Abstract
Obese and type 2 diabetic (T2DM) patients have a high prevalence of nonalcoholic fatty liver disease (NAFLD). NAFLD is a continuum of chronic liver diseases ranging from benign hepatosteatosis to nonalcoholic steatohepatitis (NASH), cirrhosis and primary hepatocellular cancer (HCC). Because of its strong association with the obesity epidemic, NAFLD is rapidly becoming a major public health concern worldwide. Surprisingly, there are no FDA approved NAFLD therapies; and current therapies focus on the co-morbidities associated with NAFLD, namely, obesity, hyperglycemia, dyslipidemia, and hypertension. The goal of this review is to provide background on the disease process, discuss human studies and preclinical models that have examined treatment options. We also provide an in-depth rationale for the use of dietary ω3 polyunsaturated fatty acid (ω3 PUFA) supplements as a treatment option for NAFLD. This focus is based on recent studies indicating that NASH patients and preclinical mouse models of NASH have low levels of hepatic C20-22 ω3 PUFA. This decline in hepatic PUFA may account for the major phenotypic features associated with NASH, including steatosis, inflammation and fibrosis. Finally, our discussion will address the strengths and limitations of ω3 PUFA supplements use in NAFLD therapy.
1. Introduction
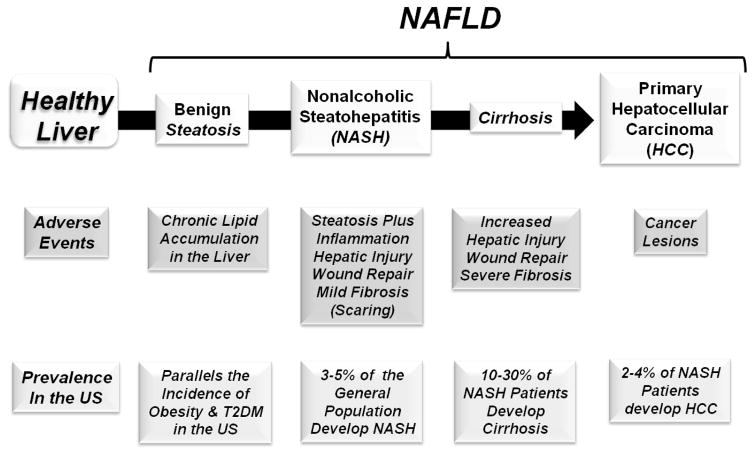
Nonalcoholic fatty liver disease (NAFLD) is a continuum of chronic liver diseases ranging from benign hepatosteatosis to nonalcoholic steatohepatitis (NASH), cirrhosis and primary hepatocellular cancer (HCC) (Lauby-Secretan, 2016; Vernon et al., 2011) (Fig 1). NAFLD is the most common chronic fatty liver disease in developed countries (Bellentani et al., 2010); and is strongly associated with obesity (Cohen et al., 2011; Farrell, 2006; Lauby-Secretan, 2016). The Centers for Disease Control and Prevention estimates that ~80 million adults (2015a) and ~13 million children (2015b) in the US are obese (BMI ≥ 30); and all have elevated risk for developing NAFLD. Considering its strong association with obesity, NAFLD is rapidly becoming a major public health problem worldwide (Chalasani et al., 2012a; Kleiner et al., 2005; Loomba, 2013).
Figure 1. NAFLD is a continuum of hepatic fatty liver diseases.
See text for description.
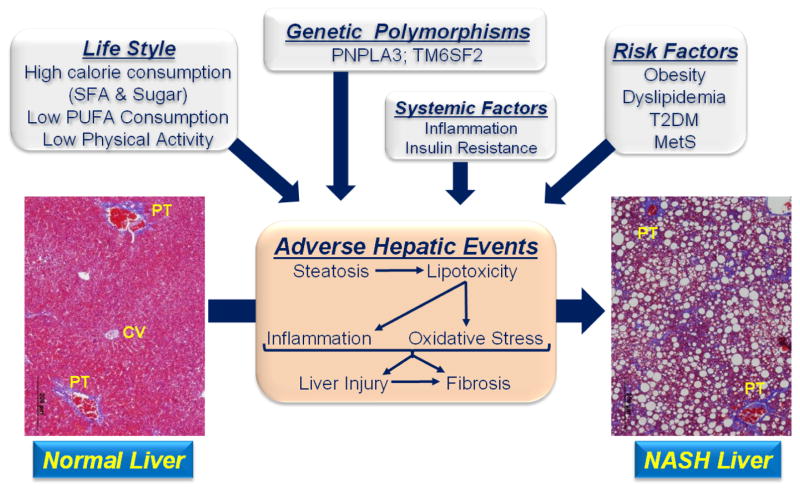
NASH, the progressive form of NAFLD was first described in 1980 (Ludwig, 1980). Multiple factors contribute to the onset of NAFLD and its progression to NASH, including lifestyle, gender, ethnicity, genetic polymorphisms (Browning, 2004; Lian et al., 2016; Park, 2008; Smagris et al., 2015; Smagris et al., 2016), health status and systemic factors linked to inflammation and metabolic control (Browning et al., 2004; Cohen et al., 2011; Kanth, 2016; Romeo et al., 2008; Smagris et al., 2015) (Fig 2). The top four risk factors for NAFLD are obesity, dyslipidemia, type 2 diabetes (T2DM) and metabolic syndrome (MetS) (Alberti et al., 2005; Chalasani et al., 2012b). The prevalence of steatosis and NASH in the obese and T2DM populations is high (≥60%) (Prashanth et al., 2009). T2DM and chronic liver disease are also risks factors for other chronic diseases, such as cardiovascular disease (Adams et al., 2005; Ekstedt et al., 2006; Sessa, 2017; Soderberg et al., 2010). NASH patients often have elevated blood lipids (dyslipidemia) and metabolic disturbances in cholesterol metabolism similar to patients with coronary artery disease (Kerr, 2012; Lonardo, 2015; Min et al., 2012; Min, 2012; Misra, 2009; Wouters et al., 2008).
Figure 2. Factors contributing to the onset and progression of NASH.
Histology from livers of Ldlr −/− mice fed a western diet for 30 wks (Lytle, 2017). Livers were fixed in formalin, imbedded sliced and stained with trichrome. Steatosis in the NASH liver appears as white circles, while fibrosis appears as blue branching strands of extracellular matrix. CV, central vein; PT, portal track; magnification = 4 x.
Ten to 30% of patients with benign steatosis develop NASH; and NASH patients have higher mortality rates than individuals with benign steatosis; and both are higher than the general population. Twenty to 30% of NASH patients progress to cirrhosis; and over a 10 year period, cirrhosis and liver related deaths occur in 20% and 12% of NASH patients, respectively (Heimbach, 2014; McCullough, 2004). Cirrhosis is a risk factor for HCC (Cohen et al., 2011; McCullough, 2006), the 5th and 7th most common cancer in men and women, respectively (Bosetti, 2014; Forner et al., 2012; Sanyal et al., 2010). Cirrhosis resulting from NASH is anticipated to be the leading cause of liver transplantation in the United States by 2020. Health care costs linked to NASH are predicted to reach $35 billion by 2025 (McCollough, 2011).
Government, academia and the pharmaceutical industry have made significant investments in the development of safe and efficacious treatments for NAFLD. Current therapies, however, rely on lifestyle modifications (diet & exercise) and treating the co-morbidities associated with NAFLD (Banini, 2017). The goal of this review is to provide background on the disease process and briefly discuss human studies and preclinical models that have examined the onset and progression of NAFLD as well as specific treatment options. Clinical and preclinical studies have established that NAFLD is associated with significant changes in the type and abundance of hepatic lipids. While hepatic saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) increase, ω3 and ω6 PUFA decrease (Arendt, 2015, 2009; Depner, 2013a, b; Petit et al., 2012; Puri et al., 2007; Zheng et al., 2012). Preclinical studies have established that the NAFLD-associated decline in hepatic ω3 PUFA is more severe than the decline in ω6 PUFA (Depner, 2013a; Lytle, 2016, 2017). These changes in lipids are also associated with major changes in urinary and hepatic oxidized lipids (oxylipins) that are generated by enzymatic and non-enzymatic mechanisms (Depner, 2012, 2013b). Such changes in the type and abundance of hepatic lipids may set the stage for the adverse events associated with NAFLD. Our discussion will include an in-depth analysis of how changes in hepatic PUFA affect NAFLD markers, i.e., steatosis, inflammation and fibrosis. We will also provide a brief review of clinical and preclinical studies assessing the impact of dietary ω3 PUFA on NAFLD. Finally, we will address the strengths and limitations of using ω3 PUFA supplementation in NAFLD therapy.
2. Why livers store excess fat
Livers store more fat when there is a breakdown of the homeostatic mechanisms controlling lipid metabolism. NAFLD is characterized by the chronic and excessive accumulation of neutral lipids [triglycerides (TAG), cholesterol esters (CE)] in liver cells (Angulo and Lindor, 2002; Neuschwander-Tetri and Caldwell, 2003) (Fig 1). The key metabolic pathways controlling hepatic lipid metabolism include de novo lipogenesis (DNL), fatty acid oxidation (FAO), formation, assembly & secretion of very low density lipoproteins (VLDL), TAG catabolism and the uptake of non-esterified fatty acids (NEFA) mobilized from adipose stores.
Parks and colleagues used isotopic methods to determine the source of hepatic lipids in NAFLD patients (Lambert, 2014). Of the TAG in the liver, 59% was derived from NEFA mobilized from adipose tissue, 26% was derived from DNL and 15% was derived from the diet (Donnelly et al., 2005). Mobilization of adipose lipid stores represent the major source of NEFA entering hepatic TAG, particularly in obese patients (Zhang, 2014).
2.a. Lipid Mobilized from Adipose Tissue
Mobilization of adipose tissue lipid involves the catabolism of TAG stored in lipid droplets and the export of NEFA to the circulation. This process is mediated by several proteins, including adipocyte triglyceride lipase (ATGL), comparative gene identification-58 (CGI58), hormone sensitive lipase, monoacylglycerol lipase and perilipin (Brasaemle, 2007; Lass et al., 2011). NEFA released from lipid droplets bind fatty acid binding proteins (FABP) and are exported to the systemic circulation as NEFA where they are transported in association with albumin and other proteins. NEFA enter cells via transporters (e.g., fatty acid transport protein-1, FATP1; cluster of differentiation-36, CD36) or diffusion. Adipocyte TAG catabolism increases in adrenergic stimulation, fasting, exercise and insulin resistance; and decreases with feeding and increases in plasma insulin in healthly individuals.
After entry into cells, NEFA are rapidly converted to fatty acyl-CoAs and assimilated into complex lipids, i.e., neutral (TAG & CE) and membrane lipids. Excess neutral lipid is stored in lipid droplets and contributes to the micro- and macro-steatosis seen in histology of biopsied liver (Fig 2). Obese individuals have a large pool of adipose tissue contributing to blood NEFA. As such, obesity, coupled with T2DM and insulin resistance exacerbates lipid mobilization and NEFA flux from adipose tissue to various tissues, including the liver.
2.b. De Novo Lipogenesis
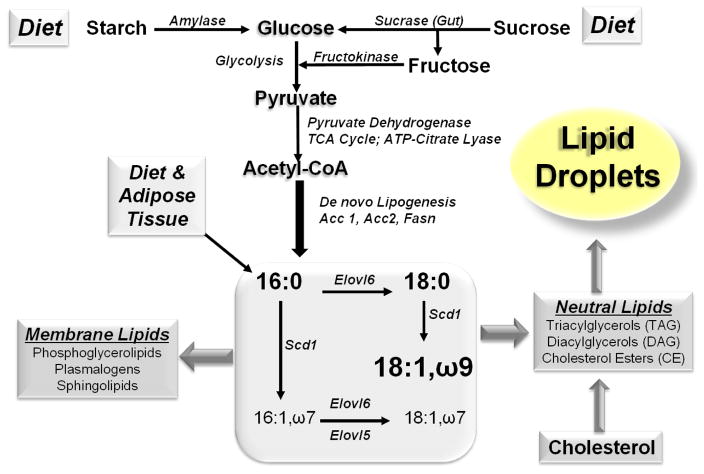
The core enzymes involved in DNL and very long chain fatty acid synthesis include ATP citrate lyase (Acyl), acetyl CoA carboxylase (Acc1), fatty acid synthase (Fasn) and fatty acid elongases (Elovl 1, 3, 5, 6 & 7), while the key enzyme involved in MUFA synthesis is stearoyl CoA desaturase (e.g., Scd1) (Fig. 3). Scd1 is a highly regulated enzyme that desaturates saturated fatty acids (SFA) to form monounsaturated fatty acids (MUFA, e.g., 16:1,ω7; 18:1,ω7 and 18:1,ω9) (Fig 3). Palmitate (16:0), stearate (18:0) and oleate (18:1,ω9) are prominent hepatic SFA and MUFA. Humans with NAFLD have increased DNL and palmitoleic acid (16:1,ω7) reflecting increased MUFA synthesis (Lambert, 2014; Lee, 2015). SFA and MUFA are substrates for complex lipid synthesis, including triglycerides, diacylglycerols, cholesterol esters, phosphoglycerolipids, plasmalogens and sphingolipids. TAG and CE are stored in lipid droplets, while phosphoglycerolipids, plasmalogens and sphingolipids are membrane lipids.
Figure 3. Dietary factors and pathways leading to fat storage.
The diagram illustrates how dietary carbohydrate and fat is assimilated into complex lipids. Lipids are assimilated into membrane lipids or stored in lipid droplets. The diagram does not illustrate the impact of fatty acid oxidation or TAG export as VLDL.
High sugar consumption from sugary beverages, for example, has been implicated as a major contributor to the obesity epidemic (Chung, 2014; Stanhope and Havel, 2008). Glucose induces DNL through insulin-dependent and insulin-independent mechanisms. Increased blood glucose resulting from sugar (glucose-fructose) or starch (glucose) consumption stimulate insulin secretion from pancreatic β-cells. Insulin, acting through its membrane receptor, stimulates the conversion of glucose to neutral lipids which are stored in adipose tissue and liver (Fig 3) (Lim et al., 2010). Feeding rodents diets high in simple sugar (sucrose) and low in PUFA elevates expression of enzymes involved in hepatic DNL and MUFA synthesis (Jump et al., 1994; Landschulz et al., 1994; Liimatta et al., 1994). Diets supplemented with PUFA (ω3 or ω6) suppress pathways involved in DNL (e.g., fatty acid synthase, Fasn) and glycolysis (e.g, L-pyruvate kinase, L-PK) (Filhoulaud, 2013; Jump et al., 1994; Jump et al., 2013; Landschulz et al., 1994; Towle, 2005).
