using a Reichert SPR system SR7500DC (Buffalo, New York, USA) at 25°C. The rhMUC1-EC192 protein was captured on individual flow cell surface of a sensor chip. The anti-hMUC1 monoclonal antibody was injected at a flow rate of 50 µL/min. Data were evaluated using Reichert SPR evaluation software.
Cell culture
Human breast cancer cell lines MCF-7 (ATCC® HTB-22™) and MDA-MB-231 (ATCC® CRM-HTB-26™) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). ATCC characterized the cell lines with tests for morphology, post-freeze viability, interspecies determination (isoenzyme analysis), cytogenetic analysis, mycoplasma contamination, and bacterial and fungal contamination. T47D and ZR75-1 were purchased from Korean Cell Line Bank (Seoul, Korea). The cell lines were characterized by the cell bank using DNA fingerprinting analysis, species verification test, mycoplasma contamination test, and viral contamination test. MCF-7 cells were cultured in Eagle's Minimum Essential Medium (ATCC) supplemented with 0.01 mg/mL human recombinant insulin; MDA-MB-231 cells were cultured in Leibovitz's L-15 medium (Thermo Fisher Scientific, Waltham, MA, USA); and T47D and ZR75-1 cells were cultured with RPMI-1640 medium (Thermo Fisher Scientific). All media were supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 mg/mL streptomycin. MCF-7, T47D, and ZR75-1 cells were incubated at 37°C in an atmosphere of 5% CO2 and MDA-MB-231 cells were incubated at 37°C in the absence of CO2.
Antibodies
For detection of MUC1 protein in cells by western blotting and immunoprecipitation, commercially-available anti-MUC1-cytoplasmic tail (CT) (anti-MUC1-CT Ab, Catalog No. ab109185, rabbit monoclonal antibody to MUC1) and anti-MUC1-CT2 (anti-MUC1-CT2 Ab, Catalog No. ab80952, Armenian hamster monoclonal antibody to MUC1) antibodies were obtained from Abcam (Cambridge, UK). These antibodies recognize the cytoplasmic tail region of MUC1. Anti-β-actin antibody was purchased from Sigma-Aldrich.
Western blotting analysis
Cells were lysed with lysis buffer and centrifuged at 16,000 × g at 4°C for 20 min. Equal amount of proteins were resolved in a 4-12% Bis-Tris gradient gel (Thermo Fisher Scientific), and transferred onto nitrocellulose membranes, which were blocked with 3% BSA in PBS-T for 1 h at room temperature. Membranes were incubated with anti-MUC1-CT, anti-MUC1-CT2, monoclonal anti-hMUC1, or anti-β-actin antibodies overnight at 4°C. Immuno-reactive protein band intensities were measured after visualization using a HRP-conjugated secondary antibody (donkey anti-mouse IgG for anti-hMUC1 monoclonal antibody, goat anti-rabbit IgG for anti-MUC1-CT Ab, rabbit anti-Armenian hamster IgG for anti-MUC1-CT2 Ab, Jackson ImmunoResearch, West Grove, PA, USA) and an enhanced chemiluminescence reagent (Thermo Fisher Scientific).
Immunoprecipitation analysis
Cell lysates were incubated with mouse anti-hMUC1 monoclonal antibody overnight at 4ºC. Protein A bead was added to the mixture and incubated at 4oC for 1 h. The immunocomplexes collected by centrifugation were washed and analyzed by western blotting. Membranes were incubated with anti-MUC1-CT antibody, anti-MUC1-CT2 antibody, anti-hMUC1 monoclonal antibody, or anti-β-actin antibody.
Deglycosylation assay
Cell lysates from T47D cells were extracted with lysis buffer (0.5% SDS, 1% β-mercaptoethanol) and boiled at 100°C for 10 min. The samples were then incubated with peptide-N-glycosidase (PNGase) F (Elpis Biotech, Taejeon, Korea) at 37°C for 2 h and boiled at 100°C for 10 min. The samples were diluted with lysis buffer and immunoprecipitation was performed with the anti-hMUC1 monoclonal antibody. The immunocomplex was analyzed by western blotting with anti-MUC-CT or anti-MUC1-CT2 antibodies.
Confocal microscopy
Cells were cultured on poly-L-lysine-coated glass cover slips in 12-well culture plates. After cells were cultured for 48 h, cells were fixed with 4% paraformaldehyde for 10 min. For detection of cell surface MUC1-C, mouse anti-hMUC1 monoclonal antibody, anti-MUC1-CT2 antibody, or mouse normal IgG were treated for 2 h on ice. For intracellular staining, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 3% BSA, and stained with anti-hMUC1 monoclonal antibody for 2 h at room temperature. After washing in PBS-T containing 1% BSA, the samples were incubated with Alexa Flour-conjugated secondary antibody such as Alexa Flour 488-conjugated goat anti-mouse IgG antibody (for anti-hMUC1 monoclonal antibody, Invitrogen) or Alexa Flour 594-conjugated goat anti-Armenian hamster IgG antibody (for anti-MUC1-CT2 antibody, Invitrogen) for 1 h. Nuclei were stained with Hoechst 33258. All samples were mounted and observed using the confocal laser scanning microscope system (CLSM system; LSM 710, Carl Zeiss, Jena, Germany) 30.
Internalization assay
The monoclonal anti-hMUC1 antibody was labeled with DyLight 488 according to the manufacturer's instructions (Thermo Fisher Scientific). The human breast cancer cells were treated with DyLight 488-labeled anti-hMUC1 antibody and incubated at 37°C for the indicated time periods. Fluorescence signals from the internalized cells were detected with CLSM (LSM 710, Carl Zeiss).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The growth of cancer cells treated with anti-hMUC1 monoclonal antibody was monitored with an MTT (Sigma-Aldrich) assay solution as described previously 28. The MTT solution was added to each well at the indicated time and the plates were incubated for an additional 4 h at 37°C. After the removal of the medium, the formazan crystals were solubilized in dimethyl sulfoxide. The color development at 595 nm was monitored using a spectrophotometer, with a reference wavelength of 650 nm.
Biodistribution imaging in vivo
Four-week-old female BALB/c nu/nu mice were implanted subcutaneously with a 17β-estradiol pellet (0.72 mg/pellet, 60-day release; Innovative Research of America, Sarasota, FL, USA). Next day, 5 × 106 cells (MCF-7, T47D, or ZR75-1) in 50% Matrigel were inoculated subcutaneously into right flank of the mice. When tumor volumes reached an average of about 100 mm3, mouse normal IgG-DyLight 755 (5 mg/kg) or anti-hMUC1 monoclonal antibody-DyLight 755 (5 mg/kg) was intravenously injected into the mice. Subsequently, the tumor-targeting efficacy of the antibody was monitored using an in vivo imaging system (IVIS 200; Xenogen Corporation, MA, USA) at 0, 24, and 48 h. For detection of intracellular localization of the anti-hMUC1 monoclonal antibody in vivo, the mice were intravenously injected with DyLight 488-labeled anti-hMUC1 monoclonal antibody (5 mg/kg). After 2 days, the tumor tissues were collected, and then frozen tissue sections were prepared. Internalization of the antibody was detected in the tumor sections with CLSM (LSM 710, Carl Zeiss).
