Abstract
Asthma is a complex inflammatory disease characterized by airway inflammation and hyperresponsiveness. The mechanisms associated with the development and progression of asthma have been widely studied in multiple populations and animal models, and these have revealed involvement of various cell types and activation of intracellular signaling pathways that result in activation of inflammatory genes. Significant contributions of Toll-like-receptors (TLRs) and transcription factors such as NF-κB, have been reported as major contributors to inflammatory pathways. These have also recently been associated with mechanisms of oxidative biology. This is of important clinical significance as the observed inefficacy of current available treatments for severe asthma is widely attributed to oxidative stress. Therefore, targeting oxidizing molecules in conjunction with inflammatory mediators and transcription factors may present a novel therapeutic strategy for asthma. In this review, we summarize TLRs and NF-κB pathways in the context of exacerbation of asthma pathogenesis and oxidative biology, and we discuss the potential use of polyphenolic flavonoid compounds, known to target these pathways and possess antioxidant activity, as potential therapeutic agents for asthma.
Keywords: asthma, inflammation, NF-κB, toll-like receptors, oxidative stress, polyphenolic flavonoid compounds
1. Introduction: Immunology of allergic airway inflammation
The pulmonary system acts as the primary site of contact for pathogens and environmental pollutants. In its uncompromised state, this system exhibits a well-orchestrated interplay of components of both innate and adaptive immunity (Cruz & Koff, 2015; Martin & Frevert, 2005). The airways and alveolar epithelial cells are the first line of defense of the lung immune system, which not only presents the physical barrier against environmental insults, but also instructs the professional immune cells to protect from lung injury; whereas immune cells such as alveolar macrophages and neutrophils recognize and act to neutralize pathogens and foreign particles (Hoffmann, et al., 2016).
Asthma is a complex inflammatory disease characterized by airway inflammation and remodeling, and airway hyperresponsiveness (AHR). The airways of asthmatic patients often present with infiltration of eosinophils, degranulated mast cells, and lymphocytes along with hyperplasia of goblet cells and altered epithelial cell tight junctions (Curtis, 2005; Hoffmann, et al., 2016). In this compromised state, the antigen presenting dendritic cells (DCs) recognize allergens and migrate to lymph nodes, where they present antigens to naïve CD4 T cells and induce their differentiation into various T helper (Th) cell types (e.g. Th1, Th2, Th17). In the case of allergic asthma, a predominance of Th2 cells is associated with the disease pathogenesis and progression (K. Chen & Kolls, 2013; Gaurav & Agrawal, 2013). A number of cytokines present in the environment influence the direction of T cell differentiation through interactions with receptors and activation of intracellular signaling cascades. Of these, toll-like receptors (TLRs), and transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) play important roles in asthma pathogenesis, and in mechanisms leading to acute and chronic inflammation.
The TLR signaling pathways lead to the activation of transcription factors like NF-κB (through the IKKα/IKKβ), activator protein-1 (AP-1) (through MAPKs) and Interferon Regulatory Factor (IRF)3 (through TBK1, IKKε and IKKα). Allergic asthma exhibits a crucial interplay of innate and adaptive immune functions, where NF-κB is considered as the master regulator of both innate and adaptive immune responses. Thus, the present review describes the role of TLRs and NF-κB in the context of asthma pathogenesis and oxidative biology as well as the use of polyphenolic flavonoid compounds targeting oxidants and signaling pathways as potential therapeutic agents for asthma.
2. Toll like receptors (TLRs)
The TLRs are a family of pattern recognition receptors (PRRs) known to possess key functions in innate immune responses (Aderem & Ulevitch, 2000; Akira, Takeda, & Kaisho, 2001). Toll-Like Receptors act by recognizing pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) by the distinct structural domains, and result in the activation of immune cells and pro-inflammatory cytokines. Long before the discovery of the TLRs, interleukin-1 (IL-1), a pleiotropic proinflammatory cytokine, was found to contribute to T cell activation and pyrogenicity (Dinarello, 1991). Although the IL-1 receptor (IL-1R) was already discovered, and the gene encoding the IL-1 receptor (IL-1R) was sequenced by the 1980s, the exact signaling mechanism of IL-1R was not yet known. The Drosophila melanogaster Toll receptor was discovered in the 1980s, where Toll, a mutant gene was shown to be critical in Drosophila embryonic development (Stein, Roth, Vogelsang, & Nusslein-Volhard, 1991). The Toll gene was cloned in 1988 and was shown to encode a transmembrane receptor with the presence of an intracytoplasmic domain with marked similarities to that of the IL-1R, and is referred as Toll/IL-1R receptor domain (TIR). While the IL-1R consists of a Ig-like domain in their ectodomain and an intracellular TIR domain, the ectodomain of Toll consists of characteristic leucine rich motifs (LRRs) (Imler & Hoffmann, 2002). Thus, each mammalian TLR is composed of an ectodomain with LRRs, which facilitates interactions with PAMPs, and a transmembrane domain responsible for downstream signaling activation. To date, ten functional mammalian TLRs are classified in humans (TLR1-10), and 13 TLRs in mouse (TLR1-9 and TLR11-13) (Takeda & Akira, 2005, 2015). Although nearly all the TLRs share common pathways, they differ greatly with the type of agonists that activate them, and in several characteristics of the subsequent immune and inflammatory response. Table 1 enlists the known human TLRs with their respective pathways and known endogenous and synthetic agonists.
Table 1.
Human Toll like receptors, cellular localization, and ligands.
| TLR | Cellular localization | Pathway | Endogenous ligands | Synthetic ligands |
|---|---|---|---|---|
| TLR1-2 | Surface | MyD88 | Not Known | Pam3CSK4 |
| TLR2 | Surface | MyD88 | Not Known | MALP-2, Pam2Cys, FSL-1, Hib-OMPC, CFA |
| TLR3 | Endosome | TRIF | Not Known | Poly I:C, poly A:U |
| TLR4 | Surface or endosome | MyD88 or TRIF | Hsp60, Hsp70, fibronectin domain A, surfactant protein A, hyaluronan, HMGB-1 | AGP, MPL A, RC-529, MDF2β, CFA |
| TLR5 | Surface | MyD88 | Not known | Flagellin |
| TLR2-6 | Surface | MyD88 | Not Known | MALP-2 Pam2Cys, FSL-1 |
| TLR7 | Endosome | MyD88 | Human RNA | Guanosine analogs, imidazoquinolines |
| TLR8 | Endosome | MyD88 | Human RNA | Imidazoquinolines, Loxoribine, ssPolyU 3M-012 |
| TLR9 | Endosome | MyD88 | Human DNA/chromatin | CpG-oligonucleotides |
| TLR10 | Not Known | Not Known | Not Known | Not Known |
2.1. TLR signaling pathways
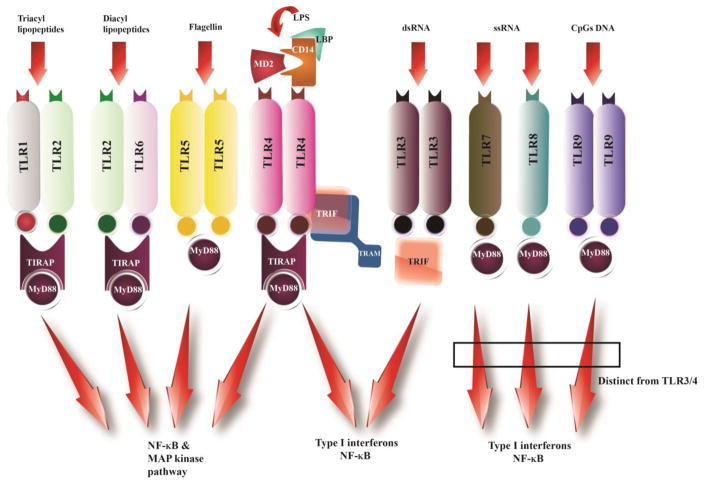
TLRs recognize PAMPs or DAMPs, and this event results in the activation of signaling pathways for the induction of cytokines, chemokines, and co-stimulatory molecules. Upon engagement of TLRs by PAMPs or DAMPs, TLRs hetero or homo dimerize, which further brings a conformational change in the TIR domain allowing the recruitment of cytoplasmic adapter proteins. Individual TLRs selectively recruit an explicit set of TIR domain adapter proteins. Myeloid differentiation primary response protein MyD88 (MyD88), TIR domain containing adaptor protein (TIRAP, MAL), TIR-domain-containing adapter-inducing interferon-β (TRIF, TICAM1) and TRAM are the four adaptor proteins that are identified to anchor the TIR domain (Takeda & Akira, 2005). However, based on the preferences of the TIR domain adapter protein, TLR signaling can be broadly classified in two distinct categories, the MyD88-dependent, which is utilized by all the TLRs except TLR3, and the MyD88-indpendent pathway or the TIR-domain-containing adapter-inducing interferon-β dependent (TRIF) pathway. This selection of MyD88 and TRIF leads to the activation of distinct signaling pathways. For example, the MyD88-dependent pathway activates NF-κB and Mitogen Activated Protein (MAP) kinases for the induction of the inflammatory cytokines genes, while the TRIF-dependent pathway leads to the activation of IRF3, NF-κB and MAPKs for induction of type I interferon and inflammatory cytokine genes (Figure 1) (Akira, Uematsu, & Takeuchi, 2006). The adapter protein TIRAP recruits MyD88 to the TLR2 and TLR4 at the plasma membrane and at the endosome for TLR9, whereas TRAM recruits TRIF to the TLR4 and results in the activation of the IRF3 (Barton & Kagan, 2009; Kawasaki & Kawai, 2014). Moreover, a number of molecules also negatively regulate TLR signaling. Molecules like Suppressor of cytokine signaling 1 (SOCS1) and casitas B-lineage lymphoma-b (Cbl-b), in combination with tyrosine kinase Syk, act on the MyD88 dependent pathway, while sterile α- and armadillo-motif-containing protein (SARM) and its splice variant TAG negatively influence the TRIF-dependent pathway (C. Han, et al., 2010; Mansell, et al., 2006; Palsson-McDermott, et al., 2009; Peng, et al., 2010).
Figure 1. Schematic of TLR signaling pathways.
TLRs differentially recruit specific sets of TIR domain-containing adaptor molecules like MyD88, TRIF, TIRAP/MAL, or TRAM. The MyD88-dependent pathway is utilized by all TLRs except TLR3, where TLR1-2 dimers, TLR2-6 dimers, or TLR5 and TLR4 self-dimerization lead to the activation of NF-κB and MAPKs for the induction of inflammatory cytokine genes. TLR3 utilizes TRIF adapter molecules and leads to the activation of IRF3, NF-κB, and MAPKs for induction of type I IFNs and inflammatory cytokine genes.
3. Nuclear factor κB (NF-κB) proteins and their regulation
NF-κB was discovered in 1986 (Sen & Baltimore, 1986b), as one of the few nuclear transcription factors capable of binding to both the heavy and the kappa (κ) light chain enhancers in B cells. Of these factors, NF-κB was found to bind only to the κ light chain enhancer. In the subsequent decades, it was established that NF-κB not only was a regulator of B cell development (Baeuerle & Baltimore, 1988; Sen & Baltimore, 1986a), but was also found in the cytoplasm of virtually all cells, and in all animals from Drosophila to humans.
In mammalian cells, there are five known NF-κB family members, RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2) (Ghosh, May, & Kopp, 1998; Gilmore, 2006). These proteins interact combinatorically to form a family of homodimers and heterodimers that constitute the transcriptionally active proteins binding to the 9–10 base pair highly variable DNA sites, also known as κB sites, (50-GGGRNWYYCC-30; R, A or G; N, any nucleotide; W, A or T; Y, C or T). All five of the NF-κB family members share a characteristic highly conserved amino-terminal Rel homology domain (RHD), which contains a nuclear localization sequence (NLS) and is responsible for dimerization, nuclear translocation, DNA-binding, and interaction with inhibitory IκB proteins.
