Abstract
The urgent need for more effective analgesic treatment options has prompted a re-evaluation of the behavioral tests used to assess pain in pre-clinical research, with an emphasis on inclusion of more voluntary, un-evoked behavioral assessments of pain. In order to validate voluntary gait analysis and a voluntary mechanical conflict-avoidance assay, we tested mouse models of neuropathy (spared nerve injury) and inflammation (complete Freund’s adjuvant) alongside reflexive measures of mechanical and thermal hypersensitivity. To establish whether the observed changes in behavioral responses were pain-related, known analgesics (buprenorphine, gabapentin, carprofen) were also administered. Spared nerve injury persistently altered several gait indices, whereas complete Freund’s adjuvant caused only transient changes. Furthermore, known analgesics could not reverse these gait changes, despite demonstrating their previously established efficacy in reflexive measures of mechanical and thermal hypersensitivity. In contrast, the mechanical conflict-avoidance assay demonstrated aversion in mice with neuropathy and inflammation-induced hypersensitivity, which could both be reversed by analgesics. We conclude that voluntary gait changes in rodent neuropathic and inflammatory pain models are not necessarily indicative of pain-related adaptations. On the other hand, mechanical conflict-avoidance represents a valid operant assay for quantifying pain-related behaviors in mice that can be reversed by known analgesics.
Keywords: Gait, Conflict-Avoidance, Inflammatory Pain, Neuropathic Pain, Catwalk
1. INTRODUCTION
Chronic pain in humans is a complex sensory and affective experience. A shift in pain-detection threshold and hypersensitivity to thermal/mechanical stimuli are key components of this experience. Historically, these aspects of stimulus-evoked assessment of pain-related behaviors - such as Hargreaves’ and von Frey filament-based assessment of thermal and mechanical sensitivity (Hargreaves, Dubner et al. 1988, Chaplan, Bach et al. 1994), tail-flick, paw flinching, licking/biting and guarding assessments (Cargill, Steinman et al. 1985, Seltzer, Dubner et al. 1990, Wheeler-Aceto, Porreca et al. 1990) - have primarily been detected and quantified in preclinical animal models of painful pathologies. However, the ongoing failure to develop efficacious, new-generation analgesic drugs has led to a re-evaluation of the field’s reliance on these evoked and/or reflexive assessments of pain-related behaviors in preclinical animal models (Mao 2009, Berge 2011, Percie du Sert and Rice 2014, Clark 2016).
In recent years in the area of pain research, newer assays of pain in rodent models, such as wheel running, burrowing, gait, ultrasonic vocalization, conditioned place preference etc. have been developed (Vrinten and Hamers 2003, Li, Rhodes et al. 2004, Williams, Riskin et al. 2008, King, Vera-Portocarrero et al. 2009, Truin, van Kleef et al. 2009, Kurejova, Nattenmuller et al. 2010, Mogil, Graham et al. 2010, Andrews, Legg et al. 2012, Cobos, Ghasemlou et al. 2012, Huehnchen, Boehmerle et al. 2013, Parvathy and Masocha 2013, Ruan, Patel et al. 2013, Chiang, Sheu et al. 2014, Muramatsu, Sasho et al. 2014, Rutten, Robens et al. 2014, Sahbaie, Sun et al. 2014, Gould, Doods et al. 2016, Pitzer, Kuner et al. 2016, Wodarski, Delaney et al. 2016, Sheahan, Siuda et al. 2017, Sugiyama, Kang et al. 2017) in an effort to improve the translational potential of preclinical findings (Barrot 2012, Tappe-Theodor and Kuner 2014, Clark 2016). However, most of these newer and operant-based assays haven’t shown universality across different pain types, such as inflammatory, neuropathic, and specific disease-related. In addition, while extensively tested, the added complexity of drug conditioning/addictive behavioral components, such as in conditioned place preference (Porreca and Navratilova 2017) has led to questions on what facets of pain-related behavioral alterations are being measured in rodents. Therefore, it is vital to establish whether these newer measures of pain are capable of detecting quantifiable behavioral changes and/or adaptations that are unequivocally pain-related, as well as develop and validate newer methods to obtain such outcomes. One way to do this is to test an assay with well-established analgesic compounds, at appropriate doses, as positive controls.
Our study evaluated voluntary changes in gait and mechanical conflict-avoidance in a representative rodent model of neuropathic pain: spared nerve injury – SNI (Decosterd and Woolf 2000), and inflammatory pain: complete Freund’s Adjuvant – CFA (Larson, Brown et al. 1986). While we observed sustained changes in several voluntary gait indices in SNI mice, the effects of CFA administration on gait patterns were much less drastic and more transient. Furthermore, administration of the opioid buprenorphine (Christoph, Kogel et al. 2005), gabapentin (Decosterd, Allchorne et al. 2004) or the NSAID carprofen (Adamson, Kendall et al. 2010, Smeester, Lee et al. 2017) did not alter SNI or CFA-induced gait changes, respectively. This was despite these drugs being administered at doses shown to be effective in the more classical, evoked assays of hypersensitivity (von Frey testing for SNI, and von Frey and Hargreaves for CFA). In contrast, the voluntary mechanical conflict-avoidance (MCA) test did show significant SNI- and CFA-induced aversion behaviors, which were reversed by the same sets of well-established analgesics. Our findings suggest that gait data and specific changes in paw imaging-based gait indices must be analyzed carefully in rodent models of painful pathologies. Also, any alterations in gait should be validated with proven analgesic drugs at recommended doses. On the other hand, the MCA test constitutes a promising operant assay for quantifying pain-related behaviors in mice with pathologies that could be potentially painful.
2. MATERIALS AND METHODS
2.1. Mice
All experiments and procedures were approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine, and in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of mice used and their suffering. 8–14 week-old mice were group-housed (5 per cage) and maintained on a 12:12 light/dark cycle (06:00 to 18:00 h) with access to food and water ad libitum. Age-matched male and female C57BL/6J mice were purchased from Jackson Labs. Based on our preliminary findings on no differences in the behavioral readouts in male versus female mice using the assays utilized in this study, mice of each sex were used in all the experiments performed.
2.2. Spared Nerve Injury (SNI)
The surgical procedure for the SNI-induced model of neuropathic pain was performed as described previously (Decosterd and Woolf 2000, Decosterd, Allchorne et al. 2002). Mice were anesthetized with 2% isoflurane and the left hind limb was clipped of fur and appropriately disinfected. A 10–15mm incision was made in the skin proximal to the knee, such that the biceps femoris muscle was accessible. Separation of the muscle allowed visualization of the trifurcation of the sciatic nerve. The common peroneal and tibial branches were ligated with 6–0 silk suture, and 1 mm of nerve was excised distal to the ligature, leaving the sural branch intact. Following wound closure and post-operative analgesia, mice were monitored for signs of distress and allowed to fully recover in a cage placed on a heating pad prior to return to the home cage. The sham operation procedure omitted the ligation and severing of the peroneal and tibial branches of the sciatic nerve, but was otherwise identical. Behavioral testing of these animals began on post-operative day 5, and wound clips were removed from the healed incision after testing was complete on post-operative day 7. Experimenters were blinded to mouse sex and sham/surgery conditions during experimental data collection/recording and subsequent analysis.
2.3. Complete Freund’s Adjuvant (CFA) Injection
Intraplantar (i.pl.) injection of Complete Freund’s Adjuvant was performed as described previously (Larson, Brown et al. 1986, Shutov, Warwick et al. 2016). Mice were restrained with the aid of a cloth held in one hand, such that the plantar surface of the hindpaw to be injected could be exposed and held in place while minimizing distress. 10μl of sterile saline (as a control), or 1 mg/ml CFA (10 μg; MilliporeSigma, St. Louis, MO) was injected into the plantar surface of the hindpaw with a 30-gauge needle coupled to a Hamilton syringe. Experimenters were blinded to mouse sex, details of injected agents, and injection laterality during experimental data collection/recording and subsequent analysis.
