Abstract
The fetal brain exhibits exquisite alcohol-induced regional neuronal vulnerability. A candidate mechanism for alcohol-mediated brain deficits is disruption of amino acid (AA) bioavailability. AAs are vitally important for proper neurodevelopment, as they comprise the most abundant neurotransmitters in the brain and act as neurotransmitter precursors, nitric oxide donors, antioxidants, and neurotrophic factors, which induce synaptogenesis, neuronal proliferation, and migration. We hypothesized that gestational alcohol alters brain AA concentrations, disrupts AAs associated with neuropathogenesis, and that alterations are region-specific. We assigned pregnant Sprague-Dawley rats to either a pair-fed control or a binge alcohol treatment group on gestational day (GD) 4. Alcohol animals acclimatized via a once daily orogastric gavage of a 4.5 g/kg alcohol dose from GD 5-10, and progressed to a 6 g/kg alcohol dose from GD 11-20. Pair-fed animals received isocaloric maltose dextrin (once daily; GD 5-20). Fetal cerebral cortex, cerebellum, and hippocampus were collected on GD 21. Following collection, Fluorometric High Performance Liquid Chromatography (HPLC) involving pre-column derivatization with o-phthaldialdehyde quantified regional content of 22 AAs. Chronic binge alcohol administration to pregnant dams regionally altered AA concentrations in all three structures, with the cerebral cortex exhibiting least vulnerability and the hippocampus exhibiting maximal vulnerability. We conjecture that the AA imbalances observed in this study are critically implicated in pathological and compensatory processes occurring in the brain in response to gestational alcohol exposure.
Keywords: Alcohol, Teratology, Gestation
Introduction
Alcohol is an established teratogen responsible for a range of physical, physiological, neuroanatomical, and behavioral deficits collectively termed fetal alcohol spectrum disorders (FASD; Riley & McGee, 2005; Sokol, Delaney-Black, & Nordstrom, 2003). In the United States, it is currently estimated that 1 in 10 pregnant women consume alcohol, and 1 in 33 pregnant women report binge drinking in the past 30 days (Tan, Denny, Cheal, Sniezek, & Kanny, 2015). Alcohol consumption during pregnancy affects virtually every developing fetal organ system, of which the most-studied target is the developing brain. The deficits reported include whole and regional brain volume reductions, cortical dysmorphology, neuronal depletion, disruption of neuronal differentiation and migration, and neurobehavioral deficits including learning, memory, and attention impairments (Gautam et al., 2015; Lebel, Roussotte, & Sowell, 2011). Since 1968, an extensive body of work has catalogued the impact of alcohol on the developing brain (Berman & Hannigan, 2000; Burd, 2004; Lebel et al., 2011; Lemione, Harasseau, Borteryu, & Menuet, 1968). However, delineation of candidate mechanisms underlying alcohol-induced neurological deficits remain indeterminate, attributed in part to the complexity of alcohol’s cellular targets and pharmacokinetics, as well as varying temporal and regional vulnerability observed in the brain throughout gestation (G. F. Hamilton, Whitcher, & Klintsova, 2010; Livy, Miller, Maier, & West, 2003; Ramadoss, Lunde, Chen, West, & Cudd, 2007).
Gestational alcohol exposure significantly impairs AA bioavailability in both the mother and the developing fetus. In rodent models, maternal plasma threonine, serine, glutamine, glycine, alanine, and methionine are reduced following an acute alcohol exposure (Padmanabhan, Ibrahim, & Bener, 2002), and plasma proline diminishes following chronic exposure (Marquis, Leichter, & Lee, 1984). Our group, demonstrated maternal and fetal plasma AA dysregulation including glutamine and glutamate in response to alcohol (Ramadoss, Wu, & Cudd, 2008; Washburn, Sawant, Lunde, Wu, & Cudd, 2013). In addition, impairment of maternal uterine artery blood flow and placental uptake of AAs are observed following maternal alcohol consumption, and these factors all critically regulate fetal AA bioavailability (Chung, Teng, Timmerman, Meschia, & Battaglia, 1998). Maternal alcohol consumption and AA disruption are directly associated with intrauterine growth restriction (IUGR), which may potentiate the risk for a myriad of adult-onset diseases (Lunde et al., 2016; Ramsay, 2010; Guoyao Wu, Bazer, Cudd, Meininger, & Spencer, 2004). Despite this evidence regarding the effects of alcohol on AA homeostasis, to the best of our knowledge, no information is available on the AA profile in the alcohol-sensitive developing brain, regionally or holistically in FASD.
