Abstract
WHIM-09 is the first patient described with WHIM syndrome, an autosomal dominant form of neutropenia related to bone marrow retention of neutrophils. Originally diagnosed incorrectly with autoimmune neutropenia, the patient underwent splenectomy at age 9, but the absolute neutrophil count (ANC) did not rise. Subsequently, she was spontaneously cured by chromothripsis (chromosome shattering), which deleted the disease allele CXCR4R334X, and 163 other genes, on chromosome 2 in a single hematopoietic stem cell (HSC). Chromothriptic CXCR4+/o HSCs replaced CXCR4+/R334X WHIM HSCs, and the ANC rose to a new sustained and benign baseline ~2–3-fold above normal that had remained unexplained. Here, we show that splenectomized Cxcr4+/0 mice had sustained and benign neutrophilia, phenocopying neutrophilia in WHIM-09. In addition, WHIM-09’s granulocyte-macrophage precursor cells possessed increased granulocyte colony-forming activity ex vivo. Thus, WHIM-09’s neutrophilia may be multifactorial, involving neutrophil-extrinsic factors (splenectomy), as well as CXCR4 haploinsufficiency-dependent neutrophil-intrinsic factors (increased myeloid precursor cell differentiation). The strong bone marrow retention signal for neutrophils conferred by the WHIM mutation may have prevented neutrophilia after splenectomy until the mutation was deleted by chromothripsis.
Keywords: Neutrophilia, CXCR4, WHIM syndrome, Chromothripsis, HSC, mutation
Introduction
WHIM syndrome is a rare autosomal dominant primary immunodeficiency disease characterized by warts (HPV infection), hypogammaglobulinemia, recurrent infections and myelokathexis (neutropenia due to failure of neutrophils to egress from the bone marrow) (1, 2). Patients often also have panleukopenia and mild thrombocytopenia, but anemia is typically absent. Almost all known cases of WHIM syndrome are caused by gain-of-function mutations in the C-terminus of the chemokine receptor CXCR4 (3). Among other activities, CXCR4 normally functions as a neutrophil retention factor in the bone marrow, and myelokathexis has been interpreted as a pathologic exaggeration of this activity (4). Once released to the blood, WHIM neutrophils appear to chemotax and kill microbes normally, but they frequently have abnormal nuclear morphology and increased cytoplasmic vacuolization consistent with accelerated apoptosis (5). Senescent neutrophils upregulate CXCR4 expression and home back to the bone marrow for destruction, further exacerbating neutropenia (6).
WHIM-09 is a 63 year-old white female and the first patient ever reported with WHIM syndrome (1, 7, 8). In an attempt to correct her severe neutropenia, she underwent splenectomy at age 9, but, while this doubled the ALC in a sustained manner, the absolute neutrophil and monocyte counts (ANC, AMC) were unaffected. Later, in her mid-30’s, the converse changes occurred to the blood leukocyte distribution, but this time spontaneously: both ANC and AMC, not ALC, increased into the normal range, and, importantly, this was associated with spontaneous clearance of warts and cessation of recurrent infections. Thus, she no longer met the criteria for WHIM syndrome and appeared ‘cured’. Still, she was not hematologically normal, since over the next ~10 years both the ANC and AMC continued to rise to a new and sustained plateau ~2–3-fold greater than the upper limits of normal. Thus, she had a stable leukocytosis driven predominantly by neutrophilia with a small contribution from monocytosis. At age 59, ~20 years after her apparent clinical cure, we first saw WHIM-09 at the National Institutes of Health (NIH) and initiated an investigation into the cure mechanism. Using a combination of genomic methods, we found that the copy of chromosome 2 carrying the disease allele had undergone chromothripsis, or ‘chromosome shattering’, in HSCs as well as in the myeloid but not the lymphoid lineage, deleting 35 megabases of DNA in 17 segments and randomly rearranging the rest to form a derivative chromosome lacking the entire mutant copy of CXCR4, as well as 163 other genes (8). Taken together, the data support a cure mechanism in which a single chromothriptic HSC acquired a selective growth advantage in the myeloid lineage, whereas differentiation into the lymphoid lineage, which continued to possess the WHIM allele, was blocked (8). Consistent with an important specific role for the CXCR4R334X deletion, we have demonstrated that Cxcr4 haploinsufficiency per se was sufficient to confer a marked bone marrow engraftment advantage to mouse bone marrow cells as compared to bone marrow cells from wild type or Cxcr4+/WHIM mice. WHIM-09 is the only person known to have had a beneficial effect from chromothripsis, which is mainly known as a cause of a small percentage of cancers (9, 10).
Despite this insight, mechanisms underlying the patient’s sustained benign neutrophilia have remained elusive. In particular, there was no evidence at presentation to NIH of leukemia, myelodysplasia or chronic infection. The many other possibilities fall into two main classes: 1) specific effects on myelopoiesis of haploinsufficiency for one or more combinations of the 164 genes lost by chromothripsis (8); and 2) effects due to splenectomy. Previously in humans, splenectomy has been reported to result in post-operative leukocytosis that is mainly composed of significant permanent increases in ALC and AMC and a transient increase in ANC (11, 12). In addition, inhibiting CXCR4 signaling with a specific antagonist, which should mimic what occurs in CXCR4 haploinsufficiency, is known to cause mobilization of all leukocyte subsets from bone marrow to blood (13) as well as to prevent the homing of aged neutrophils back to bone marrow for clearance (6, 14). CXCR4 signaling has also been shown to regulate cell apoptosis (5). Many cytokines and chemokines also affect leukocyte production, proliferation, distribution, survival, clearance and recruitment (15–22). In the present study, we have used patient samples and animal models to identify potential mechanisms of sustained neutrophilia in WHIM-09.
Materials and Methods
Patients
Consistent with the Declaration of Helsinki, all human subjects signed informed consent to participate in NIAID Institutional Review Board-approved clinical protocols at the NIH Clinical Center.