Tissue array and immunohistochemistry
Paraffin-embedded human breast cancer tissue sections were purchased from ISU ABXIS (Seoul, Korea). The tissue sections were deparaffinized with xylene for 30 min, and rehydrated with graded ethanol, and then incubated with 3% hydrogen peroxide solution for 10 min. Antigen retrieval was performed in citrate solution (pH 6.0). The sections were blocked with normal horse serum for 30 min, and then incubated with anti-hMUC1 monoclonal antibody (1 µg/slide) or anti-MUC1-CT2 antibody (1 µg/slide) for 2 h at room temperature. The sections were washed and incubated with biotinylated anti-mouse IgG antibody (Vector Laboratories, Burlingame, CA, USA) or biotinylated anti-Armenian hamster IgG antibody (Vector Laboratories) for 1 h. This was followed by washing and incubation with HRP-streptavidin for 30 min. The immunoreactivities were detected with 3,3ʹ-diaminobenzidine (DAB, Thermo Fisher Scientific) and the samples were then counterstained with hematoxylin (Muto Pure Chemicals, Tokyo, Japan). All images were captured using a microscope (Eclipse E-200; Nikon, and Tokyo, Japan).
Statistical analysis
Results are presented as mean ± standard deviation. Statistical significance of comparisons of differences between two samples was evaluated using the Student's t-test; differences were considered to be significant for values of P <0.05.
Results
Production and purification of anti-hMUC1 monoclonal antibody
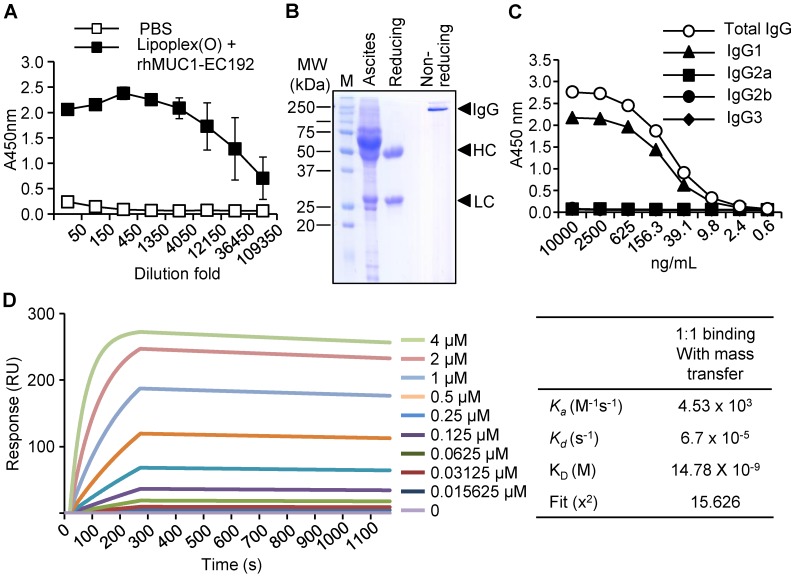
To generate a monoclonal antibody against the extracellular region of the MUC1 C-terminal subunit, expression of His-tagged rhMUC1-EC192 protein was induced in E. coli using IPTG. The recombinant protein was purified from bacterial lysates using a Ni-NTA column and examined by western blotting using anti-His tag antibody. Then we immunized mice with PBS or rhMUC1-EC192 and Lipoplex (O). After the third boosting immunization, mouse sera were collected and the presence of antibody against rhMUC1-EC192 was verified using ELISA. The results showed significant induction of rhMUC1-specific IgG (Fig. 1A). Next, we isolated hybridoma cells producing the rhMUC1-EC192-specific monoclonal antibody, purified the anti-hMUC1 monoclonal antibody from mouse ascites, and analyzed the purified antibody using SDS-PAGE and Coomassie staining (Fig. 1B). ELISA against several IgG isotypes revealed that the isotype of the anti-hMUC1 monoclonal antibody was IgG1 (Fig. 1C). The binding affinity of the antibody to rhMUC1-EC192 protein was determined by SPR analysis and the equilibrium dissociation constant (Kd) of the antibody was 15 nM (Fig. 1D). Thus, we can conclude that the monoclonal antibody produced using our procedure efficiently recognizes rhMUC1-EC192 protein.
Figure 1.
Production and characterization of the human anti-hMUC1 monoclonal antibody. (A) Mice were immunized with PBS or rhMUC1-EC192 plus Lipoplex (O) and sera were analyzed for the presence of rhMUC1-EC192-specific antibody using ELISA. (B) Anti-hMUC1 monoclonal antibody was purified from ascites and analyzed using SDS-PAGE followed by Coomassie blue staining. HC: heavy chain; LC: light chain. (C) Isotype of IgG in the purified antibody fraction was determined using ELISA. (D) Determination of binding affinity for the rhMUC1-EC192 protein using an SPR system. The rhMUC1-EC192 protein was immobilized on a sensor chip, and increasing amounts of antibody were applied. Kinetic parameters are shown in the right panel.
Examination of the specificity of anti-hMUC1 monoclonal antibody in various breast cancer cells
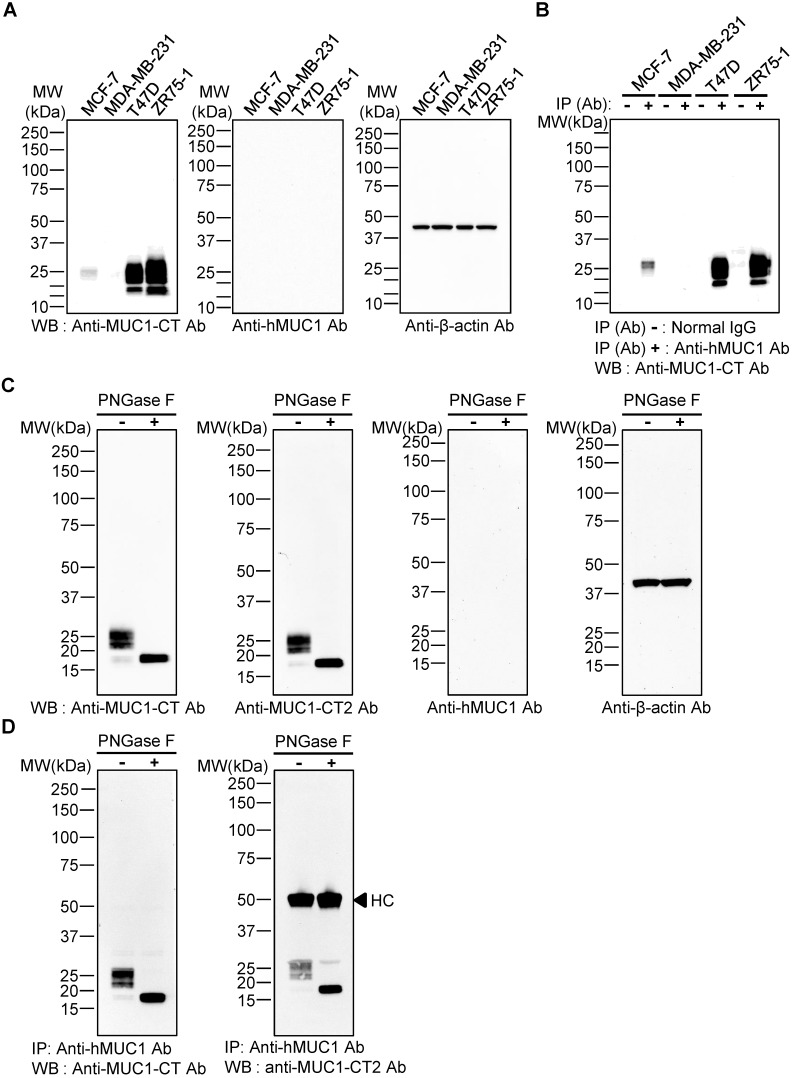
To evaluate whether the anti-hMUC1 monoclonal antibody recognizes intact MUC1 protein, western blotting using lysates of MCF-7, MDA-MB-231, T47D, and ZR75-1 breast cancer cells was carried out. The results showed that the commercially-available anti-MUC1-CT antibody detected MUC1 protein bands in MCF-7, T47D and ZR75-1, but not in MDA-MB-231 cell lysates, but the anti-hMUC1 monoclonal antibody was unable to recognize MUC1 protein in any of the samples (Fig. 2A). Since no MUC-1 protein was detected in MDA-MB-231cells, this cell line was used as a negative control in all of our subsequent studies. Considering the molecular weight of the detected MUC1 protein bands, we surmised that the multiple bands represent variously glycosylated MUC1-C. To investigate whether the anti-hMUC1 monoclonal antibody could recognize the endogenous MUC1 protein in its native state, we performed immunoprecipitation from various breast cancer cell lysates, followed by immunoblotting using the anti-MUC1-CT antibody. The data showed that the anti-hMUC1 monoclonal antibody efficiently immunoprecipitates native-state MUC1 protein in MCF-7, T47D, and ZR75-1 cell lysates (Fig. 2B). As it appears that the antibody detects conformational antigen in MUC1 protein, we performed SDS-free native PAGE and western blotting and confirmed that the anti-hMUC1 antibody recognized native MUC1 protein in T47D cell lysates (Fig. S1).
