Abstract
Rationale
Previous studies suggest that group II metabotropic glutamate receptors (mGluR2/3) are involved in regulating ethanol seeking and consumption.
Objective
The mGluR2/3 agonist LY379268 (LY37) and selective mGluR2 positive allosteric modulator biphenyl-indanone A (BINA) were used to investigate the relative contribution of mGlu2 and mGlu3 receptors on ethanol and sucrose seeking and consumption. A microinjection study was then performed to examine the role of nucleus accumbens (NAc) core mGluR2/3 on ethanol-seeking.
Methods
For the systemic experiments, separate groups of male Wistar rats [LY37 (0–2.0 mg/kg); BINA (0–20 mg/kg)] were trained to complete a response requirement (RR) resulting in access to 10% ethanol or 2% sucrose (in separate groups) for a 20-minute drinking period. Animals then underwent consummatory testing (weekly drug injections with RR1) followed by appetitive testing (weekly drug injections followed by extinction session). A separate group of male Wistar rats was surgically implanted with bilateral guide cannulae directed towards the NAc core and had weekly microinjections followed by an extinction session.
Results
Systemic administration of the mGluR2/3 agonist LY37 significantly reduced ethanol- and sucrose-seeking. The same treatment also reduced sucrose consumption and body weight (24-hours post injection). Systemic administration of the selective mGluR2 PAM BINA, however, had no effect on either seeking or consumption of ethanol or sucrose. Intra-accumbens core LY37 significantly reduced ethanol-seeking.
Conclusions
These findings suggest that systemic mGluR2/3 agonism, but not allosteric modulation of mGluR2, reduces reinforcer seeking. In particular, NAc core group II mGluR may be involved in regulating ethanol-seeking.
Keywords: Metabotropic Glutamate Receptors, LY379268, BINA, LY341495, Alcohol, Self-Administration
Introduction
Group II metabotropic glutamate receptors (mGluRs), mGlu2 and mGlu3, are predominately presynaptically located Gαi/o associated G-protein coupled receptors highly expressed in the cortex, nucleus accumbens (NAc), striatum, amygdala, and hippocampus (Ohishi et al. 1998; Tamaru et al. 2001; Xi et al. 2002). Group II mGluRs negatively regulate synaptic glutamate release (Scanziani et al. 1997; Xi et al. 2002). Given their expression in brain regions associated with drug reinforcement and regulation of excitatory neurotransmission, group II mGluR agonists have been examined for possible involvement in regulating drug reinforcement. The non-selective group II mGluR agonist LY379268 (LY37) has been shown to reduce both operant self-administration and cue-induced reinstatement for several drugs of abuse including cocaine and ethanol (Baptista et al. 2004; Bossert et al. 2005; Bossert et al. 2004; Kufahl et al. 2011; Liechti et al. 2007). Mixed findings have been reported for the effects of LY37 on alternative/natural reinforcers with either no effect (Baptista et al. 2004; Zhao et al. 2006) or a reduction in alternative reinforcer seeking and/or consumption at the highest LY37 dose tested (Jin et al. 2010; Kufahl et al. 2011; Liechti et al. 2007; Peters and Kalivas 2006). However, this high dose of LY37 has been shown to reduce locomotor behavior (Backstrom and Hyytia 2005; Kufahl et al. 2011) suggesting that the effect of LY37 on natural reinforcers may be due to sedative effects of LY37 at high doses. Further examination of the effects of systemic administration of LY37 on alternative reinforcer seeking and consumption is needed to clarify the specificity of mGluR2/3 in modulating ethanol reinforcement.
Growing evidence suggests that the effects of non-selective Group II mGluR agonists, such as LY37, on decreasing drug self-administration and reinstatement of drug-seeking are not due to equal contributions of mGlu2 and mGlu3 receptor agonism. Mice lacking mGluR3 display normal cocaine self-administration, extinction, and reinstatement responding (Cannella et al. 2013) while mGluR2 deficient mice display increased cocaine place preference (Morishima et al. 2005) and increased preference for and consumption of ethanol (Zhou et al. 2013). This suggests that loss of mGluR2, but not mGluR3, results in increased preference for and intake of drugs of abuse. Here the mGluR2 positive allosteric modulator Biphenyl indanone-A (BINA) was used to examine the role of mGluR2 in regulating ethanol-seeking and consumption.
In the present study, the effect of moderate doses of systemic LY37 and BINA administration on ethanol-seeking and consumption were assessed using the sipper tube method (e.g., Czachowski et al. 2001). In this method, the seeking response (lever press) is procedurally separated from the consummatory response (drinking) allowing for discrete analysis of the effect of LY37 and BINA on each behavior independently. Given that prior systemic LY37 and BINA studies show either similar or reduced effects when comparing self-administration to reinstatement responding (Backstrom and Hyytia 2005; Bossert et al. 2005; Jin et al. 2010; Liechti et al. 2007; Sidhpura et al. 2010), we hypothesized that systemic modulation of group II mGluRs, by either orthosteric agonism or mGlu2 positive allosteric modulation, would preferentially reduce ethanol seeking versus consumption in non-deprived Wistar rats using the sipper tube model. Furthermore, given the increasing evidence that the reduced ethanol-seeking observed with systemic LY37 is due to agonism of mGlu2 but not mGlu3 receptors, we hypothesized that modulation of mGlu2 receptors via systemic administration of the selective mGluR2 PAM BINA would result in a similar efficacy in attenuating ethanol-seeking as observed with the mGluR2/3 agonist LY37.