Animals
C57BL/6 mice were obtained from Taconic (Derwood, MD). CD45.1 Cxcr4+/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Cxcr4+/0 mice on a homozygous CD45.2 background were generated as previously described (8). Mice were maintained in a specific pathogen-free NIH facility; and all studies were reviewed and approved by the Animal Care and Use Committee of the NIAID, NIH.
Splenectomy
Splenectomy was performed as we have previously described (20).
Mouse blood cell analysis
Mice were bled and after dilution the total WBC counts were determined by automated cell counter (Cellometer Auto 2000, Nexcelon Bioscience LLC., Lawrence, MA). For leukocyte differential counts, 70 μl of whole blood were Fc-blocked with anti-mouse CD16/32 and stained with the following mouse-specific mAbs (BioLegend, San Diego, CA): CD3-FITC, CD8 –PE, CD9-APC, CD4-PECy7, CD11b-PercpCy5.5 and Ly6G-Pacific Blue followed by red blood cell lysis, two washes and analysis by flow cytometry. We identified CD11b+ Ly6G+ as neutrophils and CD11b+ Ly6G− cells as monocytes. For T and B cells, cells were gated on the lymphocyte population. The ALC, AMC and ANC were calculated as the cell percentage times the total WBC count.
Homing of neutrophils from blood to bone marrow
CD45.2 WT Cxcr4+/+ and Cxcr4+/0 mice were sacrificed and femoral bone marrow cells were collected. Neutrophils were isolated from bone marrow by gradient centrifugation with Histopaque 1119 and 1107 (23). The purity of neutrophils before and after isolation was checked by anti-mouse CD11b vs. Ly6G staining. Five million neutrophils were injected intravenously into CD45.1 WT mice. Since neutrophils have a short half-life, recipient mice were sacrificed 3 hours later, and bone marrow cells were collected. The homing of donor neutrophils to recipient bone marrow was examined by anti-mouse CD45.2 vs. Ly6G staining.
Analysis of CD34+ progenitor cells in peripheral blood
PBMCs from human donors were Fc-blocked with rat anti-human CD16/32 and stained for 30 min at 4°C with PE-anti-human CD34 mAb (BioLegend San Diego, CA). Data were acquired on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (version 9.4.2; Treestar, Ashland, OR).
Colony formation assay
PBMCs from healthy donors, WHIM patients and WHIM-09 were resuspended in IMDM + 2% FBS medium at 106 cells/ml. 0.4 ml of the cell suspension were transferred to a tube containing 4 ml methocult medium. Cells were vortexed, incubated at room temperature for 5 minutes, injected into 3 wells of a 6-well plate (1.1 ml/well, in triplicate for each subject), and cultured at 37°C and 100% humidity for 14 days. The formation of BFU-E, CFU-GM and CFU-GEMM were enumerated according to the company’s instruction manual (STEMCELL Technologies, Cambridge, MA). CMPs and GMPs were purified from bone marrow aspirates from healthy donors and WHIM-09 as previously reported (8), and suspended in in IMDM + 2% FBS medium at 2×105 or 1×105 cells/ml, respectively. Then 0.4 ml of CMPs or GMPs were transferred into a tube containing 4 ml of methocult medium, mixed well, injected into 3 wells of a 6-well plate (1.1 ml/well) and cultured for 14 days. The colonies generated were counted.
Gene expression assay
Neutrophils from HDs, WHIM patients and WHIM-09 were isolated from peripheral blood and stored in 1 ml Trizol (Thermo Fisher Scientific, Waltham, MA) in liquid nitrogen. The cells were thawed and total RNA was isolated. cDNA was generated by using the Sensi FACS TM cDNA Synthesis Kit (Bioline, Taunton, MA). Real-time PCR kits (Applied Biosystems, Foster City, CA), specific for G-CSF receptor, CCL15 or GAPDH, were used to determine quantitative differences in gene expression, and all data were normalized to constitutive GAPDH values.
Apoptosis assay
Neutrophils were isolated from the peripheral blood of healthy donors, WHIM-09 and her daughter WHIM-10, and cultured cultured in RPMI-1640 + 10% FBS + pen/strep medium at 37°C for 3 hours. Cells were washed in cold PBS two times, suspended in 1× binding buffer and stained with Annexin V-FITC and PI at room temperature for 15 minutes. Cells were analyzed by flow cytometry within an hour on an LSRII and analyzed by Flow Jo.
Cytokine ELISA assay
Blood samples were collected from healthy donors, WHIM patients and WHIM-09. Sera were isolated and stored in liquid nitrogen, thawed, diluted and tested for CXCL12, CXCL1/Groα, and CCL15/MIP-1δ levels using the duoset ELISA mAbs from R&D Systems (Minneapolis, MN), according to the manufacturer’s instructions.
Statistics
Data were analyzed using unpaired parametric t-tests (two-tailed) with Prism 6 (GraphPad Software, La Jolla, CA) and are presented as the mean ± SEM of summary data (the data were approximately normally distributed). The cut-off for statistical significance was defined as p < 0.05. In the figures approximate significance values for the pairwise comparisons marked by the bars are denoted by the number of stars as follows: ****p < 0.0001; ***p < 0.005; **p < 0.01; *p < 0.05; ns, p ≥ 0.05.
Study approval
Human subjects research in this study was governed by a clinical research protocol approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, and written informed consent was obtained from all participants with WHIM syndrome prior to inclusion in the study. Blood from anonymous volunteer healthy donors was obtained from the Department of Transfusion Medicine, Clinical Center, National Institutes of Health. Mouse studies were governed by an Animal Study Protocol approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases.
