Abstract
AIM
To determine whether fructo-oligosaccharide (FOS) affects visceral sensitivity, inflammation, and production of intestinal short-chain fatty acids (SCFA) in an irritable bowel syndrome (IBS) mouse model.
METHODS
Mice were randomly assigned to daily oral gavage of saline solution with or without FOS (8 g/kg body weight) for 14 d. Mice were further assigned to receive either daily one-hour water avoidance stress (WAS) or sham-WAS for the first 10 d. After 2 wk, visceral sensitivity was measured by abdominal withdrawal reflex in response to colorectal distension and mucosal inflammation was evaluated. Gas chromatography, real-time reverse transcription PCR, and immunohistochemistry assays were used to quantify cecal concentrations of SCFA, intestinal cytokine expression, and number of intestinal mast cells per high-power field (HPF), respectively.
RESULTS
Mice subjected to WAS exhibited visceral hypersensitivity and low-grade inflammation. Among mice subjected to WAS, FOS increased visceral hypersensitivity and led to higher cecal concentrations of acetic acid (2.49 ± 0.63 mmol/L vs 1.49 ± 0.72 mmol/L, P < 0.05), propionic acid (0.48 ± 0.09 mmol/L vs 0.36 ± 0.05 mmol/L, P < 0.01), butyric acid (0.28 ± 0.09 mmol/L vs 0.19 ± 0.003 mmol/L, P < 0.05), as well as total SCFA (3.62 ± 0.87 mmol/L vs 2.27 ± 0.75 mmol/L, P < 0.01) compared to saline administration. FOS also increased ileal interleukin (IL)-23 mRNA (4.71 ± 4.16 vs 1.00 ± 0.99, P < 0.05) and colonic IL-1β mRNA (2.15 ± 1.68 vs 0.88 ± 0.53, P < 0.05) expressions as well as increased mean mast cell counts in the ileum (12.3 ± 2.6 per HPF vs 8.3 ± 3.6 per HPF, P < 0.05) and colon (6.3 ± 3.2 per HPF vs 3.4 ± 1.2 per HPF, P < 0.05) compared to saline administration in mice subjected to WAS. No difference in visceral sensitivity, intestinal inflammation, or cecal SCFA levels was detected with or without FOS administration in mice subjected to sham-WAS.
CONCLUSION
FOS administration intensifies visceral hypersensitivity and gut inflammation in stress-induced IBS mice, but not in the control mice, and is also associated with increased intestinal SCFA production.
Keywords: Fructo-oligosaccharide, Stress, Irritable bowel syndrome, Visceral hypersensitivity, Intestinal inflammation, Short chain fatty acids, FODMAP
Core tip: Fructo-oligosaccharide is a component of Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP), which has been associated with triggering symptoms in patients with irritable bowel syndrome (IBS). In a stress-induced IBS mouse model, daily fructo-oligosaccharide (FOS) administration further intensified visceral hypersensitivity and low-grade intestinal inflammation compared to saline. FOS administration also led to increased intestinal production of individual and total short-chain fatty acids (SCFA) in mice subjected to stress. However, no difference in visceral sensitivity, intestinal inflammation, or cecal concentrations of SCFA was observed among sham-stressed mice receiving FOS or saline. Our findings suggest a mechanism of FODMAP-induced gastrointestinal symptoms associated with increased production of SCFA specific to IBS.
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by chronic abdominal pain associated with changes in bowel habit and frequency affecting more than a tenth of the general population[1,2]. Many factors contribute to the development of IBS, including altered visceral pain perception, low-grade intestinal inflammation, change in microbiota, and psychosocial factors[3]. The complex pathophysiology of IBS has posed challenges to developing effective interventions, and therapeutic gains with conventional pharmacologic therapies have been marginal at less than 15%[4].
Importance of dietary factors in triggering symptoms is increasingly being recognized in patients with IBS. Specifically, poorly absorbed, fermentable carbohydrates categorized as Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) have been studied closely[5]. Consumption of food high in FODMAP content triggers abdominal pain, bloating, and flatulence in patients with IBS. Furthermore, several randomized trials have demonstrated that low FODMAP diet reduces gastrointestinal symptoms in patients with IBS[6-9]. Although largely unexplored, the accumulation of intestinal fluid from osmotic load of poorly digested carbohydrates and excessive colonic gas production associated with ingestion of FODMAP have been proposed as a mechanism for development of gastrointestinal symptoms[10,11]. Intestinal dysmotility, visceral hypersensitivity, altered microbiota, and change in metabolic output also likely contribute to the pathophysiology of gastrointestinal symptoms associated with ingestion of FODMAP in IBS patients[11-13]. In addition, the production of short-chain fatty acids (SCFA), such as acetic, propionic, and butyric acids, may also be important in the development of symptoms in IBS[14].
Fructo-oligosaccharide (FOS) is one of the most frequently consumed FODMAP components in the general diet. The aim of our study was to investigate the effects of FOS on visceral sensitivity, intestinal SCFA production, and intestinal inflammation in a stress-induced IBS mouse model. Water avoidance stress (WAS) was utilized to simulate psychological stress in IBS, and a WAS mouse model was used to evaluate the effects of FOS administration on visceral hypersensitivity and intestinal immune activation[15]. Individual (acetic, propionic, and butyric acids) as well as total SCFA concentrations were also quantified in the cecum to determine the effects of FOS administration in a stress-induced IBS mouse model.
MATERIALS AND METHODS
Animals
Three-week-old female C57BL/6 mice (Slac Laboratory Animal Co. Ltd. Shanghai, China) were used as described in a previous study using WAS to develop a stress-induced IBS mouse model[16]. Mice were housed in pathogen-free conditions with temperature (21 ± 1 °C) and light/dark cycle (12/12 h) regulation. A purified rodent diet (AIN-76A) without any FODMAP content and demineralized water were supplied freely on demand.