Ethanol has been shown to influence glutamatergic signaling following both acute and chronic administration, particularly within the NAc and ventral tegmental area (VTA). For instance, acute administration of low to moderate ethanol doses (0.5 – 1 g/kg) results in increased extracellular glutamate concentrations in the VTA, NAc, and hippocampus (Ding et al. 2012; Moghaddam and Bolinao 1994; Selim and Bradberry 1996). Elevated NAc extracellular glutamate concentrations have also been observed during withdrawal following experimenter administered ethanol (Melendez et al. 2005) and home cage ethanol drinking (Ding et al. 2013). Intra-accumbens administration of LY37 in “post-dependent” C57BL/6J mice reduced 2-hour limited access home cage ethanol drinking (Griffin et al. 2014). Since inactivation of the NAc core but not NAc shell has been shown to reduce responding to an ethanol-conditioned stimulus in a novel context (Chaudhri et al. 2010), the NAc core subregion was selected for our initial microinjection experiment to begin clarifying the loci of action of mGluR2/3 agonists in regulating ethanol-seeking. As intra-accumbens LY37 administration produced nonspecific reductions in locomotor activity in alcohol-preferring P rats (Besheer et al. 2010), in the present experiment the non-sedative group II antagonist LY341495 (LY34) (Chi et al. 2006) was microinjected into NAc core following systemic agonist LY37 administration. We hypothesized that NAc core administration of mGluR2/3 antagonist LY34 would attenuate the suppressive effects of systemic LY37 administration on ethanol-seeking suggesting the involvement of NAc core mGluR2/3 in the regulation of ethanol-seeking.
Material and methods
Animals
Male Wistar rats (Hsd:WI, Harlan Labs, Indianapolis, IN), weighing 165 – 210 g at the beginning of the experiment, were single housed on a 12-hour light/dark cycle (lights on at 0500). Animals had ad libitum access to both food and water except for a mild water restriction during the first week of training. Animal care and procedures were in accordance with NIH Guidelines for the Care and Use of Laboratory Animals (2011) and approved by the IUPUI Institutional Animal Care and Use Committee (IACUC).
Apparatus
Sessions were conducted daily (5 days/week) in operant chambers (30×30×24.5 cm; Med-Associates, St Albans, VT). Chambers were located in sound attenuated enclosures with exhaust fans to mask external noise. The chambers were equipped with a house light, a single retractable lever, and a single retractable graduated sipper tube located on the wall opposite the lever. The sipper tube consisted of a graduated cylinder tube with a rubber stopper and stainless steel tube with two ball bearings to prevent leakage. Med-Associates software was used to control input and output from each chamber.
Systemic Experiment: Training
Upon arrival, animals were weighed and handled twice during the week preceding initial training (see Figure 1a for an overview of the entire experiment). Sessions were conducted at the same time daily during the lights on portion of the light/dark cycle. During initial training, animals underwent a brief (14–18 hr) water deprivation prior to the first training session, followed by a mild 2–4 day water restriction to facilitate acquisition of lever-press responding. Food and water were available ad libitum for the remainder of the testing.
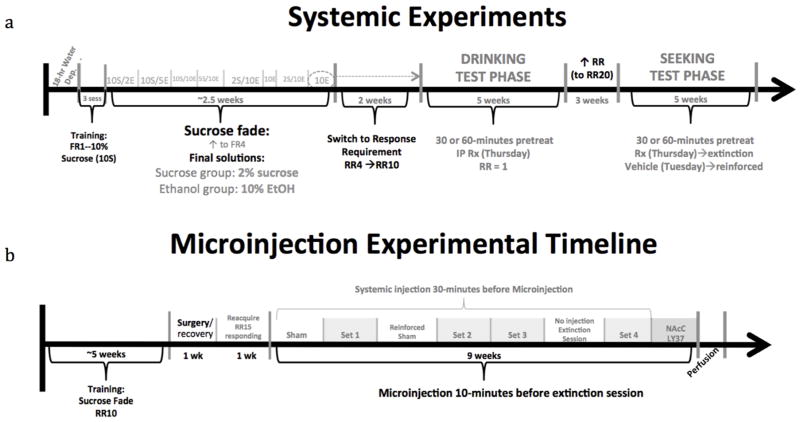
Fig. 1.
Overview systemic experiments training and testing phases (a) and microinjection timeline (b).
Separate groups of rats (LY37 and BINA) were initially trained to lever press on a FR1 schedule for 15 seconds of access to a 10% oral sucrose reinforcer. Once lever press was acquired (1–3 sessions), the schedule was increased gradually over sessions to a final FR4 schedule while the sucrose was gradually reduced using a modified sucrose-fade procedure (Samson 1986). For the sucrose-fade, over a 3-week period, the sucrose concentration was gradually reduced over sessions while ethanol was gradually faded into the solution (for ethanol groups). Final reinforcer concentrations were 2% sucrose (sucrose groups) and 10% ethanol (ethanol groups). The FR4 schedule was then discontinued and a response requirement (RR) was implemented allowing for procedural separation of seeking from consumption. For this, animals had 20 minutes to complete the RR (initially 4 lever presses). Once the RR was met, the lever was retracted and the sipper tube was inserted into the chamber. Animals then had 20 minutes of unrestricted access to the reinforcer. The RR was gradually increased over sessions to a final RR of 10 lever presses.
Systemic Experiment: Drinking Test Phase
Following training, animals underwent a six-week Drinking Test Phase. Animals had once weekly test sessions on Thursday with a RR of 1 lever-press so that minimal effort was required to gain access to the reinforcer. The other four sessions were non-injection sessions with a RR of 10. Animals were first habituated to the test procedure with a systemic vehicle injection then received IP drug injections (0.0, 0.3, 1.0, 1.5, and 2.0 mg/kg LY37; 0, 5, 10, and 20 mg/kg BINA) in a balanced design (random dose order across injection sessions with doses balanced across animals: 3 animals/dose and 3 doses/injection session). LY37 and BINA doses used were selected based on the literature for drug doses not shown to influence locomotor behavior (Backstrom and Hyytia 2005; Jin et al. 2010; Kufahl et al. 2011). Following the drinking test phase, animals had a three-week period during which no drugs were administered and the RR was gradually increased from 10 to 20 lever presses.
Systemic Experiment: Seeking Test Phase
Animals then underwent a six-week Seeking Test Phase using the same vehicle habituation, followed by weekly drug injections with doses administered in a balanced design. During the weekly test session, systemic drug injection was followed by a non-reinforced extinction session. During the extinction session, animals had 20 minutes of access to the lever, but did not gain access to the reinforcer. To control for possible scent cues, filled bottles were placed on the retracted holders. Animals had weekly reinforced vehicle injection sessions (on Tuesdays) to reduce the likelihood of systemic injection predicting an extinction session. The other three sessions were normal reinforced sessions.
