Abstract
Nucleic acid polymers (NAPs) block the release of HBsAg from infected hepatocytes. These compounds have been previously shown to have the unique ability to eliminate serum surface antigen in DHBV-infected Pekin ducks and achieve multilog reduction of HBsAg or HBsAg loss in patients with chronic HBV infection and HBV/HDV coinfection. In ducks and humans, the blockage of HBsAg release by NAPs occurs by the selective targeting of the assembly and/or secretion of subviral particles (SVPs). The clinically active NAP species REP 2055 and REP 2139 were investigated in other relevant animal models of HBV infection including woodchucks chronically infected with WHV, HBV transgenic mice and HBV infected SCID-Hu mice. The liver accumulation of REP 2139 in woodchucks following subcutaneous administration was examined and was found to be similar to that observed in mice and ducks. However, in woodchucks, NAP treatment was associated with only mild (36–79% relative to baseline) reductions in WHsAg (4/10 animals) after 3–5 weeks of treatment without changes in serum WHV DNA. In HBV infected SCID-Hu mice, REP 2055 treatment was not associated with any reduction of HBsAg, HBeAg or HBV DNA in the serum after 28 days of treatment. In HBV transgenic mice, no reductions in serum HBsAg were observed with REP 2139 with up to 12 weeks of treatment. In conclusion, the antiviral effects of NAPs in DHBV infected ducks and patients with chronic HBV infection were weak or absent in woodchuck and mouse models despite similar liver accumulation of NAPs in all these species, suggesting that the mechanisms of SVP assembly and or secretion present in rodent models differs from that in DHBV and chronic HBV infections.
Keywords: Nucleic acid polymer, HBV, HBsAg, woodchuck, mouse
1. Introduction
Several models of hepadnaviral infection have been established to investigate the viral replication and pathogenesis of HBV infection in humans (Dandri et al. 2017; Innacone and Guidotti, 2015). Infection of Pekin ducks with duck hepatitis B virus (DHBV), like the infection of woodchucks with woodchuck hepatitis virus (WHV), is an naturally occurring infection which, like WHV-infected woodchucks, establishes a reservoir of cccDNA similar to HBV infection in humans (Cova and Zoulim, 2004; Dandri et al., 2017; LeMire et al., 2005). Additionally, natural WHV or DHBV infection in woodchucks and ducks respectively also results in an abundant excess of circulating surface antigen derived from subviral particles (SVP) (Franke et al., 2007; Summers et al. 1978) similar to that observed in HBV infection (Chai et al., 2008). The antiviral responses of various direct acting antiviral agents in the duck model have mirrored their effects in human infection (Nicoll et al., 1998; Foster et al., 2003; Scougall et al., 2012).
The evaluation of the antiviral activity of a variety of investigational antiviral agents have also used many other models, including mice expressing the HBV genome from an integrated transgene (Julander et al. 2002; Julander et al., 2003),from hydrodynamically injected plasmid or adenovirus (Billioud et al., 2016; Martin et al., 2015;Qiu et al., 2016) or from injected HBV-infected HepAD38 cells (Feitelson et al., 2007; Schinazi et al., 2012), HBV infected mice with mouse / human chimeric livers (Murakami et al., 2016; Hayashi et al., 2015) or in woodchucks chronically infected with woodchuck hepatitis virus (WHV) (Menne et al., 2008; Menne et al., 2015).
Nucleic acid polymers (NAPs) have a broad spectrum antiviral activity against several enveloped viruses including HBV and hepatitis delta virus (HDV) (Vaillant, 2016). NAPs display potent prophylactic activity in preventing the establishment of persistent DHBV infection in the duck model, an effect which was shown to be driven by a post-entry activity and was effective at doses as low as 1mg/kg/day (Noordeen et al., 2013a). In established DHBV infection, the post-entry activity of the NAPs REP 2055 and REP 2139 resulted in the reduction of DHBsAg after one week of treatment with clearance of serum DHBsAg achieved as soon as two weeks after the start of treatment (Noodeen et al., 2015; Quinet et al., 2016).
This antiviral effect was shown to result from the inhibition of release of DHBsAg from the liver, an effect apparently driven by the inhibition of SVP assembly and/or release (Noordeen et al., 2015). The selective inhibition of SVP assembly and/or secretion by NAPs was recently confirmed in vitro in HepG2.2.15 cells (Blanchet et al., 2017). The clearance of DHBsAg during NAP therapy is accompanied by clearance of DHBV DNA from the blood and reduced viral replication in the liver (Roehl et al., 2017), an effect which persists after treatment was withdrawn and was accompanied by the absence of detectable DHBcAg, DHBsAg, and multilog reduction of cccDNA in the liver (Noordeen et al., 2015; Quinet et al., 2016). The antiviral effects of NAPs observed in DHBV infection in vivo have mirrored those observed in subsequent clinical trials in HBV infection. Both REP 2055 and REP 2139 rapidly reduce or clear HBsAg in patients with HBeAg-positive and HBeAg-negative chronic HBV infection and in patients with HBV / HDV co-infection with weekly doses as low as ~3mg/kg/week (Al-Mahtab et al., 2016, Bazinet et al., 2017a, Bazinet et al., 2017b). HBsAg clearance is accompanied by seroconversion of HBeAg (in HBeAg positive patients), unmasking of anti-HBs and clearance of HBV DNA. Notably NAPs also display a direct antiviral activity against HDV distinct from their effects on SVPs (Bazinet et al., 2017b). Most importantly, the clearance of HBsAg in patients has resulted in dramatically improved antiviral response to immunotherapy, with its use in the absence of serum HBsAg associated with dramatic increases in anti-HBs, the onset of therapeutic liver flares and the establishment of functional control of HBV and HDV infection persisting after treatment has been withdrawn.
NAPs are phosphorothioate oligonucleotides, a chemical class which enter hepatocytes and efficiently accumulate in the liver of mice and non-human primate species (Geary et al., 2015a). These conserved pharmacokinetic behaviours also observed for NAPs in these species are also conserved in Pekin ducks (Roehl et al., 2017). This study investigated the pharmacological behaviour of NAPs in woodchucks and their antiviral activity in other well established rodent models to better understand the mechanism of action of NAPs in hepadnaviral replication and secretion.
2. Material and methods
2.1 NAP synthesis and formulation
REP 2055 and REP 2139 are 40mer phosphorothioate oligonucleotides with the sequences (dAdC)20 and (2’OMeA, 2’OMe-5-MeC)20, respectively (Vaillant, 2016). REP 2031 is a 40mer phosphorothioate oligonucleotide with the sequence dC40 (Noordeen et al., 2013a). REP 2055 and REP 2139 were prepared under cGMP as described previously (Al-Mahtab et al., 2016). REP 2031 was prepared under cGMP-like conditions (Noordeen et al., 2013a). REP 2055 and REP 2031 were prepared as stocks in normal saline prior to administration. REP 2139 was prepared either in normal saline or as a calcium chelate complex (REP 2139-Ca, Bazinet et al., 2016) prior to administration.
2.2 Tissue distribution of NAPs in woodchucks
Woodchucks without WHV infection (n=3) were purchased from the Institute of Experimental Animal Science, CAMS (Beijing, China) and maintained according to the guidelines of the animal facility of Huazhong University of Science and Technology in Wuhan. Woodchucks were dosed with 16mg/kg of REP 2139-Ca via bolus subcutaneous (s.c.) injection. One day following injection, serum and tissue samples were obtained during sacrifice and held at −80°C until analysis. The concentrations of REP 2139 in serum, liver and kidney were determined by a previously validated fluorescence-HPLC based method (Roehl et al., 2017). Specificity of NAP detection was verified on tissues matrices from an untreated animal (data not shown).
2.3 Treatment and monitoring of WHV infected woodchucks
Chronically woodchuck hepatitis virus (WHV) infected woodchucks (Marmota monax) were purchased from North Eastern Wildlife (Ithaca, New York, United States of America) and maintained according to the guidelines of the animal facility of the University Hospital Essen. For blood sampling, woodchucks were anesthetized by intramuscular injection with 4ml of 10% Ketamine mixed with 1ml 2% of xylazine (Ceva, Tiergesundheit, Germany) and blood was taken from the hind limb vein (vena saphena).
In the first experiment REP 2055 and REP 2139 (each n=2) was dosed at 10mg/kg three times per week s.c. for three weeks. Sampling at baseline (week 0) weeks 1, 2 and on week 4 just after completion of treatment. In the second experiment, REP 2139-Ca (n=6), dosed at ~15mg/kg was administered three times per week s.c. for five weeks. Sampling at baseline, weeks 1 and week 5.
Serum was frozen at −20°C until processing. WHV DNA was quantified by real time qPCR using WHc specific primers wc1 and wc149s (Meng et al., 2014) (wc1; sense primer: TGGGGCCATGGATATAGATCCTTA; WC149S; anti-sense primer: AAGATCTCTAAATGACTGTATGTTCCG). WHsAg was monitored by electroimmunodiffusion (Laurell electrophoresis) as described previously (Gerlich et al., 2004). In brief, 10μl of the woodchuck sera (or suitable dilutions thereof) were applied to 3mm holes on glass slides that were covered with 6 ml of 0.6 % agarose and, containing 80 μl of rabbit polyclonal anti-WHs antiserum and run for 16 hours at 5mA per slide. Using a standardized serum and purified WHsAg from WHV-infected woodchucks, the length of the precipitation arc was converted into mg WHsAg/ml.
2.4 Treatment and monitoring of HBV infected SCID-Hu chimeric mice
The use of SCID-Hu mice and the experimental procedures used to treat these animals was approved by the Animal Ethics Committee of Phoenix Bio (Resolution No.: 0973). Male uPA+/+/SCID-Hu mice were prepared and infected with HBV genotype D as previously described (Tsuge et al., 2005; Utoh et al., 2008) and the viability of the liver chimera was monitored during the treatment by serum levels of human albumin (LX Reagent “Eiken” Alb II, Eiken Chemical Co., Ltd.). Animals were 21–24 weeks of age and were verified to have well established HBV infection at the start of treatment by assessment of viremia and received 28 days of treatment with REP 2055 or REP 2031 (10mg/kg/day via intraperitoneal injection (i.p.)) or entecavir (ETV) (0.03mg/kg/day via oral gavage). Control animals received volume matched, daily i.p. administration of normal saline. Antiviral effects during treatment were assessed by monitoring HBsAg (Abbott Architect quantitative), HBeAg (Abbott Architect) and HBV DNA (in-house qPCR) in blood taken before administration every 3 days during treatment.
2.5 Treatment and monitoring of HBV transgenic mice
Studies in transgenic mice were conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University, which approval has an expiration date of 30 June 2017. Two strains of adult homozygous male and female transgenic HBV mice were used: one obtained from Dr. Frank Chisari (Guidotti et al., 1995), and another from Dr. Patricia Marion (Marion et al., 2003). Whole blood was collected by submandibular cheek bleeding or cardiac exsanguination at necropsy, and sera was collected and stored at −80°C until use. Mice were pre-screened for active HBV infection and randomized into treatment and control groups (where applicable) using HBsAg prior to treatment. Animals were treated for 3 or 12 weeks with REP 2139-Ca (10mg/kg, 3 times per week via i.p. injection) and HBsAg was monitored regularly before and during treatment by quantitative ELISA (Immuno Diagnostics, Foster City, CA).
3. Results
3.1 Effects of NAPs in chronically WHV-infected woodchucks
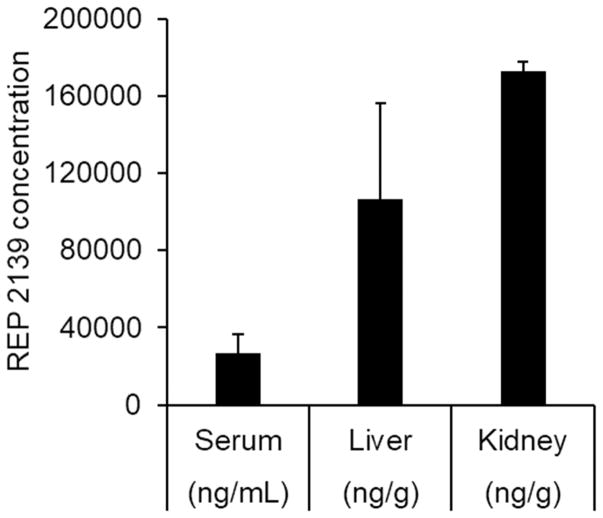
The uptake of NAPs into hepatocytes is a prerequisite for their effects on viral replication, therefore, the biodistribution of REP 2139 24 hours following s.c. injection in woodchucks was examined and demonstrated liver accumulation in this rodent species (Fig. 1). S.c. administered REP 2139 was observed to accumulate in the woodchuck liver and kidney with concentrations of 106.4 ±49.5 and 172.6±5.01 μg/g tissue respectively, consistent with the behaviour of parenterally administered REP 2139 in mice, ducks and cynomolgus monkeys (Roehl et al., 2017). The plasma concentration of REP 2139 was significantly lower (2.6±0.97μg/mL) at 24 hours post administration, consistent with the known plasma clearance behaviour of subcutaneously administered phosphorothioate oligonucleotides and other NAPs (Leeds et al., 2000; data not shown).
Figure 1.
Biodostribution of REP 2139 in serum, liver and kidney of woodchucks, 24 hours after a bolus s.c. injection of 10mg/kg of REP 2139-Ca. Plotted values are mean ± standard deviation (n=3).
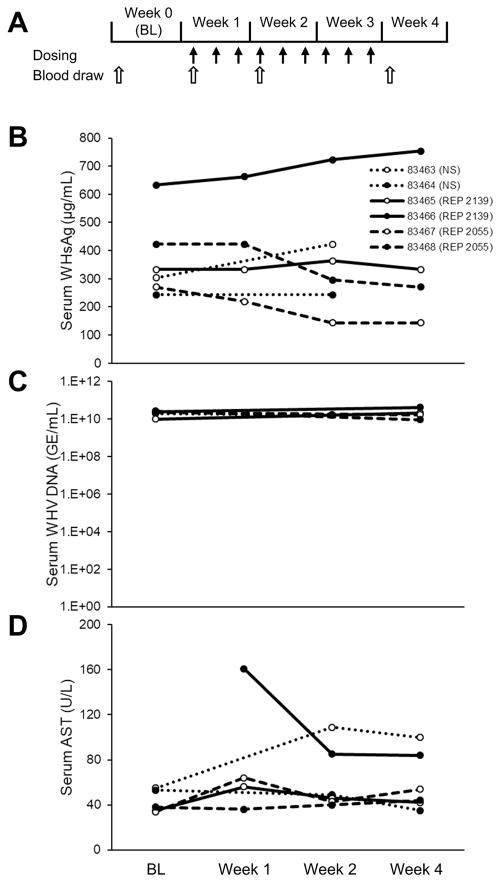
The first experiment in woodchucks consisted of testing the two clinically active NAP species, REP 2055 and REP 2139, each in two chronically WHV-infected animals. Both NAPs were administered three times per week at 10mg/kg for three weeks and the effects on WHsAg, WHV DNA and AST were evaluated during treatment (Fig. 2A). WHsAg reductions of 36 and 47%from baseline to week 3 were observed in the two animals receiving REP 2055 whereas no reduction in WHsAg occurred in the two animals receiving REP 2139 (Fig 2B). No changes in WHV DNA or AST were observed (Fig. 2C, 2D) except for AST decline observed in one animal during REP 2139 treatment.
Figure 2.
Antiviral effects of REP 2055 and REP 2139 in woodchucks with chronic WHV infection (n=4). Experimental design is indicated in (A). Changes during treatment in serum WHsAg (B), WHV DNA (C) and AST (D) are presented. BL = baseline.
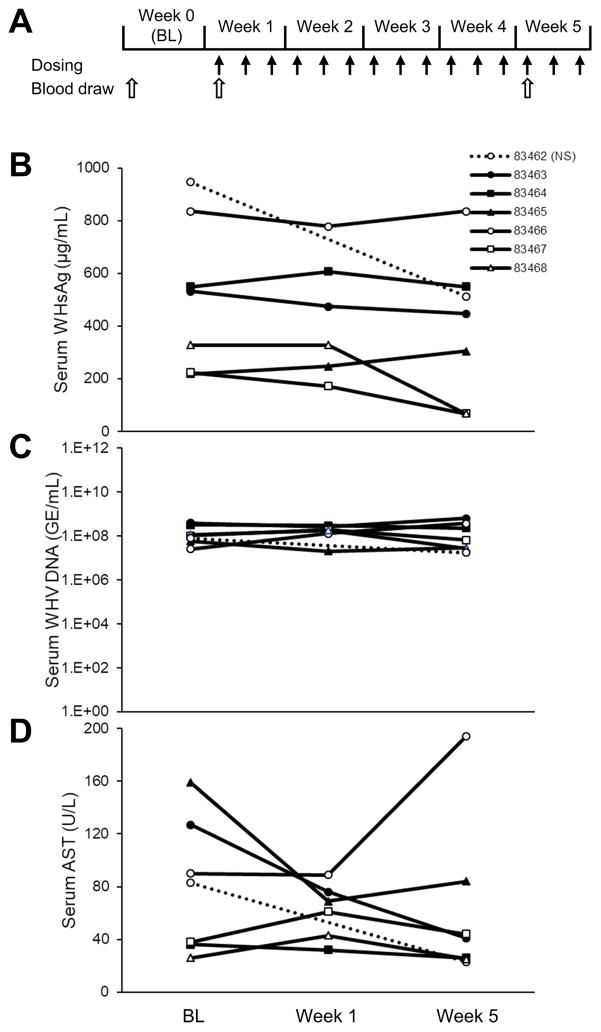
The second experiment used the same REP 2139-Ca formulation previously used in clinical trials (28, 29) with the same dosing duration and frequency as in the first woodchuck experiment but with higher dose (~15mg/kg) and longer exposure (Fig. 3A) in six WHV infected animals. In two animals, WHsAg reductions of 69 and 79% from baseline were observed animals 83467 and 83468 but no WHsAg reduction were observed in the other four animals (Fig. 3B). No changes in WHV DNA (Fig. 3C) or AST (Fig. 3D) were observed, except in one woodchuck (83466) receiving REP 2139-Ca with AST elevation at week 5, which was not accompanied by any antiviral response. WHsAg reduction was observed in the control animal (83462) in this experiment, a rare effect also reported in other studies (Meng et al., 2016).
Figure 3.
Antiviral effects of REP 2139-Ca in woodchucks with chronic WHV infection (n=6). Experimental design is indicated in (A). Changes during treatment in serum WHsAg (B), WHV DNA (C) and AST (D) are presented. BL = baseline
3.2 Effects of NAPs in HBV infected SCID-Hu mice
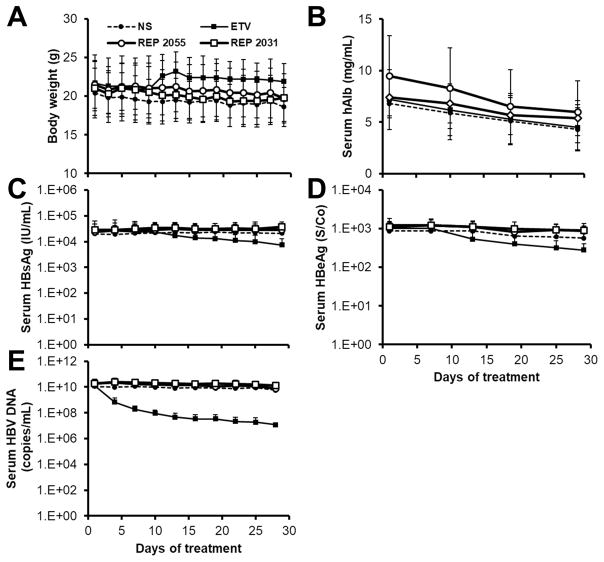
The NAPs REP 2055 and REP 2031 were tested in SCID-Hu mice with pre-established HBV (genotype D) infection. REP 2031 is a control NAP which has shown to have a mild entry-inhibition activity in vitro against HBV and DHBV but no post-entry activity in DHBV and negligible antiviral effect against DHBV infection in vivo (Guillot et al., 2017; Noordeen et al., 2013a; 2013b). Dosing in these mice consisted of daily administration of 10mg/kg REP 2055 or REP 2031 dailyfor 28 days. Separate groups of mice were treated with entecavir (ETV) as a positive control or normal saline as a negative control. Animal weights were not altered in any treatment group throughout dosing (Fig. 4A) and the human chimeric livers in these mice were stable, as evidenced by consistent production of human albumin throughout treatment (Fig. 4B). However, neither REP 2055 nor REP 2031 had any effect on HBsAg or HBeAg (Fig. 4C, 4D) or HBV DNA (Fig. 4E). Animals in the ETV group experienced mild reductions in both HBsAg (0.45log) and HBeAg (0.31log) and more substantial reductions in viremia (2.68log) compared to mice in the normal saline group.
Figure 4.
Antiviral effects of entecavir, REP 2055 and REP 2031 in HBV infected SCID-Hu mice. Changes in body weight (A), serum human albumin (B), HBsAg (C), HBeAg (D) and HBV DNA (E) are presented. Plotted values are mean ± standard deviation (n=5).
3.3 Effects of NAPs in HBV transgenic mice
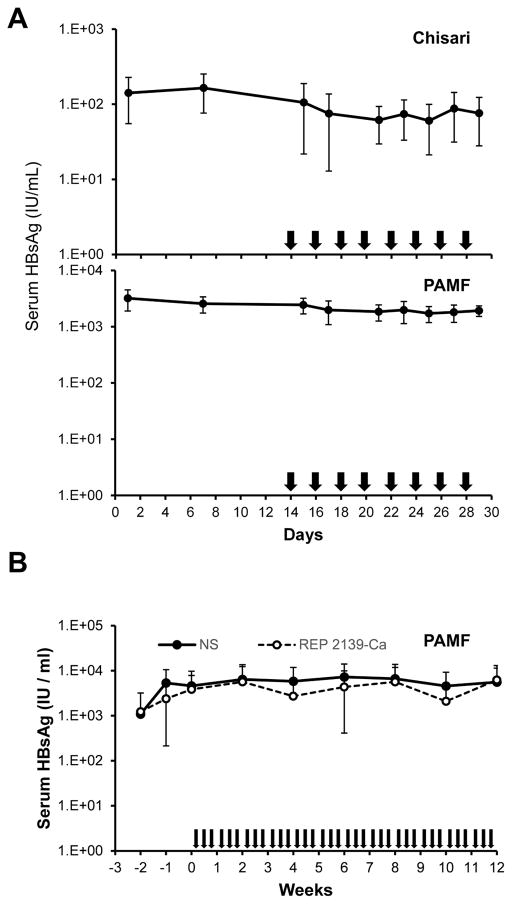
The antiviral effects of REP 2139-Ca were assessed in a preliminary experiment in two different strains of transgenic mice, the “Chisari” strain (n=6) and the “PAMF” strain (n=7). In this experiment, the stability of serum HBsAg levels was monitored for 2 weeks prior to dosing, which consisted of 10mg/kg every other day for 16 days. During this treatment, no reduction of serum HBsAg was observed (Fig. 5A). Since the PAMF mice produced significantly higher levels of circulating HBsAg, a second experiment was conducted assessing the effects of 12 weeks of therapy with REP 2139-Ca (10mg/kg, 3 times per week) in 11 animals with a matched control group receiving normal saline. In this second, long term dosing experiment, no reduction in HBsAg was observed and HBsAg levels were similar to those in normal saline treated animals (Fig. 5B).
Figure 5.
Antiviral effects of REP 2139-Ca in HBV transgenic mice. A) changes in serum HBsAg levels in “Chisari” (top) and “PAMF” (bottom) HBV transgenic mice during two weeks of treatment with REP 2139-Ca. B) changes in serum HBsAg levels in PAMF HBV transgenic mice during 12 weeks of treatment with REP 2139-Ca. Arrows indicate treatment days. Plotted values are mean ± standard deviation (n=6 [Chisari] and n=7 [PAMF] in (A), n=11 in (B)).
4. Discussion
The pharmacokinetic and biodistribution properties of NAPs are similar in mice, ducks and non-human primates and consists of clearance of NAPs from the plasma with accumulation primarily in the kidney and liver (Roehl et al., 2017). The accumulation of NAPs in the liver and kidney after subcutaneous administration of REP 2139 in the woodchuck as demonstrated in this study is consistent with the pharmacokinetic behaviour of NAPs observed in other animal models. In preclinical studies of the antiviral effects of NAPs in DHBV infected Pekin ducks, the onset of DHBsAg reduction with NAPs is reproducibly rapid, with clearance occurring within one week of daily 10mg/kg dosing (Noordeen et al., 2013a, 2015). This antiviral effect is retained in ducks with NAP dosing as low as 1mg/kg/day (Noordeen et al., 2013a). In contrast, reductions in WHsAg during NAP treatment of chronic WHV infected woodchucks were very mild, ranging from 36–79% in both experiments, was slow in onset and only occurred in 4/10 treated woodchucks. These reductions in WHsAg were not accompanied by any reduction in WHV DNA.
In mice, antiviral activity of NAPs in the liver against CMV is readily achieved in mice with daily i.p. or s.c. dosing as low as 2mg/kg (Cardin et al., 2009) and in HCV infected SCID-Hu mice with i.p. dosing every other day at 10mg/kg (Matsumura et al., 2009). However, neither REP 2055 nor REP 2139 had any effect on antigenemia or viremia in two different mouse models of HBV infection using dosing regimens demonstrated to achieve abundant levels of NAPs in the liver in this species (Roehl et al., 2017). Similar dosing regimens have resulted in rapid surface antigen clearance in DHBV infected ducks or have demonstrated antiviral activity against other hepatotropic viruses in infected normal mice or HCV infected SCID-Hu mice (Cardin et al., 2009; Matsumura et al., 2009). The weak or absent surface antigen reductions with REP 2055 and/or REP 2139 in woodchucks and mice is striking as both these NAPs have been shown to reliably achieve rapid clearance of DHBsAg in DHBV infection in vivo (Noordeen et al., 2015; Quinet et al., 2016) and HBsAg in chronic HBV infection in diverse patient populations in several clinical trials (Al-Mahtab et al., 2016; Bazinet et al., 2017a, Bazinet et al., 2017b). This lack of efficacy of NAPs in rodent models cannot be due to a pharmacokinetic or dosing level issue as described above. Moreover, it is well documented that the systemic exposure of phosphorothioate oligonucleotides (PS-ONs) in rodents and non-human primates is similar with any route of parenteral administration. Whether administered via s.c, i.p or intravenous routes (i.v.) routes, this class of compounds are rapidly cleared from the circulation and accumulate primarily in the kidney and liver and to a lesser extent in the spleen, lungs and other organs. These common behaviours have been exhaustively confirmed with numerous different species of PS-ONs in mice, rats, non-human primates and humans (Yu et al., 2007; Levin et al., 2007) and have been previously verified for NAPs in mice and non-human primates (Roehl et al., 2017). As such, achievement of pharmacological activity in the liver with other PS-ONs (antisense oligonucleotides) in human patients is similar whether these compounds are administered i.v. or s.c. (Graham et al., 2013; Janssen et al., 2013; Geary et al., 2015b).
The verification of this conserved pharmacological behaviour of NAPs in woodchucks with parenteral administration in the current study (rapid clearance from the circulation with concomitant accumulation in the kidney and liver) clearly demonstrates that the pharmacokinetic behaviour of NAPs (i.e. PS-ONs) in woodchucks is (as expected) the same as in other rodent species and non-human primates. Further, s.c. administered NAPs clearly result in their accumulation in the liver of woodchucks (an effect which would clearly be no different with i.v. administered NAPs) with little or no antiviral effect. As such, the discrepancy between the antiviral effects of NAPs in human HBV infection and rodent models of HBV infection is not due to different routes of administration used. Instead, these differences may be related to the absence in rodents of some element involved in the secretion of SVPs present in chronic HBV infection and in DHBV infected ducks.
The molecular mechanisms of SVP assembly and secretion are currently unknown and the manner in which the assembly and or secretion of SVPs is inhibited by NAPs is still under investigation. However, the current data in HepG2.2.15 cells in vitro, in DHBV infection in vivo and the close correlation of these antiviral effects with those in HBV infected patients clearly establish that NAPs inhibit SVP morphogenesis and or secretion in the human disease. This novel antiviral mechanism is highly relevant for therapeutic impact in human patients. As such, the lack of activity of NAPs in rodent models of HBV infection may provide clues as to how NAPs are acting.
SVPs from patients with chronic HBV infection contain serum components and are closely related to HDL in both lipid and cholesterol composition (Neurath et al., 1974; Burrell et al., 1975; Gavilanes et al., 1982), suggesting that the assembly and or secretion of SVPs may rely on some aspect of HDL lipid metabolism. More recent studies also support a role for lipid / HDL metabolism in SVP assembly / trafficking (Lin et al., 2003; Satoh et al., 2000). HDL are remarkably similar in avian species and humans (Kruski et al., 1975), however the cholesteryl ester transfer protein (CETP), an important enzyme which shuttles cholesterol esters from HDL to LDL and VLDL in humans, is also abundant in avian species but is absent in rodent species (Guyard-Dangremont et al., 1998). This important biochemical difference in rodents results in the bulk of plasma cholesterol being carried in HDL instead of LDL and VLDL as in avian species and humans (Camus et al., 1983). The fact that SVPs are highly similar to HDL, combined with the correlation between the lack of surface antigen response to NAPs in rodent species versus ducks and humans and the differences between in HDL metabolism between rodents and avians/humans suggests that HDL metabolism may be important in SVP production. Moreover, NAPs may somehow target an aspect of host HDL metabolism involved in SVP morphogenesis and or secretion not present in rodent species. Additional investigation will be required to validate this hypothesis.
The SCID-Hu model has an advantage over other rodent models in that the human chimeric liver present in these mice supports the infection and propagation of HBV infection with inoculation of virus derived from human sera. Importantly, the establishment of liver chimeras in SCID-Hu mice with a replacement index of < 70% is accompanied by the establishment of a human lipoprotein profile and the presence of CETP (Steenbergen et al., 2010). Human albumin expression levels in the SCID-Hu mice used in this study indicated a replacement index of > 70% but NAPs were still ineffective in this model, despite the fact that NAPs are active against HCV infection in these mice (Matsumura et al., 2009). In this model, NAPs were active in blocking HCV entry into the liver but had no antiviral effect against established HCV infection. However, the restriction to entry inhibitory activity observed in vivo is consistent with entry inhibitory properties of NAPs against HCV described in this study and as the activity of NAPs in patients with HCV infection is unknown, lack of post-entry activity in this setting is difficult to interpret.
On the other hand, it has been well established that the antiviral effect of NAPs is to work at post entry to block the release of subviral particles (Blanchet et al., 2017). NAPs with no entry activity (i.e. REP 2139, Guillot et al., 2017) are fully active in ducks and humans (Bazinet et al., 2016; Quinet et al., 2016) and a control NAP (REP 2031) capable of blocking entry (Guillot et al., 2017) but having no post-entry effect (Noordeen et al., 2013b) has no activity in vivo the duck model (Noordeen et al., 2013a) and is also inactive in Scid-Hu mice as shown in the current study. As such, the lack of activity of NAPs in the therapeutic setting in rodents (where post-entry effects are best evaluated) clearly indicates the absence of antiviral activity whether administered before or after infection.
The inactivity of NAPs in HBV infected SCID-Hu mice appears at odds with the presence of HBV infected human hepatocytes in this model. Moreover, the selective targeting of SVP assembly and or secretion recently demonstrated in HepG2.2.15 cells is achieved in the absence of any human supplements (Blanchet et al., 2017), demonstrating that the antiviral activity of NAPs is not dependent on factors from other human tissues. Therefore, that the lack of other human cell types in the SCID-Hu model is an unlikely reason for the lack of activity of NAPs. However, the human regions of the chimeric livers in SCID-Hu mice display prominent steatosis, lack of sinusoids and the absence of bile canalicular formation between human and mouse hepatocytes (Peterson et al., 2010), indicating altered lipid metabolism and a potentially incomplete biosynthetic functioning of these hepatocytes. These defects may affect SVP morphogenesis and or trafficking in this model despite the presence of HBV infected human hepatocytes.
5. Conclusions
Several studies with investigational agents against HBV infection that target HBV replication upstream of SVP assembly and or secretion have resulted in HBsAg reductions in rodent models (Billioud et al., 2016; Martin et al., 2015; Menne et al., 2015), indicating that rodent-based models of HBV infection have had and will continue to have an important utility in evaluating the antiviral effects of investigational agents against HBV infection. However, these studies suggest that the aspects of SVP assembly and or secretion targeted by NAPs in patients with chronic HBV infection are not well modeled in currently available rodent-based models of HBV infection. This possibility should be considered when interpreting the antiviral effects (especially HBsAg reduction) of new investigational agents directly targeting SVP assembly and or secretion in these models.
Highlights.
NAPs display similar liver accumulation in ducks, mice, non-human primates and woodchucks.
In woodchuck and mouse models of HBV infection, WHsAg or HBsAg response to NAP treatment is weak or absent.
SVP assembly / secretion occurring chronic HBV infection may differ from that in rodent models of HBV infection.
Acknowledgments
This work was supported by Replicor Inc., the German Research Foundation (DFG, grant TRR60), the National Natural Science Foundation of China (8146113009), the Chinese National Key Technology R&D Program (2015BAI09B06) and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Replicor Inc. has utilized the non-clinical and pre-clinical services program offered by NIAID under contract HHSN272201000039I/HHSN27200001/A19.
Glossary
- CMV
cytomegalovirus
- DHBsAg
duck hepatitis B virus surface antigen
- DHBV
duck hepatitis B virus
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDL
high density lipoprotein
- HDV
hepatitis delta virus
- LDL
low density lipoprotein
- WHsAg
woodchuck hepatitis virus surface antigen
- WHV
woodchuck hepatitis virus
- SCID-Hu
[uPA+/+: B6SJL-TgN(Alb1Plau)144Bri, SCID: C.B-17/Icr-scid/scid Jcl] mice containing human hepatocytes
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLOS ONE. 2016;11:e0156667. doi: 10.1371/journal.pone.0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Krawczyk A, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alpha 2a in patients with chronic hepatitis B virus and hepatitis D virus coinfection: a phase 2 study. Lancet Gastroenterol Hepatol. 2017a;2017 doi: 10.1016/S2468-1253(17)30288-1. (in press) [DOI] [PubMed] [Google Scholar]
- Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L2, Smesnoi V, Musteata T, Jucov A, Krawczyk A, Vaillant A. Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated Interferon alpha-2a in treatment naïve caucasian patients with chronic HBeAg negative HBV infection. J Hepatol. 2017b;66:S256. [Google Scholar]
- Billioud G, Kurse RL, Carrillo M, Whitten-Bauer C, Gao D, Kim A, Chen L, McCaleb ML, Crosby JR, Hamatake R, Hong Z, Garaigorta U, Swazye E, Bisig KD, Wieland S. In vivo reduction of hepatitis B virus antigenemia and virema by antisense oligonucleotides. J Hepatol. 2016;64:781–789. doi: 10.1016/j.jhep.2015.11.032. [DOI] [PubMed] [Google Scholar]
- Blanchet M, Vaillant A, Labonte P. Post-entry antiviral effects of nucleic acid polymers against hepatitis B virus infection in vitro. J Hepatol. 2017;66:S257. [Google Scholar]
- Burrell CJ. Host components in hepatitis B antigen. J Gen Virol. 1975;27:117–126. doi: 10.1099/0022-1317-27-2-117. [DOI] [PubMed] [Google Scholar]
- Camus MC, Chapman MJ, Forgez P, Laplaud PM. Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J Lipid Res. 1983;24:1210–1212. [PubMed] [Google Scholar]
- Cardin RD, Bravo FJ, Sewell AP, Cummins J, Flamand L, Juteau JM, Bernstein DI, Vaillant A. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virol J. 2009;6:214. doi: 10.1186/1743-422X-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N, Chang HE, Nicolas E, Han Z, Jarnik M, Taylor J. Properties of subviral particles of hepatitis B virus. J Virol. 2008;82:7812–7817. doi: 10.1128/JVI.00561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova L, Zoulim F. Duck hepatitis B virus model in the study of hepatitis B virus. Methods Mol Med. 2004;96:261–268. doi: 10.1385/1-59259-670-3:261. [DOI] [PubMed] [Google Scholar]
- Dandri M, Petersen J. Animal models of HBV infection. Best Pract Res Clin Gastroenterol. 2017;31:273–279. doi: 10.1016/j.bpg.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Feitelson MA, Clayton MM, Sun B, Schinazi RF. Development of a novel mouse model to evaluate drug candidates against hepatitis B virus. Antivir Chem Chemother. 2007;18:213–23. doi: 10.1177/095632020701800405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C, Matschl U, Bruns M. Enzymatic treatment of duck hepatitis B virus: topology of the surface proteins for virions and non-infectious subviral particles. Virology. 2007;359:26–136. doi: 10.1016/j.virol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Foster WK, Miller DS, Marion PL, Colonno RJ, Kotlarski I, Jilbert AR. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrob Agents Chemother. 2003;47:2624–2635. doi: 10.1128/AAC.47.8.2624-2635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes F, Gonzalez-Ros JM, Peterson DL. Structure of hepatitis B surface antigen. J Biol Chem. 1982;257:7770–7777. [PubMed] [Google Scholar]
- Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015a;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Geary RS, Baker BF, Crooke ST. Clinical and Preclinical Pharmacokinetics and Pharmacodynamics of Mipomersen (Kynamro®): A Second-Generation Antisense Oligonucleotide Inhibitor of Apolipoprotein B. Clin Pharmocokinet. 2015;54:133–146. doi: 10.1007/s40262-014-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich WH, Wend U, Glebe D. Quantitative assay of hepatitis B surface antigen in serum or plasma using laurel electrophoresis. Meth Mol Med. 2004;95:57–63. doi: 10.1385/1-59259-669-X:57. [DOI] [PubMed] [Google Scholar]
- Graham MJ, Lee RG, Bell TA, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, Baker BF, Burkey J, Crooke ST, Cooke RM. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–90. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot C, Martel N, Berby F, Bordes I, Hantz O, Blanchet M, Sureau C, Vaillant A, Chemin I. Inhibition of hepatitis viral entry by nucleic acid polymers in HepaRG cells and primary human hepatocytes. PLOS ONE. 2017;12:e0179697. doi: 10.1371/journal.pone.0179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyard-Dangremont V, Desrumaux C, Gambert P, Lallemant C, Lagrost L. Phospholipid and cholesteryl ester transfer activity in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp Biochem Physiol Biochem Mol Biol. 1998;120:517–525. doi: 10.1016/s0305-0491(98)10038-x. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Murakami S, Omagari K, Matsui T, Lio E, Isogawa M, Watanabe T, Karino Y, Tanaka Y. Characterization of novel entecavir resistance mutations. J Hepatol. 2015;63:546–553. doi: 10.1016/j.jhep.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Innacone M, Guidotti G. Mouse models of hepatitis B virus pathogenesis. Cold Spring Harb Perspect Med. 2015;5:a021477. doi: 10.1101/cshperspect.a021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Julander JG, Sidwell RW, Morrey JD. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antivir Res. 2002;55:27–40. doi: 10.1016/s0166-3542(01)00223-6. [DOI] [PubMed] [Google Scholar]
- Julander JG, Colonno RJ, Sidwell RW, Morrey JD. Characterization of antiviral activity of entecavir in transgenic mice expressing hepatitis B virus. Antivir Res. 2003;59:155–161. doi: 10.1016/s0166-3542(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Kruski AW, Scanu AM. Properties of rooster serum high density lipoproteins. Biochim Biophys Acta. 1975;409:26–38. doi: 10.1016/0005-2760(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Le Mire MF, Miller DS, Foster WK, Burrell CJ, Jilbert AR. Covalently Closed Circular DNA Is the Predominant Form of Duck Hepatitis B Virus DNA That Persists following Transient Infection. J Virology. 2005;79:12242–12252. doi: 10.1128/JVI.79.19.12242-12252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds JM, Henry SP, Geary R, Burckin T, Levin AA. Comparison of the pharmacokinetics of subcutaneous and intravenous administration of a phosphorothioate oligodeoxynucleotide in cynomolgus monkeys. Antisense Nucleic Acid Drug Dev. 10:435–441. doi: 10.1089/oli.1.2000.10.435. [DOI] [PubMed] [Google Scholar]
- Levin AA, Yu RZ, Geary RS. Basic principles of the pharmacokinetics of antisense oligonucleotide drugs. In: Crooke ST, editor. Antisense drug technology: principles, strategies, and applications. 2. Boca Raton: CRC Press; 2007. pp. 183–216. [Google Scholar]
- Lin YL, Shiao MS, Mettling C, Chou CK. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology. 2002;314:253–260. doi: 10.1016/s0042-6822(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Marion PL, Salazar FH, Littschwage MA, Bordier BB, Seeger C, Winters MA, Cooper AD, Cullen JM. A transgenic mouse lineage useful for testing antivirals targeting hepatitis B virus. In: Schinazi RF, Sommadossi J-P, Rice CM, editors. Frontiers in Viral Hepatitis. Elsevier B.V; 2003. pp. 197–210. [Google Scholar]
- Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, Kratzer R, Lelu-Santolaria K, Evlachev A, Meritet JFD, Schlesinger Y, Villeval D, Strub JM, Van Dorsselaer A, Marchand JB, Geist M, Brandley R, Findeli A, Boukhebza H, Menguy T, Silvestre N, Michel ML, Inchauspé G. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T-cells and exerts an antivital effect in HBV-persistent mice. Gut. 2015;64:1961–1971. doi: 10.1136/gutjnl-2014-308041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Hu Z, Kato T, Dreux M, Zhang Y-Y, Imamura M, Hiraga N, Juteau J-M, Cosset F-L, Chayama K, Vaillant A, Liang TJ. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterol. 2009;137:673–681. doi: 10.1053/j.gastro.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Ma Z, Zhang E, Kosinska AD, Liu J, Zhang X, Zhou T, Wu J, Dahmen U, Dirsch O, Yang D, Roggendorf M, Lu M. Novel woodchuck hepatitis virus (WHV) transfene mouse models show sex-dependent WHV replicative activity and development of spontaneous immune responses to WHV proteins. J Virol. 2014;288:1573–1581. doi: 10.1128/JVI.02086-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Zhang X, Pei R, Kemper T, Vollmer J, Davis HL, Glebe D, Gerlich W, Roggendorf M, Lu M. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res. 2016;125:14–24. doi: 10.1016/j.antiviral.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Menne S, Butler SD, George AL, Tochkov IA, Zhu Y, Xiong S, Gerin JL, Cote PJ, Tennent BC. Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother. 2008;52:3617–3632. doi: 10.1128/AAC.00654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne S, Tunas DB, Liu KH, Thampi L, Al-Deghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GHI, Tennent BC. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237–1245. doi: 10.1016/j.jhep.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E, Tsuge M, Hiraga N, Kan H, Uchida T, Masaki K, Nakahara T, Ono A, Miki D, Kawaoka T, Abe H, Imamura M, Aikata H, Ochi H, Hayes CN, Akita T, Tanaka J, Chayama K. Effect of tenofovir disoproxil fumarate on drug-resistant HBV clones. J Infect. 2016;72:91–102. doi: 10.1016/j.jinf.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Neurath AR, Prince AM, Lippin A. Hepatitis B antigen: Antigen sites related to human serum proteins revealed by affinity chromatography. Proc Natl Acad Sci USA. 1974;71:2662–2667. doi: 10.1073/pnas.71.7.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll AJ, Colledge DL, Toole JJ, Angus PW, Smallwood RA, Locarnini SA. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl) adenine, an acyclic phosphonate nucleoside analogue. Antimicrob Agents Chemother. 1998;42:3130–3135. doi: 10.1128/aac.42.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis virus infection in vivo. Antimicrob Agents Chemother. 2013a;57:5299–5306. doi: 10.1128/AAC.01005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob Agents Chemother. 2013b;57:5291–5298. doi: 10.1128/AAC.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen F, Scougall CA, Grosse A, Qiao Q, lian BB, Reaiche-Miller G, Finnie J, Werner M, Broering R, Schlaak JF, Vaillant A, Jilbert AR. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLOS ONE. 2015;10:e0140909. doi: 10.1371/journal.pone.0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Krull DL, Brown HR, de Serres M. Morphologic characterization of PhoenixBio (uPA+/+/SCID) humanized liver chimeric mouse model. Drug Metab Lett. 2010;4:108–184. doi: 10.2174/187231210791698456. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Lin X, Zhou M, Liu Y, Zhu W, Chen W, Zhang W, Gou L, Liu H, Wu G, Huang M, Jiang M, Xu Z, Zhou Z, Qin N, Ren S, Qiu H, Zhong S, Zhang Y, Zhang Y, Wu X, Shi L, Shen F, Mao Y, Zhou X, Yang W, Wu JZ, Yang G, Mayweg AV, Shen HC, Tang G. Design and synthesis of orally bioavailable 4-methyl heteroaryldihydropyrimidine based hepatitis B virus (HBV) capsid inhibitors. J Med Chem. 2016;59:7651–7666. doi: 10.1021/acs.jmedchem.6b00879. [DOI] [PubMed] [Google Scholar]
- Quinet J, Jamard C, Vaillant A, Cova L. Achievement of surface antigen clearance in the liver by combination therapy with REP 2139-Ca and nucleoside analogs against chronic hepatitis B. J Hepatol. 2016;64:S385. [Google Scholar]
- Roehl I, Seiffert S, Brikh C, Quinet J, Jamard C, Dorfler N, Lockridge JA, Cova L, Vaillant A. Nucleic acid polymers with accelerated plasma and tissue clearance for chronic hepatitis B therapy. Mol Ther Nuc Acids. 2017;8:1–12. doi: 10.1016/j.omtn.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh O, Imai H, Yoneyama T, Miyamura T, Utsumi H, Inoue K, Umeda M. Membrane structure of the hepatitis B virus surface antigen particle. J Biochem. 2000;127:543–550. doi: 10.1093/oxfordjournals.jbchem.a022639. [DOI] [PubMed] [Google Scholar]
- Schinazi RF, Bassit L, Clayton MM, Sun B, Kohler JJ, Obikhod A, Arzumanyan A, Feitelson MA. Evaluation of single and combination therapies with tenofovir disoproxil fumarate and emtricitabine in vitro and in a robust mouse model supporting high levels of hepatitis B virus. Antimicrob Agents Chemother. 2012;56:6186–6191. doi: 10.1128/AAC.01483-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scougall C, Noordeen F, Reaiche GY, Feng F, Low HC, Kitrinos KM, Jilbert AR. To study the ability of tenofovir and emtricitabine in monotherapy and combination therapy to treat chronic duck hepatitis B virus infection in vivo. Presented at the Australian Center for HIV and Hepatitis Virology Research Workshop; June 4th, 2012; Adelaide, Australia. 2012. [Google Scholar]
- Steenbergen RH, Joyce MA, Lund G, Lewis J, Chen R, Barsby N, Douglas D, Zhu LF, Tyrell DL, Kneteman NM. Lipoprotein profiles in SCID/uPA mice transplanted with human hepatocytes become human-like and correlate with HCV infection success. Am J Physiol Gastrointest Liver Physiol. 2010;299:G844–G854. doi: 10.1152/ajpgi.00200.2010. [DOI] [PubMed] [Google Scholar]
- Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge M, Hiraga N, Takaishi H, Noguchi C, Oga H, Imamura M, Takahashi S, Iwao E, Fujimoto Y, Ochi H, Chayama K, Tateno C, Yoshizato K. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology. 2005;42:1046–1054. doi: 10.1002/hep.20892. [DOI] [PubMed] [Google Scholar]
- Utoh R, Tateno C, Yamasaki C, Hiraga N, Kataoka M, Shimada T, Chayama K, Yoshizato K. Susceptibility of chimeric mice with livers repopulated by serially subcultured human hepatocytes to hepatitis B virus. Hepatology. 2008;47:435–446. doi: 10.1002/hep.22057. [DOI] [PubMed] [Google Scholar]
- Vaillant A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res. 2016;113:32–40. doi: 10.1016/j.antiviral.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Yu RZ, Geary RS, Siwkowski A, Levin AA. Pharmacokinetic/pharmacodynamic properties of phosphorothioate 2'-O-(2-Methoxyethyl)-modified antisense oligonucleotides in animals and man. In: Crooke ST, editor. Antisense drug technology: principles, strategies, and applications. 2. Boca Raton: CRC Press; 2007. pp. 305–26. [Google Scholar]