Abstract
Background:
Bisphenol A (BPA) is an endocrine-disrupting chemical that may contribute to development of obesity and metabolic disorders. Humans are constantly exposed to low concentrations of BPA, and studies support that the developmental period is particularly sensitive.
Objectives:
The aim was to investigate the effects of low-dose developmental BPA exposure on metabolic parameters in male and female Fischer 344 (F344) rat offspring.
Methods:
Pregnant F344 rats were exposed to BPA via their drinking water, corresponding to (BPA0.5; ) or (BPA50; ), from gestational day (GD) 3.5 until postnatal day (PND) 22, and controls were given vehicle (). Body weight (BW), adipose tissue, liver (weight, histology, and gene expression), heart weight, and lipid profile were investigated in the 5-wk-old offspring.
Results:
Males and females exhibited differential susceptibility to the different doses of BPA. Developmental BPA exposure increased plasma triglyceride levels ( compared with , females BPA50 ; compared with , males BPA0.5 ) in F344 rat offspring compared with controls. BPA exposure also increased adipocyte cell density by 122% in inguinal white adipose tissue (iWAT) of female offspring exposed to BPA0.5 compared with controls ( number of adipocytes/HPF compared with number of adipocytes/HPF; ) and by 123% in BPA0.5 females compared with BPA50 animals ( number of adipocytes/high power field (HPF) compared with number of adipocytes/HPF; ). In iWAT of male offspring, adipocyte cell density was increased by 129% in BPA50-exposed animals compared with BPA0.5-exposed animals ( number of adipocytes/HPF compared with number of adipocytes/HPF; ). Furthermore, the expression of genes involved in lipid and adipocyte homeostasis was significantly different between exposed animals and controls depending on the tissue, dose, and sex.
Conclusions:
Developmental exposure to of BPA, which is 8–10 times lower than the current preliminary EFSA (European Food Safety Authority) tolerable daily intake (TDI) of and is within the range of environmentally relevant levels, was associated with sex-specific differences in the expression of genes in adipose tissue plasma triglyceride levels in males and adipocyte cell density in females when F344 rat offspring of dams exposed to BPA at were compared with the offspring of unexposed controls. https://doi.org/10.1289/EHP505
Introduction
Debate continues regarding whether developmental exposure to bisphenol A (BPA) can induce metabolic effects, and results from in vivo studies are contradictory. Numerous studies on rodents have reported that developmental exposure to BPA may disturb normal metabolic features, such as early adipogenesis, body weight (BW), lipid levels, liver metabolism, and glucose homeostasis (Rubin et al. 2016; Somm et al. 2009; Susiarjo et al. 2015; Wei et al. 2011); therefore, BPA has been suggested as a potential obesogen (Lind et al. 2016). A few other studies have reported no effects on BW following early exposure to BPA (Kabuto et al. 2004; Newbold et al. 2007a; Roepke et al. 2016) (Table 1).
Table 1.
Metabolic disturbances observed in animal studies following developmental exposure to bisphenol A.
| Paper | Doses () | Exposure window | Exposure route | Strain, species | Outcomes |
|---|---|---|---|---|---|
| (Cabaton et al. 2013) | 0.025, 0.25, 25 | GD8–PND16 | Osmotic pump | CD-1 mice | Disrupted global metabolism () PND2, 21; significant doses: 0.025, 0.25, |
| 1, 10 | Perinatal | Water | S-D rats | Increased BW and visceral adipose tissue, abnormal lipid levels, lower adiponectin levels; significant doses: 1 and | |
| (García-Arevalo et al. 2014) | 10 | GD9–GD16 | Subcutaneous | OF-1 mice | Increased BW and increased weight of fat pad mass increased hepatic triglyceride levels, alterations of mRNA gene expression of genes involved in lipogenesis and liver metabolism () PND196; significant dose: |
| (Kabuto et al. 2004) | 5, 10 () | Embryonic/fetal and throughout lactation | Water | ICR mice | No effect on BW () |
| (Miyawaki et al. 2007)a | 1, 10 () | GD10–throughout lactation | Water | ICR mice | Increased BW (, ) adipose tissue weight, total cholesterol levels () and triacylglycerol levels () PND31; significant doses: 1 and |
| (Newbold et al. 2007a) | 10, 100, 1,000 | Perinatal | Subcutaneous | CD-1 mice | No effect on BW () |
| (Roepke et al. 2016) | 50, 5,000 | Embryonic day 18–21 and PND0–PND7 | i.p to dams, subcutaneous to pups | FCDC rats | No effect on BW, decreased levels of adipoR1, no change in ER1, 2 or levels () PND50–60; significant doses: 50 and |
| (Rubin et al. 2016) | 0.25, 2.5, 25, 250 | Perinatal (P) or perinatal and peripubertally () | Osmotic pump | CD-1 mice | Increased BW (P and ) PND28 and 35; elevated insulin levels (P and ) PND196 and 238; and elevated glucose levels () PND238; significant doses: 0.25 and |
| (Ryan and Vandenbergh 2006) | 2, 200 | GD3-PND21 | Gavage | C57/Bl-6 mice | No effect on BW (, ) |
| (Ryan et al. 2010) | 0.25 | GD1–PND21 | Diet | CD-1 mice | Increased BW and length that did not persist throughout adulthood (, ) PND21; significant dose: |
| (Somm et al. 2009) | 70 | GD6–PND21 | Water | S-D rats | Increased BW PND1 (, ), PND21 (); increased pWAT and BAT mass, adipocyte hypertrophy and alterations of mRNA gene expression of genes involved in metabolism and lipogenesis PND21(); significant dose: |
| (Susiarjo et al. 2015) | 10, 10,000 | Perinatal | Diet | C57BL/6 mice | Decreased BW PND1; increased BW, higher body fat content, and impaired glucose homeostasis () PND98–117; significant dose: |
| (Tremblay-Franco et al. 2015) | 0.25, 2.5, 25, 250 | Perinatal | Osmotic pump | S-D rats | Metabolic changes in liver and serum composition (, ) PND21, 50, 90, 140 and 200; significant doses: 0.25, 2.5, 25, and |
| (van Esterik et al. 2014)b | 3, 10, 30, 100, 300, 1,000, 3,000 | Gestation and lactation | Diet | Hybrid C57BL/6J mice | Increased () and decreased () BW, decreased fat pad weights, adipocyte size (increased in , not dose-dependent), and levels of serum triglycerides, leptin, and adiponectin () PND147 (effects were dose-dependent) |
| (Wei et al. 2011) | 50, 250, 1,250 | GD0–PND21 | Oral gavage | Wistar rats | Increased body fat percentage (, ), increased levels of triglycerides and size of adipocytes () PND189; significant dose: |
| This study | 0.5, 50 | GD3.5–PND22 | Water | F344 rats | No effect on BW. Increased plasma triglycerides, adipocyte density (decreased adipocyte size), and alterations of mRNA expression of genes involved in lipogenesis, adipocyte adiponectin signaling, and liver metabolism (e.g, increased levels of adipoR1, no change in ER1, 2, or levels) (, ) PND22; significant doses: 0.5 and |
Note: Adipor1, adiponectin receptor 1; BAT, brown adipose tissue; BW, body weight; ER, estrogen receptor; FCDF, Fischer CDF; F344, Fischer 344; GD, gestational day; i.p, intraperitoneal; OF-1, Oncins France 1; PND, postnatal day; PPARγ, peroxisome proliferator-activated receptor gamma; pWAT, perigonadal adipose tissue; S-D, Sprague-Dawley. Significant doses are statistically significant changes compared with controls.
Animals were challenged with a high-fat diet or fructose.
The benchmark dose approach was used in this study.
BPA is used in the manufacturing of many products, including polycarbonate and epoxy plastic food packaging material, and it has been shown that BPA leaches from containers into foodstuff (Sajiki and Yonekubo 2004; Vandenberg et al. 2007). Geens et al. (2012) estimated a daily human exposure level of from dietary and nondietary sources. Recently, LaKind and Naiman (2015) estimated a median daily intake of for the general U.S. population in 2011–2012, and Covaci et al. (2015) reported estimated geometric mean intakes of and for children and their mothers, respectively, from six European countries. (Covaci et al. 2015; LaKind and Naiman 2015). Several studies have revealed measurable urinary BPA concentrations in of humans in numerous different countries throughout the world (Calafat et al. 2008; Guidry et al. 2015; LaKind and Naiman 2015; Zhang et al. 2011).
