Abstract
Background
Pediatric sepsis causes significant global morbidity and mortality and low- and middle-income countries (LMICs) bear the bulk of the burden. International sepsis guidelines may not be relevant in LMICs, especially in sub-Saharan Africa (SSA), due to resource constraints and population differences. There is a critical lack of pediatric sepsis data from SSA, without which accurate risk stratification tools and context-appropriate, evidence-based protocols cannot be developed. The study’s objectives were to characterize pediatric sepsis presentations, interventions, and outcomes in a public Emergency Medicine Department (EMD) in Tanzania.
Methods
Prospective descriptive study of children (28 days to 14 years) with sepsis [suspected infection with ≥2 clinical systemic inflammatory response syndrome (SIRS) criteria] presenting to a tertiary EMD in Dar es Salaam, Tanzania (July 1 to September 30, 2016). Outcomes included: in-hospital mortality (primary), EMD mortality, and hospital length of stay. We report descriptive statistics using means and SDs, medians and interquartile ranges, and counts and percentages as appropriate. Predictive abilities of SIRS criteria, the Alert-Verbal-Painful-Unresponsive (AVPU) score and the Lambaréné Organ Dysfunction Score (LODS) for in-hospital, early and late mortality were tested.
Results
Of the 2,232 children screened, 433 (19.4%) met inclusion criteria, and 405 were enrolled. There were 247 (61%) subjects referred from an outside facility. Approximately half (54.1%) received antibiotics in the EMD, and some form of microbiologic culture was collected in 35.8% (n = 145) of subjects. In-hospital and EMD mortality were 14.2 and 1.5%, respectively, median time to death was 3 days (IQR 1–6), and median length of stay was 6 days (IQR 1–12). SIRS criteria, the AVPU score, and the LODS had low positive (17–27.1, 33.3–43.9, 18.3–55.6%, respectively) and high negative predictive values (88.6–89.8, 86.5–91.2, 86.8–90.5%, respectively) for in-hospital mortality.
Conclusion
This pediatric sepsis cohort had high and early in-hospital mortality. Current criteria and tested clinical scores were inadequate for risk-stratification and mortality prediction in this population and setting. Pediatric sepsis management must take into account the local patient population, etiologies of sepsis, healthcare system, and resource availability. Only through studies such as this that generate regional data in LMICs can accurate risk stratification tools and context-appropriate, evidence-based guidelines be developed.
Keywords: global health, resource-limited, low-resource setting, pediatric critical care, pediatric emergency medicine, pediatric sepsis
Introduction
Sepsis represents a spectrum of disease involving a systemic inflammatory response syndrome (SIRS) in the setting of infection, escalating in septic shock to cardiovascular and organ system dysfunction (1). It is the final common inflammatory pathway for most infectious disease-related deaths (2, 3) and incurs significant pediatric morbidity and mortality worldwide (1–9). Globally, there were 2.6 million deaths due to infectious diseases in children and adolescents in 2015, and 66% of these deaths occurred in sub-Saharan Africa (SSA) (10). A recent, global point prevalence study in children in pediatric intensive care units with severe sepsis from 128 sites in 26 countries, showed an aggregated in-hospital mortality rate of 25% (11). The identification and initial care of children with sepsis can significantly impact survival (12–14), and both delays in presentation (15) and delays in diagnosis have been shown to be risk factors for poor outcomes in low- and middle-income countries (LMICs) (14). The Surviving Sepsis Campaign’s international guidelines set standards for early identification, resuscitation, and protocol-based management, which have been shown to substantially impact outcomes (16). Much of the recommended sepsis management, especially regarding goal-directed interventions, is dependent on frequent or invasive monitoring that is not reliably available in limited resource settings and healthcare providers in LMICs often face resource constraints that limit capacity to implement these guidelines (13).
Beyond resource gaps that may limit the usability of international management guidelines, there is recent evidence suggesting that the clinical effectiveness of some interventions may be context-dependent. In 2011, Maitland et al., published results from a large, multicenter, randomized controlled trial: Fluid Expansion as Supportive Therapy (FEAST) (8). In this trial, African children with severe febrile illness who received fluid bolus resuscitation had a significantly higher relative risk (RR) of mortality compared to children who received only maintenance fluids (RR for any bolus vs. control, 1.45; 95% CI, 1.13–1.86; P = 0.003) (8). Current international guidelines recommend fluid bolus resuscitation for severe sepsis and septic shock (1, 16–20), but the unexpected results from the FEAST trial call into question whether practice guidelines primarily developed in high-income countries (HICs) are appropriate for LMICs. This may be especially true in malaria endemic regions given the increased possibility of shock secondary to severe anemia as compared to septic shock due to bacteremia (21, 22).
There is a critical lack of region-specific and relevant pediatric sepsis data, and few pediatric sepsis cohorts exist in SSA (7). While we recognize pediatric sepsis to be a global burden, we lack a robust understanding of how best to identify and manage sepsis in settings where triage and goal-directed therapy are challenging given the scarcity of invasive monitoring, laboratory tests, and medical personnel and where HIV, malaria, malnutrition, and limited access to care frequently complicate management. Tanzania is one such setting with a paucity of data on the profile and outcomes of children with sepsis. Thus, the objective of this prospective study was to characterize the clinical presentation, emergency interventions received, and outcomes among children with sepsis presenting to Muhimbili National Hospital (MNH) in Tanzania. The generation of region-specific data is needed to accurately risk stratify patients, develop appropriate protocols for early recognition, and implement evidence-based treatment protocols.