Results
Neutrophilia in patient WHIM-09 is benign and sustained
Since WHIM-09 has had sustained neutrophilia for ~20 years, we have been closely following her peripheral blood counts and bone marrow for evidence of transition to myelodysplasia (24). From her first visit to the NIH in 2012 to the present time, her total WBC count and ANC and AMC have been stable (Table 1). Her leukocytosis has been accompanied by mild macrocytic hyperchromic anemia; however, her B12 and folate levels have consistently been normal (data not shown), and we have attributed macrocytosis to leukocytosis, which is a known association, although the mechanism is undefined. Serial bone marrow biopsy every 6 months has not revealed an abnormally high frequency of blast cells and blast cells have never been identified in peripheral blood. Thus, we excluded the possibility that her neutrophilia is caused by myelodysplasia or leukemia. Moreover, there is no evidence of chronic infection: she has remained afebrile, and has had stable body weight and a normal erythrocyte sedimentation rate over the five years of follow-up at NIH (data not shown).
Table 1.
Serial complete blood counts for WHIM-09 over five years’ follow-up at the NIH Clinical Center.
| Date | WBC (X103/μl) 3.98–10.04a |
ANC (X103/μl) 1.56–6.13a |
AMC (X103/μl) 0.24–0.86a |
ALC (X103/μl) 1.18–3.74a |
RBC (X106/μl) 3.93–5.22a |
MHC (13) 79.4–94.8a |
MCV (pg) 25.6–32.2a |
Platelets (X103/μl) 175–369a |
|---|---|---|---|---|---|---|---|---|
| 4/30/12 | 20.24b | 16.26b | 1.9b | 1.62 | 3.48c | 104.3b | 33.9b | 507b |
| 5/2/12 | 16.13b | 9.98b | 1.86b | 3.82b | 3.33c | 104.2b | 33.9b | 499b |
| 1/28/13 | 16.2b | 9.01b | 1.65b | 5.11b | 3.27c | 101.5b | 32.1b | 480b |
| 11/13/13 | 16.06b | 11.49b | 1.29b | 2.64 | 3.63c | 101.4b | 32.8b | 467b |
| 3/18/14 | 19.57b | 13.5b | 2.45b | 3.13 | 3.36c | 103.9b | 33.3b | 571b |
| 9/29/15 | 21.14b | 16.26b | 2b | 1.88 | 2.94c | 98.6b | 30.3b | 607b |
| 5/25/16 | 15.62b | 11.93b | 1.84b | 1.34 | 3.79c | 107.1b | 34.8b | 285 |
| 7/13/16 | 13.96b | 10.22b | 1.64b | 1.61 | 4.21 | 104.8b | 35.4b | 322 |
| 9/13/16 | 15.48b | 10.56b | 1.88b | 2.36 | 3.08c | 112.7b | 37.3b | 435b |
| 1/5/17 | 13.92b | 10.0b | 1.63b | 1.87 | 3.26c | 110.1b | 37.1b | 353 |
Normal ranges based on the values of 40 healthy blood donors seen at the NIH Clinical Center.
Value above the upper limit of normal.
Value below the lower limit of normal.
WBC: White blood cell
ANC: Absolute neutrophil count
AMC: Absolute monocyte count
ALC: Absolute lymphocyte count
RBC: Red blood cell
MHC: Mean hemoglobin concentration
MCV: Mean corpuscular volume
Splenectomy in Cxcr4+/0 mice mimics WHIM-09 and phenocopies neutrophilia
Since chromothripsis caused a multigenic deletion in WHIM-09, most likely in adulthood, and since she was also lacking a spleen due to therapeutic splenectomy as a child (8), we used experiments in mice to dissect individual and combinatorial effects of splenectomy and Cxcr4+/0 genotype on the steady state blood ANC. Results from two independent experiments, one lasting 6 months the other 12.5 months after surgery followed the same pattern (Figure 1, and Supplemental Figure 1). The ANC was elevated in splenectomized Cxcr4+/+ mice compared to sham-operated Cxcr4+/+ mice (Fig. 1, left panel). The magnitude of increase was ~50%. The increase was first apparent at 3 months after surgery and was durable. The ANC was also elevated in sham-operated Cxcr4+/0 mice as compared with sham-operated Cxcr4+/+ mice. The increase was first apparent at 2 months after surgery and also was durable (Fig. 1, middle panel). The magnitude of increase was variable, but in general slightly greater than for splenectomy alone. Finally, the ANC for splenectomized Cxcr4+/0 mice, designed to mimic WHIM-09 without hemizygosity for the other 163 genes affected in the patient, was also durably elevated as compared with sham-operated Cxcr4+/+ mice (Fig. 1, right panel). In this case, the increase was apparent at one month after surgery, the earliest time point tested. The magnitude of increase varied from 0.5–2.5-fold over control. Overall, the results phenocopy neutrophilia in WHIM-09 with apparent independent contributions from both splenectomy and Cxcr4+/0 genotype.
Figure 1. Mimicking WHIM-09 by splenectomy in Cxcr4+/0 mice phenocopies neutrophilia.
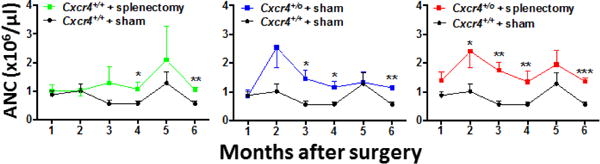
Cxcr4+/+ and Cxcr4+/0 mice received splenectomy or sham surgery at 8 weeks of age, as indicated by the code at the top of each panel, and were bled at the indicated time points for six months post-operatively for determination of absolute neutrophil count (ANC). For clarity, comparative results are shown separately in three panels against the same sham-operated Cxcr4+/+ control data. Data are the mean ± SEM of results from one experiment representative of two independent experiments with a similar pattern. The number of animals in each group was as follows: sham-operated Cxcr4+/+, 7; splenectomized Cxcr4+/+, 6; sham-operated Cxcr4+/0, 7; splenectomized Cxcr4+/0, 8. *p<0.05, **p<0.01,***p<0.005, ****p<0.001 two-tailed unpaired parametric t-test.