Figure 2.
Specificity of anti-hMUC1 monoclonal antibody. (A) Lysates from MCF-7, MDA-MB-231, T47D, and ZR75-1 breast cancer cell lines were resolved by SDS-PAGE and western blotting was performed with anti-MUC1-CT, anti-hMUC1 monoclonal antibody (anti-hMUC1), and anti-β-actin antibodies. (B) Cell lysates from MCF-7, MDA-MB-231, T47D, and ZR75-1 cells were immunoprecipitated with mouse normal IgG or anti-hMUC1 monoclonal antibody and immunoblotted using the anti-MUC1-CT antibody. (C) T47D lysates were treated with PBS (-) or PNGase F (+) and analyzed by western blotting using anti-MUC1-CT, anti-MUC1-CT2, anti-hMUC1, or anti-β-actin antibodies. (D) T47D cell lysates, with or without PNGase F treatment, were immunoprecipitated with the anti-hMUC1 monoclonal antibody and then immunoblotted with anti-MUC1-CT or anti-MUC1-CT2 antibodies. HC: heavy chain.
There are five potential sites of N-linked glycosylation in MUC1 (asparagine residues) and one such site is in the extracellular region of MUC1-C 41. PNGase F removes the N-linked glycans. Depending on the N-glycosylation extent, the size of MUC1-C is estimated to be between 23 and 25 kDa. Without N-glycosylation, MUC1-C may have a molecular weight of 17 kDa. Therefore, we then explored whether the anti-hMUC1 monoclonal antibody could recognize MUC1-C when the protein is deglycosylated at the asparagine residue. After treatment with PNGase F, the size of the MUC1 protein band became homogeneous and smaller than 20 kDa, suggesting that this protein band represents deglycosylated MUC1-C. Western blotting of the PNGase-treated T47D cell lysate showed that the anti-hMUC1 monoclonal antibody was unable to detect the MUC1 protein (Fig. 2C). However, the antibody was able to immunoprecipitate MUC1 protein from T47D cells, irrespective of the N-glycosylation status (Fig. 2D). It suggests that the N-linked glycosylation in the MUC1-C is not required for the reactivity of the antibody.
To further investigate whether the anti-hMUC1 monoclonal antibody recognizes mouse MUC1-C, we produced recombinant mouse MUC1 C-terminal protein named rmMUC1-EC187 (Supplementary information). The sequences of the two proteins have 58% identity. Then, immunoprecipitation and western blotting using purified mouse and human MUC1 C-terminal proteins were carried out. The anti-hMUC1 monoclonal antibody recognized human MUC1 C-terminal protein but not mouse MUC1 C-terminal protein (Fig. S2). These results demonstrate that the anti-hMUC1 monoclonal antibody can successfully recognize human MUC1-C in its native state.
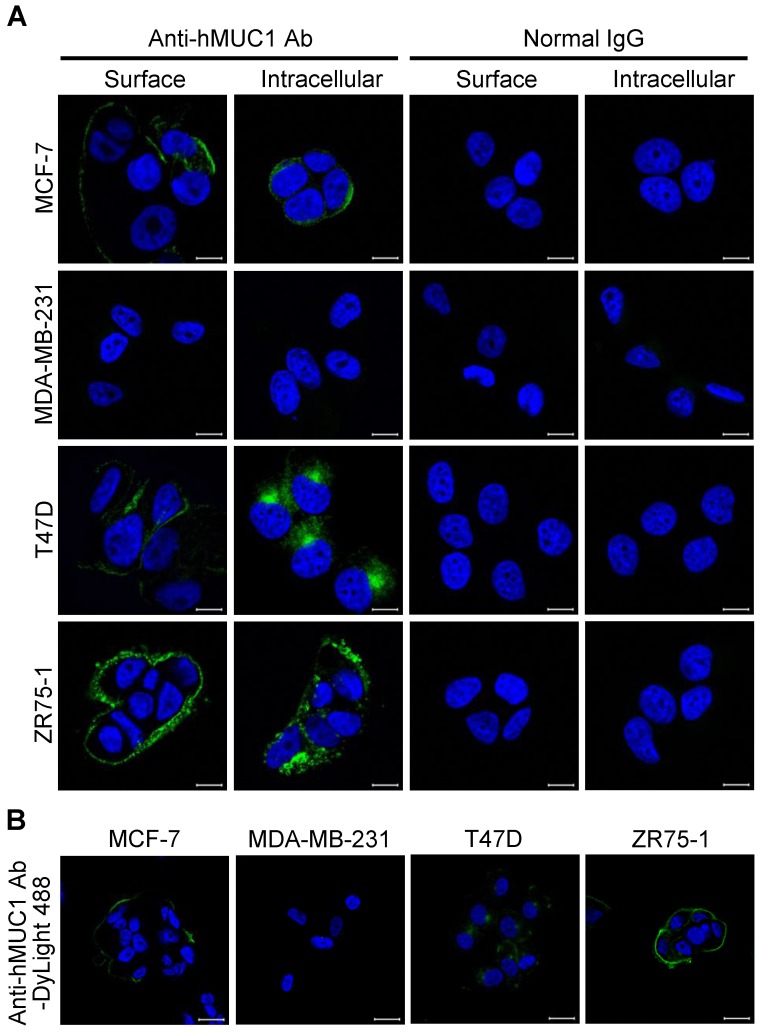
Anti-hMUC1 monoclonal antibody recognizes cell-surface and intracellular MUC1 protein
The specificity and binding affinity of an antibody to the antigen is a key determinant of its efficiency 31, 32. To determine whether the anti-hMUC1 monoclonal antibody is able to recognize and bind to MUC1 protein in the intact cells, immunofluorescence staining of MCF-7, MDA-MB-231, T47D, and ZR75-1 was performed. The confocal microscopy images show that the anti-hMUC1 monoclonal antibody prominently stains MUC1 in MCF-7, T47D, and ZR75-1 cells, both on the cell surface and in intracellular locations (Fig. 3A), suggesting that the antibody recognizes the extracellular region of MUC1-C. We also used anti-MUC1-CT2 antibody to compare the ability of the anti-hMUC1 antibody and anti-MUC1-CT2 antibody to recognize MUC1. As the anti-MUC1-CT2 antibody recognizes intracellular domain of MUC1, it was not able to recognize and bind to MUC1 protein in the intact cells (Fig. S3). We could see staining of the antibody only when we permeabilized the cells before staining.