BPA is an endocrine disruptor with the capacity to bind to several receptors (Casals-Casas and Desvergne 2011) and has been reported to act as a selective estrogen receptor modulator (SERM), meaning that BPA can execute other modes of action than through classical estrogenic pathways, and, additionally, signaling may vary across different cell types and tissues (Nagel et al. 2001). BPA interacts with both membrane-bound and nuclear estrogen receptors (ERs), and it also activates nongenomic ER pathways (Vandenberg et al. 2009) and further it binds to the orphan receptor human estrogen-related receptor gamma, , with high affinity (Takayanagi et al. 2006). Although BPA was previously believed to be a weak estrogen, more recent studies reveal that in certain contexts, BPA is a potent ER activator (Alonso-Magdalena et al. 2012; Welshons et al. 2006). Further, BPA has been shown to be a weak thyroid hormone receptor antagonist in Sprague DawleyTM (S-D) rats exposed to BPA during pregnancy and lactation (Zoeller et al. 2005); it also has anti-androgenic and aromatase inhibiting properties and binds to the aryl hydrocarbon receptor (AhR) in a human breast cancer cell line (Bonefeld-Jørgensen et al. 2007). AhR is also involved in cross-talk with several other endocrine receptor types (Pocar et al. 2005). The fact that BPA was originally considered a solely estrogenic compound limited which end points were studied, concentrating on, for example, uterotrophic response (Markey et al. 2001; Schmidt et al. 2006). At the present time, other end points are being included, better reflecting the ability of BPA to affect various cell signaling pathways. An analysis of ToxCast™ data used to screen and prioritize 309 environmental chemicals for their potential to act as endocrine disruptors ranked BPA as having the third-highest Toxicological Priority Index (ToxPi), reflecting its capacity to interfere with several different signaling systems (Reif et al. 2010).
Sex-specific effects of BPA exposure have been reported in both epidemiological and experimental studies (Caporossi and Papaleo 2015). One example of a study that showed evident sex-specific differences is that by van Esterik et al. (2014), in which hybrid mice (C57BL/6JxFVB) were prenatally exposed to BPA. A dose-dependent increase in body and liver weight was reported in adult male offspring, whereas a dose-dependent decrease in body and liver weight was seen in female offspring, suggesting that BPA can program different metabolic phenotypes in male and female mouse offspring.
During development, hormones in minute concentrations (pico- to nanomolar) regulate the differentiation and growth of cells, and this delicate regulation may thus be sensitive to disruption by endocrine active compounds. What should be considered low-dose exposure to endocrine-disrupting compounds has been debated, sometimes defined as below the lowest-observed-adverse-effect-level (LOAEL), the no-observed-adverse-effect-level (NOAEL), or tolerable daily intake (TDI), but is now more often defined as environmentally relevant levels (Vandenberg 2014), that is to say, the level of the specific compound to which the population is generally exposed. In 2015, the TDI of BPA was reduced by the European Food Safety Authority (EFSA) from to a preliminary TDI of owing to new data and refined methodologies (EFSA 2015). However, several low-dose animal studies have reported biological effects of endocrine-disrupting chemicals (EDCs), including BPA, at doses below the current preliminary EFSA TDI. (Vandenberg et al. 2013; vom Saal and Hughes 2005). Hass and colleagues have proposed that the preliminary EFSA TDI of may not sufficiently protect humans from endocrine-disrupting effects based on experimental evidence of effects on behavior, early sexual and mammary gland development, and sperm count in rats (Christiansen et al. 2014; Hass et al. 2016; Mandrup et al. 2016).
The aim of the present study was to examine the influence of developmental low-dose BPA exposure on adipose tissue and metabolic biomarkers in young Fischer 344 (F344) rats. We used the F344 rat because it may be more sensitive to hormone disruption than the frequently used S-D rat (Long et al. 2000; Steinmetz et al. 1997; Steinmetz et al. 1998), and we evaluated two exposure doses in the TDI range: , a dose 8–10 times lower than the preliminary EFSA TDI, and a higher dose corresponding to the U.S Food and Drug Administration (FDA) (2008), reference dose (RfD) of . Outcomes were examined in 5-wk-old female and male F344 offspring, including BW, liver weight, adipose tissue weight, heart weight and heart somatic index (HSI), gene expression, circulating metabolic markers, and adipose and liver tissue morphology.
Materials and Methods
Chemicals
BPA (CAS 80-05-7, , purity) (Sigma Aldrich) was dissolved in ethanol (1% of final solution) and diluted with well-flushed tap water to defined concentrations.
Animals and Housing
This study adheres to the ARRIVE guidelines for animal research (Kilkenny et al. 2010). The completed ARRIVE guidelines checklist is available upon request from the authors. The Uppsala Ethical Committee on Animal Research approved this study (C26/13) following guidelines laid down by the European Union Legislation (Council of Europe 1986 and European Parliament and the Council of the European Union 2010). All animals were treated humanely and with regard for alleviation of suffering.
Forty-five time-mated 9-wk-old female F344/DuCrl rats (Charles River) were weighed and chip-marked upon arrival in our laboratory on gestational day (GD)3.5. The study was performed using seven blocks (separated by 1 wk), and all dose groups were equally distributed among blocks. The dams were randomly distributed into three dosing groups [0 (), 0.5 () or 50 () ], with dams assigned per group aimed at retrieving 12 offspring per dose and sex. The dams arrived during 7 wk, and because some animals were not pregnant (see Table S1), an allocation of animals to groups that were lacking pregnant animals was made, explaining the difference in the number of dams in each dosing group. The manufacturer provided information on the microbiological status of the purchased animals. The rats were kept at an Uppsala University animal facility in enriched polysulfone cages (Euro Standard IV) with glass water bottles to minimize background BPA exposure and were housed in a temperature- () and humidity-controlled () room with a 12-h light/dark cycle and air turnover ten times per hour. Dams were randomly assigned to the different treatment groups and were housed one dam per cage. Litters were adjusted to six pups per dam (3 males and 3 females) on PND4. On PND22, the dams were sacrificed, and one male and one female from each litter was selected at random, chip-marked, and moved to a new cage that contained 3 offspring of the same sex and treatment group (each of which had a different mother to avoid litter effects). However, in a few cases, one pup (sibling) not included in the experiment was allocated to the cage to obtain 3 animals per cage. In total, there were 26 control offspring (13 males, 13 females), 21 BPA0.5 offspring (dams exposed to ; 11 males, 10 females), and 16 BPA50 offspring (dams exposed to ; 9 males, 7 females). Animals were surveyed on a daily basis. The offspring were weighed at PND22, PND29, and before sacrifice at PND35. Animals were anesthetized using a cocktail of ketamine () and xylazine () (intraperitoneal injection) according to Institutional Animal Care and Use Committee anesthesia guidelines for rats (IACUC 2014). The anogenital distance (AGD) and body length of the offspring were measured, and all animals were sacrificed through aortic exsanguinations. Experiments were carried out during daytime in a dedicated laboratory neighboring the animal facility.
Food and water were available ad libitum, and intake was registered per cage. Rats were fed a standard breeding chow [RM3 (NOVA-SCB)] until weaning and a maintenance diet [RM1 (NOVA-SCB)] after weaning. The manufacturer specified the nutrient and phytoestrogen content of feed provided to the dams and newborn pups [RME3, batch 9,987: 11.2 and of genistein and daidzein, respectively, and total genestein equivalents [] and to offspring after PND22 (RME1, batch 1,028: of both genistein and daidzein, and TGE). All values were well below the Organisation for Economic Co-operation and Development’s (OECD’s) upper limit. (Owens et al. 2003).
Exposure
To mimic the most likely route of human exposure, dams were exposed to BPA via their drinking water ad libitum from GD3.5 until PND22. Consumed water volume was recorded. Control females received water containing 1% ethanol (vehicle). Based on the volume consumed by the dams in our pilot study, we aimed for average doses of (denoted BPA0.5) and (denoted BPA50) (see Table S2). The main routes of BPA exposure were via the placenta in utero and via lactation. BPA concentrations were verified at the Division of Occupational and Environmental Medicine in Lund, Sweden, using the modified method described in (Bornehag et al. 2015). The division in Lund is a reference laboratory chosen for the European biomonitoring project [Consortium to Perform Human Biomonitoring on a European Scale (COPHES); http://www.eu-hbm.info/democophes].
Blood and Organ Sampling
Blood and organ samples were collected from 26 control rats (13 males, 13 females), 21 BPA0.5 rats (11 males, 10 females), and 16 BPA50 rats (9 males, 7 females). Blood was collected in ethylenediaminetetraacetic acid (EDTA)/protease inhibitor–treated tubes and centrifuged (,10 min, ) to prepare plasma. Aliquots were stored at for blood lipid analyses and at for all other analyses.