The synthesis of each NF-κB family member is a highly regulated process. NF-κB1 and NF-κB2 are synthesized as large precursors, p105 and p100, that are post-translationally processed to DNA-binding subunits p50 and p52, respectively (Serasanambati & Chilakapati, 2016). Both p50 and p52 lack a transcription activation domain, but they have a DNA binding domain. However, these proteins can form hetero-dimers with the RelA, RelB, and c-Rel, which have the transcription activation domain. Thus, the NF-κB protein homo- and hetero-dimers regulate target gene transcription differentially, that in turn contributes to the specificity of biological responses (L.-F. Chen & Greene, 2004). For instance, p50 and p52 homodimers function as repressors of NF-κB specific transcription (Ghosh, et al., 1998), whereas the heterodimers that contain RelA or c-Rel are transcriptional activators. RelB does not form homodimers but it forms stable heterodimers with either p50 or p52 (Vu, Huang, Vemu, & Ghosh, 2013), which exhibit a greater regulatory flexibility, and can function as both an activator and repressor (Fusco, et al., 2009; Marienfeld, et al., 2003; Ryseck, et al., 1992). Following nuclear translocation, the NF-κB dimer binds to the 10-basepair DNA binding sites, which have a great amount of target gene (Kawai & Akira, 2007).
NF-κB1 and NF-κB2 precursor proteins have long C-terminal domains that contain multiple copies of ankyrin repeats, which act as sites for protein-protein interaction, to inhibit these proteins. Similarly, the inhibitory IκB proteins are characterized by the presence of 6 or 7 ankyrin repeats to mediate protein-protein interactions, which binds to the RHD of p65 and masks the nuclear localization sequence, causing sequestration of NF-κB in the cytoplasm (F. E. Chen & Ghosh, 1999; Grimm & Baeuerle, 1993). The IκB family consists of seven members: IκBα, IκBβ, IκBγ, IκBε, Bcl-3, IκBζ and IκBNS. Of these, four members (IκBα, IκBβ, IκBγ, and IκBε) interact with the NF-κB in the cytoplasm to inhibit nuclear translocation of NF-κB, and the remaining three members (Bcl-3, IκBζ, and IκBNS) are present in the nucleus and interact with NF-κB to regulate transcription (Kawai & Akira, 2007). The nuclear translocation of NF-κB is regulated by interaction with IκB proteins through phosphorylation and proteasome degradation mediated by the IκB kinase (IKK) complex (Karin, 1999). The IKK complex is composed of three subunits: IKKα, IKKβ, and IKKγ. The two catalytic subunits, IKKα (IKK1) and IKKβ (IKK2) contain the kinase domain, a ubiquitin-like domain (ULD) and α-helical scaffold/dimerization domain (SDD), and the third subunit, IKKγ/NEMO (NF-κB essential modulator), is the regulatory subunit, which consists of a coiled coil (CC) domain and a leucine zipper (LZ) domain, responsible for regulating the interaction of the two catalytic subunits through their NEMO-binding domain (NBD). Thus, activation of the NF-κB pathway typically involves the stimulus-mediated phosphorylation of an IκB member primarily by the IKK (IκB kinase) complex. The phosphorylated IκB then undergoes ubiquitination and subsequently proteasome dependent proteolysis, leading to the release and translocation of NF-κB into the nucleus, where it affects gene transcription.
NF-κB activation is mediated through either the canonical (classic) or the non-canonical (non-classic, alternative) pathways, leading to specific activation of subunits and thus producing a specific genetic response (Brasier, 2006). The canonical pathway is crucial for the activation of innate immunity and inflammation signaling, and inhibition of apoptosis. Activation of cell surface receptors or intracellular stress signals proinflammatory cytokines, such as TNFα, IL-1, leading to the activation of the IKK complex. The activated IKK complex, predominantly acting through the signalsome (consisting of IKKα, IKKβ and NEMO), catalyzes phosphorylation of IκBα (at sites Ser32 and Ser36), followed by polyubiquitination and subsequent degradation of this inhibitor in the cytoplasm by the 26S proteasome complex. The degradation of IκBα results in release of NF-κB dimers (p50:RelA dimer) and unmasks the nuclear localization signal in RelA, resulting in rapid translocation of the NF-κB heterodimer to the nucleus, where it binds to DNA to activate gene transcription (Ghosh & Hayden, 2012).
The alternative pathway is mainly involved in lymphoid organogenesis as well as B-cell survival and maintenance, under normal physiological conditions. Aberrant activation of this pathway is involved in human malignancies, auto-immunity and metabolic diseases (Brasier, 2006; Keats, et al., 2007). In the alternative pathway, the stimulus dependent activation of NF-κB heterodimer (p52:RelB) is dependent on IKKα, which is induced by the NF-κB-inducing kinase (NIK), a mitogen-associated protein 3 kinase (MAP3K) originally thought to mediate NF-κB activation by cytokines including TNF-α and IL-1 (S.-C. Sun, 2011). Upon phosphorylation by NIK, activated IKKα further phosphorylates p100 at two C-terminal sites, followed by its ubiquitination and proteasomal processing to p52 (L.-F. Chen & Greene, 2004). In contrast to the p50-RelA heterodimers of the canonical pathway, the non-canonical pathway involves creation of transcriptionally competent NF-κB heterodimer, p52/RelB complexes that translocate to the nucleus and induce target gene expression (Cildir, Low, & Tergaonkar). Notably, while both IKKβ and NEMO are essential in the canonical pathway, noncanonical NF-κB signaling does not require these IKK components, but instead requires IKKα and NIK. NIK protein levels are critically regulated under normal cellular environment by continuous degradation by TRAF3 E3 ubiquitin ligase complex, comprising of TRAF3, TRAF2, and cellular inhibitor of apoptosis 1 and 2 (cIAP1/2). Upon stimulation, TRAF3 is degraded leading to NIK stabilization and accumulation. As a negative regulatory mechanism, NIK can also be phosphorylated by IKKα or TANK-Binding Kinase 1 (TBK1) and degraded to limit the activation of the pathway under different conditions.
Typically, the canonical pathway is involved in the rapid immune response towards inflammation or in response to pathogens, and the non-canonical pathway is activated through slow developmental signals. Although, both the canonical and non-canonical pathways were generally thought to occur in response to independent signaling mechanisms, and respond to separate physiological conditions, recently crosstalk interactions have been reported interconnecting the two pathways (Shih, Tsui, Caldwell, & Hoffmann, 2011). This crosstalk involves expression control of NF-κB monomers, interdependent proteolytic processing of precursors, and interrelated regulatory mechanisms (Cildir, et al.). One example is the newly identified IκBδ activity that is enhanced by one pathway and disrupted by the other (Shih, et al., 2011).
4. Expression and function of TLRs in non-infectious lung diseases
The expression of TLRs is not restricted to antigen processing and presenting cell types. Rather, TLR expression is detected in various cell populations and tissue types (Iqbal, Philbin, & Smith, 2005). In the lung, TLRs are widely expressed on resident cells such as alveolar macrophages, and infiltrating cells of myeloid and lymphoid origin. Human and animal studies have also reported high levels of expression of nearly all the TLRs in the lung (Muir, et al., 2004; Tirumurugaan, et al., 2010; Zarember & Godowski, 2002). TLRs are widely researched in infectious lung diseases, where a number of TLRs were found to be associated with pathogen clearance by amplifying immune responses (Abel, et al., 2002; Albiger, et al., 2007; Biondo, et al., 2005; Chai, et al., 2011; Eder, et al., 2004; Krutzik & Modlin, 2004; Yoshimura, et al., 1999). In the context of non-infectious lung diseases, limited studies have been done, however they indicate strong and pharmacologically important involvement of TLRs, especially in inflammatory processes activated by exposure to ambient air pollution and cigarette smoke (Bauer, Diaz-Sanchez, & Jaspers, 2012).
The severity and degree of allergic asthma exacerbations has been associated with environmental factors such as allergens, but is also dependent on allergen type, duration, degree of exposure, and the patient’s overall health and genetics. Studies have identified several susceptible genes that contribute to the onset of allergic asthma, and the overall disease progression (Ober & Yao, 2011). Among these, a predominant role of TLRs and their polymorphisms (in particular TLR2, TLR4 and TLR7, TLR8 and TLR9) has been implicated in the physiopathology of asthma (Lazarus, et al., 2003; Noguchi, et al., 2004; Reijmerink, et al., 2010; Tesse, Pandey, & Kabesch, 2011; Vercelli, 2008). Along with polymorphic variation in the TLR genes, genetic variation in regulatory and intracellular molecules involved in TLR signaling pathways are also known to exhibit functions in allergic asthma onset and exacerbations. In this regard, evolutionary genetic studies have suggested a genetic predisposition in human TLR-related pathway genes, such as IL1RL1, BPI, NOD1, NOD2 and MAP3K7IP1 with the development of asthma (Eder, et al., 2004).
5. Activators of NF-κB in noninfectious lung diseases
In the past decades, multiple studies have demonstrated that NF-κB is vigorously involved in a myriad of mechanisms. Thus, the name “central switch” justifies its role in the regulation of expression of over 300 genes in a cell-specific and stimulus-specific manner (Pahl, 1999; Thanos & Maniatis, 1995). This broad spectrum of regulatory activities of NF-κB ranges from normal cell physiological processes (O’Neill & Kaltschmidt, 1997), inflammation (Baeuerle & Baichwal, 1997), infection (Hiscott, Kwon, & Génin, 2001), metabolism of ROS (Turillazzi, et al., 2016) and DNA damage, to cancer and auto immune diseases (Davoudi, et al., 2014; De Simone, et al., 2015).
In addition to the physiological stress caused by infectious diseases, the human body is also exposed to environmental hazards and therapeutic drugs, which lead to non-infectious stress. Due to the exposure of the respiratory system to the environment, the lung is vulnerable to infectious agents causing acute pulmonary diseases and noninfectious chronic lung diseases, such as asthma, bronchopulmonary dysplasia, and chronic obstructive pulmonary disease (Parker & Prince, 2011). However, infectious agents play a role in chronic lung diseases in addition to acute pulmonary diseases and contribute to worsening of airflow obstruction and/or lung parenchymal damage. As airway injury persists due to allergen exposure, smoke, or other environmental irritants such as ozone (O3) and NO2, it causes not only impairment of innate immunity, but also increased susceptibility of the lower respiratory tract to pathogens (Bochkov, et al., 2010; Iwasaki & Medzhitov, 2010; Opitz, van Laak, Eitel, & Suttorp, 2010). These non-infectious stress conditions lead to the amplification of inflammatory pathways that are critical to the host defense. This is the case of chronic obstructive pulmonary disease (COPD) and asthma, as well as other chronic inflammatory diseases. The inflammatory response in the airway is associated with symptoms such as mucus secretion, plasma exudation, bronchoconstriction, and airway hyperresponsiveness. The inflammatory immune response in asthma only differs in severity and longevity, but not in effect, in comparison to the response against infectious agents, as the immune response persists as long as the allergen exposure continues.
Airway inflammation is one of the central features of COPD and asthma. Exacerbations of COPD are associated with elevated airway levels of proinflammatory mediators, and there have been important advances in understanding the underlying inflammatory processes in the past decade (Iwasaki & Medzhitov, 2004; Irfan Rahman, Gilmour, Jimenez, & MacNee, 2002; Rom, Avezov, Aizenbud, & Reznick, 2013). Many inflammatory cells are involved in airway inflammation, and a large number of inflammatory mediators such as cytokines, chemokines, enzymes, receptors and adhesion molecules have been identified as key players (Huertas & Palange, 2011). Inflammatory mediators play a crucial role in the chronic inflammatory process, as they appear to determine the nature of the inflammatory response by directing the selective recruitment and activation of inflammatory cells, such as eosinophils, T-lymphocytes, mast cells, DCs, and macrophages (Fischer, Pavlisko, & Voynow, 2011). Moreover, in addition to the inflammatory cells, structural cells of the airways, such as epithelial cells, fibroblasts, airway smooth muscle cells, and endothelial cells, also release inflammatory mediators. These mediators perpetuate the inflammatory response within the airways by further increasing the expression of inducible genes that encode inflammatory proteins. These inducible genes are regulated by transcription factors, which are activated in response to various stimuli and bind to the promoter region of the inflammatory genes to increase their rate of transcription. One such transcription factor is NF-κB, which is activated in response to release of pro-inflammatory cytokines, viruses, activators of protein kinase C (PKC), and in response to environmental stress, such as exposure to heavy metals, reactive oxygen intermediates, cigarette smoke and by various therapeutic drugs, including chemotherapeutic agents.
6. TLRs and NF-κB in allergic asthma: effects on epithelial and immune cell function
TLRs are expressed in a variety of airway cells including epithelial cells, macrophages, mast cells, and DCs. Stimulation of TLRs by infectious agents activates antigen presenting cells (APC), and control T helper (Th1, Th2, and Th17) immune cell differentiation in a context-dependent manner, along with activation of mast cells for cytokine production (Pulendran, et al., 2001). During allergic airway inflammation, engagement of TLRs on DCs favors Th2 polarization and eosinophilia, a characteristic of allergic lung diseases. However, studies have reported Th17 response driven neutrophilic airway inflammation in asthmatics with poor response to inhaled glucocorticoids, which primarily target eosinophilic inflammation (Barczyk, Pierzchala, & Sozanska, 2003; Bullens, et al., 2006; McGrath, et al., 2012). Cumulatively, the selection of Th2 or Th17 responses depends on factors including response of different airway cell types to different allergens, and recruitment of TLRs with specific sets of TIR domain adapter proteins. Classically, during Th2 responses, DCs play a very important role by activating allergen-specific Th2 cells and inducing their proliferation, leading to the release of a particular set of cytokines, emergence of helper functions and generation of memory cells. Regardless of site, neutrophilic inflammation tends to show a direct relationship with lipopolysaccharides (LPS) exposure, a major component of the outer cell wall of Gram-negative bacteria, by activation of the Th17 response with the assistance of DCs (McAleer, et al., 2010; L. Wang, et al., 2012; Wilson, et al., 2009). Importantly, TLR2 and TLR4 are highly expressed in cells exposed to LPS, where TLR4 is the only known TLR that can signal through both MYD88 and TRIF-dependent pathways. Interestingly, animal studies have shown that LPS-induced MYD88-dependent TLR4 activation leads to Th2 response activation and asthma, whereas TRIF-dependent activation of TLR4 leads to Th17 response and neutrophilia (Hsia, et al., 2015; Piggott, et al., 2005). Furthermore, a recent study by McAlees et al. showed variation in the response to a same set of allergens among different lung cell types. These investigators found that TLR4 expression in airway epithelial cells exposed to LPS support eosinophilic airway inflammation, whereas TLR4 expression by hematopoietic cells was essential for Th17-driven neutrophilic airway inflammation (McAlees, et al., 2015).
Taken together, these studies raise important questions about the mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma, and more importantly, how different lung cell types and their structural and molecular milieu behave when exposed to different sets of allergens. In the following section, we will further focus on the role of TLRs and NF-κB in different airway cell types during allergic asthma exacerbations.
6.1. TLRs and NF-κB in airway epithelial cells
Respiratory epithelial cells form the first line of defense against dust, microorganisms, gases and allergens. These cells form a highly regulated and impermeable barrier through the formation of tight junctions and adherent junction proteins, which may lead to epithelial barrier dysfunction upon repeated exacerbations and insults. Both the human bronchus epithelial cell line BEAS-2B and primary airway epithelial cells are reported to express all the TLRs with the exception of TLR8 in BEAS-2B cells (Ioannidis, Ye, McNally, Willette, & Flano, 2013; Mayer, et al., 2007; Muir, et al., 2004; Platz, et al., 2004; Sha, Truong-Tran, Plitt, Beck, & Schleimer, 2004). Further studies on the distribution of TLRs shed light on the importance of the integrity of the lung endothelial barrier system, as TLR3 was reported to be distributed on both apical and basolateral surfaces, TLR2 and TLR6 mostly on basolateral, and TLR1, TLR4, TLR5, TLR7, TLR9 and TLR10 were reported to have luminal distribution on airway epithelial cells (Ioannidis, et al., 2013). Nevertheless, the majority of these studies were performed on airway epithelial cells from healthy individuals, or non-asthmatic patients. Furthermore, it is worth noting that the airway epithelium of asthmatics is reported to have increased shedding and mucin production, and the compromise in the integrity and stress on airway epithelium is further augmented with increased activation of nuclear factor-κB (NF-κB), increased expression of heat-shock proteins, AP-1 and other transcription factors (Holgate, et al., 2003). This leads an open debate on the offerings of TLRs in conjunction with airway epithelial cells in the pathophysiology of asthma. Infection on the lung epithelium tends to increase the production of pro-inflammatory mediators such as IL-6, IL-8, Chemokine (C-C motif) ligand 5 (CCL5), Tumor necrosis factor (TNF) and type I IFNs (Interferon), which can further promote advance adaptive immune responses. Studies on the intestinal epithelium and dermal micro-vessel endothelial cells portray an opposite phenomenon and tend to limit inflammatory responses to infection by expressing TLR-attenuating molecules like toll/interleukin-1 resistance family member, TIR8, and transforming growth factor-β (Garlanda, et al., 2004; Naiki, et al., 2005).
Allergic asthma is triggered by multiple specific and non-specific stimuli, where bronchial epithelial cells produce a variety of cytokines and chemokines that aid in the recruitment of inflammatory cells and antigen presenting cells to the airways. Remarkably, NF-κB regulates the expression of many of these pro-inflammatory mediators, adhesion molecules, respiratory mucins, and growth and angiogenic factors. In 1997, Stacey et al. reported for the first time that primary cultures of bronchial epithelial cells of asthmatic patients who were allergic to house dust mites, resulted in NF-κB activation when exposed to Der p1 (the major allergen from mite, Dermatophagoides Pteronyssinus) (Stacey, et al., 1997). In another study, receptors for advanced glycation end-products (RAGE), the cell-surface receptors expressed by alveolar type I (ATI) epithelial cells, were upregulated by diesel particulate matter (DPM) in ATI cells, as shown by RAGE mRNA and protein synthesis (Reynolds, Wasley, & Allison, 2011). Furthermore, both DPM-induced activation of NF-κB and the secretion of two NF-κB targets, IL-8 and MCP-1, were significantly influenced by RAGE signaling. Rahman et al. later explored the effect of oxidative stress and the pro-inflammatory mediator TNF-α, on histone acetylation/deacetylation and activation of NF-κB and AP-1, leading to the release of the pro-inflammatory cytokine IL-8 in human alveolar epithelial cells (A549) (Irfan Rahman, et al., 2002). Furthermore, very strong advocating evidence for the involvement of TLRs in the progression of asthma arose from the studies on Thymic stromal lymphoprotein (TSLP). TSLP, an IL-7-like cytokine which is mainly expressed by lung epithelial cells, has been shown to activate DCs and prime the Th2 cell response along with playing a crucial role in the maintenance of Th2 central memory cells (Y. J. Liu, 2006; Wang, et al., 2006; Zhou, et al., 2005; Ziegler & Liu, 2006). Upon activation of DCs, TSLP also promotes the expression of mRNA for the Th2 attracting chemokine CCL17 (Y. J. Liu, 2006), known for its properties to accelerate pulmonary fibrosis (Belperio, et al., 2004). In addition, the increased expression of TSLP induces chemotactics like eotaxin-2 for eosinophils and IL8 for neutrophils (Y. J. Liu, 2006). Finally, strong evidence for involvement of TLRs in asthma inflammation and progression is coming from bacterial (TLR2and TLR9) and viral (TLR3 and TLR8) models, in which these were found to initiate TSLP expression by epithelial cells (Allakhverdi, et al., 2007; H. C. Lee & Ziegler, 2007).
6.2. TLRs and NF-κB in dendritic cells
As antigen presenting cells that are in close contact with the airway epithelium, DCs are crucial in determining the outcome of allergen encounters in the lung. In addition, epithelial cell derived mediators can influence the DC function on T cells polarization. Anatomically, DCs form a dense network above and beneath the respiratory epithelium and extend their dendrites through the epithelial layer to sample the luminal environment. Lung DCs exist in an immature state, where they recognize and capture inhaled foreign antigens (Cella, Sallusto, & Lanzavecchia, 1997), and undergo maturation prior to migrating to draining lymph nodes where they activate the naïve T cells. The migration of DCs to draining lymph nodes increases considerably in response to inflammatory signals. However, the activation of naïve T cells requires maturation of DCs, which in turn require an array of appropriate signals from the ingested foreign antigen. Fully mature DCs exhibit high surface expression of the major histocompatibility complex (MHC) and costimulatory molecules that influence both innate and adaptive immune responses. In contrast, immature DCs cannot activate naïve T cells and induce immunogenic tolerance to antigens. This functional heterogeneity among lung DCs is crucial for the regulation of tolerogenic or inflammatory responses against inhaled antigens.
The process of DC maturation involves direct signals derived from receptor-mediated antigen uptake, and accessory signals from the host PAMPs or DAMPs recognition through PRRs. Lung DCs exhibit an array of surface and intracellular PRRs, including TLRs, nucleotide-binding domain/leucine-rich repeat receptors, C-type lectin receptors and a variety of other receptor molecules which differ considerably among different human lung DC subsets (Demedts, Brusselle, Vermaelen, & Pauwels, 2005; Masten, et al., 2006; Schlecht, et al., 2004). Among the most widely studied human lung DC subsets are the myeloid DC type 1 (mDC1, expressing BDCA1 and MHCII), myeloid DC type 2 (mDC2, expressing BDCA3 and MHCII), and plasmacytoid DC (pDC, expressing BDCA2 and CD123) (Demedts, et al., 2005). The expression pattern of TLRs among these three DC subsets varies considerably. While mDC1 and mDC2 cells exhibit presence of TLR1-4, TLR6 and TLR8, pDCs differ greatly to the other two DC subsets with expression of TLR1, TLR6-7 and TLR9 (Demedts, et al., 2005; Masten, et al., 2006; Schlecht, et al., 2004). The TLR9 expression by pDCs is unique, as the B cells are the only other human cells known to express TLR7 and TLR9 (Fuchsberger, Hochrein, & O’Keeffe, 2005). Notably, naïve pDCs are known to express very low quantities of TLR4 (Castellaneta, Sumpter, Chen, Tokita, & Thomson, 2009). This marked variation in the expression pattern of PRRs, in particular of TLRS, in pDCs is of high importance as pDCs have been implicated in mechanisms of tolerance induction to the inhaled antigens. Direct evidence for the involvement of pDCs and TLRs in asthma is coming from animal studies evaluating Treg cells. These studies indicate involvement of two types of Treg cells in the suppression of asthmatic lung inflammation: the natural or thymic-derived Foxp3+ Treg (nTreg) cells, and the inducible Treg (iTreg) cells that develop in the periphery in response to antigen exposure (Curotto de Lafaille, et al., 2008; Duan, So, & Croft, 2008; Ostroukhova, et al., 2004). Interestingly, iTreg cells are induced and prevail when mice are exposed to pure protein antigens, whereas CD4+ T cells are primarily induced with fewer iTreg cells when mice are exposed to protein allergens mixed with TLR4 ligands (Castellaneta, et al., 2009; Duan, et al., 2010; Duan, et al., 2008; Duan, So, Mehta, Choi, & Croft, 2011; Soroosh, et al., 2013). Furthermore, studies using antibody-based depletion of pDCs during inhalation of inert soluble antigens showed asthma development with characteristic immunoglobulin E sensitization, airway eosinophilia, goblet cell hyperplasia, and Th2 cell cytokine production (de Heer, et al., 2004).
The enzymatically active allergens activate protease-activated receptors (PARs) expressed in epithelial cells, an event followed by NF-κB activation and the perpetuation of chemokines and cytokines by epithelial cells that further attract and activate DCs (Lambrecht & Hammad, 2009). The involvement of NF-κB in the control of DC development has been established in earlier studies, in which inhibition of NF-κB activation blocked maturation of DCs in terms of upregulation of MHC and costimulatory molecules (Rescigno, Martino, Sutherland, Gold, & Ricciardi-Castagnoli, 1998). In the case of atopic diseases, in which there is a predominance of Th2 responses to environmental exposures, it is known that pollen-derived allergens are often accompanied by proinflammatory and immunomodulatory lipids, termed pollen-associated lipid mediators (PALMs). These PALMs include E1-phytoprostanes (PPE1), which were identified to modulate dendritic cell (DC) function. PPE1 inhibit the DC’s capacity to produce IL-12, and enhance the DC-mediated Th2 polarization of naïve T-cells via PPAR-γ-dependent pathways that lead to inhibition of NF-κB activation and result in reduced IL-12 production by DCs and consecutive Th2 polarization (Gilles, et al., 2009).
Interestingly, incubation with one of the most commonly used analgesic and antiinflammatory agents (aspirin, a salicylate), reduced neither DC viability nor their number, but changed their surface marker phenotype via suppression of expression of antigen-presenting and costimulatory molecules. This mechanism involved secretion of the p40 subunit of IL-12 and activation of NF-κB (Matasić, Dietz, & Vuk-Pavlović, 2000). In addition, treatment with pharmacologically active concentrations of salicylates markedly reduced the levels of activated NF-κB in DCs without triggering apoptosis, thus inhibiting DC differentiation with the consequent loss of their immunostimulatory function (Hackstein, et al., 2001), as similarly observed with other many agents that suppress DC maturation via NF-κB inhibition.
6.3. TLRs and NF-κB in eosinophils
Eosinophils are considered one of the most important cell types involved in the pathobiology of asthma. As early as the 1920s, postmortem studies of asthmatics revealed hyper-inflated lungs showing desquamated lung epithelial cells and massive infiltration of eosinophils and neutrophils in the airway mucosa (Cardell & Pearson, 1959; Huber & Koessler, 1922). Subsequent experimental animal studies, and studies with human lung biopsies also indicated increased infiltration of eosinophils in asthmatics, however their function in asthma development and severity remained largely elusive (Beasley, Roche, Roberts, & Holgate, 1989; Bradding, et al., 1994; Hogan, et al., 1998; Hogan, et al., 2008). Many different molecules including IL-3, IL-4, IL-5, IL-13, and GM-CSF are known to activate eosinophils to release various chemokine and cytokine mediators including TGF-β, IL-5, IL-3, IL-4 and IL-13 (Bradding, Feather, Wilson, Holgate, & Howarth, 1995; Bruijnzeel, et al., 1992; Dubucquoi, et al., 1994; Schmid-Grendelmeier, et al., 2002). It has been proposed that these mediators play a dynamic role in asthma airway modulation and disease progression. Out of many mediators, the Th2 cell-associated cytokine IL-5 has a crucial impact on eosinophils. A very strong evidence came from a study by Sehmi et al. on the contribution of the hematopoietic progenitor cells in allergic airway diseases. In this study, the authors demonstrated that atopic asthmatic subjects had large numbers of eosinophil/basophil progenitor cells expressing IL-5 receptor alpha in the bone marrow, and when these progenitor cells were cultured with IL-5 ex vivo, they developed into eosinophils (Sehmi, et al., 1996). Another convincing study in which anti-IL-5 was administered to asthmatics in a double-blind placebo-controlled trial, demonstrated a significant reduction in airway remodeling and BAL eosinophil counts, despite no significant improvement in asthma symptoms (Flood-Page, et al., 2003).
Like many other myeloid innate immune cells, eosinophils exhibit various PRRs, including TLRs (Mansson & Cardell, 2009; Nagase, et al., 2003; O’Flaherty, et al., 2017; Porsbjerg, Baines, Sverrild, Backer, & Gibson, 2014; Sabroe, Jones, Usher, Whyte, & Dower, 2002; Wong, Cheung, Ip, & Lam, 2007), nucleotide-binding domain/leucine-rich repeat receptors (Kvarnhammar, Petterson, & Cardell, 2011), and C-type lectin receptors (Acharya & Ackerman, 2014; Willment, et al., 2005; Yoon, Ponikau, Lawrence, & Kita, 2008). Upon PRRs-assisted activation, they also secrete cytokines and chemokines for activation of other immune cells. Among the TLRs expressed in eosinophils, significant levels of TLR1-2, TLR4-7 and TLR9 are expressed constitutively. However, a few studies have also find expression of TLR3 mRNA and protein in eosinophils from bone marrow and peripheral blood (Kvarnhammar & Cardell, 2012). Studies with TLR agonists have indicated varied but strong associations of eosinophils with allergic asthma development and progression. In an animal model, TLR7 and TLR9 agonists not only were shown to diminish airway eosinophilia in a dose-dependent manner, but also to reduce levels of IL-4 and IL-5, while TLR2 and TLR4 agonists were found to promote eosinophilia (Duechs, et al., 2011). Contrasting findings from other researchers, however, indicated beneficial effects of TLR2 and TLR4 stimulation in allergic asthma (Akdis, et al., 2003; Taylor, Richmond, & Upham, 2006). Therefore, further investigation is required to determine whether TLRs play favorable or adverse effects in eosinophils during allergic asthma.
Asthmatics show a characteristic accumulation and persistence of eosinophils in their lungs (Vignola, et al., 2000). Studies have demonstrated that apoptosis of lung eosinophils is considerably delayed in asthmatic patients in comparison to healthy individuals (Ilmarinen & Kankaanranta, 2014). Yang et al. have also reported that mice deficient in the p50 subunit of NF-κB are incapable of mounting eosinophilic airway inflammatory responses, and have decreased IL-5 and chemokine-eotaxin gene expression when compared to wild-type mice; both of which are key mediators of eosinophilic inflammation in the airways (Yang, et al., 1998). Correspondingly, Das et al. observed that p50-deficient mice have reduced eosinophilic responses to aerosolized allergens due to lack of production of Th2 cytokines, IL-13, IL-4 and IL-5 (Das, et al., 2001). Similarly, c-Rel knockout mice, an allergen-induced asthma model, have decreased airway hyper-responsiveness and eosinophil infiltration as well as lower levels of serum IgE when compared to wild-type mice (Donovan, et al., 1999). Treatment with NF-κB inhibitors such as MG-132, a peptide aldehyde type of proteasome inhibitor, is reported to reduce the number of eosinophils and decrease allergic inflammation (Edwards, et al., 2009). Together, these observations support the notion that NF-κB is a central inflammatory factor in severe eosinophilic asthma, and can be used as a potential therapeutic target to prevent eosinophilia.
7. TLRs, NF-κB and lung oxidative biology
The imbalance between oxidants like reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidants in favor of oxidants, is being termed as oxidative stress, a condition known to result in biological damage. Although, generation of oxidative molecules is part of the normal metabolism, the intracellular levels of ROS and RNS are maintained at low concentrations under normal physiological conditions (Rahal, et al., 2014). The lungs are continuously exposed to a variety of oxidants that differ in type and degree, and can easily overcome the resulting oxidative stress with the help of a network of existing enzymatic and non-enzymatic antioxidants. While airway inflammation and asthma tend to increase the production of ROS and RNS, mostly contributed by eosinophils and neutrophils (Kinnula, 2005; Suzuki, et al., 2008), accumulating evidence suggests the involvement of ROS and RNS, and oxidative stress in the genesis and modulation of asthma (Al-Harbi, et al., 2015; Comhair & Erzurum, 2010; Erzurum, 2016; Ghosh & Erzurum, 2011, 2012; Jiang, et al., 2014; N. Li, Hao, Phalen, Hinds, & Nel, 2003; Riedl & Nel, 2008; Sugiura & Ichinose, 2008; Uchida, et al., 2017). Moreover, the effects of potent oxidants, such as cigarette smoke and O3, have validated the relationship between TLRs and oxidative stress in the genesis of lung inflammation. In-vitro studies have shown that inhalation of particulate matter components can stimulate TLR2 and TLR4 (Becker, Fenton, & Soukup, 2002). An interesting correlation between TLRs expression and lung inflammation was observed in studies of O3 exposure in mice. Kleeberger et al. (Kleeberger, Reddy, Zhang, & Jedlicka, 2000) performed a genome screening of mouse strains susceptible and resistant to O3 inflammation, and reported an association with a quantitative trait locus on chromosome 4, which contains the candidate gene for TLR4. Furthermore, they observed an increase in TLR4 expression in mice susceptible to O3 compared to strains resistant to O3. Another study by Hollingsworth et al. performed on TLR4-deficient mice reported an involvement of TLR4 in pulmonary inflammation triggered by environmental challenges (LPS, particulate matter, and O3), in a toxin and exposure dependent manner (Hollingsworth, et al., 2004). Similarly, studies with human bronchial airway epithelial cells and alveolar macrophages exposed to ambient air particulate matter also showed involvement of both TLR2 and TLR4 (Becker, Mundandhara, Devlin, & Madden, 2005). Further corroboration for the involvement of these receptors in lung inflammation was shown by a study on the effects of O3 exposure on TLR2-, TLR4-, and MyD88-deficient mice, where TLR2, TLR4, and MyD88 were associated with inflammation and airway hyperresponsiveness, and Myd88 was associated with the neutrophilic response to O3 (Williams, et al., 2007). In addition, our group has reported sex differences and a significant upregulation of Myd88 in response to O3 exposure along with three other genes, Cxcl2, C4b and Ccll9 (Cabello, et al., 2015).
Cigarette smoke increases the expression of TLR4 and TLR9 along with cytokine expression on lung CD8+ T cells (Mortaz, et al., 2010; Nadigel, et al., 2011). However contrasting results were obtained in a study comparting alveolar macrophages from COPD patients, healthy smokers, and non-smokers, where a decrease in TLR2 expression was also found (Droemann, et al., 2005). Several studies have shown that TLRs, and in particular TLR4 contribute significantly to the redox balance of the lung (Cosio, Saetta, & Agusti, 2009; X. Zhang, Shan, Jiang, Cohn, & Lee, 2006). Using TLR4 and MyD88 knockout mice, Zhang et al. identified TLR4 as an essential factor for lung homeostasis, and its protective effects against oxidative stress through regulation of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) activity. Taken together, these studies indicate that oxidant production is associated with different TLRs in different lung cell types.
In the previous section, we have discussed the pathways that lead to the activation of NF-κB. NF-κB is a redox-sensitive transcription factor, and the context dependent activation/inhibition of NF-κB by oxidizing agents has been studied for decades. Notably, the NF-κB canonical or classic pathway relies on activation of the IKK complex, in particular IKKβ, which in turn dictates the phosphorylation of IκBα to regulate mechanisms of cell proliferation, differentiation, apoptosis, immunity and stress responses (Siomek, 2012). The alternative pathway, in which IKKα is a major player, is important for homeostasis and adaptive immunity; whereas the IKK-independent atypical pathway is mainly triggered in response to hypoxia, reoxygenation, ionizing radiations, and H2O2.
