Abstract
Little is known about the experiences of individuals donating peripheral blood stem cells (PBSCs) or marrow for a second time. To study this, unrelated donors making a second donation through the National Marrow Donor Program (NMDP) between 2004 and 2013 were evaluated. Experiences of second-time donors giving marrow (n=118) (first donation was PBSC in 76; marrow in 42) were compared with those making only one marrow donation (n=5,829). Experiences of second-time donors giving PBSCs (n=602) (first donation was PBSCs in 362; marrow in 240) were compared to first-time PBSC donors (n=16,095). For donors giving a second PBSC or marrow donation there were no significant differences in maximum skeletal pain, maximum symptoms measured by an established modified toxicity criteria (MTC) and recovery time compared to those who donated only once. Notably, the yield of marrow nucleated cells and PBSC CD34+ cells with second donations was less. As previously noted with single first time donations, females (PBSC and Marrow) and obese donors (PBSC) had higher skeletal pain and/or toxicity with a second donation. PBSC donors who experienced high levels of pain or toxicity with the first donation also experienced high levels of these symptoms with their second donation as well as slower recovery times. In conclusion, for the large majority of donors second donation experiences were similar to first donation experiences but CD34+ yields were less. Knowledge of the donor’s first experience and stem cell yields may help centers decide whether second donations are appropriate and institute measures to improve donor experiences.
Keywords: bone marrow, peripheral blood stem cells, unrelated donor, hematopoietic cell transplantation
INTRODUCTION
Most hematopoietic stem cell transplants involving HLA-compatible related and unrelated donors using marrow or peripheral blood stem cells (PBSCs) result in engraftment, and require a single donation procedure. However, some donors are asked to donate a second time to treat graft failure or disease relapse in the same recipient or to treat another recipient(1). The effects of a single anesthetic exposure and marrow collection or single granulocyte colony-stimulating factor (G-CSF)-mobilization and an apheresis procedure for PBSC donation on symptoms, complications and time to recovery have been well documented(2–5), but less is known about the effects of a second marrow or PBSC donation on the donor experience and collection yield(6–9).
The donation of marrow or G-CSF-mobilized PBSCs by healthy subjects is commonly associated with mild/moderate pain and other symptoms, less commonly with complications, and rarely with severe adverse events (SAEs)(2, 10–19). Marrow donation involves the aspiration of up to 20 mL per kg of marrow from the posterior iliac crests while the donor is under general or regional anesthesia. Following the donation, marrow donors experience pain in the hips and back(10). Pain related to anesthesia is also common with approximately one third reporting throat pain and one sixth experiencing headaches(10). Marrow donors also experience fatigue, insomnia, nausea and dizziness(10).
When healthy subjects are given 5 days of G-CSF to mobilize hematopoietic stem cells prior to apheresis, they frequently experience headache, bone pain, myalgia, nausea, and insomnia(2, 10, 11, 13). Generally, these symptoms are mild and disappear within a few days of the collection(11, 13), but up to 10% experience severe or intolerable pain(2). During the collection of PBSCs, healthy subjects can experience citrate toxicity, thrombocytopenia, bleeding or hematoma at intravenous line insertion sites(2, 10).
In comparison to first donations, less is known about second marrow and PBSC donations. Studies of healthy subjects who have donated PBSCs twice have noted that when the first and second donations are separated by more than 3 months, the pre-apheresis concentration of CD34+ cells and mononuclear cells, and collections yield for second donations are similar or slightly less than first donations(6–9). However, donor symptoms, and adverse events associated with second PBSC donations have not been investigated. Even less is known about second marrow donations.
This study sought to explore donor symptoms, adverse events and collection yields of second donations by NMDP donors. It compared second donations with a larger cohort of individuals donating only once and then assessed with multivariate analysis factors that may be associated with different outcomes in those giving second donations.
STUDY POPULATION AND METHODS
Study Population
NMDP donors between 2004 and 2013 who donated marrow or PBSCs once were compared to those who donated either product twice. Donors who made three donations, who donated at international centers, or who received G-CSF and then donated bone marrow were excluded. All donors included in this study provided written informed consent for participation in Center for International Blood and Marrow Transplant Research (CIBMTR) studies which were approved by the NMDP Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki.
Marrow Donation
Marrow was collected in an operating room from the posterior iliac crests under general or regional anesthesia following NMDP standards. NMDP standards require that no more than 20 mL/kg (donor weight) of marrow be aspirated, the duration of anesthesia should not exceed 150 minutes and the duration of the collection should be less than 120 minutes(4).
PBSC Donation
All PBSC collections were performed according to the NMDP-sponsored and Institutional Review Board-approved protocols for the manufacture of PBSC products, operated under an investigational new drug application with the US Food and Drug Administration. Collection of the G-CSF-mobilized PBSCs has been described previously(4). Briefly, G-CSF was administered subcutaneously for 4 to 5 days at a dose of approximately 10 micrograms/kg (donor weight) each day. PBSCs were collected by apheresis over 1 or 2 days. The volume of whole blood processed by the apheresis procedure was targeted to be between 12 to 24 L. The total volume of whole blood processed, whether the PBSCs were collected over 1 or 2 days, was limited to 24 L. When PBSCs could not be collected using peripheral veins a central venous catheter (CVC) was used.
Data Collection
Data collection began at the time of the donor’s medical evaluation to determine suitability to donate hematopoietic stem cells and continued throughout the time of donation and long term as described below. Both marrow and PBSC donors were contacted by the donor center at 2 days after donation, then at 1 week, and weekly thereafter until complete recovery. “Complete recovery” was judged by the donor center coordinator or medical director based on reports of return to baseline and no ongoing symptoms associated with the collection procedure as ascertained by the weekly follow up call with the donor. Further contact with the donor occurred at 1 month, 6 months, and annually to assess for the presence of any new or residual symptoms. Detailed questions using the toxicity criteria modeled on National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) were used to assess specific symptoms commonly associated with donation (see endpoints below for list of symptoms) and to capture any toxicity the donor may have experienced as a result of the hematopoietic stem cell donation process. In addition, a complete blood count and white cell differential were performed at the initial medical evaluation, on the first day of G-CSF, the day(s) of collection, and, for some donors, at annual follow-ups.
End Points
The primary objective of this study was to compare symptoms related to the first and second PBSC and marrow donation, as well as adverse events and collection yields. The following end points were analyzed in multivariate models: incidence of grade 2 to 4 skeletal pain, fatigue, and highest toxicity level across selected body symptoms frequently associated with collection (fever in the absence of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope). Skeletal pain was defined as pain in at least 1 of the following sites: back, bone, headache, hip, limb, joint, or neck. The severity of skeletal pain was defined as the maximum grade among these pain sites. End points were analyzed at the following time points: the day with the highest level of toxicity (day + 6 from start of G-CSF for PBSCs and first assessment after marrow collection, 1 to 2 days after collection); and at 1 week and at 1 month after donation. Time to recovery from donation was defined as the time in days from the marrow collection or first day of PBSC collection to report of complete recovery as defined herein.
Statistical Methods
Comparison of second donation experiences to those donating once
The number of apheresis procedures, incidence of adverse events, presence of long-term pain or disability, and occurrence of grade 2–4 or 3–4 peak toxicity using the Modified Toxicity Criteria were compared between first and second donation using chi-square tests. The volume of blood collected and collection yields per liter of blood processed were compared using Kruskal-Wallis test. The time to complete recovery from donation was compared using the log-rank test. Linear, logistic, or Cox regression analysis depending on the outcome variable was used to adjust for differences in donor characteristics (age, sex, BMI, and race).
First donation variables as risk factors for second donation outcome
First donation outcomes were used as covariates in the linear, logistic, or Cox regression models to determine if they are associated with better or worse second donation outcomes. These covariates were only applied to the group where donors donated twice.
RESULTS
Second Donations
Second donation experiences were studied in 720 donors who first donated between 2004 and 2013. Two groups were studied; one whose second donation was marrow and another whose second donation was PBSC. Second marrow donation experiences for 118 unrelated donors were compared to the donation experiences of 5,829 unrelated donors who donated marrow once. Among those whose second donation was marrow, in 76 the first donation was PBSCs (PBSC-Marrow) and in 42 it was marrow (Marrow-Marrow). The second PBSC donation experience for 602 unrelated donors was compared to the donation experiences of 16,095 unrelated donors who donated PBSCs once. Among the 602 donors for whom the second donation was PBSCs; the first donation was marrow for 240 (Marrow-PBSC) and PBSCs for 362 (PBSC-PBSC). Among those whose second donation was marrow, those whose first donation was marrow more often donated to the same person than those whose first donation was PBSCs (69% vs 46%; p<0.016). The time between donations was less for PBSC-Marrow donors than for Marrow-Marrow donors [(median = 1.3 months (range = 0.1–24.8 months) vs 7.8 months (1.6–39.6 months); p<0.001)]. For those whose second donation was PBSCs, donors whose first donation was marrow were also more likely to donate for the same person than those whose first donation was PBSCs (85% vs 68%; p<0.001). The time between donations was less for Marrow-PBSC donors than for PBSC-PBSC donors [4.3 months (0.4–78.1 months) vs 6.1 months (0.3–52.1 months); p<0.001].
Second Donor Demographics
There were no significant differences among characteristics of single and two-time donation populations except that people whose second donation was PBSCs were significantly older at the time of the first donation than those who donated PBSCs once (Table 1). However, there was no difference in age at the time of the second donation among Marrow-PBSC and PBSC-PBSC donors (data not shown).
Table 1.
Characteristics of donors who donated PBSCs and marrow between 2004 and 2013 by products donated.
| Variable | PBSC Marrow-PBSC | PBSC-PBSC N (%) | P-valuea | Marrow N (%) | PBSC-Marrow N (%) | Marrow-Marrow N (%) | P-valuea | |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||||
| Number of donors | 16095 | 240 | 362 | 5829 | 76 | 42 | ||
| Sex | 0.434 | 0.588 | ||||||
| Female | 5942 (37) | 84 (35) | 123 (34) | 2291 (39) | 27 (36) | 14 (33) | ||
| Male | 10151 (63) | 156 (65) | 239 (66) | 3538 (61) | 49 (64) | 28 (67) | ||
| Unknown | 2 (N/A) | 0 (N/A) | 0 (N/A) | |||||
| Race | 0.403 | 0.947 | ||||||
| Caucasian | 11932 (74) | 170 (71) | 273 (75) | 3916 (67) | 55 (72) | 31 (74) | ||
| Hispanic | 1383 (9) | 27 (11) | 35 (10) | 697 (12) | 9 (12) | 3 (7) | ||
| African/African American | 680 (4) | 12 (5) | 18 (5) | 395 (7) | 3 (4) | 3 (7) | ||
| Asian/Pacific Islander | 824 (5) | 13 (5) | 11 (3) | 318 (5) | 5 (7) | 2 (5) | ||
| Native American | 146 (1) | 4 (2) | 5 (1) | 67 (1) | 0 | 1 (2) | ||
| Multiple races/other | 1012 (6) | 11 (5) | 19 (5) | 391 (7) | 4 (5) | 2 (5) | ||
| Unknown/declined | 118 (1) | 3 (1) | 1 (<1) | 45 (1) | 0 | 0 | ||
| Age at donation, first donation | 0.009 | 0.063 | ||||||
| 18 to 29 years old | 6599 (41) | 77 (32) | 139 (38) | 2371 (41) | 28 (37) | 12 (29) | ||
| 30 to 39 years old | 4602 (29) | 75 (31) | 94 (26) | 1760 (30) | 23 (30) | 19 (45) | ||
| 40 to 49 years old | 3425 (21) | 69 (29) | 86 (24) | 1269 (22) | 15 (20) | 11 (26) | ||
| 50 years old and older | 1466 (9) | 19 (8) | 43 (12) | 428 (7) | 10 (13) | 0 | ||
| Unknown | 3 (N/A) | 0 (N/A) | 0 (N/A) | 1 (N/A) | 0 (N/A) | 0 (N/A) | ||
| Median (range) | 33 (18–61) | 35 (20–60) | 34 (19–61) | 0.012 | 33 (19–61) | 34 (20–58) | 34 (20–49) | 0.603 |
| Body Mass Index BMI (kg/m2), first donation | 0.887 | 0.791 | ||||||
| Underweight, <18.5 | 110 (1) | 1 (<1) | 2 (1) | 30 (1) | 0 | 0 | ||
| Normal, 18.5–24.9 | 5147 (32) | 74 (32) | 104 (29) | 1837 (32) | 29 (38) | 12 (31) | ||
| Overweight, 25–29.9 | 6148 (38) | 87 (38) | 148 (41) | 2162 (38) | 30 (39) | 16 (41) | ||
| Obese, 30+ | 4684 (29) | 70 (30) | 108 (30) | 1715 (30) | 17 (22) | 11 (28) | ||
| Unknown | 6 (N/A) | 8 (N/A) | 0 (N/A) | 85 (N/A) | 0 (N/A) | 3 (N/A) | ||
| Body Mass Index BMI (kg/m2), second donation | 0.370 | 0.489 | ||||||
| Underweight, <18.5 | (N/A) | 2 (1) | 0 | (N/A) | 0 | 0 | ||
| Normal, 18.5–24.9 | (N/A) | 71 (30) | 106 (30) | (N/A) | 27 (36) | 12 (29) | ||
| Overweight, 25–29.9 | (N/A) | 90 (38) | 141 (39) | (N/A) | 24 (32) | 18 (43) | ||
| Obese, 30+ | (N/A) | 77 (32) | 112 (31) | (N/A) | 24 (32) | 12 (29) | ||
| Unknown | (N/A) | 0 (N/A) | 3 (N/A) | (N/A) | 1 (N/A) | 0 (N/A) | ||
| Year of donation, first donation | <0.001 | <0.001 | ||||||
| 2004–2007 | 4309 (27) | 123 (51) | 163 (45) | 1910 (33) | 35 (46) | 24 (57) | ||
| 2008–2010 | 4920 (31) | 61 (25) | 127 (35) | 1682 (29) | 21 (28) | 14 (33) | ||
| 2011–2013 | 6866 (43) | 56 (23) | 72 (20) | 2237 (38) | 20 (26) | 4 (10) | ||
| Year of donation, last donation | <0.001 | 0.132 | ||||||
| 2004–2007 | 4309 (27) | 88 (37) | 80 (22) | 1910 (33) | 18 (24) | 13 (31) | ||
| 2008–2010 | 4920 (31) | 72 (30) | 141 (39) | 1682 (29) | 21 (28) | 17 (40) | ||
| 2011–2013 | 6866 (43) | 80 (33) | 141 (39) | 2237 (38) | 37 (49) | 12 (29) | ||
The Pearson chi-square test was used for comparing discrete variables; the Kruskal-Wallis test was used for comparing continuous variables.
Second Donation Timing
The year of first donation by those whose second donation was marrow was more likely to occur earlier in the study period than donations by people donating marrow once, but there was no difference in the time of the last donation among Marrow-Marrow and PBSC-Marrow donors (Table 1). There were also significant differences in the year of donation by those whose second donation was PBSCs and the year of donation for people donating PBSCs only once (Table 1). The first donation by those whose second donation was PBSCs was more likely to occur early in the study period than donations by people donating PBSCs once. The year of last donation for PBSC-PBSC donors was more likely to occur later in the study period than for Marrow-PBSC donors (Table 1).
Second Marrow Collection Procedures and Yields
The duration of anesthesia for first donations by Marrow-Marrow donors was less than that of marrow donor who only donated once (Table 2). There was no difference in duration of anesthesia for second marrow donations by Marrow-Marrow and PBSC-Marrow donors compared to those who donated marrow only once (Table 2). For all groups, more than 95% of the donations involved the use of general anesthesia and there was no difference in anesthesia type among groups (data not shown).
Table 2.
Marrow collection procedures and yields.
| Variable | Marrow N (%) |
PBSC-Marrow N (%) |
Marrow-Marrow N (%) |
P-valuea |
|---|---|---|---|---|
| Number of donors | 5829 | 76 | 42 | |
|
| ||||
| Duration of anesthesia in minutes, first collection | ||||
| N Eval | 5706 | 0 | 39 | |
| Less than 60 minutes | 626 (11) | (N/A) | 12 (31) | <0.001 |
| Less than 120 minutes | 4548 (80) | (N/A) | 35 (90) | 0.120 |
| Less than 150 minutes, NMDP recommendation | 5295 (93) | (N/A) | 38 (97) | 0.263 |
| Less than 200 minutes | 5646 (99) | (N/A) | 39 (100) | 0.520 |
| Median (range) | 90 (25–355) | (N/A) | 71(50–198) | <0.001 |
| Duration of anesthesia in minutes, last collection | ||||
| N Eval | 5706 | 76 | 42 | |
| Less than 60 minutes | 626 (11) | 12(16) | 7 (17) | 0.211 |
| Less than 120 minutes | 4548 (80) | 59 (78) | 36(86) | 0.566 |
| Less than 150 minutes, NMDP recommendation | 5295 (93) | 71 (93) | 39 (93) | 0.978 |
| Less than 200 minutes | 5646 (99) | 75(99) | 41 (98) | 0.689 |
| Median (range) | 90 (25–355) | 86 (36–210) | 87(42–246) | 0.341 |
| Collection volume, first donation | 0.041 | |||
| <1 L | 2516 (44) | (N/A) | 25 (64) | |
| 1–1.5 L | 2447 (43) | (N/A) | 10(26) | |
| ≥1.5 L | 733 (13) | (N/A) | 4 (10) | |
| Unknown | 133 (N/A) | (N/A) | 3 (N/A) | |
| Median (range), in mL | 1063.2 (103.0–2323.0) | (N/A) | 668.0 (193.0–1921.0) | <0.001 |
| Collection volume, last donation | 0.014 | |||
| <1 L | 2516 (44) | 27(36) | 27 (68) | |
| 1–1.5 L | 2447 (43) | 41 (54) | 10 (25) | |
| ≥1.5 L | 733 (13) | 8 (11) | 3 (8) | |
| Unknown | 133 (N/A) | 0 (N/A) | 2 (N/A) | |
| Median (range), in mL | 1063.2 (103.0–2323.0) 111 | 6.0 (364.0–1780.0) | 813.0 (276.0–1648.0) | 0.010 |
| Collection volume per kg of donor weight, first donation | 0.005 | |||
| <10/kg | 1700 (30) | (N/A) | 22 (56) | |
| 10 to <15/kg | 1834 (32) | (N/A) | 8 (21) | |
| 15 to <20/kg | 1769 (31) | (N/A) | 7 (18) | |
| ≥20/kg | 375 (7) | (N/A) | 2 (5) | |
| Unknown | 151(N/A) | (N/A) | 3 (N/A) | |
| Median (range) | 13.2 (1.2–29.0) | (N/A) | 8.5 (2.2–20.5) | <0.001 |
| Collection volume per kg of donor weight, last donation | 0.089 | |||
| <10/kg | 1700 (30) | 20(26) | 21 (53) | |
| 10 to <15/kg | 1834 (32) | 25 (33) | 10 (25) | |
| 15 to <20/kg | 1769 (31) | 27(36) | 7 (18) | |
| ≥20/kg | 375 (7) | 4 (5) | 2 (5) | |
| Unknown | 151(N/A) | 0 (N/A) | 2 (N/A) | |
| Median (range) | 13.2 (1.2–29.0) | 13.9 (4.2–21.6) | 9.7 (3.2–22.6) | 0.007 |
| TNC (×108), first donation | ||||
| N Eval | 2887 | 0 | 19 | |
| Median (range) | 24194.3 (2596.8–26657440) | (N/A) | 19669.0 (5747.8–40750.0) | 0.024 |
| TNC (×108), last donation | ||||
| N Eval | 2887 | 40 | 17 | |
| Median (range) | 24194.3 (2596.8–26657440) | 26832.9 (8095.5–54504.9) | 13276.9 (6206.0–23708.4) | <0.001 |
The quantity of marrow collected was assessed by measuring its volume and TNC content. The volume of marrow collected, volume collected per kg of donor weight, and quantity of TNCs for marrow donors who only donated once was significantly higher than that of the first collections for Marrow-Marrow donors (Table 2). There was a difference in total volume of marrow collected, volume collected per kg donor weigh and quantity of TNCs for those who donated marrow once and the last donation by Marrow-Marrow and PBSC-Marrow donors (Table 2).
Second PBSC Collection Procedures and Yields
The collection procedures for second donations that were PBSCs were similar to procedures by those who donated PBSCs once but the CD34+ cell yields of the second PBSC donations was less than the yields from those who donated only once (Table 3). There was no difference in G-CSF dose, need for a 2-day collection procedure and the use of a central venous collection catheter between the first donation values of PBSC-PBSC donors and those who donated PBSCs once and among second PBSC donations for PBSC-PBSC and Marrow-PBSC donors (Table 3).
Table 3.
PBSC collection procedures and yields.
| Variable | PBSC N (%) |
Marrow-PBSC N (%) |
PBSC-PBSC N (%) |
P-valuea |
|---|---|---|---|---|
| Number of donors | 16095 | 240 | 362 | |
| Total G-CSF dose per donor weight (μg/kg), first donation | ||||
| N Eval | 15892 | 0 | 359 | |
| Median (range) | 52.9 (26.8–112.1) | (N/A) | 52.5 (36.8–63.9) | 0.174 |
| Total G-CSF dose per donor weight (μg/kg), last donation | ||||
| N Eval | 15892 | 233 | 354 | |
| Median (range) | 52.9 (26.8–112.1) | 52.7 (34.3–67.6) | 52.7 (40.5–68.7) | 0.435 |
| Two-day collection, first donation | 0.321 | |||
| No | 13347 (83) | (N/A) | 293 (81) | |
| Yes | 2748 (17) | (N/A) | 69 (19) | |
| Two-day collection, last donation | 0.134 | |||
| No | 13347 (83) | 207 (86) | 311 (86) | |
| Yes | 2748 (17) | 33(14) | 51 (14) | |
| Central line insertion, first donation | 0.442 | |||
| No | 14637 (91) | (N/A) | 325 (90) | |
| Yes | 1456 (9) | (N/A) | 37 (10) | |
| Unknown | 2 (N/A) | (N/A) | 0 (N/A) | |
| Central line insertion, last donation | 0.204 | |||
| No | 14637 (91) | 211 (88) | 325 (90) | |
| Yes | 1456 (9) | 29(12) | 37 (10) | |
| Unknown | 2(N/A) | 0 (N/A) | 0 (N/A) | |
| Volume of whole blood processed, first donation | 0.748 | |||
| Small, <12 L | 407 (3) | (N/A) | 11 (3) | |
| Standard, 12–18 L | 3065 (19) | (N/A) | 65 (18) | |
| Large, ≥ 18 L | 12603 (78) | (N/A) | 284 (79) | |
| Unknown | 20(N/A) | (N/A) | 2 (N/A) | |
| Volume of whole blood processed, last donation | <0.001 | |||
| Small, <12 L | 407 (3) | 10 (4) | 8 (2) | |
| Standard, 12–18 L | 3065 (19) | 71 (30) | 75(21) | |
| Large, ≥ 18 L | 12603 (78) | 158 (66) | 279 (77) | |
| Unknown | 20(N/A) | 1 (N/A) | 0 (N/A) | |
| Duration of apheresis in hours (day 5), first donation | ||||
| N Eval | 16080 | 0 | 361 | |
| Median (range) | 4.5 (0.3–23.8) | (N/A) | 4.6 (2.0–9.8) | 0.219 |
| Duration of apheresis in hours (day 5), last donation | ||||
| N Eval | 16080 | 239 | 362 | |
| Median (range) | 4.5 (0.3–23.8) | 4.4 (1.7–8.8) | 4.6 (2.0–10.3) | 0.266 |
| CD34+ at collection (×106), first donation | ||||
| N Eval | 14860 | 0 | 313 | |
| Median (range) | 657.4 (2.8–6000.0) | (N/A) | 577.7 (41.8–2480.0) | 0.001 |
| CD34+ at collection (×106), last donation | ||||
| N Eval | 14860 | 222 | 342 | |
| Median (range) | 657.4 (2.8–6000.0) | 526.5 (63.1–4525.4) | 543.1 (17.5–2822.7) | <0.001 |
| CD34+ at collection per liter of whole blood processed, first donation | ||||
| N Eval | 14853 | 0 | 313 | |
| Median (range) | 34.0 (0.2–333.3) | (N/A) | 30.2 (1.7–114.2) | 0.002 |
| CD34+ at collection per liter of whole blood processed, last donation | ||||
| N Eval | 14853 | 221 | 342 | |
| Median (range) | 34.0 (0.2–333.3) | 28.9 (2.5–188.6) | 27.8 (0.7–122.1) | <0.001 |
| CD34+ at collection per kg of donor weight, first donation | ||||
| N Eval | 14860 | 0 | 313 | |
| Median (range) | 8.0 (0.0–89.6) | (N/A) | 7.1 (0.7–27.2) | <0.001 |
| CD34+ at collection per kg of donor weight, last donation | ||||
| N Eval | 14860 | 222 | 342 | |
| Median (range) | 8.0 (0.0–89.6) | 6.7 (1.0–37.2) | 6.5 (0.2–31.0) | <0.001 |
The Pearson chi-square test was used for comparing discrete variables; the Kruskal-Wallis test was used for comparing continuous variables.
There was no difference in the volume of whole blood processed during the collection for the first donation by PBSC-PBSC donors and those who donated PBSCs once (Table 3). However, the volume of whole blood processed during the PBSC collection was less for the last donation by PBSC-PBSC and Marrow-PBSC donors than for those who donated PBSCs once.
PBSC collection yields expressed as total CD34+ cells collected, CD34+ cells per liter of blood processed during apheresis and CD34+ cell per kg of donor weight were significantly higher for PBSC donors who only donated once compared to those whose second donation was PBSCs and first donations by PBSC-PBSC donors (Table 3). A pairwise comparison of first and second PBSC collection yields for PBSC-PBSC donors revealed that individual donors’ second collections generally had lower numbers of CD34+ cells as measured by total CD34+ cells collected (mean= −49.091×106; p=0.011, n=307); CD34+ cells per liter of whole blood processed (−2.473×106/L, p=0.007); and CD34+ cells per kg donor weight (−0.676×106/kg, p=0.004).
Donor Pain, Symptoms and Time to Recovery
Second Marrow Donations
For those whose second donation was marrow, there was no difference in maximum skeletal pain at day 2 (Figure 1a), maximum MTC (Figure 1b) and time to recovery to baseline (Figure 2) compared to those who donated marrow once. Multivariate analysis found that maximum skeletal pain (grade 2–4) at day 2 was dependent on sex and race while time to recovery to baseline was dependent on sex and age (Table 4). Male donors experienced less skeletal pain than female donors and time for recovery was shorter for males than for females.
Figure 1.
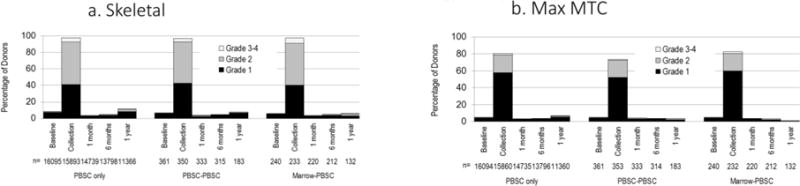
Severity of skeletal pain and maximum MTC for second donations that were marrow. The severity of maximum skeletal pain (Panel A) and maximum MTC (Panel B) scores measured at baseline, and 2 days, 1 month, 6 months and 1 year post-collection in people who donated marrow once (Marrow only), people whose first and second donations were marrow (Marrow-Marrow) and donors whose first donation was PBSCs and second donation was marrow (PBSC-Marrow). Skeletal pain was defined as pain in at least one of the following sites: back, bone, headache, hip, limb, joint, and neck. The severity of skeletal pain was defined as the maximum grade among these pain sites. MTC was the highest toxicity level of key symptoms including fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope.
Figure 2.
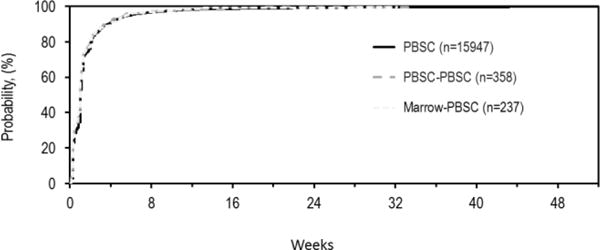
Time to recovery for second donations that were marrow. The proportion of donors who report that they had recovered to baseline levels at each time post-donation are shown for people who donated marrow once (Marrow), people whose first and second donations were marrow (Marrow-Marrow) and donors whose first donation was PBSCs and second donation was marrow (PBSC-Marrow).
Table 4.
Odds Ratios (ORs) comparing donor toxicities and recovery versus characteristics of second donations that were marrow with multivariate logistic regression analysis.
| Variable | Max skeletal pain grade 2–4 at day 2 | Recovery to baseline | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| Group | 0.315 | 0.573 | ||
| Marrow | 1.00 | 1.00 | ||
| Marrow-Marrow | 1.07 (0.53–2.15) | 0.851 | 0.87 (0.63–1.19) | 0.378 |
| PBSC-Marrow | 1.45 (0.89–2.36) | 0.131 | 0.93 (0.74–1.17) | 0.555 |
|
| ||||
| Sex | <0.001 | 0.005 | ||
| Female | 1.00 | 1.00 | ||
| Male | 0.59 (0.52–0.66) | 1.08 (1.02–1.14) | ||
|
| ||||
| Age at donation (years) | 0.013 | |||
| 18–29 | 1.00 | |||
| 30–39 | 0.90 (0.85–0.96) | 0.001 | ||
| 40–49 | 0.95 (0.89–1.02) | 0.172 | ||
| 50–59 | 0.96 (0.86–1.06) | 0.438 | ||
|
| ||||
| Race | <0.001 | |||
| Caucasian | 1.00 | |||
| Hispanic | 0.73 (0.61–0.90) | 0.001 | ||
| African/African | 1.05 (0.84–1.32) | 0.653 | ||
| American | ||||
| Asian/Pacific Islander | 0.77 (0.59–1.00) | 0.054 | ||
| Native American | 0.43 (0.23–0.81) | 0.009 | ||
| Multiple races/other | 0.81 (0.63–1.03) | 0.086 | ||
| Unknown/declined | 1.89 (0.98–3.64) | 0.056 | ||
Second PBSC Donations
For donors whose second donation was PBSCs there were no significant differences in maximum skeletal pain (grade 2–4) (Figure 3a), maximum modified toxicity criteria (MTC) (grade 2–4) (Figure 3b) and time to complete recovery (Figure 4) compared to donors who donated PBSCs once.
Figure 3.
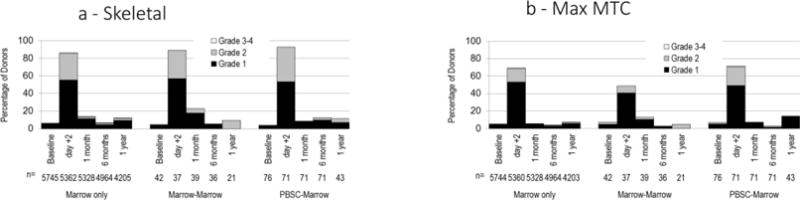
Severity of skeletal pain and MTC for second donations that were PBSCs. The severity of maximum skeletal pain (Panel A) and maximum MTC (Panel B) scores measured at baseline, at the time of collection and 1 month, 6 months and 1 year post-collection in people who donated PBSCs once (PBSC only), people whose first and second donations were PBSCs (PBSC-PBSC) and donors whose first donation was marrow and second donation was PBSCs (Marrow-PBSC). Skeletal pain represents pain in at least one of the following sites: back, bone, headache, hip, limb, joint, and neck). The severity of skeletal pain was defined as the maximum grade among these pain sites. The severity of MTC was the highest toxicity level of key symptoms including fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope.
Figure 4.
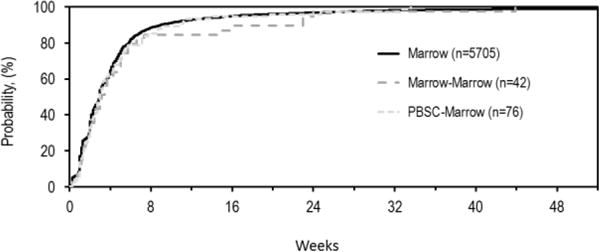
Time to recovery for second donations that were PBSCs. The proportion of donors who report that they have recovered to baseline measures at each time post-donation are shown for people who donated PBSCs once (PBSC), people whose first and second donations were PBSCs (PBSC-PBSC) and donors whose first donation was marrow and second donation was PBSCs (Marrow-PBSC).
For those whose second procedure was PBSCs multivariate analysis found that maximum MTC (grade 2–4) at collection was dependent on donor group, sex, race, age, collection year and body mass index (BMI) but not on whether the collection was the second procedure (Table 5). When the second donation was PBSCs female donors experienced lower maximum MTC grade 2–4 symptoms than females who made one PBSC donation, while male donors experienced more maximum MTC grade 2–4 than males donating PBSC only once.
Table 5.
Odds Ratios (ORs) comparing donor skeletal pain, MTC and recovery versus characteristics of second donations that were PBSCs with multivariate logistic regression analysis.
| Variable | Max skeletal pain grade 2–4 at collection | Max MTC grade 2–4 at collection | Recovery to baseline | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | HR (95% CI) | P-value | ||
|
| |||||||
| Group | 0.919 | Group*Sex | <0.001 | 0.309 | |||
| PBSC | 1.00 | F, PBSC | 1.00 | 0.009 | 1.00 | ||
| Marrow-PBSC | 1.04 (0.80–1.35) | 0.784 | F, Marrow-PBSC | 0.43 (0.24–0.78) | 0.006 | 1.06 (0.94–1.21) | 0.341 |
| PBSC-PBSC | 0.97 (0.78–1.20) | 0.764 | F, PBSC-PBSC | 0.74 (0.48–1.14) | 0.172 | 1.07 (0.96–1.19) | 0.220 |
|
|
|
||||||
| Sex | <0.001 | M, PBSC | 1.00 | 0.045 | <0.001 | ||
| Female | 1.00 | M, Marrow-PBSC | 1.53 (1.05–2.21) | 0.025 | 1.00 | ||
| Male | 0.59 (0.55–0.63) | M, PBSC-PBSC | 1.20 (0.87–1.66) | 0.264 | 1.08 (1.04–1.11) | ||
|
| |||||||
| Race | 0.003 | <0.001 | |||||
| Caucasian | 1.00 | 1.00 | |||||
| Hispanic | 1.05 (0.92–1.20) | 0.429 | 0.98 (0.92–1.03) | 0.401 | |||
| African/African | 0.77 (0.64–0.94) | 0.009 | 0.92 (0.86–1.00) | 0.047 | |||
| American | |||||||
| Asian/Pacific | 1.22 (1.03–1.44) | 0.022 | 0.90 (0.84–0.97) | <0.001 | |||
| Islander | |||||||
| Native American | 1.27 (0.89–1.82) | 0.189 | 0.89 (0.76–1.05) | 0.171 | |||
| Multiple races/other | 1.17 (1.01–1.36) | 0.038 | 0.92 (0.86–0.98) | 0.009 | |||
| Unknown/declined | 0.82 (0.50–1.34) | 0.431 | 0.70 (0.58–0.84) | <0.001 | |||
|
| |||||||
| Age at donation (years) | <0.001 | <0.001 | 0.021 | ||||
| 18–29 | 1.00 | 1.00 | 1.00 | ||||
| 30–39 | 1.11 (1.02–1.19) | 0.011 | 1.13 (1.03–1.24) | 0.010 | 0.96 (0.93–1.00) | 0.038 | |
| 40–49 | 0.79 (0.73–0.86) | <0.001 | 1.09 (0.99–1.21) | 0.078 | 0.98 (0.94–1.02) | 0.339 | |
| 50–59 | 0.61 (0.54–0.68) | <0.001 | 0.85 (0.73–0.98) | 0.021 | 1.05 (0.99–1.11) | 0.119 | |
|
| |||||||
| Collection Year | <0.001 | <0.001 | |||||
| 2004–2007 | 1.00 | 1.00 | |||||
| 2008–2010 | 0.86 (0.78–0.94) | 0.001 | 0.99 (0.95–1.03) | 0.535 | |||
| 2011–2013 | 0.76 (0.69–0.83) | <0.001 | 1.06 (1.02–1.10) | 0.003 | |||
|
| |||||||
| BMI (kg/m2) | <0.001 | <0.001 | |||||
| Underweight/normal, <24.9 | 1.00 | 1.00 | |||||
| Overweight, 25–29.9 | 1.22 (1.13–1.32) | <0.001 | 1.22 (1.11–1.34) | <0.001 | |||
| Obese, 30+ | 1.57 (1.44–1.70) | <0.001 | 1.56 (1.42–1.72) | <0.001 | |||
Maximum skeletal pain (grade 2–4) at collection was dependent on sex, age and BMI (Table 5). Males experienced less skeletal pain than females during second PBSC donations (OR=0.59, p<0.001).
During second PBSC donations obese donors experienced greater maximum skeletal pain (OR=1.57, p<0.001) and maximum MTC (grade 2–4) (OR=1.56, p<0.001) than donors that were underweight or had a lean BMI. Older second PBSC donors 50 to 59 years of age experienced less grade 2–4 skeletal pain (OR=0.61, p<0.001) and grade 2–4 MTC (OR=0.85, p=0.021) than the youngest donors, 18–29 years of age.
Time to recovery to baseline was dependent on donor sex, race, age and collection year (Table 5). For females the time to recovery to baseline after a second PBSC donation was longer than males.
Prognostic factor analysis of PBSC-PBSC and Marrow-PBSC donations revealed several important points concerning second PBSC donations (Table 6). There was an increased risk of maximum MTC (grade 2–4) or maximum skeletal pain (grade 2–4) for the second donation if the first donation was marrow and second was PBSCs. Among second-time PBSC donors high maximum MTC with the first donation predicted high maximum MTC (OR=3.29, p<0.001) and skeletal pain (OR=1.93, p=0.011) with second donation and longer recovery time (HR=0.76, p=0.009). High first donation skeletal pain predicted high second donation pain (OR=2.74, p<0.001) and slower first PBSC donation recovery predicted slower second donation recovery. A obese BMI predicts high maximum MTC with the second donation (OR=2.99, <0.001). More than 12 months between donations also predicted slower recovery after the second PBSC donation (HR=0.79, p=0.006).
Table 6.
Prognostic factor analysis for selected outcomes of second PBSC donations.
| Variable | MTC grade 2–4 during collection | Skeletal pain grade 2–4 during collection | Recovery to Baseline | |||
|---|---|---|---|---|---|---|
| OR (95 %CI) | p-value | OR (95 %CI) | p-value | HR (95 %CI) | p-value | |
|
| ||||||
| Group | 0.040 | 0.031 | ||||
| PBSC-PBSC | 1.00 | 1.00 | ||||
| Marrow-PBSC | 1.70 (1.02–2.82) | 1.50 (1.04–2.17) | ||||
|
| ||||||
| Group/time to recovery after 1st donation | <0.001 | |||||
| PBSC-PBSC, Recovery ≤3d | 1.00 | |||||
| PBSC-PBSC, Recovery 4–14d | 0.69 (0.54–0.89) | 0.004 | ||||
| PBSC-PBSC, Recovery >14d | 0.44 (0.33–0.59) | <0.001 | ||||
| Marrow-PBSC | 0.60 (0.48–0.77) | <0.001 | ||||
|
| ||||||
| Max MTC grade during collection of 1st donation | <0.001 | 0.040 | 0.031 | |||
| 0–1 | 1.00 | 1.00 | 1.00 | |||
| 2–4 | 3.29 (2.06–5.26) | <0.001 | 1.93 (1.16–3.20) | 0.011 | 0.75 (0.61–0.93) | 0.009 |
| Unknown | 0.69 (0.19–2.48) | 0.574 | 1.16 (0.07–20.05) | 0.916 | 0.91 (0.58–1.43) | 0.686 |
|
| ||||||
| Skeletal pain grade during collection of 1st donation | <0.001 | |||||
| 0–1 | 1.00 | |||||
| 2–4 | 2.74 (1.86–4.04) | <0.001 | ||||
| Unknown | 1.16 (0.07–20.05) | 0.916 | ||||
|
| ||||||
| BMI (kg/m2) | <0.001 | |||||
| Underweight/normal, <24.9 | 1.00 | |||||
| Overweight, 25–29.9 | 2.79 (1.58–4.92) | <0.001 | ||||
| Obese, 30+ | 2.99 (1.67–5.36) | <0.001 | ||||
|
| ||||||
| Interval between collections | 0.006 | |||||
| ≤12 months | 1.00 | |||||
| >12 months | 0.79 (0.66–0.93) | |||||
DISCUSSION
Although rare, approximately 3% of unrelated hematopoietic stem cell donors are asked to donate a second time to the same or a different person. This analysis of NMDP data found that the levels of pain and donation related symptoms of those donating a second time were similar to first donation experiences. For second donations that were either marrow or PBSCs, pain, peak MTC and time to recovery were no different than that of those who donated once. These results provide reassurance that second donations of either marrow or PBSCs do not present an increase in donation associated risk to unrelated donors.
Second donation factors associated with greater toxicity were similar to those previously identified in donors who only donated marrow or PBSCs once(2, 3). For those whose second donation was PBSCs grade 2–4 maximum skeletal pain and grade 2–4 maximum MTC were more likely in younger donors and in those with greater BMI. For those whose second donation was marrow, grade 2–4 maximum skeletal pain was more likely to occur in females. Time to recovery was longer in females and younger donors after second donations for both PBSC and marrow donors.
A previous analysis of 2,408 NMDP PBSC donors who donated once found that risk factors for incidence of bone pain on day 4 of G-CSF administration were female sex and being obese(2). Female donors and very heavy donors experienced higher MTC symptoms during mobilization and donation(2). A more recent comparison of 2,726 NMDP marrow and 6,768 PBSC donors who donated once found that for both marrow and PBSC donations, women were more likely to experience pain, toxicities and fatigue in the peri-collection period and in the post donation recovery period(3). This study also found that older donors were at less risk for grade 2 to 4 pain in the peri-collection period. It also found that older donors were at a greater risk for grade 2 to 4 toxicities and fatigue 1 week after the collections. Females were also less likely than males to experience complete recovery from both marrow and PBSC donations.
This study found that several first donation factors were predictive of second donation experiences. Among people whose second donation was PBSCs, longer recovery time from first donations predicted longer recovery time for second donations, greater skeletal pain during the first PBSC donation predicted greater skeletal pain during second donation and greater MTC during the first donation predicted greater MTC during the second donation.
It is notable that yields of second marrow and PBSC collections were shown to be lower than first collections. For people that donated PBSCs twice, the CD34+ cell yield from both their first and second donations were less than the CD34+ cell yields for PBSC donors who only donated once. In addition, among those who donated PBSCs twice, the CD34+ cell collection yield was less for the second donation than the first. Circulating levels of CD34+ cells were not measured prior to the apheresis collection so it is not known if the response to G-CSF differs among PBSC-PBSC donors and those who only donated once. However, since there was no difference in G-CSF dose, duration of apheresis or volume of blood processed during the first apheresis procedure for PBSC-PBSC donors and those donating PBSCs once, the people donating PBSCs twice appear to mobilize CD34+ cells less well in response to G-CSF. Others have found that several donor factors effect G-CSF mobilization of CD34+ cells including age, sex, and race(20, 21). Previous studies of people donating twice found that the quantity of CD34+ cells collected during the second donation was similar or lower to the quantity collected during first donation (6–9). With the much larger numbers we have in this study showing that second mobilizations result in lower CD34+ yields, clinicians should carefully consider whether a second PBSC collection will give adequate cells, especially if the yield from the first collection was low. In addition, transplant centers may want to be informed if a donor under consideration for a second donation had poor yields previously. Because this information is relevant to the choice of a given individual for a second donation, donor registries should make this information available to centers for use as part of their approach to choosing a donor. The findings of the variability among first and second collections were unexpected and we are planning another study to further investigate second donation collection yields.
In conclusion, policies of many registries of unrelated hematopoietic stem cell donors allow people to donate a second time for either the same person or a different person. This study found no contraindications to this practice. Second donation experiences were similar to first donation experiences, but yields of second grafts were lower. Knowledge of the donor’s first experience should help donor centers adjust management of the second donation to improve the donor experience and obtain the needed stem cell dose. To facilitate this, donor registries should provide information regarding the first donation experience and stem cell yields. The results of this study can also be used to assist donors in making a more informed decision concerning whether or not to donate marrow or PBSCs after a first donation.
Key points.
Unrelated donors donating for a second time have a similar experience to those donating once only
Experiences during the first donation may predict experiences during a subsequent donation
Highlights.
Second marrow and PBSC donation experiences are similar to those of the first donation.
First donation factors are predictive of second donation experiences.
Yields of second marrow and PBSC collections were lower than first collections.
No contraindications to second donations were identified.
Acknowledgments
This study was supported in part by research funding from the NIH Clinical Center, National Institute of Health, Bethesda, Maryland, USA [DFS].
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; two contracts HHSH250201200016C (MCW) and HHSH250201200024C (NMDP) with the Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–17–1-2388 and N00014–16–1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTIONS
DFS, BES, BRL, DMK, PVO, GES, PC, NS, DLC, and MAP were involved with the design of the study, analysis, and interpretation of the data. DFS, BES, and MAP wrote the manuscript. All authors were involved in critical review of the protocol and results. The final manuscript was read and approved by all authors.
DISCLOSURE OF CONFLICT OF INTEREST
The authors have no relevant conflicts to declare.
References
- 1.Bolan CD, Hartzman RJ, Perry EH, Trainor L, Miller J, Miller R, et al. Donation activities and product integrity in unrelated donor allogeneic hematopoietic transplantation: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):23–8. doi: 10.1016/j.bbmt.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Pulsipher MA, Chitphakdithai P, Miller JP, Logan BR, King RJ, Rizzo JD, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113(15):3604–11. doi: 10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, Lazarus HM, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121(1):197–206. doi: 10.1182/blood-2012-03-417667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Chitphakdithai P, Logan BR, Navarro WH, Levine JE, Miller JP, et al. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123(23):3655–63. doi: 10.1182/blood-2013-12-542464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredeson C, Leger C, Couban S, Simpson D, Huebsch L, Walker I, et al. An evaluation of the donor experience in the canadian multicenter randomized trial of bone marrow versus peripheral blood allografting. Biol Blood Marrow Transplant. 2004;10(6):405–14. doi: 10.1016/j.bbmt.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Platzbecker U, Bornhauser M, Zimmer K, Lerche L, Rutt C, Ehninger G, et al. Second donation of granulocyte-colony-stimulating factor-mobilized peripheral blood progenitor cells: risk factors associated with a low yield of CD34+ cells. Transfusion. 2005;45(1):11–5. doi: 10.1111/j.1537-2995.2005.04107.x. [DOI] [PubMed] [Google Scholar]
- 7.Stroncek DF, Clay ME, Herr G, Smith J, Ilstrup S, McCullough J. Blood counts in healthy donors 1 year after the collection of granulocyte-colony-stimulating factor-mobilized progenitor cells and the results of a second mobilization and collection. Transfusion. 1997;37(3):304–8. doi: 10.1046/j.1537-2995.1997.37397240213.x. [DOI] [PubMed] [Google Scholar]
- 8.Tichelli A, Passweg J, Hoffmann T, Gregor M, Kuhne T, Favre G, et al. Repeated peripheral stem cell mobilization in healthy donors: time-dependent changes in mobilization efficiency. British journal of haematology. 1999;106(1):152–8. doi: 10.1046/j.1365-2141.1999.01518.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderlini P, Lauppe J, Przepiorka D, Seong D, Champlin R, Korbling M. Peripheral blood stem cell apheresis in normal donors: feasibility and yield of second collections. Br J Haematol. 1997;96(2):415–7. doi: 10.1046/j.1365-2141.1997.d01-2013.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller JP, Perry EH, Price TH, Bolan CD, Jr, Karanes C, Boyd TM, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):29–36. doi: 10.1016/j.bbmt.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Stroncek DF, Clay ME, Petzoldt ML, Smith J, Jaszcz W, Oldham FB, et al. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36(7):601–10. doi: 10.1046/j.1537-2995.1996.36796323059.x. [DOI] [PubMed] [Google Scholar]
- 12.Stroncek DF, Clay ME, Smith J, Ilstrup S, Oldham F, McCullough J. Changes in blood counts after the administration of granulocyte-colony-stimulating factor and the collection of peripheral blood stem cells from healthy donors. Transfusion. 1996;36(7):596–600. doi: 10.1046/j.1537-2995.1996.36796323058.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderlini P, Przepiorka D, Seong D, Miller P, Sundberg J, Lichtiger B, et al. Clinical toxicity and laboratory effects of granulocyte-colony-stimulating factor (filgrastim) mobilization and blood stem cell apheresis from normal donors, and analysis of charges for the procedures. Transfusion. 1996;36(7):590–5. doi: 10.1046/j.1537-2995.1996.36796323057.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderlini P, Przepiorka D, Seong D, Champlin R, Korbling M. Transient neutropenia in normal donors after G-CSF mobilization and stem cell apheresis. Br J Haematol. 1996;94(1):155–8. doi: 10.1046/j.1365-2141.1996.d01-1778.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderlini P, Donato M, Chan KW, Huh YO, Gee AP, Lauppe MJ, et al. Allogeneic blood progenitor cell collection in normal donors after mobilization with filgrastim: the M.D. Anderson Cancer Center experience. Transfusion. 1999;39(6):555–60. doi: 10.1046/j.1537-2995.1999.39060555.x. [DOI] [PubMed] [Google Scholar]
- 16.Murata M, Harada M, Kato S, Takahashi S, Ogawa H, Okamoto S, et al. Peripheral blood stem cell mobilization and apheresis: analysis of adverse events in 94 normal donors. Bone Marrow Transplant. 1999;24(10):1065–71. doi: 10.1038/sj.bmt.1702038. [DOI] [PubMed] [Google Scholar]
- 17.Beelen DW, Ottinger H, Kolbe K, Ponisch W, Sayer HG, Knauf W, et al. Filgrastim mobilization and collection of allogeneic blood progenitor cells from adult family donors: first interim report of a prospective German multicenter study. Ann Hematol. 2002;81(12):701–9. doi: 10.1007/s00277-002-0553-5. [DOI] [PubMed] [Google Scholar]
- 18.Favre G, Beksac M, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, et al. Differences between graft product and donor side effects following bone marrow or stem cell donation. Bone Marrow Transplant. 2003;32(9):873–80. doi: 10.1038/sj.bmt.1704245. [DOI] [PubMed] [Google Scholar]
- 19.Ordemann R, Holig K, Wagner K, Rautenberg U, Bornhauser M, Kroschinsky F, et al. Acceptance and feasibility of peripheral stem cell mobilisation compared to bone marrow collection from healthy unrelated donors. Bone Marrow Transplant. 1998;21(Suppl 3):S25–8. [PubMed] [Google Scholar]
- 20.Hsu JW, Wingard JR, Logan BR, Chitphakdithai P, Akpek G, Anderlini P, et al. Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2015;21(1):165–71. doi: 10.1016/j.bbmt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panch SR, Yau YY, Fitzhugh CD, Hsieh MM, Tisdale JF, Leitman SF. Hematopoietic progenitor cell mobilization is more robust in healthy African American compared to Caucasian donors and is not affected by the presence of sickle cell trait. Transfusion. 2016;56(5):1058–65. doi: 10.1111/trf.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]


