Abstract
Background and Aims
We assessed the feasibility of field-based directly observed therapy (DOT) with minimal monitoring to deliver HCV treatment to people with a history of drug use in Chennai, India.
Methods
50 participants were randomized 1:1 to sofosbuvir + peginterferon alfa 2a + ribavirin (SOF+PR) for 12 weeks (Arm 1) vs. sofosbuvir + ribavirin (SOF+R) for 24 weeks (Arm 2). SOF+R was delivered daily at participant chosen venues and weekly peginterferon injections at the study clinic. HCV RNA testing was done to confirm active HCV infection and sustained virologic response 12 weeks after treatment completion (SVR12). No baseline genotyping or on-treatment viral loads were performed.
Results
Median age was 46 years. All were male and 10% had significant fibrosis/cirrhosis. All self-reported history of injection drug use, 18% recent non-injection drug use and 38% alcohol dependence. Six discontinued treatment (88% completed treatment in each arm). Of 22 who completed SOF+PR, all achieved SVR12 (22/25 = 88%); 15 of 22 who completed SOF+R achieved SVR12 (15/25 = 60%; p=0.05). Among those completing SOF+R, SVR12 was significantly less common among reporting ongoing substance use (36% vs. 100%) and missed doses. Active substance use and missed doses did not impact SVR with SOF+PR.
Conclusions
Field-based DOT of HCV therapy without real-time HCV RNA monitoring was feasible; however achieving 100% adherence was challenging. SOF+PR appeared superior to SOF+R in achieving SVR12, even when doses were missed with no discontinuations due to side effects. Further exploration of short duration treatment with peginterferon plus direct acting antivirals is warranted.
Keywords: directly observed therapy, hepatitis C treatment, Hepatitis C virus, India, low-and-middle income settings, people who inject drugs
Of the approximately 70 million persons chronically infected with hepatitis C virus (HCV) globally, approximately 90% reside in low-and-middle-income countries (LMICs).(1) With the advent of direct acting antivirals (DAA), HCV infection is curable with 12 weeks of all-oral, non-toxic agents.(2–6) With these remarkable developments, the World Health Organization released the first HCV global elimination targets for 2030.(7) The goal is to achieve 90% reduction in new cases and 65% reduction in HCV-associated mortality. Achieving these ambitious goals will require massive treatment scale-up in most countries where only about 5% of persons with chronic HCV have been diagnosed and fewer than 2% treated. Elimination programs in some settings(8, 9) have been facilitated by licensing and preferential pricing agreements and production of generic versions of new DAA, which have brought costs down to less than 500 USD/treatment course.(10)
However, as elimination programs shift towards HCV treatment delivery, they must take into consideration factors other than provision of free medications. First, while costs associated with medications have decreased substantially in some places, monitoring costs remain unchanged (for example, in India it costs ~80 USD for HCV RNA and ~90 USD for HCV genotype testing). Moreover, infrastructure for viral load and genotyping are not available in many LMICs. Second, elimination strategies will need to target all persons infected including drug-using populations who bear a disproportionate HCV burden and may have adherence challenges.(11)
Directly observed therapy (DOT), the standard of care for TB(12) has been demonstrated to improve treatment completion and response rates for TB,(13, 14) HIV(15) and HCV.(16–20) Using modified DOT, HCV treatment has been successfully delivered in opioid treatment programs(16–18) and prison settings(19) consistently demonstrating improvements in adherence and cure rates.(16–20) These trials, however, were conducted in the pre-DAA era when regimens were more complicated (twice daily dosing and weekly injections). Further, none were in an LMIC. Key barriers to DOT consistently identified are transportation and patient-level inconvenience, which can lead to missed doses and dropouts,(14, 21) barriers which may be amplified in LMICs, where many patients are daily wage earners.
In India, it is estimated that there are approximately 6.3 million viremic HCV-infected persons. (1) India is also home to the largest number of opioid users globally (~3 million) with HCV prevalence of ~37% among people who inject drugs (PWID).(22) Generic sofosbuvir was licensed in India in 2015 and 11 generic forms are available at a maximum retail price of 300 USD/28 tablets.(10) However, delivery challenges remain. The goal of this trial was to leverage a rich history of DOT in India (cornerstone of the Indian National Tuberculosis Programme)(23) and the dearth of molecular testing to assess the feasibility of field-based directly observed therapy (DOT) with minimal monitoring to deliver HCV therapy to people who use drugs in Chennai, India. We directly compared the safety and efficacy of the only two pangenotypic HCV regimens available in India in 2015.(24)
MATERIALS AND METHODS
Study Setting and Population
This study operated from the YR Gaitonde Centre for Substance Abuse Related Research (YRGCSAR) in North Chennai, India. YRGCSAR was established in 2004 to explore the natural history of drug abuse and incidence and prevalence of associated blood-borne pathogens among PWID in Chennai.(25) Via this center, we have previously demonstrated high HCV burden (primarily genotype 3)(26) and liver disease(27) among PWID in Chennai. The site, which is approximately 1000 square feet, is staffed by one full-time clinician, one part-time clinician, two nurses, a site manager, a phlebotomist and three outreach workers, has provided testing and/or clinical services to >2000 PWID in Chennai(25) since inception and is currently following a cohort of ~1000.(27) Blood specimens are drawn at the center and transported to a central laboratory daily for testing.
Participants were recruited for this trial between September 2015 and March 2016 from an ongoing cohort of PWID.(27) The Chennai HIV, HCV and Eeral Study (CHHEERS) included 1,042 persons recruited through community outreach to characterize the epidemiology of liver disease among HCV-infected PWID in Chennai.(27) Participants had to be ≥18 years old, provide written informed consent, report a history of drug injection in the prior 5 years, and no intention of migrating for 2 years. At enrollment, three hundred and fifty-five participants (34.1%) were HCV antibody positive: (280) 78.9% were chronically infected and 11 (3.9%) reported prior HCV treatment.