Retroperitoneal white adipose tissue (rWAT) was collected from the dorsal wall of the abdominal cavity, and gonadal WAT (gWAT) was collected from areas surrounding the epididymis, testis, and ovary. Inguinal WAT (iWAT) was dissected from the area around the pelvis and from the hind limb thigh. The conflated interscapular brown and white adipose tissues were separated into interscapular brown (iscpBAT) and white (iscpWAT) adipose tissue. Fat depots and liver were weighed, snap frozen in liquid nitrogen, and stored at . The liver somatic index (LSI; ), anogenital index [AGDi; (Clark 1999)], and heart somatic index (HSI; ) were calculated.
Histological Analysis of Adipose Tissue and Fat Accumulation in Liver
To investigate the potential impact of BPA exposure on adipose tissue morphology, sections of gWAT, iWAT, and iscpBAT were analyzed. All analyses in the present study were performed by individuals without knowledge of dosing groups. Frozen sections ( thick) of iWAT, gWAT, iscpBAT, and liver from 36 animals (6 males and 6 females selected at random from each dose group) were cut at two levels with a distance of using a cryostat (Leica CM1860 UV; Leica Microsystems) and were stained with Oil Red O/hematoxylin. Micrographs were taken of four different areas of each section at 40× magnification using a Leica DMST camera (Leica Microsystems). Adipocyte number in adipose tissue depots and percentage of liver fat were quantified per high power field (HPF) (40× magnification) using the software package Image Processing and Analysis in Java (ImageJ; National Institutes of Health).
RNA Extraction and mRNA Quantification
Total RNA was extracted from adipose tissue and liver samples using the Trizol method (Life technologies/Thermo Fisher) according to the manufacturer’s instructions. DNase treatment was performed for all RNA preparations to remove potential contaminating DNA (Ambion® DNA-free™ DNAse Treatment and Removal Reagents, Life Technologies). RNA concentration and quality (260/280 ratio ) were measured using a Nanodrop™ ND-1000 spectrophotometer (Thermo Scientific).
The liver is a key organ for metabolism and detoxification. Therefore, alterations in the expression of genes involved in liver metabolism were measured using real-time quantitative polymerase chain reaction (RT-qPCR); alterations in gene expression were also measured in adipose tissue. We tested 26 target genes and 2 housekeeping genes. We chose these specific genes for their indicative and representative roles in de novo lipogenesis, beta-oxidation, lipid mobilization, hormonal function, and inflammation. The complete list of gene targets tested in liver and adipose tissue is presented in Table S3. For complementary DNA (cDNA) synthesis, RNA was reverse-transcribed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and random hexamer primers. To measure relative transcription levels using RT-qPCR, cDNA samples were loaded in duplicates with SsoFast EvaGreen qPCR Supermix (Bio-Rad) and exon-spanning primers, designed using Universal Probe Assay Design Center (Roche Diagnostics) and pre-validated for optimal efficiency (80–120%) (see Table S4). The RT-qPCR analyses were performed using a BioRad CFX96 Touch Real-Time Detection System (Bio-Rad). Transcriptional levels (Ct values) were normalized against ribosomal protein, large, P0 (36B4), and normalized values () were subtracted from the mean in control groups to acquire a . The logarithmic values were linearized using the method (Livak and Schmittgen 2001). Moreover, the difference between the treatment groups in expression of examined housekeeping genes 36B4 and glucuronidase beta (Gusb) was tested statistically to exclude the possibility that the treatment per se affected the expression. Overall, 36B4 was the most stable housekeeping gene (females, , , , ; males, ; ; ; ), and further, the amplification of 36B4 exhibited 105.5% efficiency (see Table S4).
Plasma Lipid Analyses
To assess whether BPA exposure affected circulating lipid levels, triglycerides (TGs), adiponectin, leptin, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol were measured in the offspring. Plasma TG and cholesterol analyses were performed using an Architect c8000/c16000 analyzer (Abbott Laboratories) and four different kits: Triglyceride Cat. No. 7D74-21, Cholesterol Cat. No. 7D62-21, LDL-Cholesterol Cat. No. 1E31-20, and HDL-Cholesterol Cat. No. 3K33-21 (Abbott Laboratories) at the Central Clinical Chemistry Laboratory, Uppsala University Hospital, Uppsala, Sweden.
Plasma adiponectin and leptin levels were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) (Rat Total Adiponectin/Acrp30 Quantikine and Mouse/Rat Leptin Quantikine kits; R&D Systems). Intra- and inter-assay precision for leptin were 5.9% and 4.8%, respectively; for adiponectin, intra- and inter-assay precision were 13.1% and 12.5%.
Statistical Analyses
Statistical analyses were performed using STATISTICA 12 (StatSoft Inc.). Males and females were primarily analyzed separately based on the hypothesis that BPA is an endocrine disruptor with likely sex-specific effects. To test this assumption, an interaction term between dose and sex was included. If the interaction term was not significant, we performed a secondary data-driven analysis, merging data from males and females.
Levene’s test for homogeneity of variance and the Shapiro–Wilk normality test (SW-W) were performed to determine whether data were normally distributed. Differences between control and exposed groups were evaluated by one-way analysis of variance (ANOVA) if normally distributed, and by the Kruskal–Wallis H (KW-H) test if not. These analyses were followed by Dunnett’s or Kruskal-Wallis ANOVA post-hoc tests. Results are expressed as the . A p-value of was considered to be statistically significant.
Results
Body Weight, Weight Gain, Food and Water Intake of Dams during Gestation
Weight gain and food and water intake of exposed dams were measured to evaluate whether BPA exposure affected maternal physiology. The average BW of dams at PND22 did not differ significantly between control and BPA-exposed dams. Further, no statistically significant differences were observed between the treatment groups regarding weight gain or food intake. The mean total water consumption was slightly lower for BPA-exposed dams; however, the difference was not statistically significant ( compared with BPA0.5: ) ( CTRL compared with BPA50: ) (see Table S1). Pups of one BPA0.5 dam were transferred to other BPA0.5 dams at PND4, which explains why for this dose group.
Effects on Body and Organ Weights of Offspring
The number of dams without litters varied among the dose groups, including 4/18, 0/12, and 6/15 in the controls, BPA0.5, and BPA50 groups, respectively, but the pairwise difference was only significant between the two treatment groups (Table S5).
In the primary analysis of outcomes according to sex, there were no significant differences in males or females in the final BW of BPA-exposed offspring compared with controls (Table 2). In addition, there were no significant differences in weight gain, BW at PN22, or the weight of the gonadal, inguinal, retroperitoneal, white, or brown interscapular fat pads; nor were there significant differences in AGD, AGDi, LSI, body length, or heart, spleen, or liver weight and liver fat infiltration in female or male offspring (Table 2). In males, however, HSI was decreased in BPA0.5-exposed offspring compared with unexposed offspring (ANOVA ) ( compared with , ); however, the pair-wise comparison (Dunnett’s test) was not statistically significant.
Table 2.
Weight parameters and other measurements in male and female offspring ().
| Outcome | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | BPA/kg/d | BPA/kg/d | ANOVA p-value | Controls | BPA/kg/d | BPA/kg/d | ANOVA p-value | |
| Number of animals | 13 | 10 | 7 | 13 | 11 | 9 | ||
| Weaning BW (g) | 0.2 | 0.7 | ||||||
| Final BW (g) | 0.6 | 0.9 | ||||||
| Weight gain, wk 3–5 (g) | 0.1 | 0.6 | ||||||
| Gonadal fat pad (g) | 0.8 | 0.8 | ||||||
| Inguinal fat pad (g) | 0.8 | 0.6 | ||||||
| Retroperitoneal fat pad (g) | 0.4 | 0.3 | ||||||
| Interscapular WAT (g) | 0.08 | 0.5 | ||||||
| Interscapular BAT (g) | 0.2 | 1.0 | ||||||
| Spleen (g) | 0.5 | 0.4 | ||||||
| Heart (g) | 0.2 | 0.2 | ||||||
| HSI | 0.2 | a | b | 0.04* | ||||
| Liver weight (g) | 0.8 | 0.5 | ||||||
| LSI | 0.7 | 0.2 | ||||||
| Liver fat (%) | 0.8 | 0.6 | ||||||
| Body length (cm) | 0.9 | 0.6 | ||||||
| AGD (mm) | 0.8 | 0.8 | ||||||
| AGDi () | 0.7 | 0.8 | ||||||
| HDL (mmol/L) | 0.3a | 0.3 | ||||||
| LDL (mmol/L) | 1.0 | 0.4 | ||||||
| Total cholesterol (mmol/L) | 0.2 | 0.05 | ||||||
| Triglycerides (mmol/L) | c | d | 0.02*e | f | g | 0.008** | ||
| Plasma adiponectin (ng/mL) | 0.5 | 0.1e | ||||||
| Plasma leptin (ng/mL) | 0.6 | 0.3 | ||||||
Note: AGD, anogenital distance; AGDi, anogenital index; ANOVA, analysis of variance; BAT, brown adipose tissue; BPA, bisphenol A; BW, body weight; HDL, high-density lipoprotein; HIS, heart somatic index; LDL, low-density lipoprotein; LSI, liver somatic index; WAT, white adipose tissue. Animals were exposed to BPA from gestational day (GD)3.5 until weaning at week 3 and were sacrificed at wk 5 (postnatal day 35). Weight gain was recorded from wk 3–5. Dams were dosed with 0.5 or (actual average doses 0.4 and , respectively). Control dams were given water with 1% ethanol (vehicle).
