Abstract
Background:
Preeclampsia (PE) is a dangerous and unpredictable pregnancy complication. A seasonal pattern of risk would suggest that there are potentially preventable environmental contributors, but prior analyses have not adjusted for confounding by PE risk factors that are associated with season of conception.
Methods:
Seasonal effects were modeled and tested by representing each day of the year as an angle on a unit circle and using trigonometric functions of those angles in predictive models, using “harmonic analysis.” We applied harmonic Cox regression to model confounder-adjusted effects of the estimated day of the year of conception on risk of PE for births from the Medical Birth Registry of Norway for deliveries between 1999 and 2009. We also examined effect measure modification by parity, latitude (region), fetal sex, and smoking.
Results:
In adjusted models, PE risk was related to season, with higher risk in spring conceptions and lower risk in autumn conceptions, with a risk amplitude (maximum compared with minimum) of about 20%. The pattern replicated across subpopulations defined by parity, latitude (region), fetal sex, and smoking.
Conclusions:
These results suggest that there is a seasonal driver for PE, with effects that are not modified by parity, latitude, fetal sex, or smoking. https://doi.org/10.1289/EHP963
Introduction
Preeclampsia (PE) is a common and life-threatening hypertensive disorder of pregnancy. Diagnosed clinically by elevation in maternal blood pressure after 20 wk gestation, it is often accompanied by proteinuria. The biological onset is insidious, due to the subtlety of the symptoms (ACOG 2002). Typically seen in women having their first pregnancy, PE can rapidly progress to eclampsia, with seizures and risk of maternal and/or fetal death. Once PE is diagnosed, medically indicated delivery is considered because such an intervention is generally curative for the mother. However, if PE is diagnosed prior to 34 wk gestation, the risks involved in delivering a very immature baby must be weighed against the risks for both mother and baby that are imposed by continuing the pregnancy.
The pathophysiology of PE is not fully understood and it is unclear whether one should look for causative conditions in early pregnancy or later, when the syndrome becomes clinically apparent (Levine et al. 2005; Thadhani et al. 2004). Although the majority of cases are diagnosed late in the third trimester, evidence suggests that in some instances an underlying placental abnormality was present much earlier in gestation, as inferred from evidence of shallow invasion of the myometrium and inadequate remodeling of spiral arteries (Powe et al. 2011). At a later stage in gestation, inadequate perfusion of the placenta may cause an increase in maternal blood pressure, at which point the condition becomes clinically apparent. For cases where placental abnormalities originate early in gestation (Powe et al. 2011), the critical period for causative exposures (and preventive measures) may be during the first trimester (Huppertz 2008), despite the fact that the condition will not be diagnosed until much later.
Based on epidemiologic research, high maternal body mass index (BMI) (Thadhani et al. 1999), polycystic ovary syndrome (Palomba et al. 2015), preexisting hypertension, primiparity (Hernández-Díaz et al. 2009), family history (Boyd et al. 2013; Esplin et al. 2001; Skjærven et al. 2005), and a history of PE (Boyd et al. 2013) are recognized risk factors. There is also evidence that risk is associated with short stature (Basso et al. 2004), high altitude (Dávila et al. 2012), subfecundity (Basso et al. 2003), and long inter-pregnancy interval (Basso et al. 2001; Skjærven et al. 2002). Maternal cigarette smoking, a risk factor for many adverse reproductive outcomes, is well known to be associated with lower risk of PE (Conde-Agudelo et al. 1999; Engel et al. 2013). Smoking protection appears to require combustion by-products and not just nicotine, based on evidence from Sweden that use of smokeless tobacco (snuff) instead increases PE risk (England et al. 2003; Karumanchi and Levine 2010; Wikström et al. 2010). Except for smoking, no strong environmental risk factors have been identified, but evidence suggests a relation between PE and either season of conception or season of birth (Beltran et al. 2013; TePoel et al. 2011).
Elucidating the basis for such seasonal variation in risk could aid in identifying preventable environmental causes, such as low maternal levels of vitamin D or season-dependent dietary factors. Some investigators have focused analysis on variations in weather, usually temperature or rainfall (Tam et al. 2008; TePoel et al. 2011; Tran et al. 2015). Infectious disease could also play a role in some environments: Indirect evidence suggests that the malaria cycle may be related to PE in sub-Saharan Africa (Hlimi 2015), and seasonal variation is characteristic of many other infectious diseases. Some studies have focused on prevailing conditions (or season) at birth, whereas others have focused on prevailing conditions (or season) at conception.
However, previous studies of season and PE have not accounted for the confounding effects of factors that influence both risk of PE and season of conception (and consequently, of birth). Additionally, preterm birth can cut short the time available for developing PE—an effective but biologically uninteresting form of protection. For example, a recent Cochrane review concluded that randomization to vitamin D plus calcium may reduce the risk of PE but that it also increases the rate of preterm birth (De-Regil et al. 2016), thus restricting the time for developing PE. Therefore, to be valid, an analysis of PE must properly account for both confounding and effects on the length of gestation.
Norway provides an ideal setting for studying seasonal effects on reproduction: Its population has universally available modern obstetric care; the country stretches north-south over a vast span of latitudes, and being far from the equator, Norwegians experience seasonal extremes in both temperature and hours of daylight. We used data from the Medical Birth Registry of Norway (see Norwegian Institute of Public Health, https://www.fhi.no/en/hn/health-registries/medical-birth-registry-of-norway/medical-birth-registry-of-norway/) to assess possible effects of season of conception on risk of PE. We also examined possible effect modification of the seasonal pattern by latitude, parity (first vs. later births), sex of the fetus, and smoking. If, for example, a seasonal pattern is seen in nonsmokers but not in smokers, it would suggest that smoking confers protection by somehow interfering with the seasonal factor that produces the seasonal pattern in nonsmokers.
Methods
We analyzed data on births recorded in the Medical Birth Registry of Norway between January 1999 and December 2009. We chose 1999 as the starting year because that was when ultrasound-based dating became widely available throughout Norway and when maternal smoking started being recorded in the birth registry. Ultrasound-based dating permits less recall-dependent and more accurate estimation of the date of conception. We assigned the day of year of conception by subtracting the estimated gestational age at birth (in days) based on ultrasound dating from the day of year of delivery, and adding 14 d. We excluded all multiple births (), and singleton births conceived through in vitro fertilization (). Stillbirths were included in the analysis and censored at the time of birth (as a proxy for the time of death). The main analysis included only pregnancies with complete data on the required covariates.
PE was identified from the birth registry and we included eclampsia or the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) because they also generally present with hypertension and proteinuria. All diagnoses were based on clinical guidelines in use at the time of delivery in Norway. A total of 13,959 of the 356,662 pregnancies (3.5%) included in our complete data analyses were recorded in the registry as being complicated by PE.