In order to be eligible for the trial, subjects had to meet the following criteria, most of which are related to eligibility for peginterferon/ ribavirin-based therapy: (1) willing/able to provide written informed consent; (2) age ≥ 18 years; (3) documented evidence of active HCV infection (HCV RNA positive); (4) resident of Chennai; (5) HCV treatment naïve; and (6) if co-infected with HIV, have a CD4 > 350 cells/mm3 and either ART naïve; if on ART, participant had to be on a tenofovir-containing regimen. Subjects also had to have the following laboratory parameters at screening: (1) alanine aminotransferase (ALT) ≤ 10 x the upper limit of normal (ULN); (2) aspartate aminotransferase (AST) ≤ 10 x ULN; (3) hemoglobin ≥ 12 g/dl for male and 11 g/dl for female subjects; (4) International normalized ratio (INR) ≤ 1.5 x ULN unless subject has known hemophilia or was stable on an anticoagulant regimen affecting INR; (5) albumin ≥ 3 g/dl; 6) direct bilirubin ≤ 1.5 x ULN; (7) Creatinine clearance ≥ 60 ml/min as calculated by the Cockroft-Gault Equation; (8) alpha fetoprotein < 50 ng/ml; (9) absolute neutrophil count (ANC) ≥ 1,500/μL; (10) platelets ≥ 90,000/μL; and (11) thyroid stimulating hormone (TSH) ≤ ULN.
Participants were excluded if they satisfied any of the following criteria: (1) women who were pregnant or nursing; (2) male participants with pregnant female partners; (3) hepatic decompensation (Childs Pugh Class B and C); (4) co-infection with hepatitis B (HBsAg positive); (5) using medications contraindicated with peginterferon/ribavirin therapy; and (6) known contraindication to either peginterferon or ribavirin. All participants of reproductive potential were counseled to use at least two forms of contraception for six months after the completion of treatment.
Study Design
C-DOT was a randomized, open-label trial of sofosbuvir+peginterferon alfa 2a + weight-based ribavirin (SOF+PR) for 12 weeks (Arm 1) vs. sofosbuvir+weight-based ribavirin (SOF+R) for 24 weeks (Arm 2). Participants were randomized at a 1:1 allocation ratio using blocked randomization with varying block sizes. Sofosbuvir (Spegra, Emcure Pharmaceuticals Ltd.) was administered at 400 mg by mouth once daily. Ribavirin was sourced from Unison Pharmaceuticals (Univirin); based on low body weight, all participants took ribavirin 800 mg (four 200 mg tablets) by mouth once daily. Peginterferon alfa-2a (Taspiance, Emcure Pharmaceuticals Ltd.) was dosed at 180 μg by subcutaneous injection once weekly. All sofosbuvir and ribavirin doses were delivered daily to participants at venues of their choosing by three outreach workers. Participants in the SOF+PR arm were also required to visit the study clinic once weekly to receive their peginterferon injection. Prior to delivering medication in the field, outreach workers had to record participant biometric data (fingerprint) daily, providing confirmation that the correct participant received treatment. Additionally, outreach workers provided a small meal.
HCV RNA testing was performed at screening (to document active HCV infection) and 12 weeks after the end-of-treatment to determine sustained virologic response (SVR12) status. Since the treatment regimen was pangenotypic, HCV genotype was not determined prior to treatment and since HCV resistance was not expected, HCV RNA was not monitored during treatment. While on treatment, participants were asked to visit the clinic every 4 weeks and then 12 weeks after end-of-treatment to assess SVR12. At each visit, there was a physical exam including an assessment for adverse events and concomitant medications, and a survey collecting information on quality of life, depressive symptoms, alcohol and drug use, and adherence barriers. Safety monitoring comprising a complete blood count was performed every 4 weeks in both arms; additionally, a hepatic function panel was performed at week 12 for participants in the SOF+R arm. HCV genotyping and end-of-treatment HCV RNA testing were conducted retrospectively on stored specimens.
Study Endpoints and Statistical Analysis
The primary endpoint was treatment completion defined as completing 12 (Arm 1) or 24 (Arm 2) weeks of therapy and attending the SVR12 visit. Secondary endpoints included 1) SVR12 defined as HCV RNA < lower limit of quantification (LLOQ) 12 weeks after the end of treatment; 2) incidence of serious adverse events related to therapy defined as either Grade 3, 4 or 5 events as per the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0;(28) and 3) change in insulin resistance measured by HOMA-IR. Changes in HOMA-IR were based on fasting laboratory assessments at baseline and SVR12 visit. We also captured information on quality of life using the EQ-5D which includes a visual analogue scale (VAS) of self-rated health quality from 0 (worst health state) to 100 (best health state).(29)
An intent-to-treat (ITT) (missing=failure) approach was used for the primary analysis. Fisher’s exact tests were used to compare categorical outcomes and Mann Whitney tests to compare continuous outcomes. Secondary analyses considered a per protocol (PP) approach and explored factors within each arm associated with SVR12 in the subset that completed treatment (n=44). Factors of interest included age, pre-treatment HCV RNA level, HCV genotype, BMI, liver stiffness and ongoing substance use (drug and alcohol use). All analyses were conducted using Stata Version 13.1 (College Station, Texas).
Ethical Clearances
This study was approved by the Johns Hopkins Medicine and YRGCARE institutional review boards and all participants provided written informed consent.
