Abstract
AIM
To assess the predictive value of the tumor-associated neutrophil-to-lymphocyte ratio in terms of the clinical outcomes of patients with gastric neuroendocrine neoplasms after radical surgery.
METHODS
Data were retrospectively collected from 142 patients who were diagnosed with gastric neuroendocrine neoplasms and who underwent radical gastrectomy at our department from March 2006 to March 2015. These data were retrospectively analyzed, and a receiver operating characteristic curve analysis was used to identify the optimal value of the tumor-associated neutrophil-to-lymphocyte ratio. Univariate and multivariate survival analyses were used to identify prognostic factors. A nomogram was then applied to predict clinical outcomes after surgery.
RESULTS
The tumor-associated neutrophil-to-lymphocyte ratio was significantly associated with tumor recurrence, especially with liver metastasis and lymph node metastasis (P < 0.05 for both), but not with clinical characteristics (P > 0.05 for all). A multivariate Cox regression analysis identified the tumor-associated neutrophil-to-lymphocyte ratio as an independent prognostic factor for recurrence-free survival and overall survival (P < 0.05 for both). The concordance index of the nomograms, which included the tumor-associated neutrophil-to-lymphocyte ratio, Ki-67 index, and lymph node ratio, was 0.788 (0.759) for recurrence-free survival (overall survival) and was higher than the concordance index of the traditional TNM staging system [0.672 (0.663)].
CONCLUSION
The tumor-associated neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with gastric neuroendocrine neoplasms. Nomograms that include the tumor-associated neutrophil-to-lymphocyte ratio, Ki-67 index, and lymph node ratio have a superior ability to predict clinical outcomes of postoperative patients.
Keywords: Gastric neuroendocrine neoplasms, Tumor-associated neutrophil-to-lymphocyte ratio, Tumor recurrence, Prognosis
Core tip: The study aimed to assess the predictive value of the tumor-associated neutrophil-to-lymphocyte ratio in terms of the clinical outcomes of 142 patients diagnosed with gastric neuroendocrine neoplasms. We demonstrated that the tumor-associated neutrophil-to-lymphocyte ratio was significantly correlated with tumor recurrence, especially with liver and lymph node metastasis. Moreover, the tumor-associated neutrophil-to-lymphocyte ratio was found to be an independent predictor of recurrence-free survival and overall survival, and combining it with the Ki-67 index and lymph node ratio could improve prognosis prediction in patients with gastric neuroendocrine neoplasms, as could applying the traditional TNM staging system.
INTRODUCTION
Gastric neuroendocrine neoplasms (g-NENs) are a highly heterogeneous and poorly understood group of relatively rare tumors that are derived primarily from enterochromaffin-like cells (ECL-cells) localized in the gastric mucosa[1]. Due to an increased understanding of g-NENs and improved diagnostic techniques, the incidence of g-NENs, which account for 6% of all neuroendocrine neoplasms, is increasing every year[2]. However, due to significant differences in the clinical pathology and biological characteristics, our knowledge regarding g-NENs is still very limited[3,4]. The World Health Organization (WHO, 2010) classifies g-NENs into the following subclasses: neuroendocrine tumors (g-NETs), neuroendocrine carcinoma (g-NEC), and mixed adenoneuroendocrine carcinoma (g-MANEC)[5]. In addition to an early diagnosis, an important and effective component of proper management is the identification of the prognostic factors in patients with g-NENs. According to the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC), the TNM staging system, which accounts for invasion depth, lymph node status, and metastases, is one of the most important prognostic factors in patients with g-NENs[6,7]. However, the prognostic factors of these tumors are complex and multifaceted and have not been clearly defined thus far[8,9]. In the past decade, increasing evidence has suggested that both tumor-associated neutrophils (TANs) and tumor-associated lymphocytes (TALs) are significantly associated with patient prognosis. Elevated TANs and reduced TALs correlate with advanced stage and poor prognosis in a variety of human tumors, including cervical cancer[10], hepatocellular carcinoma[11], and pancreatic cancer[12]. However, few studies have focused on the relationship between the tumor-associated neutrophil-to-lymphocyte ratio (TA-NLR) and the prognosis of patients with neuroendocrine neoplasms, particularly g-NENs.
This study investigated the utility of the TA-NLR as a prognostic indicator and evaluated its clinical value for the diagnosis and postoperative surveillance of patients undergoing radical surgery for g-NENs.
MATERIALS AND METHODS
General conditions
Overall, 173 patients who were diagnosed with g-NENs at Fujian Medical University Union Hospital between March 2006 and March 2015 were identified from a prospective database. The exclusion criteria for this study were as follows: metastatic disease confirmed preoperatively or during surgery (n = 11), perioperative death (n = 1), and incomplete/inaccurate medical records (n = 19). In all, 142 patients who underwent radical surgery were included in this study. The pathological data of these patients were reconfirmed by two pathologists according to the North American Neuroendocrine Tumor Society (NANETS) guidelines (2010)[13]. In total, 27 (19.0%) patients were diagnosed with g-NETs, 45 (31.7%) with g-NEC, and 70 (49.3%) with g-MANEC. The ethics committee of Fujian Union Hospital approved this retrospective study. Written consent was obtained from the patients, and their information was stored in the hospital database and used for research.