2.4. Digital Gait Analysis
Analysis of voluntary footfall and gait patterns in mice was performed using the Catwalk® XT 10.5 system (Noldus Information Technology, Leesburg VA) as described in several reports (Vrinten and Hamers 2003, Truin, van Kleef et al. 2009, Mogil, Graham et al. 2010, Huehnchen, Boehmerle et al. 2013, Parvathy and Masocha 2013, Ruan, Patel et al. 2013, Chiang, Sheu et al. 2014, Muramatsu, Sasho et al. 2014, Pitzer, Kuner et al. 2016). Briefly, green light is internally reflected into a 130 × 7 cm pane of glass, covered by an enclosed tunnel with a red backlight. A high-speed video camera looking upward beneath the glass pane detects green light only when paws contact the glass as the mouse freely traverses the tunnel (entering from one side of the camera’s field of view and exiting at the other end is considered a single ‘run’). The acquisition settings were as follows: each trial was composed of four runs per mouse, in which the camera gain was set to 15 dB, green light intensity threshold was 0.06 (arbitrary units). The criteria for a compliant run across the camera’s field of view were: run duration between 0.5 and 5 seconds, and the maximum variation in run speed was not more than 60%. These criteria were empirically determined to be optimal for exclusion of runs where mice crossed the field of view in an atypical fashion, such as extreme alteration in speed, stopping/restarting of walking, etc. Any runs that did not satisfy these criteria were deemed non-compliant and were excluded from analysis. The day prior to baseline testing, mice were habituated to the testing room and apparatus for 30 and 15 min, respectively. All mice used were able to complete four compliant runs, and no mice were excluded from analysis. All habituation and testing was always performed between 09:00 and 12:00 h. The gait indices that are reported in this manuscript are defined as follows:
Stand Duration: the average length of time that the paw is making contact with the glass, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Stand Index: a measure of the rate at which the paw loses contact with the glass, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Max Contact Area: the maximum area of a paw that contacts the glass, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Max Contact Max/Mean Intensity: the maximum/mean intensity of a paw signal at the point of maximum contact with the glass plate, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Print Length/Width/Area: the length/width/area of the complete print for that paw, i.e. the sum of all contact with the glass, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Max/Mean Intensity: the maximum/mean intensity of the complete paw print, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Swing Duration/Swing Speed: the average length of time of no contact of a particular paw with the glass, or the speed of the paw during swing phase (i.e. Swing Speed = Stride Length/Swing Duration), expressed as a ratio of the ipsilateral to contralateral hind limb values.
Stride Length: the distance between successive placements of the same paw, expressed as a ratio of the ipsilateral to contralateral hind limb values.
Step Cycle: the interval between two consecutive initial contacts of the same paw (i.e. Step Cycle = Stand Duration + Swing Duration), expressed as a ratio of the ipsilateral to contralateral hind limb values.
Run Speed: the average speed of the run, expressed in cm/s.
Run Max Variability: the maximum variation in average speed, expressed as a percentage.
In addition, a number of other gait parameters, such as paw angle, paw angle variability etc. were found not to be different in all animals, irrespective of the type and duration of induced pathological conditions, and therefore are not detailed here.
2.5. Mechanical Sensitivity with von Frey Filament Testing
Mechanical sensitivity of mouse hindpaws was assessed as described in numerous prior reports (Mickle, Shepherd et al. 2015). Animals were acclimated to the testing room and apparatus for 30 min each on the two days prior to the beginning of testing, as well as on each subsequent testing day. Mechanical sensitivity was assessed using 8 von Frey monofilaments of increasing thickness/strength (0.04 – 2 grams; North Coast Medical Inc., Gilroy, CA). Each mouse was housed in a Plexiglas box on a wire mesh platform for 30 minutes prior to testing. Beginning with the thinnest filament (0.04 grams); each filament was presented to each hindpaw a total of five times. The filament was allowed to flex once making contact with the plantar surface of the hindpaw, and held in place for 1 sec. The interval between presentation of the filament was ≥5 sec, or until behavior evoked by presentation of the filament had ceased. The number of withdrawal responses was recorded for each hindpaw across all 8 filaments (with a ≥5-min interval between testing of any one mouse with a particular filament). This filament-response plot was used to generate an area under the curve (AUC) value for each hindpaw, as detailed earlier (Mickle, Shepherd et al. 2015), and provides a total measure of paw withdrawal response sensitivity across the entire range of testing filaments.
2.6. Heat Sensitivity Testing with Hargreaves’ Procedure
Thermal sensitivity of mouse hindpaws was assessed as described previously (Loo, Shepherd et al. 2012, Mickle, Shepherd et al. 2015). Animals were acclimated to the testing room and apparatus for 30 min each on the two days prior to the beginning of testing, as well as on each subsequent days of testing. Each mouse was placed inside single-occupancy Plexiglas chambers placed on top of a raised pane of glass maintained at a constant thermo-neutral temperature (30°C). The thermal sensitivity of each hindpaw was measured by focusing a beam of light from below, onto the plantar surface of the hindpaw (IITC Life Science Inc., Woodland Hills, CA). The latency to paw withdrawal from the heat source was recorded as paw withdrawal latency (PWL), measured in seconds. The light intensity is calibrated to elicit typical PWL values of 10–14 seconds in naïve mice. The beam of light automatically shuts off at 20 seconds, to avoid any potential tissue injury due to overheating. If a hindpaw was not withdrawn within 20 seconds, a PWL value of 20 seconds was assigned. The mean of two PWL values, recorded ≥5 minutes apart for each hindpaw, are used for analysis.
2.7. Mechanical conflict-avoidance (MCA) assay
Voluntary mechanical conflict-avoidance was assessed using a variation of a recently-described Coy mechanical conflict-avoidance system approach (Harte, Meyers et al. 2016), which is itself a modification of the place escape/avoidance paradigm (LaBuda and Fuchs 2000, LaBuda and Fuchs 2000). The MCA apparatus consists of three chambers, the details of which are depicted in the supplementary Figure S1. If necessary, the authors can be contacted for further details regarding fabrication. Chamber-1 is a 12.5 cm3 white Plexiglas box lit from above with white light emitting diodes (LEDs). The LEDs provide an illuminance of approximately 4800 lux, serving as a mildly aversive stimulus in order to encourage mouse ‘escape’ into the unlit chambers. This white-lit chamber is separated from the rest of the unit by a movable barrier. Chamber-2 is the 27 cm-long mechanical conflict test chamber (unlit red Plexiglas) connecting chambers 1 and 3. Chamber-3 is a 12.5 cm3 translucent red Plexiglas box (unlit) at the opposite end to chamber-1. The floor of the central conflict test chamber is fitted with an adjustable 13×31 grid of blunt ~0.5 mm diameter probes, the effective height of which can be adjusted from 0 to 5 mm within the chamber. Mice were acclimated to the MCA unit for 5 min on the day of testing with the LEDs switched off, the barrier door open and the mechanical probe height set to zero. For testing, mice were placed into chamber 1 with the barrier to the rest of the unit in position. The LEDs were switched on after 10 sec, and the barrier was removed 20 sec thereafter. The behavior of each mouse, starting from introduction into chamber 1, until the mouse crossed the halfway point of chamber 2, was monitored by videotaping. There were two main reasons why we chose to use crossing the midpoint of chamber-2 as our experimental outcome measure. First, in our preliminary assessments 3 of 24 mice tested returned to chamber-1 before crossing the midpoint of chamber-2 during the 120 sec cutoff, and were eliminated from subsequent testing. All remaining mice completed crossing the chamber-2 after crossing its midpoint. Second, we observed that mice varied the speed at which they crossed the conflict chamber, presumably because of the aversive nature of the mechanical stimulus. Despite this, the values obtained when using entry into chamber 3 as the endpoint (i.e. completing the crossing of chamber-2) show very similar data to that shown in Fig. 6C (Supplementary Fig. S6B), suggesting that either measure is a useful readout of mechanical conflict-avoidance. Therefore, the duration between the barrier opening and the mouse crossing the midpoint of chamber-2 was quantified and expressed as ‘escape latency.’ After running all mice in a particular cohort at a probe height of 0 mm, the process was repeated with the probe height set to 2 mm, and finally 5 mm.
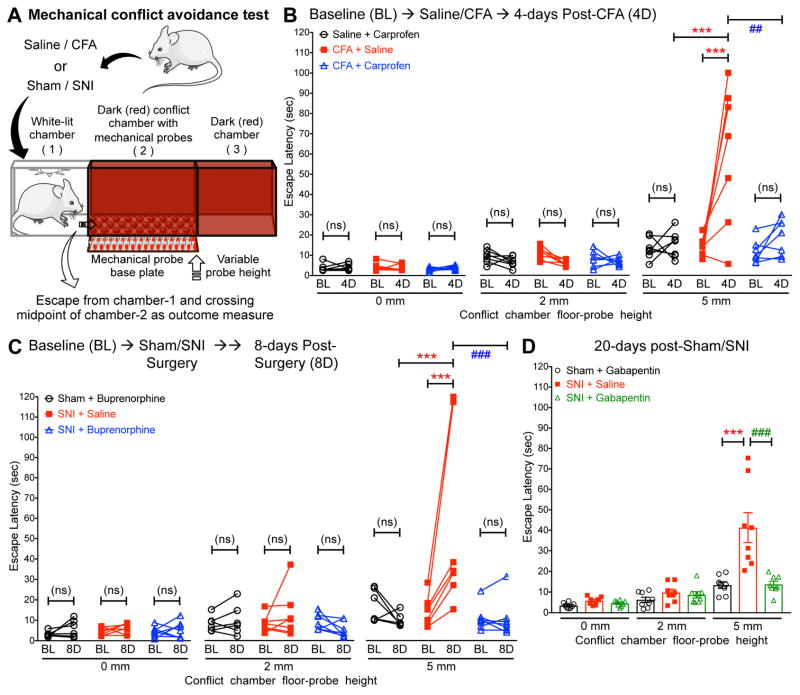
Figure 6.