Materials and Methods
This prospective descriptive study included pediatric patients presenting with sepsis to an urban, tertiary Emergency Medicine Department (EMD) at the National Referral Hospital in Dar es Salaam, Tanzania (MNH) between July 1 and September 30, 2016. The EMD is the receiving department for all acutely ill and injured patients, and is the only 24-h, full capacity public EMD in Tanzania. It is also the training site for the only emergency medicine residency in the country and cares for an average of 60,000 patients annually, and children comprise approximately 25% of the patient population. Pediatric sepsis is common at MNH; the EMD cares for an estimated 150–200 children with sepsis monthly (23, 24).
The EMD is equipped to provide plain radiographs, bedside ultrasound, continuous cardiorespiratory monitoring, low-flow oxygen, bag mask ventilation, intubation, mechanical ventilation, blood product transfusions, vasoactive medications, and resuscitation medications. MNH has a large general pediatrics ward, a high-dependency unit (HDU), and no dedicated pediatric intensive care unit. In general, the MNH ward can administer intermittent medications, intravenous fluids, low-flow oxygen, and blood products and measure vital signs every shift. The HDU is similar to the ward with a more favorable nursing ratio. Vasoactive infusions, intubation and mechanical ventilation are not routinely available for children outside of the EMD.
Children were included if they were between 28 days and 14 years of age and met criteria for sepsis, defined as having two or more clinical SIRS criteria (Box 1) with a suspected infection. At MNH, patients younger than 28 days present directly to the maternity and neonatal ward, and those over 14 years are triaged to adult rooms and admitted to the adult ward. SIRS criteria is defined per international pediatric sepsis consensus definitions (1), adapted for resource-limited settings (12). All pediatric patients presenting to the EMD were screened. Patients in cardiac arrest on presentation or with acute trauma were excluded. Written consent was obtained prior to enrollment. The primary outcome was in-hospital mortality. Secondary outcomes included EMD mortality and hospital length of stay.
Box 1. Clinical SIRS Criteria for Resource-Limited Settings (12).
Presence of ≥2 of the following 4 criteria with a suspected or proven infection:
Abnormal temperature (axillary >38 or <36.0°C) documented or by history.
Abnormal heart rate for age: tachycardia OR bradycardia (<1 year).
Respiratory insufficiency: tachypnea for age, hypoxia (SpO2 <92%), NIPPV, OR mechanical ventilation.
Ill-appearing, in distress, OR not responsive.
Patient data included: patient characteristics [age, sex, mid-upper arm circumference (MUAC), severity of illness]; patient comorbidities (HIV, malaria, TB, malnutrition); vital signs and SIRS criteria on admission; delay to care (onset of illness, facilities visited prior to MNH, duration of fever); socioeconomic status indicators (insurance status, parents’ education levels, parental literacy, number of children in the household, number of children under 5 in the household); interventions and therapies received in the EMD; relevant laboratory results; and outcomes. All diagnostic and therapeutic decisions were made at the discretion of the treating physician. Severe malnutrition was defined as a MUAC of ≤11.5 cm and severe anemia was defined as a hemoglobin ≤5 g/dL. Level of consciousness was assessed with the AVPU score (alert, responds to verbal stimuli, responds to pain, unresponsive) and severity of illness was measured with the 4-point Lambaréné Organ Dysfunction Score (LODS) (Box 2) (25). The LODS is a simple clinical prediction score for mortality validated in African children ≤15 years, including malaria endemic regions (25, 26). Early and late mortality were defined as death within 48 and ≥48 h of presentation, respectively. Microbiological investigations including cultures are not routinely performed on all patients, but results were recorded when available.
Box 2. Lambaréné Organ Dysfunction Score (LODS) (2, 6, 25).
Validated in African children ≤15 years with and without malaria. The score assigns one point for each of the following:
Prostration—defined as ≥1 of the following, depending on age: inability to breastfeed, sit, stand, or walk unsupported.
Coma—Blantyre Coma Score ≤2.
Deep breathing.
Research personnel collected data from the electronic medical record, paper chart, care provider, and guardian. Study data were managed using REDCap electronic data capture tools (version 7.2.2) hosted at MNH (27). Data were deidentified prior to analysis. This study was carried out in accordance with the recommendations and approval of the Institutional Review Boards and Committees on Human Research at Muhimbili University of Health and Allied Sciences (Ref. No. 2016-03-30/AEC/Vol.X/201) and the University of California, San Francisco (IRB # 16-18977, Ref. No. 161295). We obtained written, informed consent from all guardians and assent from subjects when appropriate in accordance with the Declaration of Helsinki.
Statistical Analysis
All data analysis and descriptive statistics were performed using Stata/MP (14.2), including means and SDs, medians and interquartile ranges, and counts and percentages as appropriate for the data type and distribution. We evaluated the ability of number of SIRS criteria, the AVPU score and the LODS to predict in-hospital mortality in this population. Scoring system discrimination was evaluated by comparing positive and negative predictive values.
Results
A total of 2,232 children presented to the EMD during the study period. There were 433 patients (19.4%) who had SIRS with a suspected infection, 28 children were excluded, and 405 subjects were successfully enrolled (Figure 1). Of those enrolled, 402 (99.3%) were followed to hospital discharge.
Figure 1.