To assess the effects of season of conception, we carried out a Cox regression in which we modeled the composite outcome of birth with a diagnosis of PE. The primary time scale was gestational time. Because the timing of birth often depends on medical decisions made after the diagnosis of PE, we would have preferred to model the time to diagnosis of PE, which, however, is not recorded in the birth registry. Nevertheless, the diagnosis is often fairly close to the time of birth, particularly after 34 wk, which is when the majority of cases of PE occur (ACOG 2013).
We began by exploring the association between time of year of conception and our set of potential confounders: smoking status, relationship status (married or living together as if married, vs. not), parity status (nulliparous vs. parous), fetal sex, educational attainment (high school or less, vs. higher), and region. Women were categorized according to whether they a) reported smoking throughout pregnancy, b) reported quitting between the first prenatal visit and delivery, or c) reported not smoking during pregnancy. The few () women who reported smoking at the time of delivery but had said they did not smoke at their first prenatal visit were not numerous enough to support separate consideration and they were categorized with those who smoked throughout pregnancy, under the presumption that they had unsuccessfully tried to quit while pregnant. We defined three regions: North (Nord-Trondelag, Nordland, Tromso, and Finmark counties), West coast (Vest-Agder, Rogaland, Hordaland, Sogn og Fjordane, and More og Romsdal counties), and Southeast (all remaining eligible counties; see https://en.wikipedia.org/wiki/Counties_of_Norway for a map). We did not have data on body mass index or prior PE, resulting in the implicit assumption that those risk factors are not predictive of season of conception.
Any periodic effect of time on an outcome, whether a circadian pattern or a time-of-year pattern, can be modeled using harmonic analysis. Harmonic analysis exploits the fact that a smooth cycling function can be approximated arbitrarily closely by a sum of rescaled sines and cosines. The “first” harmonic is a linear combination of a sine wave and a cosine wave, which approximates a cycling sine function with data-determined amplitude and phase (Hunsberger et al. 2002). When the period of interest is a calendar year, the first harmonic allows for a single peak and valley in each year. The second harmonic is based on doubling all of the angles so that there are instead two peaks and two valleys, allowing for two cycles per year; the third allows for three cycles per year, and so on.
We applied a harmonic Cox model to assess the hazard of delivery with PE. We entered as first harmonic predictors the sine and cosine of the angle corresponding to the day of year of conception [transformed to an angle based on the 365-d year, by replacing day with an angle in radians defined by ]. Similar predictors can be entered for the second harmonic, except that day is replaced by twice , which allows a contribution of a 6-mo cycle. Additional details about harmonic regression are provided elsewhere (Weinberg et al. 2015).
The association between each covariate and time of year of conception was evaluated by treating each covariate in turn as the outcome and time of year of conception as a predictor, using a harmonic logistic or linear regression model and a likelihood ratio statistic to assess the improvement in fit achieved through inclusion of the first harmonic. This approach relies on the fact that if the risk factor for PE is a confounder for assessing seasonality then it should be related to the time of year of conception.
To assess the association between time of year of conception and PE risk, we also used harmonic regression, but with a Cox proportional hazards model for birth with PE, using gestation time as the time scale and adjusting for confounders that were related to both time of year of conception and risk of PE. Although we allowed up to 12 harmonics for day of the year of conception based on a statistical step-up modeling procedure, we never needed to include more than the first harmonic (using to assess the improvement in fit offered by the second harmonic, using likelihood ratio tests). Follow-up for the Cox proportional hazards model was started at 20 wk and censored at the time of birth.
Once the primary model related to time of conception using all the data was identified, we separated the data into groups by region, smoking status, parity status, and sex of the fetus and then fit separate models for seasonal effects. This stratification was done to assess whether the seasonal patterns would replicate across independent data sets, allowing for possible effect modification based on sunlight exposures, differences in diet, proximity to health care, geography, and other factors. We also stratified the time at risk by early versus late time in gestation, defined as (or 34) completed weeks vs. more. Stratified results are displayed graphically as smoothed moving averages of the dichotomous outcome (birth with PE) across days of the year of conception (with a 61-d window, based on from the midpoint) to allow visual (but unadjusted and data-driven) assessment of the seasonal patterns. We show both the unadjusted smoothed moving average of risk across the year, which provides a sense of the raw data, and the model-based–fitted seasonal relative risk, adjusted for confounding and smoothed to be a trigonometric function.
We carried out likelihood ratio tests to assess replicability of the seasonal pattern across regions, three smoking categories, parity, sex of the baby, and preterm birth where the extended model allowed the sine and cosine coefficients to be different across strata.
In the main complete case analysis, we excluded data from two counties that were missing more than 30% of smoking data, that is, Oslo and Troms (). However, the retained counties were also missing smoking data for some 20% of pregnancies. We performed sensitivity analyses by including pregnancies from the excluded counties and all pregnancies with missing maternal smoking data by using multiple imputes by chained equations (MICE) methods. In the MICE procedure [using the R package (version 3.2.2; R Development Core Team)], the three-level smoking variable was multiply imputed based on including the first harmonic sine and cosine terms for the time of year of conception, the occurrence of PE, and the other potential confounders listed in Table 2 as predictors in a polytomous logistic model. We also carried out analyses with MICE imputing all other missing covariates.
Table 2.
Coefficients of confounders, with adjustment for other confounders, season of conception, and duration of pregnancy.
| Variable | Reference category | Coefficient | Standard error | HR | 95% CI |
|---|---|---|---|---|---|
| Smoked then quit | Nonsmoker | 0.03 | 0.91 | (0.85, 0.97) | |
| Smoked throughout or at end of pregnancy | Nonsmoker | 0.03 | 0.61 | (0.57, 0.64) | |
| North | Southeast region | 0.18 | 0.04 | 1.19 | (1.09, 1.30) |
| West coast | Southeast region | 0.03 | 0.96 | (0.90, 1.01) | |
| Years of education between 10 and 14 | Lowest education | 0.03 | 0.99 | (0.94, 1.04) | |
| Years of education | Lowest education | 0.03 | 0.78 | (0.74, 0.83) | |
| Primiparous | Parous | 0.71 | 0.03 | 2.04 | (1.94, 2.15) |
| Mother's age | 0.01 | 0.89 | (0.87, 0.92) | ||
| Mother's age squared | 0.002 | 0.0002 | 1.00 | (1.00, 1.00) | |
| Married or living together as married | Not married or living together | 0.01 | 0.03 | 1.01 | (0.95, 1.07) |
| Interaction between primiparity and north | 0.06 | 0.91 | (0.82, 1.02) | ||
| Interaction between primiparity and west coast | 0.06 | 0.04 | 1.06 | (0.99, 1.14) |
In additional sensitivity analyses, we excluded pregnancies to mothers born outside of Norway (in case their conception timing, recall of last menstrual period, or use of medical services might be systematically different), and we carried out analyses based on time of birth instead of time of conception (to assess whether one had a stronger relationship to PE than the other).