CTRL–BPA0.5: .
CTRL–BPA50: .
CTRL–BPA0.5: .
CTRL–BPA50: .
Data not normally distributed; Kruskal-Wallis p-value (and Kruskal-Wallis post hoc test) shown.
CTRL–BPA0.5: .
CTRL–BPA50: .
; **.
Associations between BPA and heart weight and HSI were not significantly different between males and females (interaction of 0.95 and 0.57, respectively). Therefore, we conducted a secondary analysis of these outcomes for both sexes combined (see Table S6). Heart weight and HSI of BPA0.5-exposed animals (males and females combined) were significantly lower than those of controls (95% and 94% of controls, respectively, both , pair-wise comparison; Dunnett’s test), but the outcomes were not significantly different from controls for the BPA50 group.
Effects on Plasma Lipids and Adipokines in Offspring
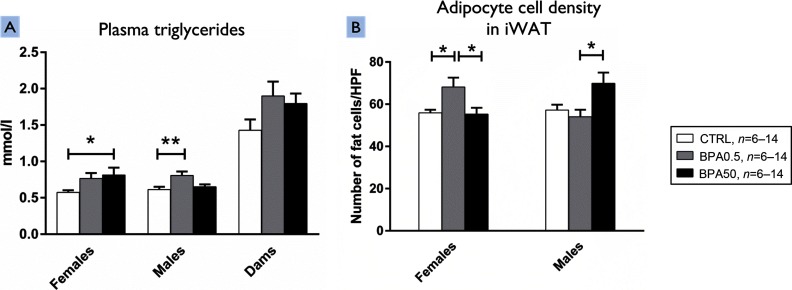
On PND35, plasma TG was significantly higher in BPA0.5 males than in controls ( compared with , ) but was not significantly different in BPA50 males compared with controls (Table 2 and Figure 1A). In females, plasma TG was significantly higher in BPA50 females than in controls ( compared with , ) but not in BPA0.5 offspring (Table 2 and Figure 1A).
Figure 1.
Plasma triglyceride levels and adipocyte density in iWAT of female and male F344 offspring following developmental bisphenol A (BPA) exposure. Effects of developmental exposure to 0.5 (BPA0.5), 50 (BPA50), or ; BPA0.5, BPA50, CTRL on (A) plasma triglyceride levels (mmol/L) in five-wk-old female and male F344 offspring and dams (females, : CTRL, ; BPA0.5, ; BPA50, ); the Kruskal–Wallis test was used to calculate the difference between groups and p-values from Dunnett's test are shown in the figure (males, : CTRL, ; BPA0.5, ; BPA50, ); analysis of variance (ANOVA)/Dunnett’s test was used to calculate the difference between groups and p-values from Dunnett's test are shown in the figure. (B) Average number of iWAT fat cells per high power field in female and male F344 offspring [postnatal day (PND)35] (females, CTRL, ; BPA0.5, ; BPA50, ; males, ; CTRL, ; BPA0.5, ; BPA50, ), ANOVA/Dunnett’s test was used to calculate the difference between groups. Values are shown as the . Note: F344, Fischer 344 rat; iWAT, inguinal white adipose tissue. * ** .
Adiponectin and leptin plasma levels were not significantly different from controls in either dose group of males or females (Table 2). No significant interaction between sex and dose of BPA was observed regarding adiponectin or leptin ( for interaction terms). Therefore, the two sexes were combined in the following analyses.
When both sexes were combined, plasma adiponectin was higher in both dose groups than in controls (124% and 123% higher for BPA0.5 and BPA50, respectively), but the differences were not statistically significant () (see Table S6).
Adipocyte Cell Density
Adipocyte cell density was significantly increased by 121.9% in iWAT of female offspring exposed to BPA0.5 compared with controls ( number of adipocytes/HPF compared with number of adipocytes/HPF, ). In addition, a 123.2% increase in adipocyte cell density was observed in iWAT of female offspring exposed to BPA0.5 compared with offspring exposed to the BPA50 dose ( number of adipocytes/HPF compared with number of adipocytes/HPF, ) (Figure 2A). In iWAT of male offspring exposed to BPA50 compared with that in male offspring exposed to BPA0.5, adipocyte cell density was increased by 129.4% ( number of adipocytes/HPF compared with number of adipocytes/HPF, ) (Figure 2B). However, no such differences were observed between offspring exposed to the BPA50 dose and controls. (Figure 1B, 2A and 2B). There were no significant differences from controls in gWAT or iscpBAT cell density for either dose group in males or females (see Table S7).
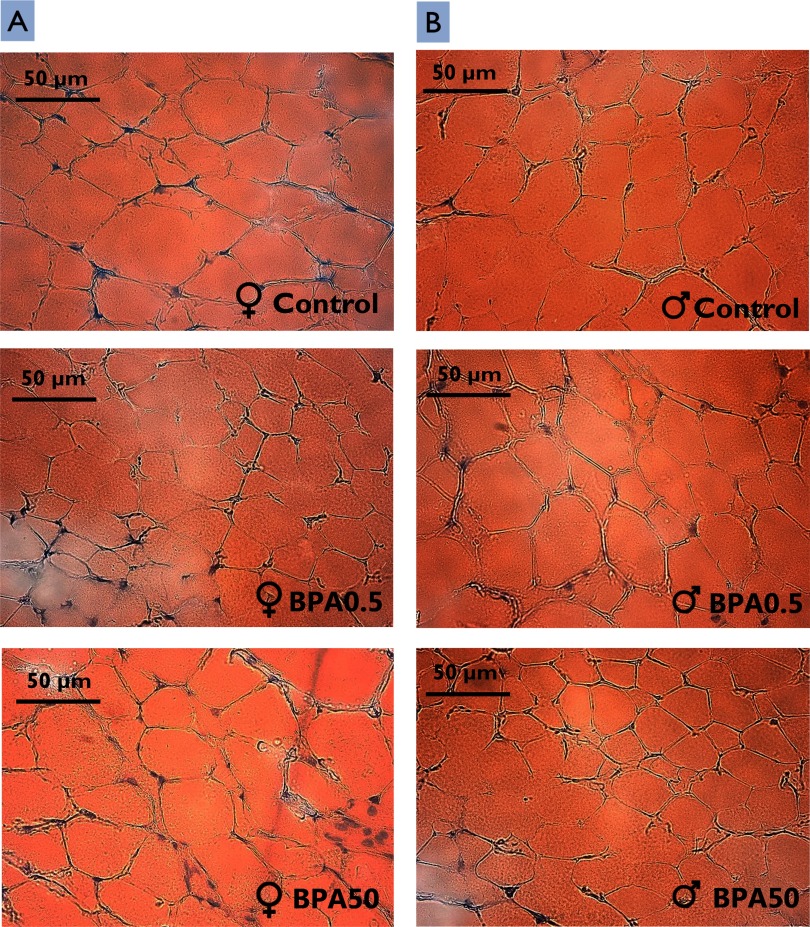
Figure 2.
Micrographs of histological sections of iWAT in control and BPA-exposed female and male F344 offspring. Representative histological sections of iWAT from 0.5 (BPA0.5) or 50 (BPA50) BPA-exposed female (A) and male (B) control and BPA-exposed five-wk-old F344 rat offspring. Sections were stained with Oil Red O. Note: F344, Fischer 344 rat; iWAT, inguinal white adipose tissue.