Microinjection experiment (Figure 1b)
The apparatus and training were identical to those of the systemic experiment. Following surgery (see below), animals were allowed to reacquire lever press responding with the RR gradually increased over sessions to a final response requirement of 15. NAc core blockade of systemic mGluR2/3 agonist induced suppression of ethanol-seeking was performed using the non-selective group II mGluR antagonist LY34. For microinjections, rats were gently restrained in a small holding tub (27 × 17 × 12 cm). Each obturator was removed and replaced with a stainless-steel injector (33 gauge) that extended 1 mm beyond the end of the guide cannulae. Drug solutions were delivered bilaterally in a volume of 0.5 μL/side over a one-minute period using 25.0 μL Hamilton syringes and KD Scientific Infusion Pumps (Model 101; KD Scientific Inc., Holliston, MA). The drug was then allowed 30 seconds to diffuse prior to removal of the injector. Following injection, obturators were replaced and the animal was returned to the animal carrier prior to the operant session. Animals had weekly microinjection extinction test sessions on Thursdays (identical to extinction sessions during seeking test phase of systemic experiment) with “normal” reinforcer sessions the remaining four days. To prevent an association of the microinjection procedure with the extinction session, a reinforced sham session and a no injection extinction session occurred the week following the first and third sets of microinjections, respectively. Animals were initially habituated to the microinjection procedure with a systemic vehicle injection plus sham microinjection (<10 mm injectors placed into guide cannulae with no fluid administered) followed by an extinction session. Animals then received each of four sets of systemic injection plus NAc core microinjection in a balanced design (systemic vehicle + NAc core vehicle, systemic LY37 + NAc core vehicle, systemic LY37 + NAc core LY34, and systemic vehicle + NAc core LY34). After the final set of systemic injection plus NAc core microinjections, animals received a microinjection of LY37 (0.5 μg/0.5 μL/side) without systemic injection to determine the effects of agonist administration into the NAc core.
Surgery
Following training, animals were surgically implanted with bilateral guide cannulae directed towards the NAc core. Thirty minutes prior to surgery, the non-steroidal anti-inflammatory drug (NSAID) carprofen was administered (5 mg/kg; sc) for pain relief. Rats were anesthetized with sodium pentobarbital (60 mg/kg, ip), the top of the head shaved, and the rat placed in the stereotaxic apparatus (Benchmark Digital Stereotaxic; myNeurolab, St. Louis, MO) with incisor bar set at 3.3 mm below the interaural line. Stainless steel guide cannulae (13 mm; 26 gauge) were implanted bilaterally terminating 1 mm dorsal to the NAc core using bregma, midline, and dura surface as reference (AP +1.6, ML ± 1.6, DV − 6.0; Paxinos and Watson 1998). Removable wire obturators (13mm length; 33 gauge) were placed into the guide cannulae to limit obstruction and maintain patency. Following surgery, animals had two days to recover prior to resuming operant sessions. Animals were checked daily to ensure proper wound healing and lack of infection.
Histology
Following the completion of the final operant session, the animals were deeply anesthetized with sodium pentobarbital (120 mg/kg, ip) and transcardially perfused with phosphate buffered saline (PBS) then 10% formalin. The brains were removed and stored in 10% formalin. The brains were sliced (60 μm sections) using a cryostat (Leica CM1950, Leica Microsystems Inc., Buffalo Grove, IL), mounted, and stained using cresyl violet. Site verification was performed using a light microscope. Only animals with confirmed bilateral cannulae placement in the NAc core were included in the analyses.
Drugs
Ethanol solutions were prepared volume/volume in water using 95% ethanol. Sucrose and sucrose/ethanol solutions were prepared weight/volume. The non-selective group II mGluR agonist LY379268 [(1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid] (Santa Cruz Biotechnology, Inc., Dallas, TX) was dissolved in sterile 0.9% saline and injected at a volume of 1.0 mL/kg body weight. The selective mGluR2 positive allosteric modulator BINA [Biphenyl-indanone A (3′-[[(2-Cyclopentyl-2,3-dihydro-6,7-dimethyl-1-oxo-1H-inden-5-yl)oxy]methyl]-[1,1′-biphenyl]-4-carboxylic acid)] (Santa Cruz Biotechnology, Inc., Dallas, TX; Tocris Bioscience, Minneapolis, MN) was dissolved in 0.5% dimethyl sulphoxide (DMSO) and 1% sodium hydroxide (NaOH) diluted with sterile water then titrated to a final pH of 7.4 using 1% lactic acid and injected at a volume of 5 mL/kg body weight. Sterile saline and sterile water plus 0.5% DMSO and 1% NaOH titrated to a final pH of 7.4 using 1% lactic acid were vehicle treatments for LY37 and BINA respectively. For systemic experiments, LY37 (0–2.0 mg/kg) and BINA (0–20 mg/kg) were administered 30 and 60 minutes prior to the operant session respectively.
For microinjections, LY37 was dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) and the non-selective group II mGluR antagonist LY341495 [(2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid] (Santa Cruz Biotechnology, Inc., Dallas, TX) was dissolved in 20% DMSO plus aCSF. Sterile aCSF plus 20% DMSO was used as the vehicle treatment for LY34. LY37 (1.5 mg/kg) was administered ip 30 minutes prior to the microinjection and LY34 (1.0 μg/side) was administered 10 minutes prior to the start of the operant session. For the final microinjection, LY37 (0.5 μg/side) was administered 10 minutes prior to the start of the operant session.