Figure 3.
Binding of anti-hMUC1 monoclonal antibody to MUC-1 protein localized on the cell surface and in intracellular regions. (A) MCF-7, MDA-MB-231, T47D, and ZR75-1 cells were surface-stained or stained in the intracellular region with anti-hMUC1 antibody (anti-hMUC1) or mouse normal IgG. The samples were then stained using Alexa 488-conjugated secondary antibody. (B) Internalization of the anti-hMUC1 monoclonal antibody in breast cancer cells. MCF-7, MDA-MB-231, T47D, and ZR75-1 cells were treated with DyLight 488-labeled anti-hMUC1 monoclonal antibody and incubated at 37°C for 6 h. The nuclei were stained with Hoechst 33258. Images were visualized by confocal microscopy. Scale bar, 10 μm.
The potency of antibodies for therapeutics further depends on internalization. Therefore, we conjugated the anti-hMUC1 monoclonal antibody with DyLight 488, an amine-reactive fluorescent label, and stained the cells with the conjugated antibody. Confocal imaging data showed that the anti-hMUC1 monoclonal antibody was most effectively internalized in T47D cells (Fig. 3B and Fig. S4). Based on these data, we suggest that the anti-hMUC1 monoclonal antibody can be used to target MUC1 protein in live cells for internalization.
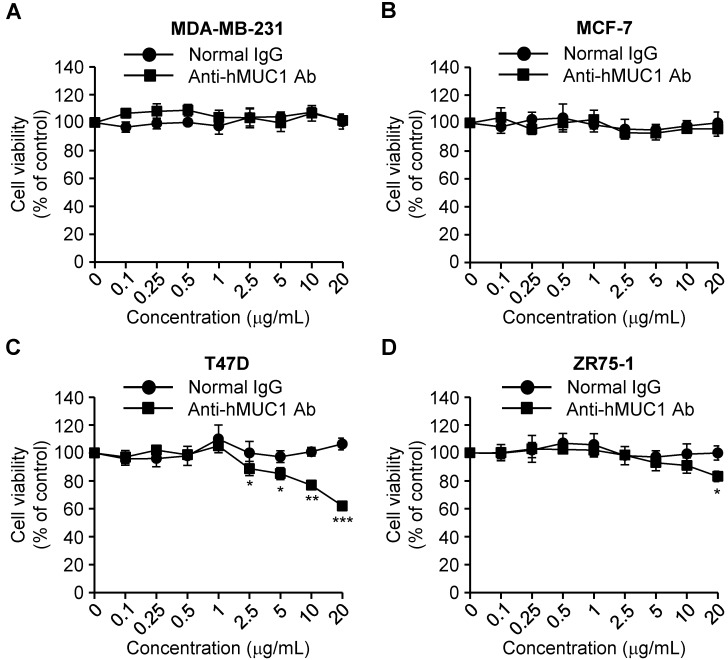
Effect of the anti-hMUC1 monoclonal antibody on proliferation of breast cancer cells
MUC-1 is overexpressed in various types of cancerous tissues and promotes cell growth 4. To investigate the anti-hMUC1 monoclonal antibody as an agent for anti-breast cancer therapy, we analyzed its impact on breast cancer cell growth. Treatment of cells with the antibody significantly delayed the proliferation of T47D cells in comparison to control IgG-treated cells. The antibody treatment slightly suppressed growth of ZR75-1 cells only at high concentration. In contrast, the anti-hMUC1 monoclonal antibody did not alter the proliferation of MCF-7 and MDA-MB-231 cells (Fig. 4). Considering that the antibody can bind to the cell surface to MCF-7, T47D, and ZR-75-1 cells but the internalization was most prominent in T47D cells (Fig. 3), this result suggests that internalization capability of anti-MUC1 antibody may be related with its anti-proliferative effect in breast cancer cells expressing MUC1. To understand the mechanism of action, we treated T47D cells with anti-hMUC1 antibody and investigated its influence on the phosphorylation levels of AKT, ERK, and p38 in T47D cells. However, there was no effect (Figure S5). There was no change in binding of Annexin V and expression of Caspase-3 and PARP suggesting that the antibody did not induce apoptosis of the cells (Figure S6). Therefore, we speculate that the antibody may inhibit the signal transduction of MUC1 but the change is very weak to be detected by western blotting or other signaling pathways are operating in the cells after antibody treatment. Furthermore, the antibody did not induce drastic change in the cell cycle, either (Figure S7). These data suggest that the anti-hMUC1 monoclonal antibody has an anti-proliferative effect on some of MUC1-expressing human breast cancer cells even though the mechanisms are not defined yet.
Figure 4.
Effect of anti-hMUC1 monoclonal antibody on proliferation of human breast cancer cells. MDA-MB-231 (A), MCF-7 (B), T47D (C), and ZR75-1 (D) cells were treated with the anti-hMUC1 monoclonal antibody or normal IgG, and cell proliferation was evaluated using an MTT assay on the fifth day after treatment. The results are representative of data from 3 independent trials. *** p < 0.001, ** p < 0.01, * p < 0.05 indicate statistical significance compared to normal IgG group.