Results
Table 1 shows the characteristics of the pregnancies included in the analysis, stratified by PE and preterm birth. As expected, a much higher fraction of first pregnancies and births to nonsmokers were complicated by PE.
Table 1.
Characteristics of pregnancies excluding births in Oslo and Troms counties.
| Characteristic | Category | No PE | PE and gestation | PE and gestation |
|---|---|---|---|---|
| Total N | 460,534 | 14,044 | 4,383 | |
| Average maternal age (y) | 29.2 | 28.6 | 29.1 | |
| Sex of baby [ (%)] | Male | 236,400 (51.3) | 7,364 (52.4) | 2,120 (48.4) |
| Female | 224,097 (48.7) | 6,680 (47.6) | 2,261 (51.6) | |
| Missing | 37 (00.0) | 0 (00.0) | 2 (0.00) | |
| Region[ (%)] | Southeast | 236,933 (51.4) | 7,115 (50.7) | 2,384 (54.4) |
| North | 49,585 (10.8) | 1,638 (11.7) | 505 (11.5) | |
| West coast | 173,420 (37.7) | 5,274 (37.6) | 1,485 (33.9) | |
| Missing | 596 (0.1) | 17 (0.1) | 9 (0.2) | |
| Maternal smoking [ (%)] | Nonsmoker | 296,552 (64.4) | 9,479 (67.5) | 2,861 (65.3) |
| Quit during pregnancy | 21,485 (4.7) | 827 (5.9) | 210 (4.8) | |
| Smoked throughout | 44,389 (9.6) | 872 (6.2) | 299 (6.8) | |
| Missing | 98,108 (21.3) | 2,866 (20.4) | 1,013 (23.1) | |
| Mother’s education [ (%)] | 0 – 9 y | 77,481 (16.8) | 2,430 (17.3) | 811 (18.5) |
| 10 – 14 y | 160,971 (35) | 5,356 (38.1) | 1,676 (38.2) | |
| 197,465 (42.9) | 5,741 (40.9) | 1,707 (38.9) | ||
| Missing | 24,617 (5.3) | 517 (3.7) | 189 (4.3) | |
| Parity [ (%)] | Parous | 256,674 (55.7) | 5,214 (37.1) | 1,623 (37) |
| Primiparous | 203,860 (44.3) | 8,830 (62.9) | 2,760 (63) | |
| Mother's birth country [ (%)] | Other | 79,116 (17.2) | 1,824 (13) | 688 (15.7) |
| Norway | 379,183 (82.3) | 12,190 (86.8) | 3,608 (82.3) | |
| Missing | 2,235 (0.5) | 30 (0.2) | 87 (2) | |
| Married or living together as married [ (%)] | No | 36,012 (7.8) | 1,215 (8.7) | 394 (9) |
| Yes | 424,522 (92.2) | 12,829 (91.3) | 3,989 (91) |
The corresponding characteristics for the two excluded counties, Oslo and Troms, can be found in Table S1. Table S2 shows results of tests of associations between covariates and season of conception. Based on harmonic logistic regression including the irst harmonic, the time of year of conception was highly predictive of maternal smoking status. The peak probability of being a smoker was among conceptions that occurred in May. Smoking status clearly has the potential to confound an assessment of seasonality of risk of PE. The other covariates that were related to seasonality of conception () were relationship status, maternal age, primiparity, education, and region (see Table S2). Sex of the fetus was not related to season of conception.
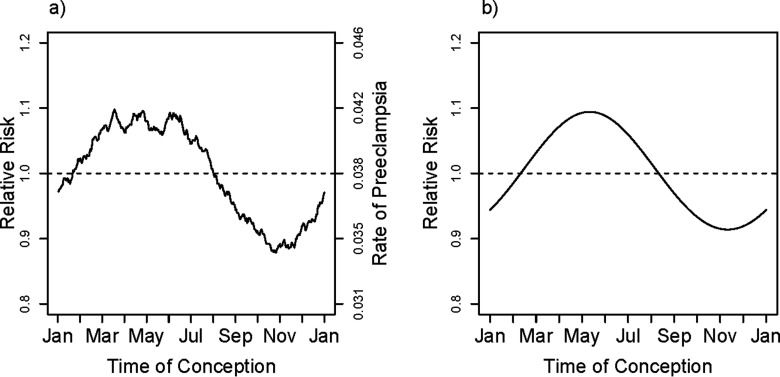
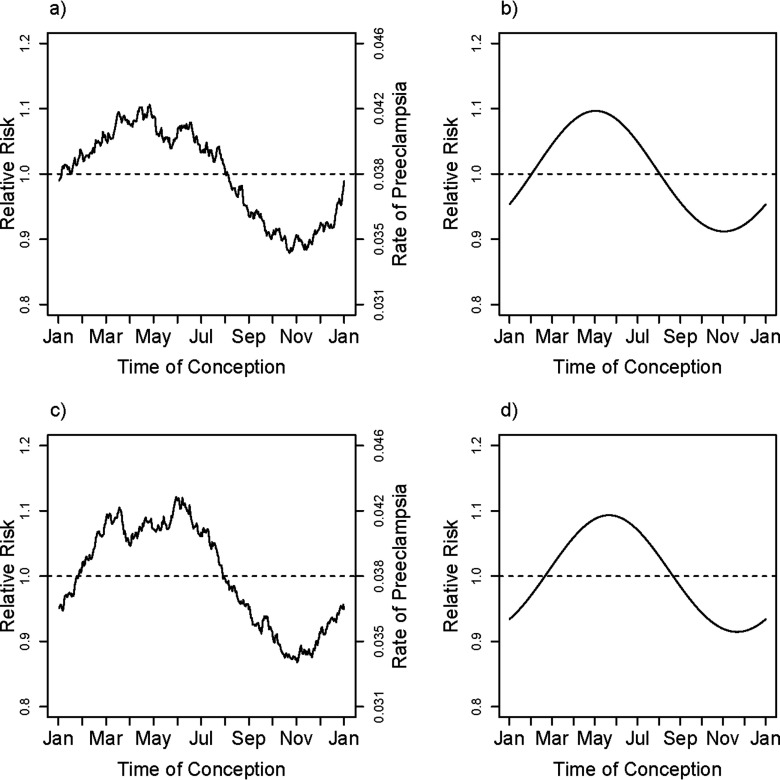
The smoothed observed rates of PE in relation to time of year of conception based on a moving average (averaged over a window) are shown in Figure 1a. Even in the raw data, with no allowance for confounding effects, the seasonality of risk is evident. Based on the multivariate model, there was a relation between the hazard of birth with PE and the time of year of conception, with inclusion of the first harmonic (). Thus, the data are inconsistent with constancy of risk across the times of year of conception, even after accounting for the mix of risk factors that is differential across seasons of conception. The fitted curve shown in Figure 1b is derived from the first harmonic for season; the displayed curve was computed by exponentiating the logarithm of the fitted adjusted hazard ratio at mean values of the covariates (entered as means of corresponding dummies for categorical variables): education, region, maternal age, maternal age squared, marital status, maternal smoking, primiparity, and interaction between region and primiparity (included because it significantly improved the fit of the model using a likelihood ratio test with ). The estimated relative hazard for birth with PE was highest for spring conceptions and lowest for fall conceptions. Inclusion of additional harmonics beyond the first did not measurably improve the fit ( for the second harmonic). The estimated amplitude (maximum risk in the spring relative to minimum risk in the fall) associated with seasonal variation in risk was about 20%.
