Abstract
This report explores the consequences of acoustic overexposures on hearing in noisy environments for two macaque monkeys trained to perform a reaction time detection task using a Go/No-Go lever release paradigm. Behavioral and non-invasive physiological assessments were obtained before and after narrowband noise exposure. Physiological measurements showed elevated auditory brainstem response (ABR) thresholds and absent distortion product otoacoustic emissions (DPOAEs) post-exposure relative to pre-exposure. Audiograms revealed frequency specific increases in tone detection thresholds, with the greatest increases at the exposure band frequency and higher. Masked detection was affected in a similar frequency specific manner: threshold shift rates (change of masked threshold per dB increase in noise level) were lower than pre-exposure values at frequencies higher than the exposure band. Detection thresholds in sinusoidally amplitude modulated (SAM) noise post-exposure showed no difference from those in unmodulated noise, whereas pre-exposure masked detection thresholds were lower in the presence of SAM noise compared to unmodulated noise. These frequency dependent results were correlated with cochlear histopathological changes in monkeys that underwent similar noise exposure. These results reveal that behavioral and physiological effects of noise exposure in macaques are similar to those seen in humans and provide preliminary information on the relationship between noise exposure, cochlear pathology and perceptual changes in hearing within individual subjects.
1. INTRODUCTION
Overexposure to loud acoustic stimuli is one of the most common forms of acquired hearing loss, and noise induced hearing loss (NIHL) has significant financial implications as one of the most commonly compensated work or military related injuries (Le Prell and Clavier 2016; Ryan et al. 2016). The cochlear consequences of noise exposure can include changes to the sensory elements of the cochlea, namely inner hair cell (IHC), outer hair cell (OHC), stereocilia, and IHC ribbon synapses (e.g., Kujawa and Liberman 2009; 2015; Sergeyenko et al. 2013; Furman et al. 2014; Valero et al. 2017) as well as damage to supporting cells, changes to the cochlear vasculature, and disruption of the cellular metabolic processes (reviewed in Saunders et al. 1985; 1991). The precise effects of noise exposure are known to be variable within individuals in many species (Davis et al. 1950; Kryter and Garinther 1965; Ward 1965; Cody and Robertson 1983; Wang et al. 2002), including primates (Moody et al. 1978; Valero et al. 2017) and humans (e.g., Davis et al. 1950). The differences in the amounts of synaptopathy and hair cell loss may explain findings for patients with NIHL who present with identical audiograms but demonstrate different functional impairments and report different perceptions of their hearing loss (Kochkin, 2007).
While the human behavioral data detailing the expressions of hearing loss in terms of audiometric thresholds, speech understanding, and speech in noise understanding is extensive (e.g. Moore 2016), our understanding of the cochlear and central changes underlying such perceptual impairments is limited, since we typically cannot combine behavioral testing with temporal bone histology. However, more specific diagnostics of cochlear pathology could potentially improve our ability to provide more targeted therapies as they become available. To circumvent these problems, NIHL has been extensively studied in a variety of animal models including mice, guinea pigs, chinchillas, and cats (e.g., Kujawa & Liberman 2015; Lin et al, 2011; Harding et al. 2004; Miller et al. 1997; Miller et al. 1963), since they provide large, homogeneous populations for studying the anatomical and physiologic effects of noise exposure. Since susceptibility to noise varies across species (Dobie and Humes 2017), it makes sense to study the species with greatest phylogenetic similarity as well as noise exposure susceptibility to humans.
Nonhuman primates provide a powerful bridge between the physiological and anatomical studies afforded by animal models and the psychoacoustic data collected from human subjects listening in complex environments. Because nonhuman primates are phylogenetically very close to humans, can be trained to perform complex behavioral tasks like humans, and show susceptibility to noise exposure that is similar to that of humans (Moody et al. 1978; Igarashi et al. 1978; Valero et al. 2017), we can combine anatomic verification of cochlear histopathology with behavioral studies to investigate the effects of controlled noise exposure as a model for human NIHL. Previous studies of NIHL in primates only reported effects on detection of tones in quiet (e.g., Moody et al. 1978), while real world hearing and a patient’s individual perception of their hearing loss typically involves hearing in noisy backgrounds. Here we report on the results of behavioral measurements of hearing in noisy environments for macaques that underwent high sound pressure level noise exposure that caused permanent threshold shifts. The current report extends previous studies of NIHL by measuring the hearing in more realistic circumstances, and comparing the clinically measurable behavioral deficits with cochlear histopathological changes in other similarly aged noise-exposed Macaca mulatta subjects recently described by Valero et al. (2017). These data form the baseline for investigating peripheral and central neuronal encoding that can underlie these changed behaviors, investigations of central anatomical changes secondary to cochlear changes, as well as for investigations of the treatment of hearing loss.
2. METHODS
All procedures were approved by the Animal Care and Use Committee at Vanderbilt University Medical Center and were in strict compliance with the guidelines for animal research established by the National Institutes of Health.
2.1 Subjects
Two male macaque monkeys were trained to perform behavioral detection experiments. Monkey L (Macaca mulatta) was 11 years old and Monkey G (Macaca radiata) was 9.5 years old at the time of noise exposure. Both behavioral and non-invasive physiological measures were obtained pre- and post-noise exposure. These trained macaques continue to be used in ongoing behavioral studies, so cochlear histology from these subjects is not available at this time. We refer to histology from a second cohort that was subject to the same noise exposure as Monkey G (Valero et al. 2017) for comparisons described in the Results.
2.2 Noise Exposure
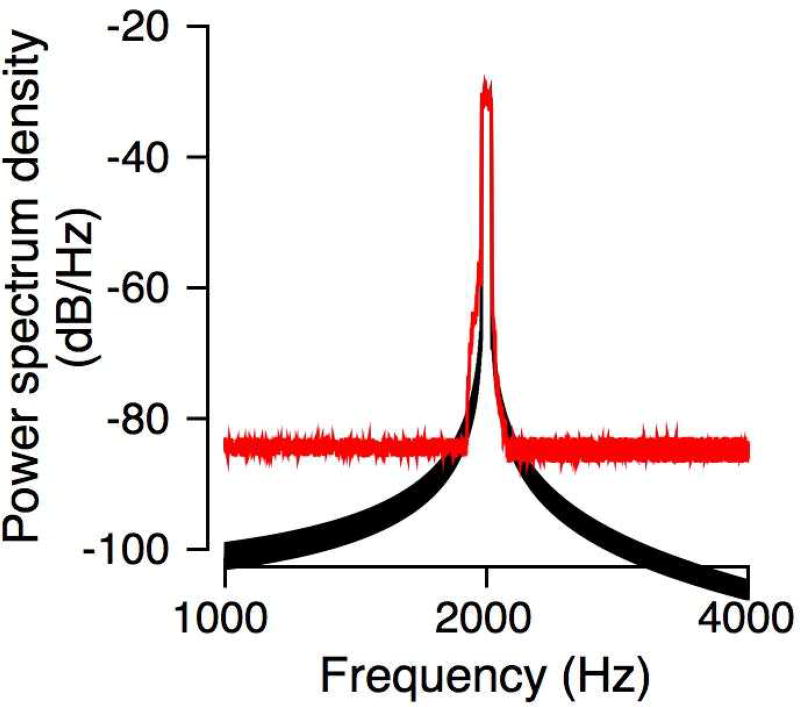
Subjects were treated with atropine (0.04 mg/kg) and sedated with an intramuscular injection of ketamine (3 – 5 mg/kg) and dexmedetomidine (10 – 40 µg/kg). They were intubated and placed in a prone position with the head slightly raised in a sound treated booth (Acoustic Systems Booth). Further procedures were conducted under isofluorane anesthesia (1 – 2%), with vital signs being monitored throughout the procedure. Pediatric ER-3 insert earphones were trimmed and inserted deep into each ear canal. The earphones were attached to closed-field loudspeakers (MF1, Tucker-David Technologies) through a 10-cm polyethylene tube. The loudspeakers were calibrated using a ¼” microphone (model 378C01, PCB Piezotronics) coupled to the tubing, insert earphones and a short tube (~1 cm) with internal diameter matching the macaque external auditory meatus (Spezio et al. 2000). The exposure stimulus was a 50-Hz wide band of noise centered at 2 kHz, and it was presented for four hours. Figure 1 shows the spectrum of the noise synthesized to create the hearing loss (black line). The output of the microphone was recorded and subject to a fast Fourier transform to compare the spectrum to the desired (synthesized) spectrum. The spectrum of the output of the microphone is shown in Figure 1 (red lines). The noise floor was higher than that of the synthesized digital signal, but overall, the bandwidth of the input and the output signal was very similar. Parallel studies suggested that exposure noise with sound pressure levels up to 140 dB SPL caused temporary threshold shifts only, but no permanent threshold shifts, as assessed by ABR (Valero et al. 2017). So, Monkey L was exposed to 141 dB SPL. Monkey G was exposed to the same stimulus at 146 dB SPL since the 141 dB exposure caused only moderate hearing loss (see Figure 2 and Figure 4 below). The level of each exposure stimulus varied by less than 0.3 dB SPL over the course of the four-hour procedure. Post-procedure, monkeys were monitored intensively for 72 hours, or longer, before they were returned to baseline daily monitoring in their home cage. Typically, behavioral data were collected starting one week after the noise exposure and the audiogram was repeatedly measured to monitor changes over time. Data shown here were obtained over the first year following noise exposure.
Figure 1.