Animal care and use statement
All animal experiment protocols were reviewed and approved by the Animal Care and Use Committee of Zhejiang University prior to initiating this study. All animals received humane care in compliance with the criteria described in “The Guide for the Care and Use of Laboratory Animals.”
Experimental design
To evaluate the effects of FOS on WAS-induced visceral hypersensitivity and intestinal inflammation, 32 mice were randomly divided into four groups of eight mice (sham-WAS + saline administration, sham-WAS + FOS administration, WAS + saline administration, and WAS + FOS administration). Mice were administered daily with oral gavage of saline solution with or without FOS (8 g/kg body weight) for 14 d. FOS dose was derived according to the Meeh-Rubner formula[8]. Mice were subjected to either WAS or sham-WAS during the first 10 d. For WAS, mice were placed on a glass platform (3 cm length × 3 cm width × 9 cm height) surrounded by water (25 °C) in the middle of a plastic container (45 cm × 30 cm × 25 cm) as previously described[15]. Control mice assigned to sham-WAS were placed in the same container without water. Food consumption quantity, body weight, and indexes were recorded daily prior to subjecting mice to daily WAS or sham-WAS.
Assessment of visceral sensitivity
Abdominal withdrawal reflex in response to colorectal distension was evaluated to assess visceral sensitivity as described previously[17]. Semi-quantitative abdominal withdrawal reflex score (0-4) was utilized to evaluate pain responses at different magnitudes of colorectal distention (20, 40, 60, and 80 mmHg)[18]. With gradual colorectal distention to 100 mmHg, the pressure eliciting abdomen lifting was recorded as pain threshold and that eliciting body arching was recorded as volume threshold. To achieve accuracy, all pressure and threshold measurements were repeated three times and recorded by two independent operators blinded to WAS or FOS assignment.
Histological evaluation of inflammation
Mice were sacrificed by cervical dislocation, and intestinal tissues were harvested for histological evaluation. Intestinal tissue was fixed in formalin and processed with hematoxylin and eosin stains. The absence or presence of neutrophil infiltration in the lamina propria and the degree of interstitial edema in the intestinal tissues were graded based on previous description[18]. Stained slides were examined by two independent observers blinded to WAS or FOS assignment.
Quantification of SCFA production
SCFA production was quantified using gas chromatography as previously described[19]. Cecal contents (50 mg) were homogenized in 0.5 mL of distilled water and 0.1 mL of 25% (w/v) metaphosphoric acid was added to the suspension. The samples were subsequently centrifuged at 14000 × g for 20 min, and the supernatant was filtered through a membrane filter (pore size 0.22 μm). SCFA in the samples were then separated with InertCap FFAP columns (0.25 mm × 30 mm × 0.25 mm), and the peaks were integrated with GC Solution software (Shimadzu, GC-2010 Plus, Japan). Single-point internal standard method was used to quantify SCFA.
Intestinal cytokine mRNA detection
Intestinal expression of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-23, IL-10, and IL-1β was evaluated. Total RNA was isolated from 100 mg of ileal and colonic tissues by using a RNA Extraction Kit (Takara, Japan) and processed with a PrimeScript RT reagent Kit (Takara, Japan) to synthesize cDNA. Primers used are listed in Table 1. Quantitative real-time PCR was performed in triplicate for each sample with Lightcycler 480 instrument (Roche Applied Science, Penzberg, Germany). Reaction conditions for amplification of DNA were as follows: 95 °C for 30 s and 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Cytokine transcript levels were normalized with β-actin, and relative gene expression was expressed as the fold change (2-ΔΔCt) relative to the expression in the control samples.
Table 1.
Primer sequences
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
| TNF-α | GGCTTTCCGAATTCACTGGAG | CCCCGCCCTTCCAAATAAA |
| β-actin | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
| IL-6 | GTATGAACAACGATGATGCACTTG | ATGGTACTCCAGAAGACCAGAGGA |
| IL-23 | AATAATGCTATGGCTGTTGC | CCTTGAGTCCTTGTGGGT |
| IL-10 | ACTGCACCCACTTCCCAGT | ATGTTGTCCAGCTGGTCCTT |
| IL-1β | TTGACGGACCCCAAAAGATG | AGAAGGTGCTCATGTCCTCA |
IL: Interleukin; TNF: Tumor necrosis factor.
Immunohistochemistry
Intestinal mucosal mast cells were estimated by immunohistochemistry. After incubating in xylene to dewax and in ethanol to rehydrate, tissue section was incubated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidase activity, followed by visualizing antigen with heat-mediation. After blocking slides with 3% goat serum at room temperature for 20 min to prevent nonspecific staining, the section was treated with mouse anti-mast cell tryptase antibody (1:20000, Abcam, Cambridge, United Kingdom) for 1 hour at room temperature. After washing, the section was treated with HRP-labeled goat anti-mouse IgG (Zhongshan Gold Bridge, Beijing, China) for 30 min. Diaminobenzidine (DAB kit, Zhongshan Gold Bridge, Beijing, China) and hematoxylin were used to visualize the reaction. Four to five non-overlapping fields were randomly selected. The number of mucosal mast cells was counted under a light microscope (400 × magnification, Leica Company, Wetzlal, Germany) by two independent observers and is expressed as cells per high power field (HPF).
