Abstract
Pritelivir, a helicase-primase inhibitor, has excellent in vitro and in vivo activity against human herpes simplex virus (HSV). Mice lethally infected with HSV type 1 or 2, including acyclovir-resistant strains, were treated 72 hours after infection for 7 days with pritelivir or acyclovir. Both drugs were administered orally twice daily either alone or in combination. Dosages of pritelivir from 0.3 to 30 mg/kg reduced mortality (P<0.001) against HSV-1, E-377. With an acyclovir resistant HSV-1, 11360, pritelivir at 1 and 3 mg/kg increased survival (P<0.005). With HSV-2, MS infected mice, all dosages higher than the 0.3 mg/kg dose of pritelivir were effective (P<0.005). For acyclovir resistant HSV-2, strain 12247, pritelivir dosages of 1 to 3 mg/kg significantly improved survival (P<0.0001). Combination therapies of pritelivir at 0.1 or 0.3 mg/kg/dose with acyclovir (10 mg/kg/dose) were protective (P<0.0001) when compared to the vehicle treated group against HSV-2, strain MS (in line with previous data using HSV-1). An increased mean days to death (P<0.05) was also observed and was indicative of a potential synergy. Pharmacokinetic studies were performed to determine pritelivir concentrations and a dose dependent relationship was found in both plasma and brain samples regardless of infection status or time of initiation of dosing. In summary, pritelivir was shown to be active when treatment was delayed to 72 hours post viral inoculation and appeared to synergistically inhibit mortality in this model in combination with acyclovir. We conclude pritelivir has potent and resistance-breaking antiviral efficacy with potential for the treatment of potentially life-threatening HSV type 1 and 2 infections, including herpes simplex encephalitis.
Keywords: antiviral therapy, murine model, herpes simplex encephalitis, combination, efficacy
1. Introduction
The seroprevalence of herpes simplex virus (HSV) infection worldwide has not changed significantly over the past several decades. Once infected, people may have either clinical and or asymptomatic recurrences resulting in person to person transmission. HSV is the most frequently reported pathogen responsible for infectious encephalitis in 2016 (Boucher, 2017). There are two distinct types of HSV infections of the central nervous system (CNS), encephalitis of older children and adults, mainly by HSV type 1 (HSV-1), and neonatal encephalitis usually caused by HSV type 2 (HSV-2) (Whitley, 2015). It is estimated that encephalitis caused by HSV occurs in approximately 1 in 250,000 to 1 in 500,000 individuals per year (Whitley, 2006). Taking into account a world population of currently 7.6 billion people (http://www.worldometers.info/world-population/, retrieved 25 Oct 2017) this equals to approximately 15,000 to 30,000 HSV encephalitis cases globally per year, with a risk of up to 70% mortality when left untreated (Whitley, 2006).
The nucleoside analog acyclovir (ACV), which needs to become activated by the viral thymidine kinase (TK) in HSV infected cells, is the only US Food and Drug Administration (FDA)-approved treatment for HSV encephalitis. However, mortality of up to 25% can still occur (Whitley, 2015).
Therefore, additional therapeutic antiviral compounds with low toxicity and non-TK dependent mechanisms of action are needed, especially in light of the increasing prevalence of ACV resistant isolates of HSV in the immunocompromised host where only limited treatment options are available (Burrel et al., 2013, Frobert et al., 2014, Piret and Boivin, 2016). Further, because of disease severity, combination therapies must be developed with drugs having differing mechanisms of action.
Pritelivir (PTV, formerly known as BAY-57-1293 or AIC316) is a non-nucleosidic inhibitor specifically targeting the viral helicase-primase complex (Kleymann et al., 2002). PTV does not need to be activated by viral or cellular enzymes such as the TK, and is active against nucleoside analog resistant strains. In two clinical trials, superior efficacy over placebo and valacyclovir (prodrug of ACV), respectively, was demonstrated in otherwise healthy persons with genital herpes (Wald et al., 2014; Wald et al., 2016). In both trials, PTV treatment led to a significant reduction in HSV shedding, genital lesions and the amounts of virus shed. PTV was also shown to be active against HSV infections in a murine model of HSV encephalitis when treatment was initiated shortly after viral inoculation (i.e. 6 h) and using dosages up to 60 mg/kg three times a day (Betz et al., 2002).
The present studies were designed to extend the time of initiation of treatment following viral inoculation and determine the lowest effective dose of PTV in these models for both HSV-1 and HSV-2, including ACV resistant strains and supported by determination of PTV plasma and brain concentrations. In addition, combination studies of PTV with ACV were conducted to evaluate the effect of these combinations in potential life-threatening HSV infections, namely herpes simplex encephalitis.
2. Materials and Methods
2.1 Experimental animals
Female BALB/c mice were purchased from Charles River Laboratories (Raleigh, North Carolina) at 3 – 4 weeks of age. Animals were quarantined and acclimated for a minimum of 3 days prior to use. Mice were group housed in microisolator cages and utilized at a quantity of 15 mice per treatment group. They were obtained, housed, utilized and euthanized according to United States Department of Agriculture (USDA) and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) regulatory policies. All animal procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee prior to initiation of studies.
2.2 Viruses and cells
The strains of HSV-1 utilized were the laboratory strain E-377 and the clinical isolate11360 and the strains of HSV- 2 were the laboratory strain MS and the clinical isolate 12247. Isolates were gifts of Jack Hill, Burroughs Wellcome. The susceptibilities of these viruses have been reported previously (Prichard et al., 2009; Tardif et al., 2014). The in vitro half maximal efficacy concentration (EC50) for ACV for strain 11360 was >100 µM and for strain 12247 was also >100 µM and both strains are considered ACV resistant. The strains have the following polymorphisms with ACV resistance-mediating mutations underlined: TK polymorphisms C6G, N23S, K36E, S181N, A192V, G251C, A265T, V267L, P268T, D286E, and N376H (relative to NC_001806) for strain 11360 and G39E, N78D, L140F and C337Y (relative to NP044492.1) and DNA polymerase polymorphisms A9T, P15S and L60P for strain 12247 (relative to NP 044500.1), and S33G, V905M, A1203T, and T1208A (NC_001806) for strain 11360. Human foreskin fibroblast (HFF) cells were prepared as primary cultures from freshly obtained newborn human foreskins and virus stocks were prepared and quantified in HFF cells for use in vivo by methods reported previously (Prichard et al., 2013).
2.3 Antiviral compounds
PTV was kindly provided by AiCuris (Wuppertal, Germany) and was suspended in 1.0% carboxymethylcellulose (CMC) in water for oral delivery to mice. ACV (Sigma Co., St. Louis, Missouri) was also suspended in 1.0% CMC for oral administration. Compounds were prepared in a 0.2 ml volume. Treatments for efficacy and drug distribution evaluations were administered to mice for 7 consecutive days beginning 24–72 h post viral inoculation by oral gavage using doses ranging from 0.03 to 45 mg/kg of PTV or 1 to 50 mg/kg of ACV given twice daily at approximately 12 h intervals depending on the specific protocol. For combination studies, data was used from previous studies which indicated that the lowest effective dose of ACV was 30 mg/kg administered orally twice daily (Prichard et al., 2011). The lowest effective dose of PTV was determined in these studies to be 0.3 mg/kg when given orally twice daily. Dose levels of 10 mg/kg for ACV and 0.3 mg/kg for PTV were selected as the highest dosages to enable the detection of improved efficacy of the combination by employing an experimental design previously reported (Quenelle et al., 2007). All mice in combination studies were dosed twice daily beginning 72 h following viral inoculation using a total volume of 0.2 ml solution of vehicle or drug solutions to equilibrate stress.
2.4 Experimental infections
Mice were manually restrained for intranasal inoculations using a total volume of 0.04 ml/mouse containing an approximate lethal dose 90% (LD90) of HSV-1, strain E-377, HSV-1, strain 11360, HSV-2, strain MS or HSV-2, strain 12247. For HSV-1, strain E-377 studies, the inoculum contained 1 × 103 plaque forming units (PFU)/mouse. For HSV-1, strain 11360 studies, the inoculum contained 3 × 105 PFU/mouse. For pharmacokinetic and efficacy studies with HSV-2, strain MS, the viral inoculum contained 1.1 × 105 PFU/mouse. The combination efficacy study used 2.1 × 104 PFU/mouse for HSV-2. For HSV-2, strain 12247 studies, the inoculum contained 8.8 × 104 PFU/mouse. For mortality experiments, animals were evaluated at least once daily for 21 days post viral inoculation and three or more times daily during peak occurrence of neurological signs so that mice could be humanely euthanized. Mice exhibiting hind limb weakness, head tilt, circling or other signs of viral encephalitis were immediately euthanized and recorded as dead the following day for statistical purposes.
For pharmacokinetic studies, five mice each from vehicle and drug-treated groups were euthanized on day 8, 9 or 10 post viral inoculation following the last treatment for aseptic collection of brain and serum. Treatments were initiated at 24, 48 or 72 h post viral inoculation and uninfected mice were included for comparison. Mice were anesthetized with ketamine-xylazine via intraperitoneal injection for retro-orbital collection of whole blood using capillary tubes into a heparinized tube setting in an ice bath. Blood was stored on ice then centrifuged 134 × g for 7 min. Plasma was collected and transferred to a new vial and immediately frozen at −80°C until assayed for PTV levels by high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) (below). Mice were euthanized via carbon dioxide-oxygen asphyxiation for aseptic retrieval of brain samples. Samples were snap frozen on ethanol-dry ice and stored frozen at −80° C until assayed for PTV concentrations by HPLC-MS (below).
2.5 Analyses of brain and plasma samples
Brains were weighed and homogenized by use of a Gentle MACS™ Octo Dissociator after addition of twice the weight of extraction medium (acidified methanol). The samples were centrifuged at 8°C for 10 minutes at ca. 1,800 × g. The supernatant was transferred into fresh tubes and centrifuged again at 8°C for 10 minutes at 13,400 × g. All extracts were stored at −80°C until subsequent analyses were performed.
Plasma and brain extract samples were diluted in the corresponding blank matrix or directly mixed with protein precipitation solution (acidified methanol) containing internal standard ([13C2H3 15N]pritelivir).
The samples were thoroughly mixed and proteins and particulates were removed by centrifugation (10 minutes at approximately 2,100 × g). The supernatant was transferred to a 96-well plate and was evaporated to dryness. The residue was reconstituted in methanol/acetonitrile/water/acetic acid (25+25+50+1, volume %) prior to analysis.
All samples were subjected to HPLC-MS (Sciex 3200 QTrap, Agilent 1200 HPLC system, and PAL HTC autosampler). Samples were separated with an Ascentis RP-amide column (Supelco), using an injection volume of 10 µl. Water containing 1% acetic acid (mobile phase A) and methanol/acetonitrile/acetic acid at 50+50+1 (volume %; mobile phase B) was used in a gradient that was held at 0% mobile phase B for 0.5 min, linearly increased to 70% mobile phase B in 2.5 min, and then held at 70% mobile phase B for 1.5 min, at a flow of 0.4 ml/min. The column was then flushed with 95% mobile phase B for 0.9 min and reequilibrated for 4.6 min. Detection was performed in the API 3200 QTrap system with a TurboIonSpray ion source operating in the positive mode, with an ionization voltage of 5,000 V and a declustering potential of 31 V. The mass transitions for the multiple-reaction monitoring were m/z 403.6 to 196.3 and 408.6 to 196.3 for PTV and its internal standard, respectively, at a collision energy of 27 eV.
The calibration range was from 2 ng/ml to 2,000 ng/ml, quality control samples were prepared as intra-batch controls. The retention time was 3.3 min for both PTV and its stable-isotope-labeled internal standard.
2.6 Statistical evaluation
Survival curves were created in GraphPad Prism® and survival was evaluated by log rank Mantel-Cox analyses. P values were considered significant when comparing each treated group to the vehicle treated group once corrections were made with Bonferroni (Dunn, 1961), using a significance level of P<0.05 as starting point for the entire family of comparisons. Mean day of death (MDD) was assessed using the Mann-Whitney U rank sum test with a P value of <0.05 considered significant.
3. Results
3.1 In vivo efficacy of single agent therapy
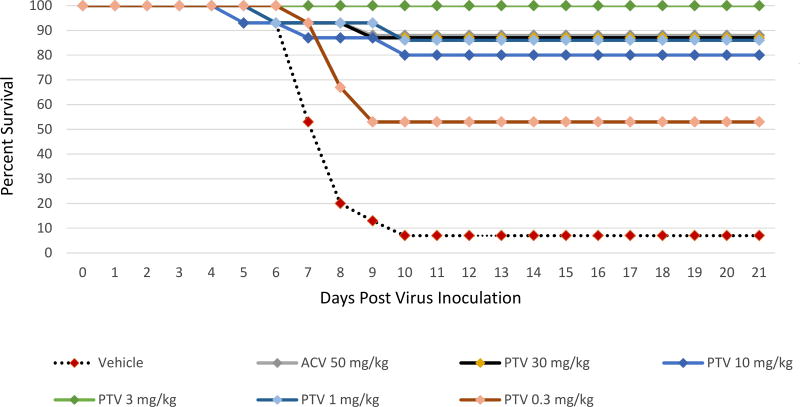
The effects on survival with PTV or ACV treatment of mice infected with the ACV sensitive HSV-1 strain E-377 are shown in Figure 1 and Supplemental Table 1. When PTV was administered twice daily orally at doses of 30, 10, 3 or 1 mg/kg, survival was significantly increased to 80–100% as compared to the vehicle treatment (7%, P<0.0001) when therapy was initiated 72 h post viral inoculation. Even the lowest PTV dose of 0.3 mg/kg twice daily was effective in increasing survival to 53% (P=0.0015). With twice daily ACV treatment at 50 mg/kg, the positive control, survival was also significantly increased to 87% (P<0.0001).
Figure 1. Survival curves for pritelivir (PTV) efficacy compared to acyclovir (ACV) against lethal intranasal inoculation using the ACV sensitive HSV-1 strain E-377.
Mice were infected intranasally with the ACV sensitive HSV-1 strain E-377 and treated twice daily with PTV, ACV, or vehicle (1% carboxymethylcellulose) as indicated for 7 days beginning 72 h after infection. Primary endpoint was mortality within 21 days post virus inoculation.
Overlapping lines are partially offset for better readability.
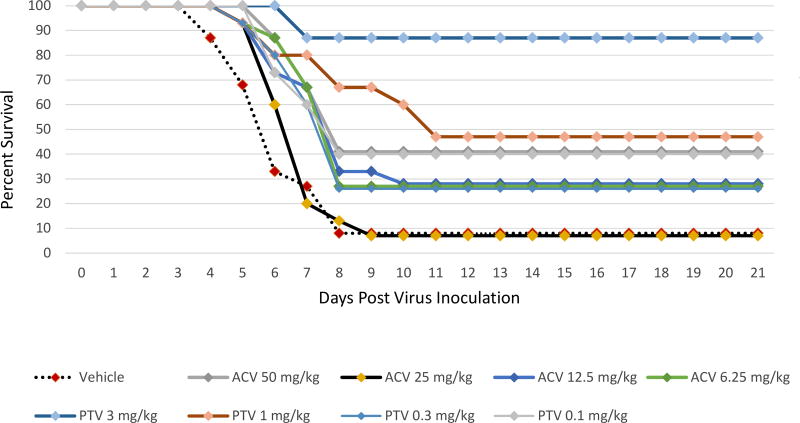
The effects of PTV or ACV on the ACV resistant HSV-1 strain 11360 survival from lethal viral infection are shown in Figure 2 and Supplemental Table 2. When PTV was orally administered twice daily at 3 or 1 mg/kg, survival was significantly increased (P<0.0001 and p=0.0025, respectively) when treatment was initiated 72 h post viral inoculation. Lower PTV doses and all ACV doses were ineffective in increasing survival as compared to the vehicle treatment with 7% survival.
Figure 2. Survival curves for pritelivir (PTV) efficacy compared to acyclovir (ACV) against lethal intranasal inoculation using the ACV resistant HSV-1 strain 11360.
Mice were infected intranasally with the ACV resistant HSV-1 strain 11360 and treated twice daily with PTV, ACV, or vehicle (1% carboxymethylcellulose) as indicated for 7 days beginning 72 h after infection. Primary endpoint was mortality within 21 days post virus inoculation.
Overlapping lines are partially offset for better readability.
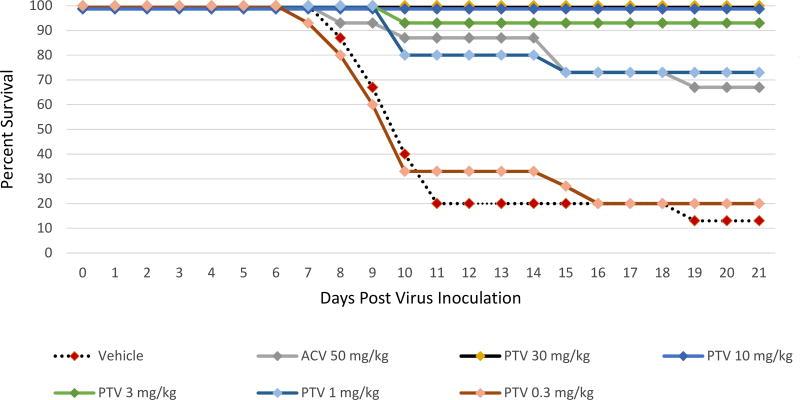
The effects on survival with PTV or ACV against the ACV sensitive HSV-2 strain MS lethal viral infection are shown in Figure 3 and Supplemental Table 3. HSV-2 survival was significantly increased to 93–100% (P<0.0001) during PTV therapy when treatments of 30, 10 or 3 mg/kg twice daily were initiated 72 h post viral inoculation in comparison to the vehicle treated group with 13% survival. The 1 mg/kg twice daily dose of PTV also increased survival (73%) compared to the vehicle treated group (P=0.0005), but not the 0.3 mg/kg twice daily dose. When twice daily treatments of ACV using doses of 50 mg/kg were started 72 h post viral inoculation, survival was also increased to 67% compared to the vehicle treated group (P=0.0009).
Figure 3. Survival curves for pritelivir (PTV) efficacy compared to acyclovir (ACV) against lethal intranasal inoculation using the ACV sensitive HSV-2 strain MS.
Mice were infected intranasally with the ACV sensitive HSV-2 strain MS and treated twice daily with PTV, ACV, or vehicle (1% carboxymethylcellulose) as indicated for 7 days beginning 72 h after infection. Primary endpoint was mortality within 21 days post virus inoculation.
Overlapping lines are partially offset for better readability.
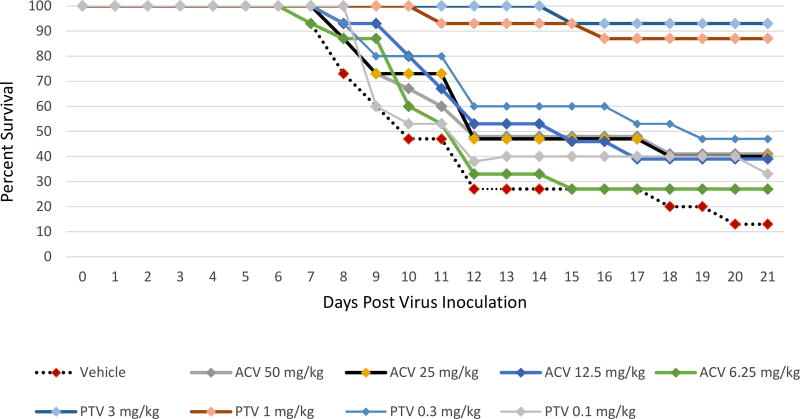
The effects of PTV or ACV against the ACV resistant HSV-2 strain 12247 on survival from lethal viral infection are shown in Figure 4 and Supplemental Table 4. HSV-2 survival was significantly increased during PTV therapy when treatments of 3 or 1 mg/kg twice daily were initiated 72 h post viral inoculation in comparison to the vehicle treated group with 13% survival (P<0.0001). The lowest doses of PTV using 0.3 or 0.1 mg/kg and all doses of ACV were ineffective.
Figure 4. Survival curves for pritelivir (PTV) efficacy compared to acyclovir (ACV) against lethal intranasal inoculation using the ACV resistant HSV-2 strain 12247.
Mice were infected intranasally with the ACV resistant HSV-2 strain 12247 and treated twice daily with PTV, ACV, or vehicle (1% carboxymethylcellulose) as indicated for 7 days beginning 72 h after infection. Primary endpoint was mortality within 21 days post virus inoculation.
Overlapping lines are partially offset for better readability.
3.2 In vivo efficacy of combined therapy
The results of PTV therapy with or without ACV against the ACV sensitive HSV-2 strain MS are shown in Table 1. ACV at the suboptimal dose of 10 mg/kg twice daily plus the suboptimal doses of PTV of 0.3 or 0.1 mg/kg twice daily showed improved survival to 47 or 40%, respectively, (P<0.0001) when compared to the vehicle treated group which had 0% survival. Also, increases in MDD from 8.0 days in the vehicle treated group to 10.9 or 10.2 days was significant (p<0.05) for the ACV 10 mg/kg with PTV at 0.3 or 0.1 mg/kg treated groups, respectively. When compared to survival with ACV alone at 10 mg/kg twice daily (27%, not significant) or PTV alone at 0.3 or 0.1 mg/kg twice daily (7 or 13%, both not significant), a combination of both treatments seems to have a beneficial effect.
Table 1.
Effect of Twice Daily Delayed Oral Treatment Using Acyclovir (ACV) in Combination with Pritelivir (PTV) on the Mortality of Mice Infected Intranasally with the ACV sensitive HSV-2 strain MS
| Treatmenta | Mortality | P-valueb | MDD ± STDEVc |
P- valued |
|
|---|---|---|---|---|---|
| Number | Percent | ||||
| Vehicle | |||||
| 1.0 % CMC | 15/15 | 100 | --- | 8.0 ± 1.5 | --- |
| ACV +72 h | |||||
| 10 mg/kg | 11/15 | 73 | NSe | 10.3 ± 4.0 | NS |
| 3 mg/kg | 12/15 | 80 | NS | 8.7 ± 1.0 | NS |
| 1 mg/kg | 15/15 | 100 | NS | 8.1 ± 1.0 | NS |
| PTV | |||||
| 0.3 mg/kg | 14/15 | 93 | NS | 9.2 ± 1.7 | NS |
| 0.1 mg/kg | 13/15 | 87 | NS | 8.5 ± 2.7 | NS |
| 0.03 mg/kg | 14/15 | 93 | NS | 8.1 ± 1.1 | NS |
| ACV + PTV | |||||
| ACV 10 + PTV 0.3 | 8/15 | 53 | <0.0001 | 10.9 ± 3.1 | <0.05 |
| ACV 10 + PTV 0.1 | 9/15 | 60 | <0.0001 | 10.2 ± 2.4 | <0.05 |
| ACV 10 + PTV 0.03 | 11/15 | 73 | NS | 9.0 ± 1.2 | NS |
| ACV 3 + PTV 0.3 | 13/15 | 87 | NS | 8.8 ± 2.1 | NS |
| ACV 3 + PTV 0.1 | 13/15 | 87 | NS | 8.4 ± 1.0 | NS |
| ACV 3 + PTV 0.03 | 12/15 | 80 | NS | 9.2 ± 2.8 | NS |
| ACV 1 + PTV 0.3 | 15/15 | 100 | NS | 8.7 ± 1.8 | NS |
| ACV 1 + PTV 0.1 | 14/15 | 93 | NS | 8.2 ± 1.1 | NS |
| ACV 1 + PTV 0.03 | 12/15 | 80 | NS | 8.3 ± 0.9 | NS |
. Animals were treated twice daily 7 days beginning 72 h after infection.
CMC=carboxymethylcellulose
. P<0.003 considered significant when compared to the vehicle control using Log rank Mantel-Cox analysis of survival curves with Bonferroni corrections (p<0.05 divided by n=15 comparisons vs. vehicle).
. MDD = Mean Day of Death. STDEV=Standard Deviation.
. MDD was assessed using the Mann-Whitney U rank sum test with a P value of <0.05 considered significant.
. NS = Not significant when compared to the vehicle control.
3.3 Pharmacokinetic analyses
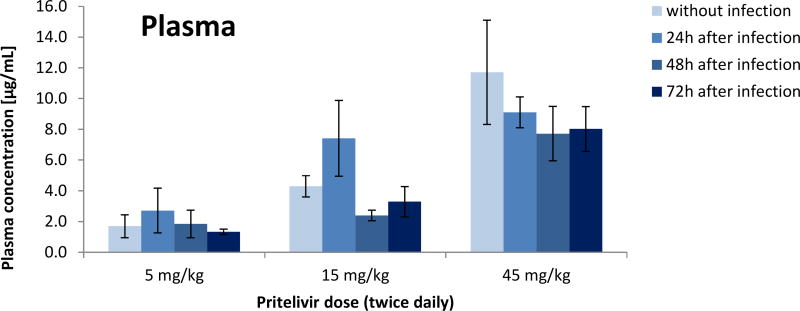
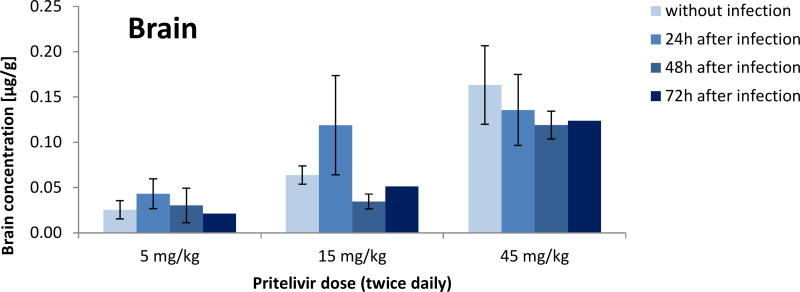
Pharmacokinetic analysis of drug concentrations showed dose dependent amounts of PTV in infected and uninfected mice. Twice daily doses of 5 mg/kg, 15 mg/kg or 45 mg/kg resulted in mean plasma concentrations of 2 µg/ml, 4 µg/ml and 9 µg/ml, respectively, independent of infection status or time of initiation of dosing (Figure 5). Similarly, brain samples showed mean concentrations of approximately 0.05 µg/g for the 5 mg/kg dose group, 0.11 µg/g for the 15 mg/kg dose group and 0.22 µg/g for the mice given 45 mg/kg dose group independent of infection status or time of initiation of dosing (Figure 6).
Figure 5. Pritelivir concentrations in the plasma of uninfected or HSV-2 infected mice.
Pritelivir concentrations from plasma samples of uninfected or HSV-2 infected mice treated at 5, 15 or 45 mg/kg twice daily for 7 consecutive days beginning 24, 48 or 72 h post viral inoculation.
Figure 6. Pritelivir concentrations in the brain of uninfected or HSV-2 infected mice.
Pritelivir concentrations from brain samples of uninfected or HSV-2 infected mice treated at 5, 15 or 45 mg/kg twice daily for 7 consecutive days beginning 24, 48 or 72 h post viral inoculation.
4. Discussion and Conclusions
In our study, PTV demonstrated potent antiviral activity in vivo against both HSV-1 and HSV-2, including ACV resistant strains, when treatment was delayed 72 h post viral inoculation exceeding the time of treatment initiation (6 h post viral inoculation) that was reported previously (Betz et al., 2002). The delayed onset of treatment of 72 h post infection is considered to closer mimic the clinical situation where patients first develop symptoms and see the doctor before getting treatment.
The activity of PTV in the combination study with ACV indicates that there is at least an additive if not a synergistic effect against HSV-2, strain MS. We previously reported potential synergy against HSV-1, E-377 in a similar study where ACV doses of 10 mg/kg combined with PTV doses of 0.3 mg/kg increased survival significantly over either agent given independently (Quenelle et al., 2016). Though further studies are needed to formally prove a synergistic effect the data demonstrate that PTV alone or in combination with ACV might be a new treatment option for HSV encephalitis as ACV is less than optimal and more effective therapy is warranted. In addition, the distinct molecular targets of the compounds (HSV helicase-primase complex for PTV and viral polymerase for ACV) would greatly reduce the risk of development of drug resistance. Furthermore, as demonstrated in our model, PTV therapy due to the different mode of action would allow for treatment of ACV-resistant infections, which also have been reported for HSV encephalitis (Kakiuchi, et. al., 2012; Schepers, et.al, 2014; Bergmann et al., 2017).
It is also of importance that we could demonstrate the presence of PTV in both plasma and brain. PTV was detected in the brain of both uninfected and infected animals and the brain exposure was proportional to the exposure in plasma. As HSV encephalitis is caused by viral replication in the brain leading to acute inflammation, congestion, and/or hemorrhage (Whitley 2006), drug delivery and activity within the CNS is vital for the treatment of this condition. In the previous study (Betz et al., 2002), it was shown that viral load could be significantly reduced in the brain cortex under PTV treatment vs. parallel placebo and valacyclovir treatment. Therefore, PTV exposure in the brain is considered to be sufficient for efficacy.
In human dose finding clinical trials for patients with genital HSV-2 infections, PTV reduced lesions and viral shedding. In addition, no resistant isolates of HSV-2 were found after 4 weeks of daily therapy (Edlefsen et al., 2016). These results and the new data presented here indicate that PTV might be useful for the treatment of HSV encephalitis in the future.
Supplementary Material
Highlights.
Pritelivir, a HSV helicase-primase inhibitor, was shown to be active in an murine model of herpes simplex encephalitis
Treatment was efficacious even when onset was delayed to 72 hours post infection with sensitive or acyclovir resistant HSV
Combination therapy with acyclovir indicated an additive (potentially synergistic) effect on drug sensitive HSV
Pritelivir crossed the blood/brain barrier with comparable exposures in HSV infected and uninfected animals
Pritelivir could be a useful treatment option for herpes simplex encephalitis
Acknowledgments
This work was supported by NIH, NIAID contracts HHSN272201000027I, A01 and A85 to the University of Alabama at Birmingham. The authors appreciate the technical support of Emma Harden for production of virus pools. The authors would like to thank Melanie Sumner, R.Ph., M.Sc. and Richard J. Whitley, M.D. for their expert review of the manuscript. The authors also thank Dr. Inmaculada Aban for statistical review and advice. Authors A.B., T.G., T.P. S.B., and H.Z. are employees of AiCuris. A.B., T.P., and H.Z. report holding stock options in AiCuris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmann M, Beer R, Kofler M, Helbok R, Pfausler B, Schmutzhard E. Acyclovir resistance in herpes simplex virus type I encephalitis: a case report. J Neurovirol. 2017;23(2):335–337. doi: 10.1007/s13365-016-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz UAK, Fischer R, Kleymann G, Hendrix M, Rūbsamen-Waigmann H. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57–1293. Antimicrob Agents Chemo. 2002;46:1766–1772. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher A, Herrmann JL, Morand P, Buzelé R, Crabol Y, Stahl JP, Mailles A. Epidemiology of infectious encephalitis causes in 2016. Med Mal Infect. 2017;47(3):221–235. doi: 10.1016/j.medmal.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Burrel S, Aime C, Hermet L, Ait-Arkoub Z, Agut H, Boutolleau D. Surveillance of herpes simplex virus resistance to antivirals: a 4-year survey. Antiviral Res. 2013;100(2):365–372. doi: 10.1016/j.antiviral.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons among means. J American Statistical Assoc. 1961;56:52–64. [Google Scholar]
- Edlefsen PT, Birkmann A, Huang M, Margaret CA, Kee JJ, Diem K, Goldner T, Timmler B, Stoelen S, Ruebsamen-Schaeff H, Zimmermann H, Warren T, Wald A, Corey L. No evidence of resistance of HSV-2 to pritelivir following four weeks of daily therapy. JID. 2016;214:258–64. doi: 10.1093/infdis/jiw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobert E, Burrel S, Ducastelle-Lepretre S, Billaud G, Ader F, Casalegno JS, Nave V, Boutolleau D, Michellet M, Lina B, Morfin F. Resistance of herpes simplex viruses to acyclovir: An update from a ten-year survey in France. Antiviral Res. 2014;111C:36–41. doi: 10.1016/j.antiviral.2014.08.013. [DOI] [PubMed] [Google Scholar]
- James SH, Kimberlin DW. Neonatal herpes simplex virus infection epidemiology and treatment. Clin Perinatal. 2015;42:47–59. doi: 10.1016/j.clp.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S, Nonoyama S, Wakamatsu H, Kogawa K, Wang L, Kinoshita-Yamaguchi H, Takayama-Ito M, Lim CK, Inoue N, Mizuguchi M, Igarashi T, Saijo M. Neonatal herpes encephalitis caused by a virologically confirmed acyclovir-resistant herpes simplex virus 1 strain. J Clin Microbiol. 2013;2013;51:356–359. doi: 10.1128/JCM.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, Handke G, Eckenberg P, Hewlett G, Pevzner V, Baumeister J, Weber O, Henninger K, Keldenich J, Jensen A, Kolb J, Bach U, Popp A, Maben J, Frappa I, Haebich D, Lockhoff O, Rubsamen-Waigmann H. New helicase-primase inhibitors as drug candidates for treatment of herpes simplex disease. Nat Med. 2002;8:392–298. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- Piret J, Boivin G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management. Curr Opin Infect Dis. 2016;29(6):654–662. doi: 10.1097/QCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Quenelle DC, Hartline CB, Harden EA, Jefferson G, Frederick SL, Daily SL, Whitley RJ, Tiwari KN, Maddry JA, Secrist JA, Kern ER. Inhibition of herpesvirus replication by 5-substituted 4'-thiopyrimidine nucleosides. Antimicrob Agents Chemother. 2009;53:5251–8. doi: 10.1128/AAC.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Hartline CB, Lanier R, Painter GR, Kern ER, Quenelle DC. CMX001 potentiates the efficacy of acyclovir in mice infected with herpes simplex virus. Antimicrob Agents and Chemother. 2011;55:4728–34. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Williams JD, Komazin-Meredith G, Khan AR, Price NB, Jefferson GM, Harden EA, Hartline CB, Peet NP, Bowlin TL. Synthesis and antiviral activities of methylenecyclopropane analogs with 6-alkoxy and 6-alkylthio substitutions that exhibit broad-spectrum antiviral activity against human herpesviruses. Antimicrob Agents Chemother. 2013;57:3518–3527. doi: 10.1128/AAC.00429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Prichard MN, Keith KA, Hruby DE, Jordan R, Painter GR, Robertson A, Kern ER. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51:4118–24. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Birkmann A, Goldner T, Pfaff T, Zimmermann H, Bonsmann S, Collins DJ, Rice TL. In vivo efficacy of twice daily delayed oral treatment with pritelivir against lethal herpes simplex virus 1 and 2 in intranasal infections of BALB/c mice; Abstract from Twenty-ninth Conference on Antiviral Research, International Society for Antiviral Research; La Jolla. 2016, April. [Google Scholar]
- Schepers K, Hernandez A, Andrei G, Gillemot S, Fiten P, Opdenakker G, Bier J, David P, Delforge M, Jacobs F, Snoeck R. Acyclovir resistant herpes simplex encephalitis in a patient treated with anti-tumor necrosis factor-α monoclonal antibodies. J Clin Virol. 2014;59:67–70. doi: 10.1016/j.jcv.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Tardif KD, Jorgensen S, Langer J, Prichard M, Schlaberg R. Simultaneous titration and phenotypic antiviral drug susceptibility testing for herpes simplex virus 1 and 2. J Clin Virol. 2014 doi: 10.1016/j.jcv.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Wald A, Corey L, Timmler B, Magaret A, Warren T, Tyring S, Johnston C, Kriesel J, Fife K, Galitz L, Stoelben S, Huang ML, Selke S, Stobernack HP, Ruebsamen-Schaeff H, Birkmann A. Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med. 2014;16;370(3):201–10. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- Wald A, Timmler B, Magaret A, Warren T, Tyring S, Johnston C, Fife K, Selke S, Huang ML, Stobernack HP, Zimmermann H, Corey L, Birkmann A, Ruebsamen-Schaeff H. Effect of pritelivir compared with valacyclovir on genital HSV-2 shedding in patients with frequent recurrences: A randomized clinical trial. JAMA. 2016 2016 Dec 20;316(23):2495–2503. doi: 10.1001/jama.2016.18189. Erratum in: JAMA. 2017 Feb 14;317(6)648. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex encephalitis: Adolescents and adults. Antiviral Res. 2006;71(2–3):14. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes Simplex Virus Infections of the Central Nervous System. Continuum (Minneap Minn) 2015;21(6):1704–1713. doi: 10.1212/CON.0000000000000243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.