Data Analyses
Daily session intakes of ethanol and sucrose were determined from the change in volume in the sipper tube (mL). Ethanol intake (g/kg) and sucrose intake (mL/kg) were calculated from session intake and daily body weight measures. Total lever presses, latency to first lever press (in sec), and latency to first lick (in sec) were recorded for each session. Ethanol and sucrose consumption data were analyzed separately using one-way within-subject repeated measures analysis of variance (RM ANOVA). To examine potential protracted effects of the acute drug administration on reinforcer intake, change in intake was computed by subtracting the intake during the reinforced session 24-hrs following the drug treatment from the intake during the reinforced session 24-hrs prior to drug treatment (i.e., Friday intake minus Wednesday intake). For systemic administration experiments, appetitive responding, lick latencies, and body weight change [body weight 24-hrs post-injection (Friday) minus body weight 1-hr prior to injection (Thursday)] were analyzed using two-way RM ANOVAs with dose and reinforcer as factors. For NAc microinjection experiment appetitive responding, lever press latency, and body weight change were analyzed using one-way within-subject RM ANOVA. The final LY37 microinjection session was not included in the original balanced design; therefore, the systemic vehicle plus NAc core vehicle and NAc core LY37 were compared using a paired samples t-test. Post-hoc comparisons were performed using Student-Newman-Keuls test (p<0.05). All analyses were conducted using SigmaStat3.5 (Systat Software, Inc., Chicago, IL).
Results
Systemic Experiment
One animal in the LY37 ethanol group had poor behavioral performance during both testing phases and was removed from analysis (LY37 ethanol n=8; LY37 sucrose n=9; BINA ethanol n=9; BINA sucrose n=9). Prior to drug injections, ethanol-reinforced animals consumed a mean of 0.64 ± 0.06 g/kg of ethanol for LY37 and 0.67 ± 0.04 g/kg for BINA. Sucrose-reinforced animals consumed a mean of 3.69 ± 0.32 mL/kg of sucrose for LY37 and 4.12 ± 0.28 mL/kg for BINA (data not shown).
Drinking Test Phase
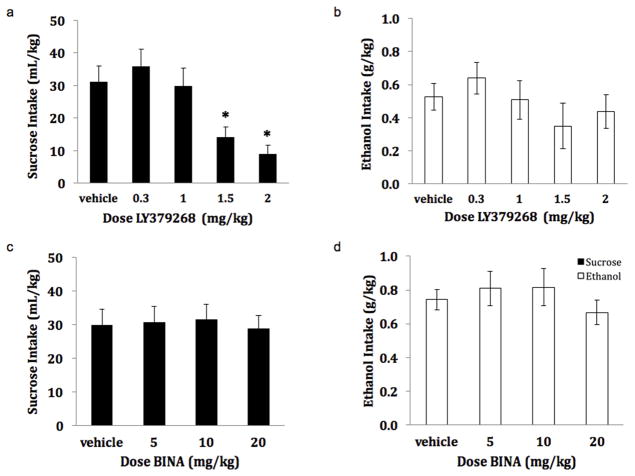
For the Drinking Test Phase, a significant effect of LY37 treatment on sucrose intake (mL/kg) was observed [F(4, 32) = 12.887, p<0.001] with post hoc analyses indicating a significant decrease in sucrose consumption at the 1.5 and 2.0 mg/kg dose (p<0.01) compared to LY37 vehicle (figure 2a). No effect of LY37 on ethanol consumption (g/kg) was observed [F(4, 28) = 1.65, p=0.19] (figure 2b). No effect of BINA on either sucrose [F(3, 24) =0.418, p=0.74] or ethanol consumption was observed [F(3, 24) =1.34, p=0.28] (figure 2c and 2d). No protracted effect of either LY37 or BINA was observed for either ethanol [LY37: F(4,28) = 1.975, p=0.13; BINA: F(3,24) = 1.154; p=0.35] or sucrose consumption [LY37: F(4,32) = 2.309, p=0.08; BINA: F(3,24) = 0.254; p=0.86] as measured by the difference in intake during reinforced session 24-hrs following drug treatment (Friday session) from intake during reinforced session 24-hrs prior to drug treatment (Wednesday session; data not shown).
Fig. 2.
Effect of LY379268 (a, b) and BINA (c, d) on Sucrose and Ethanol Consumption. Sucrose and ethanol consumption following weekly systemic drug injections (n=8–9/group). A significant reduction in sucrose consumption was observed at the 1.5 and 2.0 mg/kg doses of LY37 relative to vehicle. No effect of systemic administration LY37 on ethanol consumption was observed for any dose tested. No effect of systemic administration BINA on ethanol or sucrose consumption was observed for any dose tested. (* p<0.05)
Seeking Test Phase
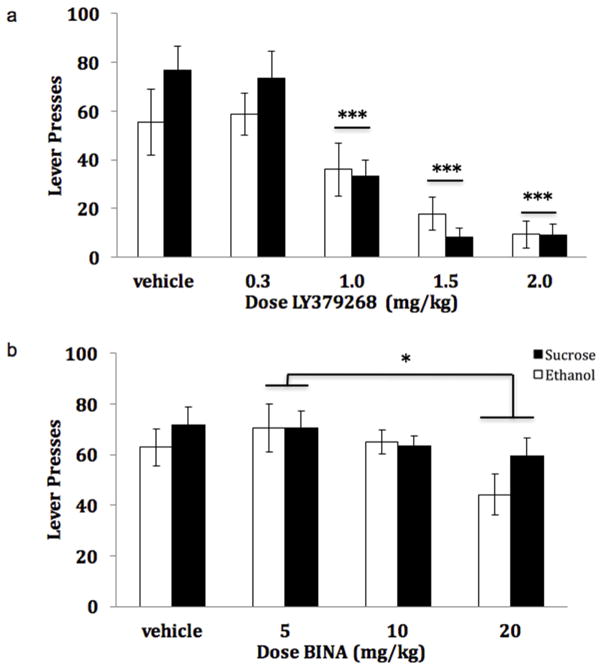
A significant main effect of LY37 on appetitive responding was observed [F(4, 60) =30.33, p<0.001]. Post hoc analyses indicate that LY37 significantly (p<0.001) decreased seeking at the 1.0, 1.5, and 2.0 mg/kg LY37 doses (figure 3a). No interaction of treatment x reinforcer was observed [F(4, 60) =1.682, p=0.17]. A main effect of BINA treatment [F(3, 48) =3.1587, p<0.05] on seeking was observed (figure 3b). Post hoc analyses indicate that the effect was due to a significant difference between the 5 mg/kg and 20 mg/kg dose (p=0.03) and a moderate decrease in seeking at the 20 mg/kg dose compared to vehicle (p = 0.055). No protracted effect of either LY37 or BINA was observed for either ethanol [LY37: F(4,26) = 1.167, p=0.35; BINA: F(3,23) = 1.072; p=0.38] or sucrose consumption [LY37: F(4,32) = 0.879, p=0.49; BINA: F(3,24) = 0.693; p=0.57] as measured by the difference in intake during reinforced session 24-hrs following drug treatment (Friday session) from intake during reinforced session 24-hrs prior to drug treatment (Wednesday session; data not shown).