Localization of the injected anti-hMUC1 monoclonal antibody in MCF-7-, T47D- and ZR75-1-derived tumors in vivo
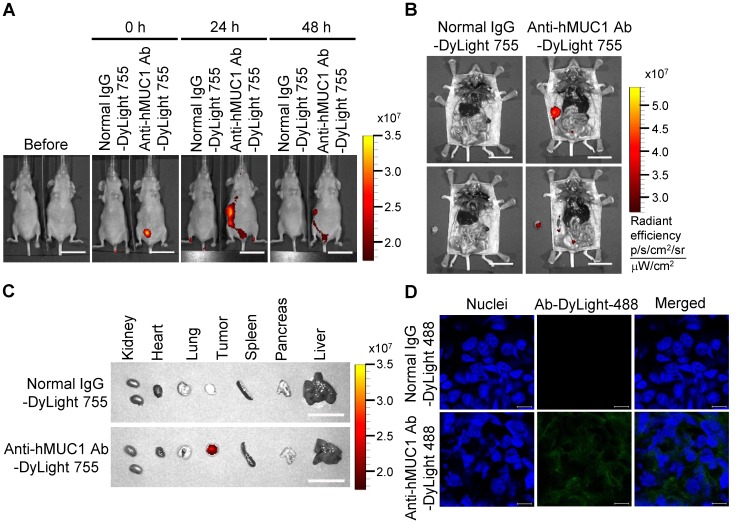
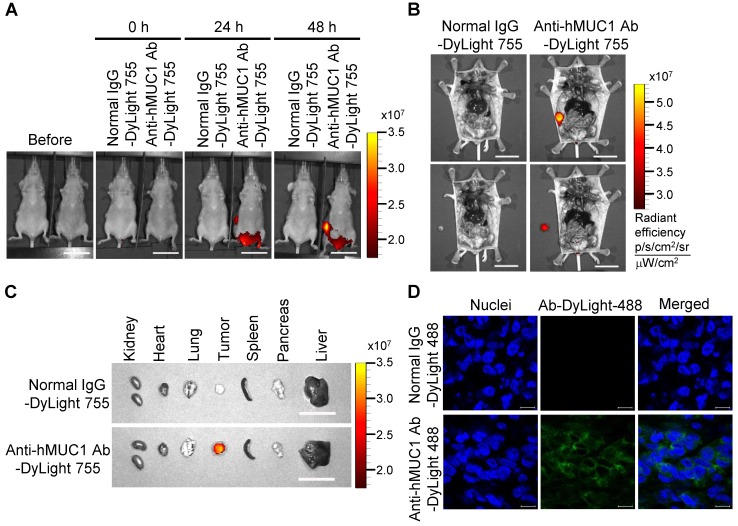
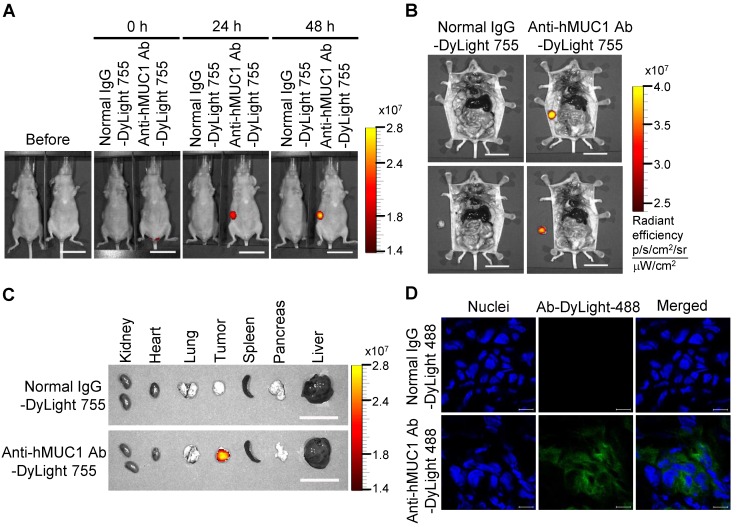
To demonstrate the effectiveness of the anti-hMUC1 monoclonal antibody in vivo, we administered DyLight 755-labeled normal IgG or anti-hMUC1 monoclonal antibody into breast tumor-bearing mice, in a mouse xenograft model established with MCF-7, T47D and ZR75-1 cells. The distribution of the labeled antibody was quantified by measuring the total flux (photons/s) of the fluorescence at 0, 24, and 48 h. The results showed that the anti-hMUC1 monoclonal antibody was localized specifically in the tumor region (Fig. 5A and B, Fig. 6A and B, and Fig. 7A and B). When the tumor and other organs were isolated, the anti-hMUC1 monoclonal antibody was detected only in tumor tissues, without affecting other vital organs (Fig. 5C, Fig. 6C, and Fig. 7C). The binding of the anti-hMUC1 monoclonal antibody to the tumor was further confirmed by confocal microscopy of sections of the MCF-7-, T47D-, and ZR75-1-derived tumors. DyLight 488-labeled anti-hMUC1 antibody staining was prominent in the sections of T47D- and ZR75-1-derived tumors (Fig. 6D and 7D) and comparatively weak in the MCF-7-derived tumors (Fig. 5D), whereas DyLight 755-labeled normal IgG was not detected in tumors (Fig. 5, Fig. 6, and Fig. 7). These results suggest that the anti-hMUC1 monoclonal antibody can be used to specifically target breast cancer cells in the animal model.
Figure 5.
Biodistribution of the anti-hMUC1 monoclonal antibody in MCF-7 cell-derived breast tumor tissue. BALB/C nu/nu mice were subcutaneously injected with MCF-7 cells to induce tumor formation. (A) The mice were injected intravenously with DyLight 755-labeled normal IgG (5 mg/kg) or anti-hMUC1 monoclonal antibody (5 mg/kg), and whole-body fluorescent imaging was performed at 0, 24, and 48 h. (B) The localization of the antibody in dissected mice. (C) Various organs and tumors were isolated and examined for antibody distribution. Scale bars, 2.5 cm (A-C). (D) The mice were injected intravenously with the DyLight 488-labeled anti-hMUC1 antibody. After 48 h, tumor sections were stained with 4',6-diamidino-2-phenylindole (DAPI) for visualizing nuclei and evaluated using confocal microscopy. Scale bars, 10 μm (D). The images are representative of data from 3 sets of mice.
Figure 6.
Biodistribution of the anti-hMUC1 monoclonal antibody in T47D cell-derived breast tumor tissue. BALB/C nu/nu mice were subcutaneously injected with T47D cells to induce tumor formation. (A) The mice were injected intravenously with DyLight 755-labeled normal IgG (5 mg/kg) or anti-hMUC1 monoclonal antibody (5 mg/kg), and whole-body fluorescent imaging was performed at 0, 24, and 48 h. (B) The localization of the antibody in dissected mice. (C) Antibody distribution in various organs and tumors. Scale bars, 2.5 cm (A-C). (D) The mice were injected intravenously with the DyLight 488-labeled anti-hMUC1 antibody. Tumor sections were stained with DAPI and evaluated using confocal microscopy. Scale bars, 10 μm (D). The images are representative of data from 3 sets of mice.
Figure 7.
Biodistribution of the anti-hMUC1 monoclonal antibody in ZR75-1 cell-derived breast tumor tissue. BALB/c nu/nu mice were subcutaneously injected with ZR75-1 cells to induce tumor formation. (A) The mice were injected intravenously with DyLight 755-labeled normal IgG (5 mg/kg) or anti-hMUC1 monoclonal antibody (5 mg/kg), and whole-body fluorescent imaging was performed at 0, 24, and 48 hours. (B) The localization of the antibody in dissected mice. (C) Antibody distribution in various organs and tumors. Scale bars, 2.5 cm (A-C). (D) The mice were injected intravenously with the DyLight 488-labeled anti-hMUC1 monoclonal antibody. Tumor sections were stained with DAPI and evaluated using confocal microscopy. Scale bars, 10 μm (D). The images are representative of data from 3 sets of mice.