Statistical analysis
Data are presented as mean ± SD or median with 5th and 95th percentiles. Differences between two groups were determined by Student’s t-test for normally distributed data or Wilcoxon two-sample otherwise. Comparisons among three or more groups were performed by one-way analysis of variance (ANOVA) for normally distributed data or Kruskal-Wallis one-way ANOVA for non-normally distributed data. Rate of weight gain was analyzed by repeated measures ANOVA using the factors of WAS administration and time. Statistical analyses were conducted using SPSS (IBM, Armonk, NY, United States; version 22) and Graphpad Prism (GraphPad Software, San Diego, CA, United States; version 6.0). A two-tailed P-value < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Professor Yunxian Yu from Department of Epidemiology and Health Statistics of Zhejiang University.
RESULTS
WAS-induced visceral hypersensitivity in the IBS mouse model
Of the 32 randomized mice, five (one in the sham-WAS + saline group, one in the sham-WAS + FOS group, two in the WAS + saline group, and one in the WAS + FOS group) died due to gavage trauma and were excluded from the outcome analysis.
During the first 10 d, mice receiving WAS had lower rate of weight gain compared to mice receiving sham-WAS (Figure 1A). No difference in quantity of consumed feed were observed between mice receiving WAS or sham-WAS. Mice subjected to WAS had higher mean abdominal withdrawal reflex scores at colorectal distention pressures of 20, 40, 60, and 80 mmHg compared to mice subjected to sham-WAS (Figure 1B). Furthermore, mice subjected to WAS had lower pain and volume thresholds compared to mice subjected to sham-WAS (Figure 1C and D).
Figure 1.
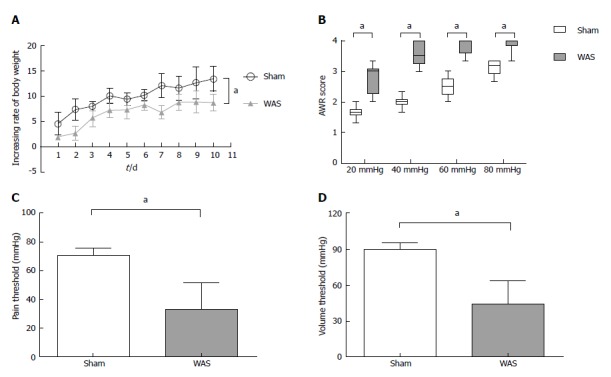
Effect of water avoidance stress on rate of weight gain and visceral sensitivity. A: Rate of weight gain (g) was lower in the water avoidance stress (WAS) group (n = 13) compared to the sham-WAS (n = 14) group. Values represent mean weight gain ± SD, repeated analysis of variance (ANOVA); B: Abdominal withdrawal reflex (AWR) scores in response to colorectal distension were increased in the WAS group compared to the sham-WAS group. Lines within the box represent the median value, ends of the box represent 25th and 75th percentiles, and the error bars represent 5th and 95th percentiles. Wilcoxon two-sample test; C: Pain threshold was decreased in the WAS group compared to the sham-WAS group. Values represent mean ± SD, Student’s t-test; D: Volume threshold was decreased in the WAS group compared to the sham-WAS group. Values represent means values ± SD, Student’s t-test. aP < 0.05, Sham vs WAS.
FOS intensifies WAS-induced visceral hypersensitivity
Among mice subjected to WAS, mice that received FOS administration for 14 d had higher mean abdominal withdrawal reflex scores at a colorectal distention pressure of 20 mmHg compared to those receiving saline administration (Figure 2A). Furthermore, mice that received FOS administration had lower pain and volume thresholds compared with those receiving saline administration following WAS (Figure 2B and C). However, no difference in mean abdominal withdrawal reflex scores, pain thresholds, or volume thresholds was observed between mice administered with FOS or saline following sham-WAS.
Figure 2.
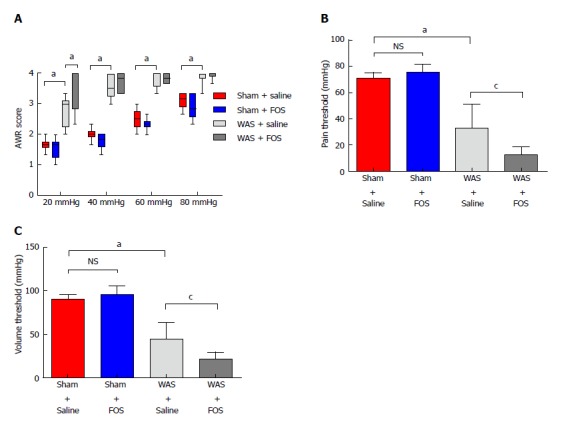
Effect of oral gavage of fructo-oligosaccharide on visceral sensitivity. A: Fructo-oligosaccharide (FOS) increased abdominal withdrawal reflex (AWR) scores in response to colorectal distension compared to saline administration following water avoidance stress (WAS). Values represent median, 25th, 75th, 5th, and 95th percentiles. Sham + saline (n = 7), sham + FOS (n = 7), WAS + saline (n = 6), WAS + FOS (n = 7). Kruskal-Wallis one-way ANOVA; B: Pain threshold decreased in FOS-administered compared to saline-administered mice following WAS. Values represent mean ± SD, one-way ANOVA; C: Volume threshold decreased in FOS compared to saline-administered mice following WAS. Values represent mean ± SD, one-way ANOVA. aP < 0.05, sham + saline vs WAS + saline; cP < 0.05, WAS + saline vs WAS + FOS.
FOS has no effect on intestinal histological score
No difference in neutrophil counts or degree of interstitial edema in the lamina propria was observed between the WAS and sham-WAS groups (Figure 3A and C). Furthermore, no difference in histologic score was observed among all four groups (sham-WAS + saline, sham-WAS + FOS, WAS + saline, WAS + FOS) at 14 d of the experiment (Figure 3B and D).
Figure 3.
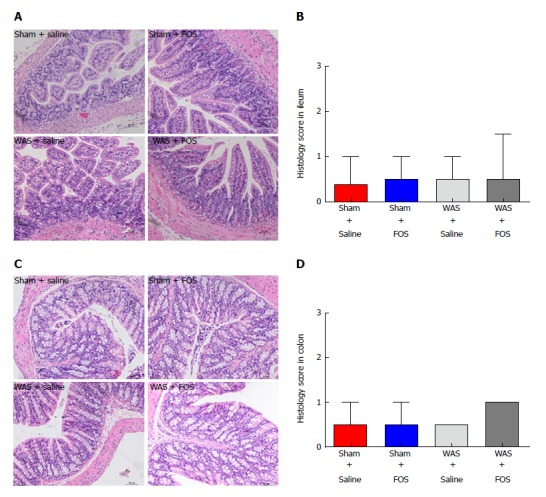
Effect of oral gavage of fructo-oligosaccharide on intestinal histological scores. A: Ileal tissues stained with hematoxylin-eosin (HE) for evaluation of inflammation score (magnification, 200×); B: No difference in structural histology among the four groups; C: Colonic tissues stained with H&E for evaluation of inflammation score (magnification, 200×); D: No difference in structural histology among the four groups. Values represent median with 5th and 95th percentiles; sham + saline (n = 7), sham + FOS (n = 7), WAS + saline (n = 6), WAS + FOS (n = 7); Kruskal-Wallis one-way ANOVA. FOS: Fructo-oligosaccharide; WAS: Water avoidance stress.
FOS increases cecal SCFA concentrations following WAS
No difference in levels of SCFA in the cecum was found between mice subjected to WAS and sham-WAS that received saline administration. Among mice subjected to WAS, mice administered with FOS had higher mean concentrations of acetic acid (2.49 ± 0.63 mmol/L vs 1.49 ± 0.72 mmol/L, P < 0.01, one-way ANOVA), propionic acid (0.48 ± 0.09 mmol/L vs 0.36 ± 0.05 mmol/L, P < 0.01, one-way ANOVA), butyric acid (0.28 ± 0.09 mmol/L vs 0.19 ± 0.003 mmol/L, P < 0.05, one-way ANOVA), and total SCFA (3.62 ± 0.87 mmol/L vs 2.27 ± 0.75 mmol/L, P < 0.01, one-way ANOVA) measured in the cecum compared to the mice administered with saline for 14 d (Figure 4). However, among mice subjected to sham-WAS, no difference in concentrations of individual or total SCFA was found between those administered with FOS or saline for 14 d.
Figure 4.
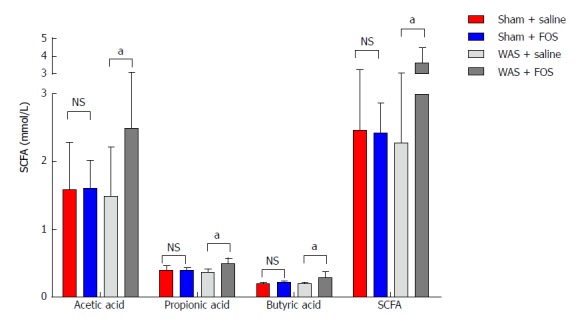
Effect of oral gavage of fructo-oligosaccharide on short chain fatty acid concentrations. The average concentrations of total SCFA, acetic, propionic, and butyric acids increased in FOS-administered mice compared to saline-administered mice following WAS intervention. No difference was observed in total SCFA, acetic, propionic, and butyric acid levels with FOS or saline administration in mice following sham-WAS. Values represent mean ± SD; sham + saline (n = 7), sham + FOS (n = 7), WAS + saline (n = 6), WAS + FOS (n = 7); one-way ANOVA. aP < 0.05, WAS + saline vs WAS + FOS. SCFA: Short chain fatty acids; FOS: Fructo-oligosaccharide; WAS: Water avoidance stress.
FOS-mediated intestinal cytokine expression following WAS
Mice subjected to WAS had higher expression of IL-6 (8.25 ± 3.95 vs 1.86 ± 1.66, P < 0.01, one-way ANOVA) and TNF-α (2.05 ± 1.73 vs 0.56 ± 0.28, P < 0.05, one-way ANOVA) mRNA in the ileal specimen, as well as higher IL-6 (1.60 ± 1.10 vs 0.46 ± 0.29, P < 0.05, one-way ANOVA) and IL-1β (0.88 ± 0.53 vs 0.34 ± 0.35, P < 0.05, one-way ANOVA) mRNA expression in the colonic specimen compared to those receiving sham-WAS (Figure 5). Among mice subjected to WAS, mice administered with FOS for 14 d had higher expression of IL-23 mRNA (4.71 ± 4.16 vs 1.00 ± 0.99, P < 0.05, one-way ANOVA) in the ileum and IL-1β mRNA (2.15 ± 1.68 vs 0.88 ± 0.53, P < 0.05, one-way ANOVA) in the colon compared to the mice administered with saline. However, among mice subjected to sham-WAS, no difference in IL-6, IL-23, TNF-α, IL-10, or IL-1β mRNA expression in the ileum or colon was found between mice administered with FOS or saline for 14 d.
Figure 5.
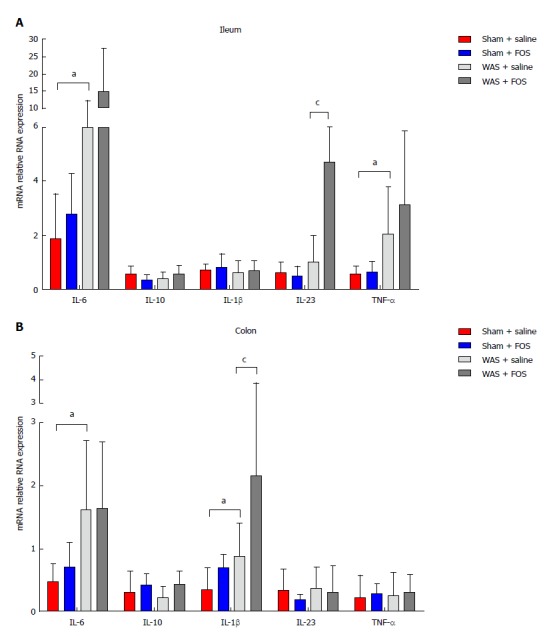
Effect of oral gavage of fructo-oligosaccharide on intestinal cytokine expression. A: Among saline-administered mice, IL-6 and TNF-α expressions increased in the ileum in the WAS group compared to the sham-WAS group. IL-23 expression increased in FOS compared to saline-administered mice following WAS; B: In saline-administered mice, colonic IL-6 and IL-1β expression increased in the WAS group compared to the sham-WAS group. IL-1β expression increased in FOS compared to saline-administered mice in the WAS group. Values represent mean ± SD; sham + saline (n = 7), sham + FOS (n = 7), WAS + saline (n = 6), WAS + FOS (n = 7); one-way ANOVA. aP < 0.05, sham + saline vs WAS + saline; cP < 0.05, WAS + saline vs WAS + FOS. FOS: Fructo-oligosaccharide; WAS: Water avoidance stress; IL: Interleukin; TNF: Tumor necrosis factor.
FOS increases the mucosal mast cell counts following WAS
Mice subjected to WAS had higher mean mast cell counts in the ileum (8.3 ± 3.6 per HPF vs 4.9 ± 1.4 per HPF, P < 0.05, one-way ANOVA) and colon (3.4 ± 1.2 per HPF vs 1.8 ± 1.5 per HPF, P < 0.05, one-way ANOVA) compared to those subjected to sham-WAS (Figure 6). Among mice subjected to WAS, mice administered with FOS for 14 d had greater mast cell infiltration in the ileum (12.3 ± 2.6 per HPF vs 8.3 ± 3.6 per HPF, P < 0.05, one-way ANOVA) and colon (6.3 ± 3.2 per HPF vs 3.4 ± 1.2 per HPF, P < 0.05, one-way ANOVA) compared to the mice administered with saline. However, among mice subjected to sham-WAS, no difference in mast cell infiltration in the ileum or colon was observed between mice administered with FOS or saline for 14 d.
Figure 6.
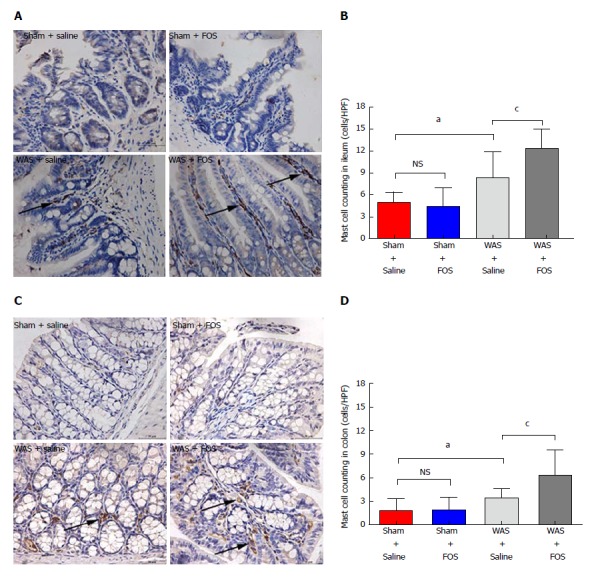
Effect of oral gavage of fructo-oligosaccharide on the number of mucosal mast cells (arrows). A: Ileal tissues stained with mast cell tryptase (magnification, 400 ×); B: In saline administered mice, mast cell counts increased in WAS compared to the sham-WAS group. Mast cell counts increased in FOS compared to saline administered mice following WAS; C: Colon stained with mast cell tryptase (magnification, 400 ×); D: In saline-administered mice, mast cell count increased in the WAS group compared to the sham-WAS group. Mast cell count increased in FOS compared to saline-administered mice following WAS. Values represent mean ± SD; sham + saline (n = 7), sham + FOS (n = 7), WAS + saline (n = 6), WAS + FOS (n = 7); one-way ANOVA. aP < 0.05, sham + saline vs WAS + saline; cP < 0.05, WAS + saline vs WAS + FOS. FOS: Fructo-oligosaccharide; WAS: Water avoidance stress.
DISCUSSION
We evaluated the effects of administration of high-dose FOS, a component of FODMAP, on visceral sensitivity and gut inflammation using a stress-induced IBS mouse model. Mice subjected to WAS exhibited visceral hypersensitivity and low-grade inflammation demonstrated by higher mucosal expression of pro-inflammatory cytokines and increased number of intestinal mast cells. Among mice subjected to WAS, FOS administration further intensified visceral hypersensitivity and also led to higher intestinal expression of IL-23 and IL-1β with increasing mucosal mast cell counts. Furthermore, FOS administration in mice subjected to WAS led to higher intestinal production of individual (acetic, propionic, and butyric acids) as well as total SCFA. However, FOS administration did not affect visceral sensitivity, intestinal inflammation, or intestinal SCFA production in control mice.
The effect of psychological stress as an inciting and/or exacerbating factor on altered brain-gut axis is central to the pathophysiology of IBS. In our study, mice subjected to WAS demonstrated visceral hypersensitivity and low-grade immune activation, characterized by increased expression of pro-inflammatory cytokines and mucosal mast cell infiltration yet without overt difference in intestinal histological scores compared to the control mice. These findings are consistent with prior studies that demonstrated the effects of stress on visceral hypersensitivity and intestinal immune activation in rodents[15,20]. Therefore, a WAS-induced IBS mouse model was used to study the effects of FOS administration on visceral sensitivity and mucosal inflammation typical in IBS.
Although the role of food intolerance-induced IBS symptoms has been long recognized, correlations with a specific food group have been difficult to demonstrate[21,22]. A key observation in our study is that FOS consumption further intensified visceral hypersensitivity already present in mice subjected to WAS. This result is consistent with the clinical studies that demonstrated adverse effects of high FODMAP diet as an individual component or as an aggregate in exacerbating gastrointestinal symptoms in IBS[6,8,23,24]. Along the same vein, our findings are concordant with studies that demonstrated the efficacy of dietary restriction of FODMAP in improving gastrointestinal symptoms, such as abdominal pain, diarrhea, bloating, flatulence, and quality of life in IBS patients[7,9,25]. Interestingly, FOS had no effect on visceral sensitivity in mice exposed to sham-WAS. Prior studies also demonstrated that high FODMAP diet-induced gastrointestinal symptoms in IBS patients but not in healthy volunteers except increased flatus[6,8]. Our findings highlight the direct effects of FODMAP on visceral hypersensitivity as a mechanism of FODMAP-induced IBS symptoms other than proposed mechanisms such as osmotic effects of poorly absorbed carbohydrates and increased colonic gas production from intestinal fermentation. A recent study indicated that hypersensitivity to colorectal distension, rather than excessive gas fermented by FODMAP, was the primary factor contributing to IBS symptoms[26]. Our finding that FOS consumption increased visceral hypersensitivity in the IBS mouse model, but not in control mice, suggests that stress-induced visceral hyperalgesia is a prerequisite for FODMAP-induced visceral hypersensitivity. Similarly, anxiety was demonstrated to be a robust predictor of inducing abdominal symptoms after ingestion of lactose, another FODMAP component, in a previous study among patients with IBS[27].
SCFA are byproducts of FODMAP fermentation. For example, IBS patients on a low FODMAP diet have altered fecal fermentation producing lower levels of stool SCFA including acetic acid and butyric acid[28-30]. Our study showed that high-dose FOS administration increased production of individual (acetic, propionic, and butyric acids) and total SCFA, which was also associated with increased visceral hypersensitivity and intestinal inflammation already present in the IBS mouse model. Although inconsistent effects, SCFA clearly play a role in the regulation of visceral pain and intestinal immune activation. For example, butyric acid reduced visceral pain in humans, but induced visceral hypersensitivity in rats[31]. Intracolonic infusion of 0.5% acetic acid led to visceral hypersensitivity in rats[32]. In addition, SCFA may also act as pro-inflammatory substrates to induce immune responses[33], but in others cases, exert anti-inflammatory properties[34]. SCFA inhibited regulatory T cell differentiation and suppressed IL-10 expression in IBS[35]. However, butyric acid exacerbated dextran sodium sulfate-induced colitis in a murine model and increased IL-23 production by stimulating dendritic cells[36].
Interestingly, administration of FOS in control mice did not increase the levels of individual or total SCFA production, highlighting the difference in fermentation of FOS between stressed and sham-stressed conditions. Stress-induced alteration in microbiota may lead to the change of fermentation products[16]. Alternatively, stress-induced release of corticotropin-releasing hormone may accelerate intestinal transit, reducing absorption of SCFA[37]. However, SCFA production was comparable between mice subjected to WAS or sham-WAS in the absence of FOS administration. Although studies have generally reported higher stool concentrations of SCFA in IBS patients, some have demonstrated similar SCFA levels in IBS and non-IBS patients, likely explained by a lack of rigorous control of dietary factors[14,38,39]. In our study, feed void of FODMAP content as an essential substrate for SCFA may account for the lack of difference in SCFA production between WAS and sham-WAS group despite possible difference in fermentation capacity of the two groups.
In our study, FOS administration in mice subjected to WAS was associated with low-grade inflammation, which is consistent with prior studies on IBS. FOS administration increased the expression of pro-inflammatory cytokines, such as IL-23 in the ileum and IL-1β in the colon, following WAS. Specifically, IL-23 is important in regulating intestinal inflammation by activating lymphocytes, as well as inducing and promoting release of other inflammatory mediators. Although FOS administration exerted anti-inflammatory effects in some studies[40], others have also demonstrated that FOS administration induced pro-inflammatory cytokine profile, including elevated IL-10 and a reduction in IL-6, typically observed in active Crohn’s disease[41]. Given the pivotal role of low-grade mucosal inflammation as a trigger of IBS symptoms[42-44], the increased pro-inflammatory cytokines may have played a role in worsening visceral hypersensitivity in FOS-administered mice following WAS. In addition to increased production of pro-inflammatory cytokines, mice subjected to WAS had further increased mucosal mast infiltration with FOS. Our findings are in line with a study that demonstrated an eight-fold reduction of urinary histamine, a measure of mast cell activation, among IBS patients receiving a low compared to high FODMAP diet[45]. Mast cells play an important role in mucosal immune activation in IBS by releasing a variety of pro-inflammatory mediators[46]. For example, tryptase released by mast cells can activate protease-activated receptor-2, which is important in inducing visceral hypersensitivity[47]. In addition to mucosal mast cell activation by WAS, FOS-induced SCFA production may also contribute to further recruitment of mucosal mast cells and secretion of histamine[48,49].
Our study has several limitations. First, FOS is only one component of FODMAP that was studied, and effects of the other FODMAP components on visceral hypersensitivity and immune activation are unknown. Second, although our study demonstrated the effects of FOS on stress-induced visceral hypersensitivity and intestinal inflammation, detailed mechanism was beyond the scope of the study and will be invaluable in future studies. Finally, although the WAS-induced mouse model exhibited visceral hypersensitivity and low-grade inflammation, experimental models are not able to fully encompass the complex biopsychosocial components of IBS, and our findings should be interpreted with caution.
In conclusion, administration of FOS, a component of FODMAP, intensified visceral hypersensitivity and gut inflammation in the stressed-induced IBS mice, but not in the control mice. A parallel increased production of intestinal SCFA was also observed with FOS administration in the IBS mice but not in the control mice. Our findings suggest a mechanism of FODMAP-induced gastrointestinal symptoms specific to IBS.
ARTICLE HIGHLIGHTS
Research background
The impact of dietary factors in exacerbating symptoms of irritable bowel syndrome (IBS) is being increasingly recognized. Specifically, abdominal pain following the consumption of Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) is common, and dietary restriction of FODMAP improves symptoms of IBS.
Research motivation
Although osmotic effects of poorly absorbed carbohydrates and increased colonic gas production from intestinal fermentation are proposed, evidence providing specific mechanism of FODMAP-induced IBS symptoms is sparse. With wide acceptance of low-FODMAP diet as a treatment for IBS, clarifying the specific mechanism is important for optimal application in clinical practice.
Research objectives
The aim of the study was to explore the effects of high-dose fructo-oligosaccharides (FOS), a component of FODMAP, on visceral sensitivity, inflammation, and production of intestinal short-chain fatty acids (SCFA) using an IBS mouse model. FOS administration intensified visceral hypersensitivity and gut inflammation already present in the stress-induced IBS mice, but not in the control mice, and was also associated with increased cecal SCFA production. The results provide a biologic framework for FODMAP-induced IBS symptoms that supports the application of low FODMAP therapy in clinical practice.
Research methods
The effects of FOS on visceral sensitivity, SCFA production, and intestinal inflammation were examined by using a water avoidance stress (WAS)-induced IBS mouse model. Mice were randomly assigned to receive daily WAS or sham-WAS for 10 d while receiving daily oral gavage of saline solution with or without high-dose FOS. After 2 wk, visceral sensitivity was measured by abdominal withdrawal reflex in response to colorectal distension and mucosal inflammation was measured by histologic analyses. Furthermore, intestinal SCFA production, cytokine expression, and mast cell counts were evaluated.
Research results
FOS administration intensified visceral hypersensitivity, increased mucosal mast cell counts, and mediated intestinal cytokine expression in the stressed-induced IBS mice, but not in the control mice. A parallel increase in cecal SCFA levels was also observed with FOS administration in the IBS mice but not in the control mice. These findings suggest that visceral hypersensitivity and gut inflammation intensified by FODMAP diet may lead to worsening IBS symptoms. Examining the effects of other FODMAP components other than FOS on visceral hypersensitivity and immune activation, as well as, detailed molecular mechanism may be invaluable in future studies.
Research conclusions
Administration of high-dose FOS, a component of FODMAP, intensified visceral sensitivity and intestinal inflammation in a stress-induced IBS mouse model, and was also associated with increased production of SCFA. These findings suggest a mechanism of FODMAP-induced gastrointestinal symptoms specific to IBS and are consistent with clinical studies that demonstrate the efficacy of low-FODMAP diet in treatment of individuals with IBS.
Research perspectives
The importance of dietary factors in triggering symptoms is increasingly being recognized in patients with IBS. FOS administration intensifies visceral hypersensitivity and gut inflammation in stress-induced IBS mice, and is also associated with increased intestinal SCFA production.
ACKNOWLEDGMENTS
We are grateful to Xin Wang, Ye-Shi Yin, Zheng-Peng Li, and Jing-Gang Chen from Zhejiang Academy of Agricultural Sciences Institute for their guidance on the experiments. We would also like to thank Professor Yun-Xian Yu from Zhejiang University for reviewing the statistical methods of this study.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by Zhejiang University Animal Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Zhejiang University.
Conflict-of-interest statement: The authors declare no conflict of interest related to this study.
Peer-review started: September 28, 2017
First decision: October 17, 2017
Article in press: November 21, 2017
P- Reviewer: Chiba T, Soares RLS S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Bin-Rui Chen, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Li-Jun Du, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Hui-Qin He, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
John J Kim, Division of Gastroenterology, Loma Linda University Medical Center, Loma Linda, CA 92354, United States.
Yan Zhao, Division of Gastroenterology, Loma Linda University Medical Center, Loma Linda, CA 92354, United States.
Ya-Wen Zhang, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Liang Luo, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Ning Dai, Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China. ndaicn@zju.edu.cn.