Fig. 3.
Effect of LY379268 (a) and BINA (b) on Sucrose and Ethanol Seeking. Appetitive responding for sucrose and ethanol following weekly systemic drug injection (n=8–9/group). A significant reduction in seeking was observed at the 1.0, 1.5, and 2.0 mg/kg doses of LY37 relative to vehicle. No difference from vehicle responding was observed for appetitive responding for any dose of BINA tested. (* p<0.05) (*** p<0.001)
Latency to First Lick
The latency to first lick is the time (in sec) following successful completion of the lever press response requirement (RR1) for the animal to turn, traverse the chamber, and make initial contact with the sipper tube. Average lick latency for vehicle administration was 2.35 ± 0.59 seconds in the LY37 groups and 1.61 ± 0.13 seconds in the BINA groups. A significant main effect of LY37 on latency to first lick was observed [F(4, 54) =3.39, p<0.05] (table I). However, post hoc analysis revealed that this effect was due to a within dose difference in first lick latency (1.5 mg/kg compared to 0.3 and 1.0 mg/kg doses, with the high dose of 2.0 mg/kg not significantly different from any other dose or vehicle). No effect of BINA administration was observed for latency to first lick [F(3, 48) =1.63, p=0.20] (table I). Overall, no dose of either the mGluR2/3 agonist LY37 or mGluR2 PAM BINA significantly increased the latency to first lick compared to the vehicle.
Table I.
Mean (±SEM) latency (in seconds) to lick or lever press response for systemic and microinjection experiments. For systemic experiments the latency to first lick is the duration of time (in seconds) for the animal to traverse the chamber and make initial contact with the sipper tube following completion of the RR1 lever response. No effect of systemic administration of LY379268 or BINA compared to vehicle baseline latency was observed for latency to first lick. For microinjection experiment the latency to first lever press following systemic injection [with non-response sessions excluded from analysis (conservative latency) and non-response as maximal latency (liberal latency)] of the non-selective group II mGluR agonist LY379268 (1.5 mg/kg) or vehicle followed by intra-accumbens core administration of non-selective group II mGluR antagonist LY341495 (1.0 μg/side) or vehicle. Additional microinjection of LY379268 (0.5 μg/side) without systemic injection was also performed. No significant effect of NAc core LY34 or LY37 administration was observed for latency to first lever press.
| Systemic Experiments (Latency to First Lick) | |||||
|---|---|---|---|---|---|
|
| |||||
| mGluR2/3 agonist LY379268 | Saline | 0.3 mg/kg | 1.0 mg/kg | 1.5 mg/kg | 2.0 mg/kg |
| Ethanol | 2.9 (±1.2) | 1.8 (±0.2) | 1.7 (±0.2) | 2.5 (±0.3) | 2.8 (±0.4) |
| Sucrose | 1.9 (±0.3) | 1.8 (±0.3) | 1.5 (±0.2) | 5.6 (±2.3) | 2.8 (±0.3) |
|
|
|||||
| mGluR2 PAM BINA | Saline | 5 mg/kg | 10 mg/kg | 20 mg/kg | |
|
|
|||||
| Ethanol | 1.7 (±0.2) | 1.7 (±0.2) | 1.7 (±0.1) | 4.4 (±1.9) | |
| Sucrose | 1.5 (±0.1) | 1.3 (±0.1) | 1.4 (±0.1) | 1.4 (±0.1) | |
|
| |||||
| Microinjection Experiment (Latency to First Lever-Press) | |||||
|
| |||||
| Systemic Injection | Vehicle | LY37 | LY37 | Vehicle | |
| NAc core Microinjection | Vehicle | Vehicle | LY34 | LY34 | LY37 |
|
| |||||
| Conservative latency | 198.5 (±156.2) | 118.6 (±81.3) | 33.4 (±12.6) | 52.4 (±22.6) | 23.7 (±10.3) |
| n=6 | n=5 | n=4 | n=5 | n=3 | |
| Liberal latency | 198.5 (±156.2) | 299.0 (±192.2) | 422.6 (±246.3) | 243.8 (±192.3) | 612.4 (±263.3) |
| n=6 | n=6 | n=6 | n=6 | n=6 | |
Body Weight
The change in body weight between injection session and subsequent session (24 hrs post-injection) during the drinking and seeking test phases was computed to examine possible nonspecific effects of systemic LY37 and BINA administration. A significant main effect of LY37 on body weight during the drinking test phase was observed [F(4, 64) =17.99, p<0.001]. Post hoc analyses indicate that LY37 significantly (p<0.01) decreased body weight 24 hours following systemic injection at the 1.0, 1.5, and 2.0 mg/kg LY37 doses relative to vehicle (table II). No interaction of treatment x reinforcer was observed [F(4, 64) =1.35, p=0.26]. Similarly, a significant main effect of LY37 on body weight during the seeking test phase was observed [F(4, 64) =10.271, p<0.001]. Post hoc analyses indicate a significant (p<0.01) reduction in body weight 24 hours following systemic LY37 administration at the 1.0, 1.5, and 2.0 mg/kg doses relative to vehicle (table II). No interaction of treatment x reinforcer was observed [F(4, 64) =0.12, p=0.98]. No effect of systemic BINA administration was observed for body weight during either the drinking test phase [F(3, 48) =0.56, p=0.65] or seeking test phase [F(3, 48) =1.337, p=0.27] (table II). Overall, systemic administration of the non-specific mGluR2/3 agonist LY37, but not the mGluR2 PAM BINA, consistently decreased body weight 24-hours following systemic administration.
Table II.
Mean (±SEM) body weight differences for systemic and microinjection experiments.
Change in body weight (g) between injection session on Thursday and subsequent session (roughly 24 hours post-injection) on Friday. For systemic experiments, a significant reduction in body weight was observed at the 1.0, 1.5, and 2.0 mg/kg doses of LY379268 for both drinking and seeking test phases. No significant effect of systemic administration of BINA on body weight was observed for any dose tested during both drinking and seeking test phases. For the microinjection experiment a significant reduction in body weight was observed for systemic LY379268 administration. (* p<0.05)
| Systemic Experiments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| mGluR2/3 agonist LY379268 | Saline | 0.3 mg/kg | 1.0 mg/kg | 1.5 mg/kg | 2.0 mg/kg | ||||||
| Drinking Test Phase | |||||||||||
| Ethanol | −1.4(±1.0) | 0.3 (±1.4) | −3.9 (±1.9) |
|
* | −11.8 (±1.7) |
|
* | −8.5 (±1.6) |
|
* |
| Sucrose | −0.4 (±1.5) | 0.2 (±0.9) | −6.0 (±1.2) | −6.4 (±1.9) | −12.2 (±2.0) | ||||||
| Seeking Test Phase | |||||||||||
| Ethanol | −3.2 (±1.2) | −2.8 (±0.5) | −8.1 (±1.4) |
|
* | −11.1 (±1.8) |
|
* | −9.8 (±1.3) |
|
* |
| Sucrose | −2.8 (±2.5) | −3.6 (±1.0) | −8.2 (±2.2) | −10.8 (±2.8) | −8.4 (±1.5) | ||||||
|
|
|||||||||||
| mGluR2 PAM BINA | Saline | 5 mg/kg | 10 mg/kg | 20 mg/kg | |||||||
|
|
|||||||||||
| Drinking Test Phase | |||||||||||
| Ethanol | −1.4(±1.4) | −0.6 (±1.9) | −3.6 (±1.6) | 1.6 (±2.1) | |||||||
| Sucrose | 0.1 (±1.6) | −2.6 (±1.3) | 0.2 (±1.1) | −1.8 (±1.3) | |||||||
| Seeking Test Phase | |||||||||||
| Ethanol | −4.1 (±1.6) | −5.0 (±1.4) | −3.9 (±1.4) | −1.4 (±1.1) | |||||||
| Sucrose | −1.7(±1.1) | −3.8 (±1.2) | −3.8 (±2.3) | −2.4 (±1.0) | |||||||
|
| |||||||||||
| Microinjection Experiment | |||||||||||
|
| |||||||||||
| Systemic Injection | Vehicle | Vehicle | LY37 | LY37 | |||||||
| NAc core Microinjection | Vehicle | LY34 | Vehicle | LY34 | |||||||
|
|
|||||||||||
| −11.5 (±3.5) | −11.7 (±2.7) | −21.8 (±3.2) | * | −21.0 (±2.8) | * | ||||||
|
|
|||||||||||
Microinjection Experiment
Four subjects were removed from the experiment prior to the start of the microinjection testing due to poor behavioral performance. Of the remaining subjects, six subjects were confirmed to have bilateral cannulae placement with injection into the NAc core (n=6). During the week prior to the sham habituation injection, animals consumed a mean of 0.44 ± 0.05 g/kg ethanol.
Appetitive Responding
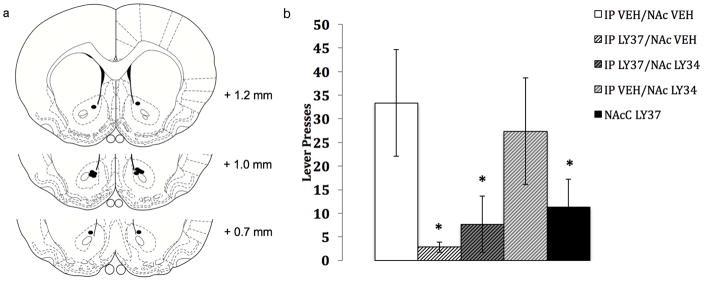
For systemic plus NAc core mGluR2/3 modulation, a significant main effect of treatment on appetitive responding was observed [F(4, 20) =12.58, p<0.001]. Post hoc analyses indicate that systemic LY37 plus NAc core vehicle decreased seeking compared to systemic vehicle plus NAc core vehicle (p<0.01) (figure 4). Systemic LY37 plus NAc core LY34 was also shown to decrease seeking compared to systemic vehicle plus NAc core vehicle (p<0.05). However, appetitive responding following systemic LY37 plus NAc core LY34 was not significantly different from responding during systemic LY37 plus NAc core vehicle (p=0.56). Administration of mGluR2/3 antagonist LY34 following systemic vehicle administration did not decrease seeking compared to systemic vehicle plus NAc core vehicle (p=0.85). Given the inability of NAc core antagonist to attenuate the systemic agonist induced suppression of ethanol-seeking, agonist (LY37) was microinjected into the NAc core to clarify whether NAc core group II mGluRs are involved in the regulation of ethanol-seeking. As this injection was not counterbalanced across animals, the data were analyzed using a paired samples t-test (NAc core LY37 vs systemic vehicle plus NAc core vehicle). Appetitive responding following NAc core LY37 administration was significantly decreased compared to systemic vehicle plus NAc core vehicle [t(5)=2.58, p<0.05].
Fig. 4.