Expression of MUC1 protein in human breast cancer tissues
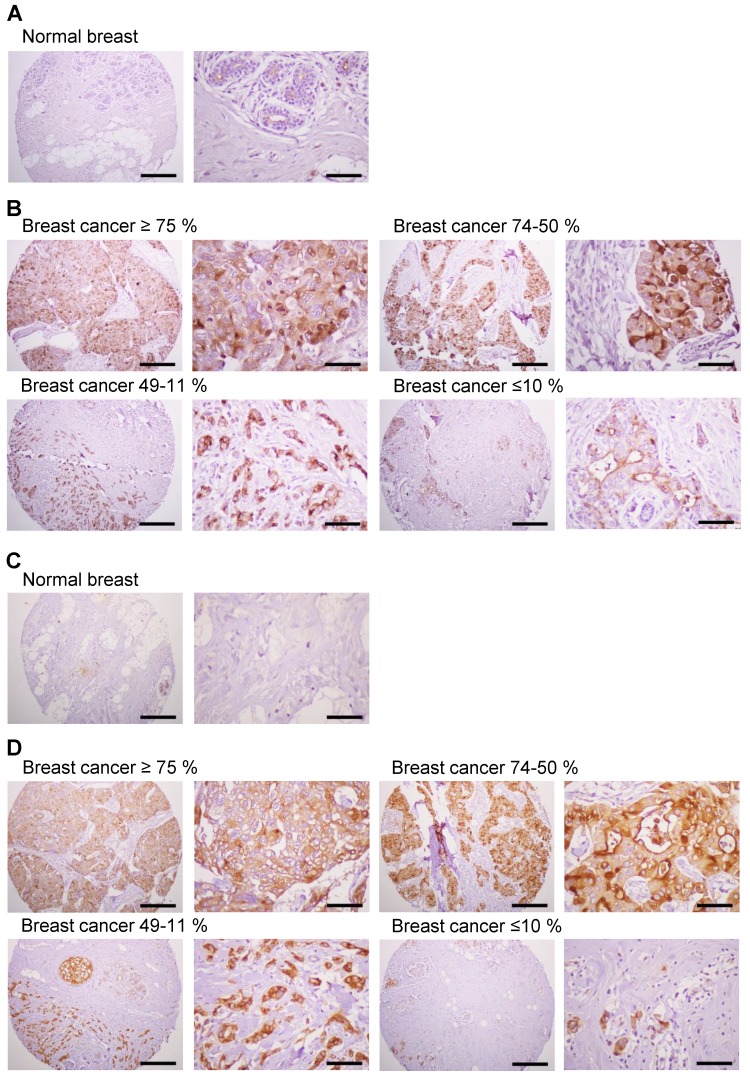
Previous studies have shown significantly enhanced expression of MUC1 in breast cancer cells 4. We investigated the expression of MUC1 protein in human breast cancer tissues by immunostaining with anti-hMUC1 monoclonal antibody and compared that with MUC1 expression in normal breast tissues. Here, MUC1 expression was detected in most of the breast cancer tissues, while no expression was detected in normal breast tissues (Fig. 8A). Analysis of 21 breast cancer specimens indicated that 24% of the samples displayed positive expression of MUC1 in ≥75% of tumor cells, and that 24% and 29% of the cancer tissue samples were positive for MUC1 expression in 50-74% and 11-49% of tumor cells, respectively (Fig. 8B, Table 1 and Table S1). However, 5% of the breast cancer specimens showed no expression of MUC1. We also performed immunostaining with anti-MUC1-CT2 antibody using the same tissue sections with sequential cuts. As shown in Fig. 8C and D, MUC1 expression was similarly detected by anti-hMUC1 monoclonal antibody and anti-MUC1-CT2 antibody. These findings suggest that MUC1 expression may have utility as a marker for breast cancer diagnostics and that the anti-hMUC1 monoclonal antibody can efficiently recognize MUC1 protein in tissue samples from patients with breast cancer.
Figure 8.
Expression of MUC1 in breast cancer tissues. Immunohistochemistry of human breast cancer tissue arrays was performed with the anti-hMUC1 monoclonal antibody (A and B) and anti-MUC1-CT2 antibody (C and D). (A, C) Normal breast tissues. (B, D) Breast cancer tissues with ≥75%, 74-50%, 49-11%, and ≤10% of tumor cells expressing MUC1. Scale bars; left panel, 100 μm and right panel, 25 μm.
Table 1.
Immunohistochemical analysis of MUC1 expression in breast cancer tissues
| Breast cancer tissue sections (AccuMax Array) |
n | MUC1 positive (%) | Number (%) of cases expressing MUC1 | ||||
|---|---|---|---|---|---|---|---|
| ≥75% | 74-50% | 49-11% | ≤10% | Negative (%) | |||
| A312(II) | 21 | 76.2 | 5 (23.8) | 5 (23.8) | 6 (28.6) | 4 (19) | 1(4.8) |
Percentages in parentheses were calculated as the number of MUC1-psoitive samples for each quartile, divided by the total number of samples in each tumor type.
Discussion
MUC1 is overexpressed in most cancer cells, including breast cancer, and MUC1 expression is correlated with disease progression and poor prognosis 3, 4. In this study, we generated a monoclonal antibody against human MUC1 and investigated the characteristics of the antibody in the milieu of breast cancer cells and tissues to evaluate its potential in anti-cancer therapeutics.
We produced a polypeptide, rhMUC1-EC192, which includes 192 amino acids corresponding to the extracellular region of MUC1 adjacent to the transmembrane domain, and successfully obtained an anti-MUC1-C specific monoclonal antibody, anti-hMUC1, by immunizing mice with rhMUC1-EC192 and Lipoplex (O) complex. Our previous study showed that immunization of mice with peptide and Lipoplex (O) is sufficient to produce high antibody titers against the peptide 28-30, 33. The anti-hMUC1 monoclonal antibody was unable to detect MUC1 protein in western blotting analysis, but efficiently immunoprecipitated MUC1-C from lysates from MCF-7, T47D, and ZR75-1 breast cancer cells, suggesting that the antibody effectively identified MUC1-C protein in the native state. Further investigation revealed that the anti-hMUC1 monoclonal antibody also recognizes MUC1-C protein on the cell surface and in intracellular regions of breast cancer cells, as assessed using confocal microscopy of MCF-7, T47D, and ZR75-1 cells.
Based on the literature, expression of MUC1-C in MDA-MB-231 cells is controversial. While some papers reported expression of MUC1-C 34-36, there was a paper showing no expression of MUC1 in these cells 37. When we cultured MDA-MB-231 cells in different conditions, the results were clearly different. The cells barely expressed MUC1-C when we cultured the cells as ATCC recommended without CO2 injection. When we cultured the cells in a regular CO2 incubator, MUC1-C expression was evident (data not shown).
Besides the binding affinity and specificity of the anti-hMUC1 monoclonal antibody, we tested whether anti-hMUC1 monoclonal antibody can be internalized after treatment. Internalization was monitored by staining cells with anti-hMUC1 conjugated with DyLight 488. Confocal microscopy imaging analysis indicated that anti-hMUC1 monoclonal antibody is most effectively internalized in T47D breast cancer cells.