RESULTS
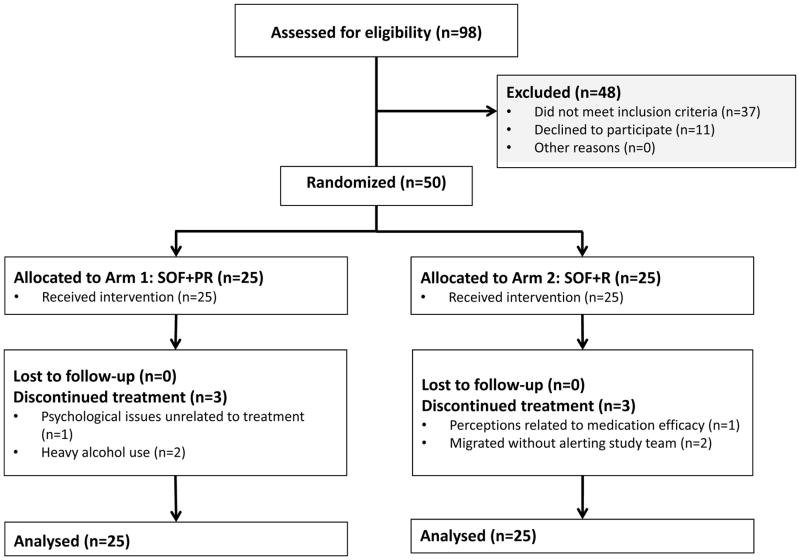
We screened 98 participants, of whom, 61 were eligible and 50 enrolled (Figure 1); Common reasons for exclusion were no active HCV infection (n=24), HIV positive status with CD4<350 or not on a tenofovir-containing regimen (n=4), and creatinine clearance<60 ml/min (n=4). The median age was 46 (interquartile range, 42 – 49). All were male and the majority (54%) had less than high school education, with a median monthly income of 90 USD/month (Table 1). 24 (48%) were daily wage earners. Two participants were co-infected with HIV; one was on ART. All participants self-reported that they had injected drugs in the past – one participant self-reported that he was actively injecting at entry into the trial. Eighteen percent reported active non-injection drug use and 38% had an AUDIT score consistent with dependence. Based on elastography (Fibroscan), the majority (58%) had no/mild liver stiffness (<8.5 kPa); 22% had moderate stiffness (8.5–12.3 kPa) and 10% severe stiffness/cirrhosis (>12.3 kPa). The median AST, ALT and FIB-4 measurements were 49 U/L, 42 U/L and 2.2, respectively. The median HCV RNA level was 6.4 log10 IU/ml. Post-treatment testing revealed that the majority of participants were infected with genotype 3 (n=42, 84%) followed by genotype 1 (n= 7, 14%).
Figure 1.
Trial Profile
Table 1.
Description of study population at baseline by treatment arm
| Arm 1 (N=25) 12 weeks SOF+PR |
Arm 2 (N=25) 24 weeks SOF+R |
|||
|---|---|---|---|---|
| Median age (years), IQR | 46 | 41 – 50 | 46 | 44 – 47 |
| Male sex, n(%) | 25 | 100 | 25 | 100 |
| Educational attainment, n(%) | ||||
| None or primary | 11 | 44.0 | 12 | 48.0 |
| Secondary | 3 | 12.0 | 1 | 4.0 |
| High school or greater | 11 | 44.0 | 12 | 48.0 |
| Median monthly income (US dollars), IQR | 90 | 68 – 120 | 90 | 72 – 150 |
| Employment status, n(%) | ||||
| Daily wages | 13 | 52.0 | 11 | 44.0 |
| Weekly/monthly wages | 10 | 40.0 | 13 | 52.0 |
| Unemployed | 2 | 8.0 | 1 | 4.0 |
| Median age at initiation of drug injection (years), IQR | 21 | 18 – 30 | 24 | 20 – 30 |
| Lifetime injection drug use, n(%) | ||||
| Heroin | 24 | 96.0 | 25 | 100.0 |
| Sedatives | 24 | 96.0 | 23 | 92.0 |
| Other opioids including buprenorphine | 19 | 76.0 | 16 | 64.0 |
| Injection drug use in prior six months, n(%) | 1 | 4.0 | 0 | 0 |
| Non-injection drug use in prior six months, n(%) | 7 | 28.0 | 2 | 8.0 |
| Marijuana use in prior six months, n(%) | 4 | 16.0 | 1 | 4.0 |
| Alcohol use in prior six months (Drinks/day), n(%) | ||||
| None | 14 | 56.0 | 13 | 52.0 |
| 1–4 drinks/day | 9 | 36.0 | 9 | 36.0 |
| >5 drinks per day | 2 | 8.0 | 3 | 12.0 |
| AUDIT category, n(%) | ||||
| No/mild alcohol use | 14 | 56.0 | 14 | 56.0 |
| Harmful/hazardous alcohol use | 1 | 4.0 | 2 | 8.0 |
| Alcohol dependence | 10 | 40.0 | 9 | 36.0 |
| HIV status, n(%) | 0 | 0 | 2 | 8.0 |
| HCV genotype, n(%) | ||||
| 3a | 22 | 88.0 | 20 | 80.0 |
| 1a | 2 | 8.0 | 5 | 20.0 |
| 6n | 1 | 4.0 | 0 | 0 |
| Median log10 HCV RNA (IU/ml), IQR | 6.5 | 6.1 – 6.6 | 6.1 | 5.5 – 6.7 |
| Liver stiffness category, n(%) | ||||
| <8.5 kPa | 15 | 60.0 | 14 | 56.0 |
| 8.5–12.3 kPa | 5 | 20.0 | 6 | 24.0 |
| >12.3 kPa | 5 | 20.0 | 5 | 20.0 |
| FIB-4 index, n(%) | ||||
| ≤1.45 | 6 | 24.0 | 7 | 28.0 |
| 1.46 – 3.25 | 16 | 64.0 | 11 | 44.0 |
| >3.25 | 3 | 12.0 | 7 | 28.0 |
| Median ALT (U/L), IQR | 38 | 32 – 64 | 45 | 29 – 69 |
| Median AST (U/L), IQR | 48 | 33 – 80 | 50 | 32 – 89 |
| Median platelet count (109/L), IQR | 174 | 147 – 210 | 155 | 132 – 184 |
| Median albumin (g/dL), IQR | 4.0 | 3.9 – 4.3 | 4.1 | 4.0 – 4.2 |
| Median total bilirubin (mg/dL), IQR | 0.8 | 0.7 – 0.9 | 0.7 | 0.6 – 1.0 |
| Median glucose (mg/dL), IQR | 84 | 81 – 104 | 90 | 85 – 107 |
| Median insulin (μU/mL), IQR | 7 | 3 – 13 | 10 | 6 – 22 |
| Median HOMA-IR, IQR | 1.3 | 0.7 – 3.4 | 2.4 | 1.1 – 5.6 |
| Median weight (kg), IQR | 55 | 49 – 62 | 65 | 54 – 70 |
| Depressive symptoms*, n(%) | 21 | 84.0 | 19 | 76.0 |
| None | 3 | 12.0 | 4 | 16.0 |
| Mild | 1 | 4.0 | 2 | 8.0 |
| Moderate / Severe | ||||