Effect of Intraaccumbens Core Antagonist on Systemic Agonist Induced Suppression of Ethanol -Seeking. Schematic diagrams adapted from the rat brain atlas (Paxinos and Watson, 1998) representing bilateral cannula placement (filled circles = microinjection site) in nucleus accumbens (fig 4a; n = 6). Each section represents approximate position in anteroposterior plane relative to bregma, and all placements were in the nucleus accumbens core. Subjects with cannula placement outside of nucleus accumbens core were not included in the data analyses and are not shown here. Appetitive responding for ethanol following systemic injection of the non-selective group II mGluR agonist LY379268 (1.5 mg/kg) or vehicle followed by intra-accumbens core administration of non-selective group II mGluR antagonist LY341495 (1.0 μg/side) or vehicle (figure 4b; n=6/group). Additional microinjection of LY379268 (0.5 μg/side) without systemic injection was also performed. A significant reduction in ethanol-seeking was observed with systemic LY37 plus NAc core vehicle, systemic LY37 plus NAc core LY34 compared to systemic vehicle plus NAc core vehicle. Ethanol-seeking was also reduced following NAc core administration of LY37 compared to systemic vehicle plus NAc core vehicle. (* p<0.05)
Latency to First Lever Press
Examination of lever press latency can be confounded for sessions in which the animal does not emit a lever press response (since this may indicate either sedation or a decrease in reinforcer seeking). Therefore, analysis of lever press latency was performed using both a conservative approach (non-response interpreted as seeking behavior and trials excluded from analysis) and liberal approach [non-response interpreted as diminished locomotion and maximum latency (1200 sec) used]. Neither the conservative [F(3,11)=0.355, p=0.79] nor liberal [F(3,15)=0.314, p=0.82] analyses indicated any effects of treatment on lever press latency (table I). The effect of NAc core LY37 administration was analyzed separately using a pair-samples t-test (NAc core LY37 vs systemic vehicle plus NAc core vehicle). Neither the conservative [t(2) =0.81, p=0.51] nor liberal [t(5) =−1.74, p=0.14] analyses yielded significant effects of treatment suggesting that NAc core administration of neither LY34 nor LY37 had a significant effect on latency to initiate responding.
Body Weight
A significant main effect of systemic treatment on the change in body weight between injection session and subsequent session was observed [F(1, 10) =8.87, p<0.01]. Post hoc analyses indicate that systemic LY37 administration significantly (p<0.01) decreased body weight 24 hours following injection (table II). The average change in body weight following systemic LY37 administration was −21.4 ± 2.1 g.
Discussion
Overall, systemic administration of the group II mGluR agonist LY37 significantly decreased reinforcer seeking and selectively decreased sucrose, but not ethanol, consumption at doses not shown to affect the latency to initiate responding. As well, in two separate experiments, systemic LY37 administration was noted to decrease body weight 24-hours following administration. Aside from the body weight change, animals were healthy and not observed to be in distress. Systemic administration of the mGluR2 PAM BINA had no effect on reinforcer consumption and no dose-related or reinforcer-specific pattern of effects on reinforcer-seeking. Systemic administration of BINA also had no effect on body weight. Finally, no protracted effect of acute administration of LY37 or BINA on either sucrose or ethanol consumption was observed.
Prior studies examining the effect of systemic group II mGluR agonists and PAM administration on operant self-administration used fixed ratio reinforcement schedules that require animals to engage in a seeking response prior to consumption of a small amount of the reinforcer across the duration of the session (Backstrom and Hyytia 2005; Jin et al. 2010; Liechti et al. 2007; Sidhpura et al. 2010). Such studies, therefore, measure a mixture of seeking and consumption. In the present study, a “sipper tube” method (e.g., Czachowski et al. 2001) was used to assess the effect of moderate doses of systemic LY37 and BINA administration on ethanol-seeking and consumption. In this method, a once-daily seeking response (lever press) is procedurally separated from the consummatory response (drinking) allowing for discrete analysis of the effect of LY37 and BINA on each behavior independently. Previous studies have reported a decrease in operant ethanol self-administration using a fixed ratio schedule following systemic LY37 administration (Backstrom and Hyytia 2005; Sidhpura et al. 2010). This is in agreement with our finding of reduced ethanol-seeking following systemic administration of the mGluR2/3 agonist LY37. However, here we observed that systemic LY37 administration does not significantly affect ethanol consumption. The lack of LY37 effect on ethanol consumption suggests a limitation of a fixed ratio procedure in that it assesses a combination of reinforcer seeking and consumption across the session while the sipper tube model allows for examination of reinforcer consumption specifically without the seeking confound. Together these findings suggest that regulation of neurotransmission via mGluR2/3 activation specifically influences ethanol-seeking with no effect on ethanol consumption.
More importantly, systemic administration of the group II mGluR agonist LY37 was observed to reduce seeking and consumption of an alternative reinforcer (sucrose) at doses not found to significantly affect start latencies. Of the previous studies that have examined the effect of LY37 on seeking and/or consumption of an alternative reinforcer, either no effect (Baptista et al. 2004; Zhao et al. 2006) or only at the highest dose of LY37 tested (3 mg/kg), was alternative reinforcer seeking and/or consumption reduced (Kufahl et al. 2011; Liechti et al. 2007; Peters and Kalivas 2006). However, this same dose of LY37 (3 mg/kg) shown to reduce seeking and consumption of food reinforcers was also shown to significantly reduce spontaneous locomotor behavior (Kufahl et al. 2011) suggesting that the observed effect of LY37 on reinforcer seeking and consumption is due to the sedative effects of LY37. However, here we demonstrated that LY37 does, in fact, reduce not only sucrose seeking but also sucrose consumption and body weight 24-hours following systemic administration at doses not observed to result in significant changes in response initiation. Further, a significant reduction in both sucrose seeking and body weight was observed following administration of 1.0 mg/kg LY37, a dose which has previously been shown to have no significant effect on spontaneous locomotor behavior (Kufahl et al. 2011). These findings indicate that the behavioral effects of group II mGluRs agonists are not specific to ethanol-seeking, but rather agonism of mGluR2/3 results in a general reduction in the incentive salience of reinforcers. This may be due to a possible transient drug-induced anhedonic-like state or malaise. Conversely, as sucrose but not ethanol consumption was reduced following LY37 administration, the effect of systemic LY37 on seeking and consumption may be due to the involvement of group 2 mGluRs in regulating feeding and satiety. Further research is needed to clarify the mechanism by which systemic mGluR2/3 agonism reduces seeking and consumption of natural reinforcers.
