Abstract
Background
This study estimated latent classes (i.e., unobserved subgroups in a population) of people who use drugs in Vancouver, Canada and examined how these classes relate to phylogenetic clustering of hepatitis C virus (HCV) infection.
Methods
HCV antibody positive people who use drugs from two cohorts in Vancouver, Canada (1996–2012) with a Core-E2 sequence were included. Time-stamped phylogenetic trees were inferred and phylogenetic clustering was determined by time to most common recent ancestor. Latent classes were estimated and the association with the phylogenetic clustering outcome assessed using an inclusive classify/analyse approach.
Results
Among 699 HCV RNA positive participants (26% female, 24% HIV+), recent drug use included injecting cocaine (80%), injecting heroin (70%), injecting cocaine/heroin (e.g. speedball, 38%), and crack cocaine smoking (28%). Latent class analysis identified four distinct subgroups of drug use typologies: 1) cocaine injecting, 2) opioid and cocaine injecting, 3) crack cocaine smoking, and 4) heroin injecting and currently receiving opioid substitution therapy. After adjusting for age and HIV infection, compared to the group defined by opioid and cocaine injecting, the odds of phylogenetic cluster membership was greater in the cocaine injecting group [adjusted OR (aOR): 3.05; 95% CI: 1.76, 5.27] and lower in the crack cocaine smoking group (aOR: 0.07; 95% CI: 0.01, 0.52).
Conclusions
Combining latent class and phylogenetic clustering analyses provides novel insights into the complex dynamics of HCV transmission. Incorporating differing risk profiles associated with drug use may provide opportunities to further optimise and target HCV treatment and prevention strategies.
Keywords: LCA, HCV, phylogenetic, clustering, distal outcome, Cocaine, Heroin, Crack cocaine
INTRODUCTION
Infection with hepatitis C virus (HCV) disproportionately affects people who use drugs (PWUD), and HCV incidence remains particularly high among drug-using youth and young adults (1–3).While the factors associated with HCV acquisition are well described, the dynamics of HCV transmission are poorly understood. Phylogenetic clustering has been used as a tool to better understand characteristics that might be associated with increased likelihood of HCV transmission (4–7). However, little is known about the association between drug use patterns and phylogenetic clustering of HCV infection PWUD. Understanding the association between drug use patterns and HCV transmission would help to better target HCV prevention strategies.
In studies of HCV transmission among cohorts of people who use drugs in Vancouver, Canada, younger age, HIV infection, recent HCV seroconversion, and syringe borrowing have been shown to be independently associated with phylogenetic clustering (4, 5). In a recently published study, methamphetamine injecting was also shown to be associated with HCV clustering among a small sample of young PWUD in Vancouver (8). Nonetheless, data investigating the association between specific patterns of drug use and HCV phylogenetic clustering are sparse. Traditional epidemiological approaches that evaluate individual factors associated with HCV transmission may not reflect the complex dynamics of drug use (particularly polysubstance use) and its relationship with HCV transmission. That is, the identification of factors associated with HCV acquisition or clustering may not fully reveal the interaction of multiple observed indicators.
Latent class analysis divides a population into mutually exclusive and exhaustive subgroups, simultaneously examining socio-demographic covariates associated with these patterns (9). The method assumes that the population is composed of distinct sub-populations (i.e., latent classes), which are not directly observed but are inferred from the observed characteristics of individuals (10). Patterns of illicit drug use defined by latent class analysis have been used to describe participant risk of HIV and HCV infection, substance abuse treatment utilization, and quality of life (11–13). Recently, latent class analysis has been combined with phylogenetic analysis to better understand the dynamics of HIV transmission in Switzerland, and to characterize the propensity for clustering both within and between socioeconomic groupings (14).
Next generation direct-acting antiviral HCV therapy are well tolerated and highly efficacious among people who actively use drugs, and may be useful in a treatment as prevention strategy (15, 16). However, limited access to these novel therapies may necessitate targeted implementation. Given the complex nature of HCV transmission, gaining a better understanding of drug use patterns and their relationship to HCV transmission may inform the development of public health interventions.
The aim of this study was to identify patterns of poly-drug use using latent class analysis, and to evaluate the association between drug uses classes and HCV phylogenetic cluster membership among two cohorts of people who use drugs in Vancouver.
MATERIALS AND METHODS
Study population and design
The study population and design, study assessments, RNA sequencing and phylogenetic analyses have been previously described (5). Briefly, people who use drugs who reported injecting drugs in the previous month were recruited into the Vancouver Injection Drug Users Study (VIDUS) from May 1996 (17), while street-involved youth who reported use of illicit drugs (or than or in addition to marijuana) in the previous month were recruited into the At Risk Youth Study (ARYS) from September 2005 (18). Participants who: 1) were HCV antibody-positive at enrolment; or 2) demonstrated recent HCV seroconversion [defined by an HCV antibody (anti-HCV) negative test at enrolment followed by an anti-HCV positive test at a subsequent study visit] between May 1996 and December 2012 and with an obtainable HCV subtype from sequencing were eligible for inclusion. The University of British Columbia/Providence Health Care Research Ethics Board approved this study. The cohort data collection procedures and instruments were harmonized such that the same information on socio-demographic characteristics, as well as information pertaining to drug use patterns and risk behaviours, were collected at enrolment and semi-annually through an interviewer-administered questionnaire.