| Quality of life index†, n(%) | 1.0 | 0.82 – 1.0 | 1.0 | 0.83 – 1.0 |
| Mobility problems | 3 | 12.0 | 1 | 4.0 |
| Self-care problems | 4 | 16.0 | 1 | 4.0 |
| Usual activities problems | 3 | 12.0 | 2 | 8.0 |
| Pain | 10 | 40.0 | 7 | 28.0 |
| Anxiety or depression | 4 | 16.0 | 5 | 20.0 |
| Median self-rated health state VAS †, n(%) | 85 | 80 – 90 | 90 | 80 – 90 |
Data are presented as n (column %) or median (interquartile range [IQR])
SOF+PR: sofosbuvir + peginterferon alfa 2a + weight-based ribavirin; SOF+R: sofosbuvir + ribavirin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HOMA-IR: homeostasis model assessment-estimated insulin resistance score; VAS: visual analogue scale with range from 0 (worst health state) to 100 (best health state)
measured using the PHQ-9 instrument;
measured using the EQ 5D-3L instrument;
Outcomes
Primary Outcome
Among the 50 participants, 6 discontinued treatment (3 per arm) for a treatment completion rate of 88% in each arm (Table 2). Of the 6 participants who discontinued treatment, 3 discontinued in the first week, and 1 each in weeks 4, 5 and 6 (Table 3). Despite these discontinuations, we obtained a specimen to examine SVR12 in one participant who received 23 days of SOF+PR before stopping. Reasons for discontinuation are in Figure 1.
Table 2.
Study outcomes by treatment arm
| Arm 1 (N=25) 12 weeks SOF+PR |
Arm 2 (N=25) 24 weeks SOF+R |
p-value | |||
|---|---|---|---|---|---|
| Primary outcome | |||||
| Treatment completion, n(%) | 22 | 88.0 | 22 | 88.0 | >0.99 |
| Secondary outcomes | |||||
| Sustained virologic response*, n(%) | 22 | 88.0 | 15 | 60.0 | 0.05 |
| Median number of serious adverse events, IQR | 0 | 0 | |||
| Median change in insulin resistance (HOMA-IR), IQR | 1.2 | -0.1, 9.1 | 0.1 | -1.3, 6.1 | 0.30 |
| Exploratory outcomes | |||||
| Percentage completed doses, n(%) | |||||
| 0–30% | 3 | 12.0 | 3 | 12.0 | |
| 75–90% | 3 | 12.0 | 2 | 8.0 | |
| >90–95% | 2 | 8.0 | 4 | 16.0 | 0.93 |
| >95%-100% | 17 | 68.0 | 16 | 64.0 | |
| Percentage observed doses received†, n(%) | |||||
| 70–90% | 0 | 0 | 3 | 12.0 | |
| >90–95% | 4 | 16.0 | 3 | 12.0 | 0.31 |
| >95–100% | 21 | 84.0 | 19 | 76.0 | |
| Median change in self-rated health state VAS †, IQR | 0 | -5, 10 | 5 | -10, 8 | 0.58 |
Intent-to-treat approach (missing=no sustained virologic response)
Out of completed doses
VAS: visual analogue scale with range from 0 (worst health state) to 100 (best health state); †measured using the EQ 5D-3L instrument
Table 3.
Characteristics of participants who either discontinued or failed treatment
| Arm | Week stopped | Age | Liver stiffness (kPa) | HIV status | Substance use in prior month | AUDIT score | Missed doses | Entry genotype | Entry HCV RNA in IU/mL | EOT HCV RNA in c/ml | Genotype at 12-week post treatment visit | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discontinued | ||||||||||||
| 1 | SOF+PR | 1 | 44 | 6.8 | Negative | Yes | 18 | 82 | 1a | 1,457,217 | - | - |
| 2 | SOF+PR | 4 | 45 | 20.9 | Negative | No | 0 | 60 | 1a | 2,866,567 | 3,318,462* | - |
| 3 | SOF+PR | 1 | 47 | 28 | Negative | No | 0 | 81 | 3a | 3,069,335 | - | - |
| 4 | SOF+R | 1 | 41 | 4.4 | Negative | No | 0 | 167 | 1a | 8,422,551 | - | - |
| 5 | SOF+R | 6 | 54 | 14.1 | Negative | No | 0 | 136 | 3a | 1,321,974 | - | - |
| 6 | SOF+R | 5 | 44 | 6.7 | Negative | Yes | 18 | 151 | 3a | 7,503,580 | - | - |
| Failed treatment | ||||||||||||
| 1 | SOF+R | 27 | 6.8 | Negative | Yes | 20 | 39 | 1a | 199,896 | 35 | 1a | |
| 2 | SOF+R | 59 | 35.8 | Negative | Yes | 8 | 4 | 1a | 6,398,338 | undetectable | 1a | |
| 3 | SOF+R | 53 | 36.8 | Negative | Yes | 29 | 3 | 1a | 2,257,839 | 5458 | 1a | |
| 4 | SOF+R | 41 | 8.4 | Negative | Yes | 34 | 16 | 3a | 1,194,654 | undetectable | 3a | |
| 5 | SOF+R | 45 | 4 | Negative | Yes | 15 | 2 | 3a | 193,013 | undetectable | 3a | |
| 6 | SOF+R | 45 | 7.8 | Negative | Yes | 21 | 12 | 3a | 68,966 | undetectable | 3a | |
| 7 | SOF+R | 46 | 11.3 | Positive | Yes | 5 | 1 | 3a | 383,733 | undetectable | 3a | |
Measured 8 weeks after last dose.