The principle behind therapeutic targeting of MUC1 is to block the MUC1-mediated signaling pathway. A therapeutic advancement in targeting MUC-1 is based on generating peptides that imitate the MUC1 domain, for example, the PTD-4 MUC1 inhibitory peptide (PMIP). Treatment with PMIP decreases breast cancer metastasis 38, 39. Another peptide, GO-201, inhibits MUC1-C dimerization by targeting the MUC1-C-CQC motif, which causes breast and prostate cancer cell death 3, 40. In our study, treatment with the anti-hMUC1 monoclonal antibody resulted in a significant reduction of the proliferative capacity in T47D breast cancer cells. Therefore, we suggest that the internalization of the antibody is somehow associated with growth suppression.
The tandem repeat region of the MUC1 is heavily decorated with O-linked mucin-type glycosylation. For N-linked glycosylation, there are five potential sites in MUC1 (asparagine residues), all located to the C-terminal of the tandem repeat. Specifically, the MUC1-N contains four sites in the degenerate sequence and the MUC1-C contains one site in the extracellular region 41. Considering that the antibody was able to detect MUC1 protein from T47D cells irrespective of the PNGase F treatment, the N-linked glycosylation in the MUC1-C is not required for the reactivity of the antibody.
The most fundamental feature of a therapeutic antibody for clinical evaluation is the capacity of the antibody to target tumors, while sparing normal cells. Our biodistribution analysis clearly showed that the anti-hMUC1 monoclonal antibody conjugated with DyLight 755 exclusively and entirely accumulated in breast tumors alone. In fact, the precise localization of the fluorescently-labeled anti-hMUC1 monoclonal antibody is remarkably specific to breast tumor tissues, given its absence of reactivity to other vital organs. The specification of biodistribution was further confirmed by confocal microscopy of tumor sections. Since MUC1 is heavily glycosylated and localized in polarized normal epithelial cells, it is likely that the antibody cannot access the protein core. However, cancer cells have dispersed and hypoglycosylated MUC1, with reduced O-linked glycosylation in the MUC1-N region 41; therefore, the antibody might be able to more efficiently access and recognize the extracellular region of MUC1-C in tumor tissue. But, there is one important reason why we have to be careful. The homology of the extracellular region of human and mouse MUC1-C is only about 58% and our antibody can't recognize recombinant mouse extracellular region of MUC1-C (Fig. S2). Therefore, whether the antibody interacts with normal human mammary epithelial cells or not is to be determined in the future work. Considering that the internalization of MUC1-C does not occur in normal epithelial cells 3 and that extent of internalization is related with suppressive effect of the antibody, it is still promising even when the antibody has background reactivity to normal cells.
An antibody with high specificity can be conjugated with various compounds to precisely target tumor cells, which could improve treatment efficacy by more specific targeting of tumor cells alone. Recent studies have focused on the development and application of MUC1 antibodies in cancer. In one study, a MUC1-specific antibody, HMFG2, was conjugated with CpG-DNA, enabling the activation of natural killer cells in the tumor tissue and reduces the tumor burden 42. In another study, a monoclonal anti-MUC1 antibody was conjugated with yttrium90 (90Y-mu-HMFG1), facilitating its binding to MUC1 epitopes and elimination of cancer cells using the radioactive yttrium moiety 43. It was also reported that mesoporous silica nanoparticle platform conjugated to tumor-specific MUC1 antibody efficiently targeted tumor-specific MUC1 protein in vitro and in a human MUC1 transgenic mouse model without short-term toxicity in liver 44. In addition, the anti-MUC1 antibody GP1.4 decreased proliferation and migration of pancreatic cells by facilitating MUC1 internalization and inhibiting EGFR signaling 45. The combined treatment with anti-MUC1 antibody GP1.4 and platinum (II) complex induced better cytotoxic and proapoptotic effects in breast cancer cell line 35, 46. A MUC1 antibody, C595, in association with docetaxel, significantly decreased tumor growth in a xenograft model of ovarian cancer by augmenting apoptosis and necrosis 47. MUC1 was also used as a therapeutic target for triple-negative breast cancer using anti-MUC1 aptamer as a drug delivery system for a radioactive polymeric nanoparticle 48. Further, dendritic cell (DC)-based vaccine has been used as an alternative approach targeting MUC1 in cancer cells. The DC loaded with MUC1-derived peptides induced an antitumor immune response resulting prolonged survival of patients with MUC1-positive refractory non-small cell lung cancer (NSCLC) 49.
While most of these antibodies target to the MUC1-N terminal domain, the anti-hMUC1 antibody we generated and characterized in this study is specific to the extracellular region of MUC1-C. Therefore, our MUC1 antibody may offer additional advantages, especially in the context of tumor cell specificity and potency (required antibody dosage). Considering that the suppressive effect of the antibody in breast cancer cells were prominent in only one of four cell lines, various conjugative strategies should be investigated to produce a more efficient therapeutic agent with this new anti-hMUC1 antibody.
We performed immunostaining of human breast cancer tissue arrays with the anti-hMUC1 monoclonal antibody, and our data showed significant expression of MUC1 in various types of human breast cancers. These results not only confirm the expression of MUC1 in human breast cancer, but also suggest the potential of the anti-hMUC1 monoclonal antibody as a clinically-useful highly-specific therapeutic agent when combined with other drugs. Further studies on other types of cancers could widen the possible utility of the anti-hMUC1 monoclonal antibody. In this study, we could not test in vivo efficacy of the anti-MUC1 monoclonal antibody as the tumors established using breast cancer cell lines did not grow over 400 mm3 in the mice. We believe that patient-derived xenograft model will be a better choice in future evaluation.
In conclusion, the anti-hMUC1 monoclonal antibody described in this study specifically recognizes MUC1 protein in breast cancer cells and tissues. Also, tumor-specific biodistribution analysis of this antibody supports its potential for further development as a therapeutic agent in breast cancer therapy.
Supplementary Material
Supplementary methods, figures and tables.
Acknowledgments
The authors are grateful to Seung-Hae Kwon and Song Rae Kim at the Chuncheon Center of the Korea Basic Science Institute for technical assistance in real-time IVIS imaging system 200. This research was supported by grants from the National Research Foundation (2013M3A9A9050126, 2016M3A9B6916708, 2017M3A9C8026781) funded by the Ministry of Science, ICT and Future Planning in the Republic of Korea.
Abbreviations
- MUC1-N
MUC1 N-terminal subunit
- MUC1-C
MUC1 C-terminal subunit
- EGFR
epidermal growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- SH2
Src homology 2
- SOS
son of sevenless
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinases
- DOPE
phosphatidyl-β-oleoyl-γ-palmitoyl ethanolamine
- CHEMS
cholesterol hemisuccinate
- Lipoplex (O)
CpG-DNA 4531(O) co-encapsulated with DOPE: CHEMS complex
- SPR
surface plasmon resonance
- PNGase
peptide-N-glycosidase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ELISA
enzyme-linked immunosorbent assay.