Hindpaw inflammation and peripheral nerve injury augment avoidance in a mechanical conflict-avoidance test, which could be attenuated by known analgesic drugs. (A) Cartoon depicting the three-chamber mechanical conflict-avoidance test system, used as a non-reflexive, voluntary, pain-indicating behavioral assay in mice. (B) Hindpaw injection of CFA (10 μl of a 1 mg/ml suspension) into mouse hindpaws significantly increased the escape latency from the white-lit chamber compared to baseline, with a 5 mm probe height in the conflict chamber, tested at 4 days (4D) post-saline/CFA injection. Systemic administration (i.p.) of carprofen (10 mg/kg) significantly attenuated this CFA-induced increase in escape latency from the white-lit chamber, with a 5 mm probe height in the conflict chamber. Mice were tested 1.5 h post saline/carprofen administration on 4D. Data are plotted as mean ± SEM (with individual latencies in overlay) of escape latency from white-lit chamber with increasing height of mechanical probes in the conflict chamber (n=7 per group). (C) Compared to baseline, induction of spared nerve injury (SNI) significantly increased the escape latency from the white-lit chamber, with a 5 mm probe height in the conflict chamber, tested at 8 days (8D) post-sham/SNI surgery. Systemic administration (i.p.) of buprenorphine (25 μg/kg) significantly attenuated the increase in escape latency from the white-lit chamber, with a 5 mm probe height in the conflict chamber. Mice were tested 1.5 h post saline/ buprenorphine administration on 8D. Data are plotted in the same format as panel B (Sham+Buprenorphine n=6; SNI+Saline/SNI+Buprenorphine n=7/group). (D) In mice tested only at 20 days post-sham/SNI surgery, SNI induced an increase in the escape latency from the white-lit chamber, with a 5 mm probe height in the conflict chamber, an increase which could be reversed by systemic administration (i.p.) of gabapentin (30 mg/kg). Mice were tested 1.5 h post saline/gabapentin administration. Data are plotted in the same format as panels B and C (n=8 per group). In panels B–D, *** denotes p<0.001, ## denotes p<0.01, and ### denotes p<0.001 for the indicated comparison groups (two-way ANOVA with Bonferroni’s post-hoc correction).
2.8. Drugs
Buprenorphine hydrochloride (Tocris Bioscience, Bristol, UK) was administered to sham and SNI surgery animals intraperitoneally (i.p.) at 25 μg/kg starting 1 hour before behavioral testing on post-operative days 8 and 11. Gabapentin (MilliporeSigma, St. Louis, MO) was administered to sham and SNI surgery animals at 30 mg/kg i.p. 1 hour prior to testing on post-operative days 11 and 20, and carprofen (Zoetis, Inc., Kalamazoo, MI) was administered Saline and CFA-injected animals at 5 mg/kg i.p., starting 1 hour prior to testing on days 2, 4 and 5 following saline/CFA injection. Drugs were dissolved in sterile saline to achieve final concentration. Control animals received equivalent volume of sterile saline administration (i.p.) as vehicle groups. These effective drug doses are derived from studies showing the effective dose of these drugs that attenuate pain-related behaviors in rodents with specific pain pathologies (Decosterd, Allchorne et al. 2004, Christoph, Kogel et al. 2005, Adamson, Kendall et al. 2010, Smeester, Lee et al. 2017).
2.9. Statistical Analysis
Data are presented as mean ± SEM. Two-way ANOVA with Bonferroni’s multiple comparisons test was performed. P<0.05 for each comparison group was considered statistically significant. All analysis was performed using GraphPad Prism 7.0 (GraphPad Software, Inc.).
3. RESULTS
3.1. Sustained alterations in gait indices in mice with nerve injury/neuropathy
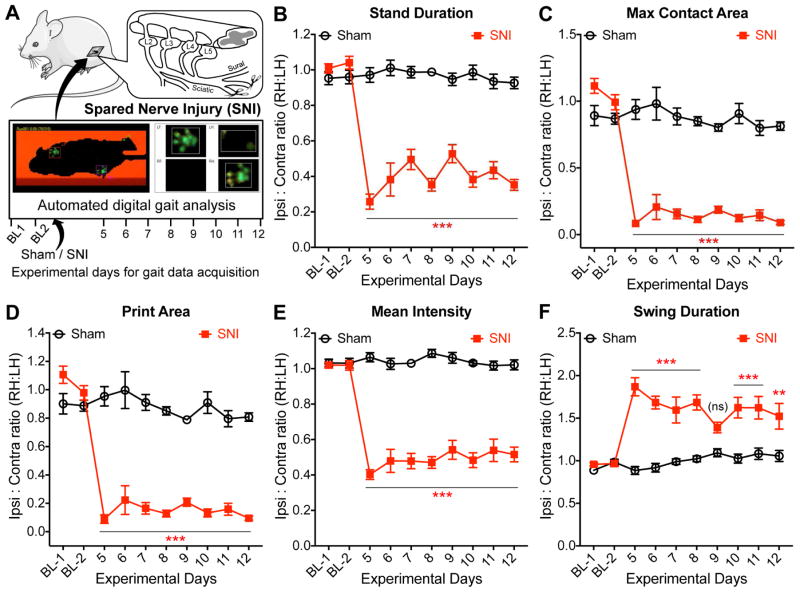
With the aim of assessing and defining non-reflexive voluntary pain-related behaviors in mice with neuropathic conditions, we performed automated digital gait analysis using the CatWalk® system. A number or previous studies have utilized gait analysis as an indicator of pain behaviors in mice that are subjected to neuropathic conditions (Truin, van Kleef et al. 2009, Mogil, Graham et al. 2010, Huehnchen, Boehmerle et al. 2013, Chiang, Sheu et al. 2014, Pitzer, Kuner et al. 2016). Mice that were subjected to nerve injury/neuropathy by SNI exhibited significant changes in several gait indices/parameters on ipsilateral hindpaws, compared to sham-operated control animals, starting 5 days post-SNI, which persisted for the remainder of the experimental duration (fig. 1, and Supplementary fig. S2). Specifically, there was a significant decrease in stand duration, without any change in the stand index (fig. 1B, and Supplementary fig. S2). Also, sustained decreases were observed in the maximum/mean intensity at the point of peak of paw contact, with a concomitant decrease in the maximal contact area of ipsilateral hindpaws of SNI mice (fig. 1C, and Supplementary fig. S2). Accordingly, a significant reduction in the length and width of ipsilateral hindpaw prints were observed in SNI mice, which contributes to an overall decrease in the paw print area (fig. 1D, and Supplementary fig. S2). Furthermore, the maximal and mean intensities in ipsilateral hindpaws of SNI mice were significantly reduced over the entire stand phase (fig. 1E, and Supplementary fig. S2). On the other hand, a significant increase in the swing duration was observed in the ipsilateral hindpaws of SNI mice, with a reciprocal decrease in swing speed (fig. 1F, and Supplementary fig. S2). However, no changes were observed in the stride length and step cycle of the ipsilateral hindpaws of SNI mice, as well as in run speed and variability (Supplementary fig. S2). To summarize these changes in gait parameters: 1) The lack of any changes in stride length, step cycle, run speed and maximal run variability indicates that mice with SNI show no gross deficit in their ability and speed of voluntary walking, as well as walking step cycle; 2) decreases in stand duration and associated parameters such as maximal and mean paw intensities, paw print length, width and area in the ipsilateral hindpaws of SNI mice indicate an overall decrease in the degree and duration of weight-bearing by the affected paw; and 3) the increase in swing duration, along with a decrease in swing speed of the ipsilateral hindpaws of SNI mice indicate that the affected paw remains off the ground for a longer duration.
Figure 1.