Secondary Outcomes
Of the 22 who completed SOF+PR, all achieved SVR12 (ITT: 22/25 = 88% [PP: 22/22 = 100%]) but only 15 of the 22 who completed SOF+R achieved SVR (15/25 = 60% [PP: 15/22 = 68%]; p-value for ITT=0.05). Of the treatment failures with SOF+R, 3 had genotype 1a and 4 had genotype 3a infection; five of the seven had HCV RNA<LLOQ at the end of treatment. The Core/E1 regions were sequenced on the 12-week post-treatment specimen and the genotype was identical to the baseline in all specimens; however, more detailed phylogenetic analyses were not conducted to distinguish relapse and re-infection. There were no treatment failures on SOF+PR, but of those that did not complete SOF+PR, 2 had genotype 1a and one had genotype 3a infection. For the one participant treated with SOF+PR from which we obtained a post-discontinuation sample, HCV RNA was 6.5 IU/mL; no serious adverse events occurred; the frequency of adverse events was comparable across arms (Supplementary Table 1). The median change in HOMA-IR in the SOF+PR arm and SOF+R arms were 1.2 and 0.1, respectively (p=0.30).
Exploratory outcomes
Of the 44 who completed treatment, the median number of missed doses of oral medication was 2 in SOF+PR (range: 0–18) and 6 in SOF+R (range: 0–39). No peginterferon injections were missed for those who completed treatment.
Factors associated with treatment completion and SVR12
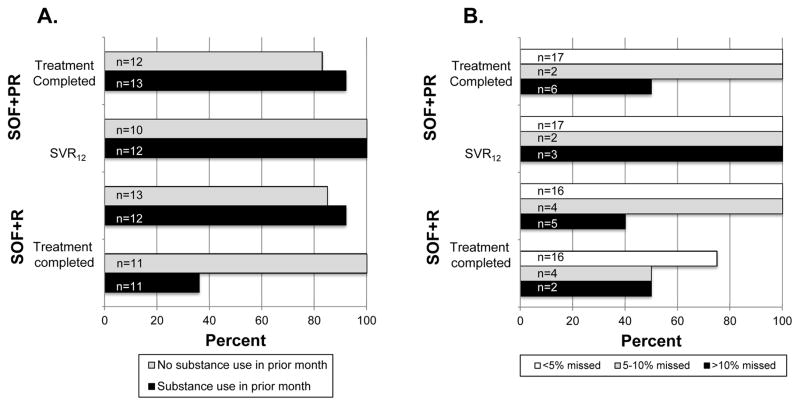
We further assessed whether treatment completion and SVR12 among those who completed treatment varied within arm by pre-treatment characteristics. Among those who completed treatment in the SOF+R arm, SVR12 was significantly lower among those with missed doses, ongoing substance use (drugs or alcohol; Figure 2) genotype 1a, lower HCV RNA and lower BMI. None of these factors including missed doses or active substance use affected SVR12 in the SOF+PR arm – all subsets achieved SVR12.
Figure 2.
Treatment completion and sustained virologic response 12 by treatment arm, substance use (Panel A) and missed doses (Panel B).
DISCUSSION
This study is among the first to evaluate directly observed delivery of DAA-based therapy in populations with a history of substance use in an LMIC setting. Our findings provide some insight into the realization of the global HCV elimination goals. First, these data support that substance using populations in a LMIC setting can be cured of HCV using a field-based DOT approach. Second, the data support that therapeutic monitoring before and during can be dramatically simplified including the removal of genotype determination. Third, HCV therapy can be delivered in LMICs with minimal infrastructure and staffing. Treatment delivery and monitoring can potentially be even further simplified with newer ribavirin-free pangenotypic regimens and advances in diagnostics (e.g., Cepheid GeneXpert HCV RNA testing).(30) Fourth, there may be a benefit of retaining peginterferon in treatment of populations where injections are perceived favorably and adherence may be challenging because: (1) it can shorten the duration of treatment; and (2) the long half-life of peginterferon can be forgiving of occasional missed doses.
Treatment completion rates were high and comparable in both groups in this trial, but SVR12 among those who completed treatment was significantly higher in those who received SOF+PR (100%) compared to those that received SOF+R (68%). Both SVR12 rates are within the range of what has been observed in prior studies of these combinations among genotype 3 populations. For example, in BOSON, a large randomized trial that compared SOF+R for 24 weeks vs. SOF+PR for 12 weeks in genotype 3 patients, SVR12 rates were 84% and 93%, respectively.(31) In VALENCE, an SVR12 of 85% was observed with 24 weeks of SOF+R.(32) SVR12 rates were lower in HCV-TARGET, a real-world clinical cohort, which reported SVR12 of 60% and 84%, for SOF+R and SOF+PR, respectively.(33) There have also been several reports evaluating sofosbuvir in India, both clinical trials and observational studies among patients with predominantly genotype 3 infection, with SVR12 rates upwards of 90%.(34–37) None of these studies in or outside India focused on persons with a history of substance use.
