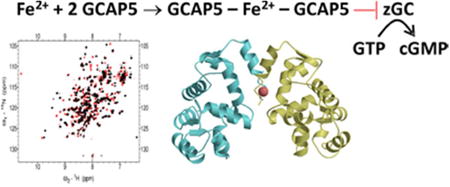
Abstract
Sensory guanylate cyclases (zGCs) in zebrafish photoreceptors are regulated by a family of guanylate cyclase activator proteins (called GCAP1-7). GCAP5 contains two non-conserved cysteine residues (Cys15 and Cys17) that could in principle bind to biologically active transition state metal ions (Zn2+ and Fe2+). Here, we present nuclear magnetic resonance (NMR) and isothermal titration calorimetry (ITC) binding analysis that demonstrate the binding of one Fe2+ ion to two GCAP5 molecules (in a 1:2 complex) with a dissociation constant in the nanomolar range. At least one other Fe2+ binds to GCAP5 with micromolar affinity that likely represents electrostatic Fe2+ binding to the EF-hand loops. The GCAP5 double mutant (C15A/C17A) lacks nanomolar binding to Fe2+, suggesting that Fe2+ at this site is ligated directly by thiolate groups of Cys15 and Cys17. Size exclusion chromatography analysis indicates that GCAP5 forms a dimer in both the Fe2+-free and Fe2+-bound states. NMR structural analysis and molecular docking studies suggest that a single Fe2+ ion is chelated by thiol side chains from Cys15 and Cys17 in the GCAP5 dimer, forming a [Fe(SCys)4] complex like that observed previously in two-iron superoxide reductases. Fe2+ binding to GCAP5 decreases its ability to activate photoreceptor human GC-E by decreasing GC-activity more than 10-fold. Our results indicate a strong Fe2+-induced inhibition of GC by GCAP5 and suggest that GCAP5 may serve as a redox sensor in visual phototransduction.
Graphical abstract
Introduction
Retinal guanylate cyclase activator proteins (GCAP11 and GCAP22) were first identified as EF-hand calcium sensor proteins in mammalian photoreceptor rod and cone cells3. Both GCAP1 and GCAP2 control Ca2+-sensitive activation of retinal guanylate cyclases (RetGCs4, 5) that is crucial for promoting the recovery phase of vertebrate visual phototransduction6, 7. Additional GCAP homologs were discovered recently in zebrafish (GCAP3-5 and 78). In situ hybridization showed that expression of zebrafish specific GCs and GCAPs starts between 3 and 4 days post fertilization coinciding with the onset of visual function9, 10. Initial in vitro studies further demonstrated that GCAPs activate mammalian GCs in a step-by-step Ca2+-relay mode fashion similar to the GC-GCAP system in mammals11-13. Interestingly, these earlier studies showed that GCAP5 is a much weaker GC activator compared to the other GCAPs under standard assay conditions13. Furthermore, GCAP5 has the most divergent amino acid sequence compared to mammalian and other zebrafish GCAP forms (Fig. 1). In particular, GCAP5 contains two non-conserved cysteine residues (Cys15 and Cys17) not found in the other GCAPs that in principle could be used to ligate biological transition metals such as Fe2+ and Zn2+. Ferrous ion has been shown to serve as a redox sensor in a variety of cell types 14, and it is tempting to speculate that redox sensing by GCAP5 might control phototransduction in zebrafish photoreceptors. Indeed, the accumulation of iron levels in the retina have been correlated to age-related macular degeneration in humans, suggesting the involvement of redox-sensitive processes in the pathogenesis of the disease15.
Figure 1. Amino acid sequence alignment of GCAP proteins.

EF-hand motifs are shaded in color (EF1 green, EF2 red, EF3 cyan and EF4 yellow). Non-conserved cysteine residues (Cys15 and Cys17) in GCAP5 are highlighted in bold and red. Swiss Protein Database accession numbers are Q90WX4 (zebrafish GCAP1), Q90WX3 (zebrafish GCAP2), Q8UUX9 (zebrafish GCAP3), Q6ZM98 (zebrafish GCAP4), and Q5MAC8 (zebrafish GCAP5).
In the current study, we characterized both the structure and binding properties of Fe2+ binding to GCAP5 in zebrafish. Our ITC binding analysis demonstrates that one Fe2+ binds to two molecules of GCAP5 with nanomolar affinity, consistent with one Fe2+ bound per GCAP5 dimer. At least one additional Fe2+ binds to GCAP5 in the micromolar range that likely binds to the second EF-hand. NMR structural studies reveal that both Cys15 and Cys17 are essential for nanomolar Fe2+ binding to GCAP5, which form a [Fe(SCys)4] complex. Our functional studies indicate that Ca2+-free/Fe2+-bound and Ca2+-bound/Fe2+-bound GCAP5 both strongly inhibit mammalian GC activity compared to that of Fe2+-free GCAP5. We suggest that Fe2+ levels in photoreceptors (and hence metabolic activity) may be controlled by light activation, and therefore propose that Fe2+ binding to GCAP5 may serve as a light-dependent redox sensor in zebrafish photoreceptors.
Materials and Methods
Cloning, expression and purification of GCAP5 forms
Recombinant myristoylated GCAP5 (hereafter designated as GCAP5) was used throughout this study and bacterial expression of myristoylated GCAP5 was accomplished by co-expressing the GCAP5 D3N mutant16 and yeast N-myristoyl CoA transferase (NMT) in E. coli strain, BL21(DE3) as described previously16 for GCAP117. Acylation of the GCAP5 D3N mutant was originally confirmed in living cells by a click-chemistry approach16. Cloning of the C15A/C17A double mutant was similar as described for other point mutants of GCAPs11, 13. Accordingly, the double mutant was prepared employing primers 5′-CTCAGCGCCgcCAAAgcCCACCAGTGG-3′ (forward) and 5′-CTCGGTGGCGGACATGCTGG-3′ (reverse). Single point mutations were introduced to GCAP5 using the same reverse primer and 5′-CTCAGCGCCgcCAAATGCCACCAGTGG-3′ (forward) for the C15A mutant and 5′-CTCAGCGCCTGCAAAgcCCACCAGTGG-3′ (forward) for the C17A mutant. Purification of GCAP5 was achieved using previously described methods with the following modifications18, 19. Briefly, myristoylated GCAP5 was expressed in the soluble fraction, in contrast to unmyristoylated GCAP5 that was expressed in inclusion bodies. The cell lysate supernatant with the addition of 0.35 M NH4SO4 (final concentration) was loaded onto a Butyl sepharose column (HiPrep Butyl FF 16/10, GE Healthcare) equilibrated with loading buffer (20 mM Tris (pH7.5), 0.35M NH4SO4, and 1mM Dithiothreitol (DTT)), and the column was washed until the column wash contained no detectable protein (A280 < 0.01). GCAP5 protein (both wild type, GCAP5WT or C15A/C17A double mutant, GCAP5C15A/C17A) was then eluted from the Butyl sepharose column with a low salt buffer (20 mM Tris (pH7.5), 2 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) and 1 mM DTT). The eluted protein fraction from the Butyl-sepharose column was then diluted 5-fold and directly applied to a Q sepharose column (HiTrap 5ml Q HP, GE Healthcare) equilibrated in low salt buffer, and GCAP5 was eluted using a standard salt gradient18, 19. The eluted protein fraction from the Q-sepharose column was concentrated to a volume of 2 mL and then applied to a size exclusion chromatography column (HiLoad 26/600 Superdex, GE Healthcare) equilibrated in low salt buffer. The final GCAP5 protein was eluted into low salt buffer. Typically, 10 mg of final purified GCAP5 protein was obtained from 1 liter of cell culture. The final protein sample was more than 95% pure as judged by SDS-PAGE. Protein myristoylation was verified using reverse phase HPLC as described20.