The black trace shows the spectrum of the digitally created signal. The red trace shows the spectrum of the signal received by the microphone after passing though the stimulus delivery apparatus and a tube that mimicked the macaque external auditory meatus.
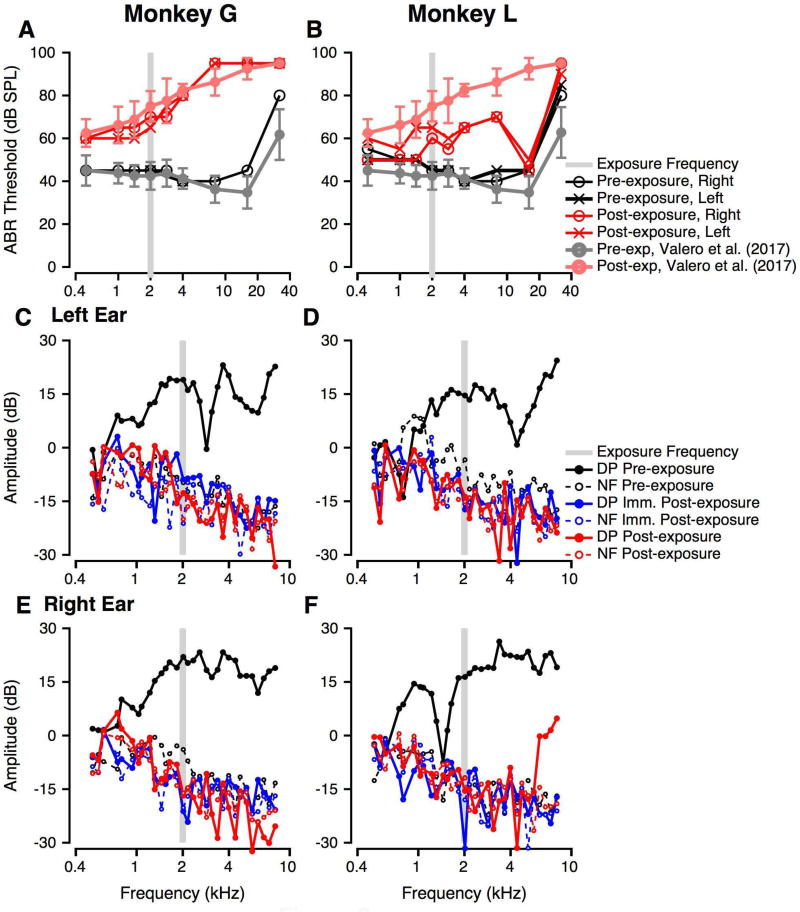
Figure 2.
Effect of noise exposure on noninvasive physiological measures of auditory function. In all panels, the grey bar shows the frequencies in the noise exposure band. A: ABR thresholds as a function of tone burst frequency pre- (black) and post-noise exposure (red, 5 weeks post) for Monkey G. Different symbols show left and right ear measures. The ABR threshold data from the anatomy cohort in Valero et al. (2017) are shown as grey (pre-exposure) and pink (post-exposure) with error bars show ±1 standard deviation. B: Similar to A, but for Monkey L. Post-exposure measures were obtained at 16 weeks. C, E: DPOAE measurements before (black), immediately post- (blue) and at 5 weeks post-exposure (red) for the left (C) and right ears (E) of Monkey G. In both panels, solid lines show distortion product (DP) magnitudes and the dashed thin line shows the noise floor (NF). D, F: Similar to C, E, but for monkey L. As in B, post-exposure measurements were obtained at 16 weeks.
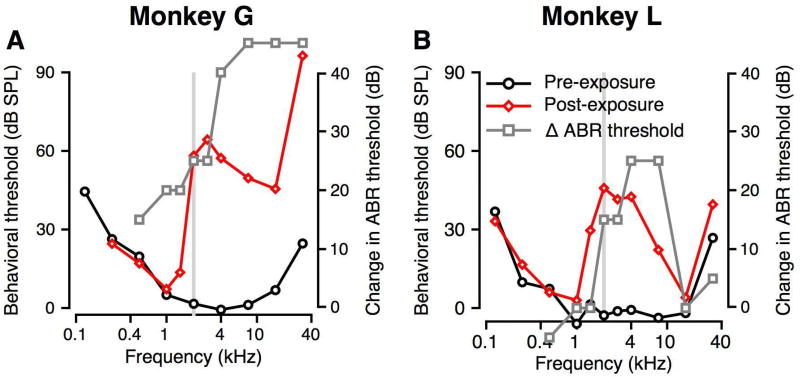
Figure 4.
Effect of noise exposure on behavioral audiograms. A, B: Behavioral thresholds are shown as a function of tone frequency for monkeys G (A) and L (B). Pre-exposure thresholds are shown in black and post-exposure thresholds are shown in red. Noise exposure band is represented with the gray bar. The change in the ABR threshold is shown by the grey squares and lines. The points above the Y axis limits are those frequencies where post-exposure ABR thresholds were over 90 dB SPL.
2.3 Physiological Testing
2.3.1 Auditory Brainstem Response
The auditory brainstem response (ABR) is a common audiological clinical measure of neural integrity. The results can be used to estimate hearing thresholds for difficult to test human subjects as well as animals (Davis and Hirsh, 1979; Heffner et al. 2008). During ABR testing, subjects were initially sedated with ketamine (10 mg/kg im) and midazolam (0.05 mg/kg im). Sedation was maintained during testing with isofluorane (1 – 2%). Monkeys were intubated and placed in a sound treated booth in a prone position with head slightly elevated and facing a calibrated free field loudspeaker, as used in the behavioral experiments (SA1 loudspeakers, Selah Audio). Ears were positioned 90 cm from the speaker, in a manner identical to that used for behavioral experiments. Subdermal needle electrodes (Rhythmlink) were placed at the forehead (reference, inverting) and shoulder (ground), and a TMTrode (Intelligent Hearing Systems, non-inverting) was placed on the tympanic membrane of the ear being tested. The TMTrodes were prepared for recording by soaking the wick in saline and coating them in Ten20 conductive paste. Impedances for subdermal needle electrodes were consistently less than 1 kΩ, and those for TMTrodes were typically 3–5 kΩ throughout the procedure. ABRs were measured for both ears.
ABRs were collected and averaged using BioSigRZ software (Tucker-Davis Technologies). Clicks and tone bursts with frequencies that spanned the audible range of macaques were used as stimuli (0.5, 1, 1.414, 2, 2.828, 4, 8, 16, and 32 kHz; Pfingst et al. 1975; Pfingst et al. 1978; Dylla et al. 2013; Bohlen et al. 2014). Tone-burst stimuli were presented at a rate of 27.7/second for two repeats of 1024 presentations of each stimulus. Stimuli had rise/fall times of 1 ms (2–32 kHz), 2 ms (1-1.414 kHz), or 4 ms (0.5 kHz) and plateau durations of 0.5 ms (2–32 kHz), 1 ms (1–1.414 kHz), or 2 ms (0.5 kHz). Recorded ABR signals were digitally filtered from 100 Hz to 3000 Hz. Stimulus level was decreased from a starting level of 90 dB SPL in 5 or 10 dB steps until there was no repeatable response with an amplitude ≥ 120 nV. ABR threshold was defined as the lowest level (to the nearest 5 dB) at which a repeatable response with amplitude greater than or equal to 120 nV could be identified. ABR thresholds were measured prior to noise exposure for both subjects (pre-exposure baseline). Post-exposure ABRs were measured at 5 weeks following noise exposure for Monkey G, and at 16 weeks following exposure for Monkey L.
2.3.2 Distortion Product Otoacoustic Emissions (DPOAEs)
DPOAEs provide an estimate of cochlear health, particularly of the OHCs (e.g., Kemp 2002), which are a necessary component for normal hearing. DPOAEs were collected during the same sedated procedure as for the ABRs, and immediately following noise exposure. A clinical OAE system (Bio-logic Scout, Natus) was used to deliver paired pure-tone stimuli into the subject’s ear canal and record emissions from the ear. DPOAEs were measured between 500 and 8000 Hz using a frequency ratio (f2/f1) of 1.22 (after Gorga et al. 1993), and presentation levels of 65 dB SPL (L1, level of f1) and 55 dB SPL (L2, level of f2) at eight frequencies per octave. A distortion product (DP) was considered to be present if it had a level of at least 0 dB SPL and was at least 6 dB above the noise floor (NF).
2.4 Behavioral Testing
Behavioral methods, stimulus generation, and analysis are identical to those described in our previous publications unless otherwise specified (Dylla et al. 2013; Bolen et al. 2014). Monkeys were prepared for behavioral procedures with surgery that implanted a head holder on the head (Dylla et al. 2013). The head holder allowed the head to be fastened to the superstructure of an acrylic primate chair that was designed to have no obstruction to sounds on either side of the head (audio chair, Crist Instrument Co., Hagerstown, MD). The head holder allowed the standardization of head position relative to the loudspeaker. A single calibrated loudspeaker (SA1 loudspeakers, Selah Audio) driven by linear studio amplifiers (SLA2, ART Pro Audio) was used to deliver tone and noise stimuli, and was located 90 cm from the ears.
The behavioral Go/No-Go task required the monkeys to hold down a lever to initiate trial. A target tone was presented on ~80% of trials after a variable hold time (signal trials). The monkey was required to release the lever within a 600 ms response window beginning at tone onset. Reaction time was calculated for each correct release as the time of lever release relative to tone onset. Correct lever release (hit) was followed by fluid delivery. There were no penalties for not releasing the lever (miss), which was taken to indicate that the tone was not detected. Tone trials were interleaved with catch trials (~20% of trials) in which no tone was played. On catch trials, monkeys were required not to release the lever (correct reject). An incorrect lever release (false alarm) resulted in a variable time-out period. Monkeys were not rewarded for correct rejections.