Interestingly and in contrast what has been observed previously,(38) we found low SVR12 among those with genotype 1 infection; only one of four genotype 1 patients who completed treatment with SOF+R achieved SVR12. However, the three patients who failed had characteristics previously associated with poor treatment response. One was actively using drugs and missed 32 doses and two had high pre-treatment viral loads and cirrhosis (liver stiffness>30 kPa). These lower response rates are consistent with the NIH SPARE trial, which included genotype 1 infected patients with unfavorable treatment predictors, and observed efficacy of 24 weeks of SOF+R to be 68% in those receiving weight-based ribavirin and 48% in those receiving low-dose ribavirin.(39)
Collectively, these data speak to the possibility of achieving cure in substance using populations using DOT, but also highlight challenges. On the one hand, the field-based DOT strategy that we used may be particularly suited for LMIC settings where human resources are abundant and salaries relatively low (the monthly salary of an outreach worker is ~250 USD). For example, if one field worker could provide DOT to 20 patients at a time for ~12 weeks, the additional treatment cost would only be 38 USD/individual. On the other hand, we did encounter challenges with this approach. In December 2015, Chennai experienced the worst flooding in over a century, receiving 16 inches of rain in 2 days making it impossible to reach participants for 2–3 days. The floods impacted 31 participants who were already on treatment, explaining 36% of all missed doses experienced. Additional challenges ensued in April and May for the 16 participants who were still receiving treatment. Extreme heat (temperatures >106 degrees Fahrenheit/ 42 degrees Celsius) impacted the ease with which contact could be made between participants and field workers. Beyond these weather-related challenges, the primary reasons for missing meetings with DOT field workers were family emergencies and unanticipated travel. A limitation of our study is that we did not have a comparison group that did not receive DOT and it is possible that such intensive intervention was not necessary for all. Subsequent studies among persons with a history of substance use should consider alternatives such as mobile phone based-DOT, clinic based-DOT (with/without opioid agonist treatment) or should compare DOT with standard 4-week prescriptions to determine the optimal strategy.
In this study, as we used pan-genotypic regimens with demonstrated efficacy and no stopping rules, we opted for a minimal number of monitoring tests. No genotyping was performed prior to treatment initiation and neither on-treatment nor end-of-treatment HCV RNA testing was performed. The only safety monitoring included a monthly complete blood count. Despite this, our treatment outcomes were comparable to other reports and, even using agents historically considered to be “highly toxic,” no participant experienced an SAE. It can be argued that some on-treatment monitoring, in particular, the 4-week HCV RNA level, may be an important adherence intervention in and of itself. In our study, the absence of this measurement likely had little impact because we maintained daily contact with participants. While we cannot rule out the value of the 4-week HCV RNA level in populations not receiving DOT, we feel these data support WHO guidance that substantial reductions in cost can be achieved by reducing monitoring tests. Further reductions (e.g., less frequent complete blood count) may be possible with newer pan-genotypic ribavirin free combinations.
Beyond molecular monitoring, we delivered treatment out of a community clinic, chosen because of its convenient location for participants, with minimal infrastructure including a small phlebotomy unit, clinical examination room and liver elastography (available through research funds). Clinicians were trained to treat hepatitis C with oversight from clinicians at the Johns Hopkins Viral Hepatitis Center. Support staff included two nurses and three outreach workers (who were also tracking participants in an ongoing cohort);(27) all were also trained to provide counseling. As LMICs begin to implement elimination programs, scaling up these types of community clinics to provide HCV treatment may prove critical. Global experience with delivering HIV treatment in similar settings has demonstrated that accessibility is a key facilitator.(40) For HCV, infrastructure required is even more minimal than what is needed for HIV and would include linkage to laboratory that can perform simple tests (e.g., FIB-4), a rapid HCV RNA measure (e.g., Cepheid GeneXpert), a clinician (nurse or doctor), and support staff (e.g., outreach workers).
We were concerned about the acceptability of peginterferon particularly because prior observational studies in India have suggested patient preference for SOF+R for 24 weeks over SOF+PR for 12 weeks due to inaccessibility of facilities providing peginterferon, financial constraints (peginterferon is expensive) and fear of side effects.(35, 36, 41) However, in this trial, no participants refused participation because of the potential of being randomized to receive peginterferon. In fact, some participants were disappointed not to have been randomized to receive “injections”. In India, particularly in lower-income groups, there is widespread belief that injections are more potent than pills. The annual per capita number of injections ranges from 3 to 6,(42, 43) one of the highest in the world.
Moreover, all those who completed therapy with SOF+PR achieved SVR12. Contrastingly, the efficacy of SOF+R appeared to have been affected by ongoing substance use and non-adherence. While few persons in our sample reported ongoing drug injection, 50% reported some substance use in the 30 days prior to initiating treatment of whom 76% had evidence of alcohol dependence. Active substance use was associated with significantly lower response to SOF+R among those who completed treatment (36% vs 100%, p=0.03). Moreover, SVR12 for SOF+R was 75% in those who missed fewer than 5% of doses it was only 50% in those who missed >10% of doses. No such differences were observed in the SOF+PR arm. Interestingly, a recent study among PWID reported SVR12 of 92% among 32 patients randomized to 4 weeks of Ledipasvir+SOF+PR vs. 77% in 32 patients randomized to 4 weeks of Ledipasvir+SOF+R in patents infected with genotype 1, 2 or 3.(44) These data lend further support to a DOT-based approach and provide rationale for further investigation into the utility of combining PR with pangenotypic regimens such as SOF+daclatasvir (SOF+DAC) or SOF+velpatasvir (SOF+VEL) for short durations (e.g., 4 or 6 weeks) in substance using populations (both alcohol and drug use) and others with potentially poor adherence.