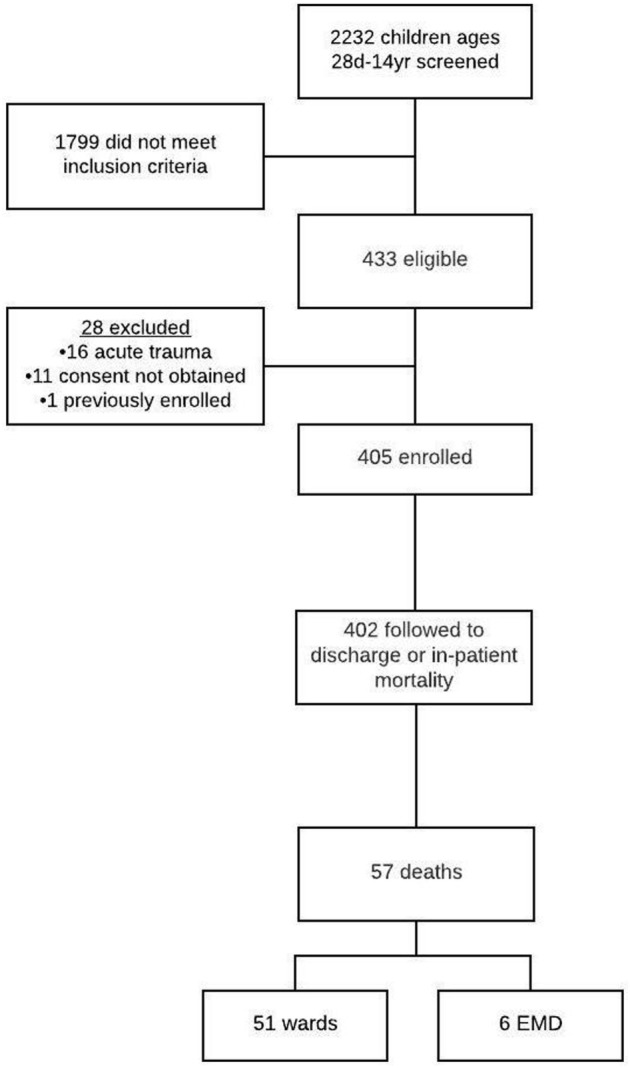
Patient screening, enrollment, and outcomes flowchart.
Baseline Patient Characteristics
The median age of children with sepsis at MNH was 25 months (IQR 11–63 months), and 73.1% were under 5 years of age (n = 296) (Table 1). Severe malnutrition was present in 12.6% (n = 51) of children, 6.9% (n = 28) tested positive for malaria by rapid diagnostic test (RDT) or microscopy, 1.7% (n = 7) had confirmed (by RDT or antibody testing) or a known history of HIV, and 12.8% (n = 52) of subjects were tested for HIV during hospitalization. The mean hemoglobin was 8.3 g/dL (n = 305), and 16.1% (n = 49) of children presented with severe anemia. The mean venous blood gas pH among those tested (n = 184) was 7.36 (SD 0.14), while the mean lactate (n = 177) was 3.2 mmol/L (SD 3.5). The majority of children (61.7%, n = 250) presented with fever: of these, 36.4% (n = 91) had fever only by history and 63.6% (n = 159) had a documented fever on arrival. Almost 66% of patients (205/311) reported a fever duration greater than 48 hours and the mean fever duration was 8.7 days (SD 27.5). Over half of the patients (61%, n = 247) were referred to MNH from an outside hospital or clinic. Among referred patients, 47.5% (116/244) had been administered antibiotics before arrival to MNH. Up-to-date immunization status was reported in 97.3% (n = 394) of patients.
Table 1.
Patient characteristics.
| Total (N = 405) | |
|---|---|
| Demographics | |
| Age (months), median (IQR) | 25 (11–63) |
| <2 years, n (%) | 195 (48.2) |
| 2–5 years, n (%) | 101 (24.9) |
| >5 years, n (%) | 109 (26.9) |
| Male sex, n (%) | 236 (58.3) |
| MUAC, mean (SD) | 14.7 (4.1) |
| ≤11.5 cm, n (%) | 51 (12.6) |
| Malaria positive, n (%) | 28 (6.9) |
| HIV positive, n (%) | 7 (1.7) |
| Fully immunized, n (%) | 394 (97.3) |
| Illness severity and level of consciousness | |
| Lambaréné Organ Dysfunction Score | |
| LODS 0, n (%) | 189 (46.7) |
| LODS 1, n (%) | 144 (35.6) |
| LODS 2, n (%) | 63 (15.6) |
| LODS 3, n (%) | 9 (2.2) |
| AVPU score | |
| Alert, n (%) | 331 (82.8) |
| Responds to verbal, n (%) | 11 (2.8) |
| Responds to pain, n (%) | 40 (10) |
| Unresponsive, n (%) | 18 (4.5) |
| Missing | 5 (1.3) |
| No. of SIRS criteria met, median (IQR) | 3 (2–3) |
| 2, n (%) | 167 (41.2) |
| 3, n (%) | 166 (41) |
| 4, n (%) | 72 (17.8) |
| Laboratory values | |
| Hemoglobin (g/dL) (n = 305), mean (SD) | 8.3 (3.0) |
| ≤5 g/dL, n (%) | 49/305 (16.1) |
| >10 g/dL, n (%) | 85/305 (27.2) |
| WBC count ×103 (n = 305), mean (SD) | 16.6 (22.4) |
| Platelet count ×103 (N = 304), mean (SD) | 322 (201.6) |
| Venous blood gas pH (n = 184), mean (SD) | 7.36 (0.14) |
| Bicarbonate (mEq/L) (n = 185), mean (SD) | 21.3 (6.5) |
| Lactate (mmol/L) (n = 177), mean (SD) | 3.2 (3.5) |
| Sodium (mEq/L) (n = 199), mean (SD) | 132.5 (7.2) |
| Potassium (mEq/L) (n = 199), mean (SD) | 4.1 (1.3) |
| Glucose (mmol/L) (n = 243), mean (SD) | 6.6 (3.6) |
| Prehospital course | |
| Fever, n (%) | 250 (61.7) |
| Documented, n (%) | 159/250 (63.6) |
| By history, n (%) | 91/250 (36.4) |
| Days of fever, mean (SD) | 8.7 (27.5) |
| >2 days, n (%) | 205/311 (65.9) |
| Hospital/clinic referral, n (%) | 247 (61.0) |
| Antibiotics prearrival, n (%) | 116/244 (47.5) |
| Socioeconomic status indicators | |
| Insured, n (%) | 172 (42.5) |
| Exempt status, n (%) | 131 (32.4) |
| Paternal literacy | 372/388 (95.9) |
| Father’s highest level of education | |
| No formal school | 12 (3) |
| Primary school | 190 (46.9) |
| Secondary school | 95 (23.5) |
| University or advanced degree | 81 (20) |
| Unknown | 27 (6.7) |
| Maternal literacy | 372/403 (92.3) |
| Mother’s highest level of education | |
| No formal school | 28 (6.9) |
| Primary school | 228 (56.3) |
| Secondary school | 78 (19.3) |
| University or advanced degree | 62 (15.3) |
| Unknown | 9 (2.2) |
| Number of children <18 in the household, median (IQR) | 2 (1–3) |
| Number of children <5 in the household, median (IQR) | 1 (1–2) |
| Electricity in the home | 283/400 (70.8) |
| Improved water source, n (%) | 403/423 (95.3) |
| Tap in the home, n (%) | 96/403 (23.8) |
| Public tap, n (%) | 214/403 (53.1) |
| Well, n (%) | 93/403 (23.1) |
| Unimproved water source | 20/423 (4.7) |
| Purchased water (bottled or truck) | 9/20 (45.0) |
| Unprotected surface water | 11/20 (55.0) |
| Toilet in the home | 230 (56.8) |