Cxcr4+/0 neutrophils have normal bone marrow homing capacity
To address whether neutrophilia in WHIM-09 and in splenectomized Cxcr4+/0 mice may be caused in part by CXCR4+/o genotype-dependent impairment of neutrophil homing back to bone marrow from the blood, we used a neutrophil transfer model in mice. In this model, CD11b+ Ly6G+ neutrophils were purified from the bone marrow of CD45.2 Cxcr4+/+ and CD45.2 Cxcr4+/0 mice (Fig. 2A), then transferred into non-conditioned CD45.1 Cxcr4+/+ recipient mice intravenously. Three hours later, homing of the transferred CD45.2 donor neutrophils to recipient bone marrow was checked by flow cytometry. Prior to transfer, the background CD45.2 staining in recipient bone marrow was essentially zero (Fig. 2B). Three hours after transfer, the frequencies of CD45.2+ Ly6G+ donor neutrophils in WT recipient bone marrow were comparable in the mice receiving Cxcr4+/0 neutrophils and the mice receiving Cxcr4+/+ neutrophils (Fig. 2B and C).
Figure 2. Cxcr4+/0 neutrophils have normal ability to home from blood to bone marrow.
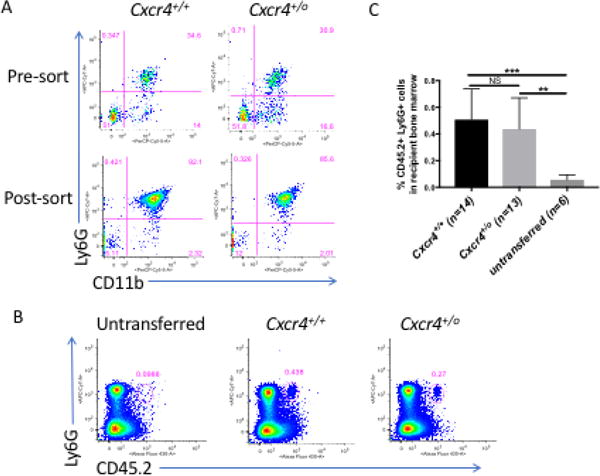
A. Neutrophil purification from donor bone marrow before transfer. Pre-sort: before sorting; Post-sort: after sorting. Neutrophils were identified by CD11b+ Ly6G+. B and C. Donor neutrophil homing to recipient bone marrow. Three hours after transfer to CD45.1 Cxcr4+/+ mice, homing of donor CD45.2 neutrophils from Cxcr4+/+ and Cxcr4+/0 mice to recipient bone marrow was determined by CD45.2 and Ly6G staining. B. The representative FACS dot plots are from the bone marrow cells of untransferred, Cxcr4+/+ neutrophil and Cxcr4+/0 neutrophil transferred recipients. In both panels, the genotype of the donor neutrophils is indicated at the top of each FACS plot. C. Summary data of donor neutrophils homing to the bone marrow in the recipient mice. Data are representative of five independent experiments with a total of 14 Cxcr4+/+ donor mice and 13 Cxcr4+/0 donor mice, with a similar pattern in each.
WHIM-09 bone marrow GMPs have increased granulocyte colony-forming unit (G-CFU) activity
Previously we reported that Cxcr4+/0 HSCs from mice are hyperproliferative and have an engraftment advantage in competitive repopulation experiments when compared to Cxcr4+/+ HSCs (8), suggesting that increased self-renewal and repopulation ability of chromothriptic Cxcr4+/0 hematopoietic stem and progenitor cells (HSPCs) may contribute to WHIM-09’s neutrophilia. To translate this mouse result to WHIM-09, we analyzed HSPCs content and function in her bone marrow and blood.
We first analyzed peripheral blood CD34+ HSPC content in purified PBMCs from healthy donors, WHIM patients, and WHIM-09. Although WHIM patients had markedly reduced frequencies and numbers of CD34+ cells compared with healthy donors, the corresponding values for WHIM-09 were normal for multiple independent samples across 5 years of observation (Figure 3A–C). Consistent with this, PBMCs from WHIM patients cultured ex vivo generated fewer BFU-E, GM-CFU and GEMM-CFU colonies than those of healthy donors, whereas the results for PBMCs from WHIM-09 were normal (Figure 3D). When the data were adjusted for the starting CD34+ cell frequency, only the BFU-E colonies remained reduced, by 50%, for WHIM patients compared to healthy donors (Fig. 3E). Thus analysis of circulating CD34+ progenitor cells did not reveal a differentiation or proliferation defect that might explain neutrophilia in WHIM-09. As an additional point, we observed that WHIM-09 had a CD34mid population that was much smaller in healthy donors and WHIM-10; however, the significance of this is unknown.
Figure 3. Frequency, number and myeloid differentiation capacity of circulating CD34+ cells are low for WHIM patients but normal for WHIM-09.