Phylogenetic analysis
HCV RNA was quantified using an in-house PCR (limit of detection: 200 IU/ml), and amplification and sequencing of a 1,514bp fragment of the HCV genome covering Core, Envelope-1, hypervariable region-1 and beginning of Envelope-2 (E2) was attempted on all samples with detectable HCV RNA (19). Sanger sequencing chromatograms were processed using RECall (20), and phylogenetic trees were inferred for participants with HCV subtypes 1a, 1b, 2a and 3a using a Bayesian phylogenetic approach implemented in BEAST v1.8.1. Initially, a genomic-partition model was employed to estimate the substitution rates of Core-E2 region from an independent HCV dataset, which exhibited a strong temporal signal (21). These rates were subsequently applied as a strong prior distribution on the molecular clock to estimate time-scaled phylogenies based on sequences generated in this study. Clusters and pairs were identified using a new method that placed an upper limit on the estimated time to most common recent ancestor (tMRCA) of potentially connected individuals (ClusterByTime, http://evolve.zoo.ox.ac.uk/Evolve/ClusterByTime.html). To account for phylogenetic uncertainty, 100 phylogenies from the posterior tree distribution were assessed with a tMRCA limit of five years (5). Sensitivity analysis of phylogenetic clustering and factors associated identified correspondence between 5-year tMRCA limit a 1.5% genetic distance threshold (5).
Latent class estimation
In this latent class analysis, the latent class variable represents categories of underlying drug use behaviour. A total of six binary observed indicators (all yes vs. no) were used to estimate the latent class variable: heroin injecting, cocaine injecting, speedball (i.e., simultaneous heroin/cocaine) injecting, crack smoking, current enrolment in opioid substitution therapy program (OST: methadone), as well as a combined variable representing non-medical use of prescription opioids (e.g., hydromorphone, morphine and/or methadone). Reported drug use relates to the past six months at time of interview. Preliminary latent class analysis models were estimated to identify a model with the optimal number of classes, increasing from 1 to eight classes without covariates, as has previously been described (22). Model fit was estimated using Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), consistent AIC (cAIC), adjusted BIC (aBIC) and model stability. The bootstrap likelihood ratio test was used to compare the fit of a latent class analysis model with k classes (k ≥ 1) to one with k + 1 classes (23). If the p-value is <0.05, the model of k classes is rejected in favour of k+1 classes and the process is repeated incrementally until the p-value is >0.05. Final model estimation for class sizes 2 to 8 was repeated with the distal outcome variable (phylogenetic clustering) included as a covariate. Due to the inclusion of a covariate, estimation of fit statistics was not possible for the final model estimation. Finally, the choice of latent class solution presented was also informed by substantive criteria, such as meaningfulness in terms of the current epidemiology of drug use (22).
Statistical analyses
The aim of this study was to assess the association between latent class membership and phylogenetic clustering. An inclusive classify-analyse estimate of the association was implemented to reduce model attenuation and bias during classification (24). Participants were assigned to the latent class corresponding to their most likely membership (i.e. their maximum posterior probability), with the class-specific proportions for the phylogenetic clustering calculated and class membership treated as an observed variable in logistic regression against phylogenetic clustering.
Descriptive analyses were performed to characterise the study sample according to being in a pair or cluster (n≥2 participants), or neither. Participant characteristics in these categories were compared using Chi-squared, Fisher’s exact and Kruskal-Wallis tests (as appropriate). Statistically significant differences were assessed at p<0.05; all p-values are two-sided. Descriptive and inclusive classify-analyse logistic regression analyses were performed using STATA software (version 12.1; StataCorp L.P., College Station, Texas, USA) and latent class analyses (classification and distal outcomes) were performed using SAS software (version 9.4; SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Study population
In total, 2,688 participants from the ARYS (n=961) and VIDUS (n=1,727) cohorts were eligible for cohort enrolment (Figure 1). At enrolment, 52% (1,390 of 2,688) were anti-HCV positive. Among participants who were anti-HCV negative at enrolment (n=1299), 185 participants demonstrated recent HCV seroconversion during follow-up, and were therefore eligible for inclusion. HCV RNA was detectable in 74% (1,112 of 1,497) of anti-HCV positive participants with available samples, and Sanger sequencing of the Core-E2 segment obtained 66% (732 of 1,112) of these participants. Recent HCV seroconversion was observed in 10% (n=76) and HIV coinfection in 23% (n=166) of participants with a sequence (Table 1). HCV genotype distribution was: 1a: 48% (n=347), 1b: 6% (n=44), 2a: 3% (n=20), 2b: 7% (n=52), 3a: 35% (n=256), 4a: <1% (n=4), 6a: 1% (n=8), 6e: <1% (n=1).
Figure 1.
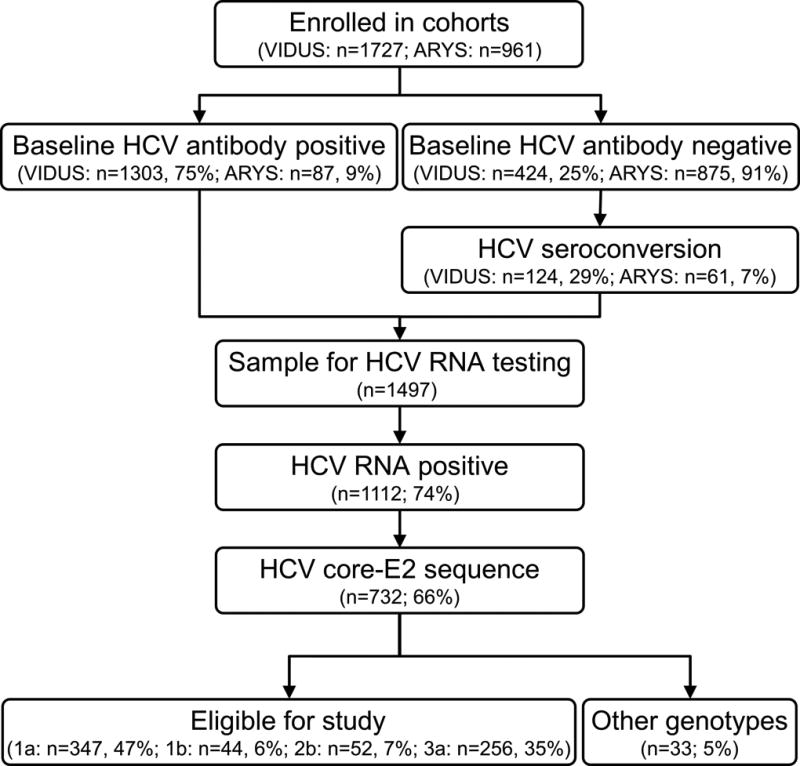
Table 1.
Characteristics of participants in the VIDUS and ARYS cohorts with HCV subtypes 1a, 1b, 2b and 3a.
| Characteristics | Total (n=699) |
No cluster (n=591) |
Cluster (n=108) |
P-value |
|---|---|---|---|---|
| Female sex (vs. male sex) | 179 (26%) | 149 (25%) | 30 (28%) | 0.574 |
| High school education or higher (vs. less than high school) | 165 (24%)^ | 140 (24%)^ | 25 (23%) | 0.889 |
| Unstable housing (vs. stable)† | 494 (71%) | 416 (70%) | 78 (72%) | 0.700 |
| Currently receiving opioid substitution therapy | 89 (13%)# | 76 (13%)# | 13 (12%) | 0.799 |
| Syringe borrowing† | 271 (39%) | 221 (37%) | 50 (46%) | 0.081 |
| Crack use† | 195 (28%) | 178 (30%) | 17 (16%) | 0.002 |
| Cocaine injecting† | 556 (80%) | 469 (79%) | 87 (81%) | 0.776 |
| Heroin injecting† | 490 (70%) | 418 (71%) | 72 (67%) | 0.397 |
| Speedball injecting† | 264 (38%) | 222 (38%) | 42 (39%) | 0.794 |
| Methamphetamine injecting† | 51 (7%) | 44 (7%) | 7 (6%) | 0.723 |
| Other opioid injecting*† | 102 (15%) | 95 (16%) | 7 (6%) | 0.009 |
| ARYS cohort | 65 (9%) | 58 (10%) | 7 (6%) | 0.273 |
| Recent HCV seroconversion | 73 (10%) | 60 (10%) | 13 (12%) | 0.556 |
| HIV infection† | 165 (24%) | 128 (22%) | 37 (34%) | 0.005 |
| HCV subtype | 0.039 | |||
| 1a | 347 (50%) | 307 (52%) | 40 (37%) | |
| 1b | 44 (6%) | 36 (6%) | 8 (7%) | |
| 2b | 52 (7%) | 43 (7%) | 9 (8%) | |
| 3a | 256 (37%) | 205 (35%) | 51 (47%) |
Other opioid injecting: hydromorphone, morphine and/or methadone,
In last six months.
Missing data: ^ two participants;
4 participants. Unstable Housing refers to living in a shelter, hostel, treatment center, or on the street
Phylogenetic analysis
As previously reported (5), phylogenetic analysis was performed on participants with 1a, 1b, 2b and 3a infection, comprising 95% of participants (699 of 732) with an identifiable subtype. Among those analysed, 22% (n = 150) were younger (aged <27 years), while 10% (n = 73) had recent HCV seroconversion. A total of 108 (15%) participants had close genetic relatedness, with 87 in pairs (2 participants, n = 44 pairs, 12%) and 21 in clusters (3 participants, n = 6 clusters, 3%) if the most common recent ancestor of the participants (inferred) was less than 5 years in the past. Pair/cluster sizes ranged from two to six participants (median: 2).
Latent class estimation
Preliminary latent class estimation identified a three class solution as optimal solution for underlying drug use behaviours according to the BIC, aBIC and cAIC model fit statistics (Table 2). The AIC statistic favoured a five class solution; while model fit statistics for the four class solution were generally close to the more favoured model. The bootstrap likelihood ratio test was >0.05 when comparing the four class solution with the three class solution, suggesting the latter provided a better fit. The final model containing the distal outcome (phylogenetic clustering) as a covariate improved model stability for the four class solution from 29% to 99%. Considering the model fit statistics and substantive knowledge of drug use epidemiology, the four class solution in the final model was selected as it provided the best description of underlying drug use patterns in this population (Figure 2).
Table 2.
Indicators of fit of models with one through eight latent classes of drug use among people who use drugs in Vancouver, Canada (n=699).
| Class | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Log Likelihood | −2214.33 | −2137.94 | −2100.24 | −2092.03 | −2084.87 | −2079.57 | −2077.13 | −2075.38 |
| G2 | 288.64 | 135.87 | 60.48 | 44.06 | 29.74 | 19.12 | 14.25 | 10.75 |
| AIC | 300.64 | 161.87 | 100.48 | 98.06 | 97.74 | 101.12 | 110.25 | 120.75 |
| BIC | 327.94 | 221.02 | 191.47 | 220.9 | 252.43 | 287.66 | 328.63 | 370.98 |
| cAIC | 333.94 | 234.02 | 211.47 | 247.9 | 286.43 | 328.66 | 376.63 | 425.98 |
| aBIC | 308.89 | 179.74 | 127.97 | 135.17 | 144.47 | 157.48 | 176.22 | 196.34 |
| Entropy | 1 | 0.77 | 0.60 | 0.72 | 0.67 | 0.70 | 0.71 | 0.71 |
| Bootstrap LRT | - | 0.01 | 0.01 | 0.07 | 0.14 | |||
| Stability | 100 | 53 | 100 | 29 | 83 | 37 | 31 | 1 |
Figure 2.
Based on the item response probabilities, the four classes were named according to the relative behaviours of the participants (Table 3). The group with the highest class probability [0.31, Standard error (SE) 0.05] was termed “Opioid and cocaine injecting”, while the class probability for “Cocaine injecting” was 0.28 (SE 0.05). A similar class probability was found for “Heroin with current OST” (0.23, SE 0.06), and the lowest class probability was the “Crack cocaine smoking” group (0.18, SE 0.06).
Table 3.
Class and item probabilities from the 4-class latent class analysis solution of drug use among people who use drugs in Vancouver, Canada (n=699)
| Latent class |
Class probabilities (SE) |
Item probabilities (SE)
|
|||||
|---|---|---|---|---|---|---|---|
| Current OST |
Heroin injecting+ |
Cocaine injecting+ |
Speedball injecting+ |
Other opioid injecting*+ |
Crack cocaine smoking+ |
||
| 1. Heroin injecting with OST | 0.23 (0.06) | 0.32 (0.07) | 1.00 (0.01) | 0.59 (0.07) | 0.09 (0.08) | 0.20 (0.06) | 0.17 (0.10) |
| 2. Cocaine injecting | 0.28 (0.05) | 0.02 (0.02) | 0.22 (0.11) | 0.88 (0.04) | 0.10 (0.03) | 0.00 (0.01) | 0.10 (0.07) |
| 3. Crack cocaine smoking | 0.18 (0.06) | 0.09 (0.07) | 0.56 (0.18) | 0.60 (0.09) | 0.20 (0.07) | 0.20 (0.09) | 0.64 (0.13) |
| 4. Opioid and cocaine injecting | 0.31 (0.05) | 0.10 (0.02) | 1.00 (0.01) | 0.99 (0.03) | 0.96 (0.10) | 0.21 (0.03) | 0.30 (0.04) |
Conditional probabilities in bold refer to one-third larger than overall and italic one-third smaller than overall.
Other opioid injecting: hydromorphone, morphine and/or methadone,
in the last six months, OST: opioid substitution therapy
Distal outcome analysis
Fifteen percent of participants (n=108 of 699) were previously found to have close viral genetic relatedness (phylogenetic clustering) (5). Latent drug use class was related to odds of phylogenetic cluster membership when assessed by an inclusive classify/analyse methodology (Table 4).
Table 4.
Inclusive classify/analyse logistic adjusted logistic regression analysis from the 4-class latent class analysis solution of drug use among people who use drugs in Vancouver, Canada (n=699)
| No cluster (n=591) |
Cluster (n=108) |
Adjusted logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | P value | |||
| Latent class | ||||
| Heroin injecting with OST | 175 (30%) | 23 (21%) | Ref | - |
| Cocaine injecting | 120 (20%) | 48 (44%) | 3.06 (1.73, 5.42) | <0.001 |
| Crack cocaine smoking | 109 (18%) | 1 (<1%) | 0.06 (0.01, 0.48) | 0.008 |
| Opioid and cocaine injecting | 187 (32%) | 36 (33%) | 1.36 (0.76, 2.42) | 0.299 |
| Age (continuous) | 0.94 (0.91, 0.96) | <0.001 | ||
| HIV infection | 128 (22%) | 37 (34%) | 1.46 (0.9, 2.37) | 0.127 |
| Recent HCV infection | 60 (10%) | 13 (12%) | 1.37 (0.65, 2.9) | 0.404 |
| ARYS cohort | 58 (8%) | 7 (6%) | 0.47 (0.18, 1.25) | 0.130 |
Other opioid injecting: hydromorphone, morphine and/or methadone,
in the last six months, OST: opioid substitution therapy.
Note: Proportions of participants not in a cluster or in a cluster relate to column total
In the inclusive classify/analyse logistic regression model, relative to those in the heroin injecting with current OST group, those in the cocaine injecting class had greater odds of phylogenetic cluster membership [odds ratio (OR): 3.04; 95% CI: 1.76, 5.27) while participants in the crack cocaine smoking group had reduce odds (OR: 0.07; 95% CI: 0.01, 0.52). There was no difference between the reference group and those in the opioid and cocaine injecting group. After adjusting for age and HIV infection, compared to the group defined by opioid and cocaine injecting, the odds of phylogenetic cluster membership was greater in the cocaine injecting group [adjusted OR (aOR): 3.05; 95% CI: 1.76, 5.27] and lower in the crack cocaine smoking group (aOR: 0.07; 95% CI: 0.01, 0.52 ).
DISCUSSION
This study characterises the association between drug use patterns and HCV phylogenetic clustering among two cohorts of people who use drugs recruited between 1996 and 2012 in Vancouver, Canada. Latent class analysis identified four distinct patterns of drug use that included: 1) cocaine injecting, 2) opioid and cocaine injecting, 3) crack cocaine smoking, and 4) heroin injecting and currently receiving OST. After adjusting for HIV infection and age, membership in the cocaine injecting class was independently associated with an increased likelihood of HCV phylogenetic clustering, while membership in the crack cocaine smoking class was associated with a decreased likelihood of phylogenetic clustering. This is the first study to combine phylogenetic clustering with latent class analysis to describe the dynamics of HCV transmission. As such, this study advances the understanding of the transmission of HCV among people who use drugs, and provides a robust methodology for understanding populations at greater risk of viral transmission.
Differences in study recruitment and indicator variables for inclusion prohibit direct comparison of latent class analyses outcomes across studies involving PWUD. However, similar to the current study, distinct classes of people who use drugs have been identified using latent class analysis in Baltimore (10, 11), San Diego (25), Montreal (26), and in other parts of Canada (27) and the USA (13). Within a multi-site cohort study of illegal opioid and other drug users outside of treatment in five Canadian cities, Monga et al. identified subgroups of participants that were composed mainly of Tylenol 3 or 4/benzodiazepine use, non-injection use of both heroin and crack, and injection of both heroin and cocaine (28). Similarly, Roy et al. identified distinct groups of people who use drugs, including those that smoke cocaine, inject cocaine or inject cocaine and opioids (26). Collectively, the application of latent class analyses to characterise drug use patterns has consistently identified participants with varying levels of risk for HIV and HCV infection, and may be important in the implementation of targeted public health interventions.
To our knowledge, this study is among the first to demonstrate that patterns of polydrug use were associated with differing risk of HCV phylogenetic clustering. Compared to participants in the heroin injection and currently receiving OST class, those in the cocaine injecting class had greater odds of HCV infection being closely genetically related to another participant. This is consistent with data demonstrating that acquisition of HCV is associated with recent injecting of cocaine, perhaps a function of the increased number of injections per day compared to heroin (29, 30). Within the VIDUS study, cocaine injecting was associated with a shorter time to HCV seroconversion (31). In the current study, the cocaine injecting class had the lowest probability of participants currently receiving OST, a harm reduction measure known to prevent HCV infection (32–34). The high number of daily injections associated with cocaine injecting and the absence of evidence-based pharmacotherapies for cocaine use disorder in this subgroup may explain the association between membership in the cocaine injecting latent class and HCV phylogenetic clustering in this cohort.
In the current study, the crack cocaine smoking class had significantly reduced odds of phylogenetic clustering. Injecting heroin and cocaine remained high in this group; however, smoking crack cocaine had the highest probability of use and injecting speedball was low. Cessation of injecting is effective at eliminating the risk of HCV acquisition (35). In addition, exclusive crack cocaine smoking is associated with cessation of injecting (36). A significant increase in crack cocaine smoking was seen in the VIDUS cohort from 1996 to 2012 (31), and previous data has demonstrated frequent cocaine injecting and methamphetamine injecting as being independent predictors of crack cocaine initiation (37). While crack cocaine smoking is associated with serious health and social problems including prevalent HCV infection (38–41), a reduction in injecting behaviour may mitigate onward transmission of HCV among this population. Similar to needle and syringe programs, improving access to safer crack-smoking equipment and facilities would likely reduce harms associated with both drug injecting and smoking and improve individual and community health outcomes (39, 42, 43).
This study has a number of limitations. Although data collection and study instruments were harmonized, the VIDUS and ARYS cohorts had differences in inclusion and exclusion criteria. To help mitigate potential cohort effects, regression-based analyses were adjusted for cohort of enrolment. Second, the VIDUS and ARYS studies are not random samples of the eligible population and the findings may not be generalizable to the broader Vancouver drug-using population or other urban settings. There also may be un-sampled additional parties involved in the transmission cluster and direction of HCV transmission cannot be determined. In addition, estimates of phylogenetic relationships refer only to the viral populations identified at the time of sampling. While reinfection or superinfection with other viruses may occur through time, their identity would not be known at the time phylogenetic inference. Third, the utilisation of PCR to amplify HCV RNA may introduce bias in the selection of participants given the nature of the methodology and the potential to insufficiently detect variant strains of the virus (including mixed infections). Care should be taken when interpreting the outcomes of latent class analysis, particularly when concluding that the classes identified represent actual individuals in the population. Nonetheless, latent class analysis provides a useful mechanism for representing the heterogeneity of factors across the population. Lastly, information on all behaviours were collected by self-report and may be subject to response biases.
The data from the current study suggest that people with ongoing cocaine injecting should be targeted with respect to treatment and prevention activities. Mathematical modelling of targeted HCV treatment and prevention interventions suggest that combining high-capacity needle and syringe program access with OST and DAA HCV therapies would significantly reduce HCV acquisition in the future (44, 45). Additionally, modelling suggests that expanding treatment to immediate injecting partners in a network of people who inject drugs (e.g. “bring a friend approach”) is favourable to a random treatment strategy (46). The outcomes from the current study should be used to inform future mathematical modelling studies and the development of treatment and prevention interventions.
In conclusion, this study of people who use drugs in Vancouver demonstrates that there are identifiable patterns of poly-drug use, with differing degrees of HCV phylogenetic clustering seen in the groups. Latent class analysis provides a framework to better understand the complex dynamics of drug use, particularly when multiple drug types are used. An enhanced understanding of HCV transmission dynamics should be used to inform future treatment and prevention strategies, particularly towards those at greatest risk of transmission.
Acknowledgments
The authors thank the study participants for their contribution to the research, current and past researchers and staff, with particular thanks to Z. Brumme, S. Dobrer, G.J. Dore, J.B. Joy, F. Lamoury, G. Magiorkinis, M-J Milloy, V. Montoya, O.G. Pybus, A. Olmstead, C. and Woods for their assistance with this project.
Conflict of interest/Disclosures: JG is a consultant/advisor and has received research grants from Abbvie, Bristol Myers Squibb, Gilead Sciences and Merck. JM has received grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. MK receives research grants from Merck, Gen-Probe (Hologic), Siemens and Roche.
Financial Support: Funding for this study was provided by the National Institutes of Health (NIH) (VIDUS- U01DA038886; R03DA033851-01) and the Canadian Institutes of Health (CIHR) (HHP-67262, RAA-79918, HES-115697; MOP-125948). NIH and CIHR had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JG is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship. VDL is supported by a Scholar Award from the Michael Institute for Health Research and a New Investigator Award from CIHR. KD is supported by a Michal Smith Foundation for Health Research/St. Paul’s Hospital-Providence Health Care Career Scholar Award and a Canadian Institutes of Health Research New Investigator Award. M-JM is supported by fellowships from the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research. BDLM is supported by an Avenir Award (No. 1DP2DA040236) from the National Institute on Drug Abuse. KH is supported by the CIHR New Investigator Award (MSH-141971). JM is supported by the British Columbia Ministry of Health and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse (NIDA), at the US National Institutes of Health (NIH). JM has also received financial support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President’s Emergency Plan for AIDS Relief (PEPfAR), UNICEF, the University of British Columbia, Simon Fraser University, Providence Health Care and Vancouver Coastal Health Authority.
List of abbreviations in the order of appearance
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- VIDUS
Vancouver Injection Drug Users Study
- ARYS
At Risk Youth Study
- RNA
ribonucleic acid
- PCR
polymerase chain reaction
- E2
envelope-2
- MCMC
Markov Chain Monte Carlo
- tMRCA
time to most common recent ancestor
- OST
opioid substitution therapy
- AIC
Akaike Information Criterion
- BIC
Bayesian Information Criterion
- cAIC
consistent AIC
- aBIC
adjusted BIC
- LRT
likelihood ratio test
References
- 1.Hagan H, Pouget ER, Williams IT, Garfein RL, Strathdee SA, Hudson SM, et al. Attribution of Hepatitis C Virus Seroconversion Risk in Young Injection Drug Users in 5 US Cities. Journal of Infectious Diseases. 2010;201(3):378–85. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. The Lancet. 2011;378(9791):571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, et al. Increases in Hepatitis C Virus Infection Related to Injection Drug Use Among Persons Aged ≤30 Years — Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Jacka B, Applegate T, Krajden M, Olmstead A, Harrigan PR, Marshall BDL, et al. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology (Baltimore, Md) 2014;60(5):1571–80. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacka B, Applegate T, Poon AF, Raghwani J, Harrigan PR, DeBeck K, et al. Transmission of hepatitis C virus infection among younger and older people who inject drugs in Vancouver, Canada. Journal of Hepatology. 2016;64(6):1247–55. doi: 10.1016/j.jhep.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, et al. Patterns and Characteristics of Hepatitis C Transmission Clusters among HIV-Positive and HIV-Negative Individuals in the Australian Trial in Acute Hepatitis C. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2011;52(6):803–11. doi: 10.1093/cid/ciq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, et al. Evidence of a large, international network of international hepatitis C virus transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136(5):1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham E, Jacka B, DeBeck K, Applegate TA, Harrigan PR, Krajden M, et al. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug and alcohol dependence. 2015;152:272–6. doi: 10.1016/j.drugalcdep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noor SB, Ross M, Lai D, Risser J. Use of Latent Class Analysis Approach to Describe Drug and Sexual HIV Risk Patterns among Injection Drug Users in Houston, Texas. AIDS Behav. 2014;18(3):276–83. doi: 10.1007/s10461-014-0713-3. English. [DOI] [PubMed] [Google Scholar]
- 10.Kuramoto SJ, Bohnert ASB, Latkin CA. Understanding subtypes of inner-city drug users with a latent class approach. Drug and Alcohol Dependence. 2011;118(2–3):237–43. doi: 10.1016/j.drugalcdep.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell PT, Mancha BE, Petras H, Trenz RC, Latimer WW. Latent classes of heroin and cocaine users predict unique HIV/HCV risk factors. Drug & Alcohol Dependence. 2012;122(3):220–7. doi: 10.1016/j.drugalcdep.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson C-A, Weng CX, French T, Anderson BJ, Nemeth C, McNutt L-A, et al. Substance Abuse Treatment Utilization, HIV Risk Behaviors, and Recruitment Among Suburban Injection Drug Users in Long Island, New York. AIDS Behav. 2014;18(3):305–15. doi: 10.1007/s10461-013-0512-2. [DOI] [PubMed] [Google Scholar]
- 13.Wu L-T, Ling W, Burchett B, Blazer DG, Yang C, Pan J-J, et al. Use of item response theory and latent class analysis to link poly-substance use disorders with addiction severity, HIV risk, and quality of life among opioid-dependent patients in the Clinical Trials Network. Drug & Alcohol Dependence. 2011;118(2):186–93. doi: 10.1016/j.drugalcdep.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avila D, Keiser O, Egger M, Kouyos R, Böni J, Yerly S, et al. Social Meets Molecular: Combining Phylogenetic and Latent Class Analyses to Understand HIV-1 Transmission in Switzerland. American Journal of Epidemiology. 2014;179(12):1514–25. doi: 10.1093/aje/kwu076. [DOI] [PubMed] [Google Scholar]
- 15.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of Hepatitis C Virus Infection Among People Who Inject Drugs Through Treatment as Prevention: Feasibility and Future Requirements. Clinical Infectious Diseases. 2013;57(7):1014–20. doi: 10.1093/cid/cit377. [DOI] [PubMed] [Google Scholar]
- 17.Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS (London, England) 1997 Jul;11(8):F59–65. doi: 10.1097/00002030-199708000-00001. Epub 1997/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. doi: 10.1186/1477-7517-3-18. Epub 2006/05/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamoury F, Jacka B, Bartlett S, Bull RA, Wong A, Amin J, et al. The influence of Hepatitis C Virus genetic region on phylogenetic clustering analysis. Plos One. 2015 doi: 10.1371/journal.pone.0131437. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods CK, Brumme CJ, Liu TF, Chui CK, Chu AL, Wynhoven B, et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. Journal of clinical microbiology. 2012;50(6):1936–42. doi: 10.1128/JCM.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RR, Parker J, Lemey P, Salemi M, Katzourakis A, Pybus OG. The mode and tempo of hepatitis C virus evolution within and among hosts. BMC Evolutionary Biology. 2011;11(1):1–10. doi: 10.1186/1471-2148-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanza ST, Rhoades BL. Latent Class Analysis: An Alternative Perspective on Subgroup Analysis in Prevention and Treatment. Prevention science : the official journal of the Society for Prevention Research. 2013;14(2):157–68. doi: 10.1007/s11121-011-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziak JJ, Lanza ST, Tan X. Effect Size, Statistical Power and Sample Size Requirements for the Bootstrap Likelihood Ratio Test in Latent Class Analysis. Structural equation modeling : a multidisciplinary journal. 2014;21(4):534–52. doi: 10.1080/10705511.2014.919819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray BC, Lanza ST, Tan X. Eliminating Bias in Classify-Analyze Approaches for Latent Class Analysis. Structural equation modeling : a multidisciplinary journal. 2015;22(1):1–11. doi: 10.1080/10705511.2014.935265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth AM, Armenta RA, Wagner KD, Roesch SC, Bluthenthal RN, Cuevas-Mota J, et al. Patterns of Drug Use, Risky Behavior, and Health Status Among Persons Who Inject Drugs Living in San Diego, California: A Latent Class Analysis. Substance use & misuse. 2015;50(2):205–14. doi: 10.3109/10826084.2014.962661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy É, Richer I, Arruda N, Vandermeerschen J, Bruneau J. Patterns of cocaine and opioid co-use and polyroutes of administration among street-based cocaine users in Montreal, Canada. International Journal of Drug Policy. 2012;24(2):142–9. doi: 10.1016/j.drugpo.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Patra J, Fischer B, Maksimowska S, Rehm J. Profiling poly-substance use typologies in a multi-site cohort of illicit opioid and other drug users in Canada-a latent class analysis. Addiction Research & Theory. 2009;17(2):168–85. [Google Scholar]
- 28.Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El-Guebaly N, et al. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug & Alcohol Dependence. 2006;88(1):1–8. doi: 10.1016/j.drugalcdep.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Roy E, Alary M, Morissette C, Leclerc P, Boudreau JF, Parent R, et al. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. International Journal of Std & Aids. 2007;18(1):23–7. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 30.Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montréal. Journal of Psychiatry and Neuroscience. 2004;29(1):40–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Grebely J, Lima VD, Marshall BDL, Milloy MJ, DeBeck K, Montaner J, et al. Declining Incidence of Hepatitis C Virus Infection among People Who Inject Drugs in a Canadian Setting, 1996–2012. PLoS ONE. 2014;9(6):e97726. doi: 10.1371/journal.pone.0097726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Medical Journal of Australia. 2014;201(6):326–9. doi: 10.5694/mja13.00153. [DOI] [PubMed] [Google Scholar]
- 33.Turner KME, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–88. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 34.Nolan S, Dias Lima V, Fairbairn N, Kerr T, Montaner J, Grebely J, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction. 2014;109(12):2053–9. doi: 10.1111/add.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection Drug Use and Hepatitis C Virus Infection in Young Adult Injectors: Using Evidence to Inform Comprehensive Prevention. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S32–S8. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox J, Maurais E, Hu L, Moodie EEM, Law S, Bozinoff N, et al. Correlates of drug use cessation among participants in the Canadian HIV-HCV Co-infection Cohort. Drug and Alcohol Dependence. 2014;137:121–8. doi: 10.1016/j.drugalcdep.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Werb DAN, DeBeck K, Kerr T, Li K, Montaner J, Wood E. Modelling crack cocaine use trends over 10 years in a Canadian setting. Drug and Alcohol Review. 2010;29(3):271–7. doi: 10.1111/j.1465-3362.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBeck K, Kerr T, Li K, Fischer B, Buxton J, Montaner J, et al. Smoking of crack cocaine as a risk factor for HIV infection among people who use injection drugs. Canadian Medical Association Journal. 2009;181(9):585–9. doi: 10.1503/cmaj.082054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivsins A, Roth E, Nakamura N, Krajden M, Fischer B. Uptake, benefits of and barriers to safer crack use kit (SCUK) distribution programmes in Victoria, Canada—A qualitative exploration. International Journal of Drug Policy. 2011;22(4):292–300. doi: 10.1016/j.drugpo.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Jauffret-Roustide M, Le Strat Y, Couturier E, Thierry D, Rondy M, Quaglia M, et al. A national cross-sectional study among drug-users in France: epidemiology of HCV and highlight on practical and statistical aspects of the design. BMC Infectious Diseases. 2009;9:113. doi: 10.1186/1471-2334-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickman M, Hope V, Brady T, Madden P, Jones S, Honor S, et al. Hepatitis C virus (HCV) prevalence, and injecting risk behaviour in multiple sites in England in 2004. Journal of Viral Hepatitis. 2007;14(9):645–52. doi: 10.1111/j.1365-2893.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 42.Shannon K, Ishida T, Morgan R, Bear A, Oleson M, Kerr T, et al. Potential community and public health impacts of medically supervised safer smoking facilities for crack cocaine users. Harm Reduction Journal. 2006;3:1. doi: 10.1186/1477-7517-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leonard L, DeRubeis E, Pelude L, Medd E, Birkett N, Seto J. “I inject less as I have easier access to pipes”: Injecting, and sharing of crack-smoking materials, decline as safer crack-smoking resources are distributed. International Journal of Drug Policy. 2008;19(3):255–64. doi: 10.1016/j.drugpo.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination Interventions to Prevent HCV Transmission Among People Who Inject Drugs: Modeling the Impact of Antiviral Treatment, Needle and Syringe Programs, and Opiate Substitution Therapy. Clinical Infectious Diseases. 2013;57(suppl 2):S39–S45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lima VD, Rozada I, Grebely J, Hull M, Lourenco L, Nosyk B, et al. Are Interferon-Free Direct-Acting Antivirals for the Treatment of HCV Enough to Control the Epidemic among People Who Inject Drugs? PLoS ONE. 2015;10(12):e0143836. doi: 10.1371/journal.pone.0143836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellard M, Rolls DA, Sacks-Davis R, Robins G, Pattison P, Higgs P, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–70. doi: 10.1002/hep.27403. [DOI] [PubMed] [Google Scholar]