Nerve injury/neuropathy-induced changes in hindpaw gait parameters in mice. (A) Cartoon depicting the experimental protocol for the acquisition of gait indices in mice, before (baseline 1 and 2 – denoted ‘BL1’ and ‘BL2’) and after the induction of spared nerve injury (SNI). (B–F) Changes in mouse hindpaw gait indices, stand duration (B), maximum contact area (C), print area (D), mean intensity (E), and swing duration (F) were observed in mice subjected to SNI, as compared to sham-operated mice. Data in panels B–F are plotted as the ratio of individual gait indices in ipsilateral (right) vs contralateral (left) hindpaws; mean ± SEM (sham n=6; SNI n=7). ** denotes p<0.01, *** denotes p<0.001, and ‘ns’ denotes not significant, compared to the respective time points in sham-operated group (two-way ANOVA with Bonferroni’s post-hoc correction). Data for additional gait indices are shown in Supplementary Fig. S2.
3.2. Nerve injury/neuropathy-induced gait changes in mice are not sensitive to known analgesics
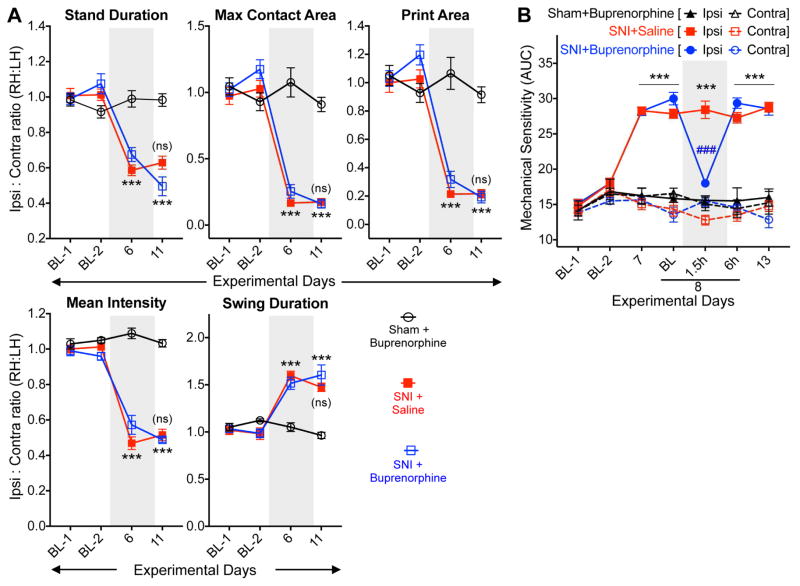
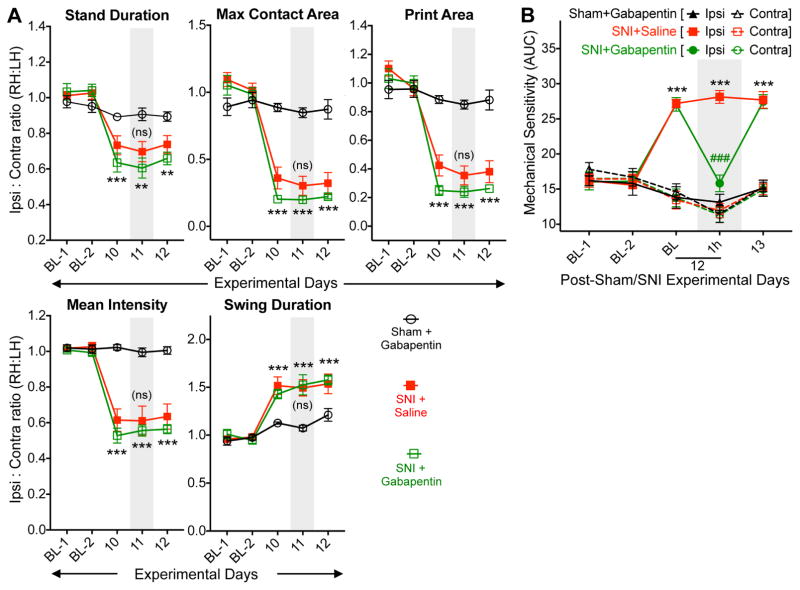
We next investigated if changes in multiple gait indices in mouse hindpaws with nerve injury/neuropathy are indicative of pain conditions. We utilized analgesics that are known to attenuate pain behaviors in mouse models of neuropathy (Decosterd, Allchorne et al. 2004, Christoph, Kogel et al. 2005). Systemic administration of buprenorphine (25 μg/kg; i.p.) did not attenuate SNI-induced changes in ipsilateral hindpaw gait indices on post-operative day 11, such as stand duration, maximum/mean intensity at the point of maximal contact, print length, print width, print area, overall maximum/mean intensity, swing duration and swing speed (fig. 2A, and Supplementary fig. S3). Buprenorphine administration in sham-operated animals did not alter any of the hindpaw gait indices (fig. 2A, and Supplementary fig. S3), indicating no direct influence of buprenorphine on mouse gait at this concentration. Similarly, systemic administration of a non-opioid analgesic, gabapentin (30 mg/kg; i.p.) on post-operative day 11 failed to reverse SNI-induced changes in ipsilateral hindpaw gait indices as mentioned above, and in sham-operated animals it did not influence hindpaw gait indices (fig. 3A, and Supplementary fig. S4). Buprenorphine and gabapentin at these doses were able to completely attenuate SNI-induced mechanical hypersensitivity, as assessed by von Frey testing (fig. 2B, and fig. 3B), verifying the anti-hyperalgesic effects of these drugs. These observations suggest that neuropathic injury-induced alterations in gait indices in mouse hindpaws may not be a manifestation of pain-related behavior.
Figure 2.
Altered hindpaw gait indices in mice with neuropathy are not influenced by the opioid analgesic buprenorphine. (A) Systemic administration (i.p.) of buprenorphine (25 μg/kg) did not attenuate altered hindpaw gait indices in SNI mice. Mice were tested 1 h post saline/buprenorphine administration. Data are plotted as the ratio of individual gait parameters in ipsilateral (right) vs contralateral (left) hindpaws; mean ± SEM (Sham+Buprenorphine n=6; SNI+Saline/SNI+Buprenorphine n=7/group). *** denotes p<0.001, and ‘ns’ denotes not significant, compared to the respective time points in the sham-operated group; and not significant for SNI+buprenorphine group, compared to the respective time point in SNI+saline group (two-way ANOVA with Bonferroni’s post-hoc correction). (B) Administration of buprenorphine (25 μg/kg; i.p.) led to complete attenuation of hindpaw mechanical hypersensitivity in SNI mice. Mice were tested 1.5 h post saline/buprenorphine administration. Data are presented as mean ± SEM of area under the curve calculated from von Frey filament strength – paw withdrawal response frequency curves for each mouse hindpaw (Sham+Buprenorphine n=6; SNI+Saline/SNI+Buprenorphine n=7/group). *** denotes p<0.001, compared to the respective time points in sham-operated group; and ### denotes p<0.001 for SNI+buprenorphine group, compared to the respective time point in SNI+saline group (two-way ANOVA with Bonferroni’s post-hoc correction). Gray boxes in A and B denote vehicle/drug administration time points. Data for additional gait indices are shown in Supplementary Fig. S3.
Figure 3.
Altered hindpaw gait indices in mice with neuropathy are not influenced by the analgesic gabapentin. (A) Systemic administration (i.p.) of gabapentin (30 mg/kg) did not significantly attenuate altered hindpaw gait indices in SNI mice. Mice were tested 1 h post saline/gabapentin administration. Data are plotted as the ratio of individual gait parameters in ipsilateral (right) vs contralateral (left) hindpaws; mean ± SEM (n=8 per group). ** denotes p<0.01, *** denotes p<0.001, and ‘ns’ denotes not significant, compared to the respective time points in sham-operated group; and ‘ns’ denotes not significant for SNI+gabapentin group, compared to the respective time point in SNI+saline group (two-way ANOVA with Bonferroni’s post-hoc correction). (B) Administration of gabapentin (30 mg/kg; i.p.) led to complete attenuation of hindpaw mechanical hypersensitivity in SNI mice. Mice were tested 1.5 h post saline/gabapentin administration. Data are presented as mean ± SEM of area under the curve calculated from von Frey filament strength – paw withdrawal response frequency curves for each mouse hindpaw (n=8 per group). *** denotes p<0.001, compared to the respective time points in sham-operated group; and ### denotes p<0.001 for SNI+gabapentin group, compared to the respective time point in SNI+saline group (two-way ANOVA with Bonferroni’s post-hoc correction). Gray boxes in A and B denote vehicle/drug administration time points. Data for additional gait indices are shown in Supplementary Fig. S4.
