Abstract
D-ɑ-tocopheryl polyethylene glycol succinate (Vitamin E TPGS or TPGS) has been approved by FDA as a safe adjuvant and widely used in drug delivery systems. The biological and physicochemical properties of TPGS provide multiple advantages for its applications in drug delivery like high biocompatibility, enhancement of drug solubility, improvement of drug permeation and selective antitumor activity. Notably, TPGS can inhibit the activity of ATP dependent P-glycoprotein and act as a potent excipient for overcoming multi-drug resistance (MDR) in tumor. In this review, we aim to discuss the recent advances of TPGS in drug delivery including TPGS based prodrugs, nitric oxide donor and polymers, and unmodified TPGS based formulations. These potential applications are focused on enhancing delivery efficiency as well as the therapeutic effect of agents, especially on overcoming MDR of tumors. It also demonstrates that the clinical translation of TPGS based nanomedicines is still faced with many challenges, which requires more detailed study on TPGS properties and based delivery system in the future.
Keywords: Vitamin E, TPGS, drug delivery, multi-drug resistance, anticancer.
Introduction
Vitamin E has been identified as an essential factor for reproduction since 1922 1. With further investigation, it has been found with other functions involving antioxidant, anti-thrombolytic and other therapeutic effects 2, 3. However, the poor water solubility of vitamin E has greatly limited its application 4. Vitamin E d-ɑ-tocopheryl poly(ethylene glycol) 1000 succinate (simply as Vitamin E TPGS or TPGS), synthesized by esterification of vitamin E succinate with poly(ethylene glycol) (PEG) 1000, is a water-soluble derivative of natural vitamin E 5. It has an amphiphilic structure comprising hydrophilic polar head portion and lipophilic alkyl tail. TPGS can be functionalized as an excellent solubilizer, emulsifier, permeation and bioavailability enhancer of hydrophobic drugs 6. Meanwhile, TPGS can act as an anticancer agent, which has been demonstrated to induce apoptogenic activity against many cancer types. It can target the mitochondria of cancer cells, resulting in the mitochondrial destabilisation for activation of mitochondrial mediators of apoptosis 7. Interestingly, it has been documented that TPGS can selectively induce apoptosis in tumor cells while exhibited nontoxicity to normal cells and tissues 8.
Multi-drug resistance (MDR) remains as a significant impediment to successful chemotherapy in clinical cancer treatment. What's worse, decades of research has identified that this phenomenon exists in nearly every effective drug, even the newest therapeutic agents 9. Therefore, how to effectively reverse drug resistance plays a critical role in achieving satisfied therapeutic effect in cancer treatment. It has been demonstrated that various mechanisms are involved in MDR including decreased drug influx, increased drug efflux, changed drug metabolism and promoted anti-apoptotic mechanism 10. Among them, the drug efflux mediated by ATP-binding cassette transporter P-glycoprotein (ABCB1) is one of the most investigated and characterized mechanisms for MDR. P-glycoprotein (P-gp) has 12 transmembrane regions to bind hydrophobic substrate drugs and two ATP-binding sites to transport drug molecules 11. It can pump out P-gp substrate drugs to the extracellular space and thus decrease the intracellular drug accumulation. Over the past few decades, considerable efforts have been devoted to exploring P-gp inhibitors for overcoming MDR. Several nonionic surfactants such as Pluronic, Tweens, Span and TPGS have been found with the ability to inhibit P-gp activity 12, 13. Though the exact mechanism of P-gp inhibition by these surfactants remains unclear, steric blocking of substrate binding 14, alteration of membrane fluidity 15 and inhibition of efflux pump ATPase 16, 17 have been proposed as the potential mechanisms. As a widely used adjuvant in drug delivery, TPGS has been shown as the most potent and commercially available P-gp inhibitor among these surfactants 18. As a membrane transporter of ATP-binding cassette family, P-gp can pump out the substrate drug via an ATP-dependent mechanism 19. TPGS can target the mitochondria and cause its dysfunction, resulting in the depletion of intracellular ATP. The reduced ATP level can then influence the activity of P-gp and decrease the drug efflux to extracellular space 20. Besides, the hydrolysis of ATP by ATPase is critical for converting the P-gp transporter to an active conformational state for substrate drug efflux 16. TPGS itself cannot stimulate ATPase activity as it is not a substrate of P-gp, but can inhibit the substrate induced ATPase activity 21. In our previous works, we have demonstrated that TPGS can significantly enhance the intracellular accumulation and cytotoxicity of chemotherapeutics to drug resistant breast adenocarcinoma cells (MCF-7/ADR) and human ovarian cancer cells (A2780/T) 22-24.
Since TPGS has been approved by the FDA as a safe pharmaceutical adjuvant, it has been extensively used in drug delivery systems as surfactant, solubilizer, stabilizer and P-gp inhibitor for enhancing bioavailability and reversing MDR. In our previous reviews 5, 6, we discussed TPGS as a molecular biomaterial and its original application in drug delivery. In this review, we focused on the progress of TPGS in drug delivery in recent five years, which took advantages of the P-gp inhibiting ability and other basic properties. We summarized the applications of TPGS based prodrugs, nitric oxide (NO) donor and polymers for overcoming MDR and delivering therapeutic agents. We also discussed the unmodified TPGS based formulations applied in reversing MDR, improving oral availability and enhancing drug permeation. We expect this review will give new inspiration for the application of TPGS in overcoming MDR and drug delivery.
TPGS properties in drug delivery systems
TPGS, a water miscible form of vitamin E, is composed of a hydrophobic vitamin E part and a hydrophilic PEG chain. It exhibits excellent drug delivery capability based on this special amphiphilic structure. With further investigations, it has been demonstrated that TPGS shows great potential in overcoming MDR tumor for the P-gp inhibition and selective anticancer effect. In this part, we will discuss about the basic properties and mainly focus on the P-gp inhibition as well as selective anticancer effect of TPGS in drug delivery systems (Figure 1).
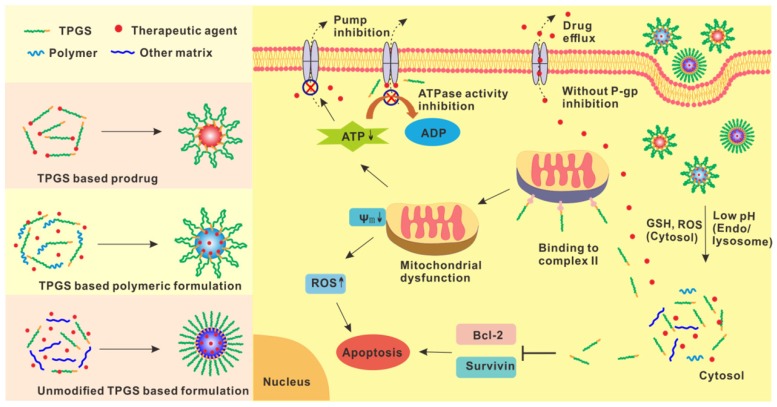
Figure 1.
Scheme of TPGS for P-gp inhibition and selective antitumor effect in drug delivery. TPGS can be easily conjugated with polymers or therapeutic agents to form TPGS based polymers or prodrugs. The resultant structure can further self-assemble into nanoparticles. Unmodified TPGS can also cooperate with other materials to form nanoformulations. After internalization by cells, the nanoparticles can be degraded in response to the specific intracellular environment (e.g. pH, GSH and ROS) to release the therapeutic agents and TPGS. Without P-gp inhibition, the drugs can be rapidly pumped out to the extracellular environment. The dissociated TPGS can bind to the mitochondrial respiratory complex II and induce mitochondrial dysfunction, resulting in the decreased mitochondrial membrane potential and increased ROS generation for cell apoptosis, and reduced ATP level for P-gp inhibition. Besides, TPGS can also inhibit the substrate induced ATPase activity to further overcome MDR. With P-gp inhibition, the intracellular accumulation of therapeutic drugs can be significantly enhanced. Meanwhile, TPGS can inhibit Bcl-2 and Survivin to promote cell apoptosis.
TPGS as a surfactant
Poor water solubility and/or poor permeability remain as the major obstacles for therapeutic drugs to exert maximum activity. TPGS can be applied as solubilizer, absorption and permeation enhancer, emulsifier as well as surface stabilizer in drug delivery. It has been widely used in fabricating nanodrugs or other formulations for many poorly water-soluble or permeable drugs, especially for biopharmaceutics classification system (BCS) class Ⅱ and Ⅳ drugs 5, 6. In addition, it has been reported that TPGS exhibited strong enhancement on the secretion of chylomicrons at low concentration and enhanced the intestinal lymphatic transport 25, which would further improve drug absorption ability.
As a surfactant, TPGS shows outstanding capability to increase drug absorption through different biological barriers. For example, TPGS was used to fabricate repaglinide nanocrystals for enhancing saturation solubility and oral bioavailability up to 25.7-fold and 15.0-fold compared with free drug, respectively 26. In Ussing chambers transport studies, TPGS can enhance drug permeation in colonic tissue 27. In addition, the influence of TPGS on the intestinal absorption ability of icariside Ⅱ was investigated in Caco-2 monolayer model and a four-site rat intestinal perfusion model. In Caco-2 monolayer model, the apparent permeability coefficients value of icariside Ⅱ was increased and the efflux ratio was remarkably reduced owing to the effect of TPGS. The four-site rat intestinal perfusion model investigation further showed significantly increased permeability of icariside Ⅱ in ileum and colon 28. Similar results were found in Caco-2 monolayer model with rhodamine123 (Rh123) in the presence of TPGS 29. Interestingly, TPGS can also act as a pore-forming agent in the fabrication of nanoparticles with high drug encapsulation efficiency, small particle size and fast drug release 30. Besides, TPGS can be used as emulsifier or surface stabilizer for the preparation of drug formulations as the hydrophobic portion can entrap hydrophobic drug and the hydrophilic part can stabilize the formulations.
TPGS as a P-gp inhibitor for overcoming MDR
Drug resistance of cancer cells can restrict the therapeutic efficacy in chemotherapeutic treatment. As the ATP dependent membrane transporter, P-gp has been one of primary causes for MDR. It can pump out the P-gp substrate drugs to decrease intracellular drug accumulation, thus reducing the cytotoxic effect of chemotherapeutic drugs in drug resistant cancer treatment. Over the past decades, there have been continuous interests to combine P-gp substrate drugs with inhibitor or some polymer with P-gp inhibiting capability in formulations for overcoming MDR 31. Rh123, a P-gp substrate, is usually used as the model drug to study the intracellular retention of drug in MDR tumor cells. TPGS can significantly increase the intracellular accumulation of Rh123 in drug-resistant tumor cells compared with free Rh123, which was evidenced from the flow cytometry and confocal microscope analysis 32. It seems that TPGS can effectively inhibit the activity of P-gp to overcome MDR.
Since the efflux transporter P-gp is ATP-dependent, the depletion of ATP plays a very important role in overcoming MDR. The MDR reversing effect of TPGS is mainly attributed to its dual actions, the inhibition of mitochondrial respiratory complex Ⅱ for shorting ATP supply and the suppression of substrate induced P-gp ATPase activity for blocking ATP utilization 20, 21, 33, 34.
Mitochondrial respiratory complex Ⅱ, also called succinate dehydrogenase, plays an important role in mitochondrial electron transport, which is an essential part in the tricarboxylic acid cycle as well as the mitochondrial respiratory chain 35. TPGS can bind with mitochondrial respiratory complex Ⅱ and induce subsequent mitochondrial dysfunction, resulting in significant depletion of intracellular energy 20, 36. TPGS can accumulate in mitochondria and inhibit the activity of complex Ⅱ, and consequently disrupt the electron transfer and activate calcium channel, which would result in the overload of calcium and ensuing dysfunction of mitochondria. Mitochondrial dysfunction is characterized by the dissipating effect on mitochondrial membrane potential, decreased ATP level and increased reactive oxygen species (ROS) generation 37. Furthermore, the mitochondrial targeting ability of TPGS may accelerate the mitochondrial dysfunction 32, 38. Substrate induced P-gp ATPase activity suppression is another mechanism for TPGS to decrease drug efflux 21. ATPase activity can be stimulated by the binding of substrate to transmembrane regions of P-gp 39. Subsequently, ATP is transformed into adenosine diphosphate (ADP) for the energy supply of drug efflux. Unlike the classical P-gp inhibitor verapamil, TPGS is not a substrate of P-gp and shows no competitive inhibition effect of substrate binding. The steric blocking function of the binding site and/or allosteric modulation of P-gp appear to be the ATPase inhibition mechanism.
TPGS as a selective anticancer agent for synergistic antitumor effects
TPGS can induce apoptosis and exhibits selective cytotoxic effects against cancer cells, which can be combined with chemotherapeutic drugs for reducing side effect and increasing treatment efficiency. There is significant different response on normal immortalized breast cells and cancer cells after TPGS treatment. TPGS can trigger the apoptotic signaling pathways and induce G1/S cell cycle arrest in breast cancer cells MCF-7 and MDA-MB-231, but no remarkable effect on non-tumorigenic cells MCF-10A and MCF-12F 40. Coincidentally, TPGS can induce apoptosis on T cell acute lymphocytic leukemia Jurkat clone E6-1 cells, but not on human peripheral blood lymphocytes. The apoptosis was evidenced by increased nuclear DNA fragmentation, enhanced cell cycle arrest and reduced mitochondrial membrane potential 41. The selective apoptosis mechanisms of cancer cells mediated by TPGS are complicated and can be listed as follows:
ROS inducer
Similar to α-tocopheryl succinate (α-TOS), TPGS can induce cancer cell apoptosis through the destruction and inhibition of mitochondrial respiratory complex Ⅱ 33, 41. The subsequent electron transfer chain disruption can promote ROS generation 20. The escalated intracellular ROS, a mediator of apoptosis, can induce DNA damage and the oxidation of lipid, protein and enzyme, leading to cell destruction 42. Besides, it has been demonstrated that ROS-mediated apoptosis mechanism was correlated with the selective anticancer activity as tumor cells could be more sensitive to ROS than normal cells 43-45. Compared with TOS, TPGS exhibited enhanced ROS generation capability 46.
Downregulation of anti-apoptotic proteins
TPGS can inhibit the phosphorylation of protein kinase B (PKB or AKT) and then downregulate the anti-apoptotic proteins Survivin and Bcl-2, which can induce the activation of caspase-3 and -7 for caspase-dependent programmed cell death 40. Concurrently, caspase-independent programmed cell death and G1/S phase cell cycle arrest also occurred 40, 41. Survivin and Bcl-2 are usually overexpressed in most cancer cells while remarkably reduced in normal cells 47. This may be the main reason for the selective cytotoxicity of TPGS.
DNA damage
TPGS can induce both caspase-dependent and caspase-independent DNA damage. This kind of DNA damage was observed in androgen receptor positive (AR+) LNCaP cells but not in AR- DU145 and PC3 cells, which was related to the cellular microenvironment 48.