IQR, interquartile range; MUAC, mid-upper arm circumference; LODS, Lambaréné Organ Dysfunction Score; AVPU, alert-verbal-pain-unresponsive score; SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
Socioeconomic Status Indicators
Regarding insurance status, 42.5% (n = 172) of subjects were uninsured, though 76.2% (n = 131) of uninsured patients were categorically exempt from paying medical expenses by Tanzanian law based on age or disease status (Table 1). Paternal and maternal literacy rates were similar at 95.9 (n = 372) and 92.3% (n = 371), respectively, though more fathers completed secondary school or higher education than mothers (43.5 and 34.6%, n = 176 and 149, respectively). The median number of children younger than 18 years in the patient household was 2 (IQR 1–3). Ninety-five percent (n = 403) of families reported having regular access to an “improved” drinking water source, defined by the WHO/UNICEF Joint Monitoring Programme as a water source that is protected from sewage (28). Almost 71% (n = 283) of families had electricity, and 56.8% (n = 230) had toilets in the home.
Illness Severity
On arrival, 82% (n = 331) of children were “alert” by the AVPU score. Using the LODS as a measure of illness severity, 46.7% (n = 189) of subjects had the lowest severity score of zero, while 17.8% (n = 72) had a score of two or more. The median number of documented SIRS criteria was 3 (IQR 2–3), with 41.2 (n = 167), 41.0 (n = 166), and 17.8% (n = 72) of subjects meeting 2, 3, and 4 criteria, respectively. Respiratory insufficiency was the most common SIRS criteria, occurring in 86.4% (n = 350) of patients, followed by abnormal temperature, abnormal heart rate, and abnormal appearance occurring in 70.9 (n = 287), 60 (n = 243), and 53.3% (n = 216) of patients, respectively (Table 2).
Table 2.
Frequency of SIRS criteria for patients enrolled in the study.
| SIRS criterion | Total (N = 405) |
|---|---|
| Abnormal temperature, n (%) | 287 (70.9) |
| Temperature, mean (SD) | 37.7 (1.0) |
| Abnormal heart rate, n (%) | 243 (60) |
| Tachycardia, n (%) | 235 (58.0) |
| Bradycardia, n (%) | 8 (2.0) |
| Respiratory insufficiency, n (%) | 350 (86.4) |
| Tachypnea, n (%) | 330 (81.5) |
| Hypoxia, n (%) | 76 (18.8) |
| SpO2, mean (SD) | 95.5 (10.3) |
| Abnormal appearance, n (%) | 216 (53.3) |
| Ill-appearing, n (%) | 203 (50.1) |
| Distress, n (%) | 21 (5.2) |
| Prostrate, n (%) | 7(1.7) |
SIRS, systemic inflammatory response syndrome.
EMD Interventions
The most common EMD interventions included: antibiotic administration (54.1%, n = 219), fluid administration (38.8%, n = 157), oxygen therapy (24.5%, n = 99), chest radiograph (11.9%, n = 48), blood transfusion (11.2%, n = 45), and antimalarial medication administration (9.2%, n = 37) (Table 3). Three percent (n = 4) of pediatric sepsis patients had a blood culture drawn in the EMD, while 75.6% (n = 306) and 4.2% (n = 17) were tested with malaria and HIV RDTs, respectively. Few patients were intubated and received mechanical ventilation (1.2%, n = 5). While patients presenting in cardiac arrest were excluded, 1.2% (n = 5) of patients decompensated to require cardiopulmonary resuscitation (CPR) in the EMD.
Table 3.
Interventions received in the EMD.
| Intervention | n (%) (N = 405) |
|---|---|
| Fluid administration | 157 (38.8) |
| Blood transfusion ordered | 45 (11.1) |
| Blood initiated in EMD | 25/45 (55.6) |
| Antibiotics received in the EMD | 219 (54.1) |
| Antibiotics received prearrival, not in the EMD | 40 (9.9) |
| Blood culture drawn in the EMD | 4 (1.0) |
| HIV rapid diagnostic test | 17 (4.2) |
| Malaria rapid diagnostic test | 306 (75.6) |
| Vasoactive infusion | 1 (0.2) |
| Hydrocortisone | 1 (0.2) |
| Antimalarial medications | 37 (9.1) |
| Oxygen | 99 (24.4) |
| Chest radiograph | 48 (11.9) |
| Ultrasound | 12 (3.0) |
| Intubation and mechanical ventilation | 5 (1.2) |
| CPR initiated | 5 (1.2) |
| ROSC | 1/5 (20) |
EMD, Emergency Medicine Department; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Some form of microbiologic culture was collected during hospitalization in 35.8% (n = 145) of patients: blood, urine and cerebral spinal fluid (CSF) cultures were collected in 29.9, 14.8, and 4.0% of patients, respectively (Table 4). Of the cultures collected, 11.6, 10, and 0% of blood, urine and CSF were positive, respectively.