Figure 1.
Overall unadjusted smoothed rate and fitted first harmonic adjusted relative rate of PE. The smoothed curve in (a) is based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with PE among pregnancies conceived at each day of the year. The fitted relative rate shown in (b) is based on the first harmonic (sine and cosine) fit to a model that is adjusted for maternal age, maternal age squared, education, primiparity, smoking (in three categories), marital status, region of Norway, and interaction between primiparity and region. The curve shown is based on entering the mean for all covariates, centering the estimated log hazard ratio and then exponentiating it.
Based on this model, which includes harmonic adjustment for time of year of conception and Cox model adjustment for duration of pregnancy, the estimated hazard ratios for the confounders included in the model, mutually adjusted for other factors in the table, are shown in Table 2. Although Westreich and Greenland (2013) cautioned against “the Table 2 fallacy,” the selection of confounders appropriate for each of these factors is reasonable except for the inclusion of time of year of conception, which cannot plausibly be a cause of any of them. Nonetheless, omitting time of year of conception yields results that are nearly identical to those shown (see Table S3). One interesting observation was that women who quit smoking during pregnancy had a PE risk that was nearly as high as that for nonsmokers.
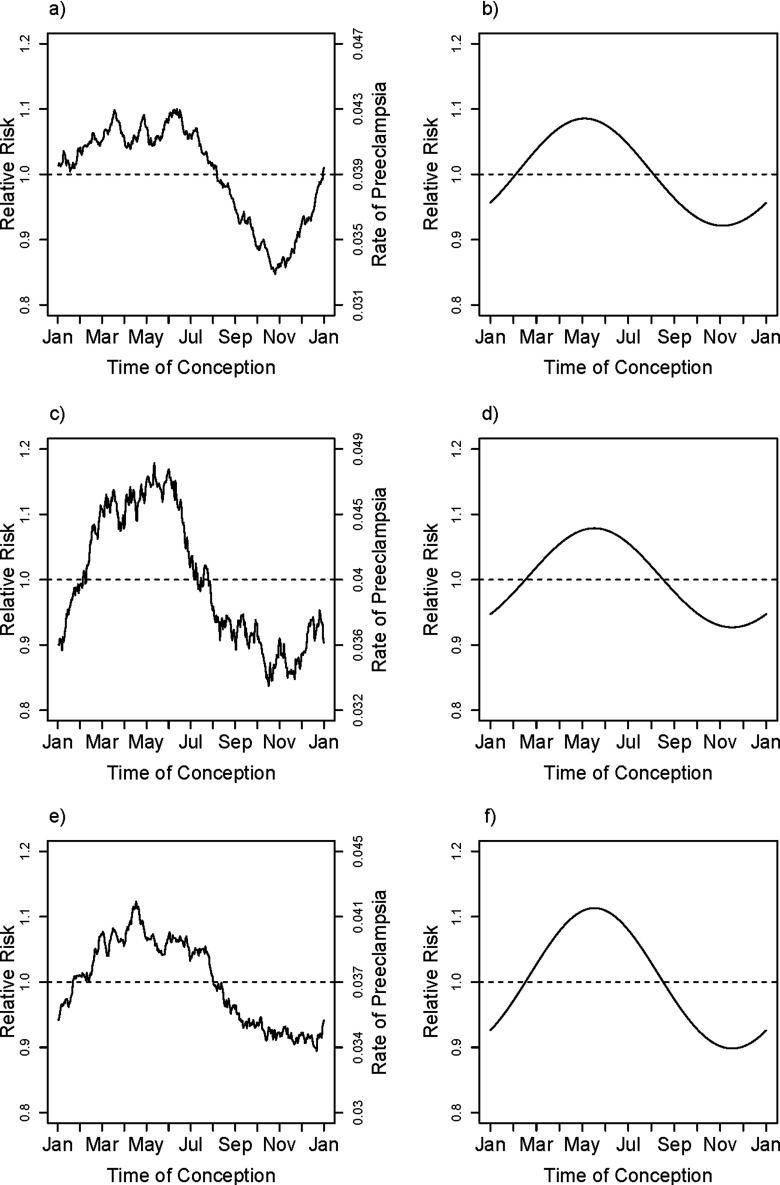
The season results were replicable across the three regions, as shown in Figure 2a,c,e, which shows the moving averages for the smoothed observed rates. The three independent relative hazard model fits for the three geographic regions are shown in Figure 2b,d,f, with the fits (again) each based on including the region-specific means for the covariates. There was little statistical evidence for heterogeneity in the seasonal patterns across regions (, based on a 4 degree-of-freedom test). The risk of PE for babies conceived in early May was estimated to be about 20–25% higher than that for those conceived in early November.
Figure 2.
fRegion-specific fitted adjusted relative rates and unadjusted smoothed rates of PE for Southeast (a,b), North (c,d) and West Coastal (e,f) regions of Norway.The smoothed curve in (a), (c), and (e) are based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with fPE among pregnancies conceived at each day of the year and within each of the three regions of Norway, Southeast (a), North (c), and West Coast (e) (see text). The fitted relative rates shown in (b), (d), and (f) are based on the first harmonic (sine and cosine) fit to models that are adjusted for covariates maternal age, maternal age squared, education, primiparity, smoking (in three categories), and marital status and within each of the three regions of Norway. The curve shown is based on entering the region-specific mean for all covariates, centering the festimated log hazard ratio and then exponentiating it.