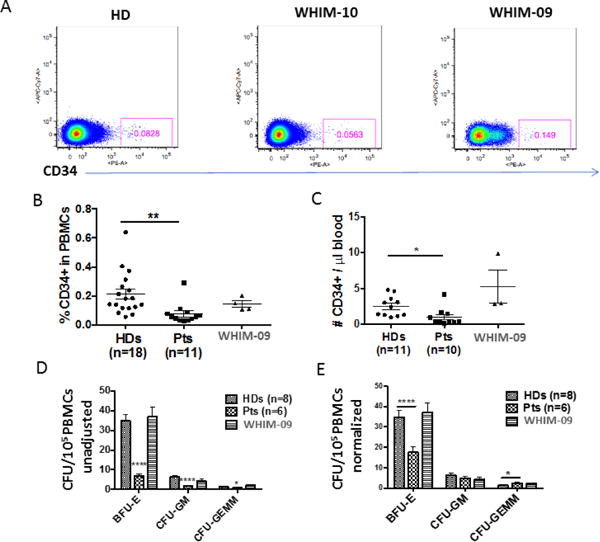
PBMCs from healthy donors (HDs), WHIM patients (Pts) and WHIM-09 were stained with anti-human CD34 mAb. A representative FACS plot (A) and summary data (HDs: n=18; Pts: n=11) (B) are shown. Cells were gated on the lymphocyte population. C. Absolute numbers (HDs: n=11; Pts: n=10). Summary data are shown. D. Colony-forming activity. Unadjusted Burst forming unit-erythroid (BFU-E), granulocyte monocyte colony-forming unit (GM-CFU) and granulocyte, erythrocyte, monocyte, megakaryocyte colony-forming unit (GEMM-CFU) colonies colonies generated from 0.1 million PBMCs were counted after 14 days. Results are triplicate summary data plotted as the mean ± SEM for four independent experiments. Each of the 8 HDs and 6 WHIM patients was tested once, in one of the four experiments, and each of four separate samples from WHIM-09 from different NIH visits were tested once in one of the four experiments. E. Adjusted colony-forming activity. The data from panel D were normalized by the CD34+ cell frequency relative to HDs. Data were combined from 4 experiments and plotted and summarized as the mean ± SEM. *p<0.05, **p<0.01, ****p<0.0001, two-tailed unpaired parametric t-test.
We next purified common myeloid precurse cells (CMPs) and granulocyte-monocyte precursor cells (GMPs) by FACS from bone marrow (8), and compared colony-forming activity for healthy donors versus WHIM-09 ex vivo. CMP and GMP frequency in bone marrow was similar for healthy donors (n=9) (CMP 0.38±0.34%, GMP 0.53±0.37%) vs WHIM-09 (CMP 0.35%, GMP 0.3%). BFU-E colonies generated from WHIM-09 CMPs (CD34+ CD38+ CD135+ CD45RA−) were reduced by ~25% compared to healthy donor CMPs, whereas the numbers of GM-CFU and GEMM-CFU colonies were similar for WHIM-09 and healthy donors (Fig. 4A).
Figure 4. WHIM-09 GMPs have increased intrinsic granulocyte differentiation activity.
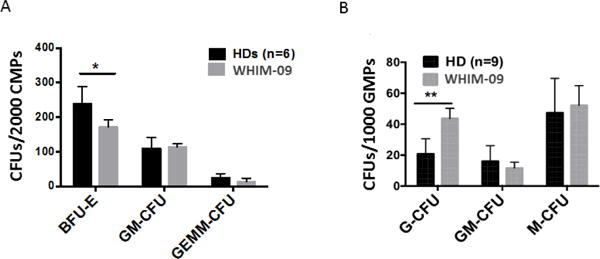
Common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs) were sorted from bone marrow of HDs and WHIM-09 and were tested in triplicate in a colony-formation assay in vitro. Burst forming unit-erythroid (BFU-E), granulocyte monocyte colony-forming unit (GM-CFU) and granulocyte, erythrocyte, monocyte, megakaryocyte colony-forming unit (GEMM-CFU) colonies generated from 2000 CMP cells (A) or 1000 GMP cells (B) were counted after 14 days in culture. Data were combined from two experiments and plotted and summarized as the mean ± SEM. Each HD was tested once, but the total for the two experiments was 6 (A) and 9 (B). WHIM-09 was tested in both experiments, and all individuals tested were tested in replicate determinations. A, *p<0.05, two-tailed unpaired parametric t-test. B, ** p<0.01, two-tailed unpaired parametric t-test.
In contrast, when bone marrow GMPs (CD34+ CD38+ CD135+ CD45RA+) were tested, we consistently observed increased G-CFU colonies for WHIM-09 compared to healthy donors (Fig. 4B). However, GM-CFU and M-CFU colony-forming activities for GMPs from WHIM-09 were not different from healthy donors. Thus, neutrophilia in WHIM-09 may also be due in part to selective effects of the chromothriptic changes on neutrophil differentiation.
Increased apoptosis in peripheral blood neutrophils from WHIM-09
To address whether decreased neutrophil apoptosis might be a factor contributing to WHIM-09’s neutrophilia, neutrophils were isolated from healthy donors, WHIM-09 and her daughter WHIM-10, then cultured in RPMI-1640 + 10% FBS + pen/strep medium for 3 hours at 37°C. Apoptosis of these cells was quantitated by Annexin V-FITC and PI staining. As shown in Fig. 5, healthy donors had low Annexin V+ PI− neutrophils (14.95 and 11.7%), whereas values for WHIM-09 were much higher (33.5%), and were highest for the WHIM patient WHIM-10 (68.5%). These results do not support decreased apoptotic clearance as a contributing factor accounting for neutrophilia in WHIM-09.
Figure 5. WHIM and WHIM-09 neutrophils have increased apoptosis.
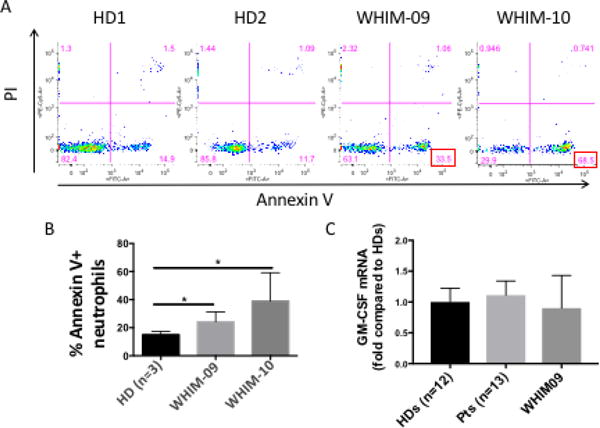
Neutrophils were isolated from the indicated subjects and cultured in vitro in RPMI-1640 + 10% FBS + pencillin/streptomycin medium without stimuli at 37°C for 3 hours. A. Representative data. B. Summary data. Data were combined from 2 individual experiments, and summarized as the mean ± SEM. Both WHIM-09 and WHIM-10 were included in both experiments. Three different healthy donors (HDs) were tested: two in one experiment, and one in the second. * p<0.05, two-tailed unpaired parametric t-test. C. PMNs were isolated from the peripheral blood of HDs (n=12), WHIM patients (n=13) and WHIM-09 (collected at 3 different times) and RNAs were isolated for G-CSF receptor mRNA expression. The data were combined from two experiments and mean and SEM were shown. There was no significant difference among the three groups.