In addition to their basic role as protein building blocks, AAs also function as precursors for neurotransmitters, nucleotides, sphingolipids, polyamines, as donors for nitric oxide, (Kwon, Spencer, Bazer, & Wu, 2003) and as potent antioxidants, regulators of hormone secretion, signaling modulators (Kwon et al., 2003). AAs are the most abundant neurotransmitters in the brain, and act as neurotrophic factors, playing major roles in synaptogenesis, neuronal proliferation, and migration (Herlenius & Lagercrantz, 2004). Outside the FASD field, AA analysis in developing brain regions has led to significant advances in understanding the mechanistic pathways leading to neuropathology.
The purpose of this study was to explore the effect of alcohol on region-specific patterns of AA abundance. We chose to explore the cerebral cortex, the hippocampus, and the cerebellum, as these regions have previously shown high alcohol-induced vulnerability, manifesting as a myriad of developmental deficits (Berman & Hannigan, 2000; D. A. Hamilton et al., 2014; Livy et al., 2003). We hypothesized that gestational alcohol exposure will alter brain AA concentrations, causing disruption to key AAs associated with neuropathogenesis, and that these alterations will be region-specific.
Materials and Methods
Animals
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) with approval by the Animal Care and Use Committee at Texas A&M University. Timed pregnant Sprague–Dawley rats were purchased from Charles River (Wilmington, MA), and were housed in a temperature-controlled room (23°C) with a 12:12-hour light–dark cycle. Rats were assigned to a pair-fed control group (n = 6) or an alcohol treatment group (n = 6) on GD 4. The alcohol animals acclimatized via a once daily orogastric gavage of a 4.5 g/kg (22.5% wt/v) alcohol dose from GD 5-10, and progressed to a 6 g/kg alcohol dose from GD 11-20 (28.5% wt/v). The pair-fed control rats were isocalorically matched to alcohol rats by daily dosing with a gavage of maltose dextrin to account for calories from alcohol. The regimen of exposure utilized in this study is based on both reported binge alcohol consumption patterns in pregnant women and binge exposure patterns implemented across FASD animal models (Caetano, Ramisetty-Mikler, Floyd, & McGrath, 2006; Church & Gerkin, 1988; Cudd, Chen, & West, 2002; May et al., 2013; Ryan, Williams, & Thomas, 2008; Thomas, Idrus, Monk, & Dominguez, 2010). All rats were weighed prior to the start of the study, and each treatment animal was yoked with a control animal of similar weight throughout the duration of the study. Feed intake in both groups was measured daily and the amount of diet consumed by the pair-fed animals was matched with the alcohol-fed animals. There was no significant maternal weight difference between the groups on GD 21. Animals were sacrificed on GD 21, one day after the last alcohol exposure. Litter size between treatment groups was not different (P = 0.77).
Fetal brain region isolation
Fetal brain tissue was collected from an equal number of male and female offspring within each treatment group. A single fetal brain per dam was utilized for sample analysis. Samples were serially washed in cold phosphate buffered saline (PBS), meninges were removed, and the bilateral hippocampi, cerebellum, and whole cerebral cortex were micro-dissected in ice-cold HEPES buffer. Individual samples were then flash frozen and stored at −80°C.
Sample Preparation
Fetal brain tissue was weighed, subsequently acidified with 50 μl of 1.5 mM HClO4, homogenized in 925 μl H2O, and then neutralized 25 μl of 2 mM K2CO3 (Go et al., 2012). The supernatant fluid was used for AA analysis by HPLC, as described previously (G. Wu, Davis, Flynn, Knabe, & Davidson, 1997). Concentrations of AAs in samples were quantified on the basis of authentic standards from Sigma Chemicals (St. Louis, MO, USA) using the Waters Millenium-32 workstation (Dai et al., 2012a, 2012b).
Statistical Analysis
The concentrations of AAs in the cortex, cerebellum, and hippocampus of control and alcohol animals were analyzed by Student’s t-test. Statistical significance was established a priori at P < 0.05.