Within each block, the background noise level was held constant, and the tone level could take one of eleven randomly interleaved values that were spaced within a ±30 dB range around an estimate of threshold. Tone levels were separated by 2.5 dB for levels within ±7.5 dB of the threshold estimate, and by 5 or 10 dB at higher and lower levels. Each of the behavioral tasks, described in more detail below, was performed before and after noise exposure.
The subjects performed tone detection in three noise conditions similar to those of Dylla et al. (2013). Tones (200 ms duration, 10 ms rise fall times) having frequencies between 125 and 32,000 Hz were presented 1) alone, or in 2) continuous broadband (40–40,000 Hz) steady white noise (SN), or 3) continuous broadband noise sinusoidally amplitude modulated (SAM) at 10 Hz. Overall noise levels are given in dB SPL; the level of the masker in a 1-Hz wide band can be obtained by subtracting 46 from the given masker level. In the SN condition, the noise level varied from 46 – 96 dB SPL or 51 – 91 dB SPL in 10 dB steps. SAM noise was created by modulating the SN at a 10-Hz modulation frequency, and 100% modulation depth. The root mean square SPL of the modulated and unmodulated maskers were equal to each other. Most of the SAM noise conditions used a root mean square level of 76 dB SPL.
2.5 Data analysis
Data analyses for behavioral experiments are identical to those used in Dylla et al. (2013) and Bohlen et al. (2014). All analyses were based on signal detection theoretical methods (Green and Swets, 1966; Macmillan and Creelman, 2005) and implemented using MATLAB (Mathworks, Natick, MA). Briefly, for each block, we determined the false alarm rate (F) and the hit rate (H) for each tone level. We converted hit rates to behavioral accuracy that would be expected in a two-alternative forced-choice task for ease of comparison to planned neurophysiological experiments. Using signal detection theory, , where the z transform converts H and F into units of standard deviation of a standard normal distribution (z-score, norminv in MATLAB; Macmillan and Creelman, 2005). The inverse z-transform (z−1) then converts a unique number of standard deviations of a standard normal distribution into a probability correct (pc, normcdf in MATLAB). Corrections were made to account for H=1, and for F=0 (see Dylla et al. 2013; after Macmillan and Creelman, 2005).
A Weibull cumulative distribution function was fitted to the plot of pc(level) for each condition (e.g. frequency, noise level, noise type) to generate a smooth relationship between behavioral accuracy and sound pressure level according to the following equation: , for x ≥ 0, where c and d represent the estimates of saturation and chance performance, respectively, x is the tone level, and λ and k represent threshold and slope parameters. To avoid levels below 0 dB SPL, the levels were translated such that all levels were greater than or equal to 0 dB, the translated values were fitted with a Weibull function, and then the levels were translated back by the same amount. From this curve fit, threshold was calculated as that tone level that corresponds to a probability correct value of 0.76 (corresponding to d’=1).
The analyses of the effects of noise on tone detection followed identical methods as described in Dylla et al. (2013). Briefly, thresholds at different SN levels were computed as above, and regressed against the noise spectrum level. Threshold shift rate was calculated as the slope of the best linear fit of threshold vs. noise spectrum level. Thresholds were not included in the linear fit if they were not significantly different from thresholds for tones in quiet (see Dylla et al. 2013). The effects of SAM noise re SN were evaluated by subtracting the tone threshold in SN from the threshold in SAM noise.
3. RESULTS
3.1 Physiological Results
Figure 2 illustrates pre- and post-exposure ABR thresholds and DPOAEs as a function of frequency. The pre- and post-exposure thresholds of the anatomy cohort from Valero et al. (2017) are shown in grey (pre-exposure) and pink (post-exposure), and the grey bar shows the noise exposure frequencies. Both monkeys showed permanent changes to frequency-specific ABR tone thresholds relative to baseline in both ears, similar to the anatomy monkeys (Fig. 2A, B). Noise exposure resulted in an elevation in ABR thresholds between 2 and 8 kHz for both subjects, with greater threshold shifts observed for Monkey G than Monkey L. Monkey G also had large threshold shifts at 16 and 32 kHz, with no measurable response at 90 dB SPL at 32 kHz (similar to the anatomy controls). Monkey L showed much smaller changes in threshold at those frequencies relative to monkey G and the anatomy subjects. Monkey G’s ABR thresholds matched the 8-week-post-exposure ABR thresholds of the 4 age-matched subjects from our previous study that were subject to 146 dB SPL exposure to the same narrowband stimulus (Valero et al. 2017). Monkey L’s ABR thresholds were lower than for other five monkeys but still showed a frequency-specific effect of noise exposure. ABR thresholds were symmetrical between ears at baseline and post-exposure for both subjects.
Baseline DPOAEs were robust for all ears tested (Figure 2C–F, black), with distortion product (DP, the amplitude at 2f2-f1) levels up to 26 dB SPL that were up to 45 dB above the noise floor (NF) at all frequencies higher than 1500 Hz. Immediately post-noise exposure, DPOAEs were completely absent for all ears at all frequencies tested (Figure 1 C–F, blue). At multiple weeks post-exposure (Figure 1 C–F, red) DPOAEs remained absent for all ears except for frequencies higher than 6.1 kHz in Monkey L’s right ear (Figure 1F, red). Even though present, these DPOAEs from 6.1–8 kHz were reduced in level re pre-exposure values. The NFs did not change with noise exposure. The reduced DPs suggest significant impairment to OHC function (Probst et al. 1991; Kemp 2002). The absence of DPOAEs in monkey G matches the results obtained for the four age-matched histological controls in our previous study (Valero et al. 2017).
3.2 Behavioral Results
3.2.1 Audiogram – Tone detection
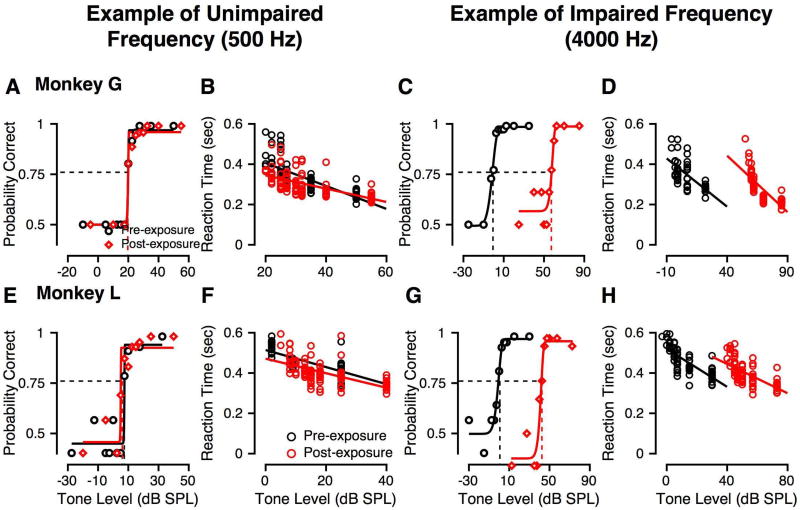
Tone alone detection was measured pre- and post-exposure for each monkey. Figure 3 shows example psychometric functions and reaction times from each subject before and after noise exposure (black and red symbols and lines, respectively) at an unaffected (500 Hz) and an affected frequency (4000 Hz) that showed large threshold changes. At 500 Hz, pre- and post-exposure psychometric functions were very similar for Monkey G (Fig. 3A) and Monkey L (Fig. 3E), with the probability of correct detection increasing in a sigmoidal fashion as a function of tone level. At 4000 Hz, one octave above the center frequency of the noise exposure band, post-exposure psychometric functions were shifted to higher levels relative to pre-exposure for both monkeys (G: Figure 3C; L: Figure 3G). The corresponding increase in behavioral thresholds is illustrated by the separation in the dashed vertical lines. Post-exposure thresholds at 4000 Hz were higher by 57 dB (Monkey G) and 43 dB (Monkey L) relative to pre-exposure values.
Figure 3.
Effect of noise exposure on psychometric functions and reaction times describing tone detection. Black symbols and lines show pre-exposure data and fits, and red symbols and lines show post-exposure data and fits. Circles show reaction time data, while diamonds show behavioral accuracy (probability correct) data. A, E: Probability correct as a function of tone SPL (psychometric function) for a 500 Hz tone for monkey G (A) and monkey L (E). The horizontal line shows probability correct of 0.76 (threshold criterion matching d′=1), and the vertical line shows the level that evoked probability correct = 0.76. B, F: Reaction times as a function of tone SPL before and after noise exposure for the data shown in A and E, respectively for monkey G (B) and monkey L (F). The line is a fit to the reaction time vs. SPL data. C, G: Probability correct as a function of tone level for a 4 kHz tone for monkeys G (C) and L (G). Format is the same as for panels A and E. D, H: Reaction times as a function of tone level before and after noise exposure for the data shown in C and G, respectively. Format is the same as for panels B and F.