Gene Transcription in Adipose and Liver
The complete list of gene targets tested in adipose tissue and liver is presented in Table S3. Compared with controls, mRNA expression in gWAT from male offspring was significantly lower in both dose groups for AdipoR2 and ACC and in BPA50 offspring for SCD1, whereas lower expression of LPL in both dose groups was significant based on one-way ANOVA only, and a significant one-way ANOVA for GATA2 reflected significantly lower and higher expression, respectively, in BPA0.5 and BPA50 offspring (Figure 3A and Table S8). In females, mRNA expression in gWAT was significantly higher compared with controls for AdipoR1 in BPA50 offspring only and significantly lower for SREBP-1c in BPA0.5 offspring only, and a significant one-way ANOVA for SCD1 reflected nonsignificant differences in lower and slightly higher expression, respectively, in BPA0.5 and BPA50 offspring (Figure 3B and Table S8). In iWAT, the only significant differences in expression were for lower expression of AdipoR1 and SCD1f in BPA0.5 males compared with controls (Figure 3C-D and Table S8). Additionally, there were no significant differences in the expression of any genes measured in iscpBAT when evaluated separately in males and females (data not shown).
Figure 3.
Transciptional levels in gWAT and iWAT of female and male F344 offspring following BPA exposure. Effects of developmental exposure to 0.5, 50, or (BPA0.5, BPA50, and CTRL, respectively) in five-wk-old F344 offspring on the relative mRNA expression of (A) AdipoR1, AdipoR2, LPL, SCD1, ACC, GATA2 and SREBP-1c in male gWAT (B) AdipoR1, AdipoR2, LPL, SCD1, ACC, GATA2 and SREBP-1c in female gWAT (C) AdipoR1 and SCD1 in male iWAT (D) AdipoR1 and SCD1 in female iWAT (females, : CTRL, ; BPA0.5, ; BPA50, ; males, : CTRL, ; BPA0.5, ; BPA50, ). ANOVA/Dunnett’s test was used to calculate the difference between groups. Values are shown as the . Note: ACC, acetyl-CoAa carboxylase; AdipoR, adiponectin receptor (1 and 2); F344, Fischer 344 rat; GATA2, G protein-coupled estrogen receptor 1; iWAT, inguinal white adipose tissue; LPL, lipoprotein lipase; SCD1, stearoyl-CoA desaturase; SREBP-1c, sterol regulatory element binding protein-1c. * ; # One-way ANOVA for all groups.
When males and females were combined, ACC and SREBP-1c analyzed in gWAT showed significantly lower expression in both dose groups compared with controls, whereas GATA2 expression was significantly different from controls based on one-way ANOVA (), reflecting nonsignificant lower and higher expression, respectively, in BPA0.5 and BPA50 offspring (see Table S6). Adiponectin expression was significantly different from controls in iscpBAT from males and females combined, reflecting nonsignificant higher and lower expression, respectively, in BPA0.5 and BPA50 offspring (see Table S6). There were no significant differences in the expression of any key genes in adipogenesis and adipocyte function in gWAT when evaluating males and females together (data not shown).
Expression of only one of the 18 genes examined in liver tissue showed a significant difference based on one-way ANOVA (see Table S8; data not shown for females or for the other genes evaluated in males). Specifically, expression was lower in male BPA0.5 and BPA50 offspring than in controls, although the difference was significant only for the BPA50 dose group (, ).
Discussion
The previous BPA TDI of , which was presumed safe for many years, was based on an NOAEL of BW/d with a 100-fold uncertainty factor (Tyl et al. 2002; Tyl et al. 2008). In January 2015, EFSA reduced the TDI to a preliminary TDI of while awaiting data from a long-term study in rats (EFSA 2015). In the present study, we evaluated developmental exposures of F344 rats to BPA [8–10 times lower than the current preliminary EFSA TDI, and consistent with human exposures (Chapin et al. 2008)] and BPA [corresponding to the former EFSA (see European Food Safety Authority, http://www.efsa.europa.eu/en/topics/topic/bisphenol) TDI and the current FDA RfD (FDA 2008)], using the oral route of exposure to be consistent with the primary route of BPA exposure in humans (Vandenberg et al. 2007). We found that these exposures were associated with significantly higher plasma triglyceride concentrations and iWAT adipocyte cell density in offspring on PND35 depending on the dose and sex of the offspring evaluated. The expression of genes involved in adiponectin signaling and lipid metabolism also differed between exposed offspring and controls depending on the gene, tissue (with most of the differences limited to iWAT), dose, and sex. Finally, when males and females were combined, heart weight and HSI were significantly lower in BPA0.5 offspring, but not BPA50 offspring, than in controls. In the present study, no significant differences in BW were seen in 5-wk-old male and female offspring exposed to BPA during development compared with controls, in accord with previous publications (Cao et al. 2015; Morrissey et al. 1987; Newbold et al. 2007a). Others have reported increased (Patisaul and Bateman 2008; Somm et al. 2009) or decreased (Negishi et al. 2003) BW in different rat strains exposed to low doses of BPA during development.
In the present study, significantly higher plasma triglyceride levels were observed in 5-wk-old BPA50 female and BPA0.5 male offspring than in controls. In experimental studies, elevated triglyceride levels at an early age in animals exposed to environmental endocrine disruptors have been associated with increased BW later in life (Newbold et al. 2007b). In the present study, we did not observe any significant differences in BW in the offspring at 5 wk of age, but we cannot rule out the possibility that differences might have occurred if follow-up had continued. Inconsistencies among studies may reflect differences in animal models, doses, routes of administration, duration of exposures, diets, developmental stage, and sex of the animals, which are all consistent with possible biological differences (Table 1). In addition, differences in sample size and power are potential noncausal explanations for differences in findings among different studies. To our knowledge, no one has attempted to reproduce findings for effects of BPA on metabolism using the same experimental model and identical conditions (Table 1).
Adipocyte cell density in iWAT (i.e., the number of unilocular cells/HPF) was significantly higher than in controls for female offspring exposed to BPA0.5 but not BPA50 BPA, suggesting adipocyte hyperplasia in response to the lower dose only. In contrast, adipocyte density in males was higher in BPA50 offspring than in BPA0.5 or control offspring (although the difference was significant only between BPA50 and BPA0.5 offspring), suggesting sex-specific differences in effects at a given dose. Adipose tissue expands through an increase in adipocyte cell number (hyperplasia), adipocyte cell size (hypertrophy), or both (Jo et al. 2009). It has been suggested that adipocyte hyperplasia occurs only at early developmental stages, implying that the number of adipocytes is programmed during childhood and remains the same throughout the whole lifetime (Spalding et al. 2008). In the present study, a larger number of cells was observed in iWAT, and one could speculate that these rats may be at a higher risk of storing more fat because of the increased number of cells; this could possibly increase the risk for these animals to develop overweight later in life.
Although adipocyte cell density was higher in iWAT samples from female BPA0.5 offspring compared with controls (and BPA50 offspring), there were no significant differences in the expression of any of the genes measured in iWAT in females (data not shown). In males, adipocyte cell density was higher in iWAT samples from BPA50 offspring than from controls (although not significantly so), whereas expression of 2 of the 30 genes measured in iWAT was lower in BPA0.5 and BPA50 offspring than in controls (significant for the BPA0.5 group only). The relationship between the expression of individual genes and adipose tissue development and regulation is complex, and expression at a single point in time may not reflect expression during critical periods of development. However, additional research will be needed to confirm our findings with regard to the effects of developmental BPA exposure on gene regulation and to determine whether there are any longer-term consequences for BW.
Expression of regulatory adipogenesis-related genes enables proliferation and differentiation of preadipocytes into lipid-storing and expanding adipocytes in a given fat depot (Drolet et al. 2008). In the present study, significant alterations in adipocyte gene expression were observed in BPA-exposed rats. Among these genes were key regulators and enzymes in adipocyte lipogenesis: Both SCD1 and ACC are regulated by the transcription factor SREBP-1c. The enzyme SCD1 converts dietary fatty acids (Mauvoisin and Mounier 2011) and has been described as having an important role in obesity development because mice lacking SCD1 are obesity-resistant and are more insulin-sensitive (Ntambi et al. 2002). Furthermore, adipose tissue-specific knockout of ACC, another central enzyme in lipogenesis, causes a reduction in adipose tissue lipid accumulation (Mao et al. 2009). Earlier reports on exposure of adult rodents to BPA demonstrate an impact on lipogenesis in female adipose tissue as well as in the liver in both sexes, where low BPA doses up-regulate SCD1 and ACC mRNA and protein (Marmugi et al. 2012; Somm et al. 2009). These findings are not consistent with and are opposite to those in the present study, where slightly but significantly reduced SCD1 and ACC mRNA levels were observed in male adipose tissue only, with either a low or high BPA dose, which unexpectedly did not correlate with reduced body or adipose tissue weights. This result could indicate that these modest BPA-induced changes on mRNA level do not influence SCD1 and ACC protein levels or function. Furthermore, discrepancies between results in the present study and those in earlier studies might be explained by, for example, differences in strain, species, choice of dose, route of administration, diet, or time of sacrifice. These discrepancies could create problems in risk assessment for chemicals if they are interpreted as inconsistent results; however, given the complexity of hormonal regulation under different circumstances, it is important to consider all available results, particularly those concerning endocrine disruption.