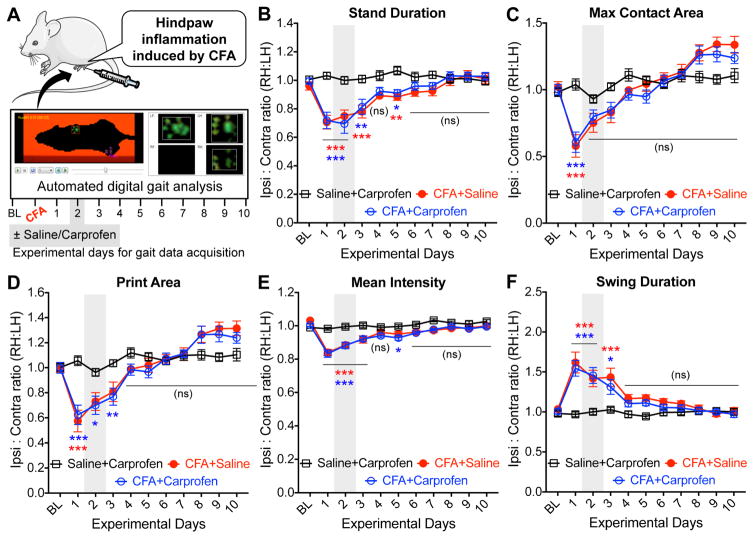
3.3. Mouse hindpaw inflammation lead to small and transient changes in gait
We next investigated if alteration in gait indices could be observed in mice with hindpaw inflammation, and the effects of a known analgesic on such gait changes (fig. 4A). Hindpaw injection of complete Freund’s adjuvant (CFA; 10 μg in a final volume of 10 μl) was utilized as model of inflammatory pain (Larson, Brown et al. 1986, Shutov, Warwick et al. 2016). Given the comparatively minor extent of gait changes observed following CFA injection, we opted to increase the number of mice in each group from 6–8 (as with SNI) to 15 to obtain sufficient statistical power. CFA-injected mice exhibited transient changes in a number of gait indices, such as decreases in stand duration, maximum contact/print area, intensity at the point of peak of paw contact, and maximal and mean intensities over the entire stand phase in the ipsilateral hindpaws, compared to saline-injected animals (fig. 4B–E, and Supplementary fig. S5). Also, a significant transient increase in the swing duration was observed in the ipsilateral hindpaws of CFA-injected mice, with a reciprocal decrease in swing speed (fig. 4F, and Supplementary fig. S5). Most of these changes in gait indices were observed 1 to 3 days post CFA-injection, which subsequently returned to the values observed for baseline and/or saline-injected conditions, and remained un-altered for the rest of the experimental duration (fig. 4B–F, and Supplementary fig. S5). Compared to SNI, CFA caused only minimal-to-moderate level changes in gait indices. We next utilized carprofen, an NSAID-type analgesic drug that is known to attenuate inflammatory pain-related hypersensitivity in mice (Adamson, Kendall et al. 2010, Smeester, Lee et al. 2017), in order to verify if CFA-induced changes in gait parameters are a manifestation of pain-like behaviors. Systemic administration of carprofen (5 mg/kg; i.p.) 2 days post-CFA injection did not significantly attenuate CFA-induced changes in ipsilateral hindpaw gait indices, such as stand duration, maximum intensity at the point of maximal contact, maximum/mean overall intensity, swing duration and swing speed (fig. 4B–F, and Supplementary fig. S5). Carprofen administration in saline-injected animals did not alter any hindpaw gait indices (fig. 4B–F, and Supplementary fig. S5), indicating that it does not directly influence mouse gait at this dose. However, carprofen was able to completely attenuate CFA-induced heat and mechanical hypersensitivity of ipsilateral hindpaws, as assessed by Hargreaves’ and von Frey testing (fig. 5A–B), verifying its known anti-hyperalgesic effects. These observations suggest that the alterations in gait indices in mouse hindpaws with inflammatory conditions also may not be a manifestation of pain-related behavior.
Figure 4.
Hindpaw inflammation leads to transient alteration in certain gait parameters in mice, which are not pain-related. (A) Cartoon depicting the experimental protocol for the acquisition of gait parameters in mice, before (baseline; denoted ‘BL’) and after the unilateral injection of Freund’s complete adjuvant (CFA; 10 μl of 1 mg/ml suspension) into the plantar region of mouse hindpaws to induce inflammation. (B–I) Hindpaw injection of CFA into mouse hindpaws led to transient alterations in gait parameters, such as stand duration (B), maximum contact area (C), print area (D), mean intensity (E) and swing duration (F). Systemic administration (i.p.) of carprofen (5 mg/kg) did not lead to any significant attenuation of altered hindpaw gait parameters in CFA-injected mice (B–F). Mice were tested 1 h post saline/carprofen administration. Data are plotted as the ratio of individual gait parameters in ipsilateral (right) vs contralateral (left) hindpaws; mean ± SEM (n=15 per group). * denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001, and ‘ns’ denotes not significant, for CFA+saline and CFA+carprofen groups, compared to the respective time points in saline+carprofen group (two-way ANOVA with Bonferroni’s post-hoc correction). Gray boxes in both panels denote vehicle/drug administration time points. Data for additional gait indices are shown in Supplementary Fig. S5.
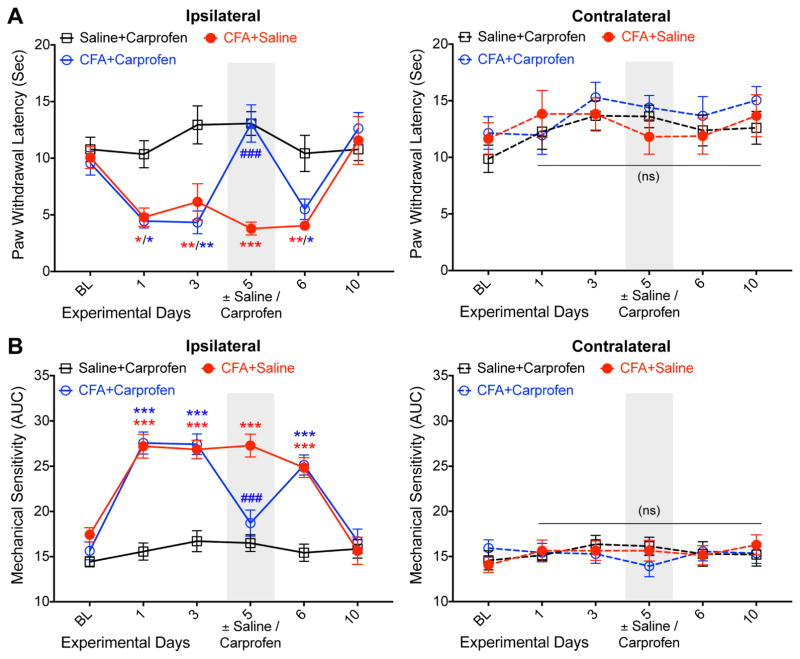
Figure 5.
Hindpaw inflammation leads to prolonged heat and mechanical hypersensitivity in mice, which could be attenuated by carprofen. (A) Hindpaw injection of CFA (10 μl of a 1 mg/ml suspension) into mouse hindpaws led to sustained heat hypersensitivity in ipsilateral hindpaws for up to 6 days. Systemic administration (i.p.) of carprofen (5 mg/kg) led to complete attenuation of ipsilateral hindpaw heat hypersensitivity in CFA-injected mice. Mice were tested 1.5 h post saline/carprofen administration. Data are plotted as mean ± SEM of paw withdrawal latency in Hargreaves’ test (n=7 per group). (B) The same cohort of mice showed the development of mechanical hypersensitivity in ipsilateral hindpaws for up to 6 days post-CFA injection. Systemic administration (i.p.) of carprofen (5 mg/kg) led to complete attenuation of ipsilateral hindpaw mechanical hypersensitivity in CFA-injected mice. Mice were tested 2 h post saline/carprofen administration. Data are plotted as mean ± SEM of area under the curve calculated from von Frey filament strength – paw withdrawal response frequency curves for each mouse hindpaw (n=7 per group). In both panels, * denotes p<0.05, ** denotes p<0.01 and *** denotes p<0.001 for CFA+saline and CFA+carprofen groups, compared to the respective time points in saline+carprofen group; and ### denotes p<0.001 for CFA+carprofen group, compared to the respective time point in CFA+saline group (two-way ANOVA with Bonferroni’s post-hoc correction). Gray boxes in both panels denote vehicle/drug administration time points.