Several limitations must be acknowledged. The sample size is small and precluded additional subgroup comparisons. Even those that were conducted should be considered exploratory. We conducted this trial prior to the availability of daclatasvir and velpatasvir in India – these combinations (SOF+DAC or SOF+VEL) are superior to SOF+R with respect to SVR and it possible that these regimens could also be more forgiving of missed doses. However, the potential to shorten duration dramatically (4 weeks) by including PEG with newer combinations such as SOF+DAC or SOF+VEL as demonstrated in the 4WIDU-C study greatly enhances the feasibility of DOT based therapy and warrants further investigation especially since short durations of PEG are associated with minimal side effects. Further if a 4-week regimen is found efficacious, DOT staff could treat three times as many patients in a 12 week period. (44)
In conclusion, these data demonstrate the feasibility of curing HCV in persons with a history of substance use in an LMIC setting with minimal use of molecular tests and limited infrastructure using a field-based DOT approach. Simplification of regimens will further facilitate delivery of these medications in such settings. Important challenges remain particularly related to ongoing substance use and non-adherence; there may still be a role for peginterferon in these sub-populations.
Supplementary Material
Supplementary Table 1. Frequency of adverse events
Acknowledgments
This research was funded in part by the National Institutes of Health, R01DA026727 and DP2DA040244 and T32AI102623 and the Johns Hopkins University Center for AIDS Research (1P30AI094189). Dr. Sulkowski was partially supported by K24DA034621.
List of Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- ALT
Alanine Aminotransferase
- ANC
Absolute Neutrophil Count
- ART
Antiretroviral Therapy
- AST
Aspartate Aminotransferase
- AUDIT
Alcohol Use Disorders Identification Test
- BMI
Body Mass Index
- CD4
Cluster of Differentiation 4
- CHHEERS
Chennai HIV, HCV and Eeral Study
- DAA
Direct-acting Antiviral Agent
- DOT
Directly Observed Therapy
- EQ-5D
EuroQOL Five Dimensions
- FIB-4
Fibrosis-4
- HBsAg
Hepatitis B Surface Antigen
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency Virus
- HOMA-IR
Homeostatic Model Assessment-Insulin Resistance
- INR
International Normalized Ratio
- ITT
Intent to Treat
- LLOQ
Lower Limit of Quantification
- LMICs
Low and Middle Income Countries
- NIH
National Institutes of Health
- PP
Per Protocol
- PWID
People Who Inject Drugs
- RNA
Ribonucleic Acid
- SAE
Severe Adverse Event
- SOF
Sofosbuvir
- SOF+PR
Sofosbuvir plus Peginterferon alfa 2a plus Ribavirin
- SOF+R
Sofosbuvir plus Ribavirin
- SVR12
Sustained Virologic Response at 12 weeks
- TB
Tuberculosis
- TSH
Thyroid Stimulating Hormone
- ULN
Upper Limit of Normal
- USD
United States Dollar
- VAS
Visual Analogue Scale
- WHO
World Health Organization
- YRGCARE
YR Gaitonde Centre for AIDS Research and Education
- YRGCSAR
YR Gaitonde Centre for Substance Abuse Related Research
Footnotes
Conflict of interest: The authors have no conflicts of interest.
Author contributions: Sunil Solomon, Mark Sulkowski and Shruti Mehta designed the trial. Aylur Srikrishnan, Pradeep Ambrose, Balakrishnan Ramasamy and Santhanam Anand implemented the trial and data collection procedures. Allison McFall and Shruti Mehta analyzed the data. David Thomas and Muniratnam Kumar provided critical input into trial design and implementation. All authors reviewed and approved the final manuscript.
Clinical trial number: NCT02541409
References
- 1.Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. The Lancet Gastroenterology & Hepatology. 2(3):161–76. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 3.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. New England Journal of Medicine. 2014;370(20):1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 5.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 6.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–23. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Combating hepatitis B and C to reach elimination by 2030. 2016 Available from: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf?ua=1.
- 8.Mitruka K, Tsertsvadze T, Butsashvili M, Gamkrelidze A, Sabelashvili P, Adamia E, et al. Launch of a Nationwide Hepatitis C Elimination Program--Georgia, April 2015. MMWR Morb Mortal Wkly Rep. 2015;64(28):753–7. doi: 10.15585/mmwr.mm6428a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Egypt steps up efforts against hepatitis C. 2014 Available from: http://www.who.int/features/2014/egypt-campaign-hepatitisc/en/
- 10.Hill A, Simmons B, Gotham D, Fortunak J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad. 2016;2(1):28–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Azar P, Wood E, Nguyen P, Luma M, Montaner J, Kerr T, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infect Dis. 2015;15:193. doi: 10.1186/s12879-015-0913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayer R, Wilkinson D. Directly observed therapy for tuberculosis: history of an idea. Lancet. 1995;345(8964):1545–8. doi: 10.1016/s0140-6736(95)91090-5. [DOI] [PubMed] [Google Scholar]
- 13.Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. JAMA. 1995;274(12):945–51. [PubMed] [Google Scholar]