, which is the master regulator in hepatocyte maturation (Tan et al. 2008), was down-regulated in the BPA50 male rat liver in the present study. A similar down-regulation of the transcription factor was reported to arise in mouse offspring after developmental BPA exposure; however, this occurred only in females (DeBenedictis et al. 2016). This reduction in , indicative of perturbations in hepatocyte development and a putative fetal origin for BPA-induced hepatic disorders, may not necessarily be sex-specific in general; instead, it may reflect a dose-dependent effect, a species-dependent effect, or both. Whether the inconsistency in expression between males and females in the different studies is incidental or reflects differences in study design needs further investigation.
In the present study, liver fat infiltration was not significantly higher in male or female offspring exposed to BPA during development. Earlier experimental studies have shown increased liver fat accumulation following BPA exposure when provoked with high-fat diet or fructose. Liver fat accumulation in male Wistar rat offspring on a high-fat diet was higher in rats developmentally exposed to BPA () than in unexposed rats on the same diet (Wei et al. 2014), and liver fat infiltration in juvenile female F344 offspring on a 5% fructose diet was higher in rats exposed to BPA than in unexposed rats on the same diet (Rönn et al. 2013). These results suggest that developmental exposure to BPA might not cause liver fat accumulation per se, but that BPA aggravates liver fat accumulation if combined with a high-calorie diet. In addition, the additive effect of a high-calorie diet has also been reported for other tissues. In rats given a high-fat diet, those exposed to BPA had a greater increase in obesity, dyslipidemia, and hyperglycemia (some of the conditions that define metabolic syndrome in humans), as well as in hyperleptinemia, hyperinsulinemia, and glucose intolerance, than unexposed rats on the same diet (Wei et al. 2011). Perinatally BPA-exposed male rats on a high-fat diet had higher body weights than male rats with perinatal BPA exposure and a normal diet, suggesting that effects of BPA on obesity may occur only when combined with a high-calorie diet (Somm et al. 2009). This finding may explain why developmental BPA exposure was not associated with increased BW in the present study because the rats were not given a high-calorie diet.
In the present study, rat offspring developmentally exposed to an environmentally relevant dose of BPA ( had significantly lower heart weight and HSI than unexposed controls, when data from males and females were combined. In addition, HSI was lower in males exposed to of BPA than in controls. We and others have reported associations between BPA and the expression of genes regulating angiogenesis, vascular tone, and cardiac structure and function, as well as epigenetic DNA methylation marks and the myocardial proteome (Klint et al. 2016; Ljunggren et al. 2016; Patel et al. 2013). In addition, Patel et al. (2015) reported that adult male mice exposed to from GD11.5 to 3 or 4 mo of age showed more inflammation and less cardiac tissue repair following an experimental myocardial infarction than unexposed mice, had lower collagen and alpha-smooth muscle actin (), and showed higher matrix metalloproteinase protein 2 (MMP2) and MMP9 expression than controls (Patel et al. 2015). Potential mechanisms are unknown, but our findings and those of previous studies suggest that the myocardium may be a target for BPA.
Adiponectin is an adipocyte-specific secreted protein essential for lipogenesis and adipocyte homeostasis (Ye et al. 2014). In contrast with previous reports of lower plasma adiponectin levels in rats with BPA exposure (Angle et al. 2013; Song et al. 2014), we found no significant differences in plasma adiponectin levels in BPA-exposed offspring compared with controls (in males, females, or both sexes combined). Although we noted significant differences in the expression of AdipoR1 and AdipoR2 in adipose tissue, associations were both positive and negative depending on the specific tissue, the dose, and the sex of the animals. In principle, effects of BPA on adiponectin signaling might contribute to the development of “adiponectin resistance,” which may result in an increase in plasma adiponectin levels or a decrease in target-tissue adiponectin receptor levels (Khan et al. 2012; Tsuchida et al. 2004).
In the present study, there were no significant differences in plasma leptin levels between male or female offspring developmentally exposed to BPA compared with controls. These findings were consistent with one previous study of developmental exposure to BPA in S-D rats (Ferguson et al. 2011), but not with a study that reported significantly lower plasma leptin levels in exposed female (but not male) mice with developmental exposure compared with controls (Anderson et al. 2013), or with a study that reported significantly higher plasma leptin levels in developmentally exposed Wistar rats on a high-fat diet compared with unexposed controls on the same diet (Wei et al. 2011).
Regulation of gene expression is complex, involving many different mechanisms. In most organs and cell types, this process can be prompted by environmental factors such as modulation of epigenetic marks (Choudhuri et al. 2010). Multiple lines of evidence from in vitro and in vivo models have shown that developmental exposure to certain environmental pollutants, including BPA, can lead to epigenetic modifications, which in turn can induce alterations in gene expression that may persist throughout the lifetime (Kundakovic and Champagne 2011; Singh and Li 2012). In addition, researchers have observed that epigenetic modifications can be inherited across generations (transgenerational epigenetic inheritance) (Guerrero-Bosagna and Skinner 2014; Xin et al. 2015). A study by Dolinoy et al. (2007) elegantly demonstrates a change in coat color and development of obesity in BPA-exposed mice (decreased methylation), and further, that this effect was negated by exposure to genistein (increased methylation). This finding showed that an EDC and a phytoestrogen can have opposite effects on epigenetic mechanisms and subsequent effects on the phenotype. The decreased methylation observed after BPA exposure gave rise to yellow coat color, diabetes, tumors, and obesity in the adult phenotype (Dolinoy et al. 2007). Alterations of gene expression following BPA exposure are often attributed to ER-mediated actions of BPA. However, BPA has been defined as a SERM, which means that BPA can act through several other pathways and can subsequently induce different effects in various cells and tissues (Nagel et al. 2001). Further, BPA has been shown to activate several other receptors with the potential to affect epigenetic mechanisms, such as the thyroid hormone and androgen receptors (Delfosse et al. 2014; Ozgyin et al. 2015).
In the present study, the simultaneous negative regulation of SCD1, AdipoR1, AdipoR2, and ACC, which are involved in prolipogenic and adiponectin-mediated antilipogenic events, may reflect skewed adipocyte gene regulation of potential epigenetic nature. Environmentally induced epigenetic changes are becoming increasingly important in understanding the etiology of health and disease; however, whether low-dose exposure to BPA altered the epigenetic landscape of adipogenesis- and lipogenesis-related genes in these young rats requires further investigation.
The results of the present study suggest a difference in susceptibility to BPA exposure between males and females. Sex-specific susceptibility to xenoestrogens may depend greatly on timing of exposure and type of xenoestrogen. Several previous studies have reported sex-specific effects following BPA exposure. For example, UDP-glucuronosyltransferase 2B1 (UGT2B1) expression in the livers of Wistar-Imamichi rats was significantly higher in female rats than in male rats, and in BPA-exposed rats, levels of glucuronidated BPA were significantly higher in liver microsomes from females than from males, whereas serum BPA concentrations were significantly higher in males than in females (Takeuchi et al. 2004). These findings suggest that sex-specific differences in BPA metabolism might have contributed to sex-specific differences in outcomes following developmental exposure to different concentrations of BPA in the present study. In another study, McCaffrey and colleagues (McCaffrey et al. 2013) demonstrated that perinatal exposure to BPA altered hypothalamic morphology in a sex-specific manner in rat offspring. Further, in S-D rats, developmental BPA exposure () was associated with significant differences in the hepatic expression of a larger number of genes in males than in females, including , which was significantly lower in males (but not females) compared with controls on PND1 following developmental exposure to BPA (Strakovsky et al. 2015); this finding is consistent with the lower hepatic expression on PND35 in BPA50 males (but not females) compared with controls that was observed in the present study.
Different strains of rodents display different sensitivity towards endocrine-disrupting substances (Hossaini et al. 2003; Kacew et al. 1995; Wiklund et al. 1981). The S-D rat strain has traditionally been used in BPA studies but is reported to be less sensitive to estrogenic substances than other rat strains (Steinmetz et al. 1998; Thigpen et al. 2007). Blood prolactin levels were higher in BPA-exposed F344 rats than in controls, but this was not the case in S-D rats (Steinmetz et al. 1997). Moreover, DNA synthesis in vaginal epithelium was higher in BPA-exposed F344 rats, but not S-D rats, compared with controls (Long et al. 2000). Thus, caution is needed when choosing an animal model for a specific end point, and the F344 rat used in the present study may be more suitable than S-D rats or other animal models for investigating effects of environmentally relevant levels of endocrine-disrupting substances (Richter et al. 2007).