Immunohistochemistry analysis
Immunohistochemical staining for CD8 or CD15 was performed using formalin-fixed, paraffin-embedded tumor tissue sections (4-μm-thick) from 142 g-NENs (Figure 1A). Briefly, the slides were baked at 65 °C for 2 h, deparaffinized with xylene, and rehydrated in graded alcohol. The slides were subjected to antigen retrieval via the high-pressure method in antigen retrieval solution. Endogenous peroxidase was inactivated using 3% H2O2 in methanol. Non-specific binding was blocked via incubation in 1% bovine serum albumin (BSA; Sigma-Aldrich; St. Louis, MO, United States) in phosphate buffered saline (PBS). Subsequently, the slides were incubated overnight at 4 °C with a primary monoclonal mouse antibody against CD8 or CD15 (1:100 dilution; Zhongshan Golden Bridge Biotech, Beijing, China). Normal goat serum was used as a negative control. After being washed with PBS, tissue sections were incubated with the secondary antibody (Zhongshan Golden Bridge Biotech, Beijing, China) for 20 min at room temperature and then stained with diaminobenzidine (DAB). Finally, the slides were counterstained in hematoxylin, dehydrated, and mounted with a coverslip.
Figure 1.
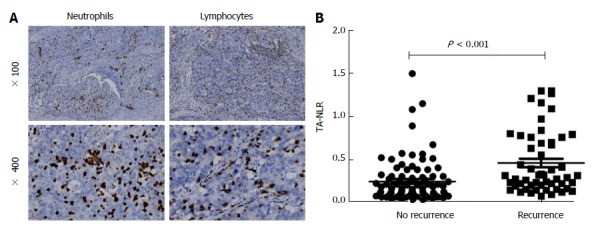
Relationship between the tumor-associated neutrophil-to-lymphocyte ratio and tumor recurrence. A: Representative immunohistochemical staining for CD15 (left) and CD8 (right); B: Significant differences in the TA-NLR were observed between the recurrence group (0.46% ± 0.05%, mean ± SE) and the non-recurrence group (0.24% ± 0.03%, P < 0.001). TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
Two pathologists who were blinded to the clinical data reviewed the immunoreactivity under a light microscope. Inflammatory cells that had infiltrated the tumor nest and tumor stroma were analyzed, and inflammatory cells that were confined to lymph vascular spaces or within the vicinity of tumor necrosis or secretions were excluded from the analysis. Cases with tumor-infiltrating inflammatory cells present in 10 non-overlapping high- power fields (× 40) were examined in representative areas on two slides of a given tumor (i.e., a total of 20 fields per neoplasm). The number of tumor-related inflammatory cells was assessed in a semiquantitative manner using the mean value of high-power fields based on a × 40 objective (magnification × 400)[14,15]. The TA-NLR was calculated as the average number of neutrophils (CD15-positive cells) divided by the average number of T lymphocytes (CD8-positive cells). A receiver operating characteristic (ROC) curve analysis was performed in relation to the occurrence of recurrence and death from any cause. For all 142 patients, a TA-NLR of 0.21 had the highest sensitivity and specificity for both outcomes. Therefore, patients were categorized into the following two groups: low TA-NLR group (≤ 0.21, 71 patients) and high TA-NLR group (> 0.21, 71 patients).
Postoperative follow-up
The patients were monitored after surgery via telephone interviews, outpatient visits, and letters. Our department follows a standardized surveillance protocol and follows patients at three-month intervals for the first two years, six-month intervals for years two to five, and at least once per year five years after surgery. The postoperative follow-up data included clinical symptoms and signs, laboratory tests, imaging examinations, and endoscopy and biopsy results. In this study, the median follow-up time was 40 mo (range, 2-106 mo). The overall survival (OS) time was calculated as the number of months from the date of surgery to the date of last contact, date of death from any cause, or the date the end point was realized. The recurrence-free survival (RFS) time was calculated as the number of months from the date of surgery to the date of identification of disease recurrence (either radiological or histological), the date of death or last contact, or the date the end point was realized.
Statistical analysis
All enumeration and measurement data were analyzed using SPSS 17.0 for Windows (SPSS, Chicago, IL, United States). χ2 test, Fisher’s exact test, or unpaired Student’s t test was utilized to compare the differences between the TA-NLR groups and the clinicopathological factors, as appropriate. A univariate survival analysis was performed using the Kaplan-Meier method. A multivariate survival analysis was performed using a Cox proportional hazards model, and the significant variables from the univariate analysis were included in the model. R software (version 3.2.0) was utilized to develop the nomograms and the forest plot. P < 0.05 was considered significant.