Reaction times (RTs) were also calculated for each of the correct responses in each of the different conditions, pre- (black) and post-exposure (red) for monkeys G (Figures 3B, D) and L (Figures 3F, H). For each tone, as the level increased, the RT decreased, consistent with previous findings in macaques (Stebbins et al. 1966; Dylla et al. 2013). The effect of tone level on RT was assessed by a linear fit of the RT vs. level, to calculate a reaction time slope. Reaction time slopes pre-and post-exposure were not statistically different at any frequency (p > 0.05, Kruskal Wallis test). The only observed effect was that the RTs and the linear fit were shifted to higher SPLs after noise exposure (compare black and red lines in Figures 3D and 2H).
Figure 4 shows the pre- and post-exposure audiograms for Monkeys G (Figure 4A) and L (Figure 4B) collected within the first 6 months after exposure, along with the changes in ABR threshold (grey lines and squares). Baseline performance showed greatest auditory sensitivity between 1–16 kHz, consistent with previous data (black lines and circles, Dylla et al. 2013; Pfingst et al. 1975; Pfingst et al. 1978; Stebbins et al. 1966; Behar et al. 1965). Following noise exposure, tone thresholds were significantly elevated at and above the exposure frequency of 2 kHz (red lines and diamonds). Monkey G (Figure 4A) showed a large threshold elevation of 62 dB at 2.828 kHz. Monkey L (Figure 4B) showed peak threshold elevations of 43 dB at 2, 2.828, and 4 kHz. Both subjects exhibited unchanged low frequency (0.125–1.0 kHz) thresholds post-exposure. Monkey G exhibited additional threshold elevation at high frequencies (16–32 kHz), with a 72 dB threshold change at 32 kHz, whereas Monkey L’s thresholds over the 16–32 kHz range were smaller, with a 10 dB change at 32 kHz. The ABR threshold changes (calculated as the minimum difference between post-exposure and pre-exposure ABR thresholds across the two ears) were different across the two monkeys. Monkey G showed significant ABR threshold changes at 0.5, 1, and 1.4 kHz, where the behavioral threshold changes were zero or minimal. There were no ABR threshold changes for monkey L at these frequencies. Both monkeys showed significant ABR threshold elevations between 2 and 8 kHz, consistent with the observed behavioral threshold elevations. ABR thresholds could not be measured in monkey G at frequencies at or above 8 kHz, and ABR threshold changes are shown above the axis limits. In contrast, monkey L showed no ABR threshold changes at 16 and 32 kHz, consistent with minimal behavioral threshold changes at these frequencies. Despite the differences in magnitude of behavioral threshold change and differences in high frequency loss, there was a characteristic peak in threshold change above the exposure frequency.
3.2.2 Detection of tones in broadband steady noise (SN)
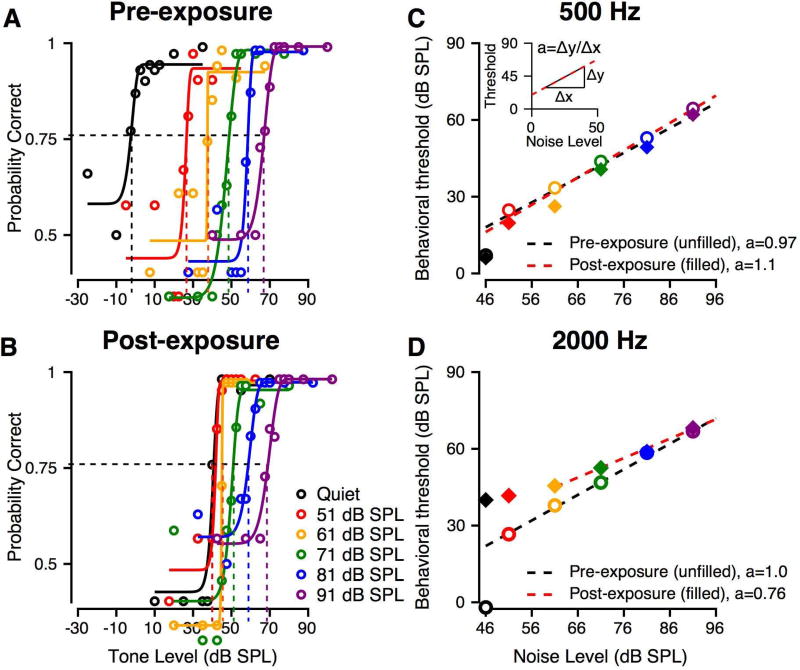
Exemplar pre- and post-exposure psychometric functions for tone detection in SN are shown in Figure 5A–B for masker levels from 51 to 91 dB SPL (shown in the different colors) for Monkey L. Pre-exposure (Figure 5A), thresholds increased progressively with increasing masker level, as illustrated by the rightward shift of the psychometric functions. Following noise exposure (Figure 5B), psychometric functions to tones in quiet were shifted to higher noise levels at frequencies with hearing impairment. In the presence of hearing loss, identified by elevated thresholds in quiet (black line in Figure 5A), the range of threshold changes was reduced, resulting in smaller changes in threshold with increasing masker level. In other words, Monkey L’s post-exposure detection threshold in quiet was higher than at baseline (compare black tracings in Figure 5B and 5A, respectively), but the post-exposure masked tone threshold in 91 dB SPL SN was not significantly different from the pre-exposure values in the same condition (compare purple traces in Figure 5B and 5A, respectively). We quantified the effect of background noise on detection thresholds by calculating a threshold shift rate (Dylla et al. 2013), or the slope of the linear fit of tone detection threshold as a function of SN level (see inset of Figure 5C) and compared pre-exposure and post-exposure shift rates. Regressions did not include thresholds that were not significantly different from tone thresholds in quiet (typically within 2.5 dB).
Figure 5.
Effect of noise exposure on masked tone detection. Example data are from Monkey L. A: Pre-exposure psychometric functions are shown for masker levels from 51 to 91 dB SPL in 10 dB steps, for a tone frequency of 2 kHz. Different colors represent data at different masker levels. The black represents the tone in quiet psychometric function. The format is similar to that for Figure 3A. B: Post-exposure psychometric functions for similar conditions as in A. The format is the same as A. C: Summary of 500 Hz tone detection thresholds as a function of background noise level. Pre-exposure thresholds are shown by open circles. The black dashed line is a linear fit. The post-exposure data are shown by filled diamonds, and the red line is a linear fit. The colors of the symbols indicate the masker level. C-Inset: Schematic of calculation of a, threshold shift rate. D: Summary of 2000 Hz tone detection thresholds as a function of background noise level, pre- and post-exposure. Format is the same as Figure 5C.
Monkey L’s threshold shift rates at 0.5 kHz (Figure 5C) and 2 kHz (Figure 5D) at baseline and post-exposure illustrate the effects of noise on frequencies unaffected and affected by noise exposure, respectively. Pre-exposure (black lines, Figure 5C, D) and at non-impaired frequencies post-exposure (red line, Figure 5C), the threshold shift rate was typically around 1 dB/dB (0.5 kHz pre-exposure: 0.97 dB/dB, 0.5 kHz post-exposure: 1.1 dB/dB, 2 kHz pre-exposure: 1.0 dB/dB). The threshold shift rate at 16 kHz for monkey G was an exception to this finding. That is, each 1 dB increment in noise level caused a 1 dB shift in threshold, consistent with observations for normal humans (Hawkins and Stevens 1950) and macaques (Dylla et al., 2013) and with the 1 dB/dB shift rate that has been postulated for ideal detection behavior (Gibson et al., 1985). At frequencies where audiometric thresholds were changed by noise exposure, such as 2 kHz (red line, Figure 5D), the threshold shift rate decreased to a value significantly less than the pre-exposure values (e.g., 2 kHz post-exposure: 0.75 dB/dB). Such shifted psychometric functions with increasing noise level were also observed for Monkey G; however, higher noise levels were required to shift thresholds in noise above the threshold for tones in quiet.
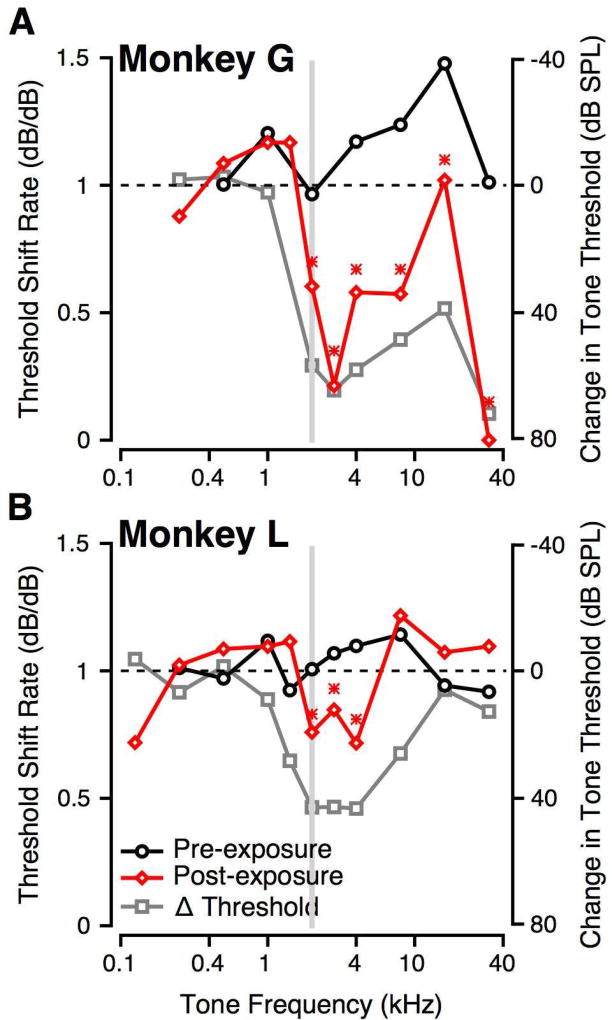
Figure 6 summarizes the threshold shift rate as a function of tone frequency pre- (black) and post-exposure (red) for Monkeys G (Figure 6A) and L (Figure 6B). Pre-exposure, threshold shift rates were generally close to 1 across frequencies for both subjects. Following noise exposure, threshold shift rates decreased by the greatest amount for frequencies around that of the noise exposure band (grey bar, Figure 6). When the exposure-related audiometric threshold changes were overlaid, the frequencies with the shift rate decrease overlapped with the frequencies showing the greatest audiometric changes (gray, Figure 6A, B). The decrease in threshold shift rates was statistically significantly at hearing impaired frequencies for both subjects (Student t-test, p<0.05). Threshold shift rates at hearing impaired frequencies were lower for Monkey G than Monkey L, consistent with greater noise-exposure related audiometric threshold shifts (see also Figure 8).