TPGS based prodrugs
Prodrug is a group of agents with low or no pharmaceutical activity and can undergo a series of in vivo biotransformation to generate parental drug 49, 50. It is designed to optimize the pharmaceutical, pharmacokinetic (PK) and pharmacodynamic (PD) properties of drugs such as improving drug solubility, permeability, stability, bioavailability, treatment efficiency and decreasing the adverse effects. Prodrug can be simply classified into carrier-based prodrug and bioprecursor prodrug. The carrier-based prodrug, which is synthesized by simply conjugating polymer with drug via a temporary linker, can easily self-assemble into nanoformulation and provide great potential in clinical application 51. For example, poly-glutamic acid (PGA)-SN38 (NK012; Nippon Kayaku, Co.) 52 and N-[2-hydroxylpropyl] methacrylamide (HPMA)-doxorubicin (PK1/FCE28068; Pfizer Inc.) 53 have been approved by FDA in clinical trials. Adagen® (Aden-osine deaminase), Onscaspar® (L-asparaginase) and Pegasys® (PEGylated IFN-α-2a) have already come into the market.
The hydroxyl of PEG in TPGS is a reactive part and can be easily conjugated with therapeutic agents to form TPGS-based prodrugs, in which TPGS can serve as a drug carrier and P-gp inhibitor to overcome MDR in cancer treatment. On account of the specific physiological characteristics in tumor cells and microenvironment (e.g. pH, GSH, ROS, hypoxia, enzyme and glucose) 54, TPGS-based stimuli-responsive prodrugs can be designed in one simple system to realize optimal cancer therapy. In this section, we will introduce the current investigations on TPGS-based prodrugs and mainly discuss about the P-gp inhibition effect for MDR in drug delivery system (Table 1).
Table 1.
TPGS based prodrugs in drug delivery
| Prodrug | Payload | Tumor model | Effects | Ref |
|---|---|---|---|---|
| TPGS-DOX | DOX | Resistant breast cancer, melanoma, hepatoma | 95-fold lower IC50 in MCF-7/ADR vs. free drug, MCF-7/ADR, B16F10, H22 tumor growth/metastasis inhibition | 23 |
| TPGS-DOX | DOX | Breast, glioma cancer | High cellular uptake and cytotoxicity | 55, 56 |
| TPGS-DOX | DOX, Ce6 | Non-small cell lung cancer | Good tumor targeting, high apoptosis, tumor growth suppression in A549 cells | 57 |
| TPGS-PTX | PTX | Resistant ovarian cancer, hepatoma | PTX accumulation in A2780/T, cytotoxicity against A2780 and A2780/T, S180 tumor inhibition | 58 |
| TPGS-cisplatin | Cisplatin | Hepatoma | High cell uptake and cytotoxicity, significant neuroprotective effects | 60 |
| TPGS-cisplatin | Cisplatin, DTX, Herceptin | Breast cancer | Enhanced cytotoxicity against SK-BR-3 cells with overexpression of HER2 | 62 |
| TPGS-5-FU | 5-FU, PTX | Resistant breast cancer, lung cancer | Cytotoxicity, P-gp inhibition in H460/TaxR cells | 64 |
| TPGS-5-FU | 5-FU, PTX | Resistant epidermal carcinoma | P-gp, β-tubulin, and p53 protein extracted from KB-8-5 cells, tumor growth inhibition in KB-3-1 and KB-8-5 tumor model | 63 |
| TPGS- mitoxantrone | Mitoxantrone | Resistant breast cancer | Cell cytotoxicity against MCF7 and MCF7/ADR cells | 65 |
| TPGS- gemcitabine |
Gemcitabine | Pancreatic cancer | Improved cytotoxicity against pancreatic cancer BxPC-3 | 66 |
| TPGS- cantharidin | Cantharidin | Colorectal breast cancer | Increased cytotoxicity on folate overexpressed HT-29 cells but not on lower expressed MCF-7 cells | 67 |
TPGS-DOX conjugate
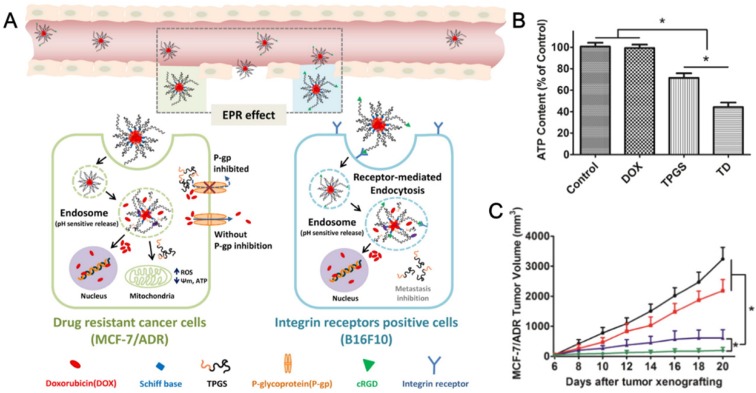
Doxorubicin (DOX) is a P-gp substrate and broad spectrum anticancer drug. However, the acquired drug resistance of DOX is an obstacle to its clinical applications in the progress of cancer therapy. Bao et al. 23 developed a pH-sensitive Schiff base-linked prodrug, TPGS-CH=N-DOX (also called TD), by conjugating DOX with TPGS for overcoming MDR. This prodrug can self-assemble into stable micelles in physiological condition and realize in vivo tumor targeting and long blood circulation by introducing a PEGylated lipid. It was the first time to provide a “molecular economical” way to combat tumor as the system combined the tumor targeting from the integrin receptor ligand peptide cyclic RGD (cRGD), long circulation property from PEGylated lipid, overcoming MDR from the material TPGS and stimuli-responsive release from Schiff based linker. The formulated hybrid micelles showed pH-sensitive drug release profile and obvious particles size change in pH 5.0 buffer which simulated the endo/lysosomal acidic environment. It also demonstrated increased DOX uptake by flow cytometry and confocal microscope analysis, and enhanced retention through in vivo pharmacokinetics compared with free drug. DOX exhibited good retention in drug sensitive MCF-7 cells during incubation. On the contrary, free drug showed much low DOX content and remarkably reduced retention in MCF-7/ADR cells even with extended incubation time. Both the P-gp inhibitors of verapamil and TPGS can increase the drug accumulation in MCF-7/ADR cells. The prodrug micelles achieved the similar drug uptake and retention trend with the admixture of TPGS and DOX in MCF-7/ADR cells. It seems that the rapidly dissociated TPGS from the internalized micelles can inhibit the P-gp activity and retain DOX for subsequent cytotoxicity against MDR tumors. The enhanced cytotoxicity and apoptosis was induced by the hybrid micelles in MCF-7/ADR cells compared with free DOX as the half-maximal inhibitory concentrations (IC50) of hybrid micelles was 95-fold lower than that of free drug after 72 h incubation. The mechanism of antitumor efficacy was further investigated through the analysis of intracellular ROS production, change of mitochondrial membrane potential (ΔΨm) and intracellular ATP level (Figure 2B). The accumulation of ROS, decreased mitochondrial membrane potential and decreased ATP generation from the hybrid micelles may contribute to the P-gp inhibition by TPGS with cutting off the energy supply from the 'cellular power plants' of mitochondria. The prodrug exhibited significant growth inhibition on MCF-7/ADR tumor (Figure 2C) and also tumor growth/metastasis inhibition on murine melanoma B16F10 and hepatocarcinoma H22 with cRGD decorated on the hybrid micelles. It provided a safe and simple prodrug platform to relieve the burden from delivery system and improve the therapeutic efficiency of nanomedicine through the rational design of prodrug for effective cancer treatment.
Figure 2.
(A). Scheme of TPGS-CH=N-DOX prodrug hybrid micelle in “Molecular Economy” way. Left, the P-gp inhibition in MCF-7/ADR cancer cells; Right, the receptor-mediated targeting and anti-metastatic effect in B16F10. (B). Intracellular ATP level depletion effect of TD micelles. (C). Tumor inhibition effect in MCF-7/ADR tumor bearing mice. Reprinted with permission from 23. Copyright 2016 Elsevier.
Some other TPGS-DOX prodrugs were also designed and constructed 55-57. Feng's group 55 developed TPGS-DOX prodrug by directly conjugating succinic anhydride modified TPGS with DOX. The prodrug showed improved cell uptake and cytotoxicity. Compared with free drug, 4.5- and 24-fold of half-life (t1/2) and area under curve (AUC) were found in TPGS-DOX prodrug, respectively. TPGS-DOX-folic acid conjugate (TPGS-DOX-FOL) was further introduced for targeted chemotherapy with higher therapeutic effects and fewer side effects 56. Moreover, the prodrug of TPGS-DOX can also be applied to package drug for combinational therapy. Hou et al. 57 constructed an acid-sensitive TPGS-DOX prodrug by firstly synthesizing a pH-sensitive cis-aconitic anhydride-modified DOX and then conjugating with TPGS. The prodrug can self-assemble into nanoparticles. Photosensitizer chlorin e6 (Ce6) was loaded in this TPGS-DOX prodrug nanoparticles for near-infrared fluorescence imaging and combination of chemotherapy and photodynamic therapy against tumor. The nanoparticles exhibited pH-responsive DOX and Ce6 release characteristics, which was caused by the acid-sensitive linker between TPGS and DOX. It also demonstrated synergistic effects on cell uptake, cancer cell apoptosis and significant growth suppression in non-small cell lung cancer (A549).
TPGS-PTX conjugate
Paclitaxel (PTX) is a BCS class Ⅳ drug with poor solubility and permeability as well as a P-gp substrate, which hinders the effective drug delivery and MDR tumor therapy. Zhang's group 58 synthesized a redox-sensitive prodrug TPGS-SS-PTX, which could be rapidly dissociated in intracellular redox environment (high GSH concentration) to release PTX for cytotoxicity against tumor and TPGS active ingredient for P-gp inhibition. The prodrug can self-assemble to stable micelles and realize the passive tumor targeting through the enhanced permeation and retention (EPR) effect. Compared with non-responsive ester bond conjugated PTX prodrug TPGS-CC-PTX, TPGS-SS-PTX exhibited better stability and in vitro sustained drug release triggered by intracellular reductive environment. The increased stability of TPGS-SS-PTX micelles may be attributed to the soft sulfurs linker between TPGS and PTX in comparison to the only two carbon linker of TPGS-CC-PTX. Compared with the clinical formulation of Taxol® and TPGS-CC-PTX, TPGS-SS-PTX micelles exhibited increased intracellular PTX accumulation for drug-resistant A2780/T cells, which may be caused by the rapid dissociated TPGS from the redox-sensitive prodrug. Rh123 was used as a model drug of P-gp substrate to evaluate the drug retention in MDR tumor. When the cells treated with verapamil or prodrugs, Rh123 fluorescence intensity was increased compared with free Rh123. In particular, much higher fluorescence intensity was exhibited in TPGS-SS-PTX compared with TPGS-CC-PTX, which further confirmed the P-gp inhibition property from dissociated TPGS. As expected, this functional prodrug micelle increased the cytotoxicity of PTX in A2780/T cells. Compared with the uncleavable TPGS-CC-PTX prodrug and Taxol®, the stimuli-responsive prodrug reduced the IC50 and increased the apoptosis/necrosis of MDR tumor. In vivo evaluation further demonstrated the potential of this prodrug micelle on cancer treatment as the increased AUC, extended t1/2, enhanced drug distribution in tumor and significant tumor growth inhibition with reduced side effects.
TPGS-cisplatin conjugate
Cisplatin is widely used in testicular, ovarian, cervical, head and neck, and non-small-cell lung cancers. However, the clinical application is limited for low solubility, nephrotoxicity, severe peripheral neurotoxicity, inherent and acquired drug resistance 59. Feng's group 60 developed TPGS-cisplatin prodrug to improve the water-solubility and reduce the neurotoxicity of cisplatin. TPGS-cisplatin can self-assemble to micelles with high drug loading capability. The higher cell uptake and cytotoxicity against HepG2 hepatocarcinoma cells were found in TPGS-cisplatin prodrug compared with free drug. The prodrug micelles also showed significant neuroprotective effects with higher IC50 value for the SH-SY5Y neuroblast-like cells in comparison to free cisplatin. In addition, TPGS is a powerful anticancer agent when dealing with breast cancer with high level of human epidermal growth factor receptor 2 (HER2) expression 61. It may be related to the inhibition effect of mitochondrial respiratory complex Ⅱ and the ensuing ROS generation, resulting in cell apoptosis via the HER2 receptor tyrosine kinase signaling pathway 33. Mi and coworkers 62 developed a targeted delivery system of TPGS-cisplatin prodrug nanoparticles for the co-delivery of cisplatin, docetaxel (DTX) and Herceptin for good tumor inhibition in HER2 overexpressed breast cancers. Poly(lactic acid) (PLA)-TPGS, TPGS-COOH and TPGS-cisplatin were mixed to fabricate nanoparticles for the multimodality treatment of breast cancer. The multidrug-loaded nanoparticles exhibited much lower IC50 value for SK-BR-3 cells with high expression of HER2 compared with the admixture of free drugs.
TPGS-5-FU conjugate
Liu's group 63, 64 developed multifunctional nanoparticles for co-delivery of hydrophobic drug PTX and hydrophilic drug 5-fluorouracil (5-FU) to overcome MDR. TPGS-5-FU was synthesized by simply conjugating succinoylated TPGS with 5-FU. The nanoparticles, composed of TPGS-5-FU prodrug and PTX, showed enhanced cytotoxicity against MDR tumor compared with individual agent treatment 64. They further developed nanoemulsions with PTX-Vitamin E and TPGS-5-FU prodrug. The nanoemulsions with drugs co-delivery exhibited synergistic effect of overcoming PTX resistance in human epidermal carcinoma cell line KB-8-5 63. The effective anticancer activity was resulted from the P-gp inhibition effect of TPGS and the synergistic effect of PTX and 5-FU which can simultaneously target diverse signaling pathways for cancer killing.
TPGS based NO donor
NO, an endogenous gaseous signaling molecule, is biologically synthesized from the oxidation of L-arginine and nicotinamide adenine dinucleotide phosphate (NADPH) via the catalytic reaction of NO synthase 68. As one of the three so far identified gasotransmitters (NO, carbon monoxide and hydrogen sulphide), NO has attracted the most considerable interests and been shown to participate in various physiological processes, such as angiogenesis, vasodilation, platelet aggregation and neurotransmission 69. Apart from its role in biological processes, NO has demonstrated great potential as a therapeutic agent in inhibiting tumor progression and metastasis 70-72. It was further evidenced that NO can promote the sensitization of resistant cancer cells to various therapeutic modalities 73. It has been proved that hypoxia was related to the resistance of tumor cells 74. NO has exhibited the specific capability to improve the oxygenation level at tumor site by normalization of tumor vasculature, which can enhance the effectiveness of chemotherapy and overcome MDR to some extent 73. As NO is a gaseous radical species with extremely short half-time (1-5 s) and chemical instability, the direct delivery of NO to tumor cells would be confronted with enormous challenges 75. It was of great importance to deliver NO using NO donors. A number of NO-releasing compounds, such as NO organic nitrates, S-nitrosothiols, nitroglycerine and N-diazeniumdiolates, have gained much attention for the efficient delivery of NO 76, 77. Co-delivery of NO donor with anticancer drugs have exhibited synergistic effects in promoting drug delivery and overcoming MDR.