RESULTS
TA-NLR is not associated with clinicopathological factors
The univariate analysis revealed that the TA-NLR was associated with the invasion depth, LNR (lymph node ratio), and postoperative complications (P < 0.05 for all; Table 1). However, the multivariate analysis revealed no significant differences in the clinicopathological factors between the two groups (P > 0.05 for all; Table 1). In addition, no significant differences were observed in the clinical symptoms, medical history, family history, active and past smoking histories, or history of heavy alcohol use between the two groups (P > 0.05 for all; Table 2).
Table 1.
Characteristics of the 142 patients with gastric neuroendocrine neoplasms with different tumor-associated neutrophil-to-lymphocyte ratios
| Clinicopathological feature |
TA-NLR |
Univariate analysis |
Multivariate analysis |
|
| ≤ 0.21 (n = 71) | > 0.21 (n = 71) | P value | P value | |
| Age (yr) | 0.322 | |||
| ≤ 70 | 57 | 52 | ||
| > 70 | 14 | 19 | ||
| Gender | 0.851 | |||
| Male | 52 | 51 | ||
| Female | 19 | 20 | ||
| Tumor site | 0.099 | |||
| Upper | 39 | 26 | ||
| Middle | 10 | 15 | ||
| Lower | 17 | 18 | ||
| Mixed | 5 | 12 | ||
| Tumor size (cm) | 0.593 | |||
| ≤ 3.5 | 25 | 22 | ||
| > 3.5 | 46 | 49 | ||
| Ki-67 index (%) | 0.081 | |||
| ≤ 2 | 13 | 6 | ||
| ≥ 3, ≤ 20 | 8 | 16 | ||
| >20 | 50 | 49 | ||
| Depth of invasion | 0.044 | 0.406 | ||
| T1 | 14 | 8 | ||
| T2 | 7 | 3 | ||
| T3 | 34 | 29 | ||
| T4 | 16 | 31 | ||
| Lymph node ratio | 0.043 | 0.355 | ||
| 0 | 25 | 13 | ||
| > 0, ≤ 0.2 | 25 | 24 | ||
| > 0.2, ≤ 0.4 | 15 | 18 | ||
| > 0.4 | 6 | 16 | ||
| Lymphovascular invasion | 0.610 | |||
| No | 43 | 40 | ||
| Yes | 28 | 31 | ||
| ASA status | 0.805 | |||
| 1 + 2 | 61 | 62 | ||
| 3 + 4 | 10 | 9 | ||
| Postoperative complication | 0.041 | 0.071 | ||
| No | 57 | 46 | ||
| Yes | 14 | 25 | ||
| Surgical approach | 0.855 | |||
| Endo/laparoscopic | 49 | 50 | ||
| Open | 22 | 21 | ||
TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
Table 2.
Characteristics of 142 patients with gastric neuroendocrine neoplasms with different levels of tumor-associated neutrophil-to-lymphocyte ratios
| Patient feature |
TA-NLR |
Univariate analysis |
|
| ≤ 0.21 (n = 71) | > 0.21 (n = 71) | P value | |
| Symptom | |||
| Abdominal pain | 46 | 41 | 0.390 |
| Dysphagia | 14 | 12 | 0.665 |
| Nausea | 12 | 9 | 0.480 |
| Vomiting | 5 | 5 | 1.000 |
| Acid-reflux | 9 | 4 | 0.156 |
| Anemia | 10 | 13 | 0.495 |
| Abdominal distention | 6 | 8 | 0.575 |
| Gastrointestinal blood loss | 12 | 14 | 0.665 |
| Weight loss | 24 | 29 | 0.386 |
| No symptoms | 2 | 7 | 0.105 |
| Medical history | |||
| Hypertension | 19 | 15 | 0.432 |
| Diabetes | 7 | 3 | 0.202 |
| Coronary heart disease | 4 | 4 | 1.000 |
| Chronic gastritis | 44 | 38 | 0.796 |
| Family history | 5 | 6 | 0.754 |
| Smoking | 26 | 27 | 0.862 |
| Drinking | 6 | 4 | 0.515 |
TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
Elevated TA-NLR is associated with a poor prognosis
As shown in Figure 2, the RFS and OS were analyzed according to age, gender, tumor site and size, lymphovascular invasion, ASA status, postoperative complications, surgical approach, invasion depth, LNR, and Ki-67 index. The hazard ratios and 95% confidence interval (CI) for the RFS and OS were compared between the subgroups. The long-term survival time, including RFS and OS, was shorter in the high TA-NLR group compared with the low TA-NLR group.
Figure 2.
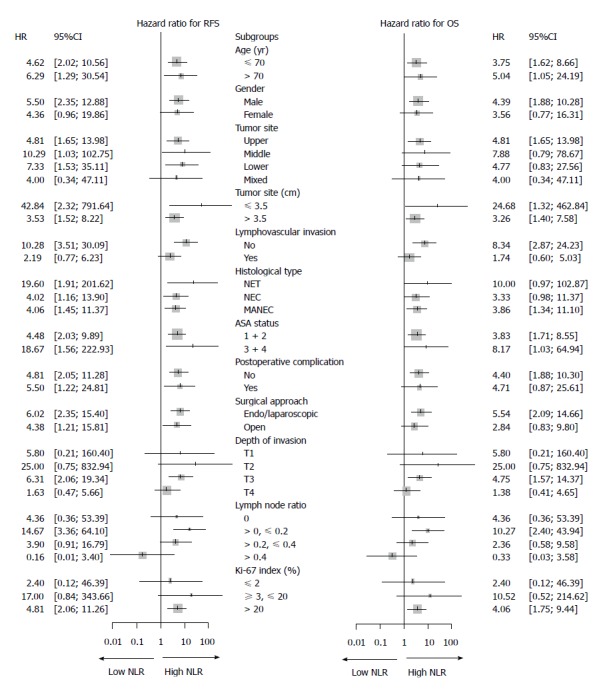
Forest plot showing the hazard ratios (oblongs) and 95%CIs (bars) for RFS (left) and OS (right) (according to subgroups) among 142 patients with gastric neuroendocrine neoplasms undergoing radical surgery. Long-term survival, including RFS and OS, was better among patients with a low TA-NLR than in patients with a high TA-NLR. g-NENs: Gastric neuroendocrine neoplasms. RFS: Recurrence-free survival; OS: Overall survival; TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
TA-NLR is an independent prognostic factor for RFS and OS
The univariate analysis found that larger tumor size, occurrence of postoperative complications, greater invasion depth, higher LNR, higher Ki-67 index, and higher TA-NLR were prognostic indicators of poorer RFS (P < 0.05 for all; Table 3). The tumor size, invasion depth, LNR, Ki-67 index, and TA-NLR were identified as prognostic indicators of OS (P < 0.05 for all; Table 4). According to the multivariate analysis, the Ki-67 index, LNR, and TA-NLR were independent prognostic factors of RFS and OS (P < 0.05 for all; Tables 3 and 4).
Table 3.
Variables associated with recurrence-free survival according to the Cox proportional hazards regression model
| Variable |
Univariate analysis |
Multivariate analysis |
||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Age (yr) | 0.790 | |||||
| ≤ 70 | Reference | |||||
| > 70 | 1.083 | 0.602-1.950 | ||||
| Gender | 0.126 | |||||
| Male | Reference | |||||
| Female | 0.608 | 0.322-1.149 | ||||
| Tumor site | 0.770 | |||||
| Upper | Reference | |||||
| Middle | 0.825 | 0.389-1.751 | ||||
| Lower | 0.885 | 0.465-1.682 | ||||
| Mixed | 1.348 | 0.588-3.091 | ||||
| Tumor size (cm) | 0.004 | 0.671 | ||||
| ≤ 3.5 | Reference | Reference | ||||
| > 3.5 | 2.740 | 1.385-5.421 | NA | NA | ||
| Lymphovascular invasion | 0.144 | |||||
| No | Reference | |||||
| Yes | 1.471 | 0.876-2.468 | ||||
| ASA status | 0.190 | |||||
| 1 + 2 | Reference | |||||
| 3 + 4 | 1.551 | 0.804-2.993 | ||||
| Postoperative complication | 0.029 | |||||
| No | Reference | Reference | 0.305 | |||
| Yes | 1.869 | 1.065-3.278 | NA | NA | ||
| Surgical approach | 0.249 | |||||
| Endo/laparoscopic | Reference | |||||
| Open | 0.733 | 0.432-1.243 | ||||
| Depth of invasion | 0.005 | 0.557 | ||||
| T1 | Reference | Reference | ||||
| T2 | 5.328 | 0.483-58.789 | NA | NA | ||
| T3 | 11.722 | 1.587-86.603 | NA | NA | ||
| T4 | 19.301 | 2.629141.682 | NA | NA | ||
| Lymph node ratio | < 0.001 | < 0.001 | ||||
| 0 | Reference | Reference | ||||
| > 0, ≤ 0.2 | 5.490 | 1.623-18.568 | 3.338 | 0.962-11.581 | ||
| > 0.2, ≤ 0.4 | 8.091 | 2.393-27.351 | 4.6 | 1.317-16.066 | ||
| > 0.4 | 17.946 | 5.239-61.480 | 10.266 | 2.906-36.266 | ||
| Ki-67 index (%) | 0.004 | < 0.001 | ||||
| ≤ 2 | Reference | Reference | ||||
| ≥ 3, ≤ 20 | 3.013 | 0.639-14.203 | 1.501 | 0.305-7.393 | ||
| > 20 | 7.047 | 1.709-29.053 | 4.999 | 1.140-21.927 | ||
| TA-NLR | < 0.001 | < 0.001 | ||||
| ≤ 0.21 | Reference | Reference | ||||
| > 0.21 | 3.366 | 1.890-5.992 | 2.974 | 1.630-5.426 | ||
TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
Table 4.
Variables associated with overall survival according to the Cox proportional hazards regression model
| Variable |
Univariate analysis |
Multivariate analysis |
||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Age (yr) | 0.566 | |||||
| ≤ 70 | Reference | |||||
| > 70 | 1.197 | 0.648- 2.211 | ||||
| Gender | 0.190 | |||||
| Male | Reference | |||||
| Female | 0.640 | 0.329-1.247 | ||||
| Tumor site | 0.190 | |||||
| Upper | Reference | |||||
| Middle | 0.687 | 0.313-1.509 | ||||
| Lower | 0.540 | 0.255-1.145 | ||||
| Mixed | 1.441 | 0.628-3.307 | ||||
| Tumor size (cm) | 0.002 | 0.214 | ||||
| ≤ 3.5 | Reference | Reference | ||||
| > 3.5 | 3.591 | 1.618-7.969 | NA | NA | ||
| Lymphovascular invasion | 0.214 | |||||
| No | Reference | |||||
| Yes | 1.417 | 0.818-2.455 | ||||
| ASA status | 0.118 | |||||
| 1 + 2 | Reference | |||||
| 3 + 4 | 1.736 | 0.870-3.465 | ||||
| Postoperative complication | 0.320 | |||||
| No | Reference | |||||
| Yes | 1.380 | 0.732-2.603 | ||||
| Surgical approach | 0.276 | |||||
| Endo/laparoscopic | Reference | |||||
| Open | 0.736 | 0.425-1.276 | ||||
| Depth of invasion | 0.024 | 0.646 | ||||
| T1 | Reference | Reference | ||||
| T2 | 5.524 | 0.501-60.954 | NA | NA | ||
| T3 | 10.793 | 1.455-80.038 | NA | NA | ||
| T4 | 15.632 | 2.116-115.464 | NA | NA | ||
| Lymph node ratio | < 0.001 | 0.002 | ||||
| 0 | Reference | Reference | ||||
| > 0, ≤ 0.2 | 4.791 | 1.402-16.370 | 2.854 | 0.813-10.027 | ||
| > 0.2, ≤ 0.4 | 6.676 | 1.956-22.790 | 3.724 | 1.054-13.162 | ||
| > 0.4 | 14.677 | 4.218-51.074 | 9.152 | 2.528-33.129 | ||
| Ki-67 index (%) | 0.002 | < 0.001 | ||||
| ≤ 2% | Reference | Reference | ||||
| ≥ 3%, ≤ 20% | 2.168 | 0.437-10.751 | 1.584 | 0.313-8.008 | ||
| > 20% | 6.582 | 1.589-27.269 | 5.535 | 1.238-24.752 | ||
| TA-NLR | < 0.001 | 0.003 | ||||
| ≤ 0.21 | Reference | Reference | ||||
| > 0.21 | 2.938 | 1.610-5.360 | 2.617 | 1.389-4.928 | ||
TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
TA-NLR is significantly correlated with recurrence site
The TA-NLR was significantly higher in the recurrence group than in the non-recurrence group (P < 0.05 for both; Figure 1B). Details regarding the recurrence site following surgery are listed in Table 5. The recurrence rate was significantly higher in the high TA-NLR group compared with the low TA-NLR group (P < 0.001). Additionally, an elevated TA-NLR was significantly associated with both liver metastasis and lymph node metastasis (P < 0.05 for both).
Table 5.
Site of recurrence after surgery
| Site of recurrence |
TA-NLR |
||
| ≤ 0.21 (n = 71) | > 0.21 (n = 71) | P value | |
| Liver | 10 | 28 | 0.001 |
| Peritoneal cavity | 6 | 9 | 0.413 |
| Lymph node | 2 | 11 | 0.009 |
| Lung | 3 | 4 | 0.721 |
| Bone | 0 | 5 | 0.058 |
| Adrenal gland | 1 | 4 | 0.366 |
| Pancreas | 2 | 2 | 1.000 |
| Locoregional recurrence | 2 | 3 | 0.683 |
| Spleen | 0 | 2 | 0.496 |
| Kidney | 1 | 1 | 1.000 |
| Brain | 0 | 1 | 1.000 |
| Number of patients with recurrence | 15 | 38 | < 0.001 |
TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
TA-NLR, combined with the Ki-67 index and LNR, is a superior prognostic prediction system
Prognostic nomograms were established using R software (Figure 3). The C-index of the nomograms for RFS (OS), which included the TA-NLR, LNR, and Ki-67 index, was 0.788 (0.759). However, the C-index of the TNM staging system for RFS (OS) was 0.673 (0.662) (Figure 4). Thus, both the TNM staging system and the nomograms had superior abilities to predict clinical outcomes for patients with g-NENs.
Figure 3.
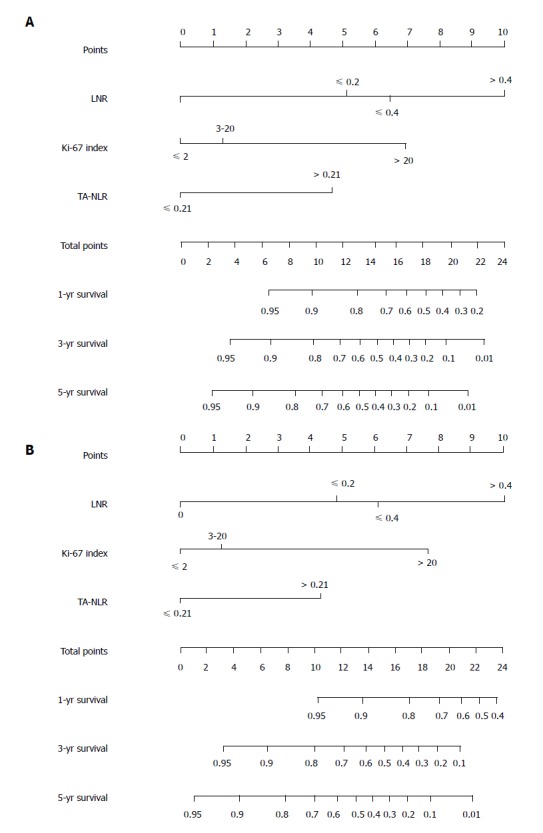
Nomograms for the prediction of recurrence-free survival (A) and overall survival (B) in patients following gastric neuroendocrine neoplasm resection; the C-index was 0.788 and 0.759 for RFS and OS, respectively. LNR: Lymph node ratio; TA-NLR: Tumor-associated neutrophil-to-lymphocyte ratio.
Figure 4.
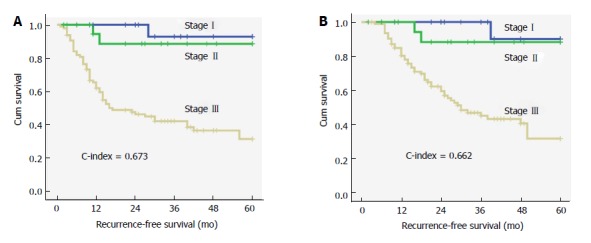
Survival curves for recurrence-free survival (A) and overall survival (B) according to the traditional TNM staging system (NCCN 2015); the C-index was 0.673 and 0.662 for RFS and OS, respectively.
DISCUSSION
Neuroendocrine neoplasms, particularly g-NENs in the digestive system, are a unique subgroup of tumors with great clinical heterogeneity and varied biology. In recent years, with the growing popularity of upper gastrointestinal endoscopy and increasing improvements in diagnostic techniques, the reported incidence of g-NENs has increased each year, and currently, the incidence is approximately 0.3 per 100 thousand[16,17]. According to previous studies, a patient’s prognosis is significantly associated with the clinical and pathological parameters as well as the biological characteristics of g-NENs[18-20]. However, the independent prognostic factors for g-NEN patients are still controversial. To our knowledge, studies have reported individual prediction models for the prognosis of g-NENs. We evaluated the prognostic value of TA-NLR in patients with g-NENs and further established a tumor prognosis prediction model to provide a basis for individual clinical therapy.
In most cases, the clinical symptoms of g-NENs are not typical because they depend on the location and invasiveness of the primary tumor or metastases. The symptoms mainly include abdominal pain, abdominal distension, difficulty swallowing, nausea, and vomiting. In this study, abdominal pain was the most common symptom, followed by weight loss, difficulty swallowing, and gastrointestinal bleeding; this finding is consistent with previous reports[21]. In addition, approximately 6% of the patients without any clinical symptoms were diagnosed via physical examinations. Among asymptomatic patients, approximately 40% were diagnosed with g-NEC or g-MANEC, although most of them were diagnosed with g-NETs. Therefore, postoperative follow-up is still essential for patients who have no clinical symptoms.
In recent years, substantial evidence has revealed that pathological stage is closely related to the prognosis of patients with g-NENs. Deep tumor invasion, lymph node metastasis, and distant metastasis were associated with decreased long-term survival[8,9,22]. The Ki-67 index, as a marker of cell proliferation, is widely used to evaluate the malignant potential of neuroendocrine tumors. The European Neuroendocrine Tumor Society (ENETS) and the WHO adopted a three-tier classification system based on the Ki-67 index for gastrointestinal pancreatic neuroendocrine tumors (G1: ≤ 2%; G2: 3%-20%; G3: > 20%). The Ki-67 index combined with a pathological staging system improves the diagnosis and prognosis prediction of patients with neuroendocrine tumors, and it is thus widely used in clinical practice. In this study, the rate of lymph node metastasis and the Ki-67 index were independent risk factors for OS and RFS in patients with g-NENs. In addition, increasing evidence has confirmed that the tumor-associated inflammatory response is closely related to the prognosis of patients with malignant tumors[12,23,24]. However, the relationship between the tumor-associated inflammatory response and g-NENs is unclear. Our study is the first to confirm that the TA-NLR is significantly associated with the prognosis of patients with g-NENs. We observed, through a univariate analysis, that the RFS and OS rates in patients with a TA-NLR > 0.21 were significantly lower than the rates in patients with a TA-NLR < 0.21. The multivariate analysis further revealed that the TA-NLR was an independent risk factor for patients with g-NENs.
Postoperative local recurrence and distant metastasis are the leading causes of death for patients with malignant tumors. Liver metastasis, peritoneal metastasis, and lymph node metastasis were the main types of tumor recurrence. The proportions of patients with these types of recurrence were 72%, 28%, and 25%, respectively. The spleen, kidney, and brain were relatively rare sites of recurrence. Our results are similar to those of previous reports[9]. In the present study, the TA-NLR was closely related to tumor recurrence, and a high incidence of liver metastasis and lymph node metastasis was observed in patients with a high TA-NLR. Thus, during the postoperative follow-up period, clinicians should utilize the prognostic value of the TA-NLR, as well as clinical characteristics, to discover potential hepatic or lymph node metastases at an earlier time point.
Nomograms, as a new type of statistical prediction model, are currently widely used in clinical practice for the majority of cancer types[25,26]. Prognostic nomograms enable the use of a combination of multiple relevant clinical predictors and can be utilized to predict RFS and OS for individual patients. For many cancers, nomograms compare favorably to the traditional TNM staging system and have been proposed as an important tool in clinical practice[13,27]. In this study, we established prognostic nomograms for g-NENs by combining the TA-NLR, Ki-67 index, and LNR. This combination had a high predictive ability, as did the traditional TNM staging system. Therefore, the combination of the TA-NLR, Ki-67 index, and LNR, as a novel prognostic system, may provide simple, more accurate prognostic predictions.
This study had some limitations. The study was uncontrolled and performed in a single institution. The results should be confirmed by subsequent prospective studies. Some heterogeneity was also present in this study, as it included multiple histological types (including NET, NEC, and MANEC), which do not represent a specific progression of a unique pathologic process. Due to the low incidence of g-NENs and the limited number of samples in the study, a statistical analysis could not be conducted for any one histological type. We will focus on each of the three histological types in the future, after more cases have been accumulated. However, to our knowledge, our study enrolled more patients with g-NENs than similar reports in the literature, and for the first time, we demonstrated that the TA-NLR was able to predict long-term survival relatively accurately in patients. Our study could be the basis for a subsequent prospective clinical study.
As a simple and inexpensive inflammatory biomarker, the TA-NLR is significantly correlated with tumor recurrence, especially with liver and lymph node metastasis. The TA-NLR is an independent predictor of RFS and OS, and its combination with the Ki-67 index and LNR could improve prognosis prediction in g-NEN patients undergoing radical surgery, as could the traditional TNM staging system.
COMMENTS
Background
The incidence of gastric neuroendocrine neoplasms (g-NENs), which account for 6% of all neuroendocrine neoplasms, is increasing every year. In addition to an early diagnosis, an important and effective component of proper management is the identification of the prognostic factors in patients with g-NENs. However, the prognostic factors for these tumors are complex and multifaceted and have not been clearly defined thus far. Few studies to date have focused on the relationship between the tumor-associated neutrophil-to-lymphocyte ratio (TA-NLR) and the prognosis of patients with g-NENs.
Research frontiers
In the past decade, increasing evidence has suggested that both tumor-associated neutrophils (TANs) and tumor-associated lymphocytes (TALs) are significantly associated with patient prognosis. Elevated TANs and reduced TALs correlate with advanced stage and poor prognosis in a variety of human tumors, including cervical cancer, hepatocellular carcinoma, and pancreatic cancer.
Innovations and breakthroughs
This study enrolled more patients with g-NENs than similar reports in the literature and, for the first time, demonstrated that the TA-NLR was able to predict long-term survival relatively accurately in patients.
Applications
This study established a novel prognostic system that included the TA-NLR, Ki-67 index, and lymph node ratio, which may provide simple, more accurate prognostic predictions. Moreover, as a simple and inexpensive inflammatory biomarker, the TA-NLR is significantly correlated with tumor recurrence, especially with liver and lymph node metastasis. Thus, during the postoperative follow-up period, clinicians should utilize the prognostic value of the TA-NLR, as well as clinical characteristics, to discover potential hepatic or lymph node metastases at an earlier time point.
Terminology
Gastric neuroendocrine neoplasms (g-NENs), a highly heterogeneous and poorly understood group of relatively rare tumors, are derived primarily from enterochromaffin-like cells (ECL-cells) localized in the gastric mucosa. The World Health Organization (WHO, 2010) classifies g-NENs into the following subclasses: neuroendocrine tumors (g-NETs), neuroendocrine carcinoma (g-NEC), and mixed adenoneuroendocrine carcinoma (g-MANEC).
Peer-review
Previous studies have established that elevated TANs and reduced TALs correlate with advanced stage and poor prognosis in a variety of human tumors, including cervical cancer, hepatocellular carcinoma, and pancreatic cancer. In this study, the authors demonstrated that the TA-NLR is an independent predictor of RFS and OS and that it is also significantly correlated with tumor recurrence, especially with liver and lymph node metastasis. However, as the authors indicate, this study was uncontrolled and was performed within a single institution. The results should therefore be confirmed in subsequent prospective studies.
ACKNOWLEDGMENTS
The authors thank Professor Yao Lin for valuable advice and discussions.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by National Key Clinical Specialty Discipline Construction Program of China, No. [2012] 649.
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Fujian Medical University Union Hospital.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest associated with the publication of this manuscript.
Data sharing statement: No additional data are available.
Peer-review started: March 11, 2017
First decision: May 9, 2017
Article in press: July 4, 2017
P- Reviewer: Harmanci O S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma YJ
Contributor Information
Long-Long Cao, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Jun Lu, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Jian-Xian Lin, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Chao-Hui Zheng, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Ping Li, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Jian-Wei Xie, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Jia-Bin Wang, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Qi-Yue Chen, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Mi Lin, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Ru-Hong Tu, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Chang-Ming Huang, Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China. hcmlr2002@163.com.
References
- 1.Burkitt MD, Pritchard DM. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24:1305–1320. doi: 10.1111/j.1365-2036.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 2.Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 3.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 5.Bosman FT, Carneiro F, Theise ND. Nomenclature and classification of neuroendocrine neoplasms of digestive system. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010. [Google Scholar]
- 6.Kulke MH, Shah MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T, Goldner WS, Halfdanarson TR, Heslin MJ, Kandeel F, Kunz PL, Kuvshinoff BW 2nd, Lieu C, Moley JF, Munene G, Pillarisetty VG, Saltz L, Sosa JA, Strosberg JR, Vauthey JN, Wolfgang C, Yao JC, Burns J, Freedman-Cass D; National comprehensive cancer network. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 7.Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY, Wiedenmann B, Papotti M, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–166. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- 8.Faggiano A, Ferolla P, Grimaldi F, Campana D, Manzoni M, Davì MV, Bianchi A, Valcavi R, Papini E, Giuffrida D, et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: the NET management study. J Endocrinol Invest. 2012;35:817–823. doi: 10.3275/8102. [DOI] [PubMed] [Google Scholar]
- 9.Lewkowicz E, Trofimiuk-Müldner M, Wysocka K, Pach D, Kiełtyka A, Stefańska A, Sowa-Staszczak A, Tomaszewska R, Hubalewska-Dydejczyk A. Gastroenteropancreatic neuroendocrine neoplasms: a 10-year experience of a single center. Pol Arch Med Wewn. 2015;125:337–346. doi: 10.20452/pamw.2832. [DOI] [PubMed] [Google Scholar]
- 10.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Sadot E, Basturk O, Klimstra DS, Gönen M, Lokshin A, Do RK, D’Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, et al. Tumor-associated Neutrophils and Malignant Progression in Intraductal Papillary Mucinous Neoplasms: An Opportunity for Identification of High-risk Disease. Ann Surg. 2015;262:1102–1107. doi: 10.1097/SLA.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS) The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 14.Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol. 2002;15:831–837. doi: 10.1097/01.MP.0000020391.98998.6B. [DOI] [PubMed] [Google Scholar]
- 15.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 17.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 18.Hu HK, Ke NW, Li A, Du XJ, Guo Q, Hu WM. Clinical characteristics and prognostic factors of gastroenteropancreatic neuroendocrine tumors: a single center experience in China. Hepatogastroenterology. 2015;62:178–183. [PubMed] [Google Scholar]
- 19.Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, Guhlke S, Biersack HJ, Sabet A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Li J, Han X, Shi C, Jin D, Lou W. Clinical characteristics and prognostic factors of patients with gastric neuroendocrine carcinoma treated with radical surgery. Chin Med J (Engl) 2014;127:2419–2422. [PubMed] [Google Scholar]
- 21.Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815–821; discussion 822-823. doi: 10.1097/00000658-199906000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota T, Ohyama S, Hiki N, Nunobe S, Yamamoto N, Yamaguchi T. Endocrine carcinoma of the stomach: clinicopathological analysis of 27 surgically treated cases in a single institute. Gastric Cancer. 2012;15:323–330. doi: 10.1007/s10120-011-0122-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Ren H, Wang L, Ning Z, Zhuang Y, Gan J, Chen S, Zhou D, Zhu H, Tan D, et al. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int J Mol Sci. 2014;15:9718–9734. doi: 10.3390/ijms15069718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7:e33655. doi: 10.1371/journal.pone.0033655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, Wang Z, Zhu Z, Deng Q, Xiong X, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24:3819–3820. doi: 10.1200/JCO.2006.07.1290. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]