3.4. Voluntary avoidance of a potentially noxious stimulus represents a non-reflexive pain behavior in mice under neuropathic and inflammatory conditions
Since our in-depth analysis showed that nerve injury/neuropathy- and inflammation-induced alterations in mouse gait are unrelated to pain, we next explored other approaches to assess non-reflexive pain behaviors in mice. Therefore, we utilized a method for pain-related quantitative behavioral assessment, wherein mice with pain-producing pathologies are monitored for voluntary avoidance of a potentially unpleasant/painful stimulus, and to investigate if any such behavioral changes could be attenuated by drugs with proven anti-hyperalgesic activity. This method combines the assessment of innate avoidance to light in mice with potential mechanical pain-producing stimuli of varying magnitudes (LaBuda and Fuchs 2000, LaBuda and Fuchs 2000, Harte, Meyers et al. 2016), as explained in detail in the methods section. With increasing mechanical probe height (0 to 5 mm) in the conflict chamber (Chamber-2; fig. 6A), mice exhibit an increase in escape latency from the white-lit chamber (Chamber-1; fig. 6A, and baseline values in fig. 6B and C; Supplementary video 1).
Hindpaw inflammation in mice with the injection of CFA significantly increased escape latency from the white-lit chamber at a probe height of 5 mm, as compared to mice with hindpaw saline injection (fig. 6B). Systemic administration of carprofen (5 mg/kg; i.p.) significantly attenuated this CFA-induced increase in escape latency from the white-lit chamber at a probe height of 5 mm (fig. 6B), suggesting such a delay is representative of pain-related behavior. Carprofen administration in mice that received hindpaw saline did not elicit any change in the escape latency from the white-lit chamber at all probe heights tested (fig. 6B).
We next investigated if a similar behavioral manifestation could also be observed in a mouse nerve injury model of experimental neuropathy. Mice that were subjected to SNI exhibited a significant increase in escape latency from the white-lit chamber at a probe height of 5 mm, as compared to sham surgery controls (fig. 6C; Supplementary video 1). Systemic administration of buprenorphine (25 μg/kg; i.p.) significantly attenuated the SNI-induced increase in escape latency from the white-lit chamber at 5 mm probe height (fig. 6C). Furthermore, we tested separate cohorts of mice at 20 days post-sham/SNI surgery, without any prior assessment of MCA behaviors before surgery, and observed similar increases in escape latency from the white-lit chamber at 5 mm probe height in SNI mice, as compared to mice with sham surgery (fig. 6D). Also, such increases in escape latency in SNI mice were attenuated by systemic administration of gabapentin (30 mg/kg; i.p.; fig. 6D), suggesting that prior experience of the mechanical stimulus in the MCA assay is not a prerequisite for observing neuropathy-related behavioral changes. Administration of buprenorphine and gabapentin in sham-operated mice did not change escape latency from the white-lit chamber at all probe heights tested (fig. 6C and D). From the same dataset, we also calculated the time taken for mice to cross the entirety of chamber 2 and enter into chamber 3, and saw no substantive differences in the data (Supplementary fig. S6). Collectively, these observations suggest that an increase in escape latency in MCA assays could represent a valid voluntary pain-related behavior in pre-clinical inflammatory and neuropathic models.
4. DISCUSSION
Development of efficacious new generation analgesics has been impeded by insufficient translatability of basic research findings on mechanisms of pain processing, as well as by the pre-clinical models used for recapitulation of human pain pathologies (Clark 2016). Historically, quantitative assessment of pain in rodents has mainly utilized measures of evoked and/or reflexive end points, such as tail-flick and hindpaw thermal stimuli, paw-withdrawal thresholds for mechanical forces, and limb guarding behavior in response to physico-chemical stimuli (Berge 2011, Percie du Sert and Rice 2014, Clark 2016) While quick to perform, sensitive and exceptionally well-validated, it has been proposed that these measures are not correlated closely enough with behaviors observed in patients with either acute or chronic pain (Wright, Goudas et al. 2004, Baron, Binder et al. 2010, Maier, Baron et al. 2010).
Recent years have seen the development of novel assays of non-reflexive and un-evoked/ongoing pain behaviors in rodents, with the goal of improving the translation of preclinical findings into clinical practice (Barrot 2012, Tappe-Theodor and Kuner 2014). Gait analysis represents one such option: prior studies have shown human gait changes with diabetic neuropathy (Katoulis, Ebdon-Parry et al. 1997) and plantar fasciitis (Wearing, Smeathers et al. 2003).
Our study shows the intensity and timeline of changes in several gait parameters in mouse models of neuropathic and inflammatory pain pathologies. Interestingly, none of these changes, which are more pronounced in neuropathic conditions, could be attenuated by known analgesic drugs, raising the possibility that gait changes observed in preclinical pain models are not attributable to altered pain sensitivity. We next utilized voluntary aversion to a potentially-painful stimulus in mice, which exhibit pronounced aversive behaviors under inflammatory and neuropathic conditions. Furthermore, such behaviors could be attenuated with the administration of known analgesic drugs, suggesting this voluntary behavior in mice could be used as an indicator of pain under pathological conditions.
Gait analysis revealed measurable changes in several gait indices in ipsilateral paws following nerve injury or inflammation, such as decreased stand duration and paw intensity/area, and increased swing duration. These changes are largely consistent with those shown in similar mouse neuropathic and inflammatory models in recent reports (Pitzer, Kuner et al. 2016). Interestingly, our data also indicate that these alterations in gait indices could not be reversed by well-established analgesics. This observation raises several possibilities; the alterations in gait parameters that we observe are not true ‘antalgic’ adaptations, but secondary, biomechanical effects of processes such as edema, denervation, and/or muscle atrophy, or perhaps pain-related changes are occurring in these models, but are undetectable by gait analysis. A number of reports have detailed gait alterations in rodents with reference to preclinical pain models, such as chronic constriction injury, spared nerve injury and osteoarthritis (Vrinten and Hamers 2003, Ruan, Patel et al. 2013, Chiang, Sheu et al. 2014, Sheahan, Siuda et al. 2017). However, when a known analgesic intervention is tested for its ability to reverse such gait changes, the results are mixed; spinal cord stimulation, morphine and gabapentin did not improve gait deficits associated with models of traumatic neuropathy, consistent with the current study (Truin, van Kleef et al. 2009, Mogil, Graham et al. 2010, Sheahan, Siuda et al. 2017), but gabapentin was shown to be effective in a chemotherapy-induced neuropathy model (Huehnchen, Boehmerle et al. 2013). Buprenorphine was not able to conclusively alter osteoarthritis-related changes in gait (Dorman, Krug et al. 2014), but administration of the extracellular matrix component hyaluronan was able to reverse similar changes (Muramatsu, Sasho et al. 2014). Furthermore, the antioxidant compound curcumin normalized gait disturbances associated with the hindpaw incision model of post-surgical pain (Sahbaie, Sun et al. 2014). Thus far, it appears that the efficacy of analgesics in gait-based measures is highly dependent upon the type of pain-inducing procedure, as well as the site, and perhaps the type of analgesic used. Clearly, our understanding of gait alterations in rodent models of inflammatory and neuropathic pain pathologies, and extensive pharmacological validation of such changes as an indicative of pain is still lacking.
Furthermore, studies utilizing knee-joint injection of carrageenan in rats and CFA in mice led to alterations in gait indices, such as decreases in stand duration and paw intensity/area, with an increase in swing duration, which could be attenuated by systemic administration of fentanyl (for carrageenan) and indomethacin (for CFA) (Vrinten and Hamers 2003, Parvathy and Masocha 2013). In our study, CFA only led to transient and low-magnitude changes in these gait parameters (fig. 4, and Supplementary fig. S5), as has been reported recently (Pitzer, Kuner et al. 2016, Sheahan, Siuda et al. 2017). Also, these changes were unaffected by the NSAID carprofen (fig. 4, and Supplementary fig. S5), suggesting these transient gait changes are not related to pain. Therefore, it could be argued that studies that invoke acute hypersensitivity in the plantar region, as opposed to the knee joint in rodents are less amenable to detect pain-related gait alterations, which may represent a problem for the large number of studies that rely upon intraplantar injection of algogenic agents. However, in mouse models of neuropathy, wherein the induction of nerve injury is proximal to hindpaw innervation, strong and sustained changes in the above-mentioned gait indices are observed [fig. 1, and Supplementary fig. S2; (Huehnchen, Boehmerle et al. 2013, Chiang, Sheu et al. 2014, Pitzer, Kuner et al. 2016, Sheahan, Siuda et al. 2017)], which remain unaffected by analgesic drugs (fig. 2–3, and Supplementary fig. S3–4).
