Abstract
AIM
To understand the influence of chronic kidney disease (CKD) on mortality, need for transfusion and rebleeding in gastrointestinal (GI) bleeding patients.
METHODS
A systematic search was conducted in three databases for studies on GI bleeding patients with CKD or end-stage renal disease (ESRD) with data on outcomes of mortality, transfusion requirement, rebleeding rate and length of hospitalization (LOH). Calculations were performed with Comprehensive Meta-Analysis software using the random effects model. Heterogeneity was tested by using Cochrane’s Q and I2 statistics. Mean difference (MD) and OR (odds ratio) were calculated.
RESULTS
1063 articles (EMBASE: 589; PubMed: 459; Cochrane: 15) were found in total. 5 retrospective articles and 1 prospective study were available for analysis. These 6 articles contained data on 406035 patients, of whom 51315 had impaired renal function. The analysis showed a higher mortality in the CKD group (OR = 1.786, 95%CI: 1.689-1.888, P < 0.001) and the ESRD group (OR = 2.530, 95%CI: 1.386-4.616, P = 0.002), and a rebleeding rate (OR = 2.510, 95%CI: 1.521-4.144, P < 0.001) in patients with impaired renal function. CKD patients required more unit red blood cell transfusion (MD = 1.863, 95%CI: 0.812-2.915, P < 0.001) and spent more time in hospital (MD = 13.245, 95%CI: 6.886-19.623, P < 0.001) than the controls.
CONCLUSION
ESRD increases mortality, need for transfusion, rebleeding rate and LOH among GI bleeding patients. Prospective patient registries and observational clinical trials are crucially needed.
Keywords: Gastrointestinal bleeding, Chronic kidney disease, Mortality, Blood transfusion, Rebleeding
Core tip: Acute gastrointestinal bleeding is a potentially life-threatening abdominal emergency that remains a common cause of hospitalization. Pre-existing chronic kidney disease (CKD) may worsen the prognosis. This is the first meta-analysis to compare CKD patients and normal renal function patients based on GI bleeding. We investigated these two groups in terms of mortality, transfusion amount, rebleeding rate and length of hospitalization.
INTRODUCTION
Acute gastrointestinal bleeding (GI) is an abdominal emergency which remains a common cause of hospitalization[1]. An accurate diagnosis of GI bleeding relies on prompt resuscitation, initial risk evaluation, and provisional clinical diagnosis followed by an appropriate definitive investigation which enables specific therapeutic interventions. GI bleeding involves any bleeding in the GI tract from the esophagus, stomach, small intestines or large intestines to the anus.
Upper GI bleeding has an annual incidence that ranges from 40 to 150 episodes per 100000 persons and a morality rate of 6%-10%[2], whereas lower GI bleeding has an annual incidence ranging from 20 to 27 episodes per 100000 persons and a mortality rate of 4%-10%[3,4]. Since GI bleeding is a potentially life-threatening acute disorder, understanding the risk factors that worsen the disease is of great importance. Scoring systems have therefore been developed to predict the outcome of therapy. The Rockall score is one of these scoring systems. It includes pre-endoscopic (age, shock and comorbidity) and post-endoscopic (diagnosis and presence or absence of endoscopic stigmata of recent haemorrhage) factors[5]. Several studies have demonstrated high mortality with higher Rockall scores[6]. However, Laeeq et al[7] have not found significantly higher mortality in patients with high pre-endoscopic Rockall score (> 5). The Rockall score only assesses the risk of mortality in patients with upper GI bleeding. The Glasgow Blatchford score is another scoring system which uses clinical and laboratory parameters. Neither scoring system makes distinction between pre-existing renal failure and acute renal failure due to haemorrhage. Both of these scoring systems have been designed for the risk assessment of upper GI bleeding. Previous studies have shown evidence of increased risk of GI bleeding in chronic kidney disease (CKD) patients and with end-stage renal disease (ESRD) requiring renal replacement therapy in comparison with the general population, but also an association with higher mortality[8-10]. Further studies have demonstrated that bleeding in CKD patients from the upper GI tract is more common than from the lower GI tract[11]. The increased prevalence of small bowel erosions, ulcers and angioectasias is also well known in CKD patients and it may be as high as 33% and it often causes obscure gastrointestinal bleeding[12-14]. However, no meta-analyses or systematic reviews have been conducted to assess the difference between CKD/ESRD patients and the normal renal function population with regard to GI bleeding.
The aim of this study was therefore to examine outcomes of GI bleeding, such as mortality, blood transfusion requirement, rebleeding rate and length of hospitalization (LOH) in CKD/ESRD patients compared to patients with normal renal functions.