A novel nitrate functionalized TPGS (TNO3) was synthesized as a NO donor which can self-assemble into stable micelles and realize the redox-sensitive release profile. After 96 h, around 90% NO content was released in cancer cells with enhanced cytotoxicity as comparison to nitroglycerine. The enhanced cell uptake and cytotoxicity was achieved when DOX was combined with TNO3, which may be due to the synergistic effects from DOX, released NO and dissociated TPGS. Co-administration of TNO3 with DOX also significantly extended the circulation time with 14.7-fold of t1/2, 6.5-fold of mean residence time (MRT) and 13.7-fold of AUC0-72h, enhanced drug accumulation in tumor and subsequently induced better antitumor efficacy compared to free DOX. The synergistic antitumor effect may be mainly resulted from EPR effect from micelles and the potent effect of NO in dilating tumor blood vessels to improve drug delivery 78.
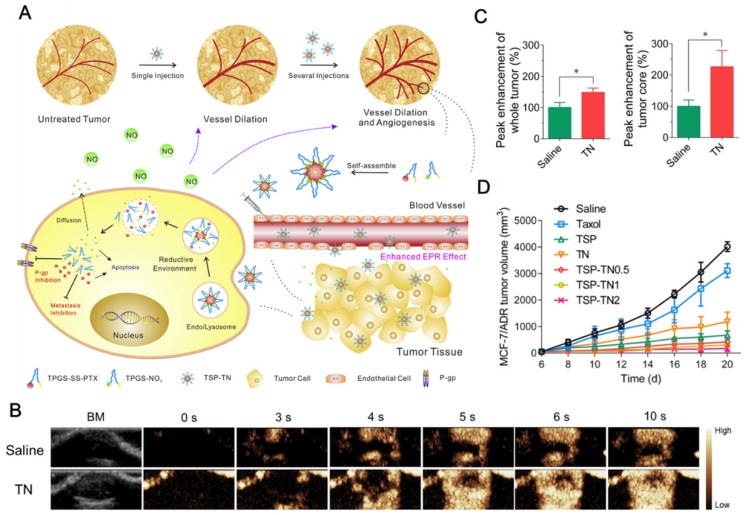
Conventional starving therapy in tumor mediated by decreasing tumor vessel density is valid in early stage of treatment but may cause hypoxia, drug resistance and metastasis. Normalization of blood vessel in tumor is becoming an attractive strategy to achieve satisfied drug distribution for cancer therapy. Yin et al. 24 constructed polymeric hybrid micelles (TSP-TN) to co-deliver PTX for tumor killing and NO for tumor angiogenesis and self-promoted drug accumulation (Figure 3A). The hybrid micelles, comprising TPGS-SS-PTX (TSP) and TNO3 (TN), were prepared via solvent-evaporation method and demonstrated stimuli-responsive drug release under reductive condition. The hybrid micelles exhibited increased blood perfusion which was confirmed by the dynamic contrast-enhanced magnetic resonance imaging and ultrasound imaging by TN single treatment (Figure 3B). The angiogenesis was further verified in the biodistribution study. Compared to saline, significantly increased vessel density and 3-fold higher TSP/Cy5 distribution in tumor frozen sections were observed in TN continuous pre-treatments, which certified the enhancing effect of NO on drug accumulation and penetration. The relative tumor angiogenesis index and drug penetration index were significantly increased (Figure 3C). Among all treatments, TSP-TN2 exhibited the best antitumor efficiency in MCF-7/ADR xenograft model with the inhibition rate of 92.1% (Figure 3D). The micelles also exhibited obvious tumor metastasis inhibition in murine melanoma. This kind of vascular-promoting strategy has great potential for inhibiting MDR and metastasis in cancer therapy.
Figure 3.
(A). Schematic representation of combinational therapy using TSP-TN for tumor angiogenesis and self-promoted drug accumulation. (B). Representative ultrasound images with single treatment of saline or TN. (C). Tumor angiogenesis index and drug penetration index of saline or TN pretreated. (D). Tumor inhibition efficacy against MCF-7/MDR tumor. Reprinted with permission from 24. Copyright 2017 Elsevier.
TPGS based polymers in drug delivery
TPGS based polymers have been extensively used in drug delivery system, which can improve the drug encapsulation efficiency, intracellular uptake and therapeutic effects in vitro and in vivo 79. Zhang et al. 80 first synthesized PLA-TPGS copolymer for drug delivery, which achieved significant antitumor efficiency. Inspired by the application of PLA-TPGS copolymer, a series of TPGS based polymers including poly(lactic-co-glycolic acid) (PLGA)-TPGS 81, polycaprolactone (PCL)-TPGS 82, hyaluronic acid (HA)-TPGS 20, poly-(beta-amino ester) (PBAE)-TPGS 22, chitosan-TPGS 83 and so on, have attracted great interests and been synthesized for medical applications (Table 2).
Table 2.
TPGS based polymers in drug delivery
| Polymer | Payload | Tumor model | Effects | Ref |
|---|---|---|---|---|
| PLA-SS-TPGS | PTX | Melanoma, Hepatoma | 3.1-fold AUC, 7.3-fold MRT vs. free drug. S180 and melannoma tumor inhibition | 86 |
| PLA-TPGS, TPGS-COOH | Iron oxides, Herceptin, DTX | HER2-positive breast cancer | More efficient cytotoxicity than the physical mixture of the corresponding single modality treatment | 88 |
| TAPP-PLA-b-TPGS | DTX | Cervical cancer | High drug loading efficiency, good cytotoxicity towards cervical carcinoma after irradiation | 89 |
| Cholic acid-PLGA-b-TPGS | DTX | Cervical cancer | Higher cellular uptake efficiency and better antitumor efficacy compared with the linear copolymer based nanoparticles | 102 |
| TAPP-PCL-b-TPGS | DTX, porphine | Cervical cancer | Enhanced cytotoxicity on MCF-7/ADR cells and synergistic antitumor effect by chemo-photodynamic therapy on cervical cancer xenograft model | 105 |
| MPEG-PCL-TPGS | PTX | Resistant lung cancer | Remarkable tumor inhibition on the resistant lung cancer | 82 |
| (PCL-ran-PGA)-b-TPGS | TRAIL, endostatin | Cervical cancer | Increased cytotoxicity on HeLa cells induced by TRAIL/endostatin-loaded nanoparticles | 108 |
| HA-conjugated TPGS | DOX | Resistant breast cancer | ROS regeneration and P-gp inhibition via TPGS | 20 |
| PBAE-b-TPGS | DTX | Resistant ovarian cancer | 22- and 100-fold lower IC50 against A2780 and A2780/T cells, respectively | 22 |
| PBAE-g-TPGS | PTX | Resistant breast cancer | Stimuli-responsive release of PTX, targeted drug delivery to tumor and remarkable MCF-7/ADR tumor inhibition | 111 |
| Chitosan-g-TPGS | DOX | Hepatoma | 2.4-fold AUC, 2.0-fold MRT vs. free drug after oral administration | 83 |
| iRGD-TPGS | PTX | Resistant lung cancer | Significant drug accumulation, downregulation of Survivin expression, and tumor apoptosis | 112 |
| PLA-PGS, Ce6-TPGS, tLyp-1-TPGS |
DOX, Ce6 | DOX-resistant breast cancer | In vivo near-infrared imaging of tumor-bearing mice and enhanced antitumor efficiency in MCF-7/ADR | 114 |
| Transferrin conjugated TPGS | DTX, gold clusters | Breast cancer | In vivo imaging and antitumor efficacy | 115 |
| 4-arm-PEG-TPGS | PTX | Hepatoma | Significant in vivo antitumor effect on S180 sarcoma-bearing mice | 118 |
PLA-TPGS polymer
PLA has been approved by FDA as a biodegradable and biocompatible agent, which is widely used in drug delivery. However, the hydrophobic characteristic and rapid clearance of PLA matrix based nanocomposites after administration have greatly limited its application 84. PLA-TPGS polymer can be synthesized by ring opening polymerization or simple conjugation of PLA with TPGS. The resultant polymer can introduce the special properties of TPGS to overcome the drawbacks of PLA to some extent. It has been extensively applied for anticancer drug delivery. The polydopamine-modified PLA-TPGS nanoparticles with galactosamine conjugation were used as a targeted drug delivery system to treat liver cancer, which showed more efficient antitumor activity than the control groups 85. In Zhang's group 86, a redox-sensitive PLA-SS-TPGS polymer was synthesized with a disulfide linkage between PLA and TPGS to realize reductive cleavage of polymer to dissociate TPGS under high concentration of intracellular GSH. The dissociated TPGS was found with P-gp inhibition activity, which enhanced intracellular drug accumulation, increased cell apoptosis and realized synergistic effect against MDR tumor. cRGD was further decorated on nanoparticles with enhanced cellular uptake and intracellular drug accumulation compared to non-targeted nanoparticles. The high cytotoxicity was also induced from the targeted nanoparticles on murine melanoma, drug-sensitive and resistant human ovarian cancer cells. Further evaluation on in vivo pharmacokinetics and tumor growth inhibition also evidenced the great potential of this redox-responsive polymer for cancer treatment. Compared with the linear copolymers, the dendrimer-like H40-PLA-TPGS nanoparticles showed better in vitro and in vivo antitumor effect 87. The combination of different therapeutic approaches has shown to be more effective than single modality in cancer treatment. The PLA-TPGS polymer can be applied in multiple drugs co-delivery for multimodality cancer therapy. Feng's group 88 blended PLA-TPGS and carboxyl group-terminated TPGS to fabricate targeted nanoparticles for the co-delivery of multiple agents, including chemotherapeutic drug DTX, biotherapy and targeting agent of Herceptin and hyperthermia therapy of iron oxides. The multimodal treatment nanoparticles exhibited synergistic antitumor effects compared with the corresponding individual or dual modality treatment. Wang et al. 89 used porphyrin-cored PLA-b-TPGS copolymer for combinational chemo-photodynamic therapy, which achieved synergistic effects against HeLa cervical cancer cells. Chemotherapy usually involves two or more drugs which are administered to the host at a same period. The metabolism of one drug can be changed by the other one or ones, resulting in the drug antagonism 90. PLA-TPGS nanoparticles were fabricated to reduce drug antagonism between DTX and tamoxifen. The nanoparticles can realize the intracellularly controlled release of the drugs for effective antitumor activity 91.
Current fabrication methods of nanoparticles are usually in lab-scale, which would limit the practical application of nanomedicine. PLA-TPGS copolymer could be applied to encapsulate DTX by the Shirasu porous glass membrane-emulsification technique with a pilot-scale. Optimization of the fabrication process was studied, including the surfactant types and concentration in the aqueous phase, organic/aqueous phase volumetric ratio, membrane-pore size, transmembrane cycles and operation pressure. DTX-loaded PLA-TPGS nanoparticles exhibited improved in vivo pharmacokinetics of 1.8-, 6.3- and 3.4-fold and 2.2-, 13.2-, 8.5-fold higher values for AUC, t1/2, and MRT, compared with those of PLGA nanoparticles and Taxotere®, respectively. The similar tendency was also seen in drug retention from the near-infrared fluorescence images and tumor growth inhibition in H22 tumor model. It would provide a feasible strategy to fabricate drug-loaded polymeric nanoparticles in a pilot-scale 92.
PLGA-TPGS polymer
PLGA is a biocompatible and non-immunogenic polymer and can be biodegraded to non-toxic product in nature 93. However, PLGA is hydrophobic and can be rapidly filtrated in liver and captured by the reticuloendothelial system. These shortages could be masterly evaded with the help of TPGS. The PLGA-TPGS polymer can be used as a polymeric matrix for nanoparticles to deliver therapeutic agents, which can achieve high drug encapsulation efficiency, sustained release behavior and improved therapeutic effects 94. Traditional Chinese medicines (TCMs) have been widely used for thousands of years. Recently, their therapeutic efficacy to cancer attracted much attention but was limited by the poor solubility. PLGA-TPGS nanoparticles can be formulated to encapsulate these TCMs to enhance the pharmacological effects. Resibufogenin 95, Emodin 96, Tanshinone IIA 97 and Quercetin 98 were loaded in PLGA-TPGS nanoparticles, resulting in enhanced antitumor efficiency for liver cancer. Gao et al. 99 combined oleanolic acid and heparin sodium separately loaded PLGA-TPGS nanoparticles, which demonstrated synergistic antitumor effects in liver cancer HCa-F cells. Fang et al. 100 fabricated the mixed micelles comprising PLGA-SS-TPGS and Pluronic F68 (F68) modified with a targeting peptide APDTKTQ for oridonin delivery which increased cellular uptake and induced apoptosis. Star-shaped polymer based drug carriers exhibited smaller hydrodynamic radius, lower solution viscosity, higher drug loading content and higher drug encapsulation efficiency in comparison to the linear polymers of same molar mass 101. DTX-loaded star-shaped cholic acid-PLGA-b-TPGS block copolymer nanoparticles showed higher cellular uptake efficiency and better antitumor efficacy compared with linear PLGA-b-TPGS copolymer based nanoparticles 102. Mei's group 103, 104 prepared the aptamer or folate conjugated nanoparticles, which could realize significantly high tumor targeting efficiency and increased therapeutic effects.
PCL-TPGS polymer
PCL-TPGS polymer can be easily synthesized by the copolymerization of PCL with TPGS and serve as a functional molecular biomaterial for drug delivery. DTX-loaded porphine functionalized star-shaped PCL-b-TPGS copolymer nanoparticles were constructed for chemo-photodynamic therapy. This system developed TPGS function as P-gp inhibitor for overcoming drug resistance of MCF7/ADR cells, and chemo-photodynamic therapy via DTX and photodynamic sensitizer of porphine for cervical cancer. As comparison to Taxotere®, the drug-loaded nanoparticles exhibited 9.4- and 56.5-fold efficiency on cancer cell cytotoxicity and significant antitumor efficacy against cervical cancer, respectively. The study also demonstrated that the TPGS component in the copolymer can enhance the drug encapsulation efficiency, cellular uptake and subsequent cytotoxic effect of MDR tumor 105. Besides, PTX-loaded MPEG-PCL-TPGS micelles exhibited remarkable tumor inhibition in the resistant A549/T tumor bearing nude mice 82. Bernabeu et al. 106 reported that PTX-loaded PCL-TPGS nanoparticles exhibited higher antitumor efficacy against both estrogen-dependent (MCF-7) and estrogen-independent (MDA-MB-231) breast cancer cells compared with drug-loaded-PCL-b-MPEG nanoparticles and commercial formulation Abraxane®. The different hydrophobic chain lengths of PCL-TPGS polymers may influence the nanoparticle properties and delivery efficiency of therapeutic agents. Suksiriworapong et al. 107 synthesized 5:1, 10:1 and 20:1 PCL-TPGS copolymers which were composed of 25.0%, 45.2% and 66.8% of PCL chains with respect to the polymer chain, respectively. They found that the 10:1 PCL-TPGS nanoparticles exhibited the best uptake efficiency and highest cytotoxicity to breast cancer cells. (PCL-ran-PGA)-b-TPGS nanoparticles modified by polyethyleneimine were applied to deliver TNF-related apoptosis-inducing ligand (TRAIL) and endostatin for synergistic anticancer treatment in cervical cancer 108.