Altered gait in humans with neuropathic and knee pathologies, such as osteoarthritis have been correlated with the magnitude of pain experienced (Divine and Hewett 2005, Don, Serrao et al. 2007). In neuropathies, such as in Charcot-Marie-Tooth disease, increased stride duration and excessive plantar flexion during swing phase and exaggerated knee swing flexion have been observed. These changes are characteristic of so-called ‘steppage’ or ‘equine’ gait, and have been suggested to represent neuropathic gait abnormalities (Don, Serrao et al. 2007). In diabetic neuropathy patients, increased stance duration and plantar pressure have been reported (Fernando, Crowther et al. 2013, Fernando, Crowther et al. 2015). However, in rodent models of experimental neuropathies, pronounced decreases in stand duration and paw print area (indicative of paw pressure) were observed. In knee osteoarthritis patients, increased stance duration, decreased stride duration, and elevated pressure load, along with reduced speed have been reported (Otsuki, Nawata et al. 1999, Astephen, Deluzio et al. 2008). In contrast, in human patients with hip osteoarthritis, opposite changes have been reported, such as decreases in stance duration and foot area, and an increase in stride duration (Cichy and Wilk 2006). It is important to note that gait analysis in humans is usually performed as a combinatorial analysis of kinematics and kinetics of walking and postural balance videos obtained from multiple focal points, such as in pelvis, hips, knee and ankle joints and feet, as well as electromyography and energy expenditure data (https://web.stanford.edu/class/engr110/2011/Rose-07b.pdf). In rodents, gait has mostly been analyzed by paw print-related assays, either manually or digitally, which provides an assessment of paw-associated gait indices only. This could preclude the quantitative assessment of pain-related changes in specific gait indices in rodent pain models. Alternately, gait changes that are observed in humans with painful pathologies might not be recapitulated in rodent models, due to fundamental differences in bipedal vs quadrupedal gait characteristics. Therefore, gait changes in mouse painful pathologies must be interpreted with caution, and require validation using established analgesics.
A number of voluntary pain-related behavioral assays in rodents have been reported, notably, conditioned place preference, burrowing, ultrasonic vocalization, and wheel-running activity (Vrinten and Hamers 2003, Li, Rhodes et al. 2004, Williams, Riskin et al. 2008, King, Vera-Portocarrero et al. 2009, Truin, van Kleef et al. 2009, Kurejova, Nattenmuller et al. 2010, Mogil, Graham et al. 2010, Andrews, Legg et al. 2012, Cobos, Ghasemlou et al. 2012, Huehnchen, Boehmerle et al. 2013, Parvathy and Masocha 2013, Ruan, Patel et al. 2013, Chiang, Sheu et al. 2014, Muramatsu, Sasho et al. 2014, Rutten, Robens et al. 2014, Sahbaie, Sun et al. 2014, Gould, Doods et al. 2016, Pitzer, Kuner et al. 2016, Wodarski, Delaney et al. 2016, Sheahan, Siuda et al. 2017, Sugiyama, Kang et al. 2017). Whilst a number of these assays measure pain-like behavior, the sensitivity of any one approach appears to be restricted to a subset of rodent pain pathological models only. Our study investigated a quantitative assay for voluntary pain-related behaviors in mouse neuropathic and inflammatory conditions.
The innate aversion to light in mice has been well documented and exploited in order to assess the extent of pain in rodents (LaBuda and Fuchs 2000, LaBuda and Fuchs 2000, Hascoet, Bourin et al. 2001). In the mechanical conflict-avoidance (MCA) assay, this behavioral characteristic is combined with a mechanical sensory stimulus of varying strength to assess avoidance of a potentially pain-inducing stimulus, as a measure of pain-related behavior in mice. Our results suggest that mice with nerve injury/neuropathy show an increase in the latency to avoid light and experience mechanical stimulation, a change which could be reversed with systemic administration of low dose buprenorphine or gabapentin (fig. 6C, D). Similar results were recently reported in rats with chronic constriction nerve injury (Harte, Meyers et al. 2016). Furthermore, our study shows that inflammatory conditions in mice also led to similar changes in the MCA assay, which could be reversed with systemic administration of the NSAID carprofen (fig. 6B). Our results suggest that the MCA assay could be utilized as a valid operant assessment of pain behavior in mice. However, the validation of this assay in multiple rodent pathological pain models must be performed, along with appropriate analgesic interventions, in order to better establish MCA as a voluntary pain-related behavior assay in rodents.
Despite the well-founded drive to reduce analgesic development’s reliance on reflexive assays of pain sensitivity, the use of such assays in rodents still has certain key strengths over un-evoked assays. The greater simplicity of the neurological circuits involved in evoked painful behavior responses likely contributes to the reduced variability and increased sensitivity of these assays, when compared to non-reflexive assays. Coupled with this is the fact that, to the best of our knowledge, widely-used rodent models of pain hypersensitivity that change un-evoked behavioral outcomes almost invariably produce a change in evoked behavioral assessments. If development and evaluation of un-evoked behavioral assessments such as gait analysis and MCA is continued and is coupled with an ongoing effort to more carefully consider the clinical relevance of the methods of pain induction themselves, we should expect to see an improvement in the rate of translation of preclinical findings into the development of efficacious pain therapeutics.
Supplementary Material
Highlights.
A neuropathic pain model causes prolonged changes in mouse voluntary gait patterns.
An inflammatory pain model causes relatively transient gait changes.
Gait changes induced by neuropathy/inflammation are not reversible with analgesics.
A mechanical conflict-avoidance (MCA) test detects changes in these pain models.
Neuropathy/inflammation-induced changes in MCA behavior are reversed by analgesics.
Acknowledgments
Funding:
This work was supported by funds from the Washington University Pain Center and Washington University School of Medicine, Department of Anesthesiology, and a Pilot and Feasibility award from a Center Core Grant [grant number P30DK056341]; awarded to the Nutrition and Obesity Research Center of Washington University School of Medicine in St. Louis (to A.J.S.).
The authors thank Dr. Judith P. Golden for help with the mouse nerve injury model, Ms. Sherri Vogt for help with mouse experiments, and Dr. Robert W. Gereau IV for discussion and constructive criticism of this work.