Table 4.
Frequency of microbiologic tests performed and culture result positivity.
| Microbiology | n (%) |
|---|---|
| Any microbiological culture performed | 145/405 (35.8) |
| Blood culture | |
| Collected at any time during hospitalization | 121/405 (29.9) |
| Culture positive | 14/121 (11.6) |
| Urine culture | |
| Collected at any time during hospitalization | 60/405 (14.8) |
| Culture positive | 6/60 (10.0) |
| CSF culture | |
| Collected at any time during hospitalization | 16/405 (4.0) |
| Culture positive | 0/16 (0) |
CSF, cerebral spinal fluid.
Outcomes
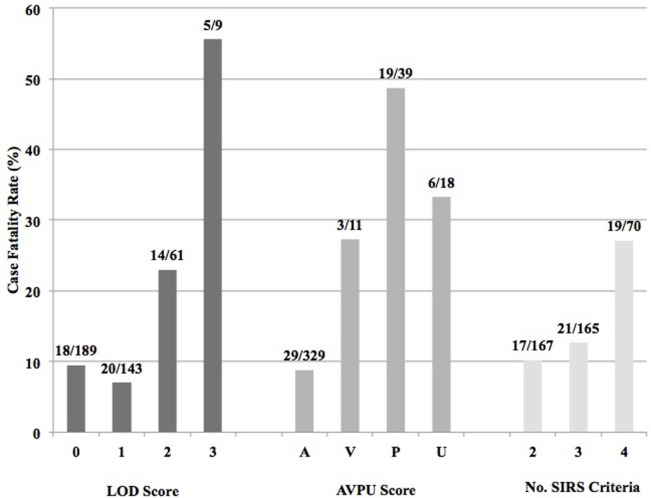
Of the 402 patients followed to discharge, 57 patients died in the hospital, yielding an in-hospital mortality of 14.2% (Table 5). Six subjects died in the EMD, representing 10.5% (6/57) of the patients who died, and the overall in-ED mortality was 1.5% (6/402). The median time to death was 3 days (IQR 1–6 days) and 35.1% (n = 20) of the patients who died did so within 48-h of presentation. The cumulative probability of mortality rose sharply until hospital day 7 (15.4%) and then rose gradually until hospital day 22 when it plateaued at 25.9% (Figure 2). The median length of stay for all patients was 6 days (IQR 1–12 days). SIRS criteria and LODS were available on all 402 subjects, while AVPU scores and in-hospital outcomes were available on 98.6% (397/402) of subjects. All five of the patients with missing AVPU scores survived to discharge. In this population, case-fatality rates increased with rising LODS and number of SIRS criteria, but less so with worsening AVPU score (Figure 3). We explored early mortality with respect to interventions received in the EMD: 80% (n = 16) received a fluid bolus, two of who were malnourished, and 85% (n = 17) received antibiotics in the EMD (Table 6). All three scores—AVPU, LODS, and SIRS—had low positive predictive values, and higher negative predictive values for in-hospital, early and late mortality (Table 7). This was also true in patient subgroups with malnutrition and severe anemia.
Table 5.
In-hospital outcomes for pediatric patients with sepsis.
| Outcome | |
|---|---|
| EMD mortality, n (%) | 6/402 (1.5) |
| In-hospital mortality, n (%) | 57/402 (14.2) |
| Ward mortality, n (%) | 51/57 (89.5) |
| EMD mortality | 6/57 (10.5) |
| <48-h mortality, n (%) | 20/57 (35.1) |
| ≥48-h mortality, n (%) | 37/57 (64.9) |
| Time to death (days), median (IQR) | 3 (1–6) |
| Length of stay all subjects (days), median (IQR) | 6 (1–12) |
IQR, interquartile range.
Figure 2.
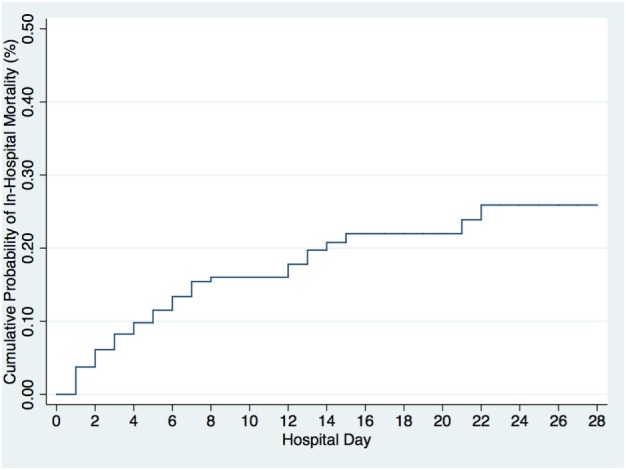
Cumulative probability of in-hospital mortality by hospital day.
Figure 3.
Case fatality rates by Lambaréné Organ Dysfunction Score, best response by the Alert-Verbal-Painful-Unresponsive (AVPU) score, and number of SIRS criteria present on admission.
Table 6.