2.c. Insulin and Glucose Control of DNL and MUFA Synthesis
The mechanisms for hormonal and nutrient control of DNL and MUFA synthesis are complex; and involve the regulation of transcription factors that alter the expression of proteins involved in DNL, fatty acid elongation and MUFA synthesis. Key transcription factors controlling DNL and MUFA synthesis include: sterol regulatory element binding protein-1c (SREBP1c) (Hua et al., 1993; Kim and Spiegelman, 1996; Vasandani et al., 2002; Yokoyama et al., 1993); and carbohydrate regulatory element binding protein (ChREBP) & its heterodimer partner, Max-like factor-X (MLX)(Stoeckman et al., 2004; Towle, 2005; Uyeda et al., 2002).
Dietary glucose stimulates insulin secretion from β-cells. Increases in blood insulin activate hepatic cellular signaling pathways through the insulin receptor and downstream signaling components, including insulin receptor substrate (IRS) and several kinases: phosphoinositol-3-kinase (PI3K), rac-β-serine-threonine kinase (Akt2), extracellular signal regulated kinase (Erk1/2) and target of rapamycin-complex 1 (mTorc1). The molecular basis for insulin control of SREBP1 and its regulation of transcription of the Acyl, Acc1, Fasn and Scd1 genes involves increases in transcription of the SREBP1c gene, SREBP1c mRNA stability, proteolytic processing of the precursor of SREBP1c to the mature (nuclear) form of SREBP1c, and inhibition of proteasomal degradation of the nuclear SREBP1 (Botolin et al., 2006; Jump et al., 2013; Li et al., 2012; Owen et al., 2012). As such, insulin increases hepatic nuclear content of SREBP1c, which stimulates transcription of genes involved in fatty acid synthesis. The net result is increased production of long chain SFA and MUFA (Jump et al., 2013).
The second key transcription factor complex controlling DNL and MUFA synthesis is the ChREBP/MLX heterodimer. Glucose functions as a feed-forward regulator of DNL through an insulin-independent pathway. Increased hepatic glucose metabolism alters the phosphorylation status of ChREBP leading to increased nuclear abundance of the CHREBP/MLX heterodimer (Baraille, 2015; Davies et al., 2008; Ma et al., 2006; Sakiyama et al., 2008; Uyeda and Repa, 2006). Like SREBP1c, the CHREBP/MLX heterodimer binds promoters of Acc1, Fasn, Scd1, L-pyruvate kinase (L-PK) leading to the increased gene transcription and increased flow of glucose-derived metabolites into SFA and MUFA synthesis (Jump et al., 2013).
Dietary PUFA, in contrast, inhibit hepatic DNL (Jump et al., 2013), an effect first described by Allman and Gibson (Allmann, 1969). Dietary PUFA also inhibit MUFA synthesis (Landschulz et al., 1994) and the elongation and desaturation of PUFA (Wang et al., 2005; Wang et al., 2006). C20-22 ω3 PUFA suppress the expression of the enzymes involved in DNL and MUFA synthesis by targeting mechanisms controlling the nuclear content of SREBP1c and the ChREBP/MLX heterodimer (Botolin and Jump, 2003; Jump, 1993; Jump et al., 2013; Landschulz et al., 1994; Liimatta et al., 1994; Mater et al., 1999; Xu et al., 2006). Interestingly, very long chain dietary ω3 PUFA (C20-22) do not interfere with insulin action or the capacity of insulin to decrease blood glucose. Docosahexaenoic acid (DHA, 22:6,ω3), the major ω3 PUFA accumulating in cells inhibits the phosphorylation of Akt2-S473 site, but not the Akt2-T308 site. The Akt-T308 site is phosphorylated by PDK1, a downstream target of insulin signaling. Akt2-S473, in contrast, is phosphorylated by multiple kinases (Jump et al., 2013). The specific kinase and/or phosphatase involved in DHA control of Akt2-S473 phosphorylation status have not been identified to date. The overall effect of DHA is to lower SREBP1c nuclear content through proteasome and Erk1/2-dependent mechanisms (Botolin et al., 2006). PUFA lower the ChREBP/MLX heterodimer by suppressing the nuclear content of MLX, an obligate heterodimer partner for ChREBP binding to promoters of target genes (Xu et al., 2006). Lowering hepatic nuclear content of SREBP1 and ChREBP/MLX suppresses the transcription of the Acc1, Fasn, Scd1 and L-PK genes leading to the reduced capacity of glucose conversion to SFA and MUFA.
Dietary PUFA also stimulate FAO by binding directly to the peroxisome proliferator activated receptor-α, (PPARα), i.e., a fatty acid-regulated nuclear receptor (Pawar and Jump, 2003; Pawar et al., 2002; Ren et al., 1997; Xu et al., 1999). PPARα regulates gene expression by binding promoters of target genes in association with the retinoid X receptor, i.e., PPARα/RXR. Binding of the PPARα/RXR heterodimeric complex to promoters stimulate the recruitment of co-regulators that are involved in controlling the transcription of the target genes. PUFA bind to and activate PPARα result in increased expression of key enzymes involved in fatty acid oxidation in mitochondria, peroxisomes and microsomes. Representative PPARα/RXR target genes in these 3 compartments include carnitine palmitoyl transferase 1, acyl CoA oxidase and cytochrome P450 4A, respectively (Jump et al., 2013; Ren et al., 1997).
More recent studies have established that dietary PUFA and endogenous production of PUFA regulate TAG catabolism (Tripathy, 2014). Both ω3 or ω6 C20-22 PUFA increase TAG catabolism by increasing hepatic abundance of ATGL (mRNA and protein) and CGI58 (protein). ATGL and CGI58 act together with perilipin, hormone sensitive lipase, monoacylglycerolipase to promote the hydrolysis of TAG to form NEFA and glycerol. While PUFA induction of FAO requires PPARα (Ren et al., 1997), PUFA regulation of TAG catabolism involves PPARβ (Tripathy, 2014). As such, the type and abundance of hepatic fatty acids play critical roles in controlling hepatic DNL & MUFA synthesis as well as FAO & TAG catabolism.
2.d. Diet
NAFLD is a disease of excess calorie consumption (Chung, 2014; Cohen et al., 2011; Leslie, 2015); particularly calories from of carbohydrates and fats (SFA and MUFA). Calorie dense diets like the “cafeteria” or “western” diet are linked to the obesity & NAFLD epidemic (Cordain, 2005; Ferolla, 2015; Malhotra, 2015; Pagliassotti et al., 1996; Pickens et al., 2009). The western diet, for example, is moderately high in fat (saturated and trans-fat), simple sugar (sucrose/fructose) and cholesterol. Excessive consumption of carbohydrate, particularly simple sugars (fructose, glucose and sucrose), leads to hexose conversion to glycerol 3-phosphate and acetyl CoA, substrates for SFA, MUFA and TAG synthesis (Fig 3).
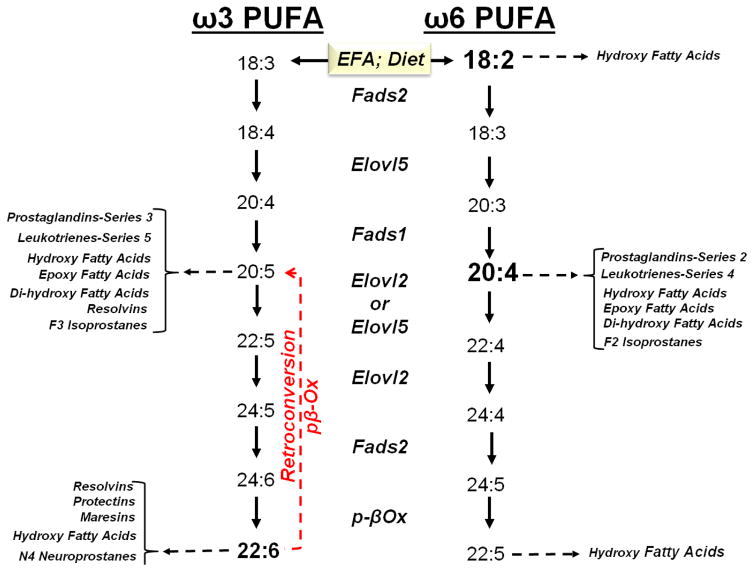
While diets like the western diet may be sufficient in essential fatty acids [EFA: linoleic acid (LA, 18:2,ω6) and α-linolenic acid (ALA, 18:3,ω3)] dietary EFAs typically represent a small fraction of total dietary fat consumed. Moreover, LA is more abundant than ALA in the western diet (Simopoulos, 2002). These EFAs are converted to C20-22 PUFA, a process that requires desaturation (Fads1, Fads2), elongation (Elovl2, Elovl5) and peroxisomal β-oxidation (Fig 4). Expression of these desaturases and elongases is regulated by SREBP1c and PPARα, but not ChREBP/MLX. Peroxisomal β-oxidation is regulated by PPARα (Jump et al., 2013; Wang et al., 2006).
Figure 4. Conversion of essential fatty acids to C20-22 ω3 and ω6 PUFA.
Essential fatty acids include linoleic acid (LA, 18:2,ω6) and α-linolenic acid (ALA, 18:3,ω3). Long chain PUFA are substrates for oxylipin formation. Oxylipins are generated by enzymatic (cyclooxygenase, lipoxygenase & CYP2C/F) and non-enzymatic processes.
ALA is the major ω3 PUFA in the human diet. Its conversion to C20-22 ω3 PUFA in humans, however, is inefficient, ~1–4% of dietary ALA is converted to C20-22 ω3 PUFA. This inefficiency is due, at least in part, to the high level of dietary LA, relative to dietary ALA, and substrate competition at the enzyme (Fads2) level. Interestingly, when DHA is in excess it is retroconverted to eicosapentaenoic acid (EPA, 20:5,ω3) and docosapentaenoic acid (ω3 DPA, 22:5,ω3) through a peroxisomal process called retroconversion (Fig 4) (Sprecher, 2000). A recent report indicates that DHA retroconversion to 20:5,ω3 predominates over Elovl2- and Fads2-mediated synthesis of EPA to tetracosahexaenoic acid (24:6,ω3) (Fig 4) (Park, 2016). While the retroconversion pathway is also inefficient, i.e., ~2% of DHA is converted to EPA in human, this pathway provides a mechanism to increase cellular EPA from dietary DHA (Plourde et al., 2011).
The retroconversion pathway is important physiologically because C20-22 ω3 PUFA are feedback inhibitors of PUFA synthesis; PUFA suppress the expression of an elongase (Elovl5) and desaturases (Fads1, Fads2) involved in PUFA synthesis (Wang et al., 2005). Clinical studies have established that supplementing the human diet with EPA increases blood levels of EPA and ω3 DPA, but not DHA (Allaire, 2016; Itakura et al., 2011). Supplementing the human diet with DHA, however, increases blood levels of DHA, ω3 DPA and EPA (Park, 2016). In fact, the only effective way to significantly increase blood and tissue levels of DHA is to supplement the diet with DHA. However, some (Asztalos, 2016; Wei, 2011), but not all investigators (Allaire, 2016) have reported that supplemental DHA increases blood levels of LDL-cholesterol, a risk factor for cardiovascular disease.
3. Progression of benign steatosis to NASH
While factors promoting benign steatosis to NASH are not fully established (McCullough, 2006; Vernon et al., 2011), aberrant hepatic metabolism associated with the excessive accumulation of lipid in liver is likely a major driver of this process. Ingestion of excess fat and simple sugar promotes lipotoxicity, oxidative stress, formation oxylipins and advance glycation end products (AGEP). Together these product contribute to liver cell damage resulting in hepatic inflammation and fibrosis (Angulo, 2002; Ito, 2007; Jialal, 2014; Neuschwander-Tetri, 2010; Sunny, 2017). Hepatic injury resulting inflammation and oxidative stress is monitored clinically by quantifying blood levels of enzymes (alanine aminotransferase, ALT; aspartate aminotransferase, AST; γ-glutamyl transferase, γGT) released from injured hepatic cells (Cohen et al., 2011; Farrell, 2006; Hashimoto et al., 2011).
In addition to these metabolic events, the progression of benign steatosis to NASH is a multi-cellular process involving hepatocytes, Kupffer cells (resident hepatic macrophage), cholangiocytes (bile duct cells), stellate cells [vitamin A storage and a source of extracellular matrix (ECM)] and inflitrating leukocytes (monocytes, macrophage and T-cells) (Day, 1998; LaBrecque et al., 2014; Schuppan, 2013; Tilg and Moschen, 2010).
3.a. Aberrant Macronutrient Metabolism
3.a.i. Lipotoxicity and Oxidative Stress
Excessive hepatic content of neutral lipid is associated with lipotoxicity (Sunny, 2017). The predominant fatty acids in neutral lipids are SFA and MUFA; these fatty acids are substrates for sphingolipid (ceramide) and diacylglycerol synthesis. Ceramides promote apoptosis, while diacylglycerols activate protein kinase C.
Turnover of hepatic lipid stores resulting from TAG catabolism or increased NEFA mobilized from adipose depots provides substrates for β-oxidation. However, NEFA can overload the capacity of mitochondria to β-oxidize fatty acids. The outcome of mitochondrial overload is an increase in acyl-carnitines, incomplete fatty acid oxidation, impaired ketogenesis and uncoupled mitochondrial respiration. Uncoupling of mitochondrial respiration generates reactive oxygen species (ROS)(Alkhouri, 2009; Sunny, 2017). ROS include highly reactive compounds, such as superoxides, hydrogen peroxide, hydroxy radicals and singlet oxygen (Koves, 2008). These compounds can form adducts with lipids, proteins and nucleic acids and alter cell function.