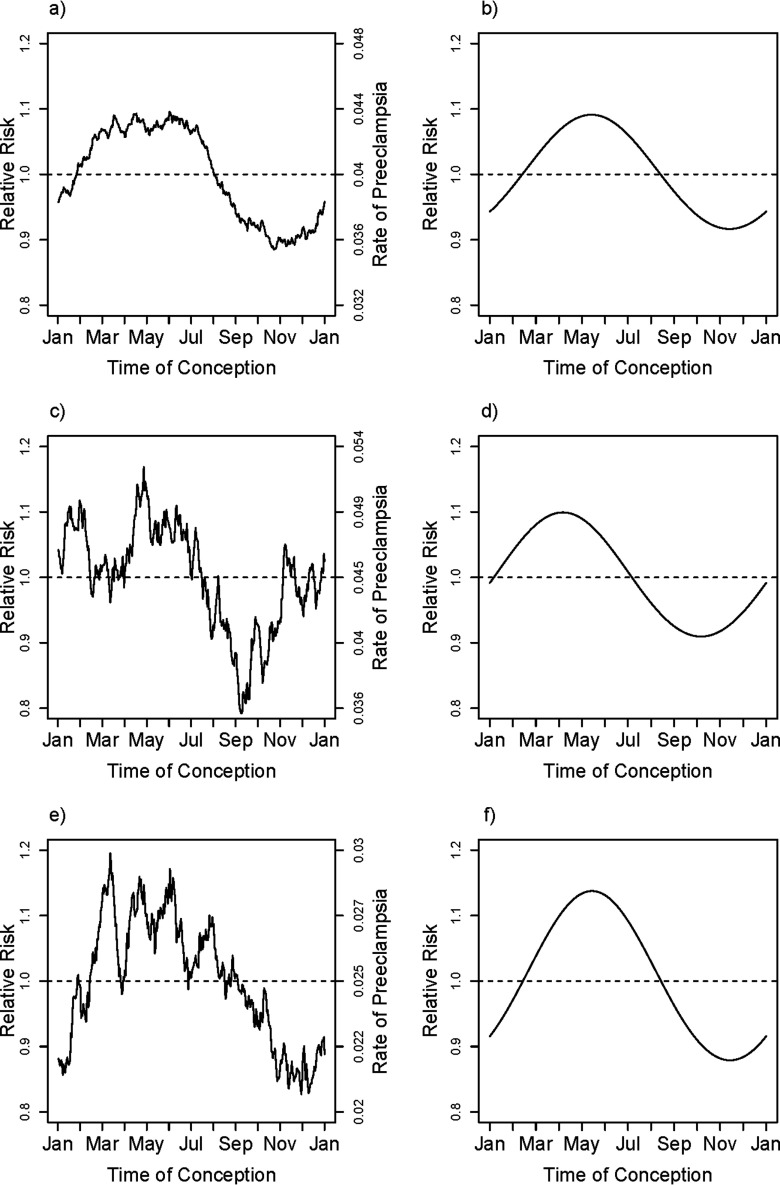
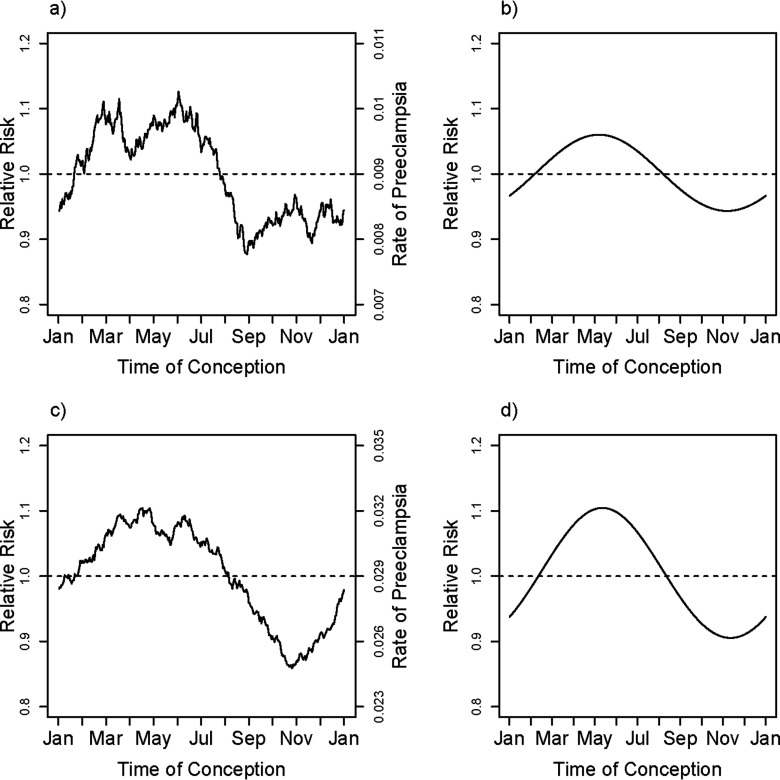
The overall estimated relative risk for smoking throughout pregnancy was 0.61 [95% confidence interval (CI): 0.57, 0.65], based on Table S3. To the extent that smoking may exert its protective effect by interfering with the mechanisms that cause higher risk for spring conceptions, one would expect to see attenuation of the amplitude of seasonal variation in risk in smokers. Separate unadjusted but smoothed rates of PE based on season of conception for the three smoking-based categories of mothers are shown in Figure 3a,c,e. Very similar seasonal patterns were evident in the three categories of smoking, based on separate and independent adjusted harmonic fits shown in Figure 3b,d,f, again using mean values of the covariates for each of the three categories ( for the test of interaction between season and smoking). Also, note that the relative amplitude of seasonal variation in risk was just as large in women who smoked throughout pregnancy, despite their substantially lower overall risk. Although quitters appear in the graph to have higher risk than the nonsmokers (note the contrasting midlines for the absolute rates), that profile may partially be explained by the fact that a much larger proportion of them are having their first pregnancy (62% vs. 44%); the estimated adjusted effect of quitting compared with nonsmokers was 0.91 (95% CI: 0.85, 0.97). The estimated adjusted effect for quitters compared with continuing smokers was 1.50 (95% CI: 1.38, 1.63).
Figure 3.
Smoking-behavior–specific unadjusted smoothed rates and fitted adjusted relative rates of PE for nonsmokers (a,b), quitters (c,d), and consistent smokers (e,f) in Norway. The smoothed curve in (a), (c), and (e) is based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with PE among pregnancies conceived at each day of the year within the three groups of smoking. The fitted relative rates shown in (b), (d), and (f) are based on the first harmonic (sine and cosine) fit separately to the three smoking categories, nonsmokers (a) and (b), women who had initially smoked but had quit by the time of delivery (c) and (d), and women who smoked throughout the pregnancy (e) and (f) with models that are adjusted for the covariates maternal age, maternal age squared, education, primiparity, marital status, region (in three categories) and interaction between primiparity and region. The curve shown is based on entering the region-specific mean for all covariates, centering the estimated log hazard ratio and then exponentiating it. Note that the absolute rates have different midlines for the three categories.