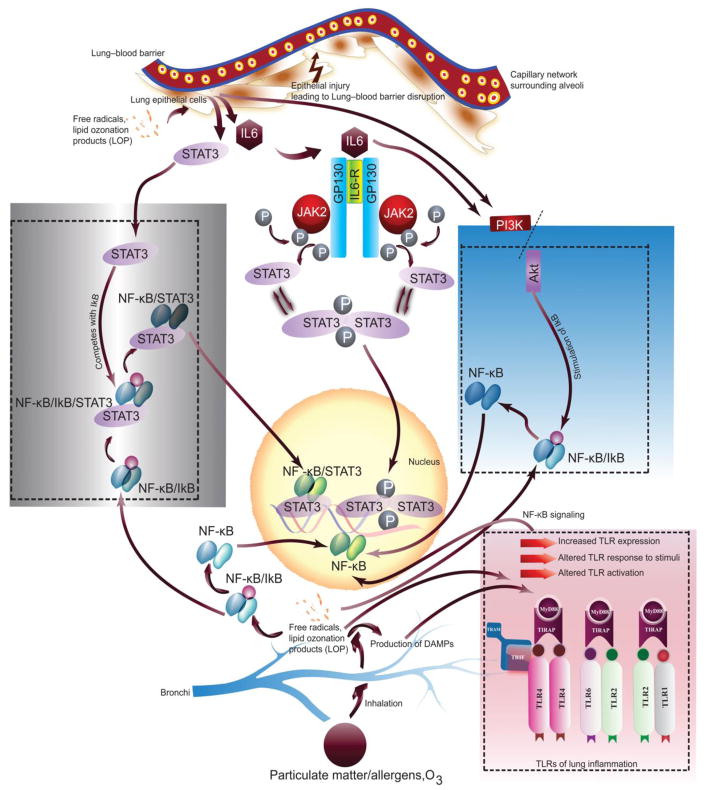
Depending on the context, ROS can be either harmful or beneficial. ROS have been shown to initiate inflammatory responses in the lung through the activation of transcription factors such as NF-κB resulting in chromatin remodeling and further gene expression of pro-inflammatory mediators (I. Rahman & MacNee, 1998; Richter, et al., 1995). A number of reports have indicated the requirement of ROS for NF-κB activation at various cellular levels and pathways (Bonizzi, et al., 1999; Chandel, Schumacker, & Arch, 2001; Hughes, Murphy, & Ledgerwood, 2005; Q. Li & Engelhardt, 2006). However, often ROS exert opposing results with NF-κB activation and inhibition, where ROS stimulates signal transduction pathways for NF-κB at cytoplasmic levels and greatly inhibit DNA binding of NF-κB at the nuclear level (Kabe, Ando, Hirao, Yoshida, & Handa, 2005). Studies on human pulmonary artery endothelial cells have shown that TNFα induces ROS, which in turn signals the activation of NF-κB, that regulates the expression of ICAM-1 and E-selectin genes (A. Rahman, Bando, Kefer, Anwar, & Malik, 1999; A. Rahman, Kefer, Bando, Niles, & Malik, 1998). Similarly, studies with NADPH oxidase, which is a main cellular source of ROS in mononuclear and granulocytic leukocytes, have shown that NADPH oxidase-derived ROS can regulate lung inflammation and injury through redox regulation of NF-κB activity (W. Han, et al., 2013; Koay, et al., 2001). Our findings with O3-induced inflammation showed differential intracellular activation of the JAK2/STAT3 and NF-κB/AKT pathway in a sex-dependent manner, and suggested a potential role of sex hormones in this regulation (Figure 2) (Mishra, DiAngelo, & Silveyra, 2016). It is noteworthy that AKT can affect IKK regulation, where AKT itself can be context-dependent regulated either positively and negatively by ROS (S. R. Lee, et al., 2002; Murata, et al., 2003).
Figure 2. Schematic of oxidative stress induced lung inflammation and role of IL-6 signaling pathways in conjunction with TLRs and NF-κB.
Oxidative stress leads to the generation of lipid peroxidation products, ROS, free radicals in lung cell types. These oxidants can further mediate activation of downstream biochemical events including increased and altered TLR expression and activation in response to allergens. Oxidants and oxidative stress also impart direct effect on NF-κB as well as on the molecules of NF-κB pathway leading to the production of inflammatory factors and lung damage. (Adapted and modified from (Mishra, et al., 2016)).
8. Pharmacological approaches targeting TLR/NF-κB and oxidative biology
The standard eosinophilic asthma treatment includes β2-agonists and systemic corticosteroids (Walford & Doherty, 2014). The oral or systemic administration of corticosteroids is known to exert strong effects on leukocyte recruitment (Cronstein, Kimmel, Levin, Martiniuk, & Weissmann, 1992), and exhibit very effective anti-inflammatory properties by modulating NF-κB and AP-1 expression (Cronstein, et al., 1992; Mittelstadt & Ashwell, 2001). Although treatment with corticosteroids is considered as the gold standard, various studies have reported a significant incidence of adverse effects with high dose and long-term use of corticosteroids (Baraket, et al., 2012; Dahl, 2006; Donohue & Ohar, 2004; Geddes, 1992).
Oxidant production by PRRs such as TLRs, or oxidant-based activation of PRRs, can initiate a cascade of events involving redox regulation of transcription factors such as NF-κB. These molecular events share several common pathways and intermediary molecules. Targeting oxidizing molecules in conjunction with PPRs and regulators of transcription factors may present a novel therapeutic strategy for asthma. Considering that ROS and RNS formation, as well as lipid peroxidation products, play key roles in the foundation and further progression of asthma, the use of free radical scavengers and antioxidant stabilizers with multifactorial effects on free radicals, PRRs and regulatory transcription factors may achieve ideal success in treatment. Molecules known to possess antioxidant activity along with ability to regulate various intracellular signaling pathways, like flavonoids, beta-carotenes, lycopene, omega-3 and omega-6 fatty acids, thiol antioxidants, melatonin, carotenoids, resveratrol, vitamin C and E may be used in targeted adjuvant treatment of asthma. However, given the vast degree of naturally occurring compounds exhibiting potent antioxidant activity and signatory intercepts in intracellular pathways, the current review will limit its approach to flavonoids, in particular to Kaempferol, Quercetin, and Isorhamnetin flavonol. Another important aspect behind the selection of flavonoids for the purpose of this review is their stronger antioxidant property compared to many other natural antioxidants including vitamin C and E (Prior & Cao, 2000). The antioxidant activity of flavonoids is dependent on their chemical structure, mainly to the catechol moiety (Van Acker, et al., 1996). Additionally, it has been reported that the number of hydroxyl (OH) substitutions correlates with higher antioxidant activity of flavonoids (Amić & Lučić, 2010; Cao, Sofic, & Prior, 1997). Natural molecules like flavonoids possess strong antioxidant activity and their ability to regulate intracellular signaling pathways could be effectively utilized for safer and effective therapeutic approaches.
Kaempferol, a flavonoid found in apples and many berries, has been shown to alleviate airway inflammation and acts as a therapeutic agent for asthmatics (Gong, et al., 2013). Niering et al. showed that Kaempferol protects rat liver hepatoma cells against cellular damage induced by H2O2 (Niering, et al., 2005). Similarly, Kaempferol has also been found to block cerebellar granule cell death, and as a potent inhibition of ROS and superoxide anion production by cerebellar granule cells (Samhan-Arias, Martin-Romero, & Gutierrez-Merino, 2004). Animal studies indicate a favorable response of Kaempferol in reduction of airway inflammation. In airway epithelial cells, Kaempferol was found to inhibit LPS-induced IL-8 production and eotaxin-1 via TLR4 activation. The same study, performed on an ovalbumin sensitized mice model treated with Kaempferol, reported reduction in the levels of CXCR2 and CCR3 along with inhibition of Tyk2-STAT1/3 signaling (Gong, et al., 2013). Similar results were obtained in a study where Astragalin, a 3-O-glucoside of Kaempferol, was found to inhibit LPS and H2O2 induced oxidative stress in airway epithelial cells through inhibition of eotaxin-1 and epithelial apoptosis, and TLR4-PKCβ2-NADPH signaling (Cho, et al., 2014). The effect of Kaempferol in NF-κB signaling was further confirmed in a model of TNF-α induced lung inflammation, where a potent inhibitory effect of Kaempferol on monocyte chemoattractant protein-1 transcription involving modulation of NF-κB signaling was reported (Gong, Shin, Han, Kim, & Kang, 2012).
Another vastly studied dietary flavonoid is Quercetin, commonly found in vegetables, fruits, seeds, nuts, tea, and wine, and is known to have strong effects on ROS metabolism and cell apoptosis (Formica & Regelson, 1995; Jeong, An, Kwon, Rhee, & Lee, 2009). In a study of sarcoidosis, an inflammatory disease that mostly affects lungs and lymph nodes, Quercetin supplementation showed a marked reduction in the levels of Malondialdehyde, TNFα/IL-10, and IL-8/IL-10, which are markers of oxidative damage (Boots, Drent, de Boer, Bast, & Haenen, 2011). The authors correlated the observed benefits with a potential role of Quercetin as a ROS and oxidant scavenger, along with its known ability to reduce inflammatory cytokine production (in particular TNFα) via modulation of NF-κβ1 and Iκβ pathways (Nair, et al., 2006). Moreover, supplementation of Quercetin in a rat model of hepatopulmonary syndrome showed anti-angiogenic effects associated with AKT/NF-κB and VEGFA/VEGFR-2 pathways (X. Li, et al., 2016). Another study with Quercetin treatment on a bleomycin-induced pulmonary fibrosis model showed that oral Quercetin revived antioxidant defense mechanisms of the lung along with restoration of lung pathology (Verma, et al., 2013). Similarly, Quercetin was able to alleviate radiation-induced oxidative stress, pneumonitis, and fibrosis (H. Liu, Xue, Li, Ao, & Lu, 2013), as well as LPS-induced lung injury (Huang, Zhong, & Wu, 2015). The beneficial effect of Quercetin involving TLR modulation was further established in a study on mouse alveolar macrophage cultures, where Quercetin attenuated TLR7-induced expression of TNF-α and IL-6 (Yasui, et al., 2015). Finally, in another study on LPS-stimulated human peripheral blood mononuclear cells (PBMCs), Quercetin was able to suppress the secretion of TNF-α, IL-1β, and IL-6 from PBMCs, and reduced the mRNA expression of TLR2 and NF-κB (M. Zhang, Lin, Li, & Li, 2016).
Isorhamnetin, a flavonol and methylated metabolite of Quercetin commonly found in numerous plant-based foods like almonds, fennel, red onion, and turnip greens, has been reported to have potent anti-inflammatory, anti-oxidative, and anti-proliferative effects. Studies using Isorhamnetin have shown that it antagonizes H2O2, reduces apoptopic damage via inhibition of cytochrome C release, and alters caspase 3 possibly via ERK inactivation in cardiomyocytes (B. Sun, et al., 2012). In addition, Isorhamnetin is a potent in vitro and in vivo inhibitor of influenza virus titer, an observation associated with reduction in ROS generation and blockage of cytoplasmic lysosome acidification (Abdal Dayem, Choi, Kim, & Cho, 2015). It also possesses photo-protective properties, and can inhibit cell damage and apoptosis caused by ultraviolet-B exposure by reducing ROS and attenuating oxidative modification of DNA, lipids, and proteins (X. Han, et al., 2015).
In a model of LPS-induced acute lung injury, Chi et al. showed that Isorhamnetin treatment is protective against oxidative damage and inflammation via inhibition of TNF-α, IL-1β and IL-6 secretion and suppression of ERK, JNK, IκBα, and NF-κB(p65) phosphorylation (Chi, et al., 2016). In a rat model of type 2 diabetes, Isorhamnetin decreased the levels of urinary osteopontin, kidney injury molecule 1 (KIM-1), and albumin, and also inhibited production of inflammatory mediators and attenuated oxidative stress possibly via modulation of NF-κB signaling activity (Qiu, Sun, Zhang, Li, & Wang, 2016). In human breast carcinoma cells, Isorhamnetin significantly inhibited adhesion, migration, and invasion of carcinoma cells by possibly downregulating the expression of MMP-2 and MMP-9 via suppression of p38 MAPK and STAT3. Notably, regulation of MMP-2 and MMP-9 have established links with NF-κB pathway modulation, and the observed reduction in MMPs may have a possible correlation with this pathway (C. Li, et al., 2015; Shishodia, Majumdar, Banerjee, & Aggarwal, 2003). Isorhamnetin also modulated TLR activity and offered protection against oxidative stress in an animal model of influenza infection, where it increased TLR2/4 expression in a dose-dependent manner (H. Wang, et al., 2012). Furthermore, in a mouse model of LPS-induced endotoxemia, Isorhamnetin reduced peritoneal macrophages NO production, and IL-6, TNF-α, CD40, COX-2 and iNOS expression (Jayashankar, Mishra, Ganju, & Singh, 2014). These observations may indicate a correlation with TLR modulation, as induction of COX-2 and iNOS are associated with TLR4, and play pivotal roles in the development of inflammatory diseases (Shweash, et al., 2011). In fact, COX-2 and iNOS have also been identified as potential therapeutic targets in other inflammatory diseases such as cancer (Murakami & Ohigashi, 2007). A study on Mycobacteria-induced lung inflammation showed that Isorhamnetin also possesses moderate anti-mycobacterial activities in the lung, and is a potent anti-inflammatory agent, reducing expression of IL-1β, IL-6, IL-12, TNF-α, and MMP-1 and inhibiting MAPK and ERK phosphorylation, a signatory fingerprint in Mycobacterial signaling pathway activation (Jnawali, et al., 2016).
Taken together, these studies indicate that flavonol compounds can be developed as therapeutic candidates for inflammatory lung disease, not only due to their direct protective effects as antioxidants for oxygen and nitrogen scavenging, but also because of their modulatory effects on cell signaling cascades related to lung inflammation and pathology.
9. Conclusion and future directions
Asthma therapy can be primarily classified in to three classes: inhaled β2-adrenoceptor (β2-AR) agonists, inhaled and systemic corticosteroids, and leukotriene synthesis inhibitors (LTSIs) together with leukotriene receptor antagonists (LTRAs) (Sayers & Hall, 2005). Corticosteroids and bronchodilators are the first line and most commonly used drugs to treat typical symptoms of asthma and exacerbations. At a cellular level, corticosteroids increase β-adrenergic responses, diminish the inflammatory cells in the airways, and offer clinical improvement. However, in severe asthma, and in COPD, corticosteroids are ineffective and offer negligible clinical improvement (Barnes, 2013; Belvisi, Hele, & Birrell, 2004; Brusselle, Joos, & Bracke, 2011; Kirkham & Rahman, 2006; I. Rahman, Morrison, Donaldson, & MacNee, 1996). The observed inefficacy of corticosteroids in the treatment of severe lung inflammation is attributed to oxidative stress, chromatin remodeling, and DNA damage that lead to decreased activity of transcriptional corepressors such as histone deacetylase-2 (HDAC-2), known to counteract histones acetylation, resulting in increased expression of inflammatory genes (Zuo, Lucas, Fortuna, Chuang, & Best, 2015). There are at least twelve known mammalian HDACs, which are differentially expressed and regulated in different cell types (Haberland, Montgomery, & Olson, 2009), and are attributed to the observed differences in responsiveness to corticosteroids. Activated pro-inflammatory transcription factors like NF-κB, which is also sensitive to corticosteroids treatment (Barnes, 2004; Barnes, Ito, & Adcock, 2004; Barnes & Karin, 1997), bind to specific sequences in DNA and subsequently interact with coactivator molecules like p300/CREB (cyclic adenosine monophosphate response element–binding protein)–binding protein (CBP) to control target gene transcription.
The β2-adrenergic receptor agonists act by binding to the β2-adrenergic receptor, a G-protein-coupled receptor protein encoded by ADRβ2 gene, which is expressed on multiple airway cell types (Ortega, Hawkins, Peters, & Bleecker, 2007). ADRβ2 is a highly polymorphic gene that can influence lung function, and also respond to β-agonist therapy (Fuso, et al., 2013; Green, Turki, Bejarano, Hall, & Liggett, 1995; Hawkins, et al., 2006; Martinez, Graves, Baldini, Solomon, & Erickson, 1997; Tan, Hall, Dewar, Dow, & Lipworth, 1997). Similarly, drugs targeting leukotriene signaling pathways are shown to decrease asthmatic exacerbations when coupled with corticosteroid therapy (Camargo, et al., 2010; Camargo, Smithline, Malice, Green, & Reiss, 2003; Dahlen, 2006; Philip, et al., 2010). However, further research on leukotrienes show that genetic polymorphisms in genes encoding target proteins of the leukotriene pathway may alter the response to LTSIs and LTRAs (Drazen, et al., 1999; In, et al., 1997; Lima, et al., 2006; Sampson, et al., 2000). In viewof advantages and disadvantages of current therapeutic regimes for asthma, and with the fast increase in the number of asthmatics as reported by the global asthma report (2014) (Network, 2014), there is a dire need for new and alternative therapeutic approaches. Considering that ROS and formation of other oxidants are common in asthma; novel therapeutics targeting redox abnormalities and signaling molecules could be effectively utilized for the treatment of asthma.
At certain concentrations, ROS and other oxidants not only possess direct cell damaging properties, but can also influence cell signaling pathways and molecules at cytoplasmic and nuclear levels. In this context, ROS modulation of PRRs like TLRs in the vicinity of airway antigens, and further modulation of intrinsic signaling molecules like NF-κB are of absolute importance in devising future strategies for better therapeutic control of the clinical features of asthma. The polyphenolic plant secondary metabolites, flavonols, are known to have powerful antioxidant, anti-allergic, anti-inflammatory and immune-modulating effects. We believe further evaluation of molecular mechanisms involved in the anti-inflammatory properties offered by various flavonols can pave the way for the development of novel therapeutic targets for asthma and other diseases characterized by airway inflammation.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- DAMPs
damage-associated molecular patterns
- DC
dendritic cells
- IFN
Interferon
- IKK (IKB)
inhibitor kappa B kinase
- IL
Interleukins
- MHC
major histocompatibility complex
- MyD88
Myeloid differentiation primary response protein MyD88
- NADPH
nicotinamide adenine dinucleotide phosphate-oxidase
- NEMO
NF-κB essential modifier
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NIK
NF-κB-inducing kinase
- O3
Ozone
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Th
T helper cell
- TIR
Toll/IL-1R receptor domain
- TIRAP
toll-interleukin 1 receptor (TIR) domain containing adaptor protein
- TLR
Toll like receptors
- TNF
Tumor necrosis factor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- TSLP
Thymic stromal lymphoprotein
Footnotes
Conflict of Interest Statement:
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdal Dayem A, Choi HY, Kim YB, Cho SG. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS One. 2015;10:e0121610. doi: 10.1371/journal.pone.0121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169:3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N, Wynn TA, Dillon S, Erb P, Baschang G, Blaser K, Alkan SS. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Al-Harbi NO, Nadeem A, Al-Harbi MM, Imam F, Al-Shabanah OA, Ahmad SF, Sayed-Ahmed MM, Bahashwan SA. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int Immunopharmacol. 2015;26:237–245. doi: 10.1016/j.intimp.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amić D, Lučić B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorganic & medicinal chemistry. 2010;18:28–35. doi: 10.1016/j.bmc.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baichwal VR. NF-kB as a frequent target for immunosuppressive and anti-inflammatory molecules. Advances in immunology. 1997;65:111–138. [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-κB transcription factor. Cell. 1988;53:211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baraket M, Oliver BG, Burgess JK, Lim S, King GG, Black JL. Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study. Respiratory research. 2012;13:11. doi: 10.1186/1465-9921-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Corticosteroid resistance in airway disease. Proc Am Thorac Soc. 2004;1:264–268. doi: 10.1513/pats.200402-014MS. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12:543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet. 2004;363:731–733. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129:14–24. doi: 10.1016/j.jaci.2011.11.004. quiz 25–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Dy M, Murray L, Burdick MD, Xue YY, Strieter RM, Keane MP. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol. 2004;173:4692–4698. doi: 10.4049/jimmunol.173.7.4692. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ, Birrell MA. New anti-inflammatory therapies and targets for asthma and chronic obstructive pulmonary disease. Expert Opin Ther Targets. 2004;8:265–285. doi: 10.1517/14728222.8.4.265. [DOI] [PubMed] [Google Scholar]
- Biondo C, Midiri A, Messina L, Tomasello F, Garufi G, Catania MR, Bombaci M, Beninati C, Teti G, Mancuso G. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur J Immunol. 2005;35:870–878. doi: 10.1002/eji.200425799. [DOI] [PubMed] [Google Scholar]
- Bochkov Y, Hanson K, Keles S, Brockman-Schneider R, Jarjour N, Gern J. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal immunology. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–512. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Bradding P, Feather IH, Wilson S, Holgate ST, Howarth PH. Cytokine immunoreactivity in seasonal rhinitis: regulation by a topical corticosteroid. Am J Respir Crit Care Med. 1995;151:1900–1906. doi: 10.1164/ajrccm.151.6.7767538. [DOI] [PubMed] [Google Scholar]
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Brasier AR. The NF-κB regulatory network. Cardiovascular toxicology. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel PL, Rihs S, Virchow JC, Jr, Warringa RA, Moser R, Walker C. Early activation or “priming” of eosinophils in asthma. Schweiz Med Wochenschr. 1992;122:298–301. [PubMed] [Google Scholar]
- Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N, Mishra V, Sinha U, DiAngelo SL, Chroneos ZC, Ekpa NA, Cooper TK, Caruso CR, Silveyra P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1150–1163. doi: 10.1152/ajplung.00018.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo CA, Jr, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, Malice MP, Legrand C, Dass SB, Knorr BA, Reiss TF. A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol. 2010;125:374–380. doi: 10.1016/j.jaci.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003;167:528–533. doi: 10.1164/rccm.200208-802OC. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical Biology and Medicine. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Cardell B, Pearson RB. Death in asthmatics. Thorax. 1959;14:341–352. [Google Scholar]
- Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annual review of immunology. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, Joosten LA, Latge JP, Netea MG. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 2011;13:151–159. doi: 10.1016/j.micinf.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276:42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-κB transcription factors: structural views. Oncogene. 1999:18. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol. 2013;31:605–633. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nature reviews Molecular cell biology. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chi G, Zhong W, Liu Y, Lu G, Lu H, Wang D, Sun F. Isorhamnetin protects mice from lipopolysaccharide-induced acute lung injury via the inhibition of inflammatory responses. Inflamm Res. 2016;65:33–41. doi: 10.1007/s00011-015-0887-9. [DOI] [PubMed] [Google Scholar]
- Cho IH, Gong JH, Kang MK, Lee EJ, Park JH, Park SJ, Kang YH. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm Med. 2014;14:122. doi: 10.1186/1471-2466-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cildir G, Low KC, Tergaonkar V. Noncanonical NF-kB Signaling in Health and Disease. Trends in Molecular Medicine. 22:414–429. doi: 10.1016/j.molmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proceedings of the National Academy of Sciences. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CSD, Koff JL. Innate and Adaptive Immunity in the Lung. In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, Siegel MD, editors. Fishman’s Pulmonary Diseases and Disorders. New York, NY: McGraw-Hill Education; 2015. p. 5e. [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Curtis JL. Cell-mediated adaptive immune defense of the lungs. Proc Am Thorac Soc. 2005;2:412–416. doi: 10.1513/pats.200507-070JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respiratory medicine. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Dahlen SE. Treatment of asthma with antileukotrienes: first line or last resort therapy? Eur J Pharmacol. 2006;533:40–56. doi: 10.1016/j.ejphar.2005.12.070. [DOI] [PubMed] [Google Scholar]
- Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nature immunology. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, Sadeghi Z, Dariushnejad H, Zarghami N. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev. 2014;15:4353. doi: 10.7314/apjcp.2014.15.10.4353. [DOI] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini M, Di Fusco D, Sica G, Sileri P, MacDonald T, Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–3503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32:177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Donohue JF, Ohar JA. Effects of corticosteroids on lung function in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2004;1:152–160. doi: 10.1513/pats.200402-003MS. [DOI] [PubMed] [Google Scholar]
- Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, De Sanctis GT, Perkins DL, Finn PW. NF-κB/Rel Transcription Factors: c-Rel Promotes Airway Hyperresponsiveness and Allergic Pulmonary Inflammation. The Journal of Immunology. 1999;163:6827–6833. [PubMed] [Google Scholar]
