Abstract
Individuals with a low initial response to alcohol (i.e., ethanol; EtOH) are at greater risk of developing alcohol abuse or dependence later in life. Similar to humans, individual differences in EtOH sensitivity also can be seen in rats, and several laboratories have used these individual differences to generate selectively bred rats that differ in acute EtOH sensitivity. We have worked with two sets of such rats (Inbred High or Low Alcohol Sensitivity strains, IHAS or ILAS, respectively; Inbred Alcohol Tolerant or Non-Tolerant strains, IAT and IANT, respectively) and have confirmed previously mapped quantitative trait loci (QTL) for these acute differences with the use of recombinant congenic lines; however, the relationship between acute sensitivity and EtOH drinking in these rats has yet to be determined. Thus, here we tested the hypothesis that QTLs underlying variation in initial low sensitivity to EtOH also will modulate variation in EtOH drinking behaviors. Separate groups of selectively inbred parent and congenic rats were tested for the loss of righting response (LORR) and also assessed for EtOH consummatory behavior using either operant self-administration or an intermittent access two-bottle choice procedure. LORR testing confirmed the presence of a LORR duration QTL in all of the congenics; however, the lack of a corresponding difference in blood EtOH concentration at the regain of the righting response suggests that these QTLs may be mediating a difference in EtOH metabolism rather than in neuronal sensitivity. IHAS/ILAS derived congenic rats did not differ from parent rats at any point during operant self-administration. IAT/IANT derived congenic rats showed small, but significant, increases in EtOH consumption relative to the parent strains only during the initial stages of operant self-administration. In contrast to operant testing, IHAS/ILAS derived congenic rats showed significantly greater EtOH consumption and preference than parent rats during intermittent access testing. There were not differences, however, between IAT/IANT congenic and parent rats during intermittent access. These data support the hypothesis that there is a genetic relationship between initial EtOH sensitivity and EtOH consumption, at least for the IHAS/ILAS derived congenic rats. Our current studies, however, cannot eliminate pharmacokinetic or taste preference factors as contributing to the rats’ responses nor can we eliminate the possibility of a linkage effect because of the fairly large size of the QTL intervals; i.e., distinct genes may be mediating the acute sensitivity and drinking responses.
Keywords: QTL, selected lines, alcoholism, ethanol, rats
Introduction
The role of genetics in the risk for alcohol use disorders (AUD) is well documented (e.g., see Schuckit, 2009). One well-supported genetically-mediated risk factor is an individual’s initial response to alcohol (e.g., Schuckit, 1980, Schuckit, 1984, Schuckit et al., 2014). For example, individuals with a low initial response to alcohol can be at a 4-fold greater risk for later development of alcoholism (Schuckit, 1994); in turn, the “low-level of response” (LR) theory has been of great interest to the alcohol research community for many years now (Quinn and Fromme, 2011).
Many laboratories have used selective breeding techniques in rodents to take advantage of individual differences in alcohol sensitivity to generate animals that express stable differences in their response to alcohol (i.e., ethanol; EtOH). There are now many strains of selected mice and rats that differ in some form of EtOH sensitivity or other EtOH-related behaviors, such as drinking (e.g., see Crabbe et al., 2010b). We have been working with two sets of selectively bred rats that are distinguished based on initial sensitivity to an intraperitoneal (i.p.) EtOH injection: namely, the Inbred High and Low Alcohol Sensitivity rats (IHAS and ILAS, respectively) and the Inbred Alcohol Tolerant and Non-Tolerant rats (IAT and IANT, respectively; Draski et al., 1992, Radcliffe et al., 2004b, Sarviharju and Korpi, 1993).
IHAS/ILAS rats were originally selected based on their sensitivity to an injection of high dose EtOH (e.g., 3.5 g/kg, i.p.) measured as the duration of the loss of righting response (LORR), or “sleep time” (ST; Draski et al., 1992). IHAS rats are more sensitive to the initial effects of EtOH than ILAS rats, displaying longer ST and lower blood EtOH concentration at regain of the righting response (BECRR; Draski et al., 1992). IAT/IANT rats were originally selected based on acute sensitivity on the tilting plane test (TPT) following injection of a moderate dose of EtOH (2 g/kg, i.p.), with IANT rats more sensitive to the initial effects of EtOH than IAT rats (Sarviharju and Korpi, 1993). The IAT/IANT rats also differ in ST and BECRR following a high dose EtOH injection (3.5 g/kg, i.p.; Radcliffe et al., 2009). In contrast to the TPT, however, IAT rats are more sensitive for ST and BECRR than IANT rats (Radcliffe et al., 2009). This finding suggests an at least partial genetic dissociation of LORR and TPT (for further discussion, see: Radcliffe et al., 2004b; Radcliffe et al., 2009). Interestingly, a highly signicant quantitaive trait locus (QTL) was mapped for TPT at the same locus as the IAT/IANT F2 LORR QTL, though it was for the baseline TPT measure, not for the TPT response to EtOH (Radcliffe et al., 2009).
