Abstract
AIM
To investigate whether morin can reduce hepatic fibrosis by activating the NF-E2-related factor 2 (Nrf2) signaling pathway.
METHODS
Twenty male Sprague-Dawley rats were randomly divided into four groups: control group, morin group, carbon tetrachloride (CCl4) group, and morin + CCl4 group. Rats in both the CCl4 and morin + CCl4 groups were injected intraperitoneally with CCl4 at a dose of 2 mL/kg twice a week. Rats in both the morin and morin + CCl4 groups were treated orally with morin at a dose of 50 mg/kg twice a week. Control rats were treated with vehicle only twice a week. At the end-point of the 8 wk of the experimental period, serum AST, ALT, and ALP were measured, and the liver specimens were obtained for pathological assessment. Real-time PCR and Western blot methods were used to analyze the expression of α-smooth muscle actin (α-SMA), collagen I, collagen III, Nrf2, heme oxygenase (HO-1), and quinone oxidoreductase 1 (NQO1) using frozen liver specimens.
RESULTS
Morin-treated rats in the morin + CCl4 group had less hyperplasia of fiber tissue, minimal inflammatory cells, and less body weight loss with favorable liver enzyme measurements compared to rats treated with CCl4 only. Additionally, morin-treated rats had significantly lower mRNA and protein expression of α-SMA, collagen I, and collagen III, but significantly higher mRNA and protein expression of Nrf2, HO-1, and NQO1 compared to rats treated with CCl4 only (P < 0.05).
CONCLUSION
Morin could play a protective role by inducing the expression of Nrf2 and its downstream antioxidant factors (HO-1 and NQO1) and reducing the expression of α-SMA, collagen I, and collagen III in CCl4-induced liver fibrosis rats.
Keywords: Liver fibrosis, Rat, Morin, Nrf2
Core tip: We constructed a liver fibrosis rat model with carbon tetrachloride (CCl4). The Sprague-Dawley rats were randomly divided into four groups: control group, morin group, CCl4 group, and morin + CCl4 group. α-SMA, collagen I, collagen III, NF-E2-related factor 2 (Nrf2), heme oxygenase (HO-1), and quinone oxidoreductase 1 (NQO1) were analyzed by real-time PCR and Western blot methods using frozen liver specimens. We found that morin could reduce hepatic fibrosis by inducing the expression of Nrf2 and its downstream antioxidant factors in the CCl4-induced rat liver fibrosis model.
INTRODUCTION
Hepatic fibrosis refers to a series of pathogenic factors and pathological changes in the pathogenesis of a variety of liver diseases with liver extracellular matrix (ECM) metabolic abnormalities[1]. Previous studies have found that the development and progression of liver fibrosis are significantly related to oxidative stress in which a large number of free radicals lead to cell metabolic disorders and subsequent destruction of normal liver cells[2-5]. Although there is currently no effective therapy for curing liver fibrosis, previous studies showed that the pathological changes in liver fibrosis could be reversed[6,7].
Oxidative stress is closely related to the occurrence of liver disease[8]. A large number of studies have shown that oxidative stress may promote the activation of hepatic satellate cells (HSCs) and increase collagen production[9]. In the past decade, numerous studies proved that NF-E2-related factor 2 (Nrf2) plays a role as an important transcription factor in normal liver cells, and its activation could increase the expression of the downstream specific genes, such as the quinone oxidoreductase 1 (NQO1), heme oxygenase (HO-1), and glutathione, which play a role against oxidative stress[10,11]. Studies have shown that Nrf2 activation could resist oxidative stress caused by hepatic ischemia and injury, liver fibrosis, and drug-induced liver damage[12-15].
Flavonoids are rich in a variety of fruits, vegetables, and components of herbal-containing dietary agents and play an important role in preventing many kinds of diseases. Morin (3, 5, 7, 2’, 4’-pentahydroxyflavone) is a kind of flavonoid that consists of a yellowish pigment found in onion and apple[16], almond (P. guajava L.)[17], fig (Chlorophora tinctoria)[18], and other moraceae, including in food and herbal medicines[19] (Figure 1). It has been shown that morin possesses biological properties, including antioxidant[20,21], anti-inflammatory[22], anti-apoptosis[23,24], and anticancer[19] activities. Morin also protects various human cells, such as myoblasts[25], hepatocytes[26], and erythrocytes, against oxidative damages[27].
Figure 1.
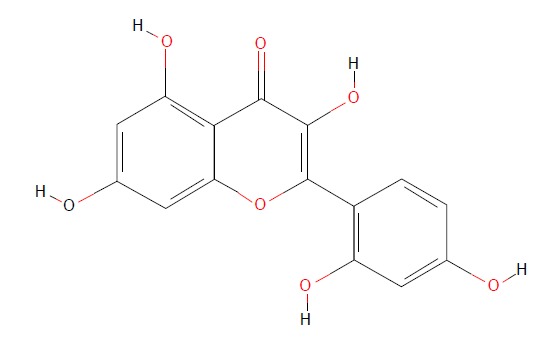
Chemical structure of morin. (https://pubchem.ncbi.nlm.nih.gov/compound/morin).
Carbon tetrachloride (CCl4) intraperitoneal injection is a classical method for establishing an animal model of hepatic fibrosis, and the toxicity of CCl4 leads to liver cell necrosis and mitochondrial damage along with aggravating oxidative stress. In addition, the abundant release of inflammatory and fibrogenic cytokines induced by CCl4 could further augment the degree of hepatic fibrosis[28]. A previous study demonstrated that morin protected against acute liver damage[29] and ameliorated liver fibrosis[20] induced by CCl4, where morin inhibited proliferation and induced apoptosis of activated HSCs by suppressing the Wnt/β-catenin and NF-kB signaling pathways. However, there is no molecular evidence of the effects of morin on the Nrf2 signaling pathway. To our knowledge, in vivo investigation of the effect of morin on the Nrf2 signaling pathway and Nrf2 expression in the CCl4-induced liver fibrosis model has not been reported. The purpose of this study was to investigate whether morin could reduce hepatic fibrosis by inducing the expression of Nrf2 and its downstream antioxidant enzymes using pathology as a gold standard in a rat model of CCl4-induced hepatic fibrosis.
MATERIALS AND METHODS
Chemicals and reagents
The chemical agents used in this study included CCl4 and olive oil (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) as well as morin (Sigma Chemical Co., St Louis, MO, United States). Serum aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The antibodies against Nrf-2, HO-1, NQO1, collagen I, collagen III, and α-SMA were obtained from Proteintech Group Inc. (Chicago, IL, United States). All other reagents used were in the purest form available commercially.
Animals and experimental design
This study was performed in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health of China (Guide for the Care and Use of Laboratory Animals, 1996) and was approved by the Animal Care and Use Committee of China Medical University. Twenty male Sprague-Dawley rats with an average body weight of 200-220 g (Changsheng Biotechnology Co., Ltd, Liaoning, China) were used in this study. All rats were fed a standard laboratory diet for a week at room temperature (20-22 °C) with a light/dark cycle of 12 h. Then, the rats were randomly divided into four groups of five rats each, i.e., control group, morin group, CCl4 group, and morin + CCl4 group. The control rats were treated with vehicle only (olive oil) equivalent to the treatment group. The rats in the morin group were treated with morin at a dose of 50 mg/kg (suspended in water as previously described[30]) by oral administration and 2 mL/kg of olive oil by intraperitoneal injection twice a week. The rats in the CCl4 group were injected intraperitoneally with CCl4 at a dose of 2 mL/kg [mixed with olive oil (40%, V/V)] twice a week. The rats in the morin + CCl4 group were treated with the same doses of morin and CCl4 via the same routes as the morin group and the CCl4 group. Body weights of animals were recorded twice per week. After 8 wk of treatment, animals were kept fasting for 24 h. Under 10% chloral hydrate anesthesia, the following procedures were performed, including obtaining blood samples from the heart for biochemical tests and resecting the liver and spleen for histopathological analysis. Liver tissues were weighted and cut in 10 mm × 10 mm × 3 mm pieces. Half of the specimen was fixed in 10% formaldehyde for histopathology and the other half was immediately frozen in -80 °C for PCR and Western blot tests.
Biochemical analysis
The blood samples were centrifuged at 3000 g for 10 min at 20 °C, and the serum was collected from the supernatant. The values of AST, ALT, and ALP were measured using commercial assay kits according to the manufacturer’s protocols.
Histopathological assessment
Specimens of the liver were embedded in paraffin and cut into 5-μm-thick sections after 24 h of fixation. Then, the samples were stained with hematoxylin and eosin (HE). The degree of liver fibrosis was analyzed and determined by an experienced pathologist. The liver fibrosis was categorized into five degrees, i.e., F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with rare septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis according to reference criteria[31].
Quantitative real-time PCR
Total cellular RNA was extracted from tissues using TRIzol (Invitrogen). Reverse transcription of 1 μg of RNA was done using RT regents (TAKARA) following the manufacturer’s instructions. Quantitative real-time PCR was done using SYBR Green PCR master mix (Applied Biosystems) in a total volume of 20 μL on the 7900HT fast Real-time PCR system (Applied Biosystems) using the following cycling parameters: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 60 s. A dissociation procedure was performed to generate a melting curve for confirmation of amplification specificity. GAPDH was used as the reference gene. The relative levels of gene expression were represented as ΔCt = Ctgene - Ctreference, and the fold change of gene expression was calculated by the 2-ΔΔCt method. Experiments were repeated in triplicate. The primer sequences are listed in Table 1.
Table 1.
Primer sequences
| Name | Primer sequence |
| Rat Collagen I for | 5'-ACTGGTACATCAGCCCAAACCC-3' |
| Rat Collagen I rev | 5'-GGAATCCATCGGTCATGCTCT-3' |
| Rat Collagen III for | 5'-GAGACTCCCCATCATAGATATCGC-3' |
| Rat Collagen III rev | 5'-AGCAAACAGGGCCAATGTCC-3' |
| Rat α-SMA for | 5'-GCTATGCTCTGCCTCATGCC-3' |
| Rat α-SMA rev | 5'-CACGCTCAGCAGTAGTCACGAA-3' |
| Rat Nrf2 for | 5'-ACACAGCATAGCCCATCTCGT-3' |
| Rat Nrf2 rev | 5'-ACCAACCTGGATGAGCGACAC-3' |
| Rat NQO1 for | 5'-CCACGCAGAGAGGACATCATT-3' |
| Rat NQO1 rev | 5'-TTCGACCACCTCCCATCCTT-3' |
| Rat HO-1 for | 5'-CTTCCCGAGCATCGACAAC-3' |
| Rat HO-1 rev | 5'-CTGTCACCCTGTGCTTGACC-3' |
| Rat Gapdh for | 5'-GCTGGTCATCAACGGGAAA-3' |
| Rat Gapdh rev | 5'-CGCCAGTAGACTCCACGACAT-3' |
Western blot analysis
Total proteins from tissues were extracted in lysis buffer (Pierce, United States) and quantified using the Bradford method. A total of 40 μg of protein were separated using 10% SDS-PAGE (80 V -120 V) and then electrophoretically transferred to a PVDF membrane (80 V 100 min) (Millipore, Bedford, MA, United States). The membrane was blocked with 5% dry milk and incubated overnight at 4 °C with antibodies against HO-1 (1:800; Proteintech), NQO-1 (1:1000; Proteintech), Nrf2 (1:800; Proteintch), collagen I (1:800, Proteintech), collagen III (1:1000, Proteintech), α-SMA (1:1000, Proteintch), and GAPDH (1:4000, Proteintech). After washing, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) at 37 °C for 2 h. Protein bands were visualized by enhanced chemiluminescence (Pierce) and detected using BioImaging Systems (UVP, Upland, CA, United States). The relative protein levels were calculated based on GAPDH protein as a loading control. Western blot images were measured with ImageJ software, and the relative gray values of protein expression were analyzed semi-quantitatively.
Statistical analysis
The experimental data are expressed as the mean ± SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) between groups, and unpaired comparisons were analyzed using the least significant difference method LSD t-test. A P-value of 0.05 or less was considered statistically significant. All analyses were conducted using SPSS version 17.0 (SPSS, Inc., Chicago, IL, United States) and Prism GraphPad software Version 6.01 (GraphPad Software Inc., San Diego, CA, United States).
RESULTS
General observation
A total of four rats died before the end-point of the study, including two in the CCl4 group, one in the morin + CCl4 group, and one in the morin group. All animals in the control group survived. Normal diet and daily activities were recorded in the control and morin groups, with body weight increasing rapidly. The CCl4 group presented poor feeding and daily activities with slow weight growth. The morin + CCl4 group presented milder symptoms compared with the CCl4 group, with increased body weight, which was, however, lower than that in the control and morin groups (Figure 2).
Figure 2.
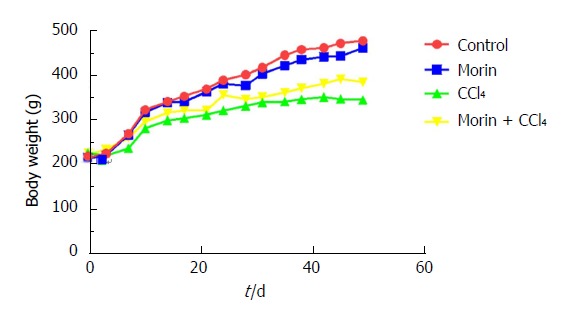
Changes in body weight among different groups. Body weight increased observably in the control and morin groups. The CCl4 group had slow weight growth, but morin treatment was associated with increased body weight.
Histological changes in the liver
The results of HE staining showed that the liver cells appeared with a normal morphology and regular lobular structure in the control and morin groups. The liver tissue of CCl4 group rats showed inflammatory cell infiltration, with portal and central veins surrounded by fibrous tissue accompanied by fibrous septa. The lobular structure was fuzzy with clearly visible false lobules. In the morin + CCl4 group, the liver tissue demonstrated less hyperplasia of fiber tissue and minimal inflammatory cells compared to the CCl4 group (Figure 3A-D).
Figure 3.
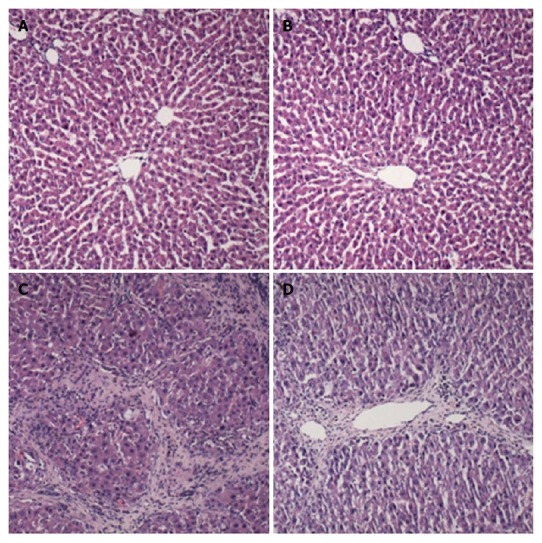
Histological changes of liver samples. A: Control group: treated with vehicle only; B: Morin group: treated with morin at a dose of 50 mg/kg twice a week; C: CCl4 group: injected with CCl4 at a dose of 2 mL/kg twice a week; D: Morin + CCl4 group: treated with the same volume of morin and CCl4 as the morin and CCl4 groups. Liver tissues were stained with H&E (× 100).
Liver-spleen ratio and liver weight index
Both the CCl4 and morin + CCl4 groups had increased liver-spleen ratio (LSR) and liver weight index (LWI) compared with the control and morin groups (P < 0.05). The LWI between the CCl4 and morin + CCl4 groups showed a significant difference (P < 0.05), while no statistically significant difference was found for LSR (P > 0.05) (Table 2).
Table 2.
Comparison of liver-spleen ratio and liver weight index among different groups
| Control | Morin | CCl4 | Morin + CCl4 | F | P value | |
| (n = 5) | (n = 4) | (n = 3) | (n = 4) | |||
| LSR | 12.27 ± 1.92 | 12.67 ± 1.60 | 16.43 ± 1.37ac | 15.11 ± 1.99ac | 4.668 | 0.022 |
| LWI% | 2.78 ± 0.25 | 2.80 ± 0.27 | 4.77 ± 0.47ac | 4.17 ± 0.39ace | 32.345 | < 0.001 |
P < 0.05 vs control group,
P < 0.05 vs morin group,
P < 0.05 vs CCl4 group. Liver-spleen ratio (LSR): Liver wet weight/spleen wet weight; liver weight index (LWI): (Liver wet weight/body weight) × l00%.
Biochemical findings
The CCl4 and morin + CCl4 groups had increased ALT, AST, and ALP levels compared to the control and morin groups (P < 0.05), and CCl4 without morin treatment dramatically increased ALT, AST, and ALP values (Table 3).
Table 3.
Serum parameters among different groups
| Control | Morin | CCl4 | Morin + CCl4 | F | P value | |
| (n = 5) | (n = 4) | (n = 3) | (n = 4) | |||
| ALT (IU/L) | 101.75 ± 15.46 | 108.00 ± 48.72 | 493.33 ± 199.38ac | 291.50 ± 111.92ace | 11.403 | 0.001 |
| AST (IU/L) | 339.25 ± 72.59 | 257.80 ± 98.22 | 1027.67 ± 206.60ac | 585.50 ± 131.85ace | 26.280 | < 0.001 |
| ALP (IU/L) | 137.75 ± 29.75 | 160.80 ± 40.90 | 377.67 ± 41.07ac | 266.50 ± 58.90ace | 22.093 | < 0.001 |
P < 0.05 vs control group,
P < 0.05 vs morin group,
P < 0.05 vs CCl4 group.
mRNA expression of α-SMA, collagen I, collagen III, Nrf2, HO-1, and NQO1
Compared with the control and morin groups, significantly higher mRNA expression of α-SMA, collagen I, and collagen III was observed in liver tissues in the CCl4 and morin + CCl4 groups (P < 0.05). However, the mRNA expression of these molecules in the morin + CCl4 group was significantly less than that in the CCl4 group (P < 0.05) (Figure 4).
Figure 4.
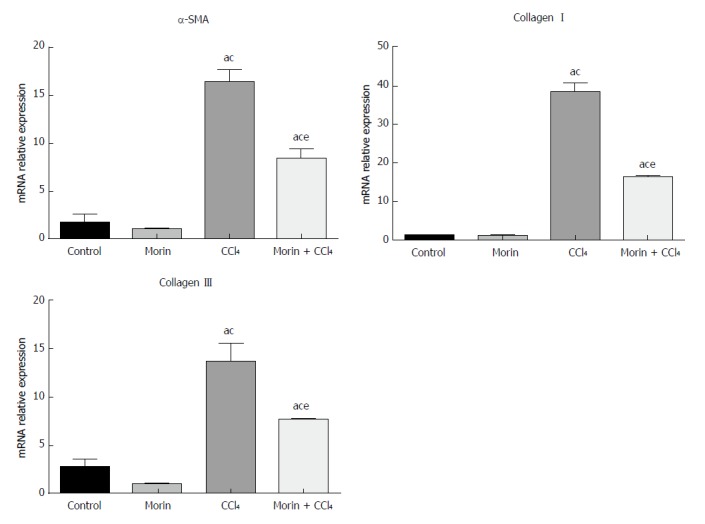
The mRNA expression of α-SMA, collagen I, and collagen III. aP < 0.05 vs control group, cP < 0.05 vs morin group, eP < 0.05 vs CCl4 group. In the control and morin groups, there was only minimal expression. The CCl4 and morin + CCl4 groups showed significantly increased expression (P < 0.05), while the expression levels in the morin + CCl4 group were lower than those of the CCl4 group (P < 0.05).
In the CCl4 and morin + CCl4 groups, mRNA expression values of NQO1, HO-1, and Nrf2 were significantly higher than those in the control and morin groups (P < 0.05), while these mRNA values of the morin + CCl4 rats were significantly different compared to those of the CCl4 group (P < 0.05) (Figure 5).
Figure 5.
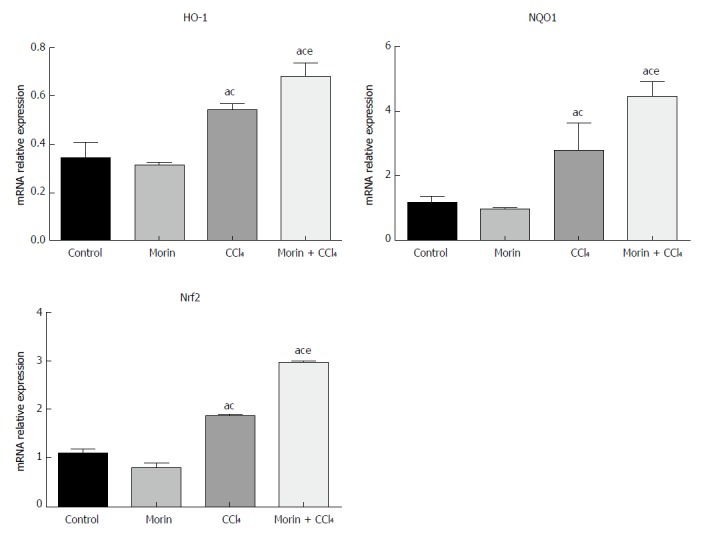
The mRNA expression of HO-1, NQO1, and Nrf2. aP < 0.05 vs control group, cP < 0.05 vs morin group, eP < 0.05 vs CCl4 group. The expression was increased obviously in the CCl4 and morin + CCl4 groups compared to the control and morin groups (P < 0.05). The expression levels in the morin + CCl4 group were significantly higher than those of the CCl4 group (P < 0.05).
Protein expression of α-SMA, collagen I, collagen III, Nrf2, HO-1, and NQO1
Compared with the control and morin groups, high expression of protein of α-SMA, collagen I, and collagen III in liver tissues in the CCl4 and morin + CCl4 groups had a statistically significant difference (P < 0.05). However, the morin + CCl4 group had less expression of these protein factors compared to the CCl4 group (P < 0.05) (Figure 6).
Figure 6.
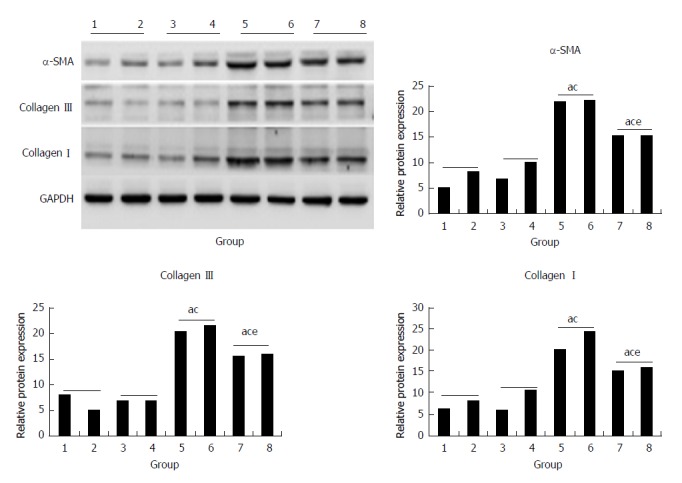
The protein expression of α-SMA, collagen III, and collagen I. (1, 2) control group, (3, 4) morin group, (5, 6) CCl4 group, (7, 8) morin + CCl4 group. aP < 0.05 vs control group, cP < 0.05 vs morin group, eP < 0.05 vs CCl4 group. The CCl4 and morin + CCl4 groups showed significantly increased expression compared to the control and morin groups (P < 0.05), and the expression levels in the morin + CCl4 group were lower than those in the CCl4 group (P < 0.05).
In the CCl4 and morin + CCl4 groups, the protein expression of Nrf2, HO-1, and NQO1 was statistically higher than that in the control and morin groups (P < 0.05), while these protein factors of the morin + CCl4 rats had more expression compared to the CCl4 group (P < 0.05) (Figure 7).
Figure 7.
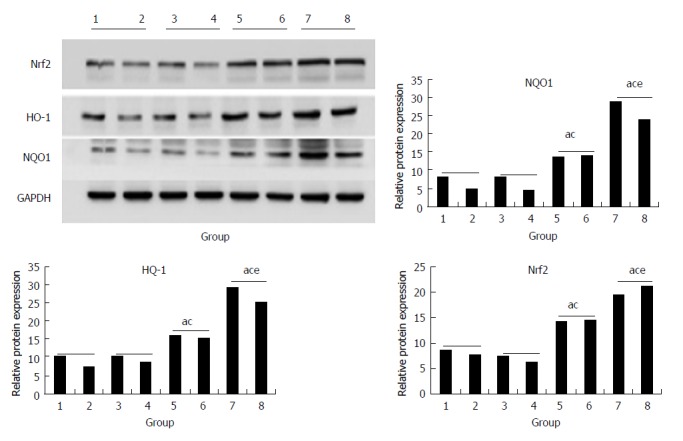
The protein expression of Nrf2, HO-1, and NQO1. (1, 2) control group, (3, 4) morin group, (5, 6) CCl4 group, (7, 8) morin + CCl4 group. aP < 0.05 vs control group, cP < 0.05 vs morin group, eP < 0.05 vs CCl4 group. In the CCl4 and morin + CCl4 groups, the protein expression was increased compared to the control and morin groups (P < 0.05); the morin + CCl4 group had a more significant change compared to the CCl4 group (P < 0.05).
DISCUSSION
Liver fibrosis is a process of continuous damage to the liver blood vessels and hepatic cells with nodule formation, which may develop into cirrhosis and cancerous lesions. Research of fibrosis at the cellular and molecular levels suggested that the progression of liver injury was closely related to oxidative stress and lipid peroxidation[32,33], leading to cell destruction and inducing hepatic fibrosis. HSCs can be activated by lipid peroxides acting as products of cell damage. After HSC activation, lipid droplets and vitamin A in the cytoplasm could be reduced or exhausted with α-SMA expression, accompanied by liver structural and functional changes resulting from redundant secretion of ECM[34]. However, it is possible to reverse liver fibrosis and early cirrhosis with effective interventions. Previous studies have shown that antioxidants have a protective effect by inhibiting the expression of α-SMA in HSC[35], thus, inhibition of oxidative stress in the liver may reduce and even reverse liver fibrosis[36].
Pathological features of liver fibrosis are reflected by fibrous tissue hyperplasia around the portal area and central vein and forming an interval of destruction of the lobular structure, accompanied by regenerative nodules and even early cirrhosis[37]. The pathological findings in this study showed that liver tissue in the CCl4 group had liver cell necrosis, fibrous tissue hyperplasia, interval widening, and pseudolobuli replacing normal lobular architecture. In the morin + CCl4 group, the liver tissue showed minimal cell necrosis with less interstitial collagen fibers and lobular structure damage compared with the CCl4 group. Thus, morin could effectively protect the liver tissue by reducing inflammation and inhibiting collagen deposition and fiber hyperplasia.
There are various enzymes that take part in liver metabolism. The damaged liver cells by pathogenic factors will produce free enzymes that are released into the bloodstream[20]. Liver function and status could be assessed by assaying the contents of serum enzymes. Aminotransferases play an important role in hepatic metabolism. When the liver cells are damaged, the serum ALT and AST levels as well as ALP level will be increased[38]. In this study, in the CCl4-induced liver fibrosis rat model, the values of serum ALT, AST, and ALP were reduced with morin administration, which implied that morin can reduce liver cell injury and thus prevent liver fibrosis. This also gives support for morin being able to condition the hepatocytes, protect against membrane frailty, and decrease the outflow of enzymes into circulation. These results are in accordance with previous studies that showed the ability of morin to inhibit hepatotoxicity[39,40].
The amount of collagen accounts for 5%-10% of the total protein in human liver tissue. If the liver injury leads to fibrosis, the collagen content in the liver protein will be significantly increased up to approximately 50%, becoming an important component of ECM[41] and ultimately leading to irreversible cirrhosis changes[42]. Liver fibrosis is a common histological change in liver disease, which is mainly manifested by excessive deposition of ECM, such as type I and type III collagen, and the expression of α-SMA[43]. At present, it is believed that the ECM actively participates in the occurrence and development of fibrosis, which has a great influence on HSC activation[44-46]. Both in vitro and in vivo experiments found that ECM synthesis was increased when liver tissue was damaged and further caused the activation of HSCs, which was based on the secretion of type I and III collagen[47-49], ultimately promoting the occurrence of liver fibrosis. In our study, using both real-time PCR and Western blot methods, it was found that the control and morin groups had only minimal expression of collagen I, collagen III, and α-SMA, which may represent normal physiological function of the liver, while their expression in the CCl4 group was significantly increased and had great relevance to the severity of liver fibrosis. With morin intervention reducing the expression of collagen I, collagen III, and α-SMA, the degree of liver fibrosis was relieved, which was evidenced by liver histopathology and serum measurements. All these results suggested that the anti-fibrotic effect of morin may be related to the down-regulation of the expression of collagen I, collagen III, and α-SMA.
Nrf2 is a key nuclear transcription factor in the oxidative stress of various cells[50]. Under normal circumstances, Nrf2 and Keapl are in a binding state in the cytoplasm[51]; they will appear dissociated when oxidative stress is occurring[52] and combine with antioxidant components as dimers, which are involved in the synthesis of antioxidase and phase II detoxification enzymes and prevent the occurrence of liver fibrosis by improving the antioxidant capacity of the liver[53]. HO-1 and NQO-1 are well characterized Nrf2-dependent antioxidant defense genes. Studies have suggested that Nrf2 and its downstream antioxidant factors HO-1 and NQO1 may contribute to improvement of liver fibrosis[54]. It has been reported that morin could promote the nuclear translocation of Nrf2 in order to play its biological role and be used as an exogenous agonist of Nrf2[55]. In this study, a CCl4 induced liver fibrosis model, along with morin as an intervention, was used to observe the expression of Nrf2 and its downstream products NQO1 and HO-1 in different groups. The results showed that the expression of Nrf2, NQO1, and HO-1 was slightly increased in the CCl4 group compared with the control and morin groups (P < 0.05). This might be due to Nrf2 activation acting as a cellular adaptive response against CCl4-induced toxicity. Nrf2 activation was initiated as soon as the subjects were challenged by CCl4-induced oxidative stress. However, it was unable to completely overcome the toxicity, while the adaptively stimulated Nrf2 might alleviate or delay the deleterious effects of CCl4. The expression of Nrf2 and its downstream products NQO1 and HO-1 was evidently increased in the morin-treated group, indicating that morin administration could enhance this effect. Additionally, this supports morin playing an important role in the prevention and treatment of liver fibrosis via the Nrf2 pathway.
This study has several limitations. First, the sample size was small, which easily led to individual differences and statistical error between the groups. Second, the anti-fibrotic mechanism of morin may be related to activation of the Nrf2 antioxidant pathway and expression of its downstream antioxidases. Further experiments are needed to confirm the specific mechanism of the morin intervention.
In summary, our current study showed that morin could play a protective role by inducing the expression of Nrf2 and its downstream antioxidant factors (HO-1 and NQO1) and reducing the expression of α-SMA, collagen I, and collagen III in a rat model of CCl4-induced hepatic fibrosis. Although further studies are required, our study demonstrated that morin could effectively alleviate chronic liver damage by activation of the Nrf2 pathway.
ARTICLE HIGHLIGHTS
Research background
Previous studies have shown that the pathological changes of liver fibrosis, which refer to a series of pathogenic factors and pathological changes in the pathogenesis of a variety of liver diseases, could be reversed. In the past decade, numerous studies demonstrated that NF-E2-related factor 2 (Nrf2) as a transcription factor plays as an important role against oxidative stress in normal liver cells. Morin possesses biological properties, including antioxidant, anti-inflammatory, anti-apoptosis, and anticancer activities. To our knowledge, in vivo investigation of the effect of morin on the Nrf2 signaling pathway and Nrf2 expression in a CCl4-induced liver fibrosis model has not been reported previously.
Research motivation
Previous studies demonstrated that morin protected acute liver damage and ameliorated liver fibrosis induced by CCl4, and morin inhibited proliferation and induced apoptosis of activated hepatic satellate cells by suppressing the Wnt/β-catenin and the NF-kB signaling pathways. However, there is no molecular evidence about the effects of morin on the Nrf2 signaling pathway.
Research objectives
The purpose of this study was to investigate whether morin can reduce hepatic fibrosis by inducing the expression of Nrf2 and its downstream antioxidant enzymes in a rat model of CCl4-induced hepatic fibrosis.
Research methods
Twenty male Sprague-Dawley rats were randomly divided into four groups: control group, morin group, carbon tetrachloride (CCl4) group, and morin + CCl4 group. At the end-point of the experimental period, serum AST, ALT, and ALP were measured, and the liver specimens were obtained for pathological assessment. α-SMA, collagen I, collagen III, NF-E2-related factor 2 (Nrf2), heme oxygenase (HO-1), and quinone oxidoreductase 1 (NQO1) were analyzed by real-time PCR and Western blot methods using frozen liver specimens.
Research results
Rats in the morin + CCl4 group had less hyperplasia of fiber tissues, minimal inflammatory cells, and less body weight loss with favorable liver enzyme measurements compared to rats treated with CCl4 only. Additionally, morin-treated rats had significantly lower mRNA and protein expression of α-SMA, collagen I, and collagen III, but significantly higher mRNA and protein expression of Nrf2, HO-1, and NQO1 compared to rats treated with CCl4 only (P < 0.05).
Research conclusions
Our study showed that morin could play a protective role by inducing the expression of Nrf2 and its downstream antioxidant factors (HO-1 and NQO1) and reducing the expression of α-SMA, collagen I, and collagen III in a rat model of CCl4-induced hepatic fibrosis.
Research perspectives
Although further studies are required, out study demonstrated that morin could effectively alleviate chronic liver damage by activation of the Nrf2 pathway.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: This study was performed in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health of China (1996), and was approved by Animal Care and Use Committee of the China Medical University (2015038R).
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: September 22, 2017
First decision: October 10, 2017
Article in press: November 14, 2017
P- Reviewer: Deepak P, Faerch K S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Liang Sang, Department of Ultrasound, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Xue-Mei Wang, Department of Ultrasound, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, China. wangxuemei@cmu1h.com.
Dong-Yang Xu, Department of Ultrasound, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Li-Xuan Sang, Department of Geriatrics, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Yang Han, Department of Pathology, China Medical University, Shenyang 110001, Liaoning Province, China.
Long-Yang Jiang, Pharmacy College, China Medical University, Shenyang 110001, Liaoning Province, China.
References
- 1.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40:633–642. doi: 10.1165/rcmb.2008-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simula MP, De Re V. Hepatitis C virus-induced oxidative stress and mitochondrial dysfunction: a focus on recent advances in proteomics. Proteomics Clin Appl. 2010;4:782–793. doi: 10.1002/prca.201000049. [DOI] [PubMed] [Google Scholar]
- 3.Clichici S, Catoi C, Mocan T, Filip A, Login C, Nagy A, Daicoviciu D, Decea N, Gherman C, Moldovan R, et al. Non-invasive oxidative stress markers for liver fibrosis development in the evolution of toxic hepatitis. Acta Physiol Hung. 2011;98:195–204. doi: 10.1556/APhysiol.98.2011.2.11. [DOI] [PubMed] [Google Scholar]
- 4.Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Lin PY, Chen CH, Wallace CG, Chen KH, Chang CL, Chen HH, Sung PH, Lin KC, Ko SF, Sun CK, et al. Therapeutic effect of rosuvastatin and propylthiouracil on ameliorating high-cholesterol diet-induced fatty liver disease, fibrosis and inflammation in rabbit. Am J Transl Res. 2017;9:3827–3841. [PMC free article] [PubMed] [Google Scholar]
- 6.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Liao JZ, Li PY. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:1964–1973. doi: 10.3748/wjg.v23.i11.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 9.Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol. 2011;251:59–69. doi: 10.1016/j.taap.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma JQ, Ding J, Zhang L, Liu CM. Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin Res Hepatol Gastroenterol. 2015;39:188–197. doi: 10.1016/j.clinre.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Cao M, Wang H, Guo L, Yang S, Liu C, Khor TO, Yu S, Kong AN. Dibenzoylmethane Protects Against CCl4-Induced Acute Liver Injury by Activating Nrf2 via JNK, AMPK, and Calcium Signaling. AAPS J. 2017;19:1703–1714. doi: 10.1208/s12248-017-0133-1. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci. 2016;185:93–100. doi: 10.1007/s11845-014-1226-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zou L, Li L, Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013;8:e53662. doi: 10.1371/journal.pone.0053662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudoh K, Uchinami H, Yoshioka M, Seki E, Yamamoto Y. Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann Surg. 2014;260:118–127. doi: 10.1097/SLA.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni YH, Huo LJ, Li TT. Antioxidant axis Nrf2-keap1-ARE in inhibition of alcoholic liver fibrosis by IL-22. World J Gastroenterol. 2017;23:2002–2011. doi: 10.3748/wjg.v23.i11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Wijeratne SS, Abou-Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem. 2006;54:312–318. doi: 10.1021/jf051692j. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Sivaramakrishnan V, Shilpa PN, Praveen Kumar VR, Niranjali Devaraj S. Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel flavonol-Morin. Chem Biol Interact. 2008;171:79–88. doi: 10.1016/j.cbi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Heeba GH, Mahmoud ME. Therapeutic potential of morin against liver fibrosis in rats: Modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ Toxicol Pharmacol. 2014;37:662–671. doi: 10.1016/j.etap.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Merwid-Ląd A, Trocha M, Chlebda-Sieragowska E, Sozański T, Szandruk M, Magdalan J, Ksiądzyna D, Pieśniewska M, Fereniec-Gołębiewska L, Kwiatkowska J, et al. The impact of morin, a natural flavonoid, on cyclophosphamide-induced changes in the oxidative stress parameters in rat livers. Adv Clin Exp Med. 2014;23:505–509. doi: 10.17219/acem/37213. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zhang DM, Gu TT, Ding XQ, Fan CY, Zhu Q, Shi YW, Hong Y, Kong LD. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochem Pharmacol. 2013;86:1791–1804. doi: 10.1016/j.bcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 23.MadanKumar P, NaveenKumar P, Devaraj H, NiranjaliDevaraj S. Morin, a dietary flavonoid, exhibits anti-fibrotic effect and induces apoptosis of activated hepatic stellate cells by suppressing canonical NF-κB signaling. Biochimie. 2015;110:107–118. doi: 10.1016/j.biochi.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Sivaramakrishnan V, Devaraj SN. Morin fosters apoptosis in experimental hepatocellular carcinogenesis model. Chem Biol Interact. 2010;183:284–292. doi: 10.1016/j.cbi.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee MH, Han MH, Lee DS, Park C, Hong SH, Kim GY, Hong SH, Song KS, Choi IW, Cha HJ, et al. Morin exerts cytoprotective effects against oxidative stress in C2C12 myoblasts via the upregulation of Nrf2-dependent HO-1 expression and the activation of the ERK pathway. Int J Mol Med. 2017;39:399–406. doi: 10.3892/ijmm.2016.2837. [DOI] [PubMed] [Google Scholar]
- 26.Hsiang CY, Wu SL, Ho TY. Morin inhibits 12-O-tetradecanoylphorbol-13-acetate-induced hepatocellular transformation via activator protein 1 signaling pathway and cell cycle progression. Biochem Pharmacol. 2005;69:1603–1611. doi: 10.1016/j.bcp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa S, Sakamoto H, Tano H. Inhibitory effects of flavonoids on free radical-induced hemolysis and their oxidative effects on hemoglobin. Chem Pharm Bull (Tokyo) 2004;52:999–1001. doi: 10.1248/cpb.52.999. [DOI] [PubMed] [Google Scholar]
- 28.Williams AT, Burk RF. Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Semin Liver Dis. 1990;10:279–284. doi: 10.1055/s-2008-1040483. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Jung KH, Hong SW, Park IS, Lee C, Han HK, Lee DH, Hong SS. Morin protects acute liver damage by carbon tetrachloride (CCl(4)) in rat. Arch Pharm Res. 2008;31:1160–1165. doi: 10.1007/s12272-001-1283-5. [DOI] [PubMed] [Google Scholar]
- 30.MadanKumar P, NaveenKumar P, Manikandan S, Devaraj H, NiranjaliDevaraj S. Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferation in vitro by suppressing Wnt/β-catenin signaling. Toxicol Appl Pharmacol. 2014;277:210–220. doi: 10.1016/j.taap.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 32.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 33.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ismail MH, Pinzani M. Reversal of liver fibrosis. Saudi J Gastroenterol. 2009;15:72–79. doi: 10.4103/1319-3767.45072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Wang XF, Shi JJ, Li YP, Yang N, Zhai S, Dang SS. Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World J Gastroenterol. 2015;21:3893–3903. doi: 10.3748/wjg.v21.i13.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J Gastroenterol. 2014;20:17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yachi R, Igarashi O, Kiyose C. Protective Effects of Vitamin E Analogs against Carbon Tetrachloride-Induced Fatty Liver in Rats. J Clin Biochem Nutr. 2010;47:148–154. doi: 10.3164/jcbn.10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prahalathan P, Kumar S, Raja B. Effect of morin, a flavonoid against DOCA-salt hypertensive rats: a dose dependent study. Asian Pac J Trop Biomed. 2012;2:443–448. doi: 10.1016/S2221-1691(12)60073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankari SG, Karthikesan K, Jalaludeen AM, Ashokkumar N. Hepatoprotective effect of morin on ethanol-induced hepatotoxicity in rats. J Basic Clin Physiol Pharmacol. 2010;21:277–294. doi: 10.1515/jbcpp.2010.21.4.277. [DOI] [PubMed] [Google Scholar]
- 41.Milani S, Herbst H, Schuppan D, Surrenti C, Riecken EO, Stein H. Cellular localization of type I III and IV procollagen gene transcripts in normal and fibrotic human liver. Am J Pathol. 1990;137:59–70. [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 43.Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 44.Shi MN, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Effect of IL-10 on the expression of HSC growth factors in hepatic fibrosis rat. World J Gastroenterol. 2005;11:4788–4793. doi: 10.3748/wjg.v11.i31.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 46.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 47.Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12:250–256. doi: 10.1016/s0955-0674(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology. 2006;44:612–622. doi: 10.1002/hep.21315. [DOI] [PubMed] [Google Scholar]
- 49.Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33–36. doi: 10.3748/wjg.v7.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan FJ, Chen YS, Azat R, Zheng XD. Mulberry Anthocyanin Extract Ameliorates Oxidative Damage in HepG2 Cells and Prolongs the Lifespan of Caenorhabditis elegans through MAPK and Nrf2 Pathways. Oxid Med Cell Longev. 2017 doi: 10.1155/2017/7956158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wortham M, He L, Gyamfi M, Copple BL, Wan YJ. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig Dis Sci. 2008;53:2761–2774. doi: 10.1007/s10620-007-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin F, Wan C, Li W, Yao L, Zhao H, Zou Y, Peng D, Huang W. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PloS One. 2017;12:e0170900. doi: 10.1371/journal.pone.0170900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Q, Zhang H, Cao Y, Li Y, Sun S, Zhang J, Zhang G. Schisandrin B attenuates CCl4-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des Devel Ther. 2017;11:2179–2191. doi: 10.2147/DDDT.S137507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang W, Jiang YF, Ponnusamy M, Diallo M. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20:13079–13087. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]