HA-TPGS polymer
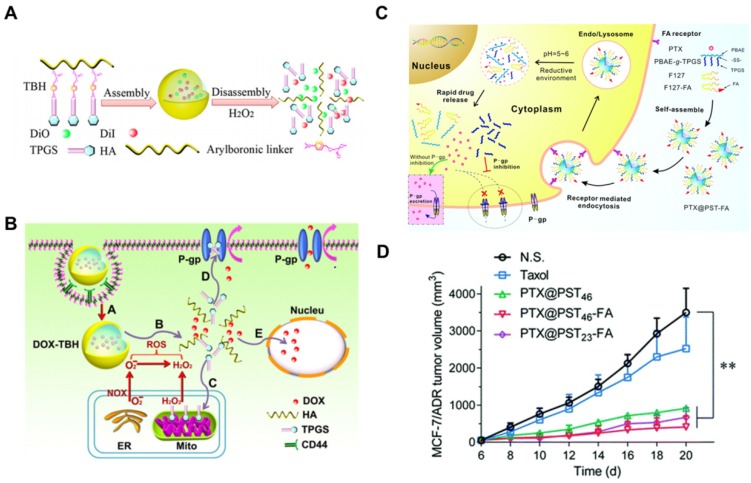
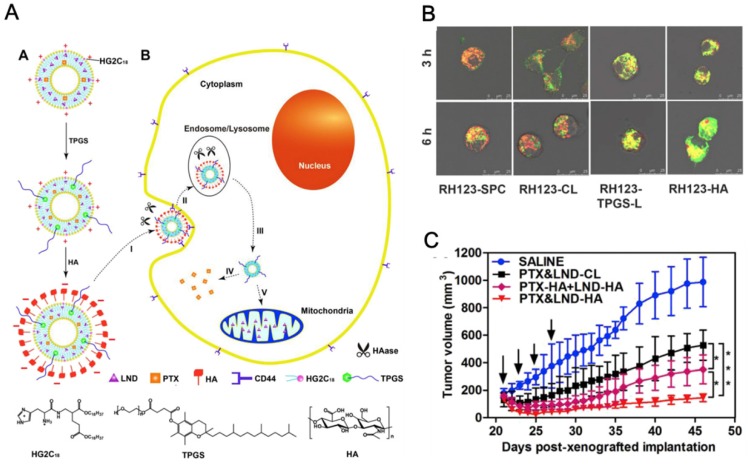
HA is a major ligand for CD44-positive cancer cells, which would facilitate the drug carriers to target this kind of malignant tumor. Su et al. 20 constructed a kind of ROS triggering and replenishing nanoparticles for DOX delivery to overcome drug resistance in MCF-7/ADR tumor (Figure 4A). TPGS was conjugated with HA via an arylboronic linker with ROS sensitivity. HA can increase tumor targeting efficiency of the nanosystem for CD44-positive malignant cells. On one hand, TPGS was applied as the P-gp inhibitor to overcome drug resistance. On the other hand, it can also promote the regeneration of ROS which can in turn replenish the consumed ROS for triggering nanoparticle dissociation (Figure 4B). The drug-loaded nanosystem can realize the targeted delivery to cancer cells, dissociate TPGS in response to intracellular ROS and enhance cytotoxicity on MCF-7/ADR cancer cells. This novel and promising system combined ROS responsive and replenishing functions to induce high drug accumulation for effective antitumor treatment.
Figure 4.
(A). Scheme of FRET-TBH assembly in water and disassembly in 1 mM H2O2. (B). The schematic presentation of ROS-triggered and regenerating antitumor nanosystem. A: TBH self-assembling nanosystem (DOX-TBH) and endocytosis mediated by HA through the overexpressed CD44 receptors; B: the breakage of arylboronic linkers by ROS, followed by DOX-TBH rapid disassembly and released DOX and TPGS; C: By acting on mitochondrial respiratory complex II, the released TPGS induced ROS generation for complete disassembly of DOX-TBH; D: TPGS as the inhibitor of P-gp efflux to overcome MDR; E: DOX and ROS induced cell death in nucleus. (C). Scheme of folate-modified redox/pH-sensitive hybrid micelles (PTX@PST-FA) for achieving targeted delivery and overcoming MDR. (D). Antitumor effect against MCF-7/ADR tumor model by hybrid micelles. A and B reprinted with permission from 20. Copyright 2014 Elsevier. C and D reprinted with permission from 111. Copyright 2017 Royal Society of Chemistry.
PBAE-TPGS polymer
PBAE, which was synthesized by Langer's group 109 via a Michael-type polymerization, has been widely used as a pH-sensitive polymer in anticancer drug delivery systems. PBAE-b-TPGS (PT) was synthesized for DTX delivery against drug sensitive A2780 and resistant A2780/T cells. PT nanoparticles were fabricated with the help of phospholipids DOPC and DSPE-PEG to increase the stability. It exhibited a pH-sensitive release behavior in weak acidic environment (pH 5.5). Compared to free drug, PT nanoparticles exhibited 22- and 100-fold lower IC50 against A2780 and A2780/T cells, respectively. Moreover, Rh123 retention and intracellular ATP assays showed that PT copolymer nanoparticles can significantly strengthen the intracellular Rh123 fluorescence intensity and decrease the ATP level compared with PBAE nanoparticles but close to that of free TPGS. It was caused by the dissociated TPGS which can inhibit the function of P-gp. The enhanced cytotoxicity and P-gp inhibition assays demonstrated that the pH-responsive copolymer can be applied in drug delivery system to increase the chemo-sensitivity of P-gp overexpressed MDR cancer cells 22. Zhang et al. 110 mixed PBAE-b-TPGS and AS1411 aptamer decorated TPGS to fabricate PTX loaded micelles for targeted delivery. The micelles can dissociate quickly in acidic pH to release PTX and showed enhanced drug accumulation in SKOV3 ovarian cancer cells. Recently, a redox/pH dual-sensitive graft copolymer, PBAE-g-TPGS, was further synthesized through a Michael-type step polymerization. To improve the biocompatibility and targeted delivery ability, Pluronic F127 (F127) and folate-F127 conjugation were then introduced to fabricate PTX loaded hybrid micelles for overcoming MDR (Figure 4C). With stimuli-responsive properties, the hybrid micelles showed redox/pH-sensitive drug release profile. The micelles exhibited good antitumor effect in MCF-7/ADR xenograft model with the help of TPGS induced P-gp inhibition and folate-mediated targeted delivery (Figure 4D) 111.
Chitosan-TPGS polymer
Chitosan-g-TPGS (CT) polymer was synthesized for overcoming P-gp induced MDR of cancer cells. The nanoparticles exhibited high encapsulation capability of DOX, pH-responsive release behavior and significant cytotoxicity on human hepatocarcinoma cells (HepG2 and BEL-7402) and MCF-7 cells. DOX-loaded CT nanoparticles also enhanced cytotoxicity and apoptosis against drug-resistant cells (MCF-7/ADR and BEL-7402/5-Fu) with significantly reduced IC50 in comparison to free drug. The inhibition of P-gp efflux was observed on MCF-7/ADR cells. DOX-loaded CT nanoparticles showed a remarkable reduction in the intracellular ATP level compared with free drug. DOX-loaded nanoparticles can induce the synergistic effect through the P-gp inhibition by TPGS and cytotoxicity by anticancer drug. The nanoparticles also improved in vivo pharmacokinetic properties and antitumor activity after oral administration 83.
Targeting ligand conjugated TPGS
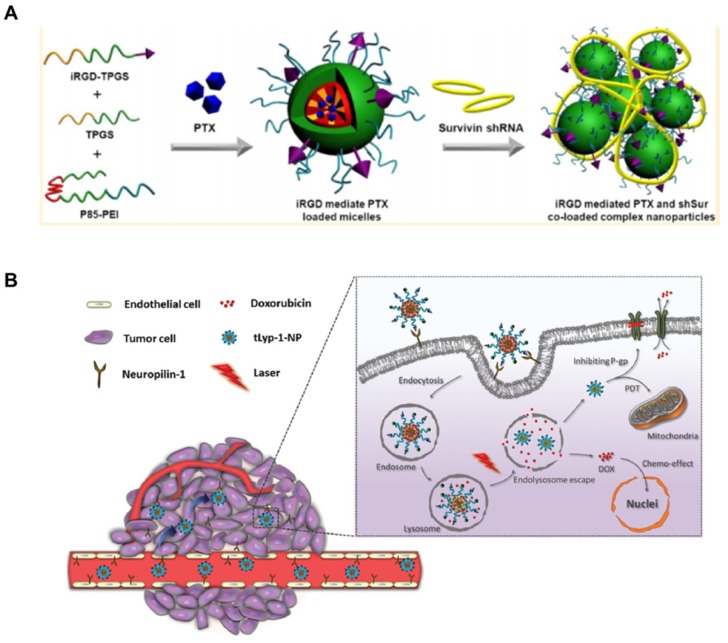
RGD has been applied as a potential targeting ligand in cancer treatment for tumors with αvβ3 integrin receptors overexpression. Li's group 112 formulated PTX and Survivin shRNA co-loaded targeted nanoparticles by mixing Pluronic P85-polyethyleneimine, TPGS and iRGD-TPGS for overcoming drug resistance (Figure 5A). The nanoparticles exhibited increased cell uptake, RNA interference effect and cytotoxicity both on A549 and A549/T cells. It was further evidenced that the targeted nanoparticles showed significant antitumor effects from the co-delivery system as comparison to Taxol®. RGD-conjugated TPGS was also used to prepare the targeted micelles by mixing with vitamin E succinate (VES)-grafted-chitosan oligosaccharide, which exhibited good growth inhibition on human glioblastoma tumor (U87MG) 113. tLyp-1 peptide has high affinity to neuropilin-1 receptor (NRP-1). It can be also used as a targeting peptide to decorate on the surface of nanoparticles as overexpressed NRP-1 on tumor and angiogenesis cells. TPGS was first conjugated with the tLyP-1 peptide and then mixed with PLA-TPGS and Ce6-conjugated TPGS to fabricate DOX loaded nanoparticles via a nanoprecipitation method. The resultant nanoparticles can enhance the vascular extravasation and improve the penetration and accumulation of drugs in tumor for synergistic antitumor effects of combinational chemo-photodynamic therapy (Figure 5B) 114. In addition, a kind of theranostic micelles of transferrin conjugated TPGS was applied for targeted co-delivery of DTX and gold clusters for simultaneous cancer imaging and therapy. The micelles exhibited enhanced cytotoxicity with 71.7-fold and 4.7-fold lower IC50 values in MDA-MB-231-luc breast cancer cells with transferrin receptors overexpression as comparison to Taxotere® and non-targeted micelles, respectively. The in vivo imaging and antitumor efficacy were further evidenced in tumor-bearing mice 115.
Figure 5.
(A). Fabrication of iRGD mediated PTX and Survivin shRNA co-loaded complex nanoparticles. (B). Design of tLyp-1-conjugated PLA-TPGS nanoparticles as a dual-targeting delivery system for co-delivery of DOX and Ce6. The nanoparticles were internalized by NRP-1 mediated endocytosis and penetrated into the inner areas of the tumor to combat drug resistant cancer via chemo-photodynamic combination therapy. A reprinted with permission from 112. Copyright 2014 American Chemical Society. B reprinted with permission from 114. Copyright 2015 Royal Society of Chemistry.
Other TPGS based polymers
Wang et al. 116 synthesized TPGS-modified reduced bovine serum albumin (TRB) for PTX delivery, which enhanced the cytotoxicity in MCF-7/ADR cells compared with Taxol® and PTX-loaded bovine serum albumin nanoparticles. The relative P-gp efflux assay also confirmed the significant inhibition on P-gp activity with TRB. Besides, lysine-linked di-tocopherol polyethylene glycol 2000 succinate (PLV2K) was synthesized for overcoming MDR in cancer treatment. The copolymer PLV2K can self-assemble into micellar system with low critical micelle concentration (CMC) of 1.14 μg/mL. DOX loaded micelles showed increased cytotoxicity and intracellular accumulation in MCF-7/ADR cells compared with free DOX, which was attributed to the uncompetitive inhibition of P-gp ATPase by PLV2K 117. To improve the stability of drug carrier in physiological condition and extend the blood circulation time, a series of 4-arm-PEG-TPGS polymers with different PEG molecular weight were synthesized. The polymers can self-assemble to nanoparticles for PTX encapsulation. The nanoparticles based on the polymer with 4-arm-PEG molecular weight of 5kDa obtained the best physiologic stability, smallest particle size and highest drug-loading efficiency. The significant in vivo antitumor effect on S180 sarcoma-bearing mice demonstrated the potential of this 4-arm-PEG5K-TPGS polymer for cancer treatment 118. Cheng et al. 119 developed TPGS-functionalized polydopamine-coated mesoporous silica nanoparticles for pH-responsive delivery of DOX. The presence of TPGS resulted in higher intracellular drug concentration and superior therapeutic effects against MDR tumor.
Unmodified TPGS based formulations
According to the basic properties mentioned above, unmodified TPGS can be used as a multifunctional material in drug delivery system. These functions can be summarized as overcoming MDR, improving oral drug bioavailability and promoting drug permeation.