Systemic administration of the selective mGluR2 PAM BINA (0–20 mg/kg, ip) did not significantly affect seeking or consumption of either ethanol or sucrose. Previously, BINA was shown to decrease both cocaine self-administration (20 and 40 mg/kg) and cue-induced reinstatement of cocaine seeking (10, 20, and 40 mg/kg) with no effect on food self-administration or cue-induced reinstatement of food-seeking (Jin et al. 2010). It is possible that the lack of BINA effect on ethanol seeking and/or consumption observed here was due to the Wistar rats used not expressing functional mGlu2 receptors given the recent findings of a premature stop codon mutation in mGluR2 (Grm2 cys407*) for some commercially available Wistar rat populations (Wood et al. 2017). However, this is unlikely as Wood et al (2017) did not observe the mutation in this strain of Harlan Wistar rats (Hsd:WI, n=48). It is also possible that a higher dose of BINA may have decreased reinforced responding and/or intake, but note that the high dose was twice the dose necessary to decrease cocaine seeking.
Interpretation of the separate contribution of mGlu2 and mGlu3 receptors in reinforcer seeking is difficult with systemic LY37 and BINA administration due to the differing mechanisms of action of the drugs (i.e., orthosteric agonism compared to positive allosteric modulation, respectively). The lack of a significant effect of systemic BINA administration on ethanol seeking could suggest that the decreased reinforcer seeking observed following systemic LY37 administration is driven primarily by agonist action at mGlu3 receptors. However, previous studies have shown that rats homozygous for a mGluR2 stop codon (Grm2 cys407*) which results in loss of functional mGluR2 expression (Corda et al. 2014; Manzo et al. 2012; Zhou et al. 2013) and mGluR2 knockout mice (Zhou et al. 2013) have increased preference for and consumption of ethanol. This suggests that loss of mGluR2 contributes to increased ethanol seeking and/or consumption. Due to mechanistic differences between BINA and LY37, the different contributions of mGluR2 and mGluR3 agonism on ethanol reinforcement is difficult to determine unequivocally from our findings. Subsequent work with yet unavailable subtype-specific orthosteric agonists may be able to disentangle the relative contributions of mGlu2 and mGlu3 receptors in ethanol reinforcement.
Appetitive responding following systemic LY37 plus NAc core LY34 was not significantly different from responding following systemic LY37 alone. This inability of NAc core mGluR2/3 blockade to alter the LY37-induced reduction in appetitive responding suggests that NAc core mGluR2/3 may not be involved in the regulation of ethanol-seeking. However, intra-accumbens core microinjection of LY37 did significantly reduce ethanol-seeking without affecting the latency to first lever press. The lack of attenuation of LY37 suppression of ethanol-seeking by NAc core LY34 may, therefore, be due to methodological factors such as the dose of LY34 chosen or the use of systemic administration of an agonist preceding the brain-site specific administration of an antagonist.
Using a behavioral model that allows for discrete separation of reinforcer seeking and consumption, we found that systemic administration of the mGluR2/3 agonist LY37, but not mGluR2 PAM BINA, decreased ethanol-seeking but not consumption contrary to previous studies that did not separate seeking from consumption. This novel finding implies that group II mGluR agonists could be an efficacious treatment approach for craving-related behaviors that generate reinforcer seeking. As well, it suggests a lack of efficacy of group II mGluR agonists for targeting drinking behavior specifically (e.g., treatment of binge drinking). Intra-accumbens core administration of LY37 was also shown to significantly reduce ethanol-seeking, further implicating group II mGluRs in ethanol-reinforced appetitive behavior. Notably, administration of LY37 also decreased sucrose consumption and body weight 24-hours following systemic administration suggesting that group II mGluRs are not specific to regulation of drug seeking but may be broadly involved in regulating incentive salience of reinforcers.
Highlights.
Systemic administration of LY379268 (LY37) and biphenyl indanone-A (BINA).
LY37 reduced ethanol seeking without influencing ethanol consumption.
Intraaccumbens core LY37 significantly reduced ethanol seeking.
BINA had no effect on seeking or consumption of either ethanol or sucrose.
LY37 significantly reduced sucrose seeking, sucrose consumption, and body weight.
Acknowledgments
Funded by T32AA07462 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–8. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baptista MAS, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: Comparison between cocaine and a potent conventional reinforcer. Journal of Neuroscience. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJM, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic Glutamate Receptor 5 Activity in the Nucleus Accumbens Is Required for the Maintenance of Ethanol Self-Administration in a Rat Genetic Model of High Alcohol Intake. Biological Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–6. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–30. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, Deroche-Gamonet V, Hansson AC, Spanagel R. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–56. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–91. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Jang JK, Kim JH, Vezina P. Blockade of group II metabotropic glutamate receptors in the nucleus accumbens produces hyperlocomotion in rats previously exposed to amphetamine. Neuropharmacology. 2006;51:986–92. doi: 10.1016/j.neuropharm.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Corda MG, Piras G, Piludu MA, Giorgi O. Differential Effects of Voluntary Ethanol Consumption on Dopamine Output in the Nucleus Accumbens Shell of Roman High- and Low-Avoidance Rats: A Behavioral and Brain Microdialysis Study. World Journal of Neuroscience. 2014;04:279–292. [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001;25:344–50. [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012;36:633–40. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–17. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–36. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–73. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–85. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo L, Gomez MJ, Callejas-Aguilera JE, Fernandez-Teruel A, Papini MR, Torres C. Oral ethanol self-administration in inbred Roman high- and low-avoidance rats: gradual versus abrupt ethanol presentation. Physiology & behavior. 2012;108:1–5. doi: 10.1016/j.physbeh.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, clinical and experimental research. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102:4170–5. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego. CA: 1998. [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–4. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: Comparison between the Lewis and Fischer 344 rat strains. Brain Research. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–11. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, Kiianmaa K, Bienkowski P, de Jong TR, Colombo G, Chastagnier D, Wafford KA, Collingridge GL, Wildt SJ, Conway-Campbell BL, Robinson ES, Lodge D. Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology. 2017;115:128–138. doi: 10.1016/j.neuropharm.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–71. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MAS, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. Journal of Neuroscience. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch MA, Hodgkinson CA, Shen PH, Lovinger DM, Edenberg HJ, Heilig M, Goldman D. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110:16963–8. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]