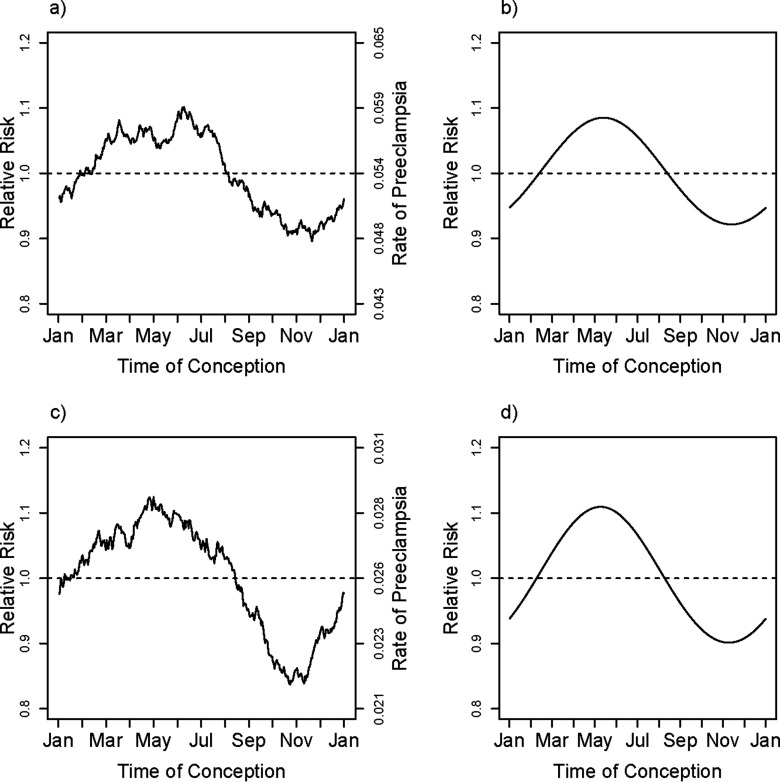
Results were also similar across the two categories of parity (Figure 4) and did not depend on sex of the fetus (Figure 5). By contrast, length of gestation did seem to make a difference. We carried out an analysis based on restricting to gestational time completed wk, implying diagnosis of PE at . There were 1,817 pregnancies with PE delivering before 34 wk and 16,610 delivering after 34 wk, and early deliveries showed little evidence for seasonal effects of time of conception ( for the first harmonic). However, the position of the estimated peak risk was similar to that for later-delivered pregnancies (see Figure S1). Comparable analyses based on the first 37 wk of gestation showed some evidence for a seasonal effect ( for the first harmonic) (fit shown in Figure 6).
Figure 4.
Parity-specific unadjusted smoothed rates and fitted adjusted relative rates of PE for primiparous (a,b) and multiparous (c,d) pregnancies in Norway. The smoothed curve in (a), and (c) is based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with PE among pregnancies conceived at each day of the year. The fitted relative rates shown in (a), and (c) are based on the first harmonic (sine and cosine) fit separately to each of the two parity categories, primiparous (b), and parous (d), with models that are adjusted for the covariates maternal age, maternal age squared, education, smoking (in three categories), marital status, and region (in three categories). The curve shown is based on entering the parity-specific mean for all covariates, centering the estimated log hazard ratio and then exponentiating it.
Figure 5.
Unadjusted smoothed rates and fitted adjusted relative rates of PE for boy births (a,b) and girl births (c,d) in Norway. The smoothed curve in (a), and (c) is based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with PE among pregnancies conceived at each day of the year. The fitted relative rates shown in (b), and (d) are based on the first harmonic (sine and cosine) fit separately to each of the fetal sex categories, with models that are adjusted for the covariates maternal age, maternal age squared, education, primiparity, smoking (in three categories), marital status, region (in three categories), and interaction between primiparity and region. The curve shown is based on entering the fetal-sex–specific mean for all covariates, centering the estimated log hazard ratio and then exponentiating it.
Figure 6.
Unadjusted smoothed rates and fitted adjusted relative rates of PE for birth before and birth after 37 wk gestation in Norway. The smoothed curve in (a), and (c) is based on a moving average of outcomes that uses a window encompassing from each day of the year, thus estimating the fraction with PE among pregnancies conceived at each day of the year. The fitted relative rates shown in (b), and (d) are based on the first harmonic (sine and cosine) fit separately to each of the two gestational ages, gestational , and gestational , with models that are adjusted for the covariates maternal age, maternal age squared, education, primiparity, smoking (in three categories), marital status, region (in three categories) and interaction between primiparity and region. The curve shown is based on entering the preterm-category–specific mean for all covariates, centering the estimated log hazard ratio and then exponentiating it.
In a final sensitivity analysis, we restricted analysis to pregnancies conceived by women who had been born in Norway (82.9%), which yielded virtually the same results as the main analysis (see Figure S2).
The sensitivity analysis carried out for the main model using multiple imputation with chained equations to handle the missing smoking data and without excluding counties showed results that were consistent with the findings already described, but with slightly smaller standard errors for the seasonal parameters. The peak risk and the relative risk for spring compared with fall conceptions changed very little. See Figure S3 for a revision of Figure 1b based on MICE.
Discussion
The risk of PE in Norway is related to season of conception, with an estimated amplitude of approximately 20%. We adjusted for confounding by factors related to the timing of conception, and for pregnancy duration by using a Cox model. With adjustment for confounding, the highest rates were in mothers conceiving in May, and the lowest in those conceiving in November. This pattern replicated across different categories of the population. For example, the pattern recurred in the three regions of Norway, despite the northern region being less urban and more subject to extremes in temperature and in the light/dark cycle, due to its high latitude. The seasonal pattern was also similar in women having their first baby and women who were parous. Finally, it was repeated across the three categories of cigarette smoking. The replicability of the pattern, with its peak in May and nadir in November, argues against this being a chance finding, consistent with the statistical test of uniformity of risk throughout the year ().
Another possible source of spurious seasonal effects has to do with preferred timing for conception, which could induce a correlation between fecundability (and its correlates) and timing (Basso et al. 1995). However, the fact that the seasonal pattern we see is repeated across subpopulations with very different preferred times to conceive argues against the observed seasonal pattern being produced by a seasonal fecundability artifact.
Because timing of conception determines the timing of all subsequent stages of pregnancy, we cannot infer from our analyses that the relevant risk factor is necessarily a seasonal exposure early in pregnancy rather than one that occurs at some later stage of gestation. Because others have found time of conception to be more predictive than time of birth (Phillips et al. 2004), we carried out a harmonic logistic regression sensitivity analysis of the Norwegian data, adjusting for the same covariates but considering the timing of birth (rather than conception) as predictor. The estimated pattern (as well as the p-value) was very similar, although shifted forward by the gestational duration of 8–9 mo, with peak risk seen for birth in the coldest months of the year and the lowest risk seen for births in the summer (see Figure S4).
A further concern is that PE is associated with small-for-gestational-age birth weight (Odegård et al. 2000), an observation that presumably signifies effects of PE on fetal growth. Because our clinical estimates for date of conception rely on ultrasound measures, they could tend to systematically understate the age of slow-growing fetuses. Such systematic bias would tend to shift the identified seasonal pattern forward. Consequently, the peak and nadir seasons for conception of PE pregnancies may be slightly earlier than what we report. However, the proportion of pregnancies where the last menstrual period-based dating differed from the U.S.-based dating by 2 wk or more was similar for PE (0.10) and non-PE (0.09) pregnancies, suggesting that differential growth in early pregnancy is not an important source of bias.
We cannot be sure that the seasonal driver in Norway would be the same as that in other populations, and thus the pattern we see may not replicate in other cultures and climates. Nevertheless, it does suggest the existence of an environmental contributor to the etiology of PE, which happens to vary seasonally in Norway.
There is growing evidence that women destined to be diagnosed with PE have lower levels of vitamin D in their first trimester of pregnancy (Achkar et al. 2015; Bodnar et al. 2007). A recent study of primiparous women found lower risk in those taking vitamin D supplements during pregnancy (Haugen et al. 2009). A randomized trial did not identify a reduced risk for PE in women taking vitamin D supplementation (Mirzakhani et al. 2016). However, in a corresponding nested case-cohort analysis, they found lower risk of PE in women with vitamin D levels of or higher at trial entry and in late pregnancy (Mirzakhani et al. 2016). There is also evidence of a seasonal pattern in vitamin D levels in Norway, with the highest levels typically in summer or fall (Brustad et al. 2007; Degerud et al. 2016; Godang et al. 2014; Johnson et al. 2012). Unfortunately, we do not have data on vitamin D. However, the seasonal patterns were similar across extremes of latitude, suggesting that ambient UV radiation, and its presumed effect on vitamin D, may not be a major contributor.
A recently published extensive review and meta-analysis (Beltran et al. 2013) considered hypertensive disorders of pregnancy in relation to season and weather patterns. Seasonal changes bring very different weather patterns in different parts of the world and some studies have assessed time of year, whereas others have considered location-specific meteorological correlates, such as temperature, humidity, precipitation, or wind speed. Also, some have focused on the time of conception, whereas others considered the time of birth. These differences, together with the fact that neither maternal smoking nor other confounders are adjusted for in those analyses, make the evidence for seasonal influences on PE difficult to harmonize and interpret. An earlier systematic review (TePoel et al. 2011) found increased risk associated with deliveries in the coldest part of the year, which is somewhat consistent with the pattern we see for Norway. An earlier report based on data from the Medical Birth Registry of Norway (Magnus and Eskild 2001) found the lowest rate of PE in births occurring in August (conceptions predominantly in autumn), which is broadly consistent with what we find with confounder-adjusted analysis of the more recent data. Beltran et al., in their recent meta-analysis (Beltran et al. 2013), found an overall positive association between temperatures at the time of conception and risk. They speculate that “exposure to heat during the first trimester of pregnancy” could be important. Our data do not seem to support a strong role for heat exposure: The peak we see is equally evident in the north of Norway, where summers are not hot. (The highest daily average temperature in Hammerfest in Finnmark County, Norway in July was , whereas that for Oslo is .)
Whatever the seasonally acting risk factor may be, its effect in Norway seems to be very similar in smokers and nonsmokers, based on the locations of the peak and nadir and the amplitude of the estimated relative effects. The apparent absence of effect modification by smoking, parity and region suggests that the differences in risk associated with those predictors are due to factors that are unrelated to season. The fact that the amplitude of the effect does not seem to be higher in the northern part of Norway suggests that the changes in the light-dark cycle and consequent effects on circadian rhythms are also not central to the seasonal effect. Research to identify the factor that drives those seasonal variations could help us devise strategies for reducing risk.
Supplemental Material
Acknowledgments
We thank D. Baird and K. Upson for helpful comments on the paper.
This work was supported by the Norwegian Research Council and in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, at the National Institutes of Health, under project Z01 AES 040006-19.
References
- Achkar M, Dodds L, Giguère Y, Forest JC, Armson BA, Woolcott C, et al. 2015. Vitamin D status in early pregnancy and risk of preeclampsia. Am J Obstet Gynecol 212:511.e1–511.e7, PMID: 25446694, 10.1016/j.ajog.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG (American College of Obstetricians and Gynecologists). 2002. ACOG practice bulletin no. 33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 99(1):159–167, PMID: 16175681, http://journals.lww.com/greenjournal/Fulltext/2002/01000/ACOG_Practice_Bulletin_No__33__Diagnosis_and.28.aspx. [DOI] [PubMed] [Google Scholar]
- ACOG. 2013. Hypertension in pregnancy: Executive summary. Obstet Gynecol 122(5):1122–1131, PMID: 24150027, http://journals.lww.com/greenjournal/Fulltext/2013/11000/Hypertension_in_Pregnancy___Executive_Summary.36.aspx. [DOI] [PubMed] [Google Scholar]
- Basso O, Christensen K, Olsen J. 2001. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology 12:624–629, PMID: 11679788, https://insights.ovid.com/pubmed?pmid=11679788. [DOI] [PubMed] [Google Scholar]
- Basso O, Olsen J, Bisanti L, Juul S, Boldsen J. 1995. Are seasonal preferences in pregnancy planning a source of bias in studies of seasonal variation in reproductive outcomes? The European Study Group on Infertility and Subfecundity. Epidemiology 6(5):520–524, PMID: 8562629, http://www.jstor.org/stable/3702124. [DOI] [PubMed] [Google Scholar]
- Basso O, Weinberg CR, Baird DD, Wilcox AJ, Olsen J, Danish National Birth Cohort. 2003. Subfecundity as a correlate of preeclampsia: A study within the Danish National Birth Cohort. Am J Epidemiol 157:195–202, PMID: 12543618, 10.1093/aje/kwf194. [DOI] [PubMed] [Google Scholar]
- Basso O, Wilcox AJ, Weinberg CR, Baird DD, Olsen J. 2004. Height and risk of severe pre-eclampsia. A study within the Danish National Birth Cohort. Int J Epidemiol 33:858–863, PMID: 15155701, 10.1093/ije/dyh116. [DOI] [PubMed] [Google Scholar]
- Beltran AJ, Wu J, Laurent O. 2013. Associations of meteorology with adverse pregnancy outcomes: A systematic review of preeclampsia, preterm birth and birth weight. Int J Environ Res Public Health 11:91–172, PMID: 24362545, 10.3390/ijerph110100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. 2007. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522, PMID: 17535985, 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd HA, Tahir H, Wohlfahrt J, Melbye M. 2013. Associations of personal and family preeclampsia history with the risk of early-, intermediate- and late-onset preeclampsia. Am J Epidemiol 178:1611–1619, PMID: 24049162, 10.1093/aje/kwt189. [DOI] [PubMed] [Google Scholar]
- Brustad M, Edvardsen K, Wilsgaard T, Engelsen O, Aksnes L, Lund E. 2007. Seasonality of UV-radiation and vitamin D status at 69 degrees north. Photochem Photobiol Sci 6:903–908, PMID: 17668121, 10.1039/b702947k. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Althabe F, Belizán JM, Kafury-Goeta AC. 1999. Cigarette smoking during pregnancy and risk of preeclampsia: A systematic review. Am J Obstet Gynecol 181:1026–1035, PMID: 10521771, 10.1016/S0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- Dávila RD, Julian CG, Browne VA, Toledo-Jaldin L, Wilson MJ, Rodriguez A, et al. 2012. Role of cytokines in altitude-associated preeclampsia. Pregnancy Hypertens 2:65–70, PMID: 22247821, 10.1016/j.preghy.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degerud E, Hoff R, Nygård O, Strand E, Nilsen DW, Nordrehaug JE, et al. 2016. Cosinor modelling of seasonal variation in 25-hydroxyvitamin D concentrations in cardiovascular patients in Norway. Eur J Clin Nutr 70:517–522, PMID: 26603883, 10.1038/ejcn.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. 2016. Vitamin D supplementation for women during pregnancy. Sao Paulo Med J 134:274–275, PMID: 27355803, 10.1590/1516-3180.20161343T2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Scher E, Wallenstein S, Savitz DA, Alsaker ER, Trogstad L, et al. 2013. Maternal active and passive smoking and hypertensive disorders of pregnancy: Risk with trimester-specific exposures. Epidemiology 24:379–386, PMID: 23429405, 10.1097/EDE.0b013e3182873a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Levine RJ, Mills JL, Klebanoff MA, Yu KF, Cnattingius S. 2003. Adverse pregnancy outcomes in snuff users. Am J Obstet Gynecol 189:939–943, PMID: 14586330, 10.1067/S0002-9378(03)00661-6. [DOI] [PubMed] [Google Scholar]
- Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, et al. 2001. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med 344:867–872, PMID: 11259719, 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- Godang K, Froslie KF, Henriksen T, Qvigstad E, Bollerslev J. 2014. Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. Eur J Endocrinol 170:609–617, PMID: 24451081, 10.1530/EJE-13-0842. [DOI] [PubMed] [Google Scholar]
- Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, et al. 2009. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 20:720–726, PMID: 19451820, 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Toh S, Cnattingius S. 2009. Risk of pre-eclampsia in first and subsequent pregnancies: Prospective cohort study. BMJ 338:b2255, PMID: 19541696, 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlimi T. 2015. Association of anemia, pre-eclampsia and eclampsia with seasonality: A realist systematic review. Health Place 31:180–192, PMID: 25555235, 10.1016/j.healthplace.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Hunsberger S, Albert PS, Follmann DA, Suh E. 2002. Parametric and semiparametric approaches to testing for seasonal trend in serial count data. Biostatistics 3:289–298, PMID: 12933619, 10.1093/biostatistics/3.2.289. [DOI] [PubMed] [Google Scholar]
- Huppertz B. 2008. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 51:970–975, PMID: 18259009, 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- Johnson LK, Hofsø D, Aasheim ET, Tanbo T, Holven KB, Andersen LF, et al. 2012. Impact of gender on vitamin D deficiency in morbidly obese patients: A cross-sectional study. Eur J Clin Nutr 66:83–90, PMID: 21792214, 10.1038/ejcn.2011.140. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Levine RJ. 2010. How does smoking reduce the risk of preeclampsia? Hypertension 55:1100–1101, PMID: 20231524, 10.1161/HYPERTENSIONAHA.109.148973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, et al. 2005. Urinary placental growth factor and risk of preeclampsia. JAMA 293:77–85, PMID: 15632339, 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- Magnus P, Eskild A. 2001. Seasonal variation in the occurrence of pre-eclampsia. BJOG 108:1116–1119, PMID: 11762648, 10.1111/j.1471-0528.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- Mirzakhani H, Litonjua AA, McElrath TF, O’Connor G, Lee-Parritz A, Iverson R, et al. 2016. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest 126:4702–4715, PMID: 27841759, 10.1172/JCI89031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegård RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. 2000. Preeclampsia and fetal growth. Obstet Gynecol 96:950–955, PMID: 11084184, http://journals.lww.com/greenjournal/Fulltext/2001/04000/PREECLAMPSIA_AND_FETAL_GROWTH.31.aspx. [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. 2015. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 21(5):575–592, PMID: 26117684, 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- Phillips JK, Bernstein IM, Mongeon JA, Badger GJ. 2004. Seasonal variation in preeclampsia based on timing of conception. Obstet Gynecol 104:1015–1020, PMID: 15516394, 10.1097/01.AOG.0000143306.88438.cf. [DOI] [PubMed] [Google Scholar]
- Powe CE, Levine RJ, Karumanchi SA. 2011. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123:2856–2869, PMID: 21690502, 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjærven R, Wilcox AJ, Lie RT. 2002. The interval between pregnancies and the risk of preeclampsia. N Engl J Med 346:33–38, PMID: 11778000, 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- Skjærven R, Vatten LJ, Wilcox AJ, Rønning T, Irgens LM, Lie RT. 2005. Recurrence of pre-eclampsia across generations: Exploring fetal and maternal genetic components in a population based cohort. BMJ 331:877, PMID: 16169871, 10.1136/bmj.38555.462685.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WH, Sahota DS, Lau TK, Li CY, Fung TY. 2008. Seasonal variation in pre-eclamptic rate and its association with the ambient temperature and humidity in early pregnancy. Gynecol Obstet Invest 66:22–26, PMID: 18230912, 10.1159/000114252. [DOI] [PubMed] [Google Scholar]
- TePoel MR, Saftlas AF, Wallis AB. 2011. Association of seasonality with hypertension in pregnancy: A systematic review. J Reprod Immunol 89:140–152, PMID: 21513987, 10.1016/j.jri.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. 1999. High body mass index and hypercholesterolemia: Risk of hypertensive disorders of pregnancy. Obstet Gynecol 94:543–550, PMID: 10511356, http://journals.lww.com/greenjournal/Fulltext/1999/10000/High_Body_Mass_Index_and_Hypercholesterolemia_.11.aspx. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. 2004. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 89:770–775, PMID: 14764795, 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- Tran TC, Boumendil A, Bussieres L, Lebreton E, Ropers J, Rozenberg P, et al. 2015. Are meteorological conditions within the first trimester of pregnancy associated with the risk of severe pre-eclampsia? Paediatr Perinat Epidemiol 29:261–270, PMID: 26053449, 10.1111/ppe.12196. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Shi M, DeRoo LA, Basso O, Skjærven R. 2015. Season and preterm birth in Norway: A cautionary tale. Int J Epidemiol 44(3):1068–1078, PMID: 26045507, 10.1093/ije/dyv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westreich D, Greenland S. 2013. The table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 177:292–298, PMID: 23371353, 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström AK, Stephansson O, Cnattingius S. 2010. Tobacco use during pregnancy and preeclampsia risk: Effects of cigarette smoking and snuff. Hypertension 55:1254–1259, PMID: 20231527, 10.1161/HYPERTENSIONAHA.109.147082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.