Early mortality due to sepsis and key EMD interventions.
| Early mortality (N = 20) | |
|---|---|
| Fluid bolus in the EMD, n (%) | 16 (80.0) |
| Malnourished, n (%) | 2/11 (18.8) |
| Blood transfusion, n (%) | 2 (10) |
| Severe anemia, n (%) | 1/14 (7.1) |
| Antibiotics prearrival, n (%) | 8/17 (34.5) |
| Antibiotics in the EMD, n (%) | 17 (85.0)a |
| Antibiotics prearrival or EMD, n (%) | 19 (95.0) |
| Antimalarial medication in the EMD, n (%) | 4 (20.0)b |
| Malaria positive, n (%) | 2 (10.0) |
aSix patients received antibiotics prearrival and in the EMD.
bTwo patients tested negative and were treated.
EMD, Emergency Medicine Department.
Table 7.
Positive and negative predictive values for clinical scores (LODS, AVPU, SIRS criteria) predicting in-hospital (subgroups malnutrition and severe anemia), early and late mortality.
| Severity of illness measure | Positive predictive value | Negative predictive value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (%) | Malnutrition (%) | Severe anemia (%) | Early mortality (%) | Late mortality (%) | Total (%) | Malnutrition (%) | Severe anemia (%) | Early mortality (%) | Late mortality (%) | |
| LODS | ||||||||||
| ≥1 | 18.3 | 21.7 | 21.4 | 40.0 | 10.6 | 90.5 | 87.5 | 90.0 | 77.8 | 92.6 |
| ≥2 | 27.1 | 37.5 | 12.5 | 47.4 | 13.9 | 88.6 | 87.0 | 82.5 | 71.8 | 91.9 |
| 3 | 55.6 | 19.4 | 50.0 | 60.0 | 22.2 | 86.8 | a | 84.8 | 67.9 | 91.2 |
| AVPU score best response | ||||||||||
| Verbal or worse | 41.2 | 42.9 | 16.7 | 39.3 | 24.6 | 91.2 | 87.5 | 83.3 | 70.0 | 94.0 |
| Pain or worse | 43.9 | 40.0 | 20.0 | 40.0 | 25.9 | 90.6 | 84.6 | 83.7 | 69.7 | 93.6 |
| Unresponsive | 33.3 | a | a | 50.0 | 16.7 | 86.5 | 78.6 | 82.6 | 67.3 | 91.1 |
| SIRS criteria | ||||||||||
| ≥3 | 17.0 | 20.0 | 19.4 | 37.5 | 10.5 | 89.8 | 81.2 | 88.2 | 72.2 | 92.8 |
| 4 | 27.1 | 25.0 | 25.0 | 42.1 | 15.3 | 88.6 | 81.5 | 87.5 | 69.2 | 92.2 |
aToo few subjects in a cell to calculate.
LODS, Lambaréné Organ Dysfunction Score; AVPU, alert-verbal-pain-unresponsive score; SIRS, systemic inflammatory response syndrome.
Discussion
This pediatric sepsis cohort had a high overall in-hospital mortality of 14.2%, substantially greater than a recent sepsis cohort in Uganda (5.4%) (29) and 48-h mortality in the FEAST trial (approximately 10%) (8). Almost 85% of deaths occurred within the first seven days of hospitalization, and over half of all deaths were within 72 h. Using SIRS criteria as a screening tool and requirement for study inclusion, the addition of the LODS, AVPU, or additional SIRS criteria were not useful in predicting in-hospital, early or late mortality in any of the tested subgroups, illustrating the difficulty of risk-stratification and mortality prediction in this patient population and setting.
The overwhelming majority of children who died did so on the ward and not in the EMD. Pediatric sepsis mortality occurring early in hospitalization, but outside of the EMD, has previously been observed in other SSA pediatric cohorts (8, 29). This may signify that despite acute stabilization, children are decompensating in the hospital, potentially due to poor monitoring, a lack of treatment resources, treatment failure, or the natural course of the disease; this study was not designed to assess the individual contribution of these factors and further investigation is needed.
Early recognition, resuscitation, and referral can be life saving in sepsis; yet, the majority of children had a fever duration over 48 h and were evaluated by providers at other health facilities prior to presentation at MNH, raising concerns about delays to definitive care. This, too, has been previously described; in South Africa, 40% of critically ill children were evaluated by >1 medical provider prior to referral to a tertiary care center, which resulted in delays in definitive care (30). Earlier recognition and targeted referral of high-risk patients by primary care providers could have a significant impact. It could be argued that the rate of antibiotic administration pre-arrival is a sign of provider recognition and treatment of sepsis, though additional exploration of referral patterns and therapies received in primary and secondary health facilities are required to address these aspects of care.
There are several limitations to this study. This cohort presented to an urban, tertiary center and may not reflect the general population in Tanzania. Parents in this cohort had a higher literacy rate (>90%) than previously reported for the general Tanzanian population (80.4%) (31) and 95% of families reported using an “improved” water source, significantly more than the 2015 national average of 55.6% (28), though there are legitimate concerns regarding tap water quality and safety (32, 33) in Tanzania. Given the inclusion criteria, this cohort was likely to be a sicker cohort than the general pediatric population; therefore, results cannot be extrapolated and applied to a general pediatric population. However, since a primary objective was to inform therapy guidelines and recommendations for the treatment of sepsis at a tertiary center, this was an ideal cohort to study.
We used SIRS criteria to identify patients with sepsis, and though these are the current, accepted criteria to identify pediatric sepsis, there has been much criticism regarding this operational definition (7, 29, 34). One criticism is that the definition of sepsis is highly context-dependent, especially in LMIC settings where diagnostic testing may be entirely unavailable or not available within a time frame relevant to guide acute care (7). In Uganda, researchers used SIRS criteria to identify sepsis in children with suspected infection; 86% met SIRS criteria, which captured 94% of all inpatient deaths, showing that SIRS criteria is highly sensitive for sepsis, but not specific, nor is it helpful in predicting mortality in this population and setting (29). The findings from this cohort study are consistent with the previous study results from Uganda.