Results
There was no significant difference in the maternal weight between the pair-fed control and alcohol-fed dams on GD 20 (Pair-fed control, 309 ± 8 g; Alcohol, 308 ± 15 g). Fetal weight was significantly decreased in the alcohol group (2.12 ± 0.11 g), compared with that in the pair-fed control (2.53 ± 0.06 g).
Concentrations of 22 AAs within each of the three brain regions of interest (cerebral cortex, hippocampus, and cerebellum) for both control and alcohol animals are summarized in Table 1 (supplementary information). The most notable changes in AA concentrations were observed in the fetal cerebellum and hippocampus, two structures established as exquisitely sensitive to prenatal alcohol exposure (Livy et al., 2003).
Fetal cerebral cortical AA dysregulation
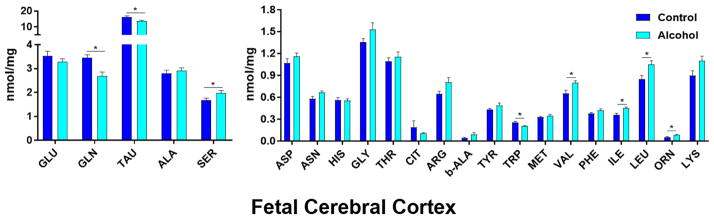
In the cerebral cortex (Figure 1), taurine was the most abundant AA, followed by glutamate, glutamine, alanine, and serine. Of the 22 AAs analyzed, concentrations of 8 AAs were significantly different (P < 0.05) between the pair-fed control and alcohol groups. Cortical isoleucine (P = 0.0093), serine (P = 0.0416), valine (P = 0.0205), leucine (P = 0.0170), and ornithine (P = 0.0174) concentrations significantly increased in the cerebral cortex of gestational alcohol exposed offspring compared with those in pair-fed controls. In contrast, glutamine (P = 0.0057), taurine (P = 0.0113), and tryptophan (P = 0.0078) were significantly decreased in the alcohol group compared to pair-fed controls. Of the three structures analyzed, the cerebral cortex had the fewest number of significantly different AA concentrations.
Figure 1. Effect of chronic gestational binge alcohol exposure on fetal cortical amino acid concentrations (nmol/mg).
Left: Most abundant fetal brain amino acids: Levels of glutamine (↓), taurine (↓), and serine (↑) were significantly different within cortical tissue in the alcohol group compared with those in the pair-fed control group. Right: Less abundant fetal brain amino acids: Isoleucine (↑), leucine (↑), valine (↑), ornithine (↑), and tryptophan (↓) were significantly different within cortical tissue in the alcohol group compared with those in the pair-fed control group. * indicates statistically significant difference in amino acid concentration, P < 0.05.
Fetal cerebellar AA dysregulation
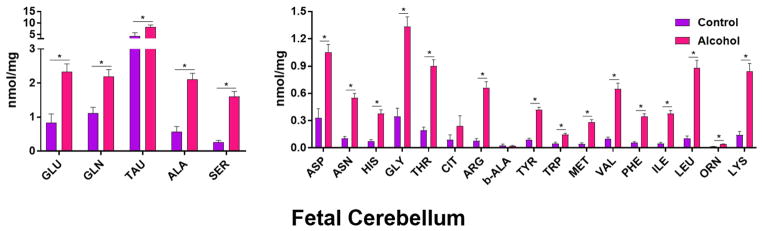
Within the cerebellum (Figure 2), taurine was the most abundant AA, followed by glutamate, glutamine, and alanine in both treatment groups. Interestingly, the fifth most abundant AA was glycine in the control group and serine in the alcohol group. Of the 22 AAs analyzed, 20 (~91%) AA concentrations were significantly different (P < .05) between the pair-fed control and alcohol groups. All 20 of these AA concentrations were increased in alcohol-exposed cerebella compared with those from pair-fed control animals. These AAs were alanine (P = 0.000069), arginine (P = 0.0000096), asparagine (P = 0.0000099), aspartate (P = 0.00026), glutamine (P = 0.0026), glutamate (P = 0.0013), glycine (P = 0.000027), histidine (P = 0.000042), isoleucine (P = 0.0000061), leucine (P = 0.0000061), lysine (P = 0.000017), methionine (P = 0.000018), ornithine (P = 0.0000033), phenylalanine (P = 0.0000068), serine (P = 0.0000059), taurine (P = 0.034), threonine (P = 0.0000046), tryptophan (P = 0.00071), tyrosine (P = 0.0000023), and valine (P = 0.0000056). The cerebellum showed more alteration than the cerebral cortex, but less than that in the hippocampus.