Figure 6.
Effect of noise exposure on threshold shift rate. In both panels, the grey bar shows the frequency range of the noise exposure. A: Threshold shift rate is shown as a function of tone frequency for monkey G. Pre-exposure shift rates are shown as black circles, and post-exposure shift rates are shown as red diamonds. Overlaid on these is the change in audiometric threshold as a consequence of noise exposure (grey squares). B: Similar to A, but for monkey L. Asterisks represent frequencies where the pre- and post-exposure shift rates were significantly different (p < 0.01, t-test for slopes).
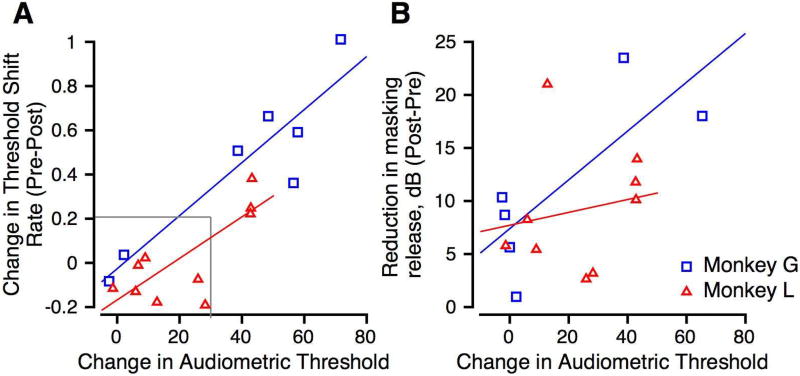
Figure 8.
A: Scatter plot of change in threshold shift rate vs. change in the audiometric threshold. Data from Monkeys G and L are shown in blue and red, respectively. Correlation coefficients were 0.515 (Monkey L) and 0.738 (Monkey G). Linear regressions are shown in blue and red for the data from Monkeys G and L, respectively. B: Scatter plot of reduction in masking release against change in the audiometric threshold. Format is similar to A. Correlation coefficients for individual subjects were 0.333 (Monkey L) and 0.371 (Monkey G).
3.2.3 Detection of tones in broadband sinusoidally amplitude modulated (SAM) noise
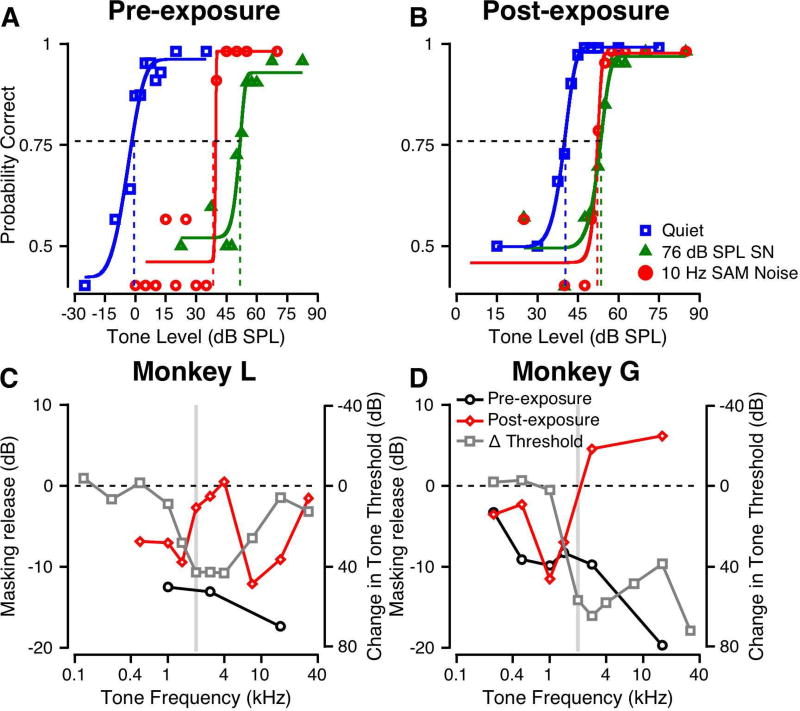
For normal hearing monkeys, tone detection thresholds were lower in SAM noise than in SN, probably due to the subject’s ability to hear the tone in SAM noise at times when the SNR is more favorable (Dylla et al. 2013). Figure 7 shows the effects of noise exposure on this masking release. In Figures 7A–B, the psychometric functions are shown for detection of a 2.828 kHz tone in quiet (blue), and in 76 dB SPL SN (green) and SAM noise (red) for Monkey L. Pre-exposure (Figure 6A), the 76 dB SPL SN produced 52 dB of masking. In 76 dB SPL SAM noise (SN modulated at 10 Hz) the tone threshold was 14 dB lower than that in SN, indicating a significant release from masking. Figure 7B shows data for Monkey L post-exposure collected under similar conditions. Monkey L’s threshold for the tone in quiet was elevated relative to the pre-exposure value (compare blue traces in Figures 7A and B). The 76 dB SPL SN produced 13 dB of masking (Figure 7B, green). The SAM noise produced about the same amount of masking, as indicated by the nearly overlapping psychometric functions for SN and SAM noise in Figure 7B (compare green and red). The thresholds were not significantly different (p>0.05, permutation test) indicating that there was no modulation-based release from masking following noise exposure. To confirm that audibility was not the factor limiting performance, we retested at 86 dB SPL such that the threshold in SN was >20 dB above the unmasked tone threshold. Even at this higher noise SPL, the threshold in SAM noise was not significantly different from that in SN.
Figure 7.
Effect of 10 Hz modulated noise on tone detection before and after noise exposure. A: Psychometric function for a 2.828 kHz tone in quiet (blue), in continuous 76 dB SPL broadband SN (green) and in 76 dB SPL broadband noise sinusoidally amplitude modulated (SAM) at 10 Hz (red). Format is similar to that of Figure 5A. B: Similar to A, but obtained post-exposure. C: Threshold change for detection of a tone in 10 Hz SAM noise relative to threshold in SN. Δ = Threshold in SAM Noise – Threshold in SN. The grey bar shows the exposure frequency range. The dashed horizontal line represents equal thresholds for SN and SAM noise. Data are shown for Monkey L. Overlaid on these is the change in audiometric thresholds as a consequence of noise exposure (grey squares and line). D: Same as C, but for Monkey G.
The masking release was quantified as the threshold change (threshold in SN – threshold in SAM noise) for each tone frequency pre- (black) and post-exposure (red) and for each monkey. As shown in Figure 7C, Monkey L had 12–17 dB release from masking pre-exposure. After noise exposure, the masking release was significantly reduced for frequencies from 2–4 kHz and 16 and 32 kHz, getting close to 0 dB masking release (dashed line). These are shown alongside the audiometric threshold changes (grey), similar to Figure 6. The reduction in masking release occurs at frequencies where the thresholds had changed as a result of noise exposure. Figure 7D shows that a lack of masking release was also observed for Monkey G. Following noise exposure, Monkey G continued to show some release from masking for frequencies below the exposure frequency (2 kHz, grey bar) but showed no release at higher frequencies. Additionally, monkey G’s post-exposure performance at some frequencies were even poorer: thresholds in SAM noise were higher than those in SN (Figure 7D). Similar to Figure 7C, we observe that the frequencies with audiometric changes overlap the frequencies with reduction in masking release (Figure 7D).
3.2.4 Correlations between behavioral tests
We wanted to know how changes in masking were related to audiometric changes. Figure 8A shows the change in audiometric threshold plotted against the change (pre-post) in threshold shift rate for Monkey L (red triangles) and Monkey G (blue squares). These were correlated for one monkey but not for the other (Spearman’s correlation; Monkey L: r=0.516, p=0.10; and Monkey G: r=0.821, p=0.031). The regression lines for the data from two monkeys are shown in different colors. This result suggests that the greater the hearing loss following noise exposure, the better the correlation with threshold shift rate.
Figure 8B shows the relationship between change in audiometric threshold and the reduction in masking release (threshold in SAM noise – threshold in SN). While it appears as though there was a strong correlation between the two variables, calculation of a Spearman’s correlation between the reduction in masking release and the change in audiometric thresholds showed that there was no correlation between the two variables (Spearman’s rho: Monkey G: ρ=0.37, p=0.49; Monkey L: ρ=0.33, p=0.37), The best linear fits for the two monkeys’ data are shown.