Abbreviations
- SNI
spared nerve injury
- CFA
complete Freund’s adjuvant
- NSAID
nonsteroidal anti-inflammatory drug
- MCA
mechanical conflict-avoidance
- AUC
area under the curve
- PWL
paw withdrawal latency
- LED
light emitting diode
- i.p
intraperitoneal
- i.pl
intraplantar
- cm
centimeter
- mm
millimeter
- SEM
standard error of the mean
- ANOVA
analysis of variance
Footnotes
Conflict of Interest:
The authors declare no conflict of interest for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci. 2010;49(5):610–616. [PMC free article] [PubMed] [Google Scholar]
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2012;16(4):485–495. doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26(3):332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164(4):1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill CL, Steinman JL, Willis WD. A fictive tail flick reflex in the rat. Brain Res. 1985;345(1):45–53. doi: 10.1016/0006-8993(85)90834-0. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Sheu ML, Cheng FC, Chen CJ, Su HL, Sheehan J, Pan HC. Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system. J Neurosurg. 2014;120(1):250–262. doi: 10.3171/2013.9.JNS13353. [DOI] [PubMed] [Google Scholar]
- Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507(1–3):87–98. doi: 10.1016/j.ejphar.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Cichy B, Wilk M. Gait analysis in osteoarthritis of the hip. Med Sci Monit. 2006;12(12):CR507–513. [PubMed] [Google Scholar]
- Clark JD. Preclinical Pain Research: Can We Do Better? Anesthesiology. 2016;125(5):846–849. doi: 10.1097/ALN.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153(4):876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Allchorne A, Woolf CJ. Progressive tactile hypersensitivity after a peripheral nerve crush: non-noxious mechanical stimulus-induced neuropathic pain. Pain. 2002;100(1–2):155–162. doi: 10.1016/s0304-3959(02)00275-0. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Allchorne A, Woolf CJ. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth Analg. 2004;99(2):457–463. doi: 10.1213/01.ANE.0000131967.69309.4F. table of contents. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Divine JG, Hewett TE. Valgus bracing for degenerative knee osteoarthritis: relieving pain, improving gait, and increasing activity. Phys Sportsmed. 2005;33(2):40–46. doi: 10.3810/psm.2005.02.48. [DOI] [PubMed] [Google Scholar]
- Don R, Serrao M, Vinci P, Ranavolo A, Cacchio A, Ioppolo F, Paoloni M, Procaccianti R, Frascarelli F, De Santis F, Pierelli F, Frascarelli M, Santilli V. Foot drop and plantar flexion failure determine different gait strategies in Charcot-Marie-Tooth patients. Clin Biomech (Bristol, Avon) 2007;22(8):905–916. doi: 10.1016/j.clinbiomech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Dorman CW, Krug HE, Frizelle SP, Funkenbusch S, Mahowald ML. A comparison of DigiGait and TreadScan imaging systems: assessment of pain using gait analysis in murine monoarthritis. J Pain Res. 2014;7:25–35. doi: 10.2147/JPR.S52195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M, Crowther R, Lazzarini P, Sangla K, Cunningham M, Buttner P, Golledge J. Biomechanical characteristics of peripheral diabetic neuropathy: A systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin Biomech (Bristol, Avon) 2013;28(8):831–845. doi: 10.1016/j.clinbiomech.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Fernando ME, Crowther RG, Cunningham M, Lazzarini PA, Sangla KS, Golledge J. Lower limb biomechanical characteristics of patients with neuropathic diabetic foot ulcers: the diabetes foot ulcer study protocol. BMC Endocr Disord. 2015;15:59. doi: 10.1186/s12902-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SA, Doods H, Lamla T, Pekcec A. Pharmacological characterization of intraplantar Complete Freund’s Adjuvant-induced burrowing deficits. Behav Brain Res. 2016;301:142–151. doi: 10.1016/j.bbr.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harte SE, Meyers JB, Donahue RR, Taylor BK, Morrow TJ. Mechanical Conflict System: A Novel Operant Method for the Assessment of Nociceptive Behavior. PLoS ONE. 2016;11(2):e0150164. doi: 10.1371/journal.pone.0150164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Nic Dhonnchadha BA. The mouse light-dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(1):141–166. doi: 10.1016/s0278-5846(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Huehnchen P, Boehmerle W, Endres M. Assessment of paclitaxel induced sensory polyneuropathy with “Catwalk” automated gait analysis in mice. PLoS One. 2013;8(10):e76772. doi: 10.1371/journal.pone.0076772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoulis EC, Ebdon-Parry M, Lanshammar H, Vileikyte L, Kulkarni J, Boulton AJ. Gait abnormalities in diabetic neuropathy. Diabetes Care. 1997;20(12):1904–1907. doi: 10.2337/diacare.20.12.1904. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurejova M, Nattenmuller U, Hildebrandt U, Selvaraj D, Stosser S, Kuner R. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain. 2010;6:18. doi: 10.1186/1744-8069-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163(2):490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci Lett. 2000;290(2):137–140. doi: 10.1016/s0304-3940(00)01340-9. [DOI] [PubMed] [Google Scholar]
- Larson AA, Brown DR, el-Atrash S, Walser MM. Pain threshold changes in adjuvant-induced inflammation: a possible model of chronic pain in the mouse. Pharmacol Biochem Behav. 1986;24(1):49–53. doi: 10.1016/0091-3057(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Li G, Rhodes JS, Girard I, Gammie SC, Garland T., Jr Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol Behav. 2004;83(3):515–524. doi: 10.1016/j.physbeh.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Loo L, Shepherd AJ, Mickle AD, Lorca RA, Shutov LP, Usachev YM, Mohapatra DP. The C-type natriuretic peptide induces thermal hyperalgesia through a noncanonical Gbetagamma-dependent modulation of TRPV1 channel. J Neurosci. 2012;32(35):11942–11955. doi: 10.1523/JNEUROSCI.1330-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Mao J. Translational pain research: achievements and challenges. J Pain. 2009;10(10):1001–1011. doi: 10.1016/j.jpain.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickle AD, Shepherd AJ, Loo L, Mohapatra DP. Induction of thermal and mechanical hypersensitivity by parathyroid hormone-related peptide through upregulation of TRPV1 function and trafficking. Pain. 2015;156(9):1620–1636. doi: 10.1097/j.pain.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu Y, Sasho T, Saito M, Yamaguchi S, Akagi R, Mukoyama S, Akatsu Y, Katsuragi J, Fukawa T, Endo J, Hoshi H, Yamamoto Y, Takahashi K. Preventive effects of hyaluronan from deterioration of gait parameters in surgically induced mice osteoarthritic knee model. Osteoarthritis Cartilage. 2014;22(6):831–835. doi: 10.1016/j.joca.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Otsuki T, Nawata K, Okuno M. Quantitative evaluation of gait pattern in patients with osteoarthrosis of the knee before and after total knee arthroplasty. Gait analysis using a pressure measuring system. J Orthop Sci. 1999;4(2):99–105. doi: 10.1007/s007760050081. [DOI] [PubMed] [Google Scholar]
- Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC Musculoskelet Disord. 2013;14:14. doi: 10.1186/1471-2474-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171(12):2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C, Kuner R, Tappe-Theodor A. EXPRESS: Voluntary and evoked behavioral correlates in neuropathic pain states under different housing conditions. Mol Pain. 2016:12. doi: 10.1177/1744806916656635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain. 2017;158(Suppl 1):S43–S49. doi: 10.1097/j.pain.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan MZ, Patel RM, Dawson BC, Jiang MM, Lee BH. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthritis Cartilage. 2013;21(9):1355–1364. doi: 10.1016/j.joca.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Robens A, Read SJ, Christoph T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18(2):213–222. doi: 10.1002/j.1532-2149.2013.00359.x. [DOI] [PubMed] [Google Scholar]
- Sahbaie P, Sun Y, Liang DY, Shi XY, Clark JD. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth Analg. 2014;118(6):1336–1344. doi: 10.1213/ANE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43(2):205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sheahan TD, Siuda ER, Bruchas MR, Shepherd AJ, Mohapatra DP, Gereau RWt, Golden JP. Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiol Pain. 2017;2:1–12. doi: 10.1016/j.ynpai.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, Woodruff TM, Clark JD, Usachev YM. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. The Journal of Neuroscience. 2016;36(18):5055–5070. doi: 10.1523/JNEUROSCI.3249-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeester BA, Lee JH, Beitz AJ. Influence of social interaction on nociceptive-induced changes in locomotor activity in a mouse model of acute inflammatory pain: Use of novel thermal assays. Brain Res Bull. 2017;134:47–54. doi: 10.1016/j.brainresbull.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Kang S, Arpey N, Arunakul P, Usachev YM, Brennan TJ. H2O2 Induces Muscle Nociception via Transient Receptor Potential Ankyrin 1 Receptors. Anesthesiology. 2017 doi: 10.1097/ALN.0000000000001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci. 2014;39(11):1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- Truin M, van Kleef M, Verboeket Y, Deumens R, Honig W, Joosten EA. The effect of Spinal Cord Stimulation in mice with chronic neuropathic pain after partial ligation of the sciatic nerve. Pain. 2009;145(3):312–318. doi: 10.1016/j.pain.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Hamers FF. ’CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102(1–2):203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- Wearing SC, Smeathers JE, Urry SR. The Effect of Plantar Fasciitis on Vertical Foot-Ground Reaction Force. Clinical Orthopaedics and Related Research. 2003;409:175–185. doi: 10.1097/01.blo.0000057989.41099.d8. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990;40(2):229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]
- Williams WO, Riskin DK, Mott AK. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci. 2008;47(1):8–10. [PMC free article] [PubMed] [Google Scholar]
- Wodarski R, Delaney A, Ultenius C, Morland R, Andrews N, Baastrup C, Bryden LA, Caspani O, Christoph T, Gardiner NJ, Huang W, Kennedy JD, Koyama S, Li D, Ligocki M, Lindsten A, Machin I, Pekcec A, Robens A, Rotariu SM, Vo BS, Segerdahl M, Stenfors C, Svensson CI, Treede RD, Uto K, Yamamoto K, Rutten K, Rice AS. Cross-centre replication of suppressed burrowing behaviour as an ethologically relevant pain outcome measure in the rat: a prospective multicentre study. Pain. 2016;157(10):2350–2365. doi: 10.1097/j.pain.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Goudas LC, Bentch A, Mehdi M, Perry PP, Carr DB. Hyperalgesia in outpatients with dermal injury: quantitative sensory testing versus a novel simple technique. Pain Med. 2004;5(2):162–167. doi: 10.1111/j.1526-4637.2004.04025.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.