Figure 2. Effect of chronic gestational binge alcohol exposure on fetal cerebellar amino acid concentrations (nmol/mg).
Left: Most abundant fetal brain amino acids: Glutamate, glutamine, taurine, alanine, and serine levels significantly increased (↑) in cerebellar tissue in the alcohol group compared with those in the pair-fed control group. Right: Less abundant fetal brain amino acids: Asparagine, aspartate, histidine, glycine, threonine, arginine, tyrosine, tryptophan, methionine, valine, phenylalanine, isoleucine, leucine, ornithine, and lysine levels significantly increased (↑) in cerebellar tissue in response to gestational alcohol exposure. * indicates statistically significant difference in amino acid concentration, P < 0.05.
Fetal hippocampal AA dysregulation
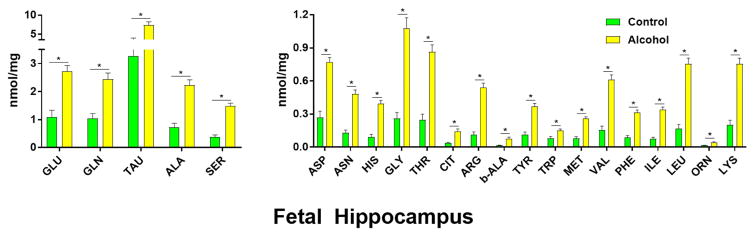
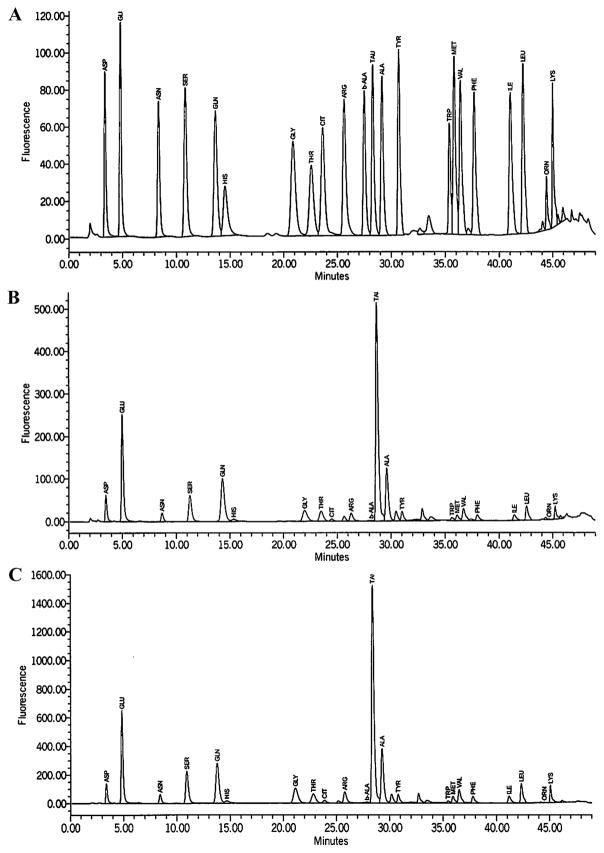
Taurine was the most abundant AA in the hippocampus (Figure 3), followed by glutamate, glutamine, alanine, and serine in both treatment groups. Representative chromatogram traces are shown from (A) standards, (B) pair-fed control, and (C) alcohol treatment groups (Figure 4). All 22 AAs analyzed were significantly different between treatment groups (P < .01). Every hippocampal AA concentration increased in the alcohol group compared with those in the pair-fed control group. Of the three structures analyzed, the hippocampus had the highest percentage of significantly different AA concentrations between treatment groups. These AAs were alanine (P = 0.00011), arginine (P = 0.0000043), asparagine (P = 0.000013), aspartate (P = 0.000033), β-alanine (P = 0.0015), citrulline (P = 0.0012), glutamine (P = 0.00052), glutamate (P = 0.00035), histidine (P = 0.000011), isoleucine (P = 0.0000039), leucine (P = 0.0000047), lysine (P = 0.000010), methionine (P = 0.000013), ornithine (P = 0.0012), phenylalanine (P = 0.0000091), serine (P = 0.0000074), taurine (P = 0.0038), threonine (P = 0.000022), tryptophan (P = 0.0052), tyrosine (P = 0.000019), and valine (P = 0.0000071).
Figure 3. Effect of chronic gestational binge alcohol exposure on fetal hippocampal amino acid concentrations (nmol/mg).
Left: Most abundant fetal brain amino acids: Glutamate, glutamine, taurine, alanine, and serine levels significantly increased (↑) in hippocampal tissue in the alcohol group compared with those in the pair-fed control group. Right: Less abundant fetal brain amino acids: Asparagine, aspartate, histidine, glycine, threonine, citrulline, arginine, β-alanine, tyrosine, tryptophan, methionine, valine, phenylalanine, isoleucine, leucine, ornithine, and lysine levels significantly increased (↑) in hippocampal tissue in the alcohol group compared with those in the pair-fed control group. * indicates statistically significant difference in amino acid concentration, P < 0.05.
Figure 4. Example chromatogram traces from fetal hippocampal tissue.
Representative chromatogram traces are shown from (A) standards, (B) pair-fed control, and (C) alcohol treatment groups.
Discussion
To our knowledge, this is the first study distinguishing dynamic, region-specific, alterations of AA concentrations in the developing fetal brain in response to gestational alcohol exposure. The cerebral cortex showed the fewest altered AA concentrations, in contrast to the cerebellum and hippocampus, which exhibited dramatic AA dysregulation. Additionally, our data demonstrate that prenatal alcohol exposure regionally increases excitatory amino acids in the fetal brain, and also increases taurine levels in these same regions. These AA imbalances may provide insight into both alcohol-mediated neuropathogenesis and the brain’s compensatory neuroprotective response to prenatal alcohol exposure.
Alcohol significantly increases hippocampal and cerebellar excitatory AAs
In alcohol-exposed offspring, considerable increases in glutamate were detected in the cerebellum (177.40%) and hippocampus (149.00%). Aspartate also increased in the cerebellum (218.54%) and hippocampus (186.73%) in the alcohol group compared with pair-fed controls. Excitatory AAs, which include glutamate and aspartate, act on voltage gated channels in plasma membranes throughout cell populations in the brain (Hsu, Chou, Chang, Chou, & Wong, 2001). Studies show increases of these AAs in the developing brain in response to traumatic brain injury (Ruppel et al., 2001), hypoxia-ischemia (Silverstein, Naik, & Simpson, 1991), and maternal stress (Peters, 1990). Excitatory AA disruption has also been observed previously in offspring brain regions following intrauterine inflammation (Lesniak et al., 2013). In the developing brain, acute increases in concentrations of excitatory neurotransmitters lead to acute neurotoxicity, as well as impairment in neurotransmitter programming, receptor expression, neuronal migration, synapse maturation, and have been linked with numerous long-term behavioral deficits and psychiatric illnesses (Herlenius & Lagercrantz, 2004). Our data indicate that alcohol induces significant regional increases in glutamate and aspartate, and increases of these AAs may be responsible for neuronal death observed in the hippocampus and cerebellum following alcohol exposure, two regions with well-documented acute alcohol vulnerability. Interestingly, branched chain AAs, valine, leucine, and isoleucine exhibited significant increases in the cerebellum and hippocampus respectively following alcohol exposure. Together, these branched chain AAs function as primary nitrogen donors in glutamate synthesis, and thus may be integral in the marked increase we observed in the excitatory AA glutamate (Daikhin & Yudkoff, 2000).
Alcohol regionally alters taurine levels, the most abundant AA in the developing brain
Our data illustrates that gestational alcohol regionally alters taurine distribution in the cerebral cortex (-16.31%, cerebellum (+88.85%, and hippocampus (+126.71%. Alcohol also increased methionine, a direct taurine precursor, in both of these structures (cerebellum: 550.88%; hippocampus: 230.95%. Taurine is the most abundant AA in the developing brain, exhibiting region-specific abundance and significantly higher levels in the developing versus mature brain (Pasantes-Morales & Hernandez-Benitez, 2010). By region, taurine concentration is highest in the olfactory bulbs (an area of sustained neurogenesis), followed by the cerebellum and then cerebral cortex. By cell type, taurine concentration is highest in the cerebellar Purkinje cells, an established target exquisitely vulnerable to prenatal alcohol exposure. Taurine is the highest free AA in milk, further suggesting a role in neuronal maturation. Across species, taurine is critically implicated in brain development and in maintaining neuronal homeostasis by acting as a model osmoregulator and intracellular calcium modulator (El Idrissi & Trenkner, 2004; Pasantes-Morales & Hernandez-Benitez, 2010). Taurine deficiency during development correlates with impaired neuronal migration, proliferation, and organization, yet the mechanisms of taurine requirements for nervous system maturation are not yet fully understood (Pasantes-Morales & Hernandez-Benitez, 2010). Taurine disruption in the developing brain correlates with impaired sensory integration and cortical processing, yet not with somatic growth impairments (Sturman, 1993; Uauy, Mena, & Peirano, 2001). This phenotype is a hallmark for many children affected with FASDs, and our working hypothesis is that taurine is a key mechanistic component involved in neuropathology underlying behavioral deficits of FASD.
Our working model
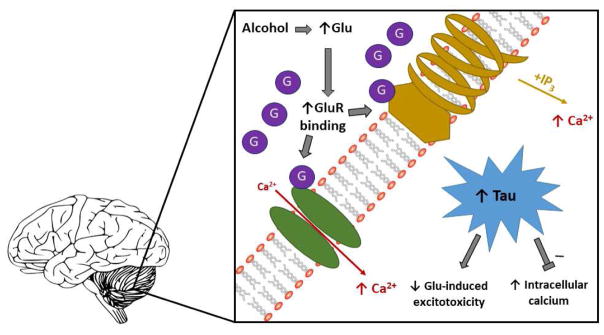
Interestingly, taurine and glutamate are linked in a unique cell-signaling pathway within the brain (Figure 5). Previous studies show that excessive glutamate release disrupts intracellular calcium homeostasis, increasing intracellular osmolality (El Idrissi & Trenkner, 1999; El Idrissi & Trenkner, 2004). In response, taurine levels increase to regulate these increases back within homeostatic levels, actively preventing neuronal death induced via excitatory neurotoxicity (Banerjee, Vitvitsky, & Garg, 2008; Nagelhus, Lehmann, & Ottersen, 1993). Based on our findings, we hypothesize that in select developing brain regions, gestational alcohol induces excessive glutamate release, potentially leading to intracellular hypertonicity; in response, taurine increases downstream in an effort to normalize glutamate-induced excitatory neurotoxicity. We further propose taurine may be acting as a compelling neuroprotectant in response to this exposure. We conjecture that AA imbalances observed in this study are critically implicated in pathological and compensatory processes occurring in the brain in response to gestational alcohol exposure.
Figure 5. Our working hypothesis.
Glutamate and taurine increase in the fetal cerebellum and hippocampus following a chronic, binge, gestational alcohol exposure paradigm. We theorize that this alcohol exposure increases glutamate and subsequent glutamate receptor (NMDA and/or metabotropic) binding in these structures, inducing calcium influx, in turn stimulating excitatory neurotoxicity leading to cell death, and potentially accounting for previously observed neuronal impairment following similar exposure paradigms. We conjecture that taurine, an ideal osmolyte, increases in response to glutamate-induced Calcium influx, counteracting osmotic disruption and offsetting alcohol-mediated damage within these distinctly vulnerable structures.
Study Limitations
It should be noted that in our chronic binge alcohol paradigm, withdrawal might play an influential role in fetal brain AA homeostasis. Our results may indicate a direct alcohol effect, alcohol withdrawal consequences, or a combined effect of both. Previous studies have ascertained that key brain excitatory amino acids increase in response to alcohol withdrawal (Dahchour, Hoffman, Deitrich, & de Witte, 2000; Dahchour, Quertemont, & De Witte, 1996; Rossetti & Carboni, 1995; Tsai & Coyle, 1998), though this is the first study analyzing an AA profile, including excitatory AAs and their precursors, following a chronic binge gestational exposure. An additional consideration worth noting is that the AA disruption observed in this study may be a reflection of alcohol-induced cellular impairment rather than alcohol teratogenicity. For instance, observed AA alterations may be an accumulative effect, reflective of alcohol-induced impairment of cellular processes, which could in turn affect AA metabolism and utilization, differentiation, and/or migration (Guerri, Bazinet, & Riley, 2009). Future studies of other brain regions and additional developmental time-points are warranted to understand how gestational alcohol exposure affects global AA homeostasis in the developing brain and to discern further mechanistic insights.