Early mortality, as compared to late mortality, reflects EMD management and acute stabilization. In children who died within 48-h, 80% received a fluid bolus and 95% received antibiotics either pre-arrival or in the EMD. These findings could signify that guidelines were more closely followed in this subgroup, but failed to alter disease progression. Another possibility is that the intervention (i.e., fluid bolus) may potentially be contributing to early mortality as was seen in the FEAST trial (8). Further analyses are required to determine guideline compliance and the association of fluid bolus with mortality.
We found that pathogen identification with conventional microbiological techniques were low-yield and not frequently used, making it difficult to determine the etiology of sepsis. A malaria RDT was far more likely to be performed than a blood culture in children with sepsis. This may be due to several factors. First, a significant percentage of children received antibiotics prior to arrival to the EMD Second, the majority of blood cultures were drawn in the ward and not in the EMD and cultures were not routinely drawn prior to initiation of antibiotics. Third, blood culture collection supplies, specifically the bottles, may not always be readily available. Finally, some clinicians expressed general distrust of the microbiologic results and did not think the results changed management.
We also found that 36% (N = 146) of children did not receive antibiotics in the EMD or prior to arrival despite antibiotic administration being an essential component of international guidelines (1, 16, 18). The low rate of antibiotic administration may have resulted from limited drug availability, failure to identify sepsis, the need for urgent treatment, incomplete documentation of drug administration, or some combination of these factors. Further work is necessary to elucidate the underlying cause of low antibiotic usage in this setting. Depending on the cause, early antibiotic administration in the EMD for children with sepsis is a potential future quality improvement project.
Some data were extracted from the chart and may not have captured all interventions performed; however, the presence of research assistants actively observing actions in the EMD likely minimized this effect. Simultaneously, having research assistants physically present in the EMD may have influenced providers’ practices, producing an observer effect. The most likely results of such an effect would be increased provider awareness of sepsis and improved guideline compliance. We did not capture a metric for provider awareness, and guideline compliance in general was poor (not reported here), though antibiotic administration and blood culture collection are two indicators, which is consistent with prior pilot data from MNH.
In summary, pediatric sepsis management must take into account the local context; the unique patient population and its comorbidities, coinfections, and etiologies of sepsis; where and how the population accesses the healthcare system; and available resources including medical personnel and monitoring capabilities. Though there are many challenges to data collection in resource-limited settings—lack of research infrastructure, incomplete documentation, severe personnel shortages, inadequate funding, and technical difficulties performing follow-up—we have generated high-quality regional data that can be used to develop an accurate risk stratification tool and inform context-appropriate, evidence-based guidelines in this setting. This is the first, critical step towards improving pediatric sepsis outcomes in SSA.
Ethics Statement
Research personnel collected data from the electronic medical record, paper chart, care provider, and guardian and then entered it into REDCap (version 7.2.2). Data were deidentified prior to analysis. This study was carried out in accordance with the recommendations and approval of the Institutional Review Boards and Committees on Human Research at Muhimbili University of Health and Allied Sciences (Ref. No. 2016-03-30/AEC/Vol.X/201) and the University of California, San Francisco (IRB # 16-18977, Ref. No. 161295). We obtained written, informed consent from all guardians and assent from subjects when appropriate in accordance with the Declaration of Helsinki.
Author Contributions
Author contributions are as follows: conception and design of the work (TK, HS, BM, WE, and TR); data acquisition (TK, HS, and BM); data analysis (TK); data interpretation (TK, HS, BM, MM, and TR); first draft of the manuscript (TK); and manuscript revision and editing, final approval of the version to be published, and agreement to be accountable for all aspects of the work (TK, HS, BM, WE, MM, and TR).
Conflict of Interest Statement
MM reports grants from Amgen and GlaxoSmithKline, personal fees from Roche-Genentec, Boerhinger-Ingelheim, Bayer, Quark Pharmaceuticals, Biogen, GlaxoSmithKline, Cerus Therapeutics, and Thesan Pharmaceuticals outside the submitted work. The other authors have no conflicts of interest to disclose; the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge Victoria Mlele for her hard work and dedication to this project, as well as Jeff Fineman, Catherine Reuben, and the research assistants at Muhimbili National Hospital for their contributions. Some of the results in this manuscript were presented at the 2017 Pediatric Academic Society meeting (35) and the 2017 Consortium of Universities for Global Health conference (36) and published in an abstract that appeared in Lancet Global Health (37).