TPGS based formulations to overcome MDR
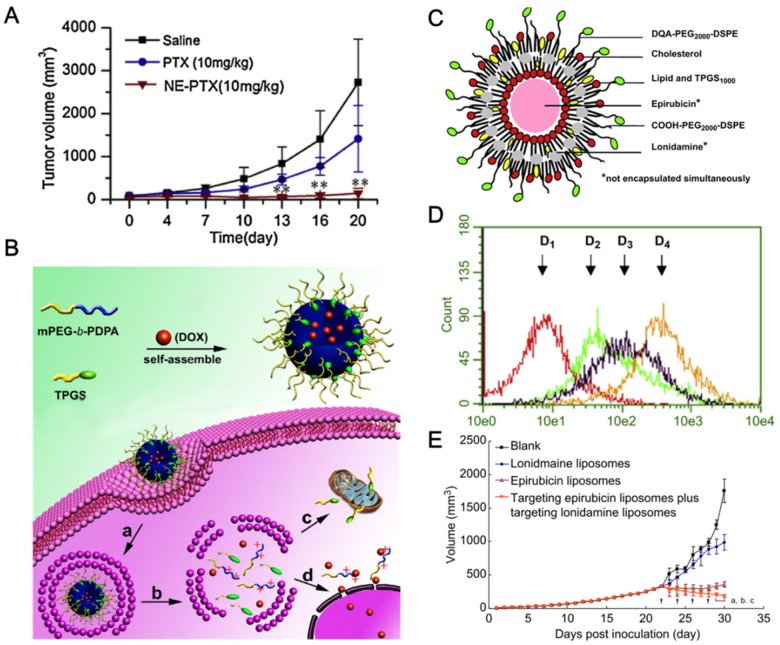
Scientific investigations have validated that TPGS can be employed as the drug carrier and P-gp inhibitor in fabricating nanodrugs for MDR reversal in cancer treatment (Table 3). Gao et al. 120 developed the TPGS stabilized PTX nanosuspensions, which exhibited 2-fold median lethal dose (LD50) of acute toxicity and improved pharmacokinetics compared to that of the marketed injection. The nanosuspensions were further evaluated on P-gp overexpressed H460 human lung cancer cells (H460/RT) with significantly high cytotoxicity and 5-fold increase on tumor inhibitory rate compared with the mixed solution of PTX and TPGS after i.v. administration 121. TPGS was also used to prepare PTX nanoemulsions for overcoming drug resistance in MCF-7/ADR cells. The nanoemulsions exhibited significant P-gp inhibition and 94% tumor inhibitory rate (Figure 6A) 18.
Table 3.
Unmodified TPGS based formulations for overcoming MDR
| Formulations | Payload | Tumor model | Effects | Ref |
|---|---|---|---|---|
| TPGS nanocrystals | PTX | Resistant lung cancer | 5-fold increase on tumor inhibitory rate on H460/RT | 120, 121 |
| TPGS/MCT/Tween 80 nanoemulsions | PTX | Resistant breast cancer | P-gp activity inhibition, 94% inhibitory rate in MCF-7/ADR bearing nude mice | 18 |
| TPGS/PEG-b-PDPA micelles | DOX | Resistant breast cancer | P-gp activity inhibition, tumor inhibition in MCF-7/ADR bearing nude mice | 38 |
| TPGS/MPEG-SS-2SA mixed micelles | PTX | Resistant breast cancer | Cytotoxicity, P-gp inhibition on MCF-7/PTX | 36 |
| TPGS/cholesterol/DSPE-PEG/DQA-PEG-DSPE liposomes | Epirubicin/LND | Resistant non-small cell lung cancer | Targeted accumulation in mitochondria, enhanced antitumor efficacy of combination of both drug-loaded liposomes in A549/cDDP cancer | 123 |
| TPGS/EPC/cholesterol/DSPE-PEG/DQA-PEG-DSPE liposomes | Topotecan | Resistant breast cancer | Mitochondrial targeting effect, tumor inhibition on MCF-7/ADR tumor bearing mice, anti-metastasis effect on melanoma | 124 |
| TPGS/HA cationic liposomes | PTX/LND | Resistant breast cancer | Inhibition on intracellular ATP production, cell apoptosis and cytotoxicity, MCF-7/MDR tumor inhibition | 32 |
| TPGS | PTX/TQR | Resistant breast cancer | IL-10 production by MCF-7/ADR cells, cell viability, P-gp expression | 125 |
| TPGS/DSPE-PEG mixed micelles | DOX/ CUR |
Resistant non-small cell lung cancer | Cytotoxicity and endocytosis on A549/ADR cells, antitumor effect on LLC-tumor bearing mice | 126 |
| TPGS/Poloxamer 407 mixed micelles | Gambogic acid | Resistant breast cancer | Increased cell uptake and 2.9-fold higher cytotoxicity | 127 |
| TPGS/Poloxamer 407 mixed micelles | CUR | Resistant breast cancer | 3-fold higher cytotoxicity of micelles in NCI/ADR-RES cells | 128 |
| TPGS/Poloxamer 407 mixed micelles | DOX | Resistant ovarian cancer | Enhanced cellular uptake and increased cytotoxicity in SKOV3 and DOX-resistant SKOV3 cells | 129 |
Figure 6.
(A). The tumor inhibition profile of PTX nanoemulsion in MCF-7/ADR tumor. (B). Scheme of the mitochondria-targeted pH-responsive PDPA/TPGS@DOX micelles for overcoming drug resistance in MCF-7/ADR tumor. (a) Cellular uptake; (b) pH-responsive dissociation of micelles in endo/lysosome; (c) the mitochondria targeting effect of TPGS; and (d) nuclear entry of DOX. (C). Schematic representation of mitochondria targeting liposomes. (D). Drug contents in mitochondrial detected by flow cytometry after 4 h incubating A549/cDDP cells. (D1) Blank control; (D2) Free Coumarin; (D3) Coumarin liposomes; (D4) Targeting Coumarin liposomes. (E). Antitumor efficiency in A549/cDDP xenografted tumor. A reprinted with permission from 18. Copyright 2014 Elsevier. B reprinted with permission from 38. Copyright 2015 Elsevier. C, D and E reprinted with permission from 123. Copyright 2013 Elsevier.
In recent years, more and more stimuli-responsive nanoparticles were designed to realize timely and spatially drug release. Yu et al. 38 developed mitochondria-targeted pH-responsive micelles with poly(ethylene glycol)-block-poly(2-(diisopropylamino)ethyl methacrylate) (PEG-b-PDPA), a pH-responsive copolymer, and TPGS for overcoming drug resistance in MCF-7/ADR tumor (Figure 6B). TPGS can increase the cytotoxicity and inhibit P-gp activity by targeting mitochondria and depleting ATP. The micelles exhibited 6-fold decrease of IC50 in MCF-7/ADR cells and more efficient tumor inhibition in MCF-7/ADR bearing nude mice compared with free DOX. Moreover, Dong et al. 36 designed PTX loaded mixed micelles, which were prepared with disulfide bond-linked MPEG and stearic acid (MPEG-SS-2SA), and TPGS. PTX can be rapidly released within 24 h under the reductive environment. The micelles demonstrated significant inhibition on mitochondrial respiratory complex Ⅱ, decrease of mitochondrial membrane potential and reduced level of ATP. Redox and pH dual-responsive lipid-coated mesoporous silica hybrid nanoparticles containing TPGS were fabricated for overcoming drug resistance. The nanoparticles exhibited higher cell uptake, cytotoxicity and intracellular accumulation in MCF-7/ADR cells as comparison to free DOX 122.
TPGS can also be combined with other functional agents for enhanced MDR cancer therapy. The major approaches are listed as follows.
TPGS can be combined with mitochondrial targeting agents to overcome drug resistance. The multifunctional liposomes were fabricated with mitochondrial targeting molecule dequalinium (DQA) and TPGS, which can circumvent the intrinsic resistance by enhancing the delivery of cytotoxic agents across mitochondrial membrane and overcome the acquired resistance by inhibiting the activity of P-gp (Figure 6C). The targeting liposomes can selectively accumulate in mitochondria and decrease the mitochondrial membrane potential (Figure 6D). The liposomes showed significantly enhanced antitumor efficacy in drug resistant A549/cDDP cancer (Figure 6E) 123. The resistance-related metastasis was also suppressed on melanoma by this kind of mitochondrial targeting liposomes loading with Topotecan 124. It seems that mitochondrial targeting strategy could be a promising way for the treatment of MDR cancers and resistance-related metastases.
TPGS can also be combined with other drug resistance inhibitor or chemosensitizer, such as lonidamine (LND) 32, tariquidar (TQR) 125 and curcumin (CUR) 126. Assanhou et al. 32 constructed TPGS-based functional liposomes for co-delivery of cytotoxic drug PTX and MDR inhibitor LND (Figure 7A). LND was used as a mitochondrial function modulator and chemosensitizing agent. TPGS was anchored on the lipid bilayer of liposomes which can serve as P-gp inhibitor for increasing the drug accumulation in MDR tumor and mitochondria-targeting agent to enhance the mitochondrial function modulating capability of LND. As expected, the intracellular retention of Rh123 was evidently increased in MCF-7/ADR cells by TPGS-contained liposomes compared with free Rh123 or liposomes without TPGS. The mitochondria-targeting property of TPGS was further evidenced as the high Rh123 signal colocalized with the MitoTracker signal and increased Rh123 content in the mitochondria after incubation of cancer cells with liposomes (Figure 7B). TPGS and LND also developed the synergistic effects on the suppression of intracellular ATP production, which enhanced P-gp inhibition and sensitized the drug-resistant tumor to PTX. The improved antitumor efficiency (Figure 7C) may provide great potential for co-delivering MDR modulator with anticancer drug to overcome MDR. TPGS-based nanoparticles with PTX and drug resistance inhibitor TQR co-delivery were constructed for overcoming drug resistance in MCF-7/ADR cells 125. In addition, DOX and chemosensitizing agent CUR co-loaded micelles were prepared with TPGS and DSPE-PEG for overcoming DOX resistance in cancer treatment. The co-loaded micelles exhibited synergistic antitumor efficacy against A549/ADR cells 126.
Figure 7.
(A). Scheme of the TPGS and HA dual-functionalized liposome for PTX and LND co-delivery to overcome MDR. A. Preparation of PTX&LND-HA. From top to bottom: PTX&LND-CL, PTX&LND-TPGS-L, and PTX&LND-HA. B. MDR reversal mechanism of PTX&LND-HA. I: tumor cell accumulation by the passive and active targeting effects; II: CD44-mediated internalization and HA degradation by HAase overexpressed in the tumor extracellular matrix and endo-lysosomes; III: endo-lysosomal escape of the liposome; IV: release of PTX for cytotoxicity and inhibition on P-gp efflux by TPGS; V: mitochondrial targeting of the liposome and release of LND to act on the mitochondrial function for sensitizing the MDR cancer cells to PTX. The mitochondrial targeting effect (B) and tumor volume change (C) in MCF-7/MDR cells and MCF-7/MDR tumor-bearing nude mice, respectively. Reprinted with permission from 32. Copyright 2015 Elsevier.
Furthermore, TPGS can be applied to combine with other polymeric inhibitors of P-gp. Saxena et al. 127 developed Poloxamer 407 and TPGS mixed micelles to overcome MDR. The micelles were designed for gambogic acid delivery with increased cell uptake and 2.9-fold higher cytotoxicity in MDR ovarian cancer cells (NCI/ADR-RES) compared with free drug. The MDR reversal effect of CUR-loaded mixed micelles was further studied. Increased uptake and 3-fold higher cytotoxicity of micelles were found in NCI/ADR-RES cells 128. In addition, pH-responsive folate-functionalized Poloxamer 407/TPGS mixed micelles were developed for selective anticancer activity, targeted cancer therapy and MDR reversal. The DOX-loaded mixed micelle, comprising TPGS and folic acid conjugated Poloxamer 407, demonstrated enhanced cellular uptake and cytotoxicity in SKOV3 and DOX-resistant SKOV3 cells, reduced drug efflux and minimal toxicity to normal cells as compared to free DOX 129.
TPGS based formulations to improve drug oral bioavailability
Oral administration is an appealing drug delivery way owing to the simplicity, convenience, high patient compliance, suitability for chronic therapy and reduced costs for physicians and industry 130, 131. However, there are still numerous inherent challenges hampering the effective delivery of drugs, such as low water solubility, limited permeability through the gastrointestinal tract, poor stability against enzymes and hydrolysis effect, which lead to poor absorption and bioavailability 132. In fact, a majority of BCS class Ⅳ drugs are substrates of P-gp and cytochrome P450 3A4 (CYP3A4), leading to poor permeability and extensive pre-systemic metabolism 133. TPGS-based formulations have many advantages to improve bioavailability of orally administered drugs. On one hand, as a nonionic surfactant, TPGS can improve drug solubility. On the other hand, TPGS can enhance drug permeation due to the P-gp inhibition effect 27. Furthermore, TPGS has been demonstrated with the capability to improve drug stability by inhibiting the CYP3A4 and CYP2C9-mediated metabolism 134. In other studies, TPGS showed little inhibition effect on CYP3A activity 135, 136, which may be related to the dosage 137. The TPGS formulations involve nanocrystals, nanosupensions, self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS), solid dispersions/tablet, solid lipid nanoparticles (SLNs), liposomes and micelles, TPGS emulsified nanoparticles and so on.
Nanocrystals and nanosupensions have been widely accepted as potent formulations to improve the poor solubility, dissolution and bioavailability of hydrophobic drugs. TPGS can be used as a stabilizer to fabricate andrographolide nanocrystals. The nanocrystals improved the intestine absorption as evidenced by the single-pass intestinal perfusion studies and oral absorption with significant inhibition on xylene induced ear swelling after oral administration 138. Moreover, TPGS was applied as stabilizer in fabricating nanocrystals of BCS class Ⅱ drug, telmisartan, with 10-fold increase in bioavailability which was due to the enhanced solubility and dissolution rate 139. Ge et al. 140 prepared the TPGS stabilized ursolic acid nanosupensions with 27.5-fold increase in oral bioavailability and 9-fold increase in peak concentration (Cmax) in comparison to raw drug.
SEDDS/SMEDDS, an isotropic mixture composed of oil, solvent, surfactant and co-solvent/surfactant, can improve oral absorption of lipophilic drugs 141. The TPGS stabilized acetylpuerarin nanoemulsions can increase drug solubilization in the gastrointestinal tract, improve lymphatic transport and thus enhance drug absorption for effective therapy on cerebral ischemic reperfusion injury 142. In addition, microemulsions of BCS Class Ⅳ drug, cefpodoxime proxetil, were prepared by using Tween 80 and TPGS as surfactants and Capmul MCM as oil phase. The SMEDDS improved the average flux, permeability and AUC of cefpodoxime proxetil for around 9-, 10.5-, and 5.4-fold compared with free drug, respectively. The improved oral bioavailability may be attributed to the enhanced solubilization of selected drug 143. Jain et al. 144 developed SEDDS containing cyclosporine A and TPGS, which exhibited good stability in simulated gastrointestinal fluids and increased the oral bioavailability around 4.48-fold compared to clinically available counterpart Bioral®. This formulation can significantly diminish ROS generation induced by cyclosporine A in human embryonic kidney cells compared with the formulation without TPGS, Bioral® and the mixture of cyclosporine A and TPGS. The nephrotoxicity was also reduced, which further evidenced the safety of TPGS containing SEDDS for cyclosporine A delivery.
TPGS was used in solid dispersions as an absorption enhancer to improve the oral bioavailability. The solid dispersion nanoparticles of dexibuprofen were prepared with Eudragit E100 and TPGS by supercritical anti-solvent technique. The nanoparticles improved the AUC0-24h and Cmax up to 4.6- and 5.7-fold, respectively 145. Additionally, fexofenadine hydrochloride dispersions containing TPGS improved the drug dissolution, permeability, oral absorption and bioavailability via investigations of in situ perfusion and in vivo pharmacokinetics 146. Moreover, the solid dispersions composed of berberine-phospholipid complex, TPGS and SiO2 were prepared to improve oral bioavailability of berberine. TPGS can act as not only a carrier to improve the drug dissolution rate but also a P-gp inhibitor for enhancing the drug intestinal absorption 147.