Analytical size exclusion chromatography (SEC)
Measurement of the molar mass of GCAP5 in the presence of Mg2+ (5 mM) and/or Fe2+ (1 mM) was performed using analytical SEC (Superdex 200 HR 10/30 column, GE Healthcare). A sample volume of 100 μL of GCAP5 (200 μM protein concentration) was applied to the column equilibrated with 30 mM MES (pH 6.6), 5 mM citrate, and 100 mM NaCl. The SEC measurements were made at 4 °C with a flow rate at 0.5 ml/min.
NMR spectroscopy
GCAP5 samples for NMR experiments consisted of 15N-labeled myristoylated and Ca2+-free GCAP5 (0.35 mM) dissolved in 30 mM MES (pH 6.6) buffer containing 1 mM DTT-d10 and 90%:10% H2O:D2O. To observe Fe2+ ion binding to GCAP5 by NMR, a series of samples of 15N-labeled GCAP5WT (and GCAP5C15A/C17A) was prepared with the addition of 0, 0.25, 0.50, 1.0, 2.0, and 5.0 equivalents of FeSO4 into the samples prepared anaerobically in a glove bag under constant purging with high purity argon gas. All NMR experiments were performed at 30°C on a Bruker 800 MHz Avance III spectrometer equipped with a triple resonance cryogenic TCI probe and pulsed field gradients. 2D 15N-1H HSQC with 2048 (1H) × 256 (15N) data points were acquired on 15N-labeled GCAP1WT or GCAP5C15A/C17A samples. Spectra were processed using NMRPipe software package21 and analyzed using SPARKY22.
Isothermal titration calorimetry (ITC)
Fe2+ binding to GCAP5WT (and GCAP5C15A/C17A) with ferrous gluconate was carried out on a VP-ITC calorimeter (Micro-Cal) at 25°C as described previously23. Metal free GCAP5 protein was prepared by first adding 5 mM EGTA into the protein sample (to remove contaminating Ca2+) and residual EGTA was removed by performing repeated buffer exchange with Amicon spin concentrator. The ITC titration buffer contained 30 mM MES (pH 6.6), 100 mM NaCl, 2 mM ferrous gluconate, and 1 mM beta-mercaptoethanol. The concentration of GCAP5 in the titration was 50 μM and Fe2+ concentration in the titrant was 2 mM. The sample was titrated with 40 injections of 5 μl aliquots.
Molecular docking calculation
The web-based docking program HADDOCK24 was used to generate a structural model of Fe2+-bound to GCAP5. First, a homology modeled structure of monomeric GCAP5 was generated based on the crystal structure of chicken GCAP1 (PDB ID: 2R2I; 63% sequence identity) using Swiss-Modeler software25. This homology model of monomeric GCAP5 was then docked to a single Fe2+ using HADDOCK. For the Fe2+ docking, an unambiguous distance restraint between the bound Fe2+ ion and sulfur atoms of cysteine residues (Cys15 and Cys17) was set to 2.35 ±0.05 Å. This distance represents the average Fe-S bond length extracted from crystal structures of rubredoxin (PDB ID: 1FHM) and rubrerythrin (PDB ID: 1LKO). The docking calculation was initiated with a rigid body energy minimization that generated 1000 structures. The best 200 structures were subjected to a semi-flexible simulated annealing step. In the final step, the 200 structures obtained from the previous simulated annealing step were refined in explicit waters. The coordinate file with the lowest HADDOCK score was selected as the final model of Fe2+-bound GCAP5. The structure of the GCAP5 dimer with one Fe2+ bound was built using HADDOCK, in which the Fe2+-bound GCAP5 monomer (calculated above) was docked onto a second GCAP5 molecule. The same HADDOCK protocol above was used to dock the dimer. The distance between Fe2+ and each sulfur atom of the four cysteine residues in the dimer was set to 2.35 ± 0.05 Å. The distance between the four sulfur atoms in the dimer was set to 3.8 ± 0.1 Å, which matched the corresponding distances observed in the crystal structure of rubredoxin. At the end of the HADDOCK dimer calculation, 195 structures formed a single cluster out of 200 water-refined structures. The coordinate file with the lowest HADDOCK score was chosen for the final structural model displayed in this study.
Guanylate cyclase assays
For testing the regulatory properties of zGCAP5 and its mutant, we reconstituted purified GCAP5 forms with cell membranes containing heterologously expressed human GC-E in HEK flip 293 cells essentially as described previously26, 27, but using a cell line that stably expressed GC-E. Cells were cultivated and harvested by centrifugation (300×g, 5 min), the supernatant was discarded and the pellet was resuspended in100 μL of 50 mM HEPES-KOH pH 7.4, 50 mM KCl, 20 mM NaCl, 1 mM DTT, mammalian protease inhibitor cocktail (1:500). Activities were measured according to a detailed protocol that was published before13, 26 with the following modifications: GCAP5 and bovine GCAP1 (control) were added at a final concentration of 5 - 10 μM and Ca2+-dependent GC activities were obtained by adjusting the free [Ca2+ ] using a K2H2EGTA/CaH2EGTA buffer system as described previously13. The free Mg2+-concentration was 1 mM. For investigating the activity profile of Fe2+ bound GCAP5 we first resuspended lyophilized purified GCAP5 forms in 30 mM Mes buffer pH 6.6, 1 mM DTT. GCAP5 was saturated with Fe2+ by incubation with 100 μM Fe2+, and unbound Fe2+ was removed by dialysis against 30 mM Mops pH 7.2, 60 mM KCl, 4 mM NaCl, 3.5 mM MgCl2, 1 mM DTT, which kept Fe2+ at a minimum concentration of 100 nM (FeSO4). Iron-loaded GCAP5 forms were reconstituted with recombinant GC-E and the activity was tested in the absence and presence of free Ca2+ in Mops-buffer (30mM) at pH 7.2, 60 mM KCl, 4 mM NaCl, 3.5 mM MgCl2, 1 mM GTP, 0.3 mM ATP, 0.16 mM Zaprinast and 5 mM DTT. To avoid interference of the Ca2+-chelating EGTA buffer with Fe2+, we lowered the EGTA concentration to 50 μM in the assay mixture.
Results
Fe2+ binding to GCAP5 monitored by ITC
The binding of Fe2+ to GCAP5 was initially detected visually when highly concentrated and purified GCAP5 protein appeared to have a faint brownish or yellow color. Two non-conserved cysteine residues in GCAP5 (Cys15 and Cys17, see Fig. 1) were suggested to participate in Fe2+ binding, because the double mutation C15A/C17A in GCAP5 (called GCAP5C15A/C17A) abolished the faint color. The UV-visible absorbance spectrum of iron-loaded GCAP5 exhibited two peaks at 325 nm and 420 nm indicative of the presence of an iron-sulfur cluster28. GCAP5 may also bind to Fe3+, and the observed faint yellow color could also be due to light absorption by Fe3+; however, Fe3+ binding to GCAP5 was not analyzed in this study, because Fe3+ under physiological conditions is not soluble enough for binding titrations by ITC or NMR.
Isothermal titration calorimetry (ITC) was used to quantitatively monitor Fe2+ binding to GCAP5 (Fig. 2). Titration of Fe2+ ions into wildtype GCAP5 (called GCAP5WT) resulted in a binding isotherm that is multiphasic and could be fit by a two-site model with 2 or more Fe2+ ions (Figs. 2A-B). The binding isotherm in Fig. 2B exhibited two distinct phases: The first phase represents high affinity binding of Fe2+ (Kd < 100 nM) with a stoichiometry of 1 Fe2+ bound to two GCAP5 molecules (stoichiometric coefficient, n = 0.4 ±0.1). The enthalpy change (∆H) for the high affinity site could not be accurately measured due to confounding enthalpy changes caused by dissociation of Fe2+ complexes with gluconate and mercaptoethanol as well as redox dependent heat of dilution. The second phase in the binding isotherm represents exothermic binding of Fe2+ (∆H = -2.8 ±0.1 kcal/mol) with a stoichiometry of at least 2 Fe2+ bound to each GCAP5 molecule (n = 2 ±0.5) and apparent dissociation constant in the micromolar range (Kd = 3 ±1 μM).