3.2.5 Correlations between behavioral tests and cochlear histology
In a previous study, we documented cochlear histopathological changes after noise exposure (Valero et al. 2017). Because the two monkeys (monkeys G and L) are still being studied, we investigated the correlation between the behavioral changes as a function of frequency reported above and the histopathological changes as a function of cochlear frequency place in the other cohort. This cohort was comprised of four male macaques that were exposed to the 146 dB SPL narrowband noise for 4 hours under identical sedation procedures. These monkeys were also male and in the same age range as Monkeys G and L (9–11 years old). Both ears of each animal in this cohort were analyzed for histology.
We were interested in the correlations between the anatomical measures and the behavioral measures. However, since monkey L was exposed to a different noise than the anatomy cohort and monkey G, comparisons with the data from the anatomy cohort are inappropriate. Table 1 documents the cross correlations between monkey G’s behavioral changes as a function of tone frequency and the histological changes observed in the IHC, OHC, and ribbon synapse counts as a function of cochlear frequency place. Monkey G’s tone detection thresholds in quiet and in SN were significantly correlated with each of the histological measures (Table 1). The IHC, OHC and synapse counts were also correlated with Monkey G’s reduction in masking release.
Table 1.
Cross-correlations between behavioral test results (change in measure vs. frequency) in monkey G and cochlear pathology vs. frequency from Valero et al. (2017)
| Monkey G | Audiogram | Threshold Shift Rate |
Change in Threshold (threshold in SN – threshold in SAM Noise) |
|---|---|---|---|
| % IHC Missing | 0.70* | 0.74* | 0.82 |
| % OHC Missing | 0.81** | 0.67* | 0.84 |
| % Synapses Missing | 0.93** | −0.94** | 0.61 |
p<0.05,
p<0.01;
Monkeys from Valero et al. (2017) were exposed to 146 dB SPL noise as part of a series of exposures that varied from animal to animal, with only one animal exposed to a single 146 dB SPL exposure as the current Monkey G.
4. DISCUSSION
4.1 Comparisons with previous studies of noise exposure in animals
Many previous studies of noise exposure in animal models are limited in their applicability to humans due to lack of behavioral measures or because of dissimilarity in auditory system neuroanatomy or physiology. Patients seen in the clinic for NIHL do not often complain of problems with detection of low-level tones in quiet, but they have difficulty with suprathreshold processing, such as understanding conversational speech in noisy environments. The macaque model of NIHL allows studies of more perceptually complex listening tasks in a model species phylogenetically very similar to humans. In addition, significantly higher noise levels were required to induce a permanent threshold shift in these macaques than were needed to create similar hearing losses in other animals (see Valero et al. 2017). Those data guided the choice of sound levels used in these studies.
There is a large literature on the behavioral effects of noise exposure in many animal models, including chinchilla (e.g. Eldredge et al. 1973; Clark et al. 1974; Slepecky et al. 1982), cats (e.g., Miller et al, 1963; Elliott and McGee 1965; Dolan et al. 1975), mice (Heffner et al. 2008), guinea pigs (Davis et al. 1953; Reudi 1954), rats (Lobarinas et al. 2017) and macaques (e.g., Hawkins et al. 1976; Moody et al. 1978; Stebbins et al. 1979). In most of these models, hearing loss was measured via ABR thresholds and detection of tones or noise presented in quiet. Similar to these studies, we showed 20 – 40 dB SPL elevations in ABR thresholds following noise exposure, with the greatest changes at frequencies higher than the exposure frequencies (Figure 2A–B). Changes in the behavioral audiogram were related to changes in the ABR thresholds, but were not identical. It is evident that although ABR thresholds can be used to assess one dimension of hearing sensitivity, they do not always accurately estimate absolute behavioral thresholds (Heffner et al. 2008). Monkey G (Figure 4A) and Monkey L (Figure 4B) showed similar audiometric changes to those shown previously for macaques exposed to a lower level of noise for a longer duration (octave-band noise, 8 hr/day for up to 20 days, 120 dB SPL; Moody et al. 1978). While the exact configuration and degree of hearing loss is different between animal models and exposure stimuli, the changes in Monkey G and Monkey L’s audiograms were similar to those found in other studies in showing a 40–60 dB hearing loss at frequencies above that of the noise exposure stimulus.
One surprising difference between our subjects on other animals is that reaction time slopes did not get significantly steeper following overexposure. Other studies have shown a relatively consistent effect of noise in increasing reaction times (e.g., Moody 1973). In the human literature, many people with hearing loss experience recruitment, an abnormally rapid growth of loudness with increasing level. Given that reaction time has been shown to be a good index of loudness perception (e.g., Stebbins 1966), animals with loudness recruitment following noise exposure should have a steeper slope of the RT versus level function. This was not the case for our subjects. It is possible that an effect would be seen with a greater sample size or a different measure of reaction time.
Because we currently only have data from one subject under each exposure condition, we are limited in our ability to draw conclusions about the variability in susceptibility between subjects. Additionally, although 141 and 146 dB SPL may sound similar, the OSHA guidelines for occupational noise exposure suggest that the 5 dB increase in noise level is equivalent to doubling the noise dose. The differences between the audiograms of monkeys G and L most likely relate to the differences in exposure level. Variability between subjects exposed to identical noise levels has been studied in guinea pigs (Cody and Robertson 1983; Wang et al. 2002) and mice (Wang et al. 2002). There is significant variability even between highly inbred strains of animals exposed to identical noise stimuli in terms of their histological, physiological, and behavioral changes (e.g., Wang et al. 2002; see Valero et al. 2017 to see histological variability in macaques exposed to the same 146 dB SPL narrowband noise).
The relationship between age-related hearing loss and noise exposure also merits some discussion. Our pre-exposure thresholds match the normative young animal data in the literature (Fowler et al. 2002; 2010). The pre-exposure ABR thresholds were a little higher than those reported by Engle et al. (2013); however, this difference can be explained by the shorter stimulus durations used in our study relative to the 10-ms tone bursts used in ABR measurements by Engle et all. (2013). Previous studies of geriatric macaques (>21.5 yrs) showed abnormalities in ABR amplitude and latency (Ng et al. 2015), with ABR thresholds becoming poorer (Fowler et al. 2010). Given that our subjects were both substantially younger than these populations, it is unlikely that age related hearing loss was a significant contributor to the changes in threshold we measured.
The audiogram is unlikely to be an accurate predictor of hearing ability in all types of noisy backgrounds. Not surprisingly, the changes in audiometric threshold were correlated strongly with the change in threshold shift rate following noise exposure for monkey G, but not for monkey L (Figure 8A). Frequencies that had large changes in audiometric thresholds also had greater deviations from pre-exposure threshold shift rate. The audiometric changes were not correlated with reduction in masking release following noise exposure for both monkeys (Figure 8B).
Measures other than the detection of tones in quiet have been studied in far fewer animal models than the audiogram. A gap-detection task has been used to measure temporal processing in noise-exposed chinchillas (Giraudi-Perry et al. 1982). Gap thresholds were identical to those for normal hearing when threshold shifts were ≤ 15 dB, were normal when sensation level (SL) was equated at threshold shifts of ≤ 30 dB, and were longer than normal even at equal SL for threshold shifts ≥40 dB. Our monkeys show significant PTS ≥ 40 dB (Figure 4), suggesting they could also show increased gap thresholds.
4.2 Comparisons with data from humans with hearing loss
In humans, noise exposure often results in a high frequency hearing loss with a “noise-notch” between 3 and 6 kHz (Davis et al. 1950). While occupational noise exposure is regulated, a person’s lifetime noise exposure is extremely difficult to quantify. The effects of noise exposure may be combined with other effects from smoking, impaired vascular flow, and genetic predisposition (Yang et al. 2016). These factors make identifying noise exposure as the sole contributor to a person’s hearing loss impossible.
The effects of noise exposure on macaques were similar to the effects seen in humans. The high-level noise exposure caused a permanent elevation in audiometric thresholds at or just above the peak noise exposure frequencies, an effect that is regularly reported in the human clinical population. Davis et al. (1950) showed the effect of narrowband noise exposure might be variable across human subjects. Monkey G and Monkey L’s “noise notch” fell at 2–4 kHz (Fig. 3). The high levels of exposure noise that caused such hearing loss are consistent with results in humans showing that even 130 dB SPL exposure stimuli mainly caused temporary threshold shifts that recovered (Davis et al. 1950; however, note that three subjects showed PTS at frequencies far removed from the exposure frequency). In our companion anatomical report, we discuss lack of effect of PTS in the ABR even for 140 dB SPL exposures (Valero et al. 2017).
For humans with hearing loss, the audiogram’s relationship to other metrics of hearing depends largely on the type of hearing loss and the speech test used (Smoorenburg 1992). While the pure tone average is typically in agreement with speech recognition threshold (SRT) in quiet, the SRT in noise was correlated with audiometric thresholds above 1 kHz, specifically the thresholds at 2 and 4 kHz (Smoorenburg 1992). Plomp (1978) found that the SRT in noise was affected not only by the lack of audibility of the signal, but also by a distortion factor (D) that represented suprathreshold processing deficits, while Lee and Humes (1993) suggested that this could potentially be accounted for by reduced audibility. The debate between the effects of audibility and the effects of suprathreshold processing deficits has become of greater interest since it has been postulated that synaptopathy may have an effect on speech processing in noisy backgrounds without affecting the audiogram (Kujawa & Liberman, 2015).