PUFA are prone to non-enzymatic oxidation. Recent metabolomic studies have established that NASH is associated with increased hepatic levels non-enzymatically derived oxylipins, such as 9,10-hydroxyoctadecenoic acid (9,10-DiHOME), 9- and 13-hydroxyoctadecadienoic acid (9- & 13-HODE). These fatty acids are generated from C18 PUFA. NASH is also associated with a decline in urinary oxylipins derived from C20-22 PUFA, including F2- and F3-isoprostanes and N4-neuroprostanes. The decline in the F2-, F3-isoprostanes & N4-neuroprostanes likely reflects the decline in hepatic (and possibly whole body) C20-22 PUFA. The appearance of non-enzymatically derived oxylipins in cells, blood or urine serve as markers of tissue and whole body oxidative stress. While some reports suggests iso- and neuro-prostanes are bio-active lipids (Milne and Morrow, 2006; Milne et al., 2011; Signorini et al., 2013) their role of in the onset and progression of NAFLD remains to be defined.
3.a.ii. Enzymatically-derived oxylipins
PUFA are substrates for enzymatic oxidation leading to the formation of a large array of bio-active oxylipins including hydroxy-, epoxy-, di- and tri- hydroxy lipids. PUFA assimilated into the sn1 and sn2-positions of membrane phosphoglycerolipids affect membrane fluidity and membrane receptor function (Jump, 2002). Excision of PUFA from membrane phosphoglycerolipids by phospholipases (PLC or PLA2) serve as substrates for oxylipin synthesis (Fig 4) (Calder, 2015; Jump, 2002; Jump et al., 2013). These oxylipins are synthesized by highly regulated enzyme-dependent pathways. The cyclooxygenases (Cox1 & Cox2) are involved in generating prostaglandins, while the lipoxygenases (Lox-5, Lox-12, Lox-15) generate hydroxy-fatty acids. Epoxygenases (Cyp2C, Cyp2J) generate epoxy-fatty acids, while epoxide hydroxylases (EPHX-1 & 2) generate di-hydroxy fatty acids.
Western diet induced NASH is associated with a significant induction of Cox2, Lox5 and Lox15 (Depner, 2013b). Metabolomic analyses established that changes in expression of these enzymes was associated with increased hepatic content of Cox and Lox products, including 6-keto-PGF1α (a non-enzymatic degradation product of prostacyclin I2) and 12-hydroxyeicosatetraenoic acid (12-HETE). In addition, NASH was associated with a decline in hepatic 17, 18-dihydroxyeicosatetraenoic acid (17, 18-DiHETE) and 18-hydroxyeicosapentaenoic acid (18-HEPE). 18-HEPE is a resolvin precursor; resolvins are involved in the resolution of inflammation (Serhan, 2015). 17,18-DiHETE is a product of epoxide hydrolase catalysis of 17,18-epoxyeicosatetraenoic acid (17,18-ETE). 17,18-ETE is the epoxy product of EPA catalyzed by Cyp2C. Changes in epoxy- and dihydroxy PUFA were associated with decreased expression of Cyp2C29, Cyp2C37, but no change in the epoxy-hydrolases (EPHX1 or EPHX2).
Enzymatically derived oxylipins regulate a vast number of pathways that, in many cases, are initiated by ligand (oxylipin) binding to membrane or nuclear receptors that regulate signaling pathways, including the control of inflammation, blood pressure, metabolism and gene expression (Calder, 2015; Farooqui, 2012; Funk, 2001; Jump et al., 2013; Milne et al., 2008; Schuck, 2014; Serhan et al., 2008; Serhan, 2015). Thus, diet-induced changes in hepatic PUFA content, coupled with changes in Cox, Lox, epoxygenase and epoxy hydrolyase activity likely play a major role in the onset and progression of NASH.
In this regard, C18-20 ω6 PUFA are pro-inflammatory, while C20-22 ω3 PUFA are anti-inflammatory fatty acids (Fig 4) (Calder, 2015). These characteristics of ω3 and ω6 PUFA are link to the capacity of PUFA to regulate Cox and Lox expression and the bioactivity of the ω6 PUFA versus ω3 PUFA products of Cox and Lox. When compared to ω6 PUFA, ω3 PUFA are poor substrates for Cox and Lox. Moreover, Cox and Lox products of ω3 PUFA typically have weak activity when compared to ω6 PUFA-derived Cox and Lox products. As such, the relative abundance of ω6 to ω3 PUFA in cells impacts the cellular inflammatory status (Calder, 2015; Simopoulos, 2002; Spadaro, 2008). Studies in humans (Lou, 2014) and mice (Depner, 2013a; Lytle, 2017) indicate that a high blood and hepatic ω6/ω3 PUFA ratio is associated with inflammation and NAFLD progression.
3.a.iii. Advanced glycation end products (AGEP)
AGEP arising from foods or from abberant carbohydrate metabolism affect the onset and progression of NAFLD (Gugliucci, 2017; Leung, 2016). For example, the formation of AGEP arising from fructose has been implicated in fructose-induced NASH (Thornalley, 1996; Wei et al., 2013). A metabolomic analysis of a preclinical model of western diet-induced NASH established that hepatic S-lactoylglutathione was decreased (Depner, 2013b), while hepatic glutathione levels were unaffected. S-lactoylglutathione is a degradation product of methylglyoxal and methylglyoxyl is involved in AGEP formation. AGEP are formed from excessive cellular hexose, triose-phosphate and ketone bodies. AGEP form adducts with proteins and lipids impairing cellular function (Gugliucci, 2017).
This brief overview reveals the diverse pathways for generating metabolites that have adverse effects on liver health status. A key goal of therapy is to minimize the substrate availability and/or the activation of these pathways to attenuate the formation of these harmful metabolites.
3.b. Inflammation
A major driver of hepatic inflammation is the combined action of circulating factors that promote inflammation (Cani et al., 2007; Depner, 2013b; Harte et al., 2010) as well as necroinflammation resulting from liver injury and cell death. Cell death can result from many of the adverse events described above, including lipotoxicity, oxidative stress (ROS and reactive nitrogen species), AGEP, ER-stress, unfolded protein response, ceramide and PKC induced apoptosis.
Circulating factors promoting inflammation can arise from the gut. For example, endotoxinemia is often observed in NASH patients (Higgins et al., 2011). Endotoxin (lipopolysaccharide) is a lipophilic cell wall component of gram negative bacteria. The appearance of microbial components in the blood can significantly affect liver health (Boursier, 2015; Goel, 2014). Multiple factors affect the appearances of gut-derived microbial components in the circulation including bacterial overgrowth and disruption of intestinal tight junctions (Boursier, 2015). Dietary fat also affects the appearance of gut derived microbial components in the circulation. In this instance, bacteria-derived lipophilic components are incorporated into chylomicron and delivered to the lymph and blood (Grunfeld, 2009). The appearance of these factors in the blood activates innate immune responses and elevated levels of circulating chemokines and cytokines (Erridge et al., 2007; Harte et al., 2010; Laugerette et al., 2011).
Circulating levels of chemokines and cytokines are mediated, at least in part, by the activation of toll-like receptors (TLR). Endotoxin is a toll-like receptor-4 (TLR4) agonist, while lipoteichoic acid, a cell wall component of gram positive bacteria, is a TLR2 agonists. These agonists bind CD14, a circulating and membrane associated protein produced in multiple cell types. Ligand-bound CD14 binds to and activates TLR2 and TLR4 signaling pathways. TLR2 heterodimerizes with TLR6 in cell membranes; these heterodimers activate caspase 8 and promote apoptosis. TLR2 also heterodimerizes with TLR1; this heterodimer activates PI3K-, Akt- and IKK/NFκB-linked mechanisms leading to increased nuclear abundance of NFκB. NFκB (p50 and p65) is a key transcription factor controlling the expression of multiple proteins involved in inflammation (Vallabhapurapu and Karin, 2009). CD14-endotoxin-activated TLR4 forms homodimers in the plasma membrane and activate pathways that increase nuclear NFκB and AP1 complexes (Fos & Jun). These events induce the expression of multiple chemokines and cytokines involved in innate immunity (Cengiz, 2015; Lee, 2011; Miura, 2014; Wada, 2016). As such, the appearance of lipophilic microbial components in the blood may play a role in the progression of benign steatosis to NASH.
TLRs are expressed in multiple cell types, including resident hepatic macrophage (Kupffer cells) and cells infiltrating the liver including leukocytes [monocytes, polarized macrophage M1 (inflammatory); M2 (anti-inflammatory), & T-cells]. The presence of these cells in the liver and their activation by systemic factors leads to increased local production of cytokines affecting the disease process. Pro-inflammatory agents derived from M1 macrophage include IL6, IL1β, TNFα, while anti-inflammatory agents derived from M2 macrophage include IL10. M1 and M2 macrophage may also play a role in ECM remodeling since these cells are a source of matrix metalloproteases (Mmp).
3.c. Fibrosis
Fibrosis (scarring) is a tissue repair and remodeling process that is induced in response to cellular injury (Chalasani et al., 2012c). This process involves hepatic stellate cell activation and recruitment of myofibrillar cells that produce ECM, including multiple collagen subtypes [e.g., collagen 1A1, 1A2, 2A; Col1A1 is the primary collagen subtype produced in human NASH], elastin, smooth muscle α2 actin (smActin), and remodeling enzymes, i.e., crosslinking enzymes [lysyl oxidases, (Lox); lysyl oxidase-like subtypes (LoxL)], protease inhibitors (Timp 1 & 2; plasminogen activator inhibitor 1 [PAI1]) (Friedman, 2008; Koyama, 2015; Rosenbloom, 2013) and macrophage-derived Mmps. Hepatic fibrosis is a risk factor for cirrhosis; and cirrhosis is a risk factor for HCC. In fact, hepatic fibrosis severity correlates with overall mortality and liver-related events, such as cirrhosis and liver transplantation (Angulo, 2015; Dulai, 2017). As such, there is considerable interest in identifying therapies that will prevent the onset, stop the progression and reverse fibrosis.
Transforming growth factor β (TGFβ) is a key regulator of fibrosis (Schuppan, 2013). TGFβ regulates fibrosis through a canonical and non-canonical pathway (Massague, 2012). In the canonical pathway, TGFβ regulates the function of the Smad [homolog of the drosophila protein, mothers against decapentaplegic and the C. elegans protein for small body size, SMA] family of transcription factors. Activated TGFβ-receptors (TGFβR1 and TGFβR2) form a membrane-associated heterodimer with serine/threonine kinase activity. TGFβ binding to receptors recruits Smads to the membrane leading to Smad2 and Smad3 phosphorylation. Phospho-Smads are translocated to nuclei in association with Smad4. Phospho-Smads bind promoters and induce expression of target genes, like collagen1A1 (Col1A1), plasminogen activated inhibitor-1 (PAI1), cMyc and Smad 7, an inhibitor of TGFβ signaling (Akhurst, 2012; Hayashi, 1998; Massague, 2012; Nyati, 2015; Yoshida, 2012).
In the non-canonical pathway, activated TGFβ receptors regulate EGF receptor signaling that activates RAS/MapK, TGFβ-activated kinase (Tak1), PI3K and Akt pathways leading to increased expression of proteins involved in fibrosis (Massague, 2012; Nyati, 2015). TGFβ also interacts with other growth factor regulated pathways, including platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF). Together, these signaling pathways regulate the production of ECM, epithelial-mesenchymal transition (EMT), cell migration and cellular invasion (Yoshida, 2012). Clearly, the onset of NAFLD and its progression to NASH is a very complex process involving multiple cell types and regulatory factors.
4. Current treatment strategies for NAFLD and NASH
NAFLD and its progressive form, NASH, are major chronic liver diseases. While these liver diseases have attracted considerable attention from the academic, medical and pharmaceutical communities, the best strategies for managing NAFLD and NASH remain to be established (Chalasani et al., 2012c; Chan et al., 2007; Leslie, 2015; Vilar-Gomez, 2015). Current strategies focus on lifestyle management (exercise and diet) (Glass, 2015; Sofi, 2014) or pharmaceutical approaches targeting mechanisms regulating weight loss [pentoxifylline, phosphodiesterase inhibitor], blood glucose [metformin, SGLT2 inhibitor, GLP1 agonist], hypertension (telmisartan), carbohydrate and lipid metabolism [ligands for nuclear receptors PPAR(α, β, γ) & FXR] and fibrosis (pirfenidone) (Musso, 2012). Some new, but unapproved pharmaceutical agents, target fatty acid synthesis (ACC inhibitor), oxidative stress (Nox4 antagonist), inflammation (TLRs and the Mcp1 receptor)/CCR2 antagonist) and collagen cross-linking (LoxL2 inhibitors) (Kappler, 2015; Koyama, 2015).
Recent meta-analyses of clinical trials have provided evidence in support of the use of weight-loss, exercise, low carbohydrate diets, PPARγ agonist (pioglitazone), FXR agonist (obeticholic acid), vitamin E and ω3-PUFA in the management of NAFLD and NASH (He, 2016a; Musso, 2017, 2012, 2010; Sato, 2015; Sawangjit, 2016; Singh, 2015a, b; Wang, 2015).
Of the pharmaceutical agents tested, all but ω3-PUFA are defined, single biochemicals. Omega-3 fatty acids used in clinical trials range from fish or seal oils to highly refined pharmaceutical grade ω3 PUFA. Pharmaceutical grade ω3 PUFA include a mix of EPA + DHA ethyl esters (Lovaza™, GlaxoSmithKline) or EPA, as ethyl esters [Vascepa ™, Amarin Corporation)] or a free fatty acid [Epanova ™, Astrazenaca)]. We are unaware of a pharmaceutical grade DHA used in NAFLD/NASH clinical trials. Instead, DHA-enriched triglycerides have been used in children with NAFLD (Nobili, 2014).
The choice of which ω3 PUFA to use in NAFLD therapy is an important one. Preclinical and clinical studies reveal that EPA and DHA do not have equivalent effects on NAFLD (Depner, 2013a), inflammation (Allaire, 2016; Depner, 2013a) or fibrosis (Depner, 2013a; Lytle, 2015). As discussed above, EPA is not efficiently converted to DHA in vivo (Depner, 2013a; Itakura et al., 2011; Lytle, 2015). Accordingly, we will examine the evidence for the use of ω3 PUFA in the NAFLD therapy. Included in this discussion is the rationale for using ω3 PUFA in children (Boyraz, 2015; Janczyk, 2015; Lassandro, 2015; Nobili, 2013, 2011, 2014; Pacifico, 2015) and adults (Capanni, 2006; Cussons, 2009; Dasarathy, 2015; Li, 2015; Musso, 2010; Nogueira, 2016; Qin, 2015; Sanyal, 2014; Scorletti, 2014; Spardaro, 2008; Zhu, 2008). We also include meta-analyses of these studies (He, 2016b; Lu, 2016; Musso, 2012; Parker, 2012).