Elevated serum CCL15 levels in WHIM-09
To address whether WHIM-09’s leukocytosis might be caused by hematopoietic cytokine dysregulation, we tested relevant candidate hematopoietic cytokines, CXCL12/SDF1α, CXCL1/Groα and CCL15/MIP-1δ, in the serum from healthy donors and WHIM patients as well as WHIM-09 (across multiple different NIH visits), all at a time when there was no apparent infection or other stress. The CXCR4-CXCL12 axis not only supports myelopoiesis, but also regulates retention of fresh neutrophils in bone marrow and homing of apoptotic neutrophils from periphery to bone marrow for clearance. CXCL1 induces neutrophil migration and also enhances IL-8 elicited calcium influx and efflux of PMNs. CCL15 is a chemotactic factor for neutrophils, monocytes and lymphocytes. In turn, neutrophils can have proteolytic activity on CCL15 to enhance its function on monocytes. As shown in Fig. 6, levels of CXCL12, the sole chemokine ligand of CXCR4, were only slightly lower for WHIM-09 than for healthy donors and WHIM patients. Levels of CXCL1/Groα, a chemokine ligand for CXCR2, which promotes neutrophil egress from bone marrow (25), were in the normal range for WHIM patients with a wide range of values but were repeatedly undetectable in WHIM-09 (and in 50% of healthy donors). Strikingly, serum levels of CCL15/MIP-1δ, which regulates hematopoietic progenitor cell retention and adhesion in bone marrow as well as HSC repopulation activity (26), were increased by 400% for WHIM-09 compared to both healthy donors and WHIM patients. We do not know the source of CCL15 in the patient. Purified neutrophil CCL15 mRNA levels were not significantly different in samples from WHIM09, other WHIM patients and healthy donors(data not shown). We also measured serum levels of G-CSF, GM-CSF and M-CSF as well as neutrophil G-CSF receptor mRNA levels for WHIM-09 but found no differences relative to healthy donors (data not shown).
Figure 6. WHIM-09 has increased serum CCL15/MIP-1δ levels.
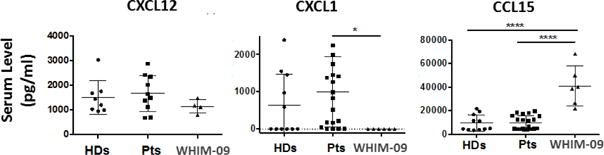
Blood was collected from HDs, WHIM patients and WHIM-09 and sera were isolated. Serum CXCL12/SDF1α (HDs: n=8, patients: n=10), CXCL1/Groα (HDs: n=11, patients: n=20) and CCL15/MIP-1δ (HDs: n=11, patients: n=20) levels were detected by quantitative ELISA. Data were plotted and summarized as the mean ± SEM. *p<0.05, **p<0.01, ****p<0.0001, two-tailed unpaired parametric t-test.
Discussion
In this study, we have shown that splenectomy in Cxcr4+/0 mice is able to phenocopy sustained benign neutrophilia in patient WHIM-09, who was cured of WHIM syndrome by chromothriptic deletions on chromosome 2(8). Both splenectomy and Cxcr4+/0 genotype had independent effects on neutrophilia. WHIM-09 underwent splenectomy as a child and is actually hemizygous for 164 genes, all on chromosome 2, one of which is the mutated copy of CXCR4, CXCR4R334X, the genetic cause of WHIM syndrome in WHIM-09 (8). Our experiments do not address whether hemizygosity for one or more of the other 163 genes deleted in WHIM-09 also promotes neutrophilia. Instead they confirm previously reported results indicating that splenectomy can cause neutrophilia in wild type mice (11, 12), and extend this to Cxcr4+/0 mice directly, and by inference to WHIM-09.
Previous work has suggested that spleen may not regulate ANC simply by sequestration, but rather is actively producing undefined factors that may affect neutrophil production, release or clearance. To understand how Cxcr4 haploinsufficiency might promote neutrophilia, effects on multiple processes including myeloid differentiation, neutrophil egress/release from bone marrow, neutrophil margination and neutrophil senescence, apoptosis and homing to bone marrow must all be considered. We have tested many of these in this report. We have previously reported that Cxcr4 haploinsufficiency promotes HSPC proliferation (8) and is associated with markedly enhanced engraftment potential in the setting of transplantation (8), which may explain, at least in part, why WHIM-09 is an ~100% chromothriptic chimera both in HSCs and in the entire myeloid lineage. We were unable to purify enough WHIM-09 HSCs to test their intrinsic differentiation potential ex vivo directly to complement our in vivo studies; however, we were able to purify sufficient numbers of myeloid progenitor cells and documented markedly increased ex vivo granulocyte colony-forming activity for her GMPs, suggesting a precursor cell-intrinsic advantage in neutrophil differentiation and production. Additional experiments in mice will be needed to judge whether this abnormality might be due to CXCR4 haploinsufficiency per se as opposed to haploinsufficiency for some combination of one or more of the 164 genes for which she is hemizygous. Not surprisingly, we found that circulating levels of CD34+ HSPCs are very low in WHIM patients, as are levels of almost all mature leukocyte subtypes that we and others have measured (13, 27), but they are normal in WHIM-09.
With regard to neutrophil release and clearance, CXCR4 is known to mediate neutrophil and HSC retention in and homing to bone marrow (14, 27–29), so it is reasonable to expect that decreased CXCR4 activity from any cause, including CXCR4 haploinsufficiency as in WHIM-09, might promote neutrophil release and inhibit neutrophil homing, resulting in neutrophilia. We have not tested release directly; however, our direct blood➔bone marrow homing experiments in mice clearly show that Cxcr4+/0 neutrophils are normal in this activity, and therefore provide evidence that defective homing and destruction may not be a mechanism accounting for neutrophilia in WHIM-09. This is inconsistent with published results from Ng and coworkers who investigated the mechanism of action by which the CXCR4 antagonist AMD3100 induces neutrophilia in mice and identified a role for decreased return of blood neutrophils to the bone marrow (14). This difference may due to different methods used. Surprisingly, they also identified a contribution from increased release of neutrophils sequestered in the lung but not from increased egress of neutrophils from the bone marrow.
A very high percentage of the CXCR4 haploinsufficient neutrophils from WHIM-09 were apoptotic when cultured briefly ex vivo, which would be expected to cause neutropenia, not neutrophilia, and thus this cannot independently explain neutrophilia in the patient. Consistent with this, previous studies have reported that CXCR4 signaling promotes cell survival (30). We also found that neutrophils from WHIM patients, which presumably have increased CXCR4 signaling, also have markedly decreased survival when cultured ex vivo. This confirms a previous report which also found impaired bcl-x expression in neutrophil precursors (5).
Interestingly, although her serum levels of myeloid growth factors were also normal, WHIM-09 had extremely high levels of CCL15, a chemokine known to induce hematopoietic progenitor cell migration and adhesion to vascular cell adhesion molecule-1 (VCAM-1), as well as to negatively regulate colony size in CFU assays using mouse Lin− Sca1+ HPCs in vitro. CCL15 also markedly enhances repopulation ability of bone marrow cells in competitive engraftment assays in vivo (26). Increased serum CCL15 levels have also been reported in patients undergoing HPC mobilization (26). Thus, CCL15 may contribute to leukocytosis in WHIM-09 through multiple effects on HSPCs. Future work will be needed to assess the functional significance of elevated CCL15 levels to neutrophilia, as well as to expand the survey of hematopoietic cytokines that may be dysregulated in WHIM-09.
We were surprised to find that serum levels of CXCL12 in WHIM-09 were normal, since receptor-mediated clearance mechanisms should be low for both her myeloid cells, due to CXCR4 hemizygosity, and her lymphoid cells, due to the known defect in CXCR4R334X WHIM receptor internalization. In contrast, serum CXCL1/Groα levels were markedly reduced in WHIM-09. This chemokine is a potent agonist at CXCR2 which promotes neutrophil egress, thereby counterbalancing CXCR4 signaling in the bone marrow (25). Thus, high not low levels of CXCL1 would be expected to result in increased neutrophil egress and neutrophilia. Additional work will be needed to explain this abnormality in WHIM-09, including measurement of additional CXC chemokines in both bone marrow and blood, as well as studies of CXCR2 expression and downregulation on patient neutrophils.
Although WHIM-09 CMPs had normal myeloid differentiation in ex vivo colony forming assays, we were surprised to find that erythroid differentiation was reduced by 25%, as measured by decreased generation of BFU-E colonies. The significance of this modest abnormality is unclear; however, it must be considered as a factor that potentially contributes to the patient’s mild macrocytic anemia. The reason for the macrocytosis is not B12 or folate deficiency, but could also be related to splenectomy (12) and leukocytosis (31, 32).
Conclusions
Splenectomy and CXCR4+/o genotype may play important roles in sustaining benign neutrophilia in WHIM-09, a patient with WHIM syndrome who underwent splenectomy as a child and became CXCR4 haploinsufficient as an adult, as chromothriptic deletion of the disease allele CXCR4R334X in the myeloid lineage mediated a functional and genetic cure. Our results suggest there other factors that may also be contributing, including increased intrinsic functionality of both her GMP precursor cells (enhanced differentiation). Neutrophilia was probably prevented in WHIM-09 at the time of splenectomy and until the mutation was deleted as an adult by the powerful bone marrow retention signal for neutrophils conferred by the WHIM mutation. WHIM-09 is the only known example of CXCR4 hemizygosity in humans. Thus, she represents an important experiment of nature and a unique opportunity for discovery of novel molecular mechanisms that may regulate neutrophil homeostasis.
Supplementary Material
Cxcr4+/+ and Cxcr4+/0 mice received splenectomy or sham surgery at 8 weeks of age, as indicated by the code at the top of each panel, and were bled at the indicated time points for 12.5 months post-operatively for determination of absolute neutrophil count (ANC). Data are the mean ± SD of results from one experiment conducted in the same manner as the experiment shown in Figure 1. The number of animals in each group was as follows: sham-operated Cxcr4+/+, 3; splenectomized Cxcr4+/+, 4; sham-operated Cxcr4+/0, 3; splenectomized Cxcr4+/0, 5. *p<0.05, **p<0.01,***p<0.005, two-tailed unpaired parametric t-test.
Acknowledgments
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases.