MATERIALS AND METHODS
Search strategy
This study was conducted using the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P)[15]. It was registered in the international prospective register of systematic reviews, PROSPERO (under registration number CRD42017077987). The meta-analysis was based on the PICO (Patient, Intervention, Comparison, Outcome) format (P: patients with GI bleeding; I: chronic renal failure; C: normal renal function; O: mortality, blood transfusion, rebleeding). A systematic search was performed in 3 databases, Pubmed, EMBASE and the Cochrane Library, with the following terms: (“GI bleeding” OR “gastrointestinal bleeding” OR “gastrointestinal hemorrhage”) AND (“chronic renal failure” OR “uremia” OR “chronic kidney failure”). The search was limited to human data and to full-text English-language articles if appropriate. The exact search term in Pubmed was: [“GI bleeding”(All Fields) OR “gastrointestinal bleeding”(All Fields) OR “gastrointestinal hemorrhage”(All Fields)] AND [“chronic renal failure”(All Fields) OR “uraemia”(All Fields) OR “uremia”(MeSH Terms) OR “uremia”(All Fields) OR “chronic kidney failure”(All Fields)] AND [“humans”(MeSH Terms) AND English(lang)]. The database search was conducted up to 10 March 2017. Reference management software (EndNote X7) was used to remove duplicates by searching overlaps between titles, authors and publication years. The reference lists in the articles obtained were also checked, and one more eligible publication was found.
Study selection
The studies were selected separately by two investigators (RH and AM). Disagreements were resolved by consulting a third reviewer (PH). Clinical studies were eligible provided they reported data on adult patients hospitalized with upper or lower GI bleeding grouped into normal renal function and CKD or ESRD groups. Articles were eligible containing data of CKD/ESRD patients and a control group in the same study. Information on mortality, transfusion, rebleeding and length of hospitalization (LOH) was manually searched. Case reports, conference abstracts, reviews and studies on paediatric patients up to age 18 alone were excluded. We found a high number of articles in which the risk of GI bleeding in CKD patients was studied, but they were not eligible for our meta-analysis, as there were no data available on outcomes of the GI bleeding in a control population without CKD/ESRD.
Data extraction, synthesis and analysis
Mortality data, number of transfused blood units, rebleeding and length of hospitalization data were extracted to analyse the influence of CKD and/or ESRD on the outcome of GI bleeding. In Sood et al[9], Tsai et al[16] and Boyle et al[17], the number of patients was calculated from percentages of mortality. Boyle et al[17] supplied information on transfusion in mean and standard error of mean, for which statistical calculation standard deviation (SD) was computed. Tsai et al[16] reported data from transfusions in the median and interquartile range (IQR), from which mean and SD were calculated with Hozo’s method[18]. All meta-analytic calculations were performed with Comprehensive Meta-Analysis software (Version 3.0, Biostat Inc.) using the random effects model (DerSimonian-Laird method[19]). Odds ratios (OR) and 95% confidence intervals (CI) were calculated for binary outcomes. In the case of LOH and transfusion for comparing mean data, a mean difference (MD) with 95%CI was calculated. All analyses were two-tailed, with an α of 0.05.
Heterogeneity was tested using Cochrane’s Q and the I2 statistics. Based on the Cochrane Handbook, I2 = 100% × (Q - df)/Q, with I2 representing the magnitude of the heterogeneity (moderate: 30%-60%; substantial: 50%-90%; considerable: 75%-100%)[20]. Only results that were available from at least 3 studies were displayed graphically with forest plots. We performed a sensitivity analysis to assess whether removing any study result in different interpretation and final conclusion[21]. To assess the effect of the year of publication on the outcome data we performed meta-regression analysis. We calculated the regression coefficient and interpreted the data with their 95%CI and r-analog.
Quality of studies and risk of bias
Because of the low number of eligible articles, publication bias was obtained with a visual inspection of the funnel plots alone according to the Cochrane Handbook[20]. The Newcastle-Ottawa Scale (NOS) adjusted to our study design was used[22] to assess the quality of nonrandomized cohort studies. The selection, comparability and outcome data were assessed based on 6 items (Table 1) with the “star system”: high-quality items with a low risk of bias received one star, while low-quality items with a high or unknown risk of bias were assigned no stars. 3 items were included during the selection process. In the case of representativeness in the study population, we assigned a star if all of the GI bleeding patients with normal or impaired renal function were included. If any selection criteria applied, we assigned no points. We used the classical definition of CKD[23], which characterizes the disease with a glomerular filtration rate (GFR) < 60 m/min lasting longer than 3 mo. ESRD was defined as a condition where haemodialysis or chronic peritoneal dialysis is performed for at least 3 mo. With regard to outcome, only the follow-up time for rebleeding was rated in articles that provided this information. Assessment of outcome and length of follow-up were not rated because most of the articles were retrospective.
Table 1.