5. Rationale for using ω3 PUFA in NAFLD and NASH therapy
Lipidomic analyses of human liver have established that NAFLD is associated with an increase in all major classes of lipids, including neutral (diacylglycerol, triacylglycerol & cholesterol esters) and membrane lipids (phosphoglycero- and sphingo-lipids) (Puri et al., 2007). The fatty acyl composition of these complex lipids, however, is enriched in SFA and MUFA and depleted of PUFA (ω3 and ω6) (Allard, 2007; Arendt, 2015; Di Minno et al., 2012; Parker et al., 2012; Petit et al., 2012; Zheng et al., 2012). Lipidomic analysis of a preclinical model have replicated these findings. In mice, ω3 PUFA (EPA, ω3 DPA and DHA) appear more vulnerable to diet-induced suppression than C20 ω6 PUFA, e.g., arachidonic acid (ARA, 20:4,ω6) (Depner, 2013a, b; Lytle, 2017). While the low PUFA content in livers of NASH patients may be due to low PUFA consumption (Allard, 2007), the depletion of hepatic PUFA in mouse models of NASH is due to the suppression of hepatic PUFA synthesis by high fat diets (Depner, 2013a, 2012, 2013b; Tripathy et al., 2010; Wang et al., 2006).
Low hepatic C20-22 ω3 PUFA sets the stage for dysregulated hepatic function affecting lipid synthesis and turnover (Jump et al., 2013) (Wang et al., 2005) (Tripathy, 2014), as well as inflammation and fibrosis (Depner, 2013a; Lytle, 2015, 2017). These changes in hepatic function are linked to changes in hepatic nuclear content (SREBP1, ChREBP/MLX, NFκB and Smad3) or function (PPARα, PPARβ) of major transcription factors controlling these pathways.
The suppression of inflammation by ω3 PUFA is more complex than just controlling NFκB content. EPA and DHA suppress inflammation by: a) competing with C20 ω6 PUFA for cyclooxygenase and lipoxygenase-mediated inflammatory eicosanoid production; b) forming Cox generated ω3 eicosanoids that bind weakly to eicosanoid receptors; and 3) forming anti-inflammatory/pro-resolving ω3-PUFA derived oxylipins, e.g., resolvins, protectins, maresins (Fig 4) (Calder, 2015; Depner, 2013a, b; Jump et al., 2013; Lytle, 2017).
As noted previously, DHA is more effective than EPA at lowering systemic inflammatory markers in humans (Allaire, 2016). In this regard, DHA, but not EPA, attenuates western diet-induced hepatic fibrosis by targeting the TGFβ-Smad3 pathway (Lytle, 2015). DHA suppresses western diet-induced phospho-Smad3 accumulation in hepatic nuclei, a key mediator of TGFβ induction of ECM production. DHA also attenuates the expression of multiple transcripts linked to fibrosis, including collagen subtypes, ECM remodeling, tissue inhibitors of metalloproteases (TIMPs), matrix metalloproteases (MMPs) and lysyl oxidases.
In contrast to targeted pharmaceutical approaches, the foregoing discussion establishes DHA as a pleiotropic regulator of cell function. DHA has broad effects on multiple signaling pathways controlling liver lipid metabolism, inflammation and fibrosis making it an ideal nutraceutical for NAFLD therapy. Equally important, C20-22 ω3 PUFA have proven to be safe and efficacious nutraceuticals. Omega-3 PUFA are used to treat severe hypertriglyceridemia (Barter and Ginsberg, 2008; Pratt et al., 2009) and DHA is included in pre- and post-natal supplements.
6. Clinical assessment of ω3 PUFA in NAFLD and NASH therapy
6.a. Children
The Centers for Disease Control and Prevention estimates 13 million children in the US are obese (2015b). This population is vulnerable to developing chronic fatty liver disease. Four clinical trials have examined the impact of ω3 PUFA supplementation on children with NAFLD. The mean age of the patient population ranged from >5 to <19 years old and included male and females. All patients were diagnosed with chronic fatty liver disease as measured by plasma ALT (> 40 U/L) (Table 1)(Boyraz, 2015; Janczyk, 2015; Nobili, 2013, 2011, 2014; Pacifico, 2015). The ω3 PUFA type and dose used to treat NAFLD varied: DHA (250 and 500 mg/d), EPA + DHA (450 – 1300 mg/d, matched to body weight) or a mix of ω3 PUFA-ethyl esters (1000 mg/d). The duration of treatment ranged from 3 to 24 months.
Table 1.
Clinical trials assessing the impact of ω3 PUFA on Children with NAFLD1.
| Study (Ref)[Year] | ClinicalTrials.gov | Population | Treatment | Treatment | Key Outcomes |
|---|---|---|---|---|---|
| NCT# | Baseline Values Patients/group |
ω3 PUFA (Dose, mg/d; | Duration (Months) | ω3 PUFA vs Placebo | |
| Nobili et al [2011] | 885313 | Age: <18 yrs 20 per group ALT> 40 U/L Biopsy Proven NAFLD |
Placebo vs DHA 250 & 500 mg/d |
6 | Plasma ALT ↓ Insulin Sensitivity ↑ Blood DHA↑ Hepatosteatosis [US]1 ↓ |
| Nobili et al [2013] | 885313 | Age: <18 yrs 8M/12F per group ALT≥57 U/L |
Placebo vs DHA 250 & 500 mg/d |
6, 12, 18, 24 | Plasma ALT ↓ Blood DHA ↑ Hepatosteatosis [US] ↓ |
| Nobili et al [2014] | 885313 | Age: <18 yrs Mean Age, 12 8M/12F per group ALT>57 U/L |
Placebo vs DHA 250 & 500 mg/d |
6, 12, 18, 24 | Serum Cytokines ↓ Blood DHA ↑ NAS ↓ Hepatic GPR120 mRNA ↑ NFκB-pSer311 ↓ |
| Janczyk, et al [2015] | 1547910 | Age: >5, <19 yrs Placebo group = 30 ω3 PUFA group = 34 ALT ≥54 U/L |
Placebo vs EPA + DHA 450–1300 mg/d Weight Adjusted |
3, 6 | Blood ω3 PUFA [NR] Plasma Triglycerides, [NC] Plasma Adiponectin↑; AST ↓ Hepatosteatosis [US]; [NC] |
| Pacifico, et al [2015] |
Placebo: Mean Age: 11 16 Males ω3 PUFA: Mean Age: 11 14 males |
Placebo vs 250 mg oil/d 39% DHA |
6 | Body weight ↓; Waist circum. ↓ Plasma ALT [NC]; Insulin ↓ Plasma Triglycerides, ↓ Blood DHA, ↑ Hepatosteatosis [H], ↓ |
|
| Boyraz, et al [2015] |
Placebo: Mean age = 13.8 yrs 27M; 29F ALT, > 56U/L ω3 PUFA Mean Age, 13.3 yrs 28 M; 24F ALT, >58 U/L |
Placebo vs ω3 PUFA Marincap, 1 g/d [FA-EE 18% EPA 12% DHA] |
12 | Plasma ALT ↓; AST ↓ Fasting Insulin ↓; HOMA-IR ↓ Plasma Triglycerides, ↓ Blood ω3 PUFA [NR] Hepatosteatosis [US] ↓ |
Abbreviations: NAS, NASH activity score; H, Histology; US, hepatic ultrasound; NR, not reported; NC, no change; FA-EE, fatty acid ethyl esters.
When compared to the placebo group, plasma markers of liver injury (ALT & AST) and dyslipidemia (TAG) were reduced; blood DHA levels increased and hepatosteatosis declined in 3 of the 4 clinical studies in the patients receiving ω3 PUFA therapy (Boyraz, 2015; Nobili, 2013, 2011, 2014; Pacifico, 2015). Janczyk et al (Janczyk, 2015) did not report blood ω3 PUFA levels prior to or following treatment. Moreover, they reported no change in plasma triglycerides or hepatic NAFLD markers in response to ω3 PUFA therapy. The lack of an effect on plasma triglycerides, a well-established marker of C20-22 ω3 PUFA action may be due to: a) no dyslipidemia in the patient population; b) high blood levels of C20-22 ω3 PUFA in the placebo and treatment groups prior to intervention or; c) failure of the placebo and treatment groups to adhere to the intervention. As such, interventions require an assessment of established markers of ω3 PUFA action, like blood TAG and DHA content, to establish efficacy of treatment as well as treatment compliance.
There are several limitations in these studies. First, most studies evaluated a low number of participants (participants ranged from 20–56 per group). Three studies used ultrasound and 2 studies used histology to assess steatosis and NASH activity score (NAS). Two studies reporting on liver histology (Nobili, 2014; Pacifico, 2015) indicated that supplemental DHA (250–500 mg/d) decreased hepatosteatosis and/or the NAS. Since no study used EPA alone, it is unclear if children would have benefited from EPA treatment. Nevertheless, the results of 3 of the 4 clinic trials indicate that DHA or the combination of EPA + DHA attenuates liver injury (plasma ALT) and reduces hepatosteatosis. These clinical studies provide reasonable evidence in support of future intervention studies to evaluate the efficacy of C20-22 ω3 PUFA therapy in children at risk for the progression of benign steatosis to NASH.
6.b. Adults
Three to five percent of the US population have some level of chronic fatty liver disease with liver injury, i.e., NASH (Fig. 1). We examined 11 clinical trials assessing the capacity of ω3 PUFA to reduce NASH markers in adults (Table 2) (Argo, 2015; Capanni, 2006; Cussons, 2009; Dasarathy, 2015; Li, 2015; Nogueira, 2016; Qin, 2015; Sanyal, 2014; Scorletti, 2014; Spardaro, 2008; Zhu, 2008). The studies included male and female patients in both placebo and treatment groups. The mean age of the participants ranged from 33 to 58 years of age. Prior to intervention, all participants had evidence of hepatosteatosis as recorded by ultrasound, proton magnetic resonance imaging, magnetic resonance imaging or histology. Most patients had evidence of liver injury with ALT ≥ 40 or NAS ≥ 4. The treatment groups received supplemental ω3 PUFA ranging in type and dose: 150 mg/d EPA + 200 mg/d DHA to 50 ml of ω3 PUFA [1:1 EPA to DHA]. One group received seal oil at 2 g/d (Zhu, 2008). The duration of treatment ranged from 2 to 18 months.
Table 2.
Clinical trials assessing the impact of ω3 fatty acids on Adults with NAFLD1.
| Study (Ref)[Year] | ClinicalTrials.gov | Population | Treatment | Treatment | Key Outcomes |
|---|---|---|---|---|---|
| NCT# | Baseline Values | ω3 PUFA (Dose, mg/d) | Duration (Months) | ω3 PUFA vs Placebo | |
| Capanni, et al [2006] |
Placebo: 9M 5F Mean Age, 58 yrs ALT, 39 U/L ω3 PUFA: 23M 19F Mean Age, 57 yrs Mean ALT, 36 U/L |
Placebo vs ω3 PUFA 375 mg EPA 625 mg/DHA/d |
12 | Blood EPA & DHA ↑ Plasma Triglycerides ↓ Plasma ALT ↓; AST ↓ Plasma Glucose ↓ Steatosis [US] ↓ |
|
| Zhu, et al [2008] |
Placebo: 50M 18F Mean Age, 51 yrs ALT,≥59 U/L ω3 PUFA: 11M 7F Mean Age, 50 yrs Mean ALT, ≥56 U/L |
Placebo vs ω3 PUFA Seal Oil 2 g/d |
2, 3, 4, 6 | Blood EPA & DHA [NR] Plasma Triglycerides ↓ Plasma ALT ↓; AST ↓ Steatosis [US] ↓ |
|
| Spadaro, et al [2008] |
Placebo: 8M 10F Mean Age, 58 yrs ALT, 39 U/L ω3 PUFA: 23M 19F Mean Age, 57 yrs Mean ALT, 36 U/L |
Placebo vs ω3 PUFA 375 mg EPA 625 mg/DHA/d |
12 | Blood EPA & DHA NR Plasma Triglycerides ↓ Plasma ALT ↓; γGT ↓ Plasma TNFα ↓ HOMA-IR ↓ Steatosis [US] ↓ |
|
| Cussons, et al [2009] | 620526 | PCOS Patients Placebo: 6F Mean Age, 33 yrs ALT,≥59 U/L ω3 PUFA: 6F Mean Age, 33 yrs Mean ALT, 32 U/L |
Placebo vs ω3 PUFA 4g/d 27% EPA, 56% DHA 7% other fatty acids |
6 | Blood EPA & DHA [NR] Plasma Triglycerides ↓ Steatosis [HMRS] ↓ |
| Scorletti, et al [2014] |
Placebo: 35M 17F Mean Age, 54 yrs ALT ≥56 U/L ω3 PUFA: 25M 26F Mean Age, 49 yrs ALT, ≥54 U/L |
Placebo vs Omacor™ [46.5% EPA-EE 37.5% DHA-EE 16% other fatty acids] | 15–18 | Blood EPA & DHA ↑ Plasma Triglycerides ↓ HDL-C ↓ Steatosis [US] ↓ Fibrosis [H] NC |
|
| Sanyal, et al [2014] | 681408 |
Placebo: 24M 51F Mean Age, 51 yrs Mean ALT 54 U/L 1.8 g/d EPA-EE 33M 54F Mean Age, 48 yrs Mean ALT, 51 U/L 2.7 g/d EPA-EE 25M 61F Mean Age, 46 yrs Mean ALT, 52 U/L |
Placebo vs EPA-EE at 1.8 g/d or 2.7 g/d | 12 | No significant effect on any blood or hepatic marker of NASH |
| Argo, et al [2015] | 681408 |
Placebo: 7M 10F Mean Age, 47 yrs ALT, 60.8 U/L Liver Fat (MRI) 11.3% NAS: 4.9 Fibrosis Score: 1.6 ω3 PUFA: 6M 11F Mean Age, 46 yrs ALT, 72.8 U/L Liver Fat (MRI) 18.3% NAS: 45.4 Fibrosis Score: 2.1 |
Placebo vs ω3 PUFA 3g/d 70% ω3 PUFA; 35% EPA, 25% DHA |
12 | Plasma ω6:ω3 ratio↓ Steatosis [MRI] ↓ Fibrosis [NC] |
| Li, et al [2015] |
Placebo: 14M 25F Mean Age, 50 yrs ALT, 91 U/L ω3 PUFA: 13M 26F Mean Age, 54 yrs Mean ALT, 89 U/L |
Placebo vs 50 ml ω3 PUFA 50% EPA 50% DHA per day |
6 | Blood EPA & DHA NR Plasma ALT ↓; AST ↓ Plasma Triglycerides ↓ Plasma Cholesterol ↓ Hepatic Steatosis [H] ↓ Hepatic Inflammation [H] ↓ Hepatic Fibrosis [H] ↓ |
|
| Dasarathy, et al [2015] | 323414 | Controlled Diabetics with NASH Placebo: 2M 17F Mean Age, 50 yrs ALT, 49 U/L NAS ≥ 4 ω3 PUFA: 6M 12F Mean Age, 52 yrs Mean ALT, 48 U/L NAS > 4 |
Placebo vs ω3 PUFA 2.2 g/d EPA 1.4 g/d DHA |
12 | ω3 PUFA provided no significant benefit over placebo in patients with diabetes |
| Qin, et al [2015] | ChiCTR-TRC 12002380 |
Placebo-Corn Oil 25M 9F Mean Age, 44 Alt 33 U/L ω3 PUFA 26M 10F Mean Age, 46 Alt 32 U/L |
Placebo Corn oil in 4 g oil capsules ω3 PUFA: 728 mg EPA + 516 mg DHA in 4g oil capsules |
3 | Blood/Plasma Parameters: EPA & DHA ↑ Total Cholesterol ↓ Triglycerides ↓ ApoB ↓ Plasma Alt ↓ Plasma γGT ↓ Adiponectin ↓ LT-B4 ↓ FGF21 ↓ CK18 ↓ PGE2 ↓ |
| Nogueira, et al [2016] | 1992809 |
Placebo: 5M 18F Mean Age, 54 yrs ALT, 47 U/L NAS 5.4 ω3 PUFA: 5M 25F Mean Age, 53 yrs Mean ALT, 45 U/L NAS 5.0 |
Placebo vs ω3 PUFA 0.6 g/d ALA 0.15 g/d EPA 0.2 g/d DHA |
12 | Blood EPA & DHA ↑ Plasma Triglycerides ↓ Plasma ALT ↓; AST ↓ Hepatic lobular Inflammation ↓ |
Abbreviations: γGT, γ-glutamyl transferase; EE, ethyl esters; US, ultrasound, HMRS, hepatic proton magnetic resonance spectroscopy; MRI, magnetic resonance imaging, H, histology; NAS, NASH activity score; NR, not reported; LT-B4, leukotriene-B4; FGF21, fibroblast growth factor 21; CK18, cytokeratin 18 fragments M30; PGE2, prostaglandin E2.