- Drazen JM, Yandava CN, Dubé L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nature genetics. 1999;22:168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mehta AK, Magalhaes JG, Ziegler SF, Dong C, Philpott DJ, Croft M. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126:1284–1293. e1210. doi: 10.1016/j.jaci.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181:8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, So T, Mehta AK, Choi H, Croft M. Inducible CD4+LAP+Foxp3- regulatory T cells suppress allergic inflammation. J Immunol. 2011;187:6499–6507. doi: 10.4049/jimmunol.1101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duechs MJ, Hahn C, Benediktus E, Werner-Klein M, Braun A, Hoymann HG, Gantner F, Erb KJ. TLR agonist mediated suppression of allergic responses is associated with increased innate inflammation in the airways. Pulm Pharmacol Ther. 2011;24:203–214. doi: 10.1016/j.pupt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, Nowak D, Martinez FD, Team AS. Toll-like receptor 2 as a major gene for asthma in children of European farmers. Journal of allergy and clinical immunology. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacology & Therapeutics. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurum SC. New Insights in Oxidant Biology in Asthma. Ann Am Thorac Soc. 2016;13(Suppl 1):S35–39. doi: 10.1513/AnnalsATS.201506-385MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Fuchsberger M, Hochrein H, O’Keeffe M. Activation of plasmacytoid dendritic cells. Immunol Cell Biol. 2005;83:571–577. doi: 10.1111/j.1440-1711.2005.01392.x. [DOI] [PubMed] [Google Scholar]
- Fusco AJ, Huang DB, Miller D, Wang VYF, Vu D, Ghosh G. NF-κB p52: RelB heterodimer recognizes two classes of κB sites with two distinct modes. EMBO reports. 2009;10:152–159. doi: 10.1038/embor.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuso L, Di Perna A, Longobardi A, Trové A, Bisceglia M, Bibi BF, Angelozzi C, Tiziano FD, Antonelli Incalzi R. Polymorphism of Beta2-adrenoceptor and regular use of formoterol in asthma: preliminary results. ISRN Pulmonology, 2013 2013 [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaurav R, Agrawal DK. Clinical view on the importance of dendritic cells in asthma. Expert Rev Clin Immunol. 2013;9:899–919. doi: 10.1586/1744666X.2013.837260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes D. Inhaled corticosteroids: benefits and risks. Thorax. 1992;47:404. doi: 10.1136/thx.47.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Erzurum SC. Nitric oxide metabolism in asthma pathophysiology. Biochim Biophys Acta. 2011;1810:1008–1016. doi: 10.1016/j.bbagen.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Erzurum SC. Modulation of Asthma Pathogenesis by Nitric Oxide Pathways and Therapeutic Opportunities. Drug Discov Today Dis Mech. 2012;9:e89–e94. doi: 10.1016/j.ddmec.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden M. Celebrating 25 years of NF-κB Research. Immunological Reviews. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annual review of immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, Jakob T, Pastore S, Behrendt H, Traidl-Hoffmann C. Pollen-Derived E1-Phytoprostanes Signal via PPAR-γ and NF-κB-Dependent Mechanisms. The Journal of Immunology. 2009;182:6653–6658. doi: 10.4049/jimmunol.0802613. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Gong JH, Shin D, Han SY, Kim JL, Kang YH. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J Nutr. 2012;142:47–56. doi: 10.3945/jn.111.150748. [DOI] [PubMed] [Google Scholar]
- Gong JH, Shin D, Han SY, Park SH, Kang MK, Kim JL, Kang YH. Blockade of Airway Inflammation by Kaempferol via Disturbing Tyk-STAT Signaling in Airway Epithelial Cells and in Asthmatic Mice. Evid Based Complement Alternat Med. 2013;2013:250725. doi: 10.1155/2013/250725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- Grimm S, Baeuerle PA. The inducible transcription factor NF-kappa B: structure-function relationship of its protein subunits. Biochemical journal. 1993;290:297. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin Inhibits In Vitro Maturation and In Vivo Immunostimulatory Function of Murine Myeloid Dendritic Cells. The Journal of Immunology. 2001;166:7053–7062. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- Han W, Li H, Cai J, Gleaves LA, Polosukhin VV, Segal BH, Yull FE, Blackwell TS. NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-kappaB activity. J Immunol. 2013;190:4786–4794. doi: 10.4049/jimmunol.1201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Piao MJ, Kim KC, Madduma Hewage SR, Yoo ES, Koh YS, Kang HK, Shin JH, Park Y, Yoo SJ, Chae S, Hyun JW. Isorhamnetin Protects Human Keratinocytes against Ultraviolet B-Induced Cell Damage. Biomol Ther (Seoul) 2015;23:357–366. doi: 10.4062/biomolther.2015.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins GA, Tantisira K, Meyers DA, Ampleford EJ, Moore WC, Klanderman B, Liggett SB, Peters SP, Weiss ST, Bleecker ER. Sequence, haplotype, and association analysis of ADRβ2 in a multiethnic asthma case-control study. American journal of respiratory and critical care medicine. 2006;174:1101–1109. doi: 10.1164/rccm.200509-1405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Kwon H, Génin P. Hostile takeovers: viral appropriation of the NF-kB pathway. The Journal of clinical investigation. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Ender F, Schmudde I, Lewkowich IP, Kohl J, Konig P, Laumonnier Y. Origin, Localization, and Immunoregulatory Properties of Pulmonary Phagocytes in Allergic Asthma. Front Immunol. 2016;7:107. doi: 10.3389/fimmu.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, Howarth P. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–29s. doi: 10.1183/09031936.03.00000803. [DOI] [PubMed] [Google Scholar]
- Hollingsworth JW, 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- Hsia BJ, Whitehead GS, Nakano K, Gowdy KM, Thomas SY, Aloor J, Nakano H, Cook DN. Trif-dependent induction of Th17 immunity by lung dendritic cells. Mucosal immunology. 2015;8:186. doi: 10.1038/mi.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Zhong T, Wu H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch Med Sci. 2015;11:427–432. doi: 10.5114/aoms.2015.50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber HL, Koessler KK. The pathology of bronchial asthma. Archives of Internal Medicine. 1922;30:689–760. [Google Scholar]
- Huertas A, Palange P. COPD: a multifactorial systemic disease. Therapeutic advances in respiratory disease. 2011;5:217–224. doi: 10.1177/1753465811400490. [DOI] [PubMed] [Google Scholar]
- Hughes G, Murphy MP, Ledgerwood EC. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J. 2005;389:83–89. doi: 10.1042/BJ20050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmarinen P, Kankaanranta H. Eosinophil Apoptosis as a Therapeutic Target in Allergic Asthma. Basic & Clinical Pharmacology & Toxicology. 2014;114:109–117. doi: 10.1111/bcpt.12163. [DOI] [PubMed] [Google Scholar]
- Imler JL, Hoffmann JA. Toll receptors in Drosophila: a family of molecules regulating development and immunity. Curr Top Microbiol Immunol. 2002;270:63–79. doi: 10.1007/978-3-642-59430-4_4. [DOI] [PubMed] [Google Scholar]
- In K, Asano K, Beier D, Grobholz J, Finn P, Silverman E, Silverman E, Collins T, Fischer A, Keith T. Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. Journal of Clinical Investigation. 1997;99:1130. doi: 10.1172/JCI119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis I, Ye F, McNally B, Willette M, Flano E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol. 2013;87:3261–3270. doi: 10.1128/JVI.01956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Philbin VJ, Smith AL. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet Immunol Immunopathol. 2005;104:117–127. doi: 10.1016/j.vetimm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashankar B, Mishra KP, Ganju L, Singh SB. Supercritical extract of Seabuckthorn Leaves (SCE200ET) inhibited endotoxemia by reducing inflammatory cytokines and nitric oxide synthase 2 expression. Int Immunopharmacol. 2014;20:89–94. doi: 10.1016/j.intimp.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Diaz PT, Best TM, Stimpfl JN, He F, Zuo L. Molecular characterization of redox mechanisms in allergic asthma. Ann Allergy Asthma Immunol. 2014;113:137–142. doi: 10.1016/j.anai.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Jnawali HN, Jeon D, Jeong MC, Lee E, Jin B, Ryoo S, Yoo J, Jung ID, Lee SJ, Park YM, Kim Y. Antituberculosis Activity of a Naturally Occurring Flavonoid, Isorhamnetin. J Nat Prod. 2016;79:961–969. doi: 10.1021/acs.jnatprod.5b01033. [DOI] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL. Production and degradation of oxygen metabolites during inflammatory states in the human lung. Curr Drug Targets Inflamm Allergy. 2005;4:465–470. doi: 10.2174/1568010054526368. [DOI] [PubMed] [Google Scholar]
- Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect Immun. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol. 2004;16:35–41. doi: 10.1016/j.smim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvarnhammar AM, Petterson T, Cardell LO. NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists. Immunology. 2011;134:314–325. doi: 10.1111/j.1365-2567.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ, Silverman EK, Martinez F, Weiss ST. Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three US ethnic groups and exploratory case–control disease association studies. Genomics. 2003;81:85–91. doi: 10.1016/s0888-7543(02)00022-8. [DOI] [PubMed] [Google Scholar]
- Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Li C, Yang D, Zhao Y, Qiu Y, Cao X, Yu Y, Guo H, Gu X, Yin X. Inhibitory Effects of Isorhamnetin on the Invasion of Human Breast Carcinoma Cells by Downregulating the Expression and Activity of Matrix Metalloproteinase-2/9. Nutr Cancer. 2015;67:1191–1200. doi: 10.1080/01635581.2015.1073763. [DOI] [PubMed] [Google Scholar]
- Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Li Q, Engelhardt JF. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem. 2006;281:1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- Li X, Chen Y, Wang L, Shang G, Zhang C, Zhao Z, Zhang H, Liu A. Quercetin alleviates pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Braz J Med Biol Res. 2016:49. doi: 10.1590/1414-431X20165326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. American journal of respiratory and critical care medicine. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xue JX, Li X, Ao R, Lu Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol Lett. 2013;6:453–459. doi: 10.3892/ol.2013.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M. RelB forms transcriptionally inactive complexes with RelA/p65. Journal of Biological Chemistry. 2003;278:19852–19860. doi: 10.1074/jbc.M301945200. [DOI] [PubMed] [Google Scholar]
- Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. Journal of Clinical Investigation. 1997;100:3184. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten BJ, Olson GK, Tarleton CA, Rund C, Schuyler M, Mehran R, Archibeque T, Lipscomb MF. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol. 2006;177:7784–7793. doi: 10.4049/jimmunol.177.11.7784. [DOI] [PubMed] [Google Scholar]
- Matasić R, Dietz AB, Vuk-Pavlović S. Cyclooxygenase-independent inhibition of dendritic cell maturation by aspirin. Immunology. 2000;101:53–60. doi: 10.1046/j.1365-2567.2000.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, Bals R, Lang R, Dalpke AH. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol. 2007;178:3134–3142. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- McAleer JP, Liu B, Li Z, Ngoi SM, Dai J, Oft M, Vella AT. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. Journal of leukocyte biology. 2010;88:21–31. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlees JW, Whitehead GS, Harley IT, Cappelletti M, Rewerts CL, Holdcroft AM, Divanovic S, Wills-Karp M, Finkelman FD, Karp CL. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal immunology. 2015;8:863. doi: 10.1038/mi.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. American journal of respiratory and critical care medicine. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, DiAngelo SL, Silveyra P. Sex-specific IL-6-associated signaling activation in ozone-induced lung inflammation. Biol Sex Differ. 2016;7:16. doi: 10.1186/s13293-016-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. Journal of Biological Chemistry. 2001;276:29603–29610. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- Mortaz E, Adcock IM, Ito K, Kraneveld AD, Nijkamp FP, Folkerts G. Cigarette smoke induces CXCL8 production by human neutrophils via activation of TLR9 receptor. Eur Respir J. 2010;36:1143–1154. doi: 10.1183/09031936.00062209. [DOI] [PubMed] [Google Scholar]
- Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- Nadigel J, Prefontaine D, Baglole CJ, Maltais F, Bourbeau J, Eidelman DH, Hamid Q. Cigarette smoke increases TLR4 and TLR9 expression and induces cytokine production from CD8(+) T cells in chronic obstructive pulmonary disease. Respir Res. 2011;12:149. doi: 10.1186/1465-9921-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem. 2005;280:5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network GA. The global asthma report 2014. Auckland, New Zealand: 2014. [Google Scholar]