Modified Newcastle-Ottawa Scale criteria
| Adapted Newcastle-Ottawa Scale Items | High-quality items carrying a low risk of bias (green) | Low-quality items carrying a high (red) or an unknown (yellow) risk of bias |
| Item 1: Representativeness of the initial study population - patients with GI bleeding and CKD/ESRD | All patients with upper or lower GI bleeding and CKD/ESRD were included. | Low: any selection criteria were applied to the study population (e.g., only transplanted patients). |
| Unknown: no data on selection process. | ||
| Item 2: Representativeness of the initial study population - patients with GI bleeding without CKD/ESRD | All patients with upper or lower GI bleeding without CKD/ESRD included. | Low: any selection criteria were applied to the study population. |
| Unknown: no data on selection process. | ||
| Item 3: Ascertainment of exposure | We defined chronic renal failure as present when eGFR was < 60 mL/min at least 3 mo. We defined end-stage renal disease as a condition where hemodialysis or chronic peritoneal dialysis is performed at least for 3 mo. | Low: CKD or ESRD is not present in all of the patients. |
| Unknown: no definitions of the conditions mentioned are provided. | ||
| Item 4: Comparability of cohorts A | Study controls for age: no significant difference was detected. | Low: significant difference was detected. |
| Unknown: no statement. | ||
| Item 5: Comparability of cohorts B | Study controls for taking ulcerogenic drugs: no significant difference was detected | Low: significant difference was detected between taking ulcerogenic drugs. |
| Unknown: no comparison made by taking ulcerogenic drugs. | ||
| Item 6: Follow-up time for rebleeding | The follow-up time is clearly defined. | Low: incomplete follow-up |
| Unknown: no follow-up time is mentioned. |
CKD: Chronic kidney disease; ESRD: End-stage renal disease.
RESULTS
Study selection
1063 articles (EMBASE: 589; PubMed: 459; Cochrane: 15) were found altogether through database searches. The flowchart (Figure 1) shows the study selection strategy. Studies in our meta-analysis were dated from 1946 to 2017. After removing duplicates, 875 publications remained. Following initial screening based on titles and abstracts, 23 articles were retrieved and screened. A further 18 were excluded because of missing outcome data or a missing control group. Patients with acute renal failure were also included in the analysis reported in Alvarez et al[24], so we did not use the data in that publication. The remaining 5[9,16,17,25,26] and one other[10] eligible record which was found in reference lists were included in the meta-analysis. The basic characteristics of the 6 eligible articles in the meta-analysis are shown in Table 2. These 6 publications contained data on 406,035 patients, of whom 51315 had impaired renal function parameters and 354720 had normal renal functions. 2 articles contained data on patients with CKD and 4 on ESRD patients. There were 2 studies involving CKD and ESRD patients, with their group identified as the CKD mixed group. The number of ESRD patients analysed was 15201, the CKD group had 36035 members, and 79 patients could be classified in the CKD mixed group.
Figure 1.
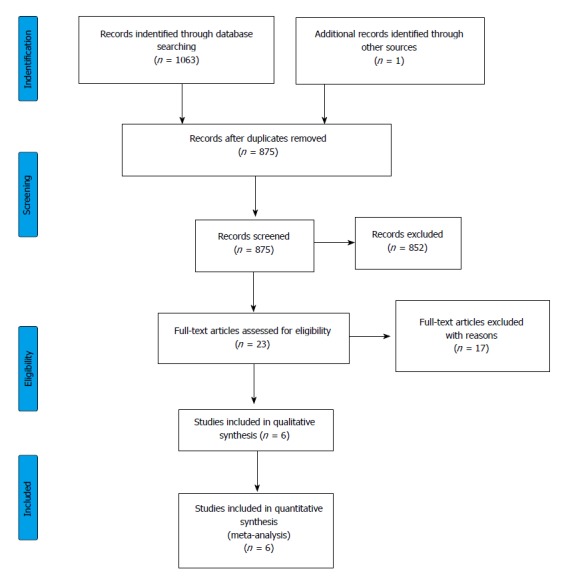
Flowchart of the study selection procedure.
Table 2.
Basic characteristics of the studies included in the meta-analysis
| Ref. | Country | Study type | Years of study | Group | Sample size | Age | Mortality | Transfusion | Rebleeding | Length of hospitalization |
| Boyle et al[17], 1983 | United States | Retrospective | 1977-1981 | Control | 40 | 54 ± 21 | √ | √ | - | √ |
| CKD (mix) | 20 | 59 ± 41 | ||||||||
| Cheung et al[10], 2010 | Canada | Retrospective | 2000-2006 | Control | 50 | 67 ± 13 | √ | √ | √ | √ |
| CKD | 50 | 71 ± 13 | ||||||||
| ESRD | 50 | 68 ± 12 | ||||||||
| Hung et al[25], 2014 | Taiwan | Retrospective | 2007 | Control | 6322 | 54.6 ± 13.3 | √ | - | - | - |
| ESRD | 110 | NR | ||||||||
| Sood et al[9], 2012 | United States | Retrospective | 2007 | Control | 347245 | NR | √ | - | - | - |
| CKD | 35985 | NR | ||||||||
| ESRD | 14983 | NR | ||||||||
| Tsai et al[16], 1996 | Taiwan | Prospective | 1991-1994 | Control | 640 | 55.7 ± 16.22 | √ | √ | √ | - |
| ESRD | 58 | 64.1 ± 11.42 | ||||||||
| Zuckerman et al[26], 1985 | United States | Retrospective | 1980-1983 | Control | 423 | 63 (16-96)3 | - | - | √ | - |
| CKD (mix) | 59 | 57 (24-84)3 |
Data expressed as mean ± SEM (standard error of mean);
Data expressed as mean ± SD (standard deviation);
Data expressed as median (interquartile range). NR: Not reported; CKD: Chronic kidney disease; ESRD: End-stage renal disease.
Mortality
Data on mortality was available in all of the articles included, but Zuckerman et al[26] reported no mortality data for the control group; we therefore removed it from the statistical analysis. Hung et al[25] reported mortality data from a 6-wk follow-up period, while the other articles contained data on an unknown follow-up period. In the subgroup analysis for CKD and ESRD, a higher mortality rate was detected compared to the control population (CKD: OR = 1.786, 95%CI: 1.689-1.888, P < 0.001; ESRD: OR = 2.530, 95%CI: 1.386-4.616, P = 0.002, Figure 2).
Figure 2.
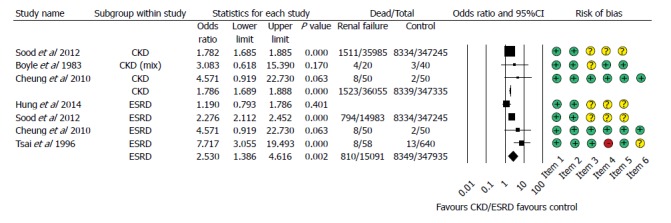
Forest plot representing the differences in mortality in gastrointestinal bleeding patients with normal and impaired renal function. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
Required units for transfusion
4 studies reported data on the transfused units of red blood cells. The required transfusion was 1.8 times higher in the patients with abnormal renal function (MD = 1.863, 95%CI: 0.812-2.915, P < 0.001, Figure 3).
Figure 3.
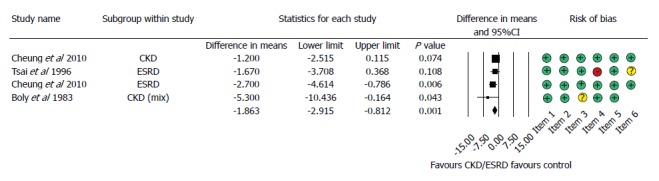
Forest plot representing the required units of transfusion in gastrointestinal bleeding patients with normal and impaired renal function. Size of squares for the difference in standardized mean values reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
Rebleeding rate
It was possible to retrieve data on the rebleeding rate from 3 articles, but Cheung et al[10] contained simultaneous data from the CKD and ESDR groups, which could be analysed. Boyle et al[17]. also presented data on rebleeding. However, this included cases of uncontrolled bleeding, so we excluded these data from our analysis. We found that patients with impaired renal function tend to bleed again 2.5 more times than patients with normal renal function (OR = 2.510, 95%CI: 1.521-4.144, P < 0.001, Figure 4).
Figure 4.
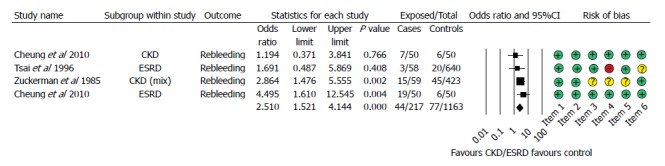
Forest plot representing the rebleeding rate in gastrointestinal bleeding patients with normal and impaired renal function. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
Length of hospitalization
Two of the six articles included reported hospital stay outcomes. Patients with impaired renal function spent significantly more time in hospital after GI bleeding (MD = 13.245, 95%CI: 6.886-19.623, P < 0.001, Figure 5).
Figure 5.
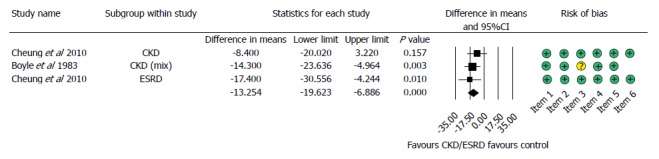
Forest plot representing the differences in length of hospitalization in gastrointestinal bleeding patients with normal and impaired renal function. Size of squares for the difference in standardized mean values reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
Heterogeneity and quality assessment of data
High heterogeneity was detected for mortality in the ESRD group (Q = 17.082; DF = 3; I2 = 82.438%; P < 0.001), while the heterogeneity for CKD was low (Q = 1.767; DF = 2; I2 = 0%; P = 0.413). However, a low heterogeneity was detected for the transfusion requirements (Q = 3.448; DF = 3; I2 = 13.003%; P = 0.328), the rebleeding rate (Q = 3.328; DF = 3; I2 = 9.845%; P = 0.344) and LOH (Q = 1.100; DF = 2; I2 = 0%; P = 0.577). To ascertain publication bias, we only made a visual assessment of the funnel plot (Figure 6) because we were only able to include 6 studies in our meta-analysis. Sensitivity analysis showed no significant difference in the OR of mortality, by removing any of the articles (Supplementary Figure 1). Meta-regression showed slight significance, in the most recent articles the OR is decreasing with the time (regression coefficient: b = -0.0548; 95%CI: -0.0968 to -0.0128; P = 0.0105; r-analog: 0.2, Supplementary Figure 2A). The number of required units for transfusion has not changed since the 1980s (b = -0.0028; 95%CI: -0.0242 to -0.0186; P = 0.7972; r-analog: 0.00, Supplementary Figure 2B). Based on data from 4 articles, no difference in rebleeding rate could be observed in the last 30 years (b = 0.0027; 95%CI: -0.0353 to 0.03; P = 0.8726; r-analog: 0.00, Supplementary Figure 2C).
Figure 6.
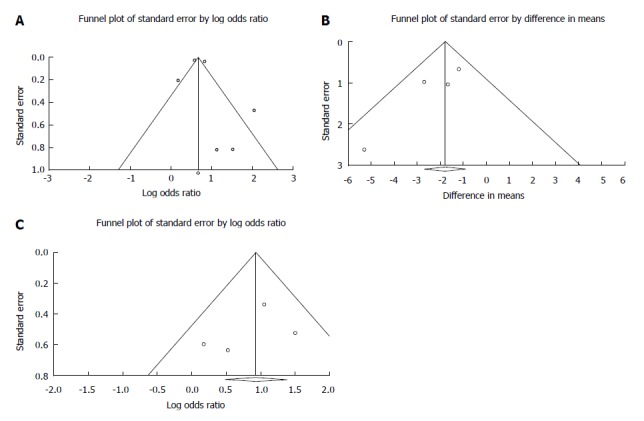
Funnel plot. A: Funnel plot of mortality; B: Funnel plot of required transfusion; and C: Funnel plot of rebleeding.
On the score based on the Newcastle-Ottawa Scale, articles were assigned between 2 and 6 stars out of a maximum of 6 stars (Table 3). There was a low risk of bias in representativeness in the study and the control population; it received 100% (Figure 7). With regard to ascertaining exposure, 33% of the articles represented a low risk of bias, while 66% had an unclear risk of bias. In these articles CKD and ESRD were not clearly defined, or patients were sorted based on a code system. With regard to a comparison of age, half of the articles contained no clear data on the groups and there was a significant difference in the ages of the ESRD and control groups in Tsai et al[16]. 50% of the articles reported data on taking ulcerogenic drugs; the other half represented an unclear risk of bias. The follow-up time for rebleeding was analysed in 3 articles; only one did not report this clearly.
Table 3.
Stars based on the Modified Newcastle-Ottawa Scale
Figure 7.
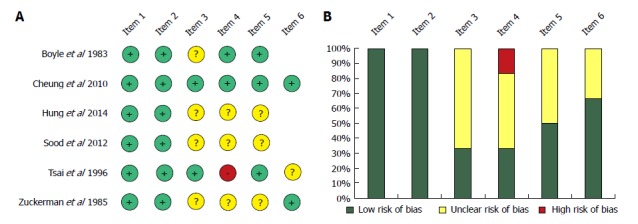
Risk assessment of articles included in the meta-analysis based on the modified Newcastle-Ottawa Scale (A); Risk of bias assessment graph (B).
DISCUSSION
CKD is a term that covers all degrees of decreased renal function (mild, moderate, and severe chronic kidney disease), where the GFR is lower than 60 mL/min for longer than 3 mo[23]. CKD is a worldwide public health problem, with both incidence and prevalence rising and the main causes being diabetes mellitus and high blood pressure. ESRD patients requiring haemodialysis or peritoneal dialysis 3 times a week represent a high burden and cost for the health care system. As the prevalence of hypertension and diabetes mellitus, the most important etiological factors for CKD and ESRD is increasing worldwide, we predict that GI bleeding with CKD will be a growing problem. According to Ohmori et al[13] the number of patients on hemodyalisis has tripled between 1990 and 2010. This is the first meta-analysis to report on the severity of complications after GI bleeding in patients with CKD or ESRD and normal renal function groups. Based on a systematic search in 3 databases, we were able to include 6 articles, which contained data on 406035 patients, of whom 51315 had impaired renal function. A higher prevalence of peptic ulcers was reported among ESRD patients undergoing long-term dialysis[27,28]. The elevated risk for GI bleeding in CKD and ESRD patients is also well known[29]. The most frequent causes of lower GI bleeding in this population have been described; diverticulosis, haemorrhoids, and ischaemic colitis have been identified in addition to angioectasias[30], but no cohort study has been conducted on this topic yet. Although we did not intend to narrow our search to upper GI bleeding, the articles eligible for our inclusion criteria contained data only on patients with upper GI bleeding, and no studies with lower GI bleeding met our inclusion criteria. Only a few of the studies detailed the endoscopic findings and cause of bleeding. Cheung et al[10] included only peptic ulcer bleeding patients, while the study of Hung et al[25] examined only esophageal variceal bleeding. Tsai et al[16] found that erosive gastritis was significantly higher in ESRD group, while Boyle et al[17] saw gastric ulcer as the most common cause of bleeding in the impaired renal function group, but it was not significant compared to controls. Zuckerman et al[26] found significantly more angiodysplasia and erosive esophagitis in the impaired renal function group.
Based on the pooled data, we found that ESRD increases mortality 2.5 times while CKD increases it 1.8 times in GI bleeding compared to the controls with normal renal function, but these ORs are not significantly different. Weng et al[31] reported that ESRD patients admitted with primary upper GI bleeding have a profoundly increased risk of in-hospital mortality. Using a large multi-centre database, Sood et al[9] reported that the in-hospital mortality risk is 50% higher in CKD patients and 3 times greater in ESRD patients. Holden et al[32] reported that the incidence rate of major bleeding episodes in haemodialyzed patients was 2.5% per person-year and that use of aspirin and/or warfarin increased this risk. Based on the result of the meta-regression the mortality-rate of GI bleeding has improved since the 1980s. It is likely one of the reasons for the heterogeneity of the data. Inhomogen patient groups also result in a significant bias. However the sensitivity analysis showed that none of the articles influences significantly the pooled OR.
Cardiovascular disease, current smoking[33] and even haemostasis disorders[34] may play a role in the background of higher risk for GI bleeding in ESRD patients. Unfortunately only few of the analysed articles detailed the other comorbidities of the GI bleeding patients. In the article of Cheung et al[10]. there was no significant difference in the comorbidities between ESRD, CKD and normal renal function group. More people in CKD and ESRD groups suffered from hypertension, diabetes mellitus and platelet abnormalities in the study of Sood et al[9], while the cirrhosis was less common than in controls. Volume replacement and blood transfusion are important parts of the therapy of GI bleeding. This meta-analysis demonstrated that patients with chronic impaired renal function develop 2.5 times more rebleeding episodes and require almost 2 more red blood cell units for transfusion than the control group. Patients with impaired renal function spent more time in hospital than the control group.
There are several limitations to this study; therefore, the results of this meta-analysis should be regarded with caution. Unfortunately, only a low number of articles was found on this topic, with half of them written in the 1980s and 1990s. In the recent articles, CKD and ESRD groups were separated, but in the earlier publications these groups were mixed, leading to a bias in our analysis, and the definition of GFR was also not mentioned. The diagnosis was based on elevated creatinine level. Hung et al[25] only involved patients with cirrhosis and the mortality rate was monitored up to 6 wk, while hospital mortalities were presumably included in the other articles. Publications with rebleeding data did not follow patients for the same time interval, and 1 paper did not report on the follow-up time. The strength of this meta-analysis is the high number of patients.
Our results have demonstrated that patients with ESRD show higher mortality during GI bleeding. CKD patients require more transfusion, and the rebleeding rate is also more elevated than that in patients with normal renal function. Because of these severe conditions, the LOH is also longer. Patients with ESRD or CKD should be observed more carefully due to the elevated complication rate. In this meta-analysis we wanted to highlight the importance of this clinical problem and we believe that it needs further scientific research. In order to understand the effect of CKD/ESRD and other comorbidities on the outcomes of GI bleeding in more details, observational trials, and registries on GI bleeding should be developed.
ARTICLE HIGHLIGHTS
Research background
Chronic kidney disease is a significant comorbidity, which can worsen the outcomes of gastrointestinal (GI) bleeding.
Research motivation
We wanted to understand the role of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the natural history of GI bleeding.
Research objectives
Our goal was to investigate the influence of CKD and ESRD on the outcomes of GI bleeding, based on all available data published in this topic.
Research methods
A comprehensive search was carried out in PubMed, Embase and Cochrane Library databases for studies detailing the outcomes of GI bleeding in the context of kidney functions. We used the PRISMA P protocol, registered our project through PROSPERO and assessed the quality of the included articles by using the Newcastle-Ottawa Scale, to ensure that this meta-analysis is done to the highest possible standards. The statistical calculations were performed with Comprehensive Meta-Analysis software, using the random effects model (DerSimonian-Laird method).
Research results
In this analysis 51315 patients with CKD and 354720 controls were included (6 articles). We found that the mortality of GI bleeding was significantly worse in CKD and ESRD with an OR of 1.79 and 2.53 respectively. Patients with kidney disease needed significantly more transfusion with a MD of 1.86 and the rebleeding rate was significantly worse in the group with impaired kidney function with an OR of 2.51. Patients with impaired kidney function needed significantly longer hospitalization with a MD of 13.25.
Research conclusions
This is the first meta-analysis and systematic review in this topic, which quantifies kidney disease as a negative risk factor in GI bleeding. GI bleeding in patients with chronic renal failure significantly increases the mortality rate, rebleeding rate, length of hospitalization, and require more blood transfusion compared to patients with normal kidney functions. Kidney disease significantly worsens the outlook of patients presenting with GI bleeding. Patients with chronic kidney disease will need to be treated with more caution due to the worse outcomes of GI bleeding. Close monitoring of the fluid balance and kidney functions, careful fluid therapy and prevention of acute kidney injury in these patients may improve the outcomes of GI bleeding.
Research perspectives
Although CKD, ESRD, and other comorbidities are major risk factors for unfavorable outcomes in GI bleeding, their roles are not well investigated nor understood and they need further scrutiny. We would better understand the role of CKD in ESRD in GI bleeding from analysis of extensive data from large multicenter and multinational observational studies and registries accurately recording the outcomes and the kidney functions.
ACKNOWLEDGMENTS
The present paper is dedicated to the 650th anniversary of the founding of the University of Pécs, Hungary.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Supported by Project Grants No. K116634 and KH125678 (to Hegyi P); Economic Development and Innovation Operative Programme Grant, No. GINOP 2.3.2-15-2016-00048 (to Hegyi P); Human Resources Development Operational Programme Grant No. EFOP-3.6.2-16-2017-00006 (to Hegyi P) of the National Research, Development; and Innovation Office and by a Momentum Grant of the Hungarian Academy of Sciences No. LP2014-10/2014 to (Hegyi P).
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this article.
Data sharing statement: No additional data are available.
Peer-review started: October 31, 2017
First decision: November 14, 2017
Article in press: December 4, 2017
P- Reviewer: Kozarek RA, Perez-Cuadrado-Robles E, Triantafyllou K S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Roland Hágendorn, Department of Gastroenterology, First Department of Medicine, University of Pécs, Pécs 7624, Hungary.
Nelli Farkas, Institute of Bioanalysis, University of Pécs, Pécs 7624, Hungary.
Áron Vincze, Department of Gastroenterology, First Department of Medicine, University of Pécs, Pécs 7624, Hungary.
Zoltán Gyöngyi, Department of Public Health Medicine, University of Pécs, Pécs 7624, Hungary.
Dezső Csupor, Department of Pharmacognosy, Faculty of Pharmacy, University of Szeged, Szeged 6720, Hungary.
Judit Bajor, Department of Gastroenterology, First Department of Medicine, University of Pécs, Pécs 7624, Hungary.
Bálint Erőss, Institute for Translational Medicine, University of Pécs, Pécs 7624, Hungary.
Péter Csécsei, Department of Neurology, University of Pécs, Pécs 7623, Hungary.
Andrea Vasas, Department of Pharmacognosy, Faculty of Pharmacy, University of Szeged, Szeged 6720, Hungary.
Zsolt Szakács, Institute for Translational Medicine, University of Pécs, Pécs 7624, Hungary.
László Szapáry, Institute for Translational Medicine, University of Pécs, Pécs 7624, Hungary.
Péter Hegyi, Institute for Translational Medicine, University of Pécs, Pécs 7624, Hungary.
Alexandra Mikó, Institute for Translational Medicine, University of Pécs, Pécs 7624, Hungary. alexandra.miko@aok.pte.hu.
References
- 1.Cutler JA, Mendeloff AI. Upper gastrointestinal bleeding. Nature and magnitude of the problem in the U.S. Dig Dis Sci. 1981;26:90S–96S. doi: 10.1007/BF01300814. [DOI] [PubMed] [Google Scholar]
- 2.Vreeburg EM, Snel P, de Bruijne JW, Bartelsman JF, Rauws EA, Tytgat GN. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236–243. [PubMed] [Google Scholar]
- 3.Hussain H, Lapin S, Cappell MS. Clinical scoring systems for determining the prognosis of gastrointestinal bleeding. Gastroenterol Clin North Am. 2000;29:445–464. doi: 10.1016/s0889-8553(05)70122-9. [DOI] [PubMed] [Google Scholar]
- 4.Zuccaro G Jr. Management of the adult patient with acute lower gastrointestinal bleeding. American College of Gastroenterology. Practice Parameters Committee. Am J Gastroenterol. 1998;93:1202–1208. doi: 10.1111/j.1572-0241.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 5.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, Wang HP, Wu MS, Ho WC, Lee H, Lin JT. The etiology and clinical characteristics of acute lower gastrointestinal bleeding in patients hospitalized for comorbid illnesses. Hepatogastroenterology. 2006;53:395–398. [PubMed] [Google Scholar]
- 7.Laeeq SM, Tasneem AA, Hanif FM, Luck NH, Mandhwani R, Wadhva R. Upper Gastrointestinal Bleeding in Patients with End Stage Renal Disease: Causes, Characteristics and Factors Associated with Need for Endoscopic Therapeutic Intervention. J Transl Int Med. 2017;5:106–111. doi: 10.1515/jtim-2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY. The risk of upper gastrointestinal bleeding in patients treated with hemodialysis: a population-based cohort study. BMC Nephrol. 2013;14:15. doi: 10.1186/1471-2369-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood P, Kumar G, Nanchal R, Sakhuja A, Ahmad S, Ali M, Kumar N, Ross EA. Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol. 2012;35:216–224. doi: 10.1159/000336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44–49. doi: 10.1016/j.gie.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Gheissari A, Rajyaguru V, Kumashiro R, Matsumoto T. Gastrointestinal hemorrhage in end stage renal disease patients. Int Surg. 1990;75:93–95. [PubMed] [Google Scholar]
- 12.Docherty E, Koulaouzidis A, Douglas S, Plevris JN. Use of small bowel capsule endoscopy in patients with chronic kidney disease: experience from a University Referral Center. Ann Gastroenterol. 2015;28:99–104. [PMC free article] [PubMed] [Google Scholar]
- 13.Ohmori T, Konishi H, Nakamura S, Shiratori K. Abnormalities of the small intestine detected by capsule endoscopy in hemodialysis patients. Intern Med. 2012;51:1455–1460. doi: 10.2169/internalmedicine.51.7190. [DOI] [PubMed] [Google Scholar]
- 14.Karagiannis S, Goulas S, Kosmadakis G, Galanis P, Arvanitis D, Boletis J, Georgiou E, Mavrogiannis C. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data) World J Gastroenterol. 2006;12:5182–5185. doi: 10.3748/wjg.v12.i32.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CJ, Hwang JC. Investigation of upper gastrointestinal hemorrhage in chronic renal failure. J Clin Gastroenterol. 1996;22:2–5. doi: 10.1097/00004836-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Boyle JM, Johnston B. Acute upper gastrointestinal hemorrhage in patients with chronic renal disease. Am J Med. 1983;75:409–412. doi: 10.1016/0002-9343(83)90341-8. [DOI] [PubMed] [Google Scholar]
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. [Google Scholar]
- 21.Viel JF, Pobel D, Carré A. Incidence of leukaemia in young people around the La Hague nuclear waste reprocessing plant: a sensitivity analysis. Stat Med. 1995;14:2459–2472. doi: 10.1002/sim.4780142114. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. 2010. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 23.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez L, Puleo J, Balint JA. Investigation of gastrointestinal bleeding in patients with end stage renal disease. Am J Gastroenterol. 1993;88:30–33. [PubMed] [Google Scholar]
- 25.Hung TH, Tseng CW, Tseng KC, Hsieh YH, Tsai CC, Tsai CC. Is end stage renal disease a risk factor for the mortality of cirrhotic patients with esophageal variceal bleeding? Hepatogastroenterology. 2014;61:1871–1875. [PubMed] [Google Scholar]
- 26.Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med. 1985;102:588–592. doi: 10.7326/0003-4819-102-5-588. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96–103. doi: 10.1038/ki.2008.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khedmat H, Ahmadzad-Asl M, Amini M, Lessan-Pezeshki M, Einollahi B, Pourfarziani V, Naseri MH, Davoudi F. Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc. 2007;39:1003–1007. doi: 10.1016/j.transproceed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Luo JC, Leu HB, Huang KW, Huang CC, Hou MC, Lin HC, Lee FY, Lee SD. Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ. 2011;183:E1345–E1351. doi: 10.1503/cmaj.110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalman RS, Pedrosa MC. Evidence-based review of gastrointestinal bleeding in the chronic kidney disease patient. Semin Dial. 2015;28:68–74. doi: 10.1111/sdi.12301. [DOI] [PubMed] [Google Scholar]
- 31.Weng SC, Shu KH, Tarng DC, Tang YJ, Cheng CH, Chen CH, Yu TM, Chuang YW, Huang ST, Sheu WH, et al. In-hospital mortality risk estimation in patients with acute nonvariceal upper gastrointestinal bleeding undergoing hemodialysis: a retrospective cohort study. Ren Fail. 2013;35:243–248. doi: 10.3109/0886022X.2012.747140. [DOI] [PubMed] [Google Scholar]
- 32.Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–110. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasse H, Gillen DL, Ball AM, Kestenbaum BR, Seliger SL, Sherrard D, Stehman-Breen CO. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64:1455–1461. doi: 10.1046/j.1523-1755.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 34.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36:34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]