All but 2 studies (Dasarathy, 2015; Sanyal, 2014) reported that the ω3 PUFA intervention improved plasma and liver parameters linked to NASH at the conclusion of the treatment. These improvements included a decline in liver injury (ALT) and hepatosteatosis. Hepatic inflammation was reported in 3 studies (Li, 2015; Nogueira, 2016; Qin, 2015); and markers of inflammation were reduced in patients receiving EPA + DHA. Only one group reported improvement in fibrosis scores (Li, 2015). One study reported no improvement in whole body, plasma or hepatic parameters of NASH following ω3 PUFA therapy. This study used a controlled diabetic patient population. The authors reported that patients on vitamin E and/or thiazolidinediones, treatment options for diabetes and NAFLD, were excluded from the study. However, the authors did not report other treatment options used to control diabetes in these patients.
Sanyal et al (Sanyal, 2014) reported no benefit of supplemental EPA (at 1.8 or 2.7 g/d) in any blood or hepatic marker of NASH. The lack of an effect on plasma TAG is disturbing since EPA is used to treat hypertriglyceridemia (Bays et al., 2011). The Japan EPA Lipid Intervention Study (JELIS) trial (Itakura et al., 2011), a multi-center, placebo-controlled, randomized, double blind 12-week study in patients with hypertriglyceridemia reported that EPA (as an ethyl ester) decreased TAG blood levels in hypertriglyceridemic patients. This group also reported that patients taking the EPA supplement had increased plasma EPA and DPA, but no increase in plasma DHA. As described previously, supplemental C20-22 ω3 PUFA inhibits expression of enzymes (Elovl5, Fads2) involved in converting EPA to DHA (Fig 4) (Wang et al., 2005; Wang et al., 2006).
6.c. Meta-Analyses
Four meta-analyses of randomized control trials of ω3 PUFA treatment of NAFLD were examined (Table 3) (He, 2016b; Lu, 2016; Musso, 2010; Parker, 2012). These meta-analyses examined 3–9 clinical trials each, with up to 561 total patients. There is considerable overlap of these meta-analyses with the clinical trials discussed in the previous section. The ω3 PUFA dose ranged from 0.83 to 6.4 g/d and the treatment period was 2 to 18 months in duration. The majority of parameters examined were plasma markers of liver damage (ALT, AST and γGT) and dyslipidemia (plasma TAG, cholesterol, LDL-C and HDL-C). Only one study reported on liver fat, i.e., hepatosteatosis (Lu, 2016). The overall outcome indicates that ω3 PUFA treatment improves plasma markers of hepatic damage and TAG, but yielded inconsistent effects on plasma markers for cholesterol (cholesterol, LDL-C and HDL-C). Supplemental ω3 PUFA lowered plasma TAG in patients with hypertriglyceridemia. While limited in the NASH markers assessed, the meta-analyses support the use of ω3 PUFA as an intervention to attenuate dyslipidemia and liver injury associated with NASH severity.
Table 3.
Summary of meta-analyses of RCTs for ω3 PUFA treatment of Adults with NAFLD1.
| Clinical Trials | Patients | ω3 PUFA Dose, g/d | Duration (Months) | Meta-Analysis | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Musso, et al (2010) | IV, Random | 95% CIL | 95% CIH | Favors | ||||
| Plasma ALT | 3 | 182 | 2 to 4 | 2 to 6 | −32.24 | −39.99 | −24.48 | ω3-PUFA |
| Parker, et al, (2012) | Hedge’s G | 95% CIL | 95% CIH | Favors | ||||
| Liver Fat | 7 | 331 | 0.83 to 5 | 2 to 12 | −0.965 | −1.348 | −0.582 | ω3-PUFA |
| Plasma ALT | −0.563 | −1.153 | −0.031 | ω3-PUFA | ||||
| Plasma AST | −0.971 | −1.815 | −0.127 | ω3-PUFA | ||||
| Lu, et al (2016) | M-H Random | 95% CIL | 95% CIH | Favors | ||||
| Liver Fat | 5 | 268 | 0.83 to 5 | 12 | 3.6 | 1.31 | 9.98 | ω3-PUFA |
| IV Random | 95% CIL | 95% CIH | Favors | |||||
| Plasma ALT | 8 | 530 | 0.83 to 4 | 2 to 18 | −4.97 | −11.14 | 1.2 | NE |
| Plasma AST | 7 | 496 | 0.83 to 4 | 2 to 18 | −2.01 | −8.72 | 4.7 | NE |
| HDL-C | 7 | 472 | 0.83 to 9 | 2 to 18 | 5.11 | −0.03 | 11 | ω3-PUFA |
| LDL-C | 6 | 433 | 0.83 to 9 | 2 to 18 | 1.28 | −4.06 | 6.63 | NE |
| Total Cholesterol | 7 | 474 | 0.83 to 4 | 2 to 18 | −3.65 | −10.4 | 3.09 | NE |
| Triglycerides | 9 | 561 | 0.83 to 4 | 2 to 18 | −35.55 | −53.9 | −17.19 | ω3-PUFA |
| He, et al (2016) | IV Fixed | 95% CIL | 95% CIH | Favors | ||||
| Plasma ALT | 6 | 396 | 0.83 to 6.4 | 6 to 18 | −7.61 | −12.38 | −2.39 | ω3-PUFA |
| Plasma γGT | 3 | 181 | 0.83 to 2.0 | 6 to 12 | −8.28 | −18.38 | 1.83 | ω3-PUFA |
| Triglycerides | 7 | 442 | 0.83 to 6.4 | 6 to 18 | −43.96 | −51.21 | −36.71 | ω3-PUFA |
| IV Random | 95% CIL | 95% CIH | Favors | |||||
| Plasma AST | 5 | 362 | 0.83 to 6.4 | 6 to 18 | −6.89 | −17.71 | 3.92 | ω3-PUFA |
| Plasma Cholesterol | 6 | 396 | 0.83 to 6.4 | 6 to 18 | −13.41 | −21.44 | −5.38 | ω3-PUFA |
| LDL-C | 6 | 360 | 0.83 to 6.4 | 6 to 18 | −7.31 | −14.26 | 0 | ω3-PUFA |
| HDL-C | 6 | 398 | 0.83 to 6.4 | 6 to 18 | 6.97 | 2.05 | 11.9 | ω3-PUFA |
Abbreviations: CIL, confidence interval-low value; CIH, confidence interval-high value; NE, no effect; ALT, alanine aminotransferase, AST, aspartate, aminotransferase, γGT, γ-glutamyl aminotransferase.
6.d. Summary of Clinical Studies
Investigators using C20-22 ω3 PUFA as fish oil, a mix of EPA + DHA ethyl esters or DHA (as TAG), but not EPA ethyl esters have shown that supplemental C20-22 ω3 PUFA attenuates plasma and hepatic markers of NASH, including plasma TAG, hepatic injury (ALT and AST), hepatosteatosis and inflammation. Attenuation of hepatic fibrosis, however, was reported in only 1 study [Table 2, (Li, 2015)]. Lovaza™ (~50:50 mix of EPA- & DHA-ethyl esters) treatment succeeded in reducing hepatic steatosis, but did not improve fibrosis scores. EPA-ethyl esters failed to reduce steatosis or fibrosis (Sanyal, 2014).
These clinical trials reveal a range of effects of ω3 PUFA on NASH which may depend on the type of ω3 PUFA used in the study. Several explanations can account for these outcomes. First, EPA and DHA are not equally effective in regulating the multiple pathways linked to NASH. Clinical and preclinical studies have established that DHA is superior to EPA in controlling steatosis, inflammation & fibrosis (Allaire, 2016; Depner, 2013a; Jump et al., 2013; Lytle, 2015). A recent clinical study established that DHA was a more robust anti-inflammatory nutraceutical than EPA (Allaire, 2016). Second, the failure of EPA to lower liver fat and fibrosis in humans (Sanyal, 2014) is likely due to its poor conversion to DHA in vivo (Itakura et al., 2011). Both EPA and DHA suppress the expression of enzymes (Elovl5, Fads1, Fads2) required for PUFA synthesis (Wang et al., 2005). In preclinical studies, DHA, but not EPA, suppressed diet-induced hepatic fibrosis by targeting the TGFβ pathway (Depner, 2013a; Lytle, 2015). Third, some clinical trials did not establish the efficacy of the ω3 PUFA treatment to increase blood levels of EPA and/or DHA or lower blood TAG (Sanyal, 2014). Fourth, fibrosis remission follows a slower timeline of remission than steatosis. Western diet-fed obese mice develop NASH. When these obese-NASH mice are placed on a low fat-low sucrose diet, obesity and hepatosteatosis resolves before fibrosis (Lytle, 2016). Finally, DHA is retroconverted to EPA in rodents and humans (Depner, 2013a; Park, 2016; Plourde, 2011; Sprecher, 2000). As such supplemental DHA increases both EPA and DHA in tissues, while EPA only increases cellular and blood levels of EPA and DPA (Depner, 2013a; Itakura et al., 2011). EPA and DPA fail to attenuate NASH markers in preclinical studies (Depner, 2013a).
7. Development of preclinical models for NAFLD and NASH
Preclinical models of human disease provide an opportunity to study the onset, progression and remission of disease in a well-controlled laboratory environment. Moreover, these models are subject to rigorous interrogation at the physiological, biochemical and molecular level. Such models can be used to evaluate clinical approaches for disease management and identify novel biomarkers to track human disease.
The goal of preclinical studies on NASH is to use an animal model that recapitulates human disease. The ideal preclinical animal model of NASH in the MetS patient should include the following characteristics, obesity, diabetes, hepatosteatosis, inflammation and fibrosis. Several models of NAFLD have been developed where liver disease is induced using specific diets or genetic manipulation (Table 4). Animal models of hepatic fibrosis induced by carbon tetrachloride (CCL4) injection or bile duct ligation are not included in this discussion because these models are not representative of NASH in the MetS patient.
Table 4.
Comparison of preclinical models of NAFLD1
| Obesity | Diabetes | Hepatosteatosis | Inflammation | Fibrosis | Reference | ||
|---|---|---|---|---|---|---|---|
| Dietary Models | |||||||
| Genetic Background | Diet | ||||||
| Mice, C57BL/6J | HFD | + | + | + | −/+ | − | Jump, et al., 2016 |
| Mice, C57BL/6J | HFHC | + | −/+ | + | + | + | Jump, et al, 2016 |
| Mice, C57BL/6J | AD + C | − | − | + | + | − | Suzuki−Kemuriyama, et al, 2016 |
| Rats, Wistar | MCD | − | − | + | + | + | Ibrahim, et al., 2016 |
| Genetic Models | |||||||
| Genetic Background | Diet | ||||||
| Ob/Ob | LFD-Chow | + | + | + | + | − | Ibrahim, et al., 2016 |
| Ldlr−/− | LFD-Chow | − | − | − | − | − | Depner, et al 2013 |
| C57BL/6J x 129AS1/Svlmj | LFD-Chow | − | − | − | − | − | Asgharpour, et al., 2016 |
| Ldlr−/− | Western | + | + | + | + | + | Depner, et al., 2013 |
| C57BL/6J x 129AS1/Svlmj | Western | + | + | + | + | + | Asgharpour, et al, 2016 |
| Mcr4−/− | Western or HFD | + | + | + | + | + |
Konuma, et al., 2015 Itoh, et al., 2011 |
Abbreviations: HFD, high fat diet, 60% energy as fat; HFHC, high fat-high cholesterol diet; LFD-Chow (≤10% calories as fat) AD + C, atherogenic diet + cholate; MCD, methionine-choline deficient diet; Ob/Ob, leptin deficient mouse; Mcr4−/−, melanocortin receptor-4 null mouse; Pten−/−, phosphatase and tensin homolog null mouse; Ldlr−/−, low density lipoprotein receptor null mouse.
Wild type C57BL/6J male mice fed a high fat diet (HFD, 60% calories as fat [91% lard; 9% soybean oil, Research Diets)] or a high fat-high cholesterol diet [HFHC, 54% calories as fat + cholesterol (0.5 % w/w)] become obese and develop glucose intolerance (diabetes), hepatosteatosis and mild hepatic inflammation (Jump, 2016). Mild hepatic fibrosis develops in the HFHC-diet fed mice, but not the HFD-fed mice, implicating a role for dietary cholesterol in the onset of hepatic fibrosis (Depner, 2012). The atherogenic + cholate (AD + C) and methionine-cholate-deficient (MCD) diets promote hepatosteatosis and inflammation without obesity or evidence of diabetes, i.e., fasting hyperglycemia (Kajikawa, 2010; Suzuki-Kemuriyama, 2016).
Ob/Ob mice are hyperphagic resulting from deficient leptin expression; leptin is a satiety hormone produced in adipose tissue. These mice become obese when fed a low fat chow (control or maintenance) diet. Livers of Ob/Ob mice become fatty (steatotic) with evidence of mild inflammation (Ibrahim, 2016). Little fibrosis develops in Ob/Ob mice because leptin plays a role in the development of fibrosis (Takahashi, 2012). Melanocortin receptor-4 null mice (Mcr4 −/−) mice, like the Ob/Ob mice, are hyperphagic and develop diabetes and NASH with fibrosis when fed a HFD (Itoh, 2011) or western diet (Konuma, 2015).
LDL-receptor null mice (Ldlr −/−) (Bieghs et al., 2012; Depner, 2013a) and the cross of C57BL/6J x 129AS1Svlmj mice [also called Diamond mice (diet-induced animal model of non-alcoholic fatty liver disease)] (Asgharpour, 2016) are lean with no evidence of hyperphagia, diabetes or liver disease when fed a low fat or chow maintenance diet. When fed a western diet, however, both genotypes develop robust obesity, diabetes, hepatosteatosis with significant inflammation and fibrosis. The western diet from Research Diets is moderately high in fat (41% total calories; 95% milk fat, 5% corn oil), hexoses (23% calories) and cholesterol (0.15% w/w). The obesity epidemic in western societies is linked, at least in part, to the consumption of high calorie diets, like the western or cafeteria diets (Arendt, 2015; Cordain, 2005; Depner, 2013a, b; Ferolla, 2015; Lytle, 2016; Malhotra, 2015; Pagliassotti et al., 1996; Pickens et al., 2009).
Of the models described above, Ldlr −/−, Mcr4−/− and Diamond mice become obese and develop diabetes and NASH when fed a western diet. These preclinical models appear to recapitulate NASH in obese humans. Although not reported in other preclinical models, Ldlr −/− mice fed a western diet also develop endotoxinemia (Depner, 2013b). These mice also have increased blood levels of agonist activating toll-like receptors (TLR2 and TLR4) (Lytle, 2015). As such, this is another feature that recapitulates human NASH (Harte et al., 2010).
8. Preclinical studies of ω3 PUFA effects on NAFLD and NASH
C57BL/6J, Ldlr −/−, Mcr4 −/− and liver-specific Pten −/− null mice (L-Pten −/−) have been used to assess the impact of EPA, DHA or the combination of EPA & DHA on liver health (Table 5). While Ldlr −/− and Mcr4 −/− mice were fed a western diet without or with ω3 PUFA; the C57BL/6J mice were fed the AD + C diet without and with ω3 PUFA. The L-Pten −/− mice were maintained on a chow maintenance diet without or with ω3 PUFA.
Table 5.
Effect of dietary ω3 PUFA on the onset and progression of NASH and primary hepatocellular carcinoma (HCC)1.
| Mouse Model | Diet | ω3 PUFA Dose2 | Treatment Duration wks | Hepatic Phenotype | ω3 PUFA Effect | Reference |
|---|---|---|---|---|---|---|
| Ldlr−/− | Western | EPA or DHA 2% energy |
16 | NASH [S, I, OS, F] | DHA>EPA Decreased S, I, OS, F |
Depner, et al [2013] |
| Mcr4−/− | Western | EPA 9.6% energy |
24 | NASH [S, I, F] | EPA Decreased S, F, NAS |
Nonuma, et al [2015] |
| C57BL/6J Wild type | AD + C | EPA or DHA 9% energy |
4 | Mild NAFLD [S, I, OS, F] | EPA = DHA Decreased S, I, OS, F |
Suzuki-Kemuriyama, et al [2016] |
| L-Pten−/− | Chow | EPA 10% energy |
40 & 72 | HCC | EPA Decreased S, I, OS, CP-A |
Ishii, et al. [2009] |
Abbreviations: AD + C, atherogenic diet + cholate; S, steatosis; I, inflammation; F, fibrosis; OS, oxidative stress; NAS, NASH activity score, CP-A, cell proliferation-apoptosis; HCC, primary hepatocellular carcinoma.
The dose of ω3 PUFA in some reports was reported as weight % of diet. To provide consistency amongst studies, the ω3 PUFA dose was recalculated and represented as % energy in the diet.
Supplemental ω3 PUFA varied from 2% to 10% of total calories. The western diet is 4.7 kcal/g. Mice consume ~3 grams of food/day or 14.1 kcals. At the 2% level mice consume ~0.28 kcal of ω3 PUFA/day. This level of consumption is similar to the level of ω3 PUFA consumed by humans (ω3 PUFA at 1.8% energy per day) ingesting 2000 kcal/day and taking 4 grams of ω3 PUFA/day to treat hypertriglyceridemia (Barter and Ginsberg, 2008; Pratt et al., 2009). The duration of feeding the test diets ranged from 4 to 72 weeks.
All preclinical models developed steatosis and hepatic inflammation. The level of hepatic fibrosis, however, varied from mild to moderate. No mice developed robust fibrosis resembling human cirrhosis. L-Pten −/− mice fed a chow diet, however, developed a HCC phenotype that was evident after 40 weeks on the chow diet.
In all 4 preclinical studies, ω3 PUFA supplementation lowered steatosis. This result is expected based on the well-established capacity of ω3 PUFA to target transcription factors controlling the expression of enzymes involved in hepatic lipid synthesis, oxidation and storage. Dietary DHA was better than EPA at lowering hepatic markers of inflammation (Depner, 2013a; Lytle, 2017), including osteopontin, IL2rn, IL1α, IL1β, Mcp1, TNFα, CD68. Interestingly, the endotoxinemia induced by the western diet was not attenuated by DHA (Depner, 2013b). Instead, DHA suppressed hepatic expression of CD14 (mRNA and protein), as well as the expression of TLR2 and TLR4. DHA also suppressed the nuclear abundance of NFκB-p50 as well as its precursor mRNA (Depner, 2013a). These studies establish that DHA attenuates the capacity of cells to respond to inflammation, rather than lowering plasma levels of TLR4 agonist (Depner, 2013b; Lytle, 2015).
At the 2% calorie dose, DHA, but not EPA, attenuated western diet-induced fibrosis as examined by gene expression, Col1A1 protein abundance and histology (Depner, 2013a; Lytle, 2015). Moreover, this effect was linked to DHA attenuation of hepatic TGFβ signaling. DHA attenuated western diet-induced phospho-Smad3 accumulation in hepatic nuclei. DHA attenuated expression of multiple genes associated with fibrosis, i.e., collagens, Timp 1 & 2, lysyl oxidase, and Lox-like oxidases, subtypes 1–3 (Lytle, 2015). At 10% energy, EPA decreased the severity of HCC in L-Pten −/− mice. Taken together, these findings established that ω3 PUFA have the capacity to decrease the severity of multiple markers of NASH including steatosis, inflammation and fibrosis.
All of the studies presented in Table 5 use ω3 PUFA in the prevention of diet induced liver disease. A recent study tested whether DHA could reverse NASH in mice with pre-existing disease (Lytle, 2017). Ldlr −/− mice were fed the western diet for 22 weeks to induce NASH. Mice were either maintained on a western diet supplemented with olive oil or switched to a western diet containing DHA at 2% energy; the two diets were isocaloric. After 8 weeks on these diet, mice had similar body weights, but blood lipids (triglycerides and cholesterol) in the western diet + DHA group were significantly lower when compared to the western diet + olive oil group. Moreover, hepatosteatosis, inflammation and fibrosis was significantly attenuated in the western diet + DHA group versus the western diet + olive oil group. In fact, the level of hepatic inflammation and fibrosis was less than that seen in mice fed the western diet for only 22 wks. As such, DHA not only blocked western diet-induced NASH progression, it also promoted disease remission.
Lytle et al also carried out a comparative study where mice fed the western diet for 22 weeks were switched to a chow diet (Lytle, 2017). In both a previous study (Lytle, 2016), and the more recent study (Lytle, 2017), switching obese mice with NASH from a western diet to a chow diet reversed nearly all adverse events associated with diet-induced NASH, including obesity, hyperglycemia, dyslipidemia, hepatic lipid content, inflammation, oxidative stress and fibrosis. While this approach can effectively treat NASH in humans, complicance to weight loss therapy may be a complicating factor (Burgess, 2017).
9. Future usage of ω3 PUFA in NASH therapy
NAFLD and NASH represent major public health concerns worldwide. While changes in diet and lifestyle are effective therapies, such therapies are often difficult to sustain over the long term (Burgess, 2017). As such, safe and efficacious pharmaceutical approaches are needed to prevent the onset of NAFLD and its progression to NASH, cirrhosis and HCC.
We have presented evidence in support of ω3 PUFA supplements containing DHA as a treatment strategy for NAFLD. This strategy is supported by data from the clinical trials in which children and adults were treated with DHA alone or EPA + DHA (Tables 1–3). In nearly every study, dietary ω3 PUFA supplementation lowered blood triglycerides and reduced liver steatosis and injury. Unfortunately, measures of hepatic inflammation and fibrosis were not included in all clinical studies. The preclinical studies of NASH (Tables 4 & 5), however, provided more compelling evidence for the use of DHA in the suppression of diet-induced steatosis, inflammation and fibrosis.
A feature of both the clinical and preclinical studies is that EPA and DHA are not equivalent in their effects on systemic inflammation (Allaire, 2016; Cottin et al., 2011) or NASH parameters (Boyraz, 2015; Janczyk, 2015; Nobili, 2013, 2011, 2014; Pacifico, 2015). The preclinical studies in mice (Depner, 2013a, 2013b; Lytle, 2017) support the findings that DHA is superior to EPA in the management of NASH markers, i.e., steatosis, inflammation and fibrosis.
While encouraging, there are limitations to ω3 PUFA usage in the obese diabetic patient. These supplements are not effective thearpies for weight loss or the control of blood glucose. As is clear from the recent study by Lytle, et al, supplemental DHA has no effect on body weight or blood glucose (Lytle, 2017). Only after returning the obese mice to a low fat-chow diet was body weight and blood glucose returned to levels seen in the lean control mice (Lytle, 2017). As such, ω3 PUFA supplementation should be combined with other therapeutic approaches to combat obesity and hyperglycemia in the obese and T2DM patient. Despite this limitation, DHA addition to the western diet blocks NAFLD progression (Lytle, 2017). This is good news for obese patients who struggle with weight loss therapy.
Finally, we have a good understanding of how ω3 PUFA lower liver lipids and attenuate inflammation (Calder, 2015; Jump et al., 2013). In contrast, we have an incomplete understanding of how ω3 PUFA attenuate fibrosis, particularly when DHA attenuates expression of a broad array of proteins involved in fibrosis (Lytle, 2015, 2017). One study suggest that DHA acts directly on human stellate cells to suppress expression of Col1A1 (Lytle, 2015). This effect, however, is not robust. DHA suppresses hepatic TGFβ signaling in a preclinical model, but this effect may be indirect and involve DHA control of other factors, such as circulating cytokines affecting TGFβ signaling. As such, future studies are needed to clarify the in vivo mechanisms for DHA suppression of TGFβ signaling and hepatic fibrosis.
Acknowledgments
This work was supported by the National Institutes of Health grant DK 094600 and the National Institute of Food and Agriculture grant 2009-65200-05846.
Abbreviations
- ACC
acetyl CoA carboxylase
- ACYL
ATP citrate lyase
- Akt2
rac-βserine-threonine kinase
- ALA
α-linolenic acid
- ALT
alanine aminotransferase
- ARA
arachidonic acid, 20:4,ω6
- AST
aspartate aminotransferase
- ATGL
adipocyte triglyceride lipase
- CD36
cluster of differentiation-36
- CE
cholesterol esters
- CGI58
comparative gene identification-58
- ChREBP
carbohydrate regulatory element binding protein
- Col1A1
collagen 1A1
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- DNL
de novo lipogenesis
- ECM
extracellular matrix
- Elovl
elongase
- EGF
epidermal growth factor
- EPA
eicosapentaenoic acid
- Erk1/2
extracellular signal regulated kinase
- FABP
fatty acid binding protein
- FASN
fatty acid synthase
- FADS
fatty acid desaturase
- FAO
fatty acid oxidation
- FXR
farnesyl X receptor
- γGT
γ-glutamyl transferase
- HCC
hepatocellular carcinoma
- LA
linoleic acid
- LDLR
low density lipoprotein receptor
- MCD
methionine choline deficient diet
- MetS
metabolic syndrome
- MLX
Max-like factor-X
- MMP
membrane metalloprotease
- mTorc
target of rapamycin-complex
- MUFA
monounsaturated fatty acids
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NASH activity score
- NEFA
non-esterified fatty acids
- PI3K
phosphoinositol-3-kinase
- PPAR
peroxisome proliferator activate receptor
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SCD1
stearoyl CoA desaturase-1
- SFA
saturated fatty acids
- Smad
homolog of the drosophila protein, mothers against decapentaplegic and the C. elegans protein for small body size, SMA
- SREBP
sterol regulatory element binding protein
- T2DM
type 2 diabetes mellitus
- TAG
triglyceride
- TGF
transforming growth factor
- TLR
toll-like receptors
- Timp
tissue inhibitor of metalloprotease
- VLDL
very low density lipoprotein
Footnotes
10. Conflict of interest statement.
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2015 a). http://www.cdc.gov/obesity/data/adult.html
- 2015 b). http://www.cdc.gov/obesity/data/childhood.html
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Hata A. Targeting the TGFb signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Exp Rev Gastroenterol Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire J, Couture P, Leclerc M, Charet A, Marin J, Lepine MC, Talbot D, Tchernof A, Lamarche B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA (ComparED) study. Am J Clin Nutr. 2016;104:280–287. doi: 10.3945/ajcn.116.131896. [DOI] [PubMed] [Google Scholar]
- Allard JB, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, Jalali P, Kandasamy T, Prayitno N, Sherman M, Guindi M, Ma DW, Heathcote JE. Nutrition assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2007;48:300–307. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Allmann DW, Gibson DW. Fatty acid synthesis during early linoleic acid deficiency in the mouse. J Lipid Res. 1969;6:51–62. [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjomsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17(Suppl):S186–190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Arendt BM, Comelli EM, Ma DW, Lou W, Teterina A, Kim T, Fung DKH, McGilvray I, Fischer SE, Allard JP. Altered hepatic gene expression in non-alcoholic fatty liver disease is associated with lower n-3 and n-6 polyunsaturated fatty acids. Hepatology. 2015;61:1565–1578. doi: 10.1002/hep.27695. [DOI] [PubMed] [Google Scholar]
- Arendt BM, Mohammed SS, Aghdassi E, Prayitno NR, Ma DWL, Nguyen A, Guindi M, Sherman M, Heathcote EJ, Allard JP. Hepatic fatty acid composition differs between chronic hepatitis C patients with and without steatosis. The Journal of nutrition. 2009;139:691–695. doi: 10.3945/jn.108.101782. [DOI] [PubMed] [Google Scholar]
- Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, Sha NL, Al-Osaimi AM, Pramoonjago P, Jayakuman S, Pinder LP, Simmons-Egolf WD, Burks SG, Bao Yongde, Taylor AG, Rodriguez J, Caldwell SH. Effects of n-3 fish oil on metabolic and histological parameters in NASH: A double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62:190–197. doi: 10.1016/j.jhep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D, Jr, Warncke UO, Wijesinghe DS, Sanyal AJ. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepato. 2016;65:579–588. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos IB, Gleason JA, Sever S, Gedik R, Asztalos BF, Horvath KV, Dansinger ML, Lamon-Vava S, Schaefer EJ. Effects of eicosapentaenoic acid and docosahexaenoic acidn on cardiovascular disease risk factors: a randomized clinical trial. Metabolism: Clinical and Experimental. 2016;65:1636–1645. doi: 10.1016/j.metabol.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Banini BA, Sanyal AJ. Current and future pharmacologic treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:134–141. doi: 10.1097/MOG.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraille F, Planchais J, Dentin R, Guilmeau S, Postic C. Integration of ChREBP-mediated glucose sensing into whole body metabolism. Physiology. 2015;30:428–437. doi: 10.1152/physiol.00016.2015. [DOI] [PubMed] [Google Scholar]
- Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040–1045. doi: 10.1016/j.amjcard.2008.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RAa, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, Placebo-controlled, Randomized, Double-Blind, 12-week study with an open-label Extension [MARINE] trial) Am J Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- Bieghs V, Van Gorp PJ, Wouters K, Hendrikx T, Gijbels MJ, van Bilsen M, Bakker J, Binder CJ, Lutjohann D, Staels B, et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7:e30668. doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C, Turati F, Vecchia CL. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterology. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Botolin D, Jump DB. Selective proteolytic processing of rat hepatic sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 during postnatal development. J Biol Chem. 2003;278:6959–6962. doi: 10.1074/jbc.M212846200. [DOI] [PubMed] [Google Scholar]
- Botolin D, Wang Y, Christian B, Jump DB. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J Lipid Res. 2006;47:181–192. doi: 10.1194/jlr.M500365-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursier JaD, AM Implications of gut microbiota in nonalcoholic fatty liver disease. PloS Pathogens. 2015;11:e1004559. doi: 10.1371/journal.ppat.1004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyraz M, Pirgon O, Dundar B, Cekmez F, Hatipoglu N. Long-term treatment with n-3 polyunsaturated fatty acids as a monotherapy in chicken with nonalcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2015;7:121–127. doi: 10.4274/jcrpe.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Burgess E, Hassmen P, Welvaert M, Pumpa KL. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: a systemic review and meta-analysis. Clin Obes. 2017;7:105–114. doi: 10.1111/cob.12180. [DOI] [PubMed] [Google Scholar]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bodogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R, Surrenti C, Casini A. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- Cengiz M, Ozenirler S, Elbeg S. Role of serum toll-like receptors 2 and 4 in non-alcoholic steatohepatis and liver fibrosis. J Gastroentero Hepatol. 2015;30:1190–1196. doi: 10.1111/jgh.12924. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012a;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, et al. American Association for the Study of Liver D, American College of G. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. The American journal of gastroenterology. 2012b;107:811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, et al. American Gastroenterological A, American Association for the Study of Liver D. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012c;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Chan HL, de Silva HJ, Leung NW, Lim SG, Farrell GC. How should we manage patients with non-alcoholic fatty liver disease in 2007? J Gastroenterol Hepatol. 2007;22:801–808. doi: 10.1111/j.1440-1746.2007.04977.x. [DOI] [PubMed] [Google Scholar]
- Chung M, Ma J, Patel, Berger S, Lau J, Lichtenstein AH. Fructose, high fructose corn syrup, sucrose and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:833–849. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Orgins and evolution of the western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc. 2011;70:215–231. doi: 10.1017/S0029665111000061. [DOI] [PubMed] [Google Scholar]
- Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementaion decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metabol. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double blind randomized placebo controlled clinical trail of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:197–144. doi: 10.1097/MCG.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem. 2008 doi: 10.1074/jbc.M801539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OFW. Steatohepatitis: a tale of two “hits”? Gastroentero. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- Depner CM, Philbrick KA, Jump DB. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(−/−) mouse model of western diet-induced nonalcoholic steatohepatitis. J Nutr. 2013a;143:315–323. doi: 10.3945/jn.112.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner CM, Torres-Gonzalez M, Tripathy S, Milne G, Jump DB. Menhaden oil decreases high-fat diet-induced markers of hepatic damage, steatosis, inflammation, and fibrosis in obese Ldlr−/− mice. J Nutr. 2012;142:1495–1503. doi: 10.3945/jn.112.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner CM, Traber MG, Bobe G, Bohren KM, Morin-Kensicki E, Milne G, Jump DB. A metabolomic analysis of omega-3 fatty acid mediated attenuation of western diet-induced non-alcoholic steatohepatitis in LDLR−/− mice. Plos One. 2013b;8(12):e83756. doi: 10.1371/journal.pone.0083756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno MN, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:5839–5847. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW-S, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis state in non-alsoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017 doi: 10.1002/hep.29085. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. The American journal of clinical nutrition. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- Farooqui AA. N-3 fatty acid-derived lipid mediators in the brain: new weapons against oxidative stress and inflammation. Curr Med Chem. 2012;19:532–543. doi: 10.2174/092986712798918851. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Ferolla SM, Silva LC, de Ferrari ML, da Cunha AS, dos Martins FS, Couto CA, Ferrari TC. Dietary approach in the treatment of nonalcoholic fatty liver disease. World J Hepatol. 2015;28:2522–2534. doi: 10.4254/wjh.v7.i24.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab. 2013;24:257–268. doi: 10.1016/j.tem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Glass LM, Dickson RC, Anderson JC, Sufiawinata AA, Putra J, Berk BS, Toor A. Total body weight loss of ≥10% is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:1024–1030. doi: 10.1007/s10620-014-3380-3. [DOI] [PubMed] [Google Scholar]
- Goel A, Gupta M, Aggarwal R. Gut microbiota and liver disease. J Gastroenterology and Hepatology. 2014;29:1139–1148. doi: 10.1111/jgh.12556. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Feingold KR. Endotoxin in the gut and chylomicrons: translocation or transportation? Journal of Lipid Res. 2009;50:1–2. doi: 10.1194/jlr.E800018-JLR200. [DOI] [PubMed] [Google Scholar]
- Gugliucci A. Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr. 2017;8:54–62. doi: 10.3945/an.116.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. Journal of inflammation. 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto E, Tokushige K, Farrell GC. Histological features of non-alcoholic fatty liver disease: What is important? J Gastroenterol Hepatol. 2011;27:5–7. doi: 10.1111/j.1440-1746.2011.06957.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qui Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1998;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- He L, Liu X, Wang L, Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: a meta-analysis of randomized clinical trials. Medicine. 2016a;95:e4947. doi: 10.1097/MD.0000000000004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XX, Wu X-L, Chen RP, Chen C, Liu XG, Wu BJ, Huang ZM. Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Plos One. 2016b;11:e0162368. doi: 10.1371/journal.pone.0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach J. Debate: A bridge too far-Liver transplatation for nonalcoholic steatohepatitis will overwhelm the organ supply. Liver Transplantation. 2014;20:S32–S37. doi: 10.1002/lt.23980. [DOI] [PubMed] [Google Scholar]
- Higgins JA, Jackman MR, Brown IL, Johnson GC, Steig A, Wyatt HR, Hill JO, Maclean PS. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutrition & metabolism. 2011;8:49. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Yokoyama C, Wu J, Briggs MR, Brown MS, Goldstein JL, Wang X. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc Natl Acad Sci U S A. 1993;90:11603–11607. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Hirsova P, Malhi H, Gores GJ. Animal models of nonalcoholic steatohepatitis: Eat, Delete, Inflame. Dig Dis Sci. 2016;61:1325–1336. doi: 10.1007/s10620-015-3977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99–107. doi: 10.5551/jat.5876. [DOI] [PubMed] [Google Scholar]
- Ito S, Yukawa T, Uetake S, Yamauchi M. Serum intracellular adhesion molecule-1 in patients with nonalcoholic steatohepatitis: comparison with alcoholic hepatitis. Alcoholism: Clin Experi Res. 2007;31:83S–87S. doi: 10.1111/j.1530-0277.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Itoh M, Suganami T, Nakagawa N, Tanaka M, Yamamoto Y, Kamei Y, Terai S, Sakaida I, Ogawa Y. Melanocortin 4 receptor-deficient mice as a mouse model of nonalcoholic steatohepatitis. Am J Pathol. 2011;179:2454–2463. doi: 10.1016/j.ajpath.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczyk W, Labensztejn D, Wierzbicka-Rucinska A, mazur A, Neuhoff-Murawaska J, Matusik P, Socha P. Omega-3 fatty acids therapy in children with nonalcoholic fatty liver disease: a randomized control trial. J Pediatr. 2015;166:1358–1363. doi: 10.1016/j.jpeds.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- Jump DB, Clarke SD, Thelen A, Liimatta M. Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J Lipid Res. 1994;35:1076–1084. [PubMed] [Google Scholar]
- Jump DB, Clarke SD, MacDougald O, Thelen A. Polyunsaturated fatty acids inhibit S14 gene transcription in rat liver and cultured hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8454–8458. doi: 10.1073/pnas.90.18.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Depner CM, Tripathy S, Lytle KA. Impact of dietary fat on the development of non-alcoholic fatty liver disease in Ldlr−/− mice. Proc Nutrition Soc. 2016;75:1–9. doi: 10.1017/S002966511500244X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Tripathy S, Depner CM. Fatty Acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33:249–269. doi: 10.1146/annurev-nutr-071812-161139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid ethyl ester prevents development of steatosis and hepatic fibrosis in rats. Dig Dis Sci. 2010;55:631–641. doi: 10.1007/s10620-009-1020-0. [DOI] [PubMed] [Google Scholar]
- Kanth VVR, Sasikala M, Sharma M, Rao PN, Reddy DN. Genetics of non-alcoholic fatty liver disease: From susceptibility and nutrient interaction to management. World J Hepatol. 2016;8:827–837. doi: 10.4254/wjh.v8.i20.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler R. From crawl to sprint: the race to treat NASH. Life Sci VC. 2015 https://lifescivc.com/2015/05/from-crawl-to-sprint-the-race-to-treat-nash/
- Kerr TA, Davidson NO. Cholesterol and NAFLD: Renewed focus on an old villain. Hepatology. 2012;56:1995–1998. doi: 10.1002/hep.26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Konuma K, Itoh M, Suganami T, Kanai S, Nakagawa N, Sakai T, Kawano H, Hara M, Kojima S, Izumi Y, Ogawa Y. Eicosapentaenoic acid ameliorates non-alcoholic steatohepatitis in a novel mouse model using melanocortin 4 receptor deficient mice. PloS One. 2015;10:e0121528. doi: 10.1371/journal.pone.0121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bane J, Stevens R, Dyck JRB, Newgard CB, Lupaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Met. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Brenner DA. New therapies for hepatic fibrosis. Clin Res Hepato Gastroentero. 2015;39:S75–S79. doi: 10.1016/j.clinre.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroentero. 2014;48:467–473. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristics of individuals with nonalcoholic fatty liver disease. Gastroentero. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz KT, Jump DB, MacDougald OA, Lane MD. Transcriptional control of the stearoyl-CoA desaturase-1 gene by polyunsaturated fatty acids. Biochem Biophys Res Commun. 1994;200:763–768. doi: 10.1006/bbrc.1994.1516. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassandro C, Banderali G, Radaelli G, Borghi E, Moretti F, Verduci E. Docosahexaenoic acid levels in blood and metabolis syndrome in obese children: Is there a link? Int J Mol Soc. 2015;16 doi: 10.3390/ijms160819989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer-viewpoint of the IARC working group. New Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugerette F, Vors C, Geloen A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22:53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhao L, Hwang DH. Modulation of pattern recognitiion receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev. 2011;68:38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lambert JE, Hovhannisyan Y, Ramos-Roman MA, Trombold JR, Wagner DA, Parks EJ. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101:34–43. doi: 10.3945/ajcn.114.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. The liver’s weight problem. Science. 2015:18–20. doi: 10.1126/science.349.6243.18. [DOI] [PubMed] [Google Scholar]