Like humans (Völkel et al. 2002), F344 rats excrete more BPA via the kidneys than S-D rats (Snyder et al. 2000), further supporting the idea that the use of the F344 rat model is more suitable for predicting effects in humans. In addition, human liver microsomes do not glucuronidate BPA as extensively as immature female rat liver microsomes, which imply that humans may be exposed to a higher burden than the rat for the same dose of BPA (Elsby et al. 2001).
Conclusions
In conclusion, in the present study of F344 rats developmentally exposed to low doses of BPA, we observed significant differences in markers of lipid and adipocyte homeostasis in exposed offspring compared with controls that varied depending on the dose received and on the sex of the offspring. However, a longer-term study is necessary to define potential late-onset effects of BPA exposure on obesity and metabolic health.
Compared with controls, F344 rat offspring exposed to BPA during development had significantly higher plasma triglyceride levels (BPA50 females and BPA0.5 males) and significantly higher adipocyte density (BPA0.5 females, with a nonsignificant increase in BPA50 males). Moreover, mRNA expression of genes central to lipogenesis and adipocyte adiponectin signaling in adipose tissue, mainly in gWAT, and one gene in the liver (), differed between BPA-exposed offspring compared with controls, depending on sex and dose. Differences in some metabolic parameters were observed in male or female offspring of dams exposed to the lowest, environmentally relevant, dose (), which is 8–10 times lower than EFSA’s current preliminary TDI of . The results of the present study add to the list of investigations describing effects from BPA exposures at concentrations lower than the present EFSA TDI, suggesting that regulatory agencies should consider lowering the TDI further.
Supplemental Material
Acknowledgments
The authors would like to thank the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, FORMAS, for providing funding [grant no. 216-2012-475]. The authors also wish to acknowledge B. Andersson and M. El-Ghezzaoui for excellent technical assistance, E. Lampa for valuable statistical guidance, and J. Örberg for valuable scientific advice.
References
- Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. 2012. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol 355:201–207, PMID: 22227557, 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. 2013. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J 27:1784–1792, PMID: 23345456, 10.1096/fj.12-223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, et al. 2013. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol 42:256–268, PMID: 23892310, 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. 2007. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: New data and a brief review. Environ Health Perspect 115 (Suppl1):69–76, PMID: 18174953, 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jönsson BA, Lindh CH, Jensen TK, Bodin A, et al. 2015. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect 123:101–107, PMID: 25353625, 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, et al. 2013. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect 121:586–593, PMID: 23425943, 10.1289/ehp.1205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116:39–44, PMID: 18197297, 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Echelberger R, Liu M, Sluzas E, McCaffrey K, Buckley B, et al. 2015. Soy but not bisphenol A (BPA) or the phytoestrogen genistin alters developmental weight gain and food intake in pregnant rats and their offspring. Reprod Toxicol 58:282–294, PMID: 26216788, 10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporossi L, Papaleo B. 2015. Exposure to bisphenol A and gender differences: From rodents to humans evidences and hypothesis about the health effects. J Xenobiotics 5:1, 10.4081/xeno.2015.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: From endocrine to metabolic disruption. Annu Rev Physiol 73:135–162, PMID: 21054169, 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE Jr, Hayward SW, Lees PS, et al. 2008. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 83:157–395, PMID: 18613034, 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Cui Y, Klaassen CD. 2010. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol 245:378–393, PMID: 20381512, 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Axelstad M, Boberg J, Vinggaard AM, Pedersen GA, Hass U. 2014. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 147:477–487, PMID: 24298045, 10.1530/REP-13-0377. [DOI] [PubMed] [Google Scholar]
- Clark RL. 1999. Endpoints of reproductive system development. In: An Evaluation and Interpretation of Reproductive Endpoints for Human Risk Assessment. Dastin G, Kimmel C, eds. Washington, DC:Health and Environmental Science Institute, 27–62. [Google Scholar]
- Council of Europe. 1986. European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. https://rm.coe.int/168007a67b [accessed 13 June 2017].
- Covaci A, Den Hond E, Geens T, Govarts E, Koppen G, Frederiksen H, et al. 2015. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ Res 141:77–85, PMID: 25440295, 10.1016/j.envres.2014.08.008. [DOI] [PubMed] [Google Scholar]
- DeBenedictis B, Guan H, Yang K. 2016. Prenatal exposure to bisphenol A disrupts mouse fetal liver maturation in a sex-specific manner. J Cell Biochem 117:344–350, PMID: 26146954, 10.1002/jcb.25276. [DOI] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, le Maire A, Bourguet W, Balaguer P. 2014. Nuclear receptor profiling of bisphenol-A and its halogenated analogues. Vitam Horm 94:229–251, PMID: 24388193, 10.1016/B978-0-12-800095-3.00009-2. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA U S A 104:13056–13061, PMID: 17670942, 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, et al. 2008. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond) 32:283–291, PMID: 17726433, 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 13:3978, 10.2903/j.efsa.2015.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsby R, Maggs JL, Ashby J, Park BK. 2001. Comparison of the modulatory effects of human and rat liver microsomal metabolism on the estrogenicity of bisphenol A: Implications for extrapolation to humans. J Pharmacol Exp Ther 297:103–113, PMID: 11259533. [PubMed] [Google Scholar]
- European Parliament and the Council of the European Union. 2010. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063, [accessed 13 June 2017].
- FDA (U.S Food and Drug Administration). 2008. “ Draft Assessment of Bisphenol A for use in Food Contact Applications”. https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf [accessed 13 June 2017].
- Ferguson SA, Law CD Jr., Abshire JS. 2011. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol Sci 124:149–160, PMID: 21813462, 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- García-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. 2014. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One 9:e100214, PMID: 24959901, 10.1371/journal.pone.0100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50:3725–3740, PMID: 22889897, 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Skinner MK. 2014. Environmentally induced epigenetic transgenerational inheritance of male infertility. Curr Opin Genet Dev 26:79–88, 10.1016/j.gde.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry VT, Longnecker MP, Aase H, Eggesbø M, Zeiner P, Reichborn-Kjennerud T, et al. 2015. Measurement of total and free urinary phenol and paraben concentrations over the course of pregnancy: Assessing reliability and contamination of specimens in the norwegian mother and child cohort study. Environ Health Perspect 123:705–711, PMID: 25782115, 10.1289/ehp.1408325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K, Axelstad M. 2016. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology, PMID: 27089241, 10.1111/andr.12176. [DOI] [PubMed] [Google Scholar]
- Hossaini A, Dalgaard M, Vinggaard AM, Pakarinen P, Larsen JJ. 2003. Male reproductive effects of octylphenol and estradiol in fischer and wistar rats. Reprod Toxicol 17:607–615, PMID: 14555199, 10.1016/j.reprotox.2003.05.001. [DOI] [PubMed] [Google Scholar]
- IACUC (Institutional Animal Care and Use Committee, University of Iowa). 2014. IACUC guidelines: Anesthesia. http://animal.research.uiowa.edu/iacuc-guidelines-anesthesia [accessed 4 May 2016].