DTX-loaded SLNs were prepared using Tween 80 or TPGS as emulsifier for oral delivery. Compared with Tween 80-emulsified SLNs, TPGS-emulsified SLNs demonstrated enhanced intestinal absorption and oral bioavailability of DTX in rats, which may be due to the P-gp inhibition from TPGS along with the increased intestinal lymphatic uptake of SLNs 148. Lumefantrine-loaded binary SLNs were constructed with stearic acid, caprylic acid, TPGS and Poloxamer 188 to improve the oral bioavailability. The SLNs exhibited 2.7-fold Cmax and 2.2-fold AUC in comparison to free drug 149. Notably, compared with CUR solution, CUR-loaded SLNs containing Brij78 and TPGS were found to improve the relative bioavailability for 9-fold and significantly increase the effective permeability through in situ intestinal absorption study 150.
Liposomes and micelles containing TPGS can improve solubility, permeability and oral bioavailability. Phospholipid complex and TPGS mixed micelles for baohuoside I delivery can enhance the solubility, permeability and inhibit drug efflux. With the increased ratio of TPGS, the drug solubility was increased and efflux was reduced. The mixed micelles increased the relative bioavailability up to 5.3-fold compared with free drug 151. In particular, MPEG-PLA/TPGS mixed micelles can increase the relative bioavailability of CUR up to 9-fold compared to CUR suspensions 152. Similarly, the micelles were prepared by mixing MPEG-PLA, TPGS and stearic acid grafted chitosan oligosaccharide, which enhanced oral bioavailability 153. Recently, the mixed micelles of Poloxamer 407 and TPGS were developed to co-deliver resveratrol and metabolism inhibitor piperine, which exhibited enhanced oral bioavailability of resveratrol up to 5.7-fold compared to free drug. Moreover, higher cytotoxicity in MCF-7 cells was evidenced from the resveratrol/piperine-loaded micelles with 14.5-fold lower of IC50 compared with that of free resveratrol 154.
TPGS based formulations to promote drug permeation
TPGS can improve drug permeation across physiological barriers such as skin or cornea. It can be applied to construct many formulations for transdermal drug delivery and the therapy of eye diseases.
The ibuprofen supersaturated solution with TPGS and hydroxypropyl methylcellulose (HPMC) exhibited higher permeation rate and longer onset of crystallization time compared with other solubilizer/polymer systems, which may be applicable in transdermal drug delivery systems 155. Following this work, the TPGS/HPMC nanosuspensions improved the permeability of ibuprofen with TPGS as solubilizer through the Skin-A Case Study 156. Moreover, TPGS nanoemulsion based nanogels were formed for topical delivery of amphotericin B to treat cutaneous fungal infections. The nanogels exhibited 3.9-fold higher skin deposition through porcine ear skin and 2.0-fold higher antifungal activity against Aspergillus niger and Candida albicans compared with the marketed topical formulation. The nanogels can penetrate into the deeper layers of skin 157. A topical formulation of griseofulvin, an antifungal agent, was prepared in the Carbopol (980 NF) base with the combination of TPGS as the penetration enhancer. The formulation exhibited enhanced drug permeation and retention in skin and showed great potential as the convenient alternative for the treatment of superficial fungal infection 158.
In parallel with transdermal drug delivery, TPGS was applied to promote drug translocation in cornea. Poor water solubility of drugs would limit the penetration and restrict pharmacological effects for eye diseases. Cholkar et al. 159 prepared dexamethasone-loaded micelles with TPGS and octoxynol-40 (Oc-40) (weight ratio 4.5:2.0). The mixed polymers exhibited lower CMC (0.012 wt%) compared with TPGS (0.025 wt%) and Oc-40 (0.107 wt%). The safety of formulations was evidenced from the cytotoxicity on rabbit primary corneal epithelial cells. Rapamycin-loaded micelles were also prepared with TPGS and Oc-40, which exhibited good tolerance as the low in vitro cytotoxicity on human retinal pigment epithelial and rabbit primary corneal epithelial cells. The micelles also exhibited feasibility in clinical application as the negligible drug partition into vitreous humor but very high drug level in targeted site of retina-choroid 160. Moreover, in situ CUR gels were prepared by dispersing an ion-sensitive Pluronic P123/TPGS mixed micelles in gellan gum solution. The gels exhibited better corneal penetration and longer ocular retention compared with CUR solution 161.
Conclusions and perspectives
In this review, we summarized the properties and recent progress of TPGS in drug delivery. The merits of TPGS for drug delivery are listed here as following ones. (i) TPGS has been approved by FDA as a safe pharmaceutical adjuvant with high biocompatibility. (ii) TPGS can serve as an effective P-gp inhibitor for overcoming MDR. (iii) TPGS itself can act as an anticancer agent with selective toxicity to tumor cells. (iv) TPGS can be easily combined with nanotechnology to develop nanomedicines, which has been shown as a promising strategy in cancer treatment with increased solubility and stability of therapeutic agents, improved PK/PD, enhanced treatment efficiency and reduced side effects. Here we discussed many examples to use TPGS based nanomedicines including TPGS based prodrugs, NO donor and polymers, and unmodified TPGS based formulations, which took advantages of the properties of TPGS in drug delivery, especially the potent effect to overcome MDR. These examples clearly illustrated the potential and promising applications of TPGS for overcoming MDR, improving oral bioavailability, promoting drug permeation and other functions.
P-gp inhibition has been widely accepted as the major mechanism of TPGS to overcome MDR. Though mitochondria dependent P-gp pump inhibition has been well characterized, there is little cue about the exact mechanism of ATPase inhibition by TPGS. It remains unclear whether the ATPase inhibition is achieved by steric blocking of substrate binding, the direct interaction of TPGS with P-gp nucleotide binding domains or indirectly influencing P-gp function via an allosteric modulation 34. Besides, TPGS may not work with the drug resistance acquired from tumor heterogenicity during tumor progression. It has been reported that TPGS can prevent tumor invasion and metastasis. The underlying mechanisms are unknown and still need to be investigated for more comprehensive application of TPGS in tumor metastasis. Furthermore, the impact of TPGS on immune system requires to be studied for the reason that TPGS can be used as an adjuvant in vaccine development for cancer immunotherapy. As to TPGS based formulations, the limitations to realize the precise stimuli-responsive property and deep penetration of nanoformulations in tumor microenvironment still remain as obstacles for the widespread application of these nanomedicines. More effective strategies should be exploited to enhance the targeted delivery efficiency, achieve the controlled drug release and increase the penetration of therapeutics in tumor. Clinical translation is the ultimate goal for the development of nanomedicines, which should be focused on simple structure with multifunctional properties. Taking advantage of the biocompatibility and multiple functions of TPGS, TPGS based nanomedicines may be promising for the property as “molecular economy” in pharmaceutics. However, the preparation of TPGS nanomedicines is still in laboratory scale and the progress on developing novel nanomedicines is relatively slow, which hinders the successful clinical translation of TPGS based nanomedicines. It may be accelerated by optimization of preparation procedures for industry production and exploration of effective models to minimize the physiological difference between animal and human.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81373360 and 81673374) and Fundamental Research Funds for the Central Universities (2015ZDTD048).
Abbreviations
- ABCB1
ATP-binding cassette transporter P-glycoprotein
- ADP
adenosine diphosphate
- α-TOS
α-tocopheryl succinate
- AR+
androgen receptor positive
- AUC
area under curve
- BCS
biopharmaceutics classification system
- Ce6
chlorin e6
- Cmax
peak concentration
- CMC
critical micelle concentration
- CP
cefpodoxime proxetil
- cRGD
cyclic RGD
- CUR
curcumin
- CYP
cytochrome P450
- DOX
doxorubicin
- DTX
docetaxel
- DQA
dequalinium
- EPR
enhanced permeation and retention
- F127
pluronic F127
- FOL
folic acid
- 5-FU
5-fluorouracil
- HA
hyaluronic acid
- HER2
human epidermal growth factor receptor 2
- IC50
the half-maximal inhibitory concentrations
- NADPH
nicotinamide adenine dinucleotide phosphate
- LD50
median lethal dose
- LND
lonidamine
- MDR
multi-drug resistance
- MPEG-SS-2SA
disulfide bond-linked MPEG and stearic acid
- MRT
mean residence time
- NO
nitric oxide
- NRP-1
neuropilin-1 receptor
- PBAE
poly-(beta-amino ester)
- PCL
polycaprolactone
- PD
pharmacodynamics
- PEG
poly(ethylene glycol)
- PGA
poly-glutamic acid
- P-gp
P-glycoprotein
- PK
pharmacokinetic
- PKB
protein kinase B
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PTX
paclitaxel
- Rh123
rhodamine123
- ROS
reactive oxygen species
- SEDDS/SMEDDS
self-emulsifying/microemulsifying drug delivery system
- SLNs
solid lipid nanoparticles
- t1/2
half-life
- TCMs
traditional Chinese medicines
- TNO3
nitrate functionalized TPGS
- TQR
tariquidar
- Vitamin E TPGS or TPGS
D-ɑ-tocopheryl polyethylene glycol succinate
- VES
vitamin E succinate
- ΔΨm
mitochondrial membrane potential.
References
- 1.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–1. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 2.Frank J. Beyond vitamin E supplementation: an alternative strategy to improve vitamin E status. J Plant Physiol. 2005;162:834–43. doi: 10.1016/j.jplph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of α-tocopherol (vitamin E) FEBS Lett. 2002;519:8–10. doi: 10.1016/s0014-5793(02)02706-0. [DOI] [PubMed] [Google Scholar]
- 4.Penn JS, Tolman BL, Bullard LE. Effect of a water-soluble vitamin E analog, trolox C, on retinal vascular development in an animal model of retinopathy of prematurity. Free Radic Biol Med. 1997;22:977–84. doi: 10.1016/s0891-5849(96)00479-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33:4889–906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49:175–86. doi: 10.1016/j.ejps.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil J, Dong LF, Ramanathapuram L, Hahn T, Chladova M, Wang XF. et al. Vitamin E analogues as a novel group of mitocans: anti-cancer agents that act by targeting mitochondria. Mol Aspects Med. 2007;28:607–45. doi: 10.1016/j.mam.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Jiri N, Marco T, Albert SM, Renata A, Brian AS, Marc B. et al. Vitamin E analogues: a new class of inducers of apoptosis with selective anti-cancer effects. Curr Cancer Drug Targets. 2004;4:355–72. doi: 10.2174/1568009043332943. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 10.Patel NR, Pattni BS, Abouzeid AH, Torchilin VP. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliver Rev. 2013;65:1748–62. doi: 10.1016/j.addr.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM. et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–9. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 12.Bogman K, Erne-Brand F, Alsenz J, Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci. 2003;92:1250–61. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 13.Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 14.Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV. Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004;21:2226–33. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugger ED, Novak BL, Burton PS, Audus KL, Borchardt RT. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J Pharm Sci. 2002;91:1991–2002. doi: 10.1002/jps.10176. [DOI] [PubMed] [Google Scholar]
- 16.Tang J, Fu Q, Wang Y, Racette K, Wang D, Liu F. Vitamin E reverses multidrug resistance in vitro and in vivo. Cancer Lett. 2013;336:149–57. doi: 10.1016/j.canlet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–93. [PubMed] [Google Scholar]
- 18.Bu H, He X, Zhang Z, Yin Q, Yu H, Li Y. A TPGS-incorporating nanoemulsion of paclitaxel circumvents drug resistance in breast cancer. Int J Pharm. 2014;471:206–13. doi: 10.1016/j.ijpharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Dong X, Mattingly CA, Tseng MT, Cho MJ, Liu Y, Adams VR. et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69:3918–26. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Z, Chen M, Xiao Y, Sun M, Zong L, Asghar S. et al. ROS-triggered and regenerating anticancer nanosystem: an effective strategy to subdue tumor's multidrug resistance. J Control Release. 2014;196:370–83. doi: 10.1016/j.jconrel.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Collnot EM, Baldes C, Wempe MF, Kappl R, Hüttermann J, Hyatt JA. et al. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4:465–74. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Tan S, Guo Y, Huang J, Chu M, Liu H. et al. pH-Sensitive docetaxel-loaded D-alpha-tocopheryl polyethylene glycol succinate-poly(beta-amino ester) copolymer nanoparticles for overcoming multidrug resistance. Biomacromolecules. 2013;14:2636–46. doi: 10.1021/bm4005113. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y, Yin M, Hu X, Zhuang X, Sun Y, Guo Y. et al. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J Control Release. 2016;235:182–94. doi: 10.1016/j.jconrel.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Yin M, Tan S, Bao Y, Zhang Z. Enhanced tumor therapy via drug co-delivery and in situ vascular-promoting strategy. J Control Release. 2017;258:108–20. doi: 10.1016/j.jconrel.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Fan Z, Wu J, Fang X, Sha X. A new function of Vitamin E-TPGS in the intestinal lymphatic transport of lipophilic drugs: enhancing the secretion of chylomicrons. Int J Pharm. 2013;445:141–7. doi: 10.1016/j.ijpharm.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 26.Gadadare R, Mandpe L, Pokharkar V. Ultra rapidly dissolving repaglinide nanosized crystals prepared via bottom-up and top-down approach: influence of food on pharmacokinetics behavior. AAPS PharmSciTech. 2015;16:787–99. doi: 10.1208/s12249-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bittner B, Bravo González RC, Bohrmann B, Kuentz M, Huwyler J. Drug-excipient interactions by Vitamin E-TPGS: in vitro studies on inhibition of P-glycoprotein and colonic drug absorption. J Drug Deliv Sci Technol. 2008;18:145–8. [Google Scholar]
- 28.Zhang Z, Lv H, Jia X, Lingling C, Xin J, Van C. et al. Influence of vitamin E tocopherol polyethylene glycol succinate 1000 on intestinal absorption of icariside II. Pharmazie. 2012;67:59–62. [PubMed] [Google Scholar]