Threshold shifts with increasing noise level like those seen in Monkey G and L pre-exposure (Figure 5A, Figure 6) or other normal hearing macaques (Dylla et al. 2013) have been described in humans (Hawkins and Stevens 1950). Such thresholds shifts in noise-exposed macaques were also affected as in humans with sensorineural hearing loss. Threshold shift rate was less than that in normal hearing subjects when the masker had a lower frequency than the signal (Murnane and Turner 1991; Stelmachowicz 1987). These studies also identified a relationship between slope of the masking function for a signal and the threshold for the same frequency signal: the greater the slope, the lower the threshold for tones in quiet. We found a similar relationship in noise-exposed macaques (Figure 6, Figure 8A).
For normal hearing animals and humans, tone detection in noise can be improved by amplitude modulating the noise (e.g., Bacon et al., 1997), presumably by the subjects listening in the dips of the noise, when the SNR is higher than for SN. This improvement in threshold is best for low frequency modulations as they provide the greatest duration of improved SNR. Prior to noise exposure, our subjects had a10–20 dB masking release. This masking release was reduced for some signal frequencies after exposure (Figure 7). However, the reduction in masking release was not related to the audiometric changes (Figure 8B).
4.3 Correlations between physiology, behavior, and histopathology
The benefit of using the nonhuman primate model of NIHL is that we can study behavior, physiology, and anatomy in the same subjects before and after controlled noise exposure. This allows for correlations and comparisons between each measure. Better understanding of the cochlear pathology that underlies difficulty in certain listening tasks would lead to more specific diagnostic evaluations and more targeted treatments. Engle et al. (2013) investigated cochlear changes with age related hearing loss and showed that changes in cochlear histology were related to the age of the animal, but because of the restricted age range of our subjects, this age effect is likely limited in our study.
Using behavioral and physiological measures to estimate cochlear pathology is important for diagnosis of human disorders affecting the cochlea because it is impossible to visualize the problem directly. OHCs are implicated in frequency tuning and creating the nonlinearities of the cochlea (Cody and Johnstone 1981; Robles and Ruggero 2001). Animal studies show that dysfunction of OHCs can account for up to 50 dB of hearing loss (Ryan and Dallos 1975, Dallos et al. 2008, Stebbins et al. 1979). Damage to OHCs is seen in reduced or absent DPOAEs, broadened auditory filters, and permanent threshold shifts. Our subjects showed absent DPOAEs following noise exposure (Figure 2C–F) and PTS in the ABR and in the behavioral audiogram. Preliminary data behavioral data from Monkey G and L also suggest broadened auditory filters in the regions of hearing loss (Burton and Ramachandran, unpublished observations).
Ribbon synapses are a specialized type of synapse responsible for the fast transmission of information from the inner hair cells to the auditory nerve fibers. Recent studies in animal models have shown that these synapses, not OHCs as previously thought, are the element most vulnerable to damage from noise (Kujawa and Liberman 2006, 2009, 2015). Our cohort of macaques that were part of the anatomy study showed significant synaptopathy in a frequency dependent manner (Valero et al. 2017). The studies of synaptopathy are currently limited to small mammals and have typically not involved behavioral tasks (however, see Lobarinas et al. 2017). The predicted consequence of synaptopathy is a decline in temporal processing and total auditory nerve response at suprathreshold SPLs, and a minimal effect near threshold (Kujawa and Liberman 2009).
While the effects of noise exposure on the cochlea are important, it is very likely that the behavioral deficits are also related to the well-described changes in more central structures. Noise exposure may cause changes in the spiral ganglion numbers, with neuropathy increasing with time after exposure (e.g., Kujawa and Liberman 2009). These changes are probably correlated with fiber degeneration in the cochlear nucleus (e.g., Morest and Bohne 1983; Morest et al. 1998) and transneuronally in the superior olive and the inferior colliculus (e.g., Morest et al. 1979; 1998). There is a change in the balance of excitation and inhibition in more central structures as a result of the noise exposure, with up-regulation of excitation and down-regulation of inhibition (e.g., Kotak et al. 2005; Sarro et al. 2008). Further, neurophysiological representations of sound are modified more centrally (e.g., Schwaber et al. 1993; Wang et al. 2002; Race et al. 2017), which could lead to altered evidence accumulation and altered percepts after noise exposure and hearing loss.
Having the ability to determine the contribution of each of the major structures in the cochlea and the brain to various auditory tasks may be useful for more accurately diagnosing a patient’s hearing loss and devising specific treatments. It is clear that clinical audiology and research used to assess auditory function in animal models or humans will have to reach beyond the traditional audiogram for comprehensive assessments of auditory function.
Highlights.
Monkeys exposed to high SPL narrowband noise showed permanent ABR and behavioral threshold shifts.
Post-exposure, masked threshold shifts increased more slowly with masker level re: pre-exposure.
Post-exposure, masking release by SAM noise relative to steady noise (SN) was reduced re: pre-exposure.
Noise exposure induced behavioral changes predicted well cochlear histological changes as a function of frequency.
Acknowledgments
The authors would like to thank Mary Feurtado for help with procedures involving anesthesia, Drs. T. A. Hackett, M. C. Liberman, and M. D. Valero for discussions, Maggie Dylla, Chase Mackey and Jessica Fuller for help with data collection, and Dr. Stephen Lisberger and Scott Ruffner for use of the FigureComposer software to make figures. This study was partly supported by the Vanderbilt Hobbs Discovery Grant to RR. JAB and SNH were partially supported by NIH NIDCD T35 DC 008763 to Linda Hood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon SP, Lee J, Peterson DN, Rainey D. Masking by modulated and unmodulated noise: Effects of bandwidth, modulation rate, signal frequency, and masker level. J Acoust Soc Am. 1997;101(3):1600–1610. doi: 10.1121/1.418175. [DOI] [PubMed] [Google Scholar]
- Behar I, Cronholm JN, Loeb M. Auditory sensitivity of the rhesus monkey. J Comp Phys Psych. 1965;59(3):426–428. doi: 10.1037/h0022047. [DOI] [PubMed] [Google Scholar]
- Bohlen P, Dylla M, Timms C, Ramachandran R. Detection of modulated tones in modulated noise by non-human primates. J Assoc Res Otolaryngol. 2014;15(5):801–821. doi: 10.1007/s10162-014-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WW, Clark CS, Moody DB, Stebbins WC. Noise-induced hearing loss in the chinchilla, as determined by a positive-reinforcement technique. J Acoust Soc Am. 1974;56(4):1202–9. doi: 10.1121/1.1903409. [DOI] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Acoustic trauma: single neuron basis for the “half-octave shift”. J Acoust Soc Am. 1981;70(3):707–711. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- Cody A, Robertson D. Variability of noise-induced damage in the guinea pig cochlea: Electrophysiological and morphological correlates after strictly controlled exposures. Hear Res. 1983;9(1):55–70. doi: 10.1016/0378-5955(83)90134-x. [DOI] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-Based Outer Hair Cell Motility Is Necessary for Mammalian Cochlear Amplification. Neuron. 2008;58(3):333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Hirsh SK. A Slow Brain Stem Response for Low-Frequency Audiometry. Int J Audiol. 1979;18(6):445–461. doi: 10.3109/00206097909072636. [DOI] [PubMed] [Google Scholar]
- Davis H, Morgan CT, Hawkins JE, Galambos R, Smith FW. Temporary Deafness Following Exposure To Loud Tones And Noise. Acta Otolaryngol. 1950;88:1–56. [PubMed] [Google Scholar]
- Davis, H. & Associates. Acoustic trauma in the guinea pig. J Acoust Soc Am. 1953;56(6):1180–1189. [Google Scholar]
- Dylla M, Hrnicek A, Rice C, Ramachandran R. Detection of tones and their modification by noise in nonhuman primates. J Assoc Res Otolaryngol. 2013;14(4):547–560. doi: 10.1007/s10162-013-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie RA, Humes LE. Commentary on the regulatory implications of noise-induced cochlear neuropathy. Int J Audiol. 2017;56(sup1):74–78. doi: 10.1080/14992027.2016.1255359. [DOI] [PubMed] [Google Scholar]
- Dolan TR, Ades HW, Bredberg G, Neff WD. Inner ear damage and hearing loss after exposure to tones of high intensity. Acta Otolaryngol. 1975;80(5–6):343–52. doi: 10.3109/00016487509121336. [DOI] [PubMed] [Google Scholar]
- Eldredge DH, Mills JH, Bohne BA. Anatomical, behavioral, and electrophysiological observations on chinchillas after long exposures to noise. Adv Otorhinolaryngol. 1973;20:64–81. doi: 10.1159/000393089. [DOI] [PubMed] [Google Scholar]
- Engle JR, Tinling S, Recanzone GH. Age-Related Hearing Loss in Rhesus Monkeys Is Correlated with Cochlear Histopathologies. PLoS ONE. 2013;8(2):e55092. doi: 10.1371/journal.pone.0055092. http://doi.org/10.1371/journal.pone.0055092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CG, Torre P, Kemnitz JW., 3rd Effects of caloric restriction and aging on the auditory function of rhesus monkeys (Macaca mulatta): The University of Wisconsin Study. Hear Res. 2002;169(1–2):24–35. doi: 10.1016/s0378-5955(02)00335-0. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Chiasson KB, Leslie TH, Thomas D, Beasley TM, Kemnitz JW. Auditory function in rhesus monkeys: Effects of aging and caloric restriction in the Wisconsin monkeys five years later. Hear Res. 2010;261(1):75–81. doi: 10.1016/j.heares.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(3):577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DJ, Young ED, Costalupes JA. Similarity of dynamic range adjustment in auditory nerve and cochlear nuclei. J Neurophysiol. 1985;53:940–958. doi: 10.1152/jn.1985.53.4.940. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. J Acoust Soc Am. 1982;72(5):1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, Beauchaine KL, Kaminski JR, Peters J, Jesteadt W. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: distortion product responses. J Acoust Soc Am. 1993;93(4 Pt 1):2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; 1966. [Google Scholar]