Figure 2. GCAP5 binds to Fe + as measured by ITC.
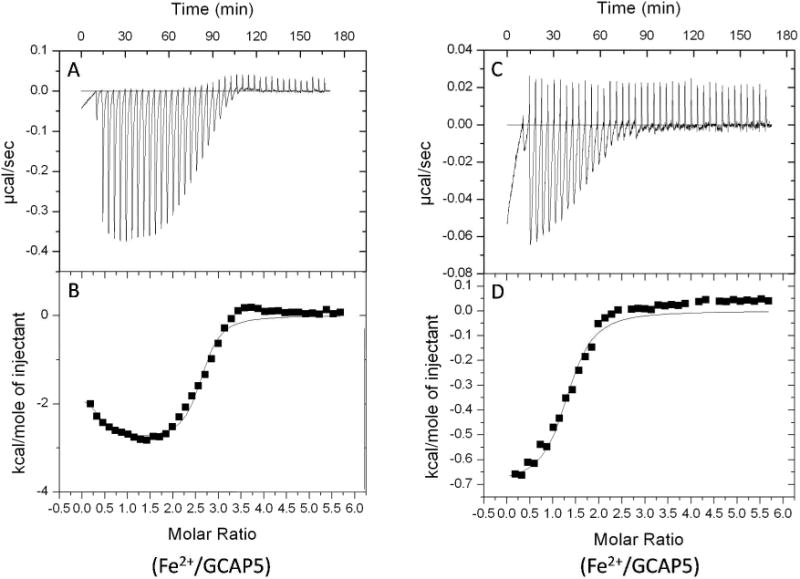
Change of heat resulting from incremental addition of Fe2+ to GCAP5WT (A) or GCAP5C15A/C17A (C) during ITC titration at 25°C. Binding isotherms of Fe2+ binding to GCAP5WT (B) or GCAP5C15A/C17A (D) were derived from the integrated heat at each injection. The binding isotherm for GCAP5WT (B) was fit to a two-site model (see Methods). The fit suggests a high affinity site with a dissociation constant (Kd) that is less than 100 nM and stoichiometry (n) equal to 0.4 ±0.1 equivalents of Fe2+ bound per GCAP5 polypeptide. The lower affinity site has an apparent Kd of 3 ±1 μM with a stoichiometric coefficient of 2 ±0.5 and ∆H = -2.8 ±0.1 kcal/mol. The binding isotherm for GCAP5C15A/C17A (D) was fit to a onesite model with dissociation constant of 3 ±1 μ M and ∆H = -0.7 ±0.1 kcal/mol.
Titration of Fe2+ ions into the C15A/C17A double mutant (GCAP5C15A/C17A) resulted in a monophasic exothermic binding isotherm that could be fit by a one-site model (Figs. 2C-D). The binding isotherm in Fig. 2D exhibited exothermic binding of Fe2+ (∆H = -0.7 ±0.1 kcal/mol) with a dissociation constant in the micromolar range (Kd = 3 ±1 μM), but the amplitude of the exothermic heat signal was approximately six-fold lower. The double mutant (GCAP5C15A/C17A) eliminated the nanomolar Fe2+ binding site observed for GCAP5WT, suggesting that Cys15 and Cys17 are both essential for nanomolar Fe2+ binding to GCAP5. The micromolar Fe2+ binding observed for GCAP5C15A/C17A is possibly due to non-specific electrostatic interaction of Fe2+ with negatively charged EF-hand loops or negatively charged residues on the protein surface.
NMR titration of Fe2+ binding to GCAP5
To probe the structural effects of Fe2+ binding to GCAP5, 15N-1H HSQC NMR spectra were recorded for 15N-labeled GCAP5 in the presence and absence of saturating Fe2+ concentration (Fig. 3). The HSQC spectrum of GCAP5 in the absence of Fe2+ (red peaks in Fig. 3) exhibits 191 well resolved peaks with uniform intensity, indicating that GCAP5 is stably folded under NMR conditions. Each peak in the HSQC spectrum represents a particular amide group with a unique chemical shift environment that provides a residue specific fingerprint of protein conformation. Thus, Fe2+-dependent spectral changes in the HSQC can be analyzed to infer particular amino acids at or near the Fe2+ binding sites. The HSQC spectrum of GCAP5 in the presence of saturating levels of Fe2+ (black peaks in Fig. 3) revealed more than a dozen peaks whose spectral frequency and/or intensity were altered by the addition of Fe2+. Fe2+-binding caused some peaks to become broadened beyond detection (see peaks at 7.6, 7.8, 8.05, 9.1, 9.3, and 10.6 ppm), whereas Fe2+ also caused a few new peaks to appear (6.4, 6.8 and 9.7 ppm). The large number of Fe2+-induced spectral changes suggests that GCAP5 has multiple Fe2+ binding sites, consistent with the Fe2+ binding heterogeneity observed in the ITC isotherm (Fig. 2B).
Figure 3. NMR spectroscopy of GCAP5 vs Fe2+.
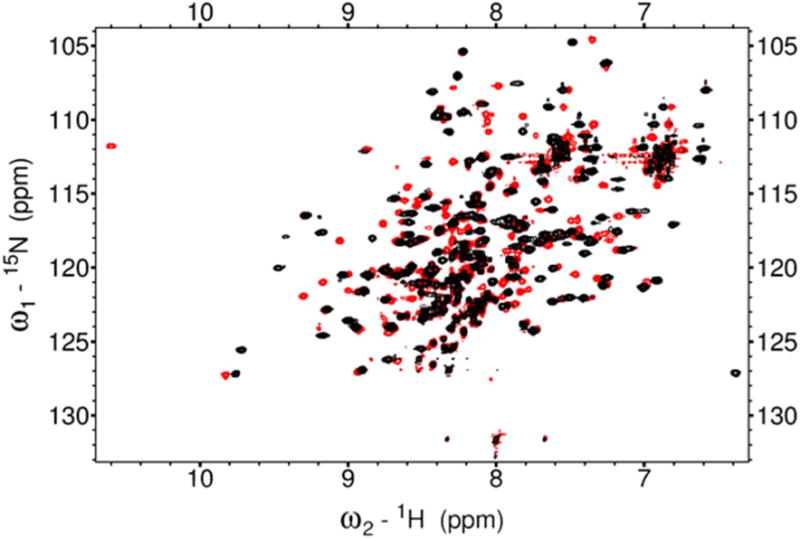
1H-15N HSQC spectra of 15N-labeled GCAP5 in the Fe2+-free (red) and Fe2+-saturated (black) states. The experimental conditions are defined under Materials and Methods and were the same as described previously for GCAP117.
To discriminate the different Fe2+ binding sites, HSQC spectra of GCAP5WT (Fig. 4A) and GCAP5C15A/C17A (Fig. 4B) were recorded at many different Fe2+ concentrations. The NMR titration of GCAP5WT (Fig 4A) revealed two types of binding sites: one type had two separate resolved peaks that represent Fe2+-free and Fe2+-bound states (see magenta solid circle and arrows in Fig. 4A), which is indicative of slow exchange kinetics on the NMR chemical shift timescale. The term “slow exchange” in this context refers to Fe2+ binding event in which the exchange rate of binding is smaller (slower) than the frequency difference between the NMR peaks (representing the free and bound states). In other words, the exchange rate is slower than the chemical shift timescale and the two states are resolved. By contrast, many other peaks in the NMR titration exhibited fast exchange kinetics in which a single peak titrates from the Fe2+-free to Fe2+-bound state (see magenta dashed circles in Fig. 4). In this case, the exchange rate is larger (faster) than the frequency difference between the free and bound peaks and the two states are not resolved. Instead, the two states are represented by a single averaged peak whose chemical shift titrates progressively from the Fe2+-free to Fe2+-bound state (see dashed circles in Fig. 4).
Figure 4. NMR titration of Fe2+ binding to GCAP5.
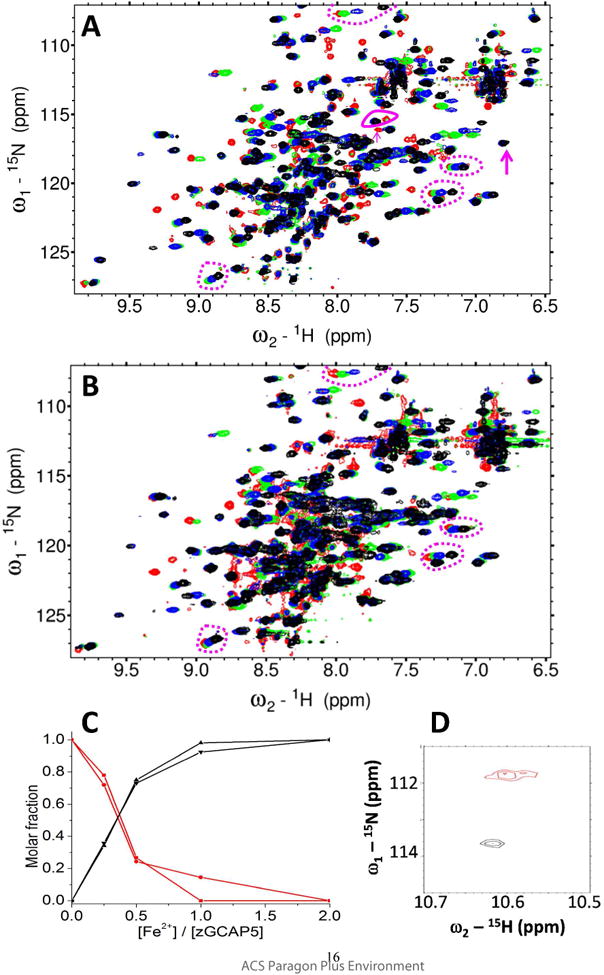
Overlay of 1H-15N HSQC spectra of 15N-labeled GCAP5WT (A) or GCAP5C15A/C17A (B) titrated with 0 (red), 0.50 (green), 1.0 (blue), and 3 (black) equivalents of Fe2+ ions. Peaks exhibiting slow exchange kinetics during the titration are marked by magenta arrows and solid circle in panel A, and are not present in the titration of GCAP5C15A/C17A (B). The slow exchange peaks at 7.6/115 and 7.7/116 ppm in the Fe2+-free GCAP5WT spectrum are tentatively assigned to Cys15 and Cys17 because these peaks are abolished in the spectrum of Fe2+-free GCAP5C15A/C17A. The slow exchange peaks at 7.7/115 and 6.8/117 ppm in the Fe2+-bound GCAP5WT spectrum are also assigned to Cys15 and Cys17 because these peaks are abolished in the spectrum of Fe2+-bound GCAP5C15A/C17A. Representative peaks exhibiting fast exchange kinetics are marked by the magenta dashed circles in panels A and B. (C) NMR intensity of the slow exchange peaks from Fe2+-free state (7.6/115 and 7.7/116 ppm, red) and Fe2+-bound state (7.7/115 and 6.8/117 ppm, black) are plotted as a function of Fe2+ concentration. (D) Expanded view of downfield region of HSQC spectrum of GCAP5C15A/C17A. The downfield peak at 10.60/111.8 ppm, assigned to Gly68 in EF2 in the Fe2+-free state (red), is shifted to 10.65/113.8 ppm in the Fe2+-bound state (black), suggesting that Fe2+ may bind to EF2.
High affinity Fe2+ binding site
The Fe2+ binding site with slow exchange kinetics (and hence high affinity) is represented by sets of two NMR peaks in Fig. 4A that have different chemical shifts in the Fe2+-free versus Fe2+-bound states (see solid magenta circle and arrows in Fig. 4A). For example, the NMR peak in the Fe2+-free state at 7.6/115 ppm is clearly resolved from its Fe2+-bound resonance at 7.7/115 ppm (see solid magenta circle in Fig. 4A). During the NMR titration, the peak at 7.6/115 ppm (Fe2+-free state) decreased in intensity as the Fe2+ concentration was increased, while the corresponding peak at 7.7/115 ppm (Fe2+-bound state) increased in intensity as Fe2+ concentration was increased (Fig. 4C). A similar situation occurs for the NMR peak at 7.7/116 ppm in Fe2+-free state that titrates into the peak at 6.8/117 ppm in the Fe2+-bound state (see magenta arrows in Fig. 4A). The NMR intensity of each slow exchange peak (magenta circle and arrows in Fig. 4A) is plotted as a function of Fe2+ concentration (Fig. 4C). The concentration profiles in Fig. 4C indicate that the Fe2+-bound peaks (7.7/115 ppm and 6.8/117 ppm) both saturate when the Fe2+:GCAP5 ratio is 0.5, consistent with a binding stoichiometry in which one Fe2+ binds to two GCAP5 molecules. A similar 1:2 stoichiometry was observed for the high affinity Fe2+-binding site detected by ITC (Fig. 2A-B).
The slow exchange peaks in the NMR titration of GCAP5WT in Fig. 4A (7.6/115 ppm and 7.7/116 ppm in Fe2+-free state; vs 7.7/115 ppm and 6.8/117 ppm in Fe2+-bound state) are not observed in the NMR titration of GCAP5C15A/C17A (Fig. 4B). The loss of these slow exchange peaks caused by the C15A/C17A mutation suggests that these peaks (7.6/115 ppm, 7.7/115 ppm, 7.7/116 ppm, and 6.8/117 ppm) are likely assigned to backbone amide resonances of Cys15 and Cys17 in the Fe2+-free and Fe2+-bound states, respectively. Thus, high affinity Fe2+ binding requires both Cys15 and Cys17, consistent with ITC observations above that indicate a lack of nanomolar Fe2+-binding to GCAP5C15A/C17A (Fig. 2D).
Low affinity Fe2+ binding sites
A number of peaks in the NMR titration exhibited fast exchange kinetics, consistent with relatively low affinity Fe2+ binding (see dashed circles in Figs. 4A-B). The NMR peaks that titrate with fast exchange kinetics are present in NMR spectra of both GCAP5WT and GCAP5C15A/C17A, and therefore represent bound Fe2+ that must be ligated by non-cysteine residues. These fast exchanging NMR peaks likely represent the multiple Fe2+ binding sites detected by ITC with micromolar affinity (Fig. 2). The multiple low affinity Fe2+ binding sites probed by NMR and ITC are likely due to non-specific electrostatic Fe2+ binding with negatively charged EF-hand loops. To test whether Fe2+ might be bound electrostatically to the EF-hand loops, we monitored Fe2+ binding to GCAP5 in the presence of saturating Ca2+ levels using ITC (Fig. S1). If Fe2+ binds electrostatically to EF-hand loops, then the presence of saturating Ca2+ should weaken or prevent Fe2+ binding to the EF-hand loops. Ca2+ binds to two EF-hands of GCAP5 with nanomolar affinity (apparent KD of 0.37 and 0.61 nM16) and to one EF-hand with an apparent KD of 2.91 μM16 compared to micromolar affinity for Fe2+. The ITC Fe2+ binding isotherm in the presence of Ca2+ showed an overall lower ∆H (∆H = -0.17 kcal/mol) for the exothermic Fe2+ binding phase (Fig. S1). Thus, the Ca2+-induced lowering of ∆H is consistent with having Ca2+ bound to at least two EF-hands in GCAP5 that may impede Fe2+ binding to the EF-hands. Indeed, the most downfield NMR peak at 10.60 ppm (assigned to Gly68 in the second EF-hand, EF2) in the Fe2+-free/Ca2+-free/Mg2+-free state undergoes a substantial Fe2+-induced spectral shift upon adding a saturating amount of Fe2+ (Fig. 4D), consistent with possible Fe2+ binding at EF2. In the presence of physiological Mg2+ levels (1 mM), Mg2+ interferes with Ca2+-binding to GCAP516 and is known to bind to EF2 in GCAP117,29. To test whether Fe2+ can bind to EF2 in place of Mg2+, an HSQC spectrum of Mg2+-bound GCAP5 was recorded in the presence and absence of saturating Fe2+ (Fig. S2). In the absence of Fe2+, the Mg2+-bound GCAP5 exhibited a downfield peak at 10.65 ppm assigned to Gly68 that represents Mg2+ binding to EF2 like that observed for GCAP117. This downfield peak becomes significantly broadened upon adding a saturating concentration of Fe2+, consistent with Fe2+ binding to EF2 in place of Mg2+ when the Fe2+ concentration exceeds that of Mg2+.
GCAP5 is a dimer
The molecular size of GCAP5 in solution was determined by size exclusion chromatography (SEC) in Fig. 5. The elution time of GCAP5, calibrated using protein standards (see black peaks in Fig. 5), corresponded to a molar mass of 40 kDa, indicating that GCAP5 is a dimer in solution. The addition of Fe2+ and/or Mg2+ had no effect on the GCAP5 elution time. Therefore, GCAP5 exists as a dimer in both the Fe2+-free and Fe2+-bound states. The addition of Ca2+ to GCAP5 caused a shorter elution time that corresponded to a molar mass of ∼60 kDa. The Ca2+-induced oligomerization of GCAP5 was also detected by NMR, and many NMR peaks of Ca2+-bound GCAP5 were broadened beyond detection (Fig. S3). The Ca2+-induced broadening of NMR peaks was also observed for unmyristoylated GCAP5, which argues against a possible Ca2+-myristoyl switch for GCAP516.
Figure 5. GCAP5 is a dimer.
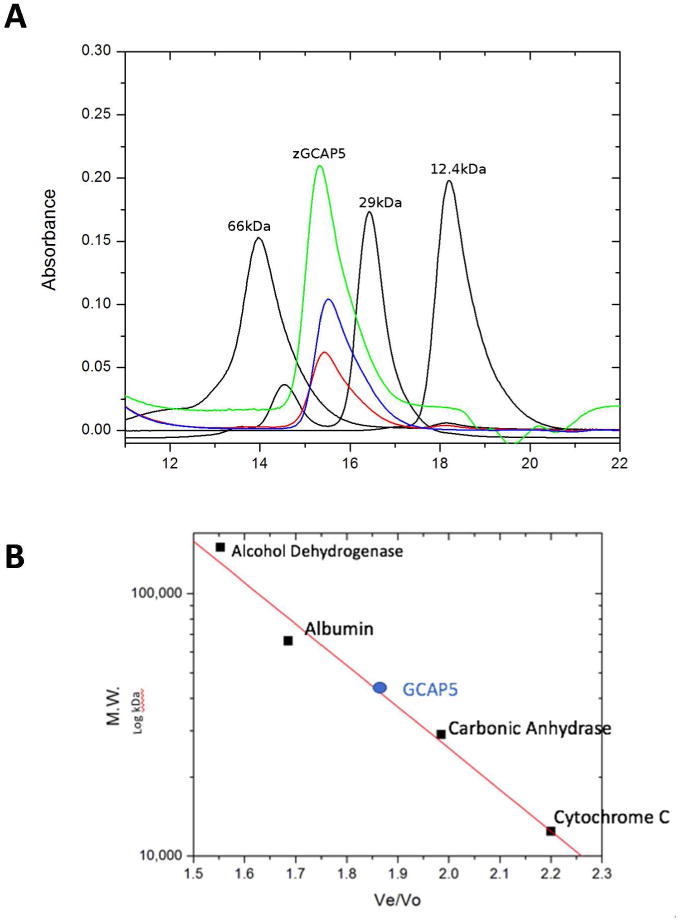
(A) SEC chromatograms of GCAP5WT in the presence of Mg2+ (green) and Fe2+ (blue), and GCAP5C15A/C17A in the presence of Fe2+ (red). SEC chromatograms of protein standards (12.4, 29, and 66 kDa) are shown in black. (B) Molar mass calibration curve. The molar mass of GCAP5 was calculated to be 42 kDa (based on the protein standard masses), consistent with GCAP5 forming a dimer in both Fe2+-free and Fe2+-bound states.
Structural model of a GCAP5 dimer in a [Fe(SCys)4] complex
The Fe2+ binding analysis by NMR and ITC above demonstrates that one Fe2+ binds with nanomolar affinity to two molecules of GCAP5. This high affinity Fe2+ binding to GCAP5 was abolished by the C15A/C17A mutation, suggesting that side-chain sulfur atoms may ligate the bound Fe2+. A structural model of GCAP5 bound to Fe2+ was generated in a stepwise fashion. First, a three-dimensional homology model of GCAP5 was generated based on the known crystal structure of chicken GCAP130. The homology modeled structure of GCAP5 was then docked (using HADDOCK, see Methods) to a single Fe2+ ion that was ligated directly to the thiolate atoms of Cys15 and Cys17 (Fig. 6A). In the structure of monomeric GCAP5 bound to Fe2+, the indole side-chain of Trp20 is located in close proximity to the bound Fe2+, which may explain the Fe2+-induced NMR spectral shift of the Trp20 indole resonance at 9.7/127 ppm (Fig. 3). A bound Mg2+ at EF2 was included based on the downfield NMR resonance assigned to Gly68 of EF2 in the HSQC spectrum of Mg2+-bound GCAP5 (Fig. S2).
Figure 6. Structural model of GCAP5 bound to Fe2+.
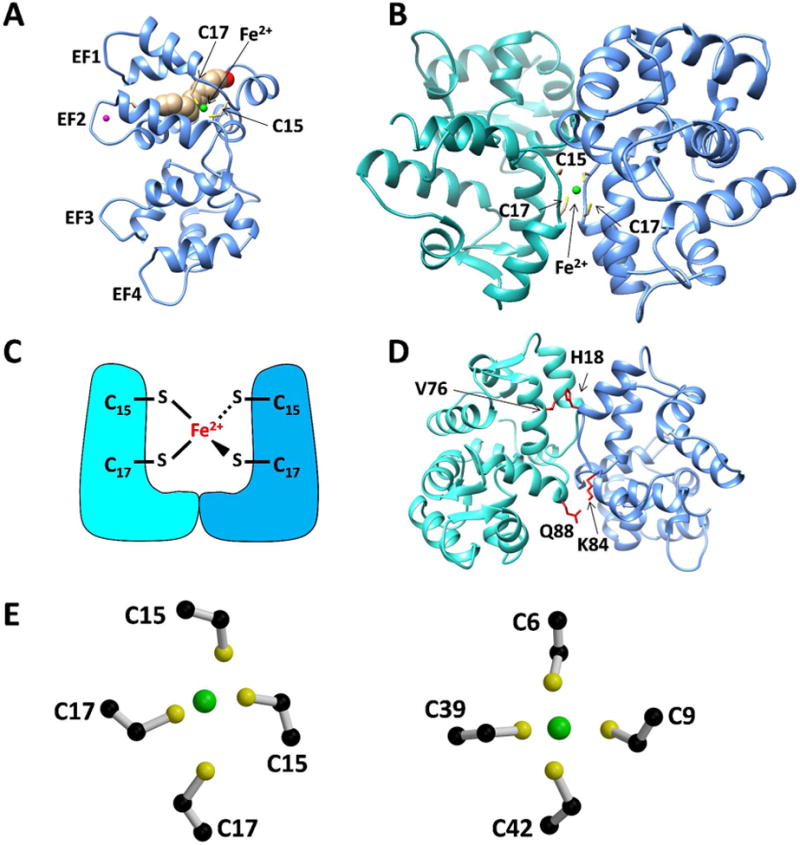
(A) Homology model structure of Ca2+-free/Mg2+-bound GCAP5 with bound Fe2+ (green) ligated by Cys15 and Cys17 (yellow). Mg2+ bound to EF2 (magenta) is supported by the downfield NMR resonance assigned to Gly68 in EF2 of Mg2+-bound GCAP5 (Fig. S2), which is similar to that observed for Mg2+-bound GCAP117. Covalently attached myristic acid is depicted by a space-filling model (brown). (B) Docked structure of GCAP5 dimer in a [Fe(SCys)4] complex. (C) Schematic model of GCAP5 dimer showing Fe2+ bound to residues Cys15 and Cys17. (D) Intermolecular residue contacts at the GCAP5 dimer interface. The H18 imidazole group interacts with side-chain methyl groups of V76, and K84 side-chain amine group is hydrogen bonded to the side-chain carbonyl group of Q88. (E) Close-up view of Fe2+ binding site in GCAP5 (left) compared to that of rubredoxin (1FMH, right). Carbon, sulfur, and Fe2+ atoms are colored black, yellow, and green.
To generate a dimeric structure of GCAP5, a second homology modeled structure of GCAP5 was docked onto the Fe2+-bound GCAP5 structure in Fig. 6A. The modeled structure of the GCAP5 dimer (Fig. 6B) contains a single bound Fe2+ that is ligated by the four cysteinyl thiolates of Cys15 and Cys17 from the two docked GCAP5 molecules (Fig. 6B). In essence, a single bound Fe2+ bridges two GCAP5 molecules in a [Fe(SCys)4] complex (Fig. 6C).
Fe2+-induced inhibition of guanylate cyclase
Fe2+-binding to GCAP5 was unexpected and had not been observed for any other GCAP form before. Therefore, we asked, whether Fe2+-bound GCAP5 differs from the Fe2+-free form in its regulatory properties. Since we had to adjust the assay conditions for the addition of Fe2+, we performed control incubations with bovine GCAP1 (well characterized under different assay conditions). GCAP5 is the weakest activator among all zebrafish GCAP forms showing only very low Ca2+-dependent activation (x-fold is 1.5 in the case of nonmyristoylated GCAP5)13. In the absence of Fe2+, GCAP5 activated recombinant human GC-E roughly by a factor of 20 at high and low Ca2+-concentration when compared to the GC-activity in the absence of either GCAP5 or bovine GCAP1 (Figure 7). However, we did not see higher activity in the absence of Ca2+ as this is typically seen with other GCAP forms and in our control incubation using bovine GCAP1 (Figure 7B). Further, Fe2+-bound GCAP5 strongly decreased GC-E activity independent of the absence or presence of Ca2+. The GCAP5 double mutant C15A/C17A activated GC-E to a similar extent as the wildtype did. However, it showed a Ca2+-dependent activation yielding an x-fold activation of 1.4 (Figure 7A). In the presence of Fe2+, we observed a slight decrease in activity, but the Ca2+-dependency was preserved (Figure 7A). In contrast, the presence of Fe2+ decreased the activation of GC-E by Ca2+-free GCAP1 reaching the same level as with Ca2+-bound GCAP1 (Figure 7B). Fe2+ did not influence GC-E activity in the absence of any GCAP (Figure 7C).
Figure 7. GC-E enzyme activity regulated by GCAPs in the presence of Fe2+.
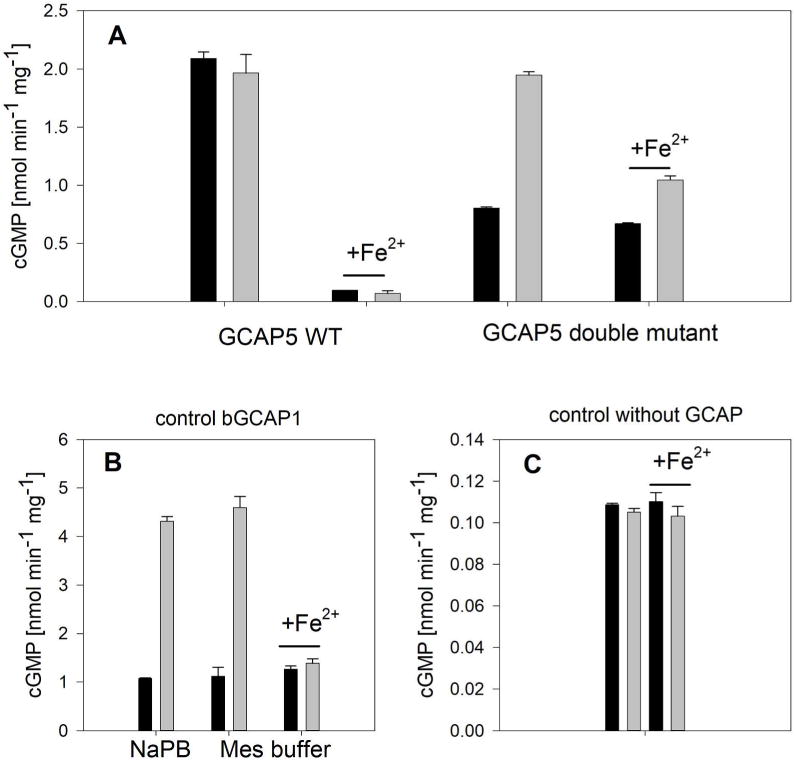
(A) Activation of recombinant human GC-E by wildtype (WT) GCAP5 or the C15A/C17A double mutant. GCAP concentration was 10 μM, black bars are incubations in the presence of 1.5 μM free [Ca2+ ], gray bars are in the presence of Ca2+-chelating EGTA (< 10 nM). Fe2+ was present at 100 nM. (B) Control incubations of human recombinant GC-E with 5 μM bovine GCAP1 in the absence and presence of 100 nM Fe2+. Lyophilized GCAP1 was either resuspended in Na-phosphate buffer (NaPB) or in Mes buffer like GCAP5. (C) Control incubation of GC-E without GCAPs in the presence and absence of Ca2+ and/or Fe2+. Basal activity showed no influence of either Ca2+ or Fe2+. Black and gray bars in (B) and (C) are as indicated in (A).
Discussion
We present ITC (Fig. 2), NMR (Figs. 3-4) and cyclase enzymatic assays (Fig. 7) that monitor and structurally characterize Fe2+ binding to GCAP5. One Fe2+ binds to two molecules of GCAP5 with nanomolar affinity, and additional Fe2+ ions bind to GCAP5 with micromolar affinity. The low affinity Fe2+ binding to EF2 (Fig. 4D) is likely not physiological, because micromolar Fe2+ levels are outside the physiological range. The highest affinity Fe2+ binding site is not observed in the GCAP5C15A/C17A mutant (Figs. 2B and 4B), consistent with the bound Fe2+ at this site being ligated by side-chain sulfur atoms in Cys15 and Cys17. A structural model of a GCAP5 dimer forming a [Fe(SCys)4] complex (Figs. 6B) shows a single bound Fe2+ ligated by the four sulfur atoms from Cys15 and Cys17 in a symmetric dimer (Fig. 6E). A similar [Fe(SCys)4] structural motif was reported previously in the crystal structures of rubredoxin31 (Fig. 6E, right panel) and a two-iron superoxide reductase32, 33. Our SEC data on GCAP5 (Fig. 5) indicate that both Fe2+-free and Fe2+-bound GCAP5 exist as a dimer in solution. A structural model of the Fe2+-bound GCAP5 dimer (Fig. 6B) suggests intermolecular protein hydrophobic contacts (between His18 and Val76) as well as an intermolecular hydrogen bond (between Lys84 and Gln88) that may stabilize GCAP5 as a pre-formed protein dimer in the Fe2+-free state (Fig. 6D). The intermolecular contact involving Val76 was also implicated in the dimerization of GCAP1, because mutating this Val residue to Glu abolishes dimerization of GCAP117, 19, 34.
The bound Fe2+ in GCAP5 is coordinated by four cysteinyl thiolate atoms (Fig. 6E) that is quite similar to the structure of a bound Zn2+ in a Cys4 zinc finger motif35. This structural similarity suggests that Zn2+ might bind to GCAP5 in place of Fe2+ at the high affinity site. Zn2+ is transported into retinal photoreceptor cells and has been suggested to play a role in phototransduction36. Future studies are needed to probe whether Zn2+ can replace Fe2+ and bind with nanomolar affinity to GCAP5. If so, then it will be interesting to test whether Zn2+ binding to GCAP5 (like Fe2+ binding) can also regulate zGCs during visual phototransduction.
Functional implications of Fe2+-binding to GCAP5
Our results suggest that Fe2+ binding to GCAP5 may stabilize and perhaps modulate the quaternary structure of the GCAP5 dimer that could play a role in regulating zebrafish GCs. However, our in vitro functional analysis in this study employed a recombinant mammalian GC, since active recombinant photoreceptor specific GCs from zebrafish are not currently available. Although zebrafish GCAP5 can regulate native zebrafish GCs in retinae membranes9, we currently do not have access to zebrafish GC samples in live animals. The addition of Fe2+ caused complex effects on the regulation of mammalian GC and showed a strong inhibitory effect mediated by both GCAP5 and GCAP1 (Figs. 7A and 7B). The Fe2+-induced GC inhibition meditated by GCAP1 is surprising, and might indicate possible Fe2+ binding to the EF-hands in GCAP1 that may mimick Ca2+ binding. The Fe2+-induced inhibition of GC was somewhat weakened by the GCAP5 double mutant (C15A/C17A), consistent with functional Fe2+ binding by Cys15 and Cys17 (Fig. 6). The GCAP5 double mutant also showed a more typical Ca2+-dependent activation of the target GC compared to the Ca2+-independent GC activation by GCAP5WT (Fig. 7A). This suggests that Fe2+ binding to GCAP5 may stabilize the inhibitory conformational state of GCAP5 even in the absence of Ca2+, and therefore Fe2+-bound GCAP5 may serve to constitutively disable GC activation. The lack of Ca2+-sensitive GC activation by GCAP5WT might also be explained by differences in our test incubations when compared to previous results9, 13. In the current study, we used myristoylated GCAP5 instead of nonmyristoylated protein that was used previously. The presence of the myristoyl group in GCAP5 appears to prevent the typical Ca2+-sensitive activation of a GC, whereas the double mutation (C15A/C17A) restored the Ca2+-dependent GC activation. These observations suggest a complex interdependence of the Fe2+ binding site (at Cys15 and Cys17), the myristoyl group, and the Ca2+-free/Mg2+-bound activator state of GCAP5. The source of GC in our test system was not critical for the outcome of the assay. We compared heterologously expressed human GC-E in HEK 293 cells with mammalian GC from native bovine rod outer segment membranes and did not observe a principal difference in the results (data not shown).
Does Fe2+-binding to GCAP5 have any physiological meaning? At this point, we can only speculate on possible physiological implications, but several observations suggest that GCAP5 might not be the main activator of GCs in the zebrafish retina. Indeed, all other zebrafish GCAPs are at least 3-fold more active than GCAP513. Furthermore, the expression and activation profiles of the other GCAPs promote a dynamic Ca2+-sensitive activation of zebrafish GCs in the absence of GCAP5, suggesting a lack of GCAP5 participation perhaps due to compensatory actions9-13. Finally, the downregulation of GCAP5 by Fe2+ further diminishes the already weak impact of GCAP5 on GC activation. Instead, we suggest that GCAP5 might bind to a different and as yet unknown target protein. Preliminary studies suggest that GCAP5 is expressed in the inner segment (K.W.K and A.S., unpublished results). Therefore, the binding of Fe2+ to GCAP5 might serve a regulatory role for a redox-sensitive process in the inner segment. Future experiments are needed to look for a GCAP5 binding partner protein expressed in the inner segment.
The Fe2+-induced inhibition of GC mediated by GCAP5 (Fig. 7A) may protect photoreceptor cells from apoptosis and possibly prevent retinal degeneration. Mutations in GCs and/or GCAPs that cause constitutive GC activation are genetically linked to retinal degeneration29, 37-41. Also, the retinal degeneration 3 (RD3) protein (that binds to GC in place of GCAPs and prevents GC activation) is important for turning off basal cyclase activity during the trafficking of GC to the outer segment42. The basal cyclase activity of GC that occurs in the absence of RD3 is thought to trigger apoptosis of photoreceptor cells that could lead to retinal degeneration43. Therefore, inhibition of basal cyclase activity by RD3 is believed to be important for preventing retinal degeneration. We suggest that Fe2+-induced inhibition of GC by GCAP5 might be a different mechanism for turning off GC activity in the inner segment, which could serve to protect zebrafish photoreceptor cells from retinal degeneration.
Supplementary Material
Acknowledgments
We thank Bennett Addison for help with NMR experiments.
†This work was supported by a grant from the National Institutes of Health (EY012347 to J.B.A. and by grants from the Deutsche Forschungsgemeinschaft, KO948/7-2 to K.W.K and GRK1885/1 to K.W.K and to the Research Training Group in Oldenburg).
Abbreviations
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- HSQC
heteronuclear single quantum coherence
- ITC
isothermal titration calorimetry
- NMR
nuclear magnetic resonance
- GC
retinal guanylate cyclase
- SEC
size exclusion chromatography
Footnotes
Conflict of Interest Disclosure. The authors declare no competing financial interest.