- 14.Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330(17):1179–84. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 15.Hart JE, Jeon CY, Ivers LC, Behforouz HL, Caldas A, Drobac PC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. 2010;54(2):167–79. doi: 10.1097/QAI.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonkovsky HL, Tice AD, Yapp RG, Bodenheimer HC, Jr, Monto A, Rossi SJ, et al. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. The American journal of gastroenterology. 2008;103(11):2757–65. doi: 10.1111/j.1572-0241.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- 17.Bruce RD, Eiserman J, Acosta A, Gote C, Lim JK, Altice FL. Developing a modified directly observed therapy intervention for hepatitis C treatment in a methadone maintenance program: implications for program replication. Am J Drug Alcohol Abuse. 2012;38(3):206–12. doi: 10.3109/00952990.2011.643975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin AH, Berg KM, Li X, Hidalgo J, Arnsten JH. Rationale and design of a randomized controlled trial of directly observed hepatitis C treatment delivered in methadone clinics. BMC Infect Dis. 2011;11:315. doi: 10.1186/1471-2334-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiz de la Hoya P, Portilla J, Marco A, Garcia-Guerrero J, Faraco I, Anton J, et al. Directly observed therapy for chronic hepatitis C: a randomized clinical trial in the prison setting. Gastroenterol Hepatol. 2014;37(8):443–51. doi: 10.1016/j.gastrohep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Waizmann M, Ackermann G. High rates of sustained virological response in hepatitis C virus-infected injection drug users receiving directly observed therapy with peginterferon alpha-2a (40KD) (PEGASYS) and once-daily ribavirin. J Subst Abuse Treat. 2010;38(4):338–45. doi: 10.1016/j.jsat.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Jereb JA, Simone PM, Onorato IM. Directly observed therapy and tuberculosis treatment completion. Jereb et al. re: Bayer et al. Am J Pub Health. 1999;89(4):603–4. doi: 10.2105/ajph.89.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon SS, Mehta SH, Srikrishnan AK, Solomon S, McFall AM, Laeyendecker O, et al. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis. 2015;15(1):36–45. doi: 10.1016/S1473-3099(14)71045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murali MS, Sajjan BS. DOTS strategy for control of tuberculosis epidemic. Indian J Med Sci. 2002;56(1):16–8. [PubMed] [Google Scholar]
- 24.Puri P, Anand AC, Saraswat VA, Acharya SK, Dhiman RK, Sarin SK, et al. Indian National Association for Study of the Liver (INASL) Guidance for Antiviral Therapy Against HCV Infection in 2015. Journal of Clinical and Experimental Hepatology. 2015;5(3):221–38. doi: 10.1016/j.jceh.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon SS, Desai M, Srikrishnan AK, Thamburaj E, Vasudevan CK, Kumar MS, et al. The profile of injection drug users in Chennai, India: identification of risk behaviours and implications for interventions. Substance use & misuse. 2010;45(3):354–67. doi: 10.3109/10826080903452447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E, et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49(3):327–32. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SS, Srikrishnan AK, McFall AM, Kumar MS, Saravanan S, Balakrishnan P, et al. Burden of Liver Disease among Community-Based People Who Inject Drugs (PWID) in Chennai, India. PLoS One. 2016;11(1):e0147879. doi: 10.1371/journal.pone.0147879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectous Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. 2014. [Google Scholar]
- 29.Gusi N, Olivares PR, Rajendram R. The EQ-5D Health-Related Quality of Life Questionnaire. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer New York; 2010. pp. 87–99. [Google Scholar]
- 30.McHugh MP, Wu AH, Chevaliez S, Pawlotsky JM, Hallin M, Templeton KE. Multicentre Evaluation of the Cepheid Xpert(R) Hepatitis C Virus (HCV) Viral Load Assay. J Clin Microbiol. 2017 doi: 10.1128/JCM.02460-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149(6):1462–70. doi: 10.1053/j.gastro.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 33.Feld JJ, Maan R, Zeuzem S, Kuo A, Nelson DR, Di Bisceglie AM, et al. Effectiveness and Safety of Sofosbuvir-Based Regimens for Chronic HCV Genotype 3 Infection: Results of the HCV-TARGET Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(6):776–83. doi: 10.1093/cid/ciw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta R, Kabrawala M, Nandwani S, Tekriwal R, Nandaniya P, Shah M, et al. Efficacy and safety of sofosbuvir-based therapy for chronic hepatitis C infection in “real-life” cohort. Indian J Gastroenterol. 2016;35(6):459–64. doi: 10.1007/s12664-016-0713-5. [DOI] [PubMed] [Google Scholar]
- 35.Satsangi S, Mehta M, Duseja A, Taneja S, Dhiman RK, Chawla Y. Dual treatment with sofosbuvir plus ribavirin is as effective as triple therapy with pegylated interferon plus sofosbuvir plus ribavirin in predominant genotype 3 patients with chronic hepatitis C. J Gastroenterol Hepatol. 2016;6(Suppl 1):S21. doi: 10.1111/jgh.13595. [DOI] [PubMed] [Google Scholar]
- 36.Sood A, Midha V, Mahajan R, Narang V, Mehta V, Wander P, et al. Results of Sofosbuvir based combination therapy for chronic hepatitis C cohort of Indian patients in real life clinical practice. J Gastroenterol Hepatol. 2016 doi: 10.1111/jgh.13628. [DOI] [PubMed] [Google Scholar]
- 37.Shah SR, Chowdhury A, Mehta R, Kapoor D, Duseja A, Koshy A, et al. Sofosbuvir plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 or 3 infection in India. J Viral Hepat. 2016 doi: 10.1111/jvh.12654. [DOI] [PubMed] [Google Scholar]
- 38.Brown RS, Jr, O’Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR, Jr, et al. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl. 2016;22(1):24–33. doi: 10.1002/lt.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. Jama. 2013;310(8):804–11. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Med. 2016;13(8):e1002107. doi: 10.1371/journal.pmed.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falade-Nwulia O, Mehta SH, Lasola J, Latkin C, Niculescu A, O’Connor C, et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat. 2016;23(5):366–74. doi: 10.1111/jvh.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IPEN Study Group. Injection practices in India. WHO South-East Asia Journal of Public Health. 2012;1(2):189–200. doi: 10.4103/2224-3151.206931. [DOI] [PubMed] [Google Scholar]
- 43.Janjua NZ, Butt ZA, Mahmood B, Altaf A. Towards safe injection practices for prevention of hepatitis C transmission in South Asia: Challenges and progress. World journal of gastroenterology : WJG. 2016;22(25):5837–52. doi: 10.3748/wjg.v22.i25.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ovrehus ALBI, Kinggaard Holm D, Moessner BK, Krarup H, Christensen PB. Four weeks of sofosbuvir, ledipasvir and ribavirin with or without interferon give high cure rates in drug users with hepatitis C - a randomized controlled trial (4WIDUC) Hepatology. 2016;64(Suppl1):462A–3A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Frequency of adverse events