- Leung C, Herath CB, Jia Z, Andrikopoulos S, Brown BE, Davies MJ, Rivera LR, Furness JB, Forbes JM, Angus PW. Dietary advanced glycation end-products aggravate non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8026–8040. doi: 10.3748/wjg.v22.i35.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2012;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Yand LH, Sha KH, Liu TG, Zhang LG, Liu XX. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalsoholic steatohepatitis. World J Gastroentero. 2015;20:7008–7013. doi: 10.3748/wjg.v21.i22.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Wei E, Groenendyk J, Das SK, Hermansson M, Li L, Watts R, Thiesen A, Oudit GY, Michalak M, et al. Ces3/TGH Deficiency Attenuates Steatohepatitis. Sci Rep. 2016;6:25747. doi: 10.1038/srep25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liimatta M, Towle HC, Clarke S, Jump DB. Dietary polyunsaturated fatty acids interfere with the insulin/glucose activation of L-type pyruvate kinase gene transcription. Mol Endocrinol. 1994;8:1147–1153. doi: 10.1210/mend.8.9.7838147. [DOI] [PubMed] [Google Scholar]
- Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nature Reviews: Gastroentero & Hepato. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism: Clinical and Experimental. 2015;65:1136–1150. doi: 10.1016/j.metabol.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10 doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- Lou D-j, Zhu Q-q, Si X-w, Guan L-l, You Q-y, Yu Z-m, Zhang A-z, Li D. Serum phospholipid omega-3 polyunsaturated fatty acids and insulin resistance in type 2 diabetes mellitus and non-alcoholic fatty liver disease. J Diabetes and its Complications. 2014;28:711–714. doi: 10.1016/j.jdiacomp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Lu W, Li S, Li J, Wang J, Zhang R, Zhou Y, Yin Q, Zheng Y, Wang F, Xia Y, Chen K, Liu T, Lu J, Zhou Y, Guo C. Effects of omega-3 fatty acid in nonalcoholic fatty liver disease: a meta analysis. Gastro Res Pract. 2016;2016:1459790. doi: 10.1155/2016/1459790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Pro. 1980;55:434–438. [PubMed] [Google Scholar]
- Lytle KA, Jump DB. Is western diet-induced nonalcoholic steatohepatitis in Ldlr−/− mice reversible? PLoS One. 2016;11:e0146942. doi: 10.1371/journal.pone.0146942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle KA, Depner CM, Wong CP, Jump DB. Docosahexaenoic acid attenuates western diet induced hepatic fibrosis in Ldlr−/− mice by targeting the TGF-beta-Smad pathway. J Lipid Res. 2015;56:1936–1946. doi: 10.1194/jlr.M061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle KA, Wong CP, Jump DB. Docosahexaenoic acid blocks progression of western diet-induced nonalcoholic steatohepatitis in obese Ldlr−/− mice. Plos One. 2017;12:e0173376. doi: 10.1371/journal.pone.0173376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- Malhotra N, Beaton MD. Management of non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;26:2962–2967. doi: 10.4254/wjh.v7.i30.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signalling in context. Nature Reviews: Molecular Cell Biology. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater MK, Thelen AP, Pan DA, Jump DB. Sterol response element-binding protein 1c (SREBP1c) is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. J Biol Chem. 1999;274:32725–32732. doi: 10.1074/jbc.274.46.32725. [DOI] [PubMed] [Google Scholar]
- McCollough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12:333–340. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. viii. doi: 10.1016/j.cld.2004.04.004. Review. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- Milne GL, Morrow JD. Isoprostanes and related compounds: update 2006. Antioxid Redox Signal. 2006;8:1379–1384. doi: 10.1089/ars.2006.8.1379. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., 2nd Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Met. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra VL, Khashab M, Chalasani N. Non-alcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50–55. doi: 10.1007/s11894-009-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Ohnishi H. Role of gut microbiota and toll-like receptors in nonalcoholic fatty liver disease. World J Gastroentero. 2014;20:7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Cassader M, Paschetta E, Gambino R. Thiozolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis, A meta-analysis. JAMA Internal Medicine. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–905. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randominzed trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Nobili V, Alisi A, Della Corte C, Rise P, Galli C, Agostoni C, Bedogni G. Docosahexaenoic acid for the treatment of fatty liver: Randomized controlled trial in children. Nutr Met Cardio Dis. 2013;23:1066–1070. doi: 10.1016/j.numecd.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomized controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- Nobili V, Carpino G, Alisi A, De Vito R, Franchitto A, Alpini G, Onori P, Gaudio E. Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. Plos One. 2014;9:e88005. doi: 10.1371/journal.pone.0088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira MA, Oliveira CP, Alves VAF, Stefano JT, dos Reis Rodrigues LS, Torrinhas RS, Cogliati B, Barbeiro H, Carrilho FJ, Waitzberg DL. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, couble-bline, placebo-controlled trial. Clinical Nutr. 2016;35:578–586. doi: 10.1016/j.clnu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Nyati S, Schinske-Sebolt Pitchiaya S, Chekhovskiy K, Chator A, Chaudhry N, Dosch J, Van Dort ME, Varambally S, Kumar-Sinha C, Nyati MK, Ray D, Walter NG, Yu H, Ross BD, Rehemtulla A. The kinase activity of the Ser/Thr kinase Bub1 promotes TGF-b signaling. Sci Signal. 2015;8:ra1. doi: 10.1126/scisignal.2005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico L, Bonci E, Di Martino M, Versacci P, Andreoli G, Silvestri LM, Chiesa C. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementaion on hepatic fat and associated cardiovascular factors in overweight children with nonalcoholic fatty liver disease. Nutr Meta Cardio Dis. 2015;25:734–741. doi: 10.1016/j.numecd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol-Regulatory, Integrative and Comparative Physiology. 1996;271:R1319–R1326. doi: 10.1152/ajpregu.1996.271.5.R1319. [DOI] [PubMed] [Google Scholar]
- Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, Kim CY, Cho YM, Kim SH, Lee Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23:900–907. doi: 10.1111/j.1440-1746.2007.05212.x. [DOI] [PubMed] [Google Scholar]
- Park HG, Lawrence P, Engel MG, Kothapalli K, Brenna JT. Metabolic fate of docosahexaenoic acid (DHA, 22:6,n-3) in human cells; direct retroconversion of DHA to eicosapentaenoic acid (20:5, n-3) dominates over elongation to tetracosahexaenoic acid(24:6,n-3) FEBS Lett. 2016;590:3188–3194. doi: 10.1002/1873-3468.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–35939. doi: 10.1074/jbc.M306238200. [DOI] [PubMed] [Google Scholar]
- Pawar A, Xu J, Jerks E, Mangelsdorf DJ, Jump DB. Fatty acid regulation of liver X receptors (LXR) and peroxisome proliferator-activated receptor alpha (PPARalpha) in HEK293 cells. J Biol Chem. 2002;277:39243–39250. doi: 10.1074/jbc.M206170200. [DOI] [PubMed] [Google Scholar]
- Petit JM, Guiu B, Duvillard L, Jooste V, Brindisi MC, Athias A, Bouillet B, Habchi M, Cottet V, Gambert P, et al. Increased erythrocytes n-3 and n-6 polyunsaturated fatty acids is significantly associated with a lower prevalence of steatosis in patients with type 2 diabetes. Clin Nutr. 2012;31:520–525. doi: 10.1016/j.clnu.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Pickens MK, Yan JS, Ng RK, Ogata H, Grenert JP, Beysen C, Turner SM, Maher JJ. Dietary sucrose is essential to the development of liver injury in the methionine-choline-deficient model of steatohepatitis. J Lipid Res. 2009;50:2072–2082. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plourde M, Chouinard-Watkins R, Vandal M, Zhang Y, Lawrence P, Brenna JT, Cunnane SC. Plasma incorporation, apparent retroconversion and beta-oxidation of 13C-docosahexaenoic acid in the elderly. Nutr Metab (Lond) 2011;8:5–9. doi: 10.1186/1743-7075-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- Pratt CM, Reiffel JA, Ellenbogen KA, Naccarelli GV, Kowey PR. Efficacy and safety of prescription omega-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: a prospective study. Am Heart J. 2009;158:163–169. e161–163. doi: 10.1016/j.ahj.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, Wu Y, Chen JL, Kang C, Shu FR, Zhang QY, Mi MT. Fish oil supplements lower serum lipids and gllucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: A randomized clinical trial. Plos One. 2015;10:e0133496. doi: 10.1371/journal.pone.0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Thelen AP, Peters JM, Gonzalez FJ, Jump DB. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. J Biol Chem. 1997;272:26827–26832. doi: 10.1074/jbc.272.43.26827. [DOI] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832:1088–1103. doi: 10.1016/j.bbadis.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Sakiyama H, Wynn RM, Lee WR, Fukasawa M, Mizuguchi H, Gardner KH, Repa JJ, Uyeda K. Regulation of nuclear import/export of carbohydrate response element-binding protein (ChREBP): interaction of an alpha-helix of ChREBP with the 14-3-3 proteins and regulation by phosphorylation. J Biol Chem. 2008;283:24899–24908. doi: 10.1074/jbc.M804308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl-eicosapentaenoic acid on histologic features of nonalcoholic steatohepatitis in a Phase 2 trial. Gastroentero. 2014;147:377–384. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa, Yoneda M. Vitamine E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized control trials. Nutrition. 2015;31:923–930. doi: 10.1016/j.nut.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Sawangjit R, Chongmelazme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, Chaiykunapruk N. Comparative efficacy of interventins on nonalcolic fatty liver disease (NAFLD). A PRISMA-compliant systematic review and network meta-analysis. Medicine. 2016;95:e4529. doi: 10.1097/MD.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck RN, Zha W, Edin ML, Gruzdev A, Vendrov KC, Miller TM, Xu Z, Lih FB, DeGraff LM, Tomer KB, Jones HM, Makowski L, Huang L, Poloyac SM, Zeldin DC, Lee CR. The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease. PloS one. 2014;9:e110162. doi: 10.1371/journal.pone.0110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorletti E, Bhatia L, McCormic KG, Clough GF, Nash K, Hodson L, Moyses HE, Calder PC, Byrne CD WELCOME Study Investigators. Effects of purified eicosapentaenoic acid and docosahexaenoic acids in nonalcoholic fatty lifer disease: Results from the WELCOME Study. Hepatology. 2014;60:1211–1221. doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa AD, Umano GR, del Giudice EM, Santoro N. From the liver to the heart: Cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J Hepatol. 2017;9:69–73. doi: 10.4254/wjh.v9.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini C, De Felice C, Durand T, Oger C, Galano JM, Leoncini S, Pecorelli A, Valacchi G, Ciccoli L, Hayek J. Isoprostanes and 4-hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxidative Med Cell Long. 2013;2013:343824. doi: 10.1155/2013/343824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Singh S, Khera R, Allen AM, Murad MH, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology. 2015a;62:1417–1432. doi: 10.1002/hep.27999. [DOI] [PubMed] [Google Scholar]
- Smagris E, BasuRay S, Li J, Huang Y, Lai KM, Gromada J, Cohen JC, Hobbs HH. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology. 2015;61:108–118. doi: 10.1002/hep.27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J Biol Chem. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- Sofi F, Casini A. Mediterranean diet and non-alcoholic fatty liver disease. W J Gastroentero. 2014;20:7339–7346. doi: 10.3748/wjg.v20.i23.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spardaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects on n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liv Dis. 2008;40:194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:1733S–1737S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends in Endocrino & Meta. 2017 doi: 10.1016/j.tem.2016.11.006. In press. [DOI] [PubMed] [Google Scholar]
- Suzuki-Kemuriyama N, Matsuzaka T, Kuba M, Ohno H, Han S, Takeuchi Y, Isaka M, Kobayashi K, Iwasaki H, Yatoh S, Suzuki H, Miyajima K, Nakae D, Yahagi N, Nakagawa Y, Sone H, Yamada N, Shimano H. Different effects of eicosapentaenoic and docosahexaenoic acids on atherogenic high fat diet-induced non-alcholic fatty liver disease in mice. PloS One. 2016;11:e0157580. doi: 10.1371/journal.pone.0157580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. W J Gastroentero. 2012;18:2300–2308. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- Towle HC. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol Metab. 2005;16:489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Tripathy S, Lytle KA, Stevens RD, Bain JR, Newgard CB, Greenberg AS, Huang LS, Jump DB. Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice. J Lipid Res. 2014;55:1448–1464. doi: 10.1194/jlr.M050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S, Torres-Gonzalez M, Jump DB. Elevated hepatic fatty acid elongase-5 activity corrects dietary fat-induced hyperglycemia in obese C57BL/6J mice. J Lipid Res. 2010;51:2642–2654. doi: 10.1194/jlr.M006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63:2075–2080. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Vasandani C, Kafrouni AI, Caronna A, Bashmakov Y, Gotthardt M, Horton JD, Spady DK. Upregulation of hepatic LDL transport by n-3 fatty acids in LDL receptor knockout mice. J Lipid Res. 2002;43:772–784. [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E, Martenez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroentero. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Wada JaMH. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephr. 2016;12:13–26. doi: 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Zhang Y, Yang Y, Qin G. Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: results from a meta-analysis. Int j Clin Exp Med. 2015;8:17221–17234. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J Lipid Res. 2005;46:706–715. doi: 10.1194/jlr.M400335-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair MG, Peters JM, Busik JV, Olson LK, et al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47:2028–2041. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MYaJ, TA Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Moran G, Estrada A, Pagliassotti MJ. Fructose-induced stress signaling in the liver involves methylglyoxal. Nutr Metab (Lond) 2013;10:32. doi: 10.1186/1743-7075-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- Xu J, Christian B, Jump DB. Regulation of rat hepatic L-pyruvate kinase promoter composition and activity by glucose, n-3 polyunsaturated fatty acids, and peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem. 2006;281:18351–18362. doi: 10.1074/jbc.M601277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- Yoshida K, Matsuzaki K. Differential regulation of TGF-beta/Smad signaling in hepatic stellate cells between acute and chronic liver injuries. Frontiers in Physiol. 2012;3:1–7. doi: 10.3389/fphys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:1–6. doi: 10.1038/srep05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JS, Xu A, Huang T, Yu X, Li D. Low docosahexaenoic acid content in plasma phospholipids is associated with increased non-alcoholic fatty liver disease in China. Lipids. 2012;47:549–556. doi: 10.1007/s11745-012-3671-4. [DOI] [PubMed] [Google Scholar]
- Zhu FS, Liu S, Chen XM, Huang Z-G, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. W J Gastroentero. 2008;14:6395–6400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]