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. 2009. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324, PMID: 19325873, 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto H, Amakawa M, Shishibori T. 2004. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci 74:2931–2940, PMID: 15051418, 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- Kacew S, Ruben Z, McConnell RF. 1995. Strain as a determinant factor in the differential responsiveness of rats to chemicals. Toxicol Pathol 23:701–714, 714–715, PMID: 8772256, 10.1177/019262339502300608. [DOI] [PubMed] [Google Scholar]
- Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, et al. 2012. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation. Circ Heart Fail 5:340–348, PMID: 22379072, 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412, PMID: 20613859, 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klint H, Lejonklou MH, Karimullina E, Rönn M, Lind L, Lind PM, et al. 2016. Low-dose exposure to bisphenol A in combination with fructose increases expression of genes regulating angiogenesis and vascular tone in juvenile Fischer 344 rat cardiac tissue. Ups J Med Sci 122:20–27, 10.1080/03009734.2016.1225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun 25:1084–1093, PMID: 21333735, 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Naiman DQ. 2015. Temporal trends in bisphenol A exposure in the United States from 2003-2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ Res 142:84–95, PMID: 26121292, 10.1016/j.envres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Lind L, Lind PM, Lejonklou MH, Dunder L, Bergman Å, Guerrero-Bosagna C, et al. 2016. Uppsala consensus statement on environmental contaminants and the global obesity epidemic. Environ Health Perspect 124:A81–A83, PMID: 27135406, 10.1289/ehp.1511115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408, PMID: 11846609, 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ljunggren SA, Iggland M, Rönn M, Lind L, Lind PM, Karlsson H. 2016. Altered heart proteome in fructose-fed Fisher 344 rats exposed to bisphenol A. Toxicology 347–349:6–16, PMID: 26930160, 10.1016/j.tox.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Long X, Steinmetz R, Ben-Jonathan N, Caperell-Grant A, Young PC, Nephew KP, et al. 2000. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect 108:243–247, PMID: 10706531, 10.2307/3454441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup K, Boberg J, Isling LK, Christiansen S, Hass U. 2016. Low-dose effects of bisphenol A on mammary gland development in rats. Andrology, PMID: 27088260, 10.1111/andr.12193. [DOI] [PubMed] [Google Scholar]
- Mao J, Yang T, Gu Z, Heird WC, Finegold MJ, Lee B, et al. 2009. aP2-Cre-mediated inactivation of acetyl-CoA carboxylase 1 causes growth retardation and reduced lipid accumulation in adipose tissues. Proc Natl Acad Sci USA 106:17576–17581, PMID: 19805143, 10.1073/pnas.0909055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. 2001. The mouse uterotrophic assay: A reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect 109:55–60, PMID: 11171525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, et al. 2012. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 55:395–407, PMID: 21932408, 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- Mauvoisin D, Mounier C. 2011. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie 93:78–86, PMID: 20713121, 10.1016/j.biochi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. 2013. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology 36:55–62, PMID: 23500335, 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. 2007. Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb 14:245–252, PMID: 17938543. [DOI] [PubMed] [Google Scholar]
- Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA. 1987. The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol 8:571–582, PMID: 3609543, 10.1016/0272-0590(87)90142-4. [DOI] [PubMed] [Google Scholar]
- Nagel SC, Hagelbarger JL, McDonnell DP. 2001. Development of an er action indicator mouse for the study of estrogens, selective er modulators (serms), and xenobiotics. Endocrinology 142:4721–4728, PMID: 11606437, 10.1210/endo.142.11.8471. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Takatori A, Ishii Y, Kyuwa S, Kuroda Y, et al. 2003. Effects of perinatal exposure to bisphenol A on the behavior of offspring in F344 rats. Environ Toxicol Pharmacol 14:99–108, PMID: 21782668, 10.1016/S1382-6689(03)00044-9. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. 2007a. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 24:253–258, PMID: 17804194, 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. 2007b. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol 23:290–296, PMID: 17321108, 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, et al. 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99:11482–11486, PMID: 12177411, 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens W, Ashby J, Odum J, Onyon L. 2003. The OECD program to validate the rat uterotrophic bioassay. Phase 2: Dietary phytoestrogen analyses. Environ Health Perspect 111:1559–1567, PMID: 12948898, 10.1289/ehp.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgyin L, Erdős E, Bojcsuk D, Balint BL. 2015. Nuclear receptors in transgenerational epigenetic inheritance. Prog Biophys Mol Biol 118:34–43, PMID: 25792088, 10.1016/j.pbiomolbio.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Patel BB, Kasneci A, Bolt AM, Di Lalla V, Di Iorio MR, Raad M, et al. 2015. Chronic exposure to bisphenol A reduces successful cardiac remodeling after an experimental myocardial infarction in male C57bl/6n mice. Toxicol Sci 146:101–115, PMID: 25862758, 10.1093/toxsci/kfv073. [DOI] [PubMed] [Google Scholar]
- Patel BB, Raad M, Sebag IA, Chalifour LE. 2013. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci 133:174–185, PMID: 23418087, 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. 2008. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav 53:580–588, PMID: 18308321, 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. 2005. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction 129:379–389, PMID: 15798013, 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, et al. 2010. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 118:1714–1720, PMID: 20826373, 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224, PMID: 17683900, 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Yang JA, Yasrebi A, Mamounis KJ, Oruc E, Zama AM, et al. 2016. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats. Reprod Toxicol 62:18–26, PMID: 27103539, 10.1016/j.reprotox.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn M, Kullberg J, Karlsson H, Berglund J, Malmberg F, Orberg J, et al. 2013. Bisphenol A exposure increases liver fat in juvenile fructose-fed Fischer 344 rats. Toxicology 303:125–132, PMID: 23142792, 10.1016/j.tox.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, et al. 2016. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod Toxicol 68:130–144, PMID: 27496714, 10.1016/j.reprotox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. 2010. Perinatal exposure to bisphenol-A and the development of metabolic syndrome in CD-1 mice. Endocrinology 151(6):2603–2612, PMID: 20351315, 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. 2006. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav 50:85–93, PMID: 16540110, 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sajiki J, Yonekubo J. 2004. Leaching of bisphenol A (BPA) from polycarbonate plastic to water containing amino acids and its degradation by radical oxygen species. Chemosphere 55:861–867, PMID: 15041290, 10.1016/j.chemosphere.2003.11.065. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Degen GH, Seibel J, Hertrampf T, Vollmer G, Diel P. 2006. Hormonal activity of combinations of genistein, bisphenol A and 17beta-estradiol in the female Wistar rat. Arch Toxicol 80:839–845, PMID: 16639590, 10.1007/s00204-006-0102-4. [DOI] [PubMed] [Google Scholar]
- Singh S, Li SS. 2012. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci 13:10143–10153, PMID: 22949852, 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SC, Fennell TR. 2000. Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168:225–234, PMID: 11042095, 10.1006/taap.2000.9051. [DOI] [PubMed] [Google Scholar]
- Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S,et al. 2009. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Perspect 117:1549–1555, PMID: 20019905, 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Zhang L, Zhang H, Wei W, Jia L. 2014. Perinatal BPA exposure induces hyperglycemia, oxidative stress and decreased adiponectin production in later life of male rat offspring. Int J Environ Res Public Health 11:3728–3742, PMID: 24705360, 10.3390/ijerph110403728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. 2008. Dynamics of fat cell turnover in humans. Nature 453:783–787, PMID: 18454136, 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. 1997. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 138:1780–1786, PMID: 9112368, 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. 1998. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 139:2741–2747, PMID: 9607780, 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, et al. 2015. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol 284:101–112, PMID: 25748669, 10.1016/j.taap.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, et al. 2015. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 156:2049–2058, PMID: 25807043, 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. 2006. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol Lett 167:95–105, PMID: 17049190, 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Nakamura N, Ikezuki Y, Takai Y, Yano T, et al. 2004. Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem Biophys Res Commun 325:549–554, PMID: 15530427, 10.1016/j.bbrc.2004.10.073. [DOI] [PubMed] [Google Scholar]
- Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, et al. 2008. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology 47:1667–1679, PMID: 18393386, 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, et al. 2007. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect 115:1717–1726, PMID: 18087589, 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay-Franco M, Cabaton NJ, Canlet C, Gautier R, Schaeberle CM, Jourdan F, et al. 2015. Dynamic metabolic disruption in rats perinatally exposed to low doses of bisphenol-A. PLoS One 10:e0141698, PMID: 26517871, 10.1371/journal.pone.0141698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. 2004. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279:30817–30822, PMID: 15123605, 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, et al. 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci 104:362–384, PMID: 18445619, 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, et al. 2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68:121–146, PMID: 12075117, 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, et al. 2014. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology 321:40–52, PMID: 24726836, 10.1016/j.tox.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, et al. 2013. Low dose effects of bisphenol A: An integrated review of in vitro, laboratory animal, and epidemiology studies. Endocrine Disruptors 1:1, e26490, 10.4161/endo.26490. [DOI] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177, PMID: 17825522, 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95, PMID: 19074586, 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN. 2014. Low-dose effects of hormones and endocrine disruptors. Vitam Horm 94:129–165, PMID: 24388189, 10.1016/B978-0-12-800095-3.00005-5. [DOI] [PubMed] [Google Scholar]
- Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. 2002. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15:1281–1287, PMID: 12387626. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. 2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933, PMID: 16079060, 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. 2011. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 152:3049–3061, PMID: 21586551, 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z, et al. 2014. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J Endocrinol 222:313–325, PMID: 25112833, 10.1530/JOE-14-0356. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147:56–69, 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Wiklund J, Wertz N, Gorski J. 1981. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology 109:1700–1707, PMID: 7297500, 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS. 2015. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation?. Semin Cell Dev Biol 43:66–75, PMID: 26026600, 10.1016/j.semcdb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Holland WL, Gordillo R, Wang M, Wang QA, Shao M, et al. 2014. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. Elife 3:, 10.7554/eLife.03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Alomirah H, Cho HS, Li YF, Liao C, Minh TB, et al. 2011. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol 45:7044–7050, PMID: 21732633, 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. 2005. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612, PMID: 15498886, 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.