Footnotes
Author contribution
Q.L., Z.L., and A.Y. generated and analyzed the experimental data; E.C., D.V., D.H.M. and P.M.M. provided patient recruitment and care; Q.L. D.H.M. and P.M.M. supervised the experiments and analyzed the data; J.L. provided mouse strains; and Q.L., and P.M.M. wrote the manuscript with participation from all of the other authors.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Zuelzer WW. “Myelokathexis”–a New Form of Chronic Granulocytopenia. Report of a Case. N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 2.Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89(5):663–72. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–4. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 4.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116(15):2803–11. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000;95(1):320–7. [PubMed] [Google Scholar]
- 6.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–93. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 7.Krill CE, Jr, Smith HD, Mauer AM. Chronic Idiopathic Granulocytopenia. N Engl J Med. 1964;270:973–9. doi: 10.1056/NEJM196405072701902. [DOI] [PubMed] [Google Scholar]
- 8.McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, et al. Chromothriptic cure of WHIM syndrome. Cell. 2015;160(4):686–99. doi: 10.1016/j.cell.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott DH, Gao JL, Murphy PM. Chromothriptic cure of WHIM syndrome: Implications for bone marrow transplantation. Rare Dis. 2015;3(1):e1073430. doi: 10.1080/21675511.2015.1073430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MJ, Jallepalli PV. Chromothripsis: chromosomes in crisis. Dev Cell. 2012;23(5):908–17. doi: 10.1016/j.devcel.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride JA, Dacie JV, Shapley R. The effect of splenectomy on the leucocyte count. Br J Haematol. 1968;14(2):225–31. doi: 10.1111/j.1365-2141.1968.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 12.Tibblin E, Dreborg S, Erikson A, Hakansson G, Svennerholm L. Hematological findings in the Norrbottnian type of Gaucher disease. Eur J Pediatr. 1982;139(3):187–91. doi: 10.1007/BF01377354. [DOI] [PubMed] [Google Scholar]
- 13.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O’Brien S, Ulrick J, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123(15):2308–16. doi: 10.1182/blood-2013-09-527226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devi S, Wang Y, Chew WK, Lima R, N AG, Mattar CN, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210(11):2321–36. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prystowsky MB, Otten G, Naujokas MF, Vardiman J, Ihle JN, Goldwasser E, et al. Multiple hemopoietic lineages are found after stimulation of mouse bone marrow precursor cells with interleukin 3. Am J Pathol. 1984;117(2):171–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf D, Johnson GR, Burgess AW. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980;55(1):138–47. [PubMed] [Google Scholar]
- 17.Lopez AF, To LB, Yang YC, Gamble JR, Shannon MF, Burns GF, et al. Stimulation of proliferation, differentiation, and function of human cells by primate interleukin 3. Proc Natl Acad Sci USA. 1987;84(9):2761–5. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, et al. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. 2016;6:33123. doi: 10.1038/srep33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newburger PE. Disorders of neutrophil number and function. Hematology Am Soc Hematol Educ Program. 2006:104–10. doi: 10.1182/asheducation-2006.1.104. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Li Z, Gao JL, Wan W, Ganesan S, McDermott DH, et al. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice. Eur J Immunol. 2015;45(6):1855–67. doi: 10.1002/eji.201445245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerschuk CM, Allard MF, Lee S, Brumwell ML, Hogg JC. Effect of epinephrine on neutrophil kinetics in rabbit lungs. J Appl Physiol (1985) 1988;65(1):401–7. doi: 10.1152/jappl.1988.65.1.401. [DOI] [PubMed] [Google Scholar]
- 22.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA. 2010;107(42):18073–8. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013;(77):e50586. doi: 10.3791/50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Germing U, Kobbe G, Haas R, Gattermann N. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int. 2013;110(46):783–90. doi: 10.3238/arztebl.2013.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter R, Ruster B, Bistrian R, Forssmann WG, Seifried E, Henschler R. Beta-Chemokine CCL15 Affects the Adhesion and Migration of Hematopoietic Progenitor Cells. Transfus Med Hemother. 2015;42(1):29–37. doi: 10.1159/000370168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118(18):4963–6. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psatha N, Sgouramali E, Gkountis A, Siametis A, Baliakas P, Constantinou V, et al. Superior long-term repopulating capacity of G-CSF+plerixafor-mobilized blood: implications for stem cell gene therapy by studies in the Hbb(th-3) mouse model. Hum Gene Ther Methods. 2014;25(6):317–27. doi: 10.1089/hgtb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa J, Migita M, Ueda T, Fukazawa R, Adachi K, Ooue Y, et al. Dextran sulfate and stromal cell derived factor-1 promote CXCR4 expression and improve bone marrow homing efficiency of infused hematopoietic stem cells. J Nippon Med Sch. 2009;76(4):198–208. doi: 10.1272/jnms.76.198. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Lopez C, Valencia J, Hidalgo L, Martinez VG, Zapata AG, Sacedon R, et al. CXCL12/CXCR4 signaling promotes human thymic dendritic cell survival regulating the Bcl-2/Bax ratio. Immunol Lett. 2008;120(1–2):72–8. doi: 10.1016/j.imlet.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Weimar V. Macrocytic anemia and leucocytosis of guinea pigs with muscular stiffness disease. Proc Soc Exp Biol Med. 1954;85(3):488–91. doi: 10.3181/00379727-85-20928. [DOI] [PubMed] [Google Scholar]
- 32.Gans RO, Stehouwer CD. Clinical thinking and decision-making in the practice. A patient with thrombophlebitis. Ned Tijdschr Geneeskd. 1999;143(46):2307–12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cxcr4+/+ and Cxcr4+/0 mice received splenectomy or sham surgery at 8 weeks of age, as indicated by the code at the top of each panel, and were bled at the indicated time points for 12.5 months post-operatively for determination of absolute neutrophil count (ANC). Data are the mean ± SD of results from one experiment conducted in the same manner as the experiment shown in Figure 1. The number of animals in each group was as follows: sham-operated Cxcr4+/+, 3; splenectomized Cxcr4+/+, 4; sham-operated Cxcr4+/0, 3; splenectomized Cxcr4+/0, 5. *p<0.05, **p<0.01,***p<0.005, two-tailed unpaired parametric t-test.