Perspectives
In the last half century, vast progress has been made discerning targets of gestational alcohol exposure, yet limited knowledge persists for the mechanisms underlying alcohol-mediated developmental pathology. Understanding foundational building blocks for pathogenesis, such as alcohol-induced disruption of AA homeostasis essential for development, is key for mechanistic discernment. Collectively, our data demonstrate that alcohol-induced dysregulation of AA concentrations in the developing brain are region-specific. Future studies are warranted in identifying mechanisms underlying the observed AA dysregulation and whether these mechanisms are region-specific. Further discernment of these mechanisms would advance the FASD field by providing pivotal insight into alcohol’s action on specific regions of the fetal brain, as well as by identifying strategic sites for therapeutic intervention.
Supplementary Material
Fetal brain concentrations (nmol/mg) of the 22 analyzed amino acids by brain region and treatment group. Values are mean ± SEM.*, P < 0.05.
Alcohol exposure regionally dysregulates amino acid homeostasis in the fetal brain
Alcohol increases excitatory amino acids, which may induce excitatory neurotoxicity
Regional taurine elevation could indicate a neuroprotective response to alcohol
Acknowledgments
Funding This work was supported by National Institutes of Health [AA19446, AA23520, AA23035] and Texas A&M University [Tier One Program] JR.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee R, Vitvitsky V, Garg SK. The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends in biochemical sciences. 2008;33(9):413–419. doi: 10.1016/j.tibs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Burd L. Fetal alcohol syndrome. Paper presented at the American Journal of Medical Genetics Part C: Seminars in Medical Genetics.2004. [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The Epidemiology of Drinking Among Women of Child-Bearing Age. Alcoholism: Clinical and Experimental Research. 2006;30(6):1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Chung M, Teng C, Timmerman M, Meschia G, Battaglia FC. Production and utilization of amino acids by ovine placenta in vivo. American Journal of Physiology-Endocrinology And Metabolism. 1998;274(1):E13–E22. doi: 10.1152/ajpendo.1998.274.1.E13. [DOI] [PubMed] [Google Scholar]
- Church M, Gerkin K. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82(2):147–154. [PubMed] [Google Scholar]
- Cudd TA, Chen WJA, West JR. Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcoholism: Clinical and Experimental Research. 2002;26(1):53–58. [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35(6):548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naive and chronically alcoholised rats. Brain Res. 1996;735(1):9–19. doi: 10.1016/0006-8993(96)00537-9. [DOI] [PubMed] [Google Scholar]
- Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids. 2012a;42(5):1597–1608. doi: 10.1007/s00726-011-0846-x. [DOI] [PubMed] [Google Scholar]
- Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Regulatory role for L-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids. 2012b;43(1):233–244. doi: 10.1007/s00726-011-1067-z. [DOI] [PubMed] [Google Scholar]
- Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130(4S Suppl):1026S–1031S. doi: 10.1093/jn/130.4.1026S. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. Journal of Neuroscience. 1999;19(21):9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochemical research. 2004;29(1):189–197. doi: 10.1023/b:nere.0000010448.17740.6e. [DOI] [PubMed] [Google Scholar]
- Gautam P, Lebel C, Narr KL, Mattson SN, May PA, Adnams CM, … Sowell ER. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Human brain mapping. 2015;36(6):2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go G, Wu G, Silvey DT, Choi S, Li X, Smith SB. Lipid metabolism in pigs fed supplemental conjugated linoleic acid and/or dietary arginine. Amino Acids. 2012;43(4):1713–1726. doi: 10.1007/s00726-012-1255-5. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol & Alcoholism. 2009;44(2):108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, … Savage DD. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behavioural brain research. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64(2):127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Experimental Neurology. 2004;190:8–21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hsu MM, Chou YY, Chang YC, Chou TC, Wong CS. An analysis of excitatory amino acids, nitric oxide, and prostaglandin E2 in the cerebrospinal fluid of pregnant women: the effect on labor pain. Anesthesia & Analgesia. 2001;93(5):1293–1296. doi: 10.1097/00000539-200111000-00053. [DOI] [PubMed] [Google Scholar]
- Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biology of reproduction. 2003;68(5):1813–1820. doi: 10.1095/biolreprod.102.012971. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the Impact of Prenatal Alcohol Exposure on the Structure of the Developing Human Brain. Neuropsychology Review. 2011;21(2):102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemione P, Harasseau H, Borteryu J, Menuet J. Les enfants de parents alcooliques: anomalies observées à propos de 127 cas [The children of alcoholic parents: anomalies observed in 127 cases] Quest Medicale. 1968:21. [Google Scholar]
- Lesniak WG, Jyoti A, Mishra MK, Louissaint N, Romero R, Chugani DC, … Kannan RM. Concurrent quantification of tryptophan and its major metabolites. Analytical biochemistry. 2013;443(2):222–231. doi: 10.1016/j.ab.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy D, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicology and teratology. 2003;25(4):447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lunde ER, Washburn SE, Golding MC, Bake S, Miranda RC, Ramadoss J. Alcohol-Induced Developmental Origins of Adult-Onset Diseases. Alcoholism: Clinical and Experimental Research. 2016 doi: 10.1111/acer.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis S, Leichter J, Lee M. Plasma amino acids and glucose levels in the rat fetus and dam after chronic maternal alcohol consumption. Neonatology. 1984;46(1):36–43. doi: 10.1159/000242030. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, … Hasken J. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug and alcohol dependence. 2013;133(2):502–512. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus E, Lehmann A, Ottersen O. Neuronal-glial exchange of taurine during hypo-osmotic stress: a combined immunocytochemical and biochemical analysis in rat cerebellar cortex. Neuroscience. 1993;54(3):615–631. doi: 10.1016/0306-4522(93)90233-6. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R, Ibrahim A, Bener A. Effect of maternal methionine pre-treatment on alcohol-induced exencephaly and axial skeletal dysmorphogenesis in mouse fetuses. Drug and alcohol dependence. 2002;65(3):263–281. doi: 10.1016/s0376-8716(01)00173-9. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Hernandez-Benitez R. Taurine and brain development: trophic or cytoprotective actions? Neurochem Res. 2010;35(12):1939–1943. doi: 10.1007/s11064-010-0262-8. [DOI] [PubMed] [Google Scholar]
- Peters DA. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacology Biochemistry and Behavior. 1990;35(4):943–947. doi: 10.1016/0091-3057(90)90383-s. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Chen WJA, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcoholism: Clinical and Experimental Research. 2007;31(10):1738–1745. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Wu G, Cudd TA. Chronic binge ethanol-mediated acidemia reduces availability of glutamine and related amino acids in maternal plasma of pregnant sheep. Alcohol. 2008;42(8):657–666. doi: 10.1016/j.alcohol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay M. Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Med. 2010;2(4):27. doi: 10.1186/gm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1–3):177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Ruppel RA, Kochanek PM, Adelson PD, Rose ME, Wisniewski SR, Bell MJ, … Graham SH. Excitatory amino acid concentrations in ventricular cerebrospinal fluid after severe traumatic brain injury in infants and children: the role of child abuse. The Journal of pediatrics. 2001;138(1):18–25. doi: 10.1067/mpd.2001.110979. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain research. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein FS, Naik B, Simpson J. Hypoxia-ischemia stimulates hippocampal glutamate efflux in perinatal rat brain: an in vivo microdialysis study. Pediatric research. 1991;30(6):587–590. doi: 10.1203/00006450-199112000-00021. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290(22):2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73(1):119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2015;64:1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annual review of medicine. 1998;49(1):173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Uauy R, Mena P, Peirano P. Mechanisms for nutrient effects on brain development and cognition. Nestle Nutr Workshop Ser Clin Perform Programme. 2001;5:41–70. doi: 10.1159/000061845. discussion 70–42. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Sawant OB, Lunde ER, Wu G, Cudd TA. Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids. 2013;45(3):543–554. doi: 10.1007/s00726-012-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. The Journal of nutrition. 2004;134(9):2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr. 1997;127(12):2342–2349. doi: 10.1093/jn/127.12.2342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fetal brain concentrations (nmol/mg) of the 22 analyzed amino acids by brain region and treatment group. Values are mean ± SEM.*, P < 0.05.