- 29.Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–46. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Chen H, Zeng X, Wang Z, Zhang X, Wu Y. et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance. Biomaterials. 2014;35:2391–400. doi: 10.1016/j.biomaterials.2013.11.086. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Liu K. P-gp inhibition-based strategies for modulating pharmacokinetics of anticancer drugs: an update. Curr Drug Metab. 2016;17:806–26. doi: 10.2174/1389200217666160629112717. [DOI] [PubMed] [Google Scholar]
- 32.Assanhou AG, Li W, Zhang L, Xue L, Kong L, Sun H. et al. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015;73:284–95. doi: 10.1016/j.biomaterials.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Dong LF, Low P, Dyason JC, Wang XF, Prochazka L, Witting PK. et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene. 2008;27:4324–35. doi: 10.1038/onc.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collnot EM, Baldes C, Schaefer UF, Edgar KJ, Wempe MF, Lehr CM. Vitamin E TPGS P-glycoprotein inihibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm. 2010;7:642–51. doi: 10.1021/mp900191s. [DOI] [PubMed] [Google Scholar]
- 35.Ackrell BA. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 2000;466:1–5. doi: 10.1016/s0014-5793(99)01749-4. [DOI] [PubMed] [Google Scholar]
- 36.Dong K, Yan Y, Wang P, Shi X, Zhang L, Wang K. et al. Biodegradable mixed MPEG-SS-2SA/TPGS micelles for triggered intracellular release of paclitaxel and reversing multidrug resistance. Int J Nanomedicine. 2016;11:5109–23. doi: 10.2147/IJN.S111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mbaya E, Oules B, Caspersen C, Tacine R, Massinet H, Pennuto M. et al. Calcium signalling-dependent mitochondrial dysfunction and bioenergetics regulation in respiratory chain Complex II deficiency. Cell Death Differ. 2010;17:1855–66. doi: 10.1038/cdd.2010.51. [DOI] [PubMed] [Google Scholar]
- 38.Yu P, Yu H, Guo C, Cui Z, Chen X, Yin Q. et al. Reversal of doxorubicin resistance in breast cancer by mitochondria-targeted pH-responsive micelles. Acta Biomater. 2015;14:115–24. doi: 10.1016/j.actbio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM. et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–9. doi: 10.1021/bi973045u. [DOI] [PubMed] [Google Scholar]
- 40.Neophytou CM, Constantinou C, Papageorgis P, Constantinou AI. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem Pharmacol. 2014;89:31–42. doi: 10.1016/j.bcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Moreno C, Jimenez-Del-Rio M, Sierra-Garcia L, Lopez-Osorio B, Velez-Pardo C. Vitamin E synthetic derivate-TPGS-selectively induces apoptosis in jurkat T cells via oxidative stress signaling pathways: implications for acute lymphoblastic leukemia. Apoptosis. 2016;21:1019–32. doi: 10.1007/s10495-016-1266-x. [DOI] [PubMed] [Google Scholar]
- 42.Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson EI, Hergenrother PJ. Runaway ROS as a selective anticancer strategy. ChemMedChem. 2011;6:1957–9. doi: 10.1002/cmdc.201100381. [DOI] [PubMed] [Google Scholar]
- 44.Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD. et al. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer. 2009;61:348–56. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 45.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M. et al. O2- and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31:487–500. doi: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youk HJ, Lee E, Choi MK, Lee YJ, Chung JH, Kim SH. et al. Enhanced anticancer efficacy of alpha-tocopheryl succinate by conjugation with polyethylene glycol. J Control Release. 2005;107:43–52. doi: 10.1016/j.jconrel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 47.O'Driscoll L, Linehan R, Clynes M. Survivin: role in normal cells and in pathological conditions. Curr Cancer Drug Targets. 2003;3:131–52. doi: 10.2174/1568009033482038. [DOI] [PubMed] [Google Scholar]
- 48.Constantinou C, Neophytou CM, Vraka P, Hyatt JA, Papas KA, Constantinou AI. Induction of DNA damage and caspase-independent programmed cell death by vitamin E. Nutr Cancer. 2012;64:136–52. doi: 10.1080/01635581.2012.630167. [DOI] [PubMed] [Google Scholar]
- 49.Albert A. Chemical aspects of selective toxicity. Nature. 1958;182:421–2. doi: 10.1038/182421a0. [DOI] [PubMed] [Google Scholar]
- 50.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T. et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 51.Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. Prodrug approach: an overview of recent cases. Eur J Med Chem. 2017;127:810–27. doi: 10.1016/j.ejmech.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 52.Hamaguchi T, Doi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y. et al. Phase I study of NK012, a novel SN-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res. 2010;16:5058–66. doi: 10.1158/1078-0432.CCR-10-0387. [DOI] [PubMed] [Google Scholar]
- 53.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R. et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 54.Li R, Xie Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J Control Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Cao N, Feng SS. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS): conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials. 2008;29:3856–65. doi: 10.1016/j.biomaterials.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Anbharasi V, Cao N, Feng SS. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol succinate and folic acid as a prodrug for targeted chemotherapy. J Biomed Mater Res A. 2010;94:730–43. doi: 10.1002/jbm.a.32734. [DOI] [PubMed] [Google Scholar]
- 57.Hou W, Zhao X, Qian X, Pan F, Zhang C, Yang Y. et al. pH-Sensitive self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale. 2016;8:104–16. doi: 10.1039/c5nr06842h. [DOI] [PubMed] [Google Scholar]
- 58.Bao Y, Guo Y, Zhuang X, Li D, Cheng B, Tan S. et al. D-alpha-tocopherol polyethylene glycol succinate-based redox-sensitive paclitaxel prodrug for overcoming multidrug resistance in cancer cells. Mol Pharm. 2014;11:3196–209. doi: 10.1021/mp500384d. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 60.Mi Y, Zhao J, Feng SS. Vitamin E TPGS prodrug micelles for hydrophilic drug delivery with neuroprotective effects. Int J Pharm. 2012;438:98–106. doi: 10.1016/j.ijpharm.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 61.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 62.Mi Y, Zhao J, Feng SS. Targeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. J Control Release. 2013;169:185–92. doi: 10.1016/j.jconrel.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y, Liu D, Wang D, Wang Y, Fu Q, Fallon JK. et al. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol Pharm. 2014;11:2623–30. doi: 10.1021/mp400778r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang D, Tang J, Wang Y, Ramishetti S, Fu Q, Racette K. et al. Multifunctional nanoparticles based on a single-molecule modification for the treatment of drug-resistant cancer. Mol Pharm. 2013;10:1465–9. doi: 10.1021/mp400022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao Y, Ping Q, Zong L. Preparation and antitumor activity of mitoxantrone conjugated D-alpha-tocopherylpolyethylene glycol 1000 succinate prodrug micelle. Journal of China Pharmaceutical University. 2016;47:311–6. [Google Scholar]
- 66.Khare V, Al Sakarchi W, Gupta PN, Curtis ADM, Hoskins C. Synthesis and characterization of TPGS-gemcitabine prodrug micelles for pancreatic cancer therapy. RSC Adv. 2016;6:60126–37. [Google Scholar]
- 67.Sheng S, Zhang T, Li S, Wei J, Xu G, Sun T. et al. Targeting vitamin E TPGS-cantharidin conjugate nanoparticles for colorectal cancer therapy. RSC Adv. 2015;5:53846–56. [Google Scholar]
- 68.Nathan CZ. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 69.Quinn JF, Whittaker MR, Davis TP. Delivering nitric oxide with nanoparticles. J Control Release. 2015;205:190–205. doi: 10.1016/j.jconrel.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 70.Sang J, Chen Y, Tao Y. Nitric oxide inhibits gastric cancer cell growth through the modulation of the Akt pathway. Mol Med Rep. 2011;4:1163–7. doi: 10.3892/mmr.2011.535. [DOI] [PubMed] [Google Scholar]
- 71.Shang ZJ, Li JR, Li ZB. Effects of exogenous nitric oxide on oral squamous cell carcinoma: an in vitro study. J. Oral Maxillofac. Surg. 2002;60:905–10. doi: 10.1053/joms.2002.33860. [DOI] [PubMed] [Google Scholar]
- 72.Harada K, Supriatno, Kawaguchi S, Tomitaro O, Yoshida H, Sato M. Overexpression of iNOS gene suppresses the tumor igenicity and metastasis of oral cancer cells. In Vivo. 2004;18:449–55. [PubMed] [Google Scholar]
- 73.Garbán HJ. Breaking resistance: role of nitric oxide in the sensitization of cancer cells to chemo-and immunotherapy. Nitric Oxide (NO) and Cancer: Springer; 2010. pp. 283–90. [Google Scholar]
- 74.Efferth T. Role of nitric oxide for modulation of cancer therapy resistance. Nitric Oxide (NO) and Cancer: Springer; 2010. pp. 265–82. [Google Scholar]
- 75.Gladwin MT, Lancaster JR, Freeman BA, Schechter AN. Nitric oxide's reactions with hemoglobin: a view through the SNO-storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 76.Al SA, Doni H, Ferro A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci. 2000;98:507–20. [PubMed] [Google Scholar]
- 77.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102:1135–54. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 78.Song Q, Tan S, Zhuang X, Guo Y, Zhao Y, Wu T. et al. Nitric oxide releasing D-alpha-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol Pharm. 2014;11:4118–29. doi: 10.1021/mp5003009. [DOI] [PubMed] [Google Scholar]
- 79.Vijayakumar MR, Muthu MS, Singh S. Copolymers of poly (lactic acid) and D-α-tocopheryl polyethylene glycol 1000 succinate-based nanomedicines: versatile multifunctional platforms for cancer diagnosis and therapy. Expert Opin Drug Deliv. 2013;10:529–43. doi: 10.1517/17425247.2013.758632. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Z, Feng SS. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27:262–70. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Lee SH, Feng SS. Folate-decorated poly (lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials. 2007;28:1889–99. doi: 10.1016/j.biomaterials.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 82.Zhang XY, Zhang YD. Enhanced antiproliferative and apoptosis effect of paclitaxel-loaded polymeric micelles against non-small cell lung cancers. Tumor Biol. 2015;36:4949–59. doi: 10.1007/s13277-015-3142-7. [DOI] [PubMed] [Google Scholar]
- 83.Guo Y, Chu M, Tan S, Zhao S, Liu H, Otieno BO. et al. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm. 2014;11:59–70. doi: 10.1021/mp400514t. [DOI] [PubMed] [Google Scholar]
- 84.Mu L, Feng S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol®) J Control Release. 2002;80:129–44. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhu D, Tao W, Zhang H, Liu G, Wang T, Zhang L. et al. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016;30:144–54. doi: 10.1016/j.actbio.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 86.Guo Y, Niu B, Song Q, Zhao Y, Bao Y, Tan S. et al. RGD-decorated redox-responsive D-alpha-tocopherol polyethylene glycol succinate-poly(lactide) nanoparticles for targeted drug delivery. J Mater Chem B. 2016;4:2338–50. doi: 10.1039/c6tb00055j. [DOI] [PubMed] [Google Scholar]
- 87.Zeng XW, Tao W, Wang ZY, Zhang XD, Zhu HJ, Wu YP. et al. Docetaxel-loaded nanoparticles of dendritic amphiphilic block copolymer H40-PLA-b-TPGS for cancer treatment. Part Part Syst Charact. 2015;32:112–22. [Google Scholar]
- 88.Mi Y, Liu X, Zhao J, Ding J, Feng SS. Multimodality treatment of cancer with herceptin conjugated, thermomagnetic iron oxides and docetaxel loaded nanoparticles of biodegradable polymers. Biomaterials. 2012;33:7519–29. doi: 10.1016/j.biomaterials.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 89.Wang T, Zhu D, Liu G, Tao W, Cao W, Zhang L. et al. DTX-loaded star-shaped TAPP-PLA-b-TPGS nanoparticles for cancer chemical and photodynamic combination therapy. RSC Adv. 2015;5:50617–27. [Google Scholar]
- 90.Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer. 2006;6:546–58. doi: 10.1038/nrc1887. [DOI] [PubMed] [Google Scholar]
- 91.Tan GR, Feng SS, Leong DT. The reduction of anti-cancer drug antagonism by the spatial protection of drugs with PLA-TPGS nanoparticles. Biomaterials. 2014;35:3044–51. doi: 10.1016/j.biomaterials.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 92.Yu Y, Tan S, Zhao S, Zhuang X, Song Q, Wang Y. et al. Antitumor activity of docetaxel-loaded polymeric nanoparticles fabricated by Shirasu porous glass membrane-emulsification technique. Int J Nanomedicine. 2013;8:2641–52. doi: 10.2147/IJN.S48214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliver Rev. 1995;16:61–73. [Google Scholar]
- 94.Ma Y, Zheng Y, Liu K, Tian G, Tian Y, Xu L. et al. Nanoparticles of poly (lactide-co-glycolide)-da-tocopheryl polyethylene glycol 1000 succinate random copolymer for cancer treatment. Nanoscale Res Lett. 2010;5:1161. doi: 10.1007/s11671-010-9620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu Q, Xu H, Gao M, Guan X, Liu H, Deng S. et al. Liver-targeting Resibufogenin-loaded poly(lactic-co-glycolic acid)-D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Int J Nanomedicine. 2016;11:449–63. doi: 10.2147/IJN.S93541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu H, Gao M, Xu H, Guan X, Lv L, Deng S. et al. A promising emodin-loaded poly (lactic-co-glycolic acid)-d-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Pharm Res. 2016;33:217–36. doi: 10.1007/s11095-015-1781-4. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, Li Y, Fang X, Zhou D, Wang Y, Chen M. TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int J Pharm. 2014;476:185–98. doi: 10.1016/j.ijpharm.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 98.Guan X, Gao M, Xu H, Zhang C, Liu H, Lv L. et al. Quercetin-loaded poly (lactic-co-glycolic acid)-D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for the targeted treatment of liver cancer. Drug Deliv. 2016;23:3307–18. doi: 10.1080/10717544.2016.1176087. [DOI] [PubMed] [Google Scholar]
- 99.Gao M, Xu H, Bao X, Zhang C, Guan X, Liu H. et al. Oleanolic acid-loaded PLGA-TPGS nanoparticles combined with heparin sodium-loaded PLGA-TPGS nanoparticles for enhancing chemotherapy to liver cancer. Life Sci. 2016;165:63–74. doi: 10.1016/j.lfs.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 100.Fang X, Xu Y, Chan H, Wang C, Zheng Q, Xiao F. et al. A redox-sensitive and RAGE-targeting nanocarrier for hepatocellular carcinoma therapy. Mol Pharm. 2016;13:3613–25. doi: 10.1021/acs.molpharmaceut.6b00116. [DOI] [PubMed] [Google Scholar]
- 101.Maglio G, Nese G, Nuzzo M, Palumbo R. Synthesis and characterization of star-shaped diblock poly (ε-caprolactone)/poly (ethylene oxide) copolymers. Macromol Rapid Commun. 2004;25:1139–44. [Google Scholar]
- 102.Zeng X, Tao W, Mei L, Huang L, Tan C, Feng SS. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials. 2013;34:6058–67. doi: 10.1016/j.biomaterials.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 103.Tao W, Zeng XW, Wu J, Zhu X, Yu XH, Zhang XD. et al. Polydopamine-based surface modification of novel nanoparticle-aptamer bioconjugates for in vivo breast cancer targeting and enhanced therapeutic effects. Theranostics. 2016;6:470–84. doi: 10.7150/thno.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao W, Zhang JX, Zeng XW, Liu D, Liu G, Zhu X. et al. Blended nanoparticle system based on miscible structurally similar polymers: a safe, simple, targeted, and surprisingly high efficiency vehicle for cancer therapy. Adv Healthc Mater. 2015;4:1203–14. doi: 10.1002/adhm.201400751. [DOI] [PubMed] [Google Scholar]
- 105.Cao W, Zeng X, Liu G, Li Z, Zeng X, Wang L. et al. Porphine functionalized nanoparticles of star-shaped poly(epsilon-caprolactone)-b-D-alpha-tocopheryl polyethylene glycol 1000 succinate biodegradable copolymer for chemophotodynamic therapy on cervical cancer. Acta Biomater. 2015;26:145–58. doi: 10.1016/j.actbio.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 106.Bernabeu E, Gonzalez L, Legaspi MJ, Moretton MA, Chiappetta DA. Paclitaxel-loaded TPGS-b-PCL nanoparticles: in vitro cytotoxicity and cellular uptake in MCF-7 and MDA-MB-231 cells versus mPEG-b-PCL nanoparticles and Abraxane®. J Nanosci and Nanotechnol. 2016;16:160–70. doi: 10.1166/jnn.2016.10739. [DOI] [PubMed] [Google Scholar]
- 107.Suksiriworapong J, Phoca K, Ngamsom S, Sripha K, Moongkarndi P, Junyaprasert VB. Comparison of poly(epsilon-caprolactone) chain lengths of poly(epsilon-caprolactone)-co-d-alpha-tocopheryl-poly(ethylene glycol) 1000 succinate nanoparticles for enhancement of quercetin delivery to SKBR3 breast cancer cells. Eur J Pharm Biopharm. 2016;101:15–24. doi: 10.1016/j.ejpb.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 108.Zheng Y, Chen H, Zeng X, Liu Z, Xiao X, Zhu Y. et al. Surface modification of TPGS-b-(PCL-ran-PGA) nanoparticles with polyethyleneimine as a co-delivery system of TRAIL and endostatin for cervical cancer gene therapy. Nanoscale Res Lett. 2013;8:161–72. doi: 10.1186/1556-276X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lynn DM, Langer R. Degradable poly (β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–8. [Google Scholar]
- 110.Zhang J, Chen R, Fang X, Chen F, Wang Y, Chen M. Nucleolin targeting AS1411 aptamer modified pH-sensitive micelles for enhanced delivery and antitumor efficacy of paclitaxel. Nano Res. 2015;8:201–18. [Google Scholar]
- 111.Yin M, Bao Y, Gao X, Wu Y, Sun Y, Zhao X. et al. Redox/pH dual-sensitive hybrid micelles for targeting delivery and overcoming multidrug resistance of cancer. J Mater Chem B. 2017;5:2964–78. doi: 10.1039/c6tb03282f. [DOI] [PubMed] [Google Scholar]
- 112.Shen J, Meng Q, Sui H, Yin Q, Zhang Z, Yu H. et al. iRGD conjugated TPGS mediates codelivery of paclitaxel and Survivin shRNA for the reversal of lung cancer resistance. Mol Pharm. 2014;11:2579–91. doi: 10.1021/mp400576f. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Feng S, Liu W, Yuan Z, Yin P, Gao F. Vitamin E succinate-grafted-chitosan oligosaccharide/RGD-conjugated TPGS mixed micelles loaded with paclitaxel for U87MG tumor therapy. Mol Pharm. 2017;14:1190–203. doi: 10.1021/acs.molpharmaceut.6b01068. [DOI] [PubMed] [Google Scholar]
- 114.Jiang D, Gao X, Kang T, Feng X, Yao J, Yang M. et al. Actively targeting D-alpha-tocopheryl polyethylene glycol 1000 succinate-poly(lactic acid) nanoparticles as vesicles for chemo-photodynamic combination therapy of doxorubicin-resistant breast cancer. Nanoscale. 2016;8:3100–18. doi: 10.1039/c5nr07724a. [DOI] [PubMed] [Google Scholar]
- 115.Muthu MS, Kutty RV, Luo Z, Xie J, Feng SS. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials. 2015;39:234–48. doi: 10.1016/j.biomaterials.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 116.Chen F, Wu J, Zheng C, Zhu J, Zhang Y, You X. et al. TPGS modified reduced bovine serum albumin nanoparticles as a lipophilic anticancer drug carrier for overcoming multidrug resistance. J Mater Chem B. 2016;4:3959–68. doi: 10.1039/c6tb00515b. [DOI] [PubMed] [Google Scholar]
- 117.Wang J, Sun J, Chen Q, Gao Y, Li L, Li H. et al. Star-shape copolymer of lysine-linked di-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials. 2012;33:6877–88. doi: 10.1016/j.biomaterials.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 118.Wu Y, Chu Q, Tan S, Zhuang X, Bao Y, Wu T. et al. D-alpha-tocopherol polyethylene glycol succinate-based derivative nanoparticles as a novel carrier for paclitaxel delivery. Int J Nanomedicine. 2015;10:5219–35. doi: 10.2147/IJN.S82847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng W, Liang CY, Xu L, Liu G, Gao NS, Tao W, TPGS-functionalized polydopamine-modified mesoporous silica as drug nanocarriers for enhanced lung cancer chemotherapy against multidrug resistance. Small; 2017. p. 13. [DOI] [PubMed] [Google Scholar]
- 120.Gao L, Liu G, Kang J, Niu M, Wang Z, Wang H. et al. Paclitaxel nanosuspensions coated with P-gp inhibitory surfactants: I. Acute toxicity and pharmacokinetics studies. Colloids Surf B Biointerfaces. 2013;111:277–81. doi: 10.1016/j.colsurfb.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Gao L, Liu G, Ma J, Wang X, Wang F, Wang H. et al. Paclitaxel nanosuspension coated with P-gp inhibitory surfactants: II. Ability to reverse the drug-resistance of H460 human lung cancer cells. Colloids Surf B Biointerfaces. 2014;117:122–7. doi: 10.1016/j.colsurfb.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 122.Han N, Zhao QF, Wan L, Wang Y, Gao YK, Wang P. et al. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl Mater Interfaces. 2015;7:3342–51. doi: 10.1021/am5082793. [DOI] [PubMed] [Google Scholar]
- 123.Li N, Zhang C-X, Wang XX, Zhang L, Ma X, Zhou J. et al. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 2013;34:3366–80. doi: 10.1016/j.biomaterials.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 124.Yu Y, Wang ZH, Zhang L, Yao HJ, Zhang Y, Li RJ. et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials. 2012;33:1808–20. doi: 10.1016/j.biomaterials.2011.10.085. [DOI] [PubMed] [Google Scholar]
- 125.Liu BY, Wu C, He XY, Zhuo RX, Cheng SX. Multi-drug loaded vitamin E-TPGS nanoparticles for synergistic drug delivery to overcome drug resistance in tumor treatment. Sci Bull. 2016;61:552–60. [Google Scholar]
- 126.Gu Y, Li J, Li Y, Song L, Li D, Peng L. et al. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int J Nanomedicine. 2016;11:5757–70. doi: 10.2147/IJN.S118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713–21. doi: 10.2147/IJN.S28745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saxena V, Hussain MD. Polymeric mixed micelles for delivery of curcumin to multidrug resistant ovarian cancer. J Biomed Nanotechnol. 2013;9:1146–54. doi: 10.1166/jbn.2013.1632. [DOI] [PubMed] [Google Scholar]
- 129.Butt AM, Amin MCIM, Katas H. Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs. Int J Nanomedicine. 2015;10:1321–34. doi: 10.2147/IJN.S78438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:340315. doi: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Portu S, Mantovani LG, Ravaioli A, Tamburini E, Bollina R, Cozzi C. et al. Cost analysis of capecitabine vs 5-fluorouracil-based treatment for metastatic colorectal cancer patients. J Chemother. 2010;22:125–8. doi: 10.1179/joc.2010.22.2.125. [DOI] [PubMed] [Google Scholar]
- 132.Morales JO, Fathe KR, Brunaugh A, Ferrati S, Li S, Montenegro-Nicolini M. et al. Challenges and future prospects for the delivery of biologics: oral Mucosal, pulmonary, and transdermal routes. AAPS J. 2017;19:652–68. doi: 10.1208/s12248-017-0054-z. [DOI] [PubMed] [Google Scholar]
- 133.Ghadi R, Dand N. BCS class IV drugs: highly notorious candidates for formulation development. J Control Release. 2017;248:71–95. doi: 10.1016/j.jconrel.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 134.Christiansen A, Backensfeld T, Denner K, Weitschies W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur J Pharm Biopharm. 2011;78:166–72. doi: 10.1016/j.ejpb.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 135.Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa. 3: effects of polyethoxylated solubilizing agents on drug permeation and metabolism. J Pharm Sci. 2010;99:1016–27. doi: 10.1002/jps.21836. [DOI] [PubMed] [Google Scholar]
- 136.Johnson BM, Charman WN, Porter CJ. An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E d-alpha-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci. 2002;4:193–205. doi: 10.1208/ps040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vasconcelos T, Marques S, Sarmento B. The biopharmaceutical classification system of excipients. Ther Deliv. 2017;8:65–78. doi: 10.4155/tde-2016-0067. [DOI] [PubMed] [Google Scholar]
- 138.Du P, Jiang Q, Yang R, Liu C, Li Y, Wang L. et al. Nanonization of andrographolide by a wet milling method: the effects of vitamin E TPGS and oral bioavailability enhancement. RSC Adv. 2016;6:101404–14. [Google Scholar]
- 139.Bajaj A, Rao MRP, Pardeshi A, Sali D. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS PharmSciTech. 2012;13:1331–40. doi: 10.1208/s12249-012-9860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ge ZQ, Du XY, Huang XN, Qiao B. Enhanced oral bioavailability of ursolic acid nanoparticles via antisolvent precipitation with TPGS1000 as a stabilizer. J Drug Deliv Sci Technol. 2015;29:210–7. [Google Scholar]
- 141.Rahman MA, Hussain A, Hussain MS, Mirza MA, Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS) Drug Dev Ind Pharm. 2013;39:1–19. doi: 10.3109/03639045.2012.660949. [DOI] [PubMed] [Google Scholar]
- 142.Sun D, Wei X, Xue X, Fang Z, Ren M, Lou H. et al. Enhanced oral absorption and therapeutic effect of acetylpuerarin based on D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoemulsions. Int J Nanomedicine. 2014;9:3413–23. doi: 10.2147/IJN.S63777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bajaj A, Rao MRP, Khole I, Munjapara G. Self-nanoemulsifying drug delivery system of cefpodoxime proxetil containing tocopherol polyethylene glycol succinate. Drug Dev Ind Pharm. 2013;39:635–45. doi: 10.3109/03639045.2012.683440. [DOI] [PubMed] [Google Scholar]
- 144.Jain S, Kambam S, Thanki K, Jain AK. Cyclosporine A loaded self-nanoemulsifying drug delivery system (SNEDDS): implication of a functional excipient based co-encapsulation strategy on oral bioavailability and nephrotoxicity. RSC Adv. 2015;5:49633–42. [Google Scholar]
- 145.Bakhsh S, Safdar M, Alam S, Ramzan M, Rashid SA, Tariq M. et al. Synthesis, characterization and in vivo assessment of dexibuprofen-eudragit solid dispersion nanoparticles with supercritical antisolvent technique. Latin American Journal of Pharmacy. 2016;35:1528–37. [Google Scholar]
- 146.Eedara BB, Veerareddy PR, Jukanti R, Bandari S. Improved oral bioavailability of fexofenadine hydrochloride using lipid surfactants: ex vivo, in situ and in vivo studies. Drug Dev Ind Pharm. 2014;40:1030–43. doi: 10.3109/03639045.2013.801984. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Z, Chen Y, Deng J, Jia X, Zhou J, Lv H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: preparation, characterization and in vivo studies. Int J Pharm. 2014;465:306–16. doi: 10.1016/j.ijpharm.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 148.Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomedicine. 2014;9:495–504. doi: 10.2147/IJN.S56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garg A, Bhalala K, Tomar DS, Wahajuddin. In-situ single pass intestinal permeability and pharmacokinetic study of developed Lumefantrine loaded solid lipid nanoparticles. Int J Pharm. 2017;516:120–30. doi: 10.1016/j.ijpharm.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 150.Ji H, Tang J, Li M, Ren J, Zheng N, Wu L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016;23:459–70. doi: 10.3109/10717544.2014.918677. [DOI] [PubMed] [Google Scholar]
- 151.Jin X, Zhang ZH, Sun E, Tan XB, Zhu FX, Jia XB. A novel drug-phospholipid complex loaded micelle for baohuoside I enhanced oral absorption: in vivo and in vitro evaluations. Drug Dev Ind Pharm. 2013;39:1421–30. doi: 10.3109/03639045.2012.719234. [DOI] [PubMed] [Google Scholar]
- 152.Duan Y, Zhang B, Chu L, Tong HHY, Liu W, Zhai G. Evaluation in vitro and in vivo of curcumin-loaded mPEG-PLA/TPGS mixed micelles for oral administration. Colloids Surf B Biointerfaces. 2016;141:345–54. doi: 10.1016/j.colsurfb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 153.Dou J, Zhang H, Liu X, Zhang M, Zhai G. Preparation and evaluation in vitro and in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf B Biointerfaces. 2014;114:20–7. doi: 10.1016/j.colsurfb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 154.Jadhav P, Bothiraja C, Pawar A. Resveratrol-piperine loaded mixed micelles: formulation, characterization, bioavailability, safety and in vitro anticancer activity. RSC Adv. 2016;6:112795–805. [Google Scholar]
- 155.Ghosh I, Michniak-Kohn B. A comparative study of Vitamin E TPGS/HPMC supersaturated system and other solubilizer/polymer combinations to enhance the permeability of a poorly soluble drug through the skin. Drug Dev Ind Pharm. 2012;38:1408–16. doi: 10.3109/03639045.2011.653363. [DOI] [PubMed] [Google Scholar]
- 156.Ghosh I, Michniak-Kohn B. Influence of critical parameters of nanosuspension formulation on the permeability of a poorly soluble drug through the skin-A case study. AAPS PharmSciTech. 2013;14:1108–17. doi: 10.1208/s12249-013-9995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaur L, Jain SK, Singh K. Vitamin E TPGS based nanogel for the skin targeting of high molecular weight anti-fungal drug: development and in vitro and in vivo assessment. RSC Adv. 2015;5:53671–86. [Google Scholar]
- 158.Aggarwal N, Goindi S, Mehta SD. Preparation and evaluation of dermal delivery system of griseofulvin containing vitamin E-TPGS as penetration enhancer. AAPS PharmSciTech. 2012;13:67–74. doi: 10.1208/s12249-011-9722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cholkar K, Hariharan S, Gunda S, Mitra AK. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech. 2014;15:1454–67. doi: 10.1208/s12249-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cholkar K, Gunda S, Earla R, Pal D, Mitra AK. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2015;16:610–22. doi: 10.1208/s12249-014-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duan Y, Cai X, Du H, Zhai G. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf B Biointerfaces. 2015;128:322–30. doi: 10.1016/j.colsurfb.2015.02.007. [DOI] [PubMed] [Google Scholar]