Supporting Information Available: Supporting information available online comprises 3 figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 2.Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Cloning, sequencing and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 3.Palczewski K, Polans AS, Baehr W, Ames JB. Ca(2+)-binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Dizhoor AM, Lowe DG, Olsevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 5.Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;6:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. [PubMed] [Google Scholar]
- 7.Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi Y, Yang L, Sokal I, Filipek S, Palczewski K, Baehr W. Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP18) in pufferfish (Fugu rubripes) J Mol Evol. 2004;59:204–217. doi: 10.1007/s00239-004-2614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries R, Scholten A, Saftel W, Koch KW. Zebrafish guanylate cyclase type 3 signaling in cone photoreceptors. PLoS One. 2013;8:e69656. doi: 10.1371/journal.pone.0069656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratscho N, Scholten A, Koch KW. Expression profiles of three novel sensory guanylate cyclases and guanylate cyclase-activating proteins in the zebrafish retina. Biochim Biophys Acta. 2009;1793:1110–1114. doi: 10.1016/j.bbamcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Fries R, Scholten A, Saftel W, Koch KW. Operation profile of zebrafish guanylate cyclase-activating protein 3. Journal of neurochemistry. 2012;121:54–65. doi: 10.1111/j.1471-4159.2011.07643.x. [DOI] [PubMed] [Google Scholar]
- 12.Koch KW. The guanylate cyclase signaling system in zebrafish photoreceptors. FEBS J. 2013;587:2055–2059. doi: 10.1016/j.febslet.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Scholten A, Koch KW. Differential calcium signaling by cone specific guanylate cyclase-activating proteins from the zebrafish retina. PLoS One. 2011;6:e23117. doi: 10.1371/journal.pone.0023117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crack JC, Green J, Thomson AJ, Le Brun NE. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Accounts of chemical research. 2014;47:3196–3205. doi: 10.1021/ar5002507. [DOI] [PubMed] [Google Scholar]
- 15.Sterling J, Guttha S, Song Y, Song D, Hadziahmetovic M, Dunaief JL. Iron importers Zip8 and Zip14 are expressed in retina and regulated by retinal iron levels. Experimental eye research. 2017;155:15–23. doi: 10.1016/j.exer.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulmann S, Vocke F, Scholten A, Koch KW. Retina specific GCAPs in zebrafish acquire functional selectivity in Ca2+-sensing by myristoylation and Mg2+-binding. Scientific reports. 2015;5:11228. doi: 10.1038/srep11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim S, Peshenko IV, Olshevskaya EV, Dizhoor AM, Ames JB. Structure of Guanylyl Cyclase Activator Protein 1 (GCAP1) Mutant V77E in a Ca2+-free/Mg2+-bound Activator State. J Biol Chem. 2016;291:4429–4441. doi: 10.1074/jbc.M115.696161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim S, Peshenko IV, Dizhoor AM, Ames JB. Effects of Ca2+, Mg2+, and myristoylation on guanylyl cyclase activating protein 1 structure and stability. Biochemistry. 2009;48:850–862. doi: 10.1021/bi801897p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim S, Peshenko IV, Dizhoor AM, Ames JB. Structural insights for activation of retinal guanylate cyclase by GCAP1. PLoS One. 2013;8:e81822. doi: 10.1371/journal.pone.0081822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizhoor AM, Ericsson LH, Johnson RS, Kumar S, Olshevskaya E, Zozulya S, Neubert TA, Stryer L, Hurley JB, Walsh KA. The NH2 terminus of retinal recoverin is acylated by a small family of fatty acids. J Biol Chem. 1992;267:16033–16036. [PubMed] [Google Scholar]
- 21.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeiffer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 22.Lee W, Tonelli M, Markley JL. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingard JN, Chan J, Bosanac I, Haeseleer F, Palczewski K, Ikura M, Ames JB. Structural analysis of Mg2+ and Ca2+ binding to CaBP1, a neuron-specific regulator of calcium channels. J Biol Chem. 2005;280:37461–37470. doi: 10.1074/jbc.M508541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zundert GC, Rodrigues JP, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond AS, van Dijk M, de Vries SJ, Bonvin AM. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. Journal of molecular biology. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 26.Koch KW, Helten A. Signal Transduction in the Retina. Taylor and Francis CRC Press; 2008. Guanylate cyclase-based signaling in photoreceptors and retina; pp. 121–143. [Google Scholar]
- 27.Zagel P, Dell'Orco D, Koch KW. The dimerization domain in outer segment guanylate cyclase is a Ca(2)(+)-sensitive control switch module. Biochemistry. 2013;52:5065–5074. doi: 10.1021/bi400288p. [DOI] [PubMed] [Google Scholar]
- 28.Huang Q, Hong X, Hao Q. SNAP-25 is also an iron-sulfur protein. FEBS letters. 2008;582:1431–1436. doi: 10.1016/j.febslet.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino V, Sulmann S, Koch KW, Dell'Orco D. Structural effects of Mg(2)(+) on the regulatory states of three neuronal calcium sensors operating in vertebrate phototransduction. Biochim Biophys Acta. 2015;1853:2055–2065. doi: 10.1016/j.bbamcr.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Stephen R, Bereta G, Golczak M, Palczewski K, Sousa MC. Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure. 2007;15:1392–1402. doi: 10.1016/j.str.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min T, Ergenekan C, Eidsness M, Ichiye T, Kang C. Leucine 41 is a gate for water entry in the reduction of Clostridium pasteurianum rubredoxin. Protein science: a publication of the Protein Society. 2001;10:613–621. doi: 10.1110/gad.34501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.deMaré F, Kurtz D, Nordlund P. The structure of Desulfovibrio vulgaris rubrerythrin reveals a unique combination of rubredoxin-like FeS4 and ferritin-like diiron domains. Nat Struct Biol. 1996;3:539–546. doi: 10.1038/nsb0696-539. [DOI] [PubMed] [Google Scholar]
- 33.Emerson JP, Cabelli DE, Kurtz DM., Jr An engineered two-iron superoxide reductase lacking the [Fe(SCys)4] site retains its catalytic properties in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3802–3807. doi: 10.1073/pnas.0537177100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peshenko IV, Olshevskaya EV, Lim S, Ames JB, Dizhoor AM. Identification of target binding site in photoreceptor guanylyl cyclase-activating protein 1 (GCAP1) J Biol Chem. 2014;289:10140–10154. doi: 10.1074/jbc.M113.540716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Q, Liu YP, Yan XX, Liang DC. Structural and functional characterization of Cys4 zinc finger motif in the recombination mediator protein RecR. DNA repair. 2014;24:10–14. doi: 10.1016/j.dnarep.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Redenti S, Ripps H, Chappell RL. Zinc release at the synaptic terminals of rod photoreceptors. Experimental eye research. 2007;85:580–584. doi: 10.1016/j.exer.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Jiang L, Baehr W. GCAP1 Mutations Associated with Autosomal Dominant Cone Dystrophy. Adv Exp Med Biol. 2010;664:273–282. doi: 10.1007/978-1-4419-1399-9_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Warren MJ, Bird AC, Bhattacharya SS. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genetics. 1998;7:273–277. doi: 10.1093/hmg/7.2.273. [DOI] [PubMed] [Google Scholar]
- 39.Koch KW, Dell'Orco D. Protein and Signaling Networks in Vertebrate Photoreceptor Cells. Frontiers in molecular neuroscience. 2015;8:67. doi: 10.3389/fnmol.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dell'Orco D, Sulmann S, Zagel P, Marino V, Koch KW. Impact of cone dystrophy-related mutations in GCAP1 on a kinetic model of phototransduction. Cell Mol Life Sci. 2014;71:3829–3840. doi: 10.1007/s00018-014-1593-4. [DOI] [PubMed] [Google Scholar]
- 41.Kitiratschky VBD, Behnen P, Kellner U, Heckenlively JR, Zrenner E, Jägle H, Kohl S, Wissinger B, Koch KW. Mutations in the GUCA1A gene involved in hereditary cone dystrophies impair calcium-mediated regulation of guanlyate cyclase. Hum Mutat. 2009;30:E782–796. doi: 10.1002/humu.21055. [DOI] [PubMed] [Google Scholar]
- 42.Zulliger R, Naash MI, Rajala RV, Molday RS, Azadi S. Impaired association of retinal degeneration-3 with guanylate cyclase-1 and guanylate cyclase-activating protein-1 leads to congenital amaurosis-1. J Biol Chem. 2015;290:3488–3499. doi: 10.1074/jbc.M114.616656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peshenko IV, Olshevskaya EV, Dizhoor AM. Functional Study and Mapping Sites for Interaction with the Target Enzyme in Retinal Degeneration 3 (RD3) Protein. J Biol Chem. 2016;291:19713–19723. doi: 10.1074/jbc.M116.742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