Footnotes
Funding. This study was supported by funds from the University of California, San Francisco (UCSF) Division of Critical Care and the UCSF Department of Pediatrics Clinical-Translational Pilot grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med (2005) 6(1):2–8. 10.1097/01.PCC.0000149131.72248.E6 [DOI] [PubMed] [Google Scholar]
- 2.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. World federation of pediatric intensive care and critical care societies: global sepsis initiative. Pediatr Crit Care Med (2011) 12(5):494–503. 10.1097/PCC.0b013e318207096c [DOI] [PubMed] [Google Scholar]
- 3.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med (2005) 6(3 Suppl):S3–5. 10.1097/01.PCC.0000161289.22464.C3 [DOI] [PubMed] [Google Scholar]
- 4.Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health (2012) 2(1):010404. 10.7189/jogh.02.010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet (2014) 388(10063):3027–35. 10.1016/S0140-6736(16)31593-8 [DOI] [PubMed] [Google Scholar]
- 6.Mangia CMF, Kissoon N, Carcillo JA. Sepsis and septic shock: a global overview. J Pediatric Infect Dis (2009) 4(2):71–6. 10.3233/JPI-2009-0157 [DOI] [Google Scholar]
- 7.Wiens MO, Kumbakumba E, Kissoon N, Ansermino JM, Ndamira A, Larson CP. Pediatric sepsis in the developing world: challenges in defining sepsis and issues in post-discharge mortality. Clin Epidemiol (2012) 4:319–25. 10.2147/CLEP.S35693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med (2011) 364(26):2483–95. 10.1056/NEJMoa1101549 [DOI] [PubMed] [Google Scholar]
- 9.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med (2013) 11:68. 10.1186/1741-7015-11-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassebaum N, Kyu HH, Zoeckler L, Olsen HE, Thomas K, Pinho C, et al. Child and adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA Pediatr (2017) 171(6):573–92. 10.1001/jamapediatrics.2017.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med (2015) 191(10):1147–57. 10.1164/rccm.201412-2323OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med (2012) 38(4):557–74. 10.1007/s00134-012-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care (2011) 15(1):R10. 10.1186/cc9410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One (2009) 4(11):e7782. 10.1371/journal.pone.0007782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Sun B, Yue H, Lin X, Li B, Yang X, et al. An epidemiologic survey of pediatric sepsis in regional hospitals in China. Pediatr Crit Care Med (2014) 15(9):814–20. 10.1097/PCC.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med (2013) 41(2):580–637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira CF. Early goal-directed therapy in treatment of pediatric septic shock. Shock (2010) 34(Suppl 1):44–7. 10.1097/SHK.0b013e3181e7e6d5 [DOI] [PubMed] [Google Scholar]
- 18.WHO. Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva: World Health Organization; (2013). [PubMed] [Google Scholar]
- 19.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med (2009) 37(2):666–88. 10.1097/CCM.0b013e31819323c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira CF, de Oliveira DSF, Gottschald AFC, Moura JDG, Costa GA, Ventura AC, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med (2008) 34(6):1065–75. 10.1007/s00134-008-1085-9 [DOI] [PubMed] [Google Scholar]
- 21.Barreiro P, Tiziano G, Fano H, Yohannes T, Gosa A, Reyes F, et al. Malaria and severe anemia over eight years at Gambo Rural Hospital, southern Ethiopia. Pathog Glob Health (2017) 111(4):195–9. 10.1080/20477724.2017.1322262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. N Engl J Med (2008) 358(9):888–99. 10.1056/NEJMoa072727 [DOI] [PubMed] [Google Scholar]
- 23.Reynolds TA, Mfinanga JA, Sawe HR, Runyon MS, Mwafongo V. Emergency care capacity in Africa: a clinical and educational initiative in Tanzania. J Public Health Policy (2012) 33(Suppl 1):S126–37. 10.1057/jphp.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UNAIDS. HIV and AIDS Estimates 2014 (2014). Available from: http://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania
- 25.Helbok R, Kendjo E, Issifou S, Lackner P, Newton CR, Kombila M, et al. The Lambarene Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis (2009) 200(12):1834–41. 10.1086/648409 [DOI] [PubMed] [Google Scholar]
- 26.Conroy AL, Hawkes M, Hayford K, Namasopo S, Opoka RO, John CC, et al. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non-malaria febrile illness. Crit Care (2015) 19:47. 10.1186/s13054-015-0773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform (2009) 42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNICEF WHO. WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation (2015). Available from: https://www.wssinfo.org/
- 29.Wiens MO, Larson CP, Kumbakumba E, Kissoon N, Ansermino JM, Singer J, et al. Application of sepsis definitions to pediatric patients admitted with suspected infections in Uganda. Pediatr Crit Care Med (2016) 17(5):400–5. 10.1097/PCC.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodkinson P, Argent A, Wallis L, Reid S, Perera R, Harrison S, et al. Pathways to care for critically ill or injured children: a cohort study from first presentation to healthcare services through to admission to intensive care or death. PLoS One (2016) 11(1):e0145473. 10.1371/journal.pone.0145473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bank W. Adult Literacy Rate, Population 15+ Years, Both Sexes (%). The World Bank; (2015). Available from: http://data.worldbank.org/indicator/SE.ADT.LITR.ZS?locations=TZ&view=chart [Google Scholar]
- 32.Kihupi CSM, Yohana L, Saria JA, Malebo HM. Fecal contamination of drinking-water in Tanzania’s commercial capital, Dar Es Salaam: implication on health of the consumers. SM J Public Health Epidemiol (2016) 2(1):1025–9. [Google Scholar]
- 33.Mohamed H, Clasen T, Njee RM, Malebo HM, Mbuligwe S, Brown J. Microbiological effectiveness of household water treatment technologies under field use conditions in rural Tanzania. Trop Med Int Health (2016) 21(1):33–40. 10.1111/tmi.12628 [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet (2013) 381(9868):774–5. 10.1016/S0140-6736(12)61815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortz TB, Murray B, Matthay M, Reynolds T. Pediatric Sepsis Risk Factors and Barriers to Care at a Referral Hospital in Tanzania: A Prospective Cohort Study. San Francisco, CA: Pediatric Academic Society; (2017). [Google Scholar]
- 36.Kortz TB, Murray B, Matthay M, Reynolds T. Surviving paediatric sepsis in Tanzania: a prospective cohort study to identify risk factors. Consortium of Universities for Global Health. Washington, DC: (2017). [Google Scholar]
- 37.Kortz TB, Murray B, Matthay M, Reynolds T. Surviving paediatric sepsis in Tanzania: a prospective cohort study to identify risk factors. Lancet Glob Health (2017) 5(S14). 10.1016/S2214-109X(17)30121-3 [DOI] [Google Scholar]