References
- 1.Rodríguez-Fandiño O, Hernández-Ruiz J, Schmulson M. From cytokines to toll-like receptors and beyond - current knowledge and future research needs in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:363–373. doi: 10.5056/jnm.2010.16.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Nakade Y, Fukuda H, Tsukamoto K, Mantyh C, Pappas TN. Daily intake of high dietary fiber slows accelerated colonic transit induced by restrain stress in rats. Dig Dis Sci. 2008;53:1271–1277. doi: 10.1007/s10620-008-0228-8. [DOI] [PubMed] [Google Scholar]
- 4.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–147. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 5.Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2014;8:819–834. doi: 10.1586/17474124.2014.917956. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407.e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 9.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 11.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66:1517–1527. doi: 10.1136/gutjnl-2017-313750. [DOI] [PubMed] [Google Scholar]
- 12.Salvioli B, Serra J, Azpiroz F, Malagelada JR. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101:1853–1857. doi: 10.1111/j.1572-0241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 13.Serra J, Villoria A, Azpiroz F, Lobo B, Santos J, Accarino A, Malagelada JR. Impaired intestinal gas propulsion in manometrically proven dysmotility and in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:401–406, e91-e92. doi: 10.1111/j.1365-2982.2009.01447.x. [DOI] [PubMed] [Google Scholar]
- 14.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519, e114-e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Zhang M, Chen CC, Gillilland M 3rd, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, Chang YM, Gumucio DL, Owyang C, Kao JY. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478–1487, 1487.e1-1487.e8. doi: 10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RC 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 19.Bai S, Chen H, Zhu L, Liu W, Yu HD, Wang X, Yin Y. Comparative study on the in vitro effects of Pseudomonas aeruginosa and seaweed alginates on human gut microbiota. PLoS One. 2017;12:e0171576. doi: 10.1371/journal.pone.0171576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Gao J, Gillilland M 3rd, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146:484–96.e4. doi: 10.1053/j.gastro.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares RL, Figueiredo HN, Santos JM, Oliveira RF, Godoy RL, Mendonca FA. Discrepancies between the responses to skin prick test to food and respiratory antigens in two subtypes of patients with irritable bowel syndrome. World J Gastroenterol. 2008;14:3044–3048. doi: 10.3748/wjg.14.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares RL, Figueiredo HN, Maneschy CP, Rocha VR, Santos JM. Correlation between symptoms of the irritable bowel syndrome and the response to the food extract skin prick test. Braz J Med Biol Res. 2004;37:659–662. doi: 10.1590/s0100-879x2004000500005. [DOI] [PubMed] [Google Scholar]
- 23.Evans PR, Piesse C, Bak YT, Kellow JE. Fructose-sorbitol malabsorption and symptom provocation in irritable bowel syndrome: relationship to enteric hypersensitivity and dysmotility. Scand J Gastroenterol. 1998;33:1158–1163. doi: 10.1080/00365529850172502. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein R, Braverman D, Stankiewicz H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr Med Assoc J. 2000;2:583–587. [PubMed] [Google Scholar]
- 25.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am J Gastroenterol. 2016;111:1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 26.Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, Gowland P, Spiller R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152:124–133.e2. doi: 10.1053/j.gastro.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Fox M, Cong Y, Chu H, Zheng X, Long Y, Fried M, Dai N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39:302–311. doi: 10.1111/apt.12582. [DOI] [PubMed] [Google Scholar]
- 28.Hustoft TN, Hausken T, Ystad SO, Valeur J, Brokstad K, Hatlebakk JG, Lied GA. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017:29. doi: 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- 29.Staudacher H, Lomer MC, Lindsay JO, Irving PM, Whelan K. The impact of low FODMAP dietary advice and probiotics on symptoms in irritable bowel syndrome: A randomised, placebo-controlled, 2 × 2 factorial trial. Gut. 2015;64:A51. [Google Scholar]
- 30.Valeur J, Røseth AG, Knudsen T, Malmstrøm GH, Fiennes JT, Midtvedt T, Berstad A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion. 2016;94:50–56. doi: 10.1159/000448280. [DOI] [PubMed] [Google Scholar]
- 31.Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate- algesic or analgesic? Neurogastroenterol Motil. 2011;23:975–979. doi: 10.1111/j.1365-2982.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e1-10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 34.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu WL, Lu G, Liang SJ, Wu XL, Pei LX, Geng H, Ning HX, Sun JH. Short chain fatty acids mediated flora-host interaction and irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi. 2015;23:5815. [Google Scholar]
- 36.Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, Veniaminova NA, Merchant JL, Chen CC, Huffnagle GB, et al. Butyrate increases IL-23 production by stimulated dendritic cells. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1384–G1392. doi: 10.1152/ajpgi.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray KA, Lam C, Rehman S, Marciani L, Costigan C, Hoad CL, Lingaya MR, Banwait R, Bawden SJ, Gowland PA, et al. Corticotropin-releasing factor increases ascending colon volume after a fructose test meal in healthy humans: a randomized controlled trial. Am J Clin Nutr. 2016;103:1318–1326. doi: 10.3945/ajcn.115.125047. [DOI] [PubMed] [Google Scholar]
- 38.Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered Colonic Bacterial Fermentation as a Potential Pathophysiological Factor in Irritable Bowel Syndrome. Am J Gastroenterol. 2015;110:1339–1346. doi: 10.1038/ajg.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids - a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016;16:51. doi: 10.1186/s12876-016-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, Kamm MA, Knight SC, Forbes A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60:923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 42.Vázquez-Frias R, Gutiérrez-Reyes G, Urbán-Reyes M, Velázquez-Guadarrama N, Fortoul-van der Goes TI, Reyes-López A, Consuelo-Sánchez A. Proinflammatory and anti-inflammatory cytokine profile in pediatric patients with irritable bowel syndrome. Rev Gastroenterol Mex. 2015;80:6–12. doi: 10.1016/j.rgmx.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 44.Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–1212. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 45.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66:1241–1251. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 46.Lee KN, Lee OY. The Role of Mast Cells in Irritable Bowel Syndrome. Gastroenterol Res Pract. 2016;2016:2031480. doi: 10.1155/2016/2031480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- 48.Yamada K, Mori M, Matsuo N, Shoji K, Ueyama T, Sugano M. Effects of fatty acids on accumulation and secretion of histamine in RBL-2H3 cells and leukotriene release from peritoneal exudate cells isolated from Wistar rats. J Nutr Sci Vitaminol (Tokyo) 1996;42:301–311. doi: 10.3177/jnsv.42.301. [DOI] [PubMed] [Google Scholar]
- 49.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]