- Harding GW, Bohne BA. Noise-induced hair-cell loss and total exposure energy: analysis of a large data set. J Acoust Soc Am. 2004;115(5 Pt 1):2207–2220. doi: 10.1121/1.1689961. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Stevens SS. The masking of pure tones and of speech by white noise. J Acoust Soc Am. 1950;22(1):6–13. [Google Scholar]
- Hawkins JE, Johnsson LG, Stebbins WC, Moody DB, Coombs SL. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol. 1976;81(3–4):337–343. doi: 10.3109/00016487609119971. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Koay G, Heffner RS. Comparison of behavioral and auditory brainstem response measures of threshold shift in rats exposed to loud sound. The J Acoust Soc Am. 2008;124(2):1093–1104. doi: 10.1121/1.2949518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Levy JK, Jerger J. Comparative Toxicity of Netilmicin and Gentamicin in Squirrel Monkeys (Saimiri sciureus) J Infec Dis. 1978;137(4):476–480. doi: 10.1093/infdis/137.4.476. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- Kochkin S. MarkeTrak VII. The Hearing Journal. 2007;60(4):24–51. [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25(15):3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryter KD, Garinther GR. Auditory effects of acoustic impulses from firearms. Acta Otolaryngol Suppl. 1965;211:1–22. [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of Age-Related Hearing Loss by Early Noise Exposure: Evidence of a Misspent Youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330(Pt B):191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Clavier OH. Effects of noise on speech recognition: Challenges for communication by service members. Hear Res. 2017;349:76–89. doi: 10.1016/j.heares.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lee LW, Humes LE. Evaluating a speech reception threshold model for hearing impaired listeners. J. Acoust Soc Am. 1993;93(5):2879–2885. doi: 10.1121/1.405807. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12(5):605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Spankovich C, Le Prell CG. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res. 2017;349:155–163. doi: 10.1016/j.heares.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. Mahwah, NJ: Lawrence Erlbaum; 2005. [Google Scholar]
- Miller JM, Watson CS, Covell WP. Deafening Effects of Noise on the Cat. J Occ Env Med. 1963;5(11):555. [Google Scholar]
- Miller RL, Schilling JR, Franck KR, Young ED. Effects of acoustic trauma on the representation of the vowel /ε/ in cat auditory nerve fibers. J Acoust Soc Am. 1997;101(6):3602–3616. doi: 10.1121/1.418321. [DOI] [PubMed] [Google Scholar]
- Moody DB. Otophysiology. Vol. 20. Karger Publishers; 1973. Behavioral Studies of Noise-Induced Hearing Loss in Primates: Loudness Recruitment1; pp. 82–101. [DOI] [PubMed] [Google Scholar]
- Moody DB, Stebbins WC, Hawkins JE, Johnsson LG. Hearing loss and cochlear pathology in the monkey (Macaca) following exposure to high levels of noise. Arch Otorhinolaryngol. 1978;220(1–2):47–72. doi: 10.1007/BF00456301. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. A review of the perceptual effects of hearing loss for frequencies above 3 kHz. Int J Audiol. 2016;55(12):707–714. doi: 10.1080/14992027.2016.1204565. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Huss M, Vickers DA, Glasberg BR, Alcántara J. A Test for the Diagnosis of Dead Regions in the Cochlea. Br J Audiol. 2000;34(4):205–224. doi: 10.3109/03005364000000131. [DOI] [PubMed] [Google Scholar]
- Morest DK, Bohne BA. Noise-induced degeneration in the brain and representation of inner and outer hair cells. Hear Res. 1983;9(2):145–151. doi: 10.1016/0378-5955(83)90024-2. [DOI] [PubMed] [Google Scholar]
- Morest DK, Ard MD, Yurgeluntodd D. Degeneration in the central auditory pathways after acoustic deprivation or over-stimulation in the cat. Anat Rec. 1979;193(3):750. [Google Scholar]
- Morest DK, Kim J, Potashner SJ, Bohne BA. Long-term degeneration in the cochlear nerve and cochlear nucleus of the adult chinchilla following acoustic overstimulation. Mic Res Tec. 1998;41(3):205–216. doi: 10.1002/(SICI)1097-0029(19980501)41:3<205::AID-JEMT4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Murnane O, Turner CW. Growth of masking in sensorineural hearing loss. Audiology. 1991;30:275–285. doi: 10.3109/00206099109072891. [DOI] [PubMed] [Google Scholar]
- Ng C, Navarro X, Engle JR, Recanzone GH. Age-related changes of auditory brainstem responses in nonhuman primates. J Neurophysiol. 2015;114(1):455–467. doi: 10.1152/jn.00663.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091(1):89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Hienz R, Miller J. Reaction time procedure for measurement of hearing. II. Threshold functions. J Acoust Soc Am. 1975;57(2):431–436. doi: 10.1121/1.380466. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Laycock J, Flammino F, Lonsbury-Martin B, Martin G. Pure tone thresholds for the rhesus monkey. Hear Res. 1978;1(1):43–47. doi: 10.1016/0378-5955(78)90008-4. [DOI] [PubMed] [Google Scholar]
- Plomp R. Auditory handicap of hearing impairment and the limited benefit of hearing aids. J Acoust Soc Am. 1978;63(2):533–549. doi: 10.1121/1.381753. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin B, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89(2027) doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Race N, Lai J, Shi R, Bartlett EL. Differences in post-injury auditory system pathophysiology after mild blast and non-blast acute acoustic trauma. J Neurophysiol. 2017 doi: 10.1152/jn.00710.2016. jn-00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüedi L. Different Types and Degrees of Acoustic Trauma by Experimental Exposure of the Human and Animal Ear to Pure Tones and Noise. Ann Otol, Rhinol Laryngol. 1954;63(3):702–726. doi: 10.1177/000348945406300308. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81(3):1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Dallos P. Effect of absence of cochlear outer hair cells on behavioral auditory threshold. Nature. 1975;253(5486):44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Kujawa SG, Hammill T, Le Prell C, Kil J. Temporary and Permanent Noise-induced Threshold Shifts: A Review of Basic and Clinical Observations. Otol. Neurotol. 2016;37(8):e271–275. doi: 10.1097/MAO.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GAB AA receptors in the auditory cortex. Cerebral Cortex. 2008;18(12):2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am. 1985;78(3):833–860. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Cohen YE, Szymko YM. The structural and functional consequences of acoustic injury in the cochlea and peripheral auditory system: a five year update. J Acoust Soc Am. 1991;90(1):136–146. doi: 10.1121/1.401307. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Otol Neurotol. 1993;14(3):252–258. [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline. J Neurosci. 2013;33(34):13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky N, Hamernik R, Henderson D, Coling D. Correlation of audiometric data with changes in cochlear hair cell stereocilia resulting from impulse noise trauma. Acta oto-laryngol. 1982;93(1–6):329–340. doi: 10.3109/00016488209130890. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF. Speech reception in quiet and in noisy conditions by individuals with noise-induced hearing loss in relation to their tone audiogram. J Acoust Soc Am. 1992;91(1):421–437. doi: 10.1121/1.402729. [DOI] [PubMed] [Google Scholar]
- Soli SD. Some thoughts on communication handicap and hearing impairment. Int J Audiol. 2008;47(6):285–286. doi: 10.1080/14992020802060656. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Keller CH, Marrocco RT, Takahashi TT. Head-related transfer functions of the Rhesus monkey. Hear Res. 2000;144(1):73–88. doi: 10.1016/s0378-5955(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Stebbins WC. Auditory reaction time and the derivation of equal loudness contours for the monkey. J Exp Anal Behav. 1966;9(2):135–142. doi: 10.1901/jeab.1966.9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC, Green S, Miller FL. Auditory sensitivity of the monkey. Science. 1966;153(3744):1646–1647. doi: 10.1126/science.153.3744.1646-a. [DOI] [PubMed] [Google Scholar]
- Stebbins WC, Hawkins JE, Johnsson LG, Moody DB. Hearing thresholds with outer and inner hair cell loss. Am J Otolaryngol. 1979;1(1):15–27. doi: 10.1016/s0196-0709(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Stelmachowicz PG, Lewis DE, Larson LL, Jesteadt W. Growth of masking as a measure of response growth in hearing-impaired listeners. J Acoust Soc Am. 1987;81(6):1881–1887. doi: 10.1121/1.394752. [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta) Hear Res. 2017;353:213–223. doi: 10.1016/j.heares.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168(1):238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3(3):248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WD. The concept of susceptibility to hearing loss. J Occ Env Med. 1965;7(12):595–607. doi: 10.1097/00043764-196512000-00001. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Mcardle R, Betancourt MB, Herring K, Lipton T, Chisolm TH. Word-Recognition Performance in Interrupted Noise by Young Listeners with Normal Hearing and Older Listeners with Hearing Loss. J Am Acad Audiol. 2010;21(2):90–109. doi: 10.3766/jaaa.21.2.4. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xu X, Jiao J, Zheng Y, He L, Yu S, Zhang Z. Genetic variation in EYA4 on the risk of noise-induced hearing loss in Chinese steelworks firm sample. Occup Environ Med. 2016;73(12):823–828. doi: 10.1136/oemed-2016-103613. [DOI] [PubMed] [Google Scholar]