- Niering P, Michels G, Watjen W, Ohler S, Steffan B, Chovolou Y, Kampkotter A, Proksch P, Kahl R. Protective and detrimental effects of kaempferol in rat H4IIE cells: implication of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2005;209:114–122. doi: 10.1016/j.taap.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, Arinami T. An association study of asthma and total serum immunoglobin E levels for Toll-like receptor polymorphisms in a Japanese population. Clinical & Experimental Allergy. 2004;34:177–183. doi: 10.1111/j.1365-2222.2004.01839.x. [DOI] [PubMed] [Google Scholar]
- O’Flaherty SM, Sutummaporn K, Haggtoft WL, Worrall AP, Rizzo M, Braniste V, Hoglund P, Kadri N, Chambers BJ. TLR stimulated eosinophils mediate recruitment and activation of NK cells in vivo. Scand J Immunol. 2017 doi: 10.1111/sji.12554. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Kaltschmidt C. NF-kB: a crucial transcription factor for glial and neuronal cell function. Trends in neurosciences. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. American journal of respiratory and critical care medicine. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- Ortega VE, Hawkins GA, Peters SP, Bleecker ER. Pharmacogenetics of the beta 2-adrenergic receptor gene. Immunol Allergy Clin North Am. 2007;27:665–684. vii. doi: 10.1016/j.iac.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999:18. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, Doyle SL, McGettrick AF, Hardy M, Husebye H, Banahan K, Gong M, Golenbock D, Espevik T, O’Neill LA. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat Immunol. 2009;10:579–586. doi: 10.1038/ni.1727. [DOI] [PubMed] [Google Scholar]
- Parker D, Prince A. Innate immunity in the respiratory epithelium. American journal of respiratory cell and molecular biology. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Yuan Q, Lin B, Panneerselvam P, Wang X, Luan XL, Lim SK, Leung BP, Ho B, Ding JL. SARM inhibits both TRIF- and MyD88-mediated AP-1 activation. Eur J Immunol. 2010;40:1738–1747. doi: 10.1002/eji.200940034. [DOI] [PubMed] [Google Scholar]
- Philip G, Pedinoff A, Vandormael K, Tymofyeyev Y, Smugar SS, Reiss TF, Korenblat PE. A phase I randomized, placebo-controlled, dose-exploration study of single-dose inhaled montelukast in patients with chronic asthma. Journal of Asthma. 2010;47:1078–1084. doi: 10.3109/02770903.2010.520100. [DOI] [PubMed] [Google Scholar]
- Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. Journal of Clinical Investigation. 2005;115:459. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, Bals R. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–1223. doi: 10.4049/jimmunol.173.2.1219. [DOI] [PubMed] [Google Scholar]
- Porsbjerg C, Baines K, Sverrild A, Backer V, Gibson P. Eosinophilic airway inflammation is associated with increased TLR2 and TLR4 expression in adult asthmatics. European Respiratory Journal. 2014;44:1716. [Google Scholar]
- Prior RL, Cao G. Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortScience. 2000;35:588–592. [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Sun G, Zhang Y, Li X, Wang R. Involvement of the NF-kappaB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. Biomed Rep. 2016;4:628–634. doi: 10.3892/br.2016.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bando M, Kefer J, Anwar KN, Malik AB. Protein kinase C-activated oxidant generation in endothelial cells signals intercellular adhesion molecule-1 gene transcription. Mol Pharmacol. 1999;55:575–583. [PubMed] [Google Scholar]
- Rahman A, Kefer J, Bando M, Niles WD, Malik AB. E-selectin expression in human endothelial cells by TNF-alpha-induced oxidant generation and NF-kappaB activation. Am J Physiol. 1998;275:L533–544. doi: 10.1152/ajplung.1998.275.3.L533. [DOI] [PubMed] [Google Scholar]
- Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-α induce histone acetylation and NF-κB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Molecular and cellular biochemistry. 2002;234:239–248. [PubMed] [Google Scholar]
- Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- Reijmerink NE, Bottema RW, Kerkhof M, Gerritsen J, Stelma FF, Thijs C, Van Schayck CP, Smit HA, Brunekreef B, Koppelman GH. TLR-related pathway analysis: novel gene–gene interactions in the development of asthma and atopy. Allergy. 2010;65:199–207. doi: 10.1111/j.1398-9995.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic Cell Survival and Maturation Are Regulated by Different Signaling Pathways. The Journal of Experimental Medicine. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PR, Wasley KM, Allison CH. Diesel Particulate Matter Induces Receptor for Advanced Glycation End-Products (RAGE) Expression in Pulmonary Epithelial Cells, and RAGE Signaling Influences NF-[kappa] B-Mediated Inflammation. Environmental health perspectives. 2011;119:332. doi: 10.1289/ehp.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, Walter P, Yaffee M. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- Rom O, Avezov K, Aizenbud D, Reznick AZ. Cigarette smoking and inflammation revisited. Respiratory physiology & neurobiology. 2013;187:5–10. doi: 10.1016/j.resp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Ryseck RP, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Molecular and cellular biology. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free Radic Biol Med. 2004;37:48–61. doi: 10.1016/j.freeradbiomed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Sampson A, Siddiqui S, Buchanan D, Howarth P, Holgate S, Holloway J, Sayers I. Variant LTC4 synthase allele modifies cysteinyl leukotriene synthesis in eosinophils and predicts clinical response to zafirlukast. Thorax. 2000;55:S28–S31. doi: 10.1136/thorax.55.suppl_2.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers I, Hall IP. Pharmacogenetic approaches in the treatment of asthma. Curr Allergy Asthma Rep. 2005;5:101–108. doi: 10.1007/s11882-005-0082-0. [DOI] [PubMed] [Google Scholar]
- Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G, Blaser K, Wuthrich B, Simon HU. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–1027. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- Sehmi R, Howie K, Sutherland DR, Schragge W, O’Byrne PM, Denburg JA. Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. Am J Respir Cell Mol Biol. 1996;15:645–655. doi: 10.1165/ajrcmb.15.5.8918371. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell. 1986a;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. cell. 1986b;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Serasanambati M, Chilakapati SR. Function of Nuclear Factor kappa B (NF-kB) in human diseases-A Review. South Indian Journal of Biological Sciences. 2016;2:368–387. [Google Scholar]
- Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- Shih VFS, Tsui R, Caldwell A, Hoffmann A. A single NFkB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- Shweash M, Adrienne McGachy H, Schroeder J, Neamatallah T, Bryant CE, Millington O, Mottram JC, Alexander J, Plevin R. Leishmania mexicana promastigotes inhibit macrophage IL-12 production via TLR-4 dependent COX-2, iNOS and arginase-1 expression. Mol Immunol. 2011;48:1800–1808. doi: 10.1016/j.molimm.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomek A. NF-kappaB signaling pathway and free radical impact. Acta Biochim Pol. 2012;59:323–331. [PubMed] [Google Scholar]
- Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kB activation through interference with IKBα function in asthmatic bronchial epithelial cells. Biochemical and biophysical research communications. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]
- Stein D, Roth S, Vogelsang E, Nusslein-Volhard C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell. 1991;65:725–735. doi: 10.1016/0092-8674(91)90381-8. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxid Redox Signal. 2008;10:785–797. doi: 10.1089/ars.2007.1937. [DOI] [PubMed] [Google Scholar]
- Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu Y, Cao L, Yang ZH, Sun XB. Isorhamnetin inhibits H(2)O(2)-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem. 2012;113:473–485. doi: 10.1002/jcb.23371. [DOI] [PubMed] [Google Scholar]
- Sun SC. Non-canonical NF-κB signaling pathway. Cell research. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Matsukura S, Takeuchi H, Kawaguchi M, Ieki K, Odaka M, Watanabe S, Homma T, Dohi K, Aruga T, Sato M, Kurokawa M, Kokubu F, Adachi M. Increase in reactive oxygen metabolite level in acute exacerbations of asthma. Int Arch Allergy Immunol. 2008;146(Suppl 1):67–72. doi: 10.1159/000126064. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109(14 12):11 –10. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between β 2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. The Lancet. 1997;350:995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Richmond P, Upham JW. Toll-like receptor 2 ligands inhibit TH2 responses to mite allergen. J Allergy Clin Immunol. 2006;117:1148–1154. doi: 10.1016/j.jaci.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Tesse R, Pandey RC, Kabesch M. Genetic variations in toll-like receptor pathway genes influence asthma and atopy. Allergy. 2011;66:307–316. doi: 10.1111/j.1398-9995.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Tirumurugaan KG, Dhanasekaran S, Raj GD, Raja A, Kumanan K, Ramaswamy V. Differential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus) Vet Immunol Immunopathol. 2010;133:296–301. doi: 10.1016/j.vetimm.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardò FP, Pomara C, Riezzo I, Fineschi V. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. Journal of cellular and molecular medicine. 2016 doi: 10.1111/jcmm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Anderson EL, Squillace DL, Patil N, Maniak P, Iijima K, Kita H, O’Grady S. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy. 2017 doi: 10.1111/all.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker SA, Tromp MN, Griffioen DH, Van Bennekom WP, Van Der Vijgh WJ, Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biology and Medicine. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Vercelli D. Discovering susceptibility genes for asthma and allergy. Nature reviews Immunology. 2008;8:169. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- Verma R, Kushwah L, Gohel D, Patel M, Marvania T, Balakrishnan S. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm Med. 2013;2013:921724. doi: 10.1155/2013/921724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola AM, Chiappara G, Gagliardo R, Gjomarkaj M, Merendino A, Siena L, Bousquet J, Bonsignore G. Apoptosis and airway inflammation in asthma. Apoptosis. 2000;5:473–485. doi: 10.1023/a:1009661406440. [DOI] [PubMed] [Google Scholar]
- Vu D, Huang DB, Vemu A, Ghosh G. A structural basis for selective dimerization by NF-κB RelB. Journal of molecular biology. 2013;425:1934–1945. doi: 10.1016/j.jmb.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. Journal of asthma and allergy. 2014;7:53. doi: 10.2147/JAA.S39119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Q, Cheng ML, Ma L, Meng QZ, Duan L, Chen Y, Tan JW, Chen M, Liang TT, Li GJ, Li JL. Effect of the Miaoyao Fanggan sachet-derived isorhamnetin on TLR2/4 and NKp46 expression in mice. J Ethnopharmacol. 2012;144:138–144. doi: 10.1016/j.jep.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Wang L, Xiao W, Zheng Y, Xiao R, Zhu G, Wang M, Li Y, Peng S, Tan X, He Y. High dose lipopolysaccharide triggers polarization of mouse thymic Th17 cells in vitro in the presence of mature dendritic cells. Cellular immunology. 2012;274:98–108. doi: 10.1016/j.cellimm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Williams AS, Leung SY, Nath P, Khorasani NM, Bhavsar P, Issa R, Mitchell JA, Adcock IM, Chung KF. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol (1985) 2007;103:1189–1195. doi: 10.1152/japplphysiol.00172.2007. [DOI] [PubMed] [Google Scholar]
- Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. Journal of Experimental Medicine. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui M, Matsushima M, Omura A, Mori K, Ogasawara N, Kodera Y, Shiga M, Ito K, Kojima S, Kawabe T. The Suppressive Effect of Quercetin on Toll-Like Receptor 7-Mediated Activation in Alveolar Macrophages. Pharmacology. 2015;96:201–209. doi: 10.1159/000438993. [DOI] [PubMed] [Google Scholar]
- Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- Zhang M, Lin JM, Li XS, Li J. Quercetin ameliorates LPS-induced inflammation in human peripheral blood mononuclear cells by inhibition of the TLR2-NF-kappaB pathway. Genet Mol Res. 2016:15. doi: 10.4238/gmr.15028297. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- Zuo L, Lucas K, Fortuna CA, Chuang CC, Best TM. Molecular Regulation of Toll-like Receptors in Asthma and COPD. Front Physiol. 2015;6:312. doi: 10.3389/fphys.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]