References
- 1.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 3.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–81. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J. et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–93. [PubMed] [Google Scholar]
- 6.Levitin F, Stern O, Weiss M, Gil-Henn C, Ziv R, Prokocimer Z. et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–86. doi: 10.1074/jbc.M506047200. [DOI] [PubMed] [Google Scholar]
- 7.Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990;265:5573–8. [PubMed] [Google Scholar]
- 8.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–76. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X. et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:13787–92. doi: 10.1073/pnas.1203339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta. 2014;1845:126–35. doi: 10.1016/j.bbcan.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;7:669–70. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- 12.Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC. et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 14.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res. 2006;8:R37. doi: 10.1186/bcr1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Masri A, Gendler SJ. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene. 2005;24:5799–808. doi: 10.1038/sj.onc.1208738. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–64. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L. et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276:35239–42. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 19.Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–12. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 21.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meerzaman D, Shapiro PS, Kim KC. Involvement of the MAP kinase ERK2 in MUC1 mucin signaling. Am J Physiol Lung Cell Mol Physiol. 2001;281:L86–91. doi: 10.1152/ajplung.2001.281.1.L86. [DOI] [PubMed] [Google Scholar]
- 23.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–3. [PubMed] [Google Scholar]
- 24.Kwon S, Choi KC, Kim YE, Ha YW, Kim D, Park BK. et al. Monoclonal antibody targeting of the cell surface molecule TM4SF5 inhibits the growth of hepatocellular carcinoma. Cancer Res. 2014;74:3844–56. doi: 10.1158/0008-5472.CAN-13-2730. [DOI] [PubMed] [Google Scholar]
- 25.Pegram MD, Borges VF, Ibrahim N, Fuloria J, Shapiro C, Perez S. et al. Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Res. 2009;11:R73. doi: 10.1186/bcr2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie AM, Broadhead TJ, Chan SY, Owen J, Farnsworth AP, Sopwith M. et al. Phase I open study of the effects of ascending doses of the cytotoxic immunoconjugate CMB-401 (hCTMO1-calicheamicin) in patients with epithelial ovarian cancer. Ann Oncol. 2000;11:735–41. doi: 10.1023/a:1008349300781. [DOI] [PubMed] [Google Scholar]
- 27.Kufe DW. Targeting the human MUC1 oncoprotein: a tale of two proteins. Cancer Biol Ther. 2008;7:81–4. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Kwon S, Rhee JW, Kim KD, Kim YE, Park CS. et al. Production of antibodies with peptide-CpG-DNA-liposome complex without carriers. BMC Immunol. 2011;12:29. doi: 10.1186/1471-2172-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park BK, Lee SI, Lee Y, Cho S, Lee YS, Kwon H-J. Effect of a monoclonal antibody against human relaxin-2 on cancer cell growth inhibition. Appl Biol Chem. 2016;59:739–46. [Google Scholar]
- 30.Wu G, Kim D, Park BK, Park S, Ha JH, Kim TH. et al. Anti-metastatic effect of the TM4SF5-specific peptide vaccine and humanized monoclonal antibody on colon cancer in a mouse lung metastasis model. Oncotarget. 2016;7:79170–86. doi: 10.18632/oncotarget.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leal M, Sapra P, Hurvitz SA, Senter P, Wahl A, Schutten M. et al. Antibody-drug conjugates: an emerging modality for the treatment of cancer. Ann N Y Acad Sci. 2014;1321:41–54. doi: 10.1111/nyas.12499. [DOI] [PubMed] [Google Scholar]
- 32.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–59. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 33.Kim YE, Kwon S, Wu G, Kim D, Park BK, Park JA. et al. Therapeutic effect of a TM4SF5-specific monoclonal antibody against colon cancer in a mouse model. Oncotarget. 2014;5:8402–15. doi: 10.18632/oncotarget.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goode G, Gunda V, Chaika NV, Purohit V, Yu F, Singh PK. MUC1 facilitates metabolomic reprogramming in triple-negative breast cancer. PloS One. 2017;12:e0176820. doi: 10.1371/journal.pone.0176820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gornowicz A, Bielawska A, Czarnomysy R, Gabryel-Porowska H, Muszynska A, Bielawski K. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol Cell Biochem. 2015;408:103–13. doi: 10.1007/s11010-015-2486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajabi H, Tagde A, Alam M, Bouillez A, Pitroda S, Suzuki Y. et al. DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene. 2016;35:6439–45. doi: 10.1038/onc.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo JK, Choi Y, Oh SH, Jeong JH, Choi DH, Seo HS. et al. Mucin 1 enhances the tumor angiogenic response by activation of the AKT signaling pathway. Oncogene. 2012;31:2187–98. doi: 10.1038/onc.2011.410. [DOI] [PubMed] [Google Scholar]
- 38.Horm TM, Bitler BG, Broka DM, Louderbough JM, Schroeder JA. MUC1 drives c-Met-dependent migration and scattering. Mol Cancer Res. 2012;10:1544–54. doi: 10.1158/1541-7786.MCR-12-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bitler BG, Menzl I, Huerta CL, Sands B, Knowlton W, Chang A. et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–9. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T. et al. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–41. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–42. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schettini J, Kidiyoor A, Besmer DM, Tinder TL, Roy LD, Lustgarten J. et al. Intratumoral delivery of CpG-conjugated anti-MUC1 antibody enhances NK cell anti-tumor activity. Cancer Immunol Immunother. 2012;61:2055–65. doi: 10.1007/s00262-012-1264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oei AL, Moreno M, Verheijen RH, Sweep FC, Thomas CM, Massuger LF. et al. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer. 2008;123:1848–53. doi: 10.1002/ijc.23725. [DOI] [PubMed] [Google Scholar]
- 44.Dreau D, Moore LJ, Alvarez-Berrios MP, Tarannum M, Mukherjee P, Vivero-Escoto JL. Mucin-1-Antibody-Conjugated Mesoporous Silica Nanoparticles for Selective Breast Cancer Detection in a Mucin-1 Transgenic Murine Mouse Model. J Biomed Nanotechnol. 2016;12:2172–84. doi: 10.1166/jbn.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hisatsune A, Nakayama H, Kawasaki M, Horie I, Miyata T, Isohama Y. et al. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun. 2011;405:377–81. doi: 10.1016/j.bbrc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Gornowicz A, Kaluza Z, Bielawska A, Gabryel-Porowska H, Czarnomysy R, Bielawski K. Cytotoxic efficacy of a novel dinuclear platinum(II) complex used with anti-MUC1 in human breast cancer cells. Mol Cell Biochem. 2014;392:161–74. doi: 10.1007/s11010-014-2018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Chen H, Pourgholami MH, Beretov J, Hao J, Chao H. et al. Anti-MUC1 monoclonal antibody (C595) and docetaxel markedly reduce tumor burden and ascites, and prolong survival in an in vivo ovarian cancer model. PloS One. 2011;6:e24405. doi: 10.1371/journal.pone.0024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos do Carmo F, Ricci-Junior E, Cerqueira-Coutinho C, Albernaz MS, Bernardes ES, Missailidis S. et al. Anti-MUC1 nano-aptamers for triple-negative breast cancer imaging by single-photon emission computed tomography in inducted animals: initial considerations. Int J Nanomedicine. 2017;12:53–60. doi: 10.2147/IJN.S118482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teramoto K, Ozaki Y, Hanaoka J, Sawai S, Tezuka N, Fujino S. et al. Predictive biomarkers and effectiveness of MUC1-targeted dendritic-cell-based vaccine in patients with refractory non-small cell lung cancer. Ther Adv Med Oncol. 2017;9:147–57. doi: 10.1177/1758834016678375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods, figures and tables.