Studies of EtOH-induced ataxia in humans has been shown to support Schuckit’s LR theory (Schuckit, 1985), but the extent to which animal models of motor impairment relate to the human condition is unclear (Crabbe et al., 2010a). Some animal genetic studies have shown the expected relationship for LORR, including in our own work, though some have not (Crabbe et al., 2006; Radcliffe et al., 2013). The negative results are likely due to a variety of reasons, not the least of which is that genes involved in EtOH-induced motor impairment do not overlap completely with genes involved in drinking behavior. TPT is not a commonly studied response to acute EtOH and very little information exists with regard to genetic effects on it in the context of the Schuckit theory with the exception of the IAT and IANT rats as noted below. Thus, the difference in LORR sensitivity between both IHAS/ILAS and IAT/IANT rats appears to make them potentially useful for investigating the LR theory, and, in particular, for determining the underlying genetics of differential LORR sensitivity.
EtOH self-administration and two-bottle choice drinking was tested previously in the HAS/LAS and AT/ANT parent strains: consistent with the Schuckit hypothesis, the low sensitivity lines self-administered or drank more EtOH than the high sensitivity lines (Files et al., 1996, Sarviharju and Korpi, 1993). QTLs for LORR subsequently have been mapped in segregating populations derived from both the IAT/IANT and IHAS/ILAS on chromosomes 1 and 2, respectively (Radcliffe et al., 2009, Radcliffe et al., 2006). Marker-assisted selection was used to breed congenic strains in which the QTL interval from one strain was bred onto the background of another to confirm the QTL effect and to reduce the size of the original interval (Radcliffe et al., 2004a, Radcliffe et al., 2006, Radcliffe et al., 2009). Although we know these QTLs alter variation in acute sensitivity to high dose EtOH in the LORR test, the effect they have on EtOH consummatory behaviors was unknown. We therefore sought to determine the relationship between initial EtOH sensitivity and EtOH-drinking behavior in the congenic lines. We bred more refined congenics, which were used to test the hypothesis that specific QTLs responsible for variation in initial sensitivity to acute EtOH also will alter variation in EtOH consumption during operant self-administration (SA) and an intermittent access (IA) two-bottle choice procedure.
Materials and methods
Animals
A total of 109 inbred parent and congenic rats were used for the EtOH consummatory experiments, and 325 rats for the LORR experiments (see Table 1 for details). Selectively bred parent strains that have been inbred included the Inbred High Alcohol Sensitive-1 (IHAS-1; Draski et al., 1992) and the IAT/IANT rats (Radcliffe et al., 2004b). The Inbred Low Alcohol Sensitive-1 strain (ILAS-1) bred poorly and died out several years ago. Note that a second set of HAS and LAS lines were generated, but never used for any of the mapping studies (HAS-2, LAS-2; Draski et al., 1992). The three congenic strains were derived from congenics used in previous studies (Radcliffe et al., 2006, Radcliffe et al., 2009) and carry either reduced introgressed intervals (i.e., the Tiia and IN6BX rats) or were more finely genotyped (i.e., the HLC2CO rats) than previous congenics. Details of their origin are shown in Table 2 and their genomic structure is shown in Figure 1. The congenics were maintained as heterozygotes through backcrossing to their parent background strain for greater than 10 generations. For the LORR experiments, the Tiia and HLC2CO were still being backcrossed and were therefore heterozygous; these lines were tested against their non-congenic littermates, which were genetically like the background strain (i.e., the IAT and IHAS, respectively). The IN6BX congenics were inbred for the LORR experiments and were compared to the parent IAT strain. All congenics were inbred for SA and IA testing. Table 1 lists the comparisons for all experiments. Genotyping was conducted as previously described (Radcliffe et al., 2006).
Table 1.
Sample sizes and planned comparisons for the rat strains used in the LORR/BECRR, self-administration (SA), and intermittent access (IA) experiments.
| Experiment | Comparisons |
|---|---|
| SA | IHAS-1 (N=8); HLC2CO (N=7) |
| SA | IAT (N=8); IANT (N=8); IN6BX (N=9); Tiia (N=8) |
| IA | IHAS-1 (N=11); HLC2CO (N=7) |
| IA | IAT (N=10); IANT (N=8); IN6BX (N=9); Tiia (N=13) |
| LORR/BECRR | HLC2CO control littermates1 (N=41); HLC2CO (N=68) |
| LORR/BECRR | IAT (N=19); IN6BX (N=17) |
| LORR/BECRR | Tiia control littermates1 (N=99); Tiia (N=73) |
For the LORR/BECRR experiments, the HLC2CO and Tiia were being backcrossed to the IHAS-1 and IAT, respectively. Therefore, the congenics were heterozygous and were compared to their control littermates which were genetically similar to their respective inbred parent line.
Table 2.
Derivation of the congenic lines.
| Congenic Strain |
Abbreviation1 | Background strain |
Introgressed strain |
Progenitor congenic2 |
RRRC #3 |
|---|---|---|---|---|---|
| IAT.IANT-Lorr1r | Tiia | IAT | IANT | T2a | 728 |
| IAT.IANT-Lorr1-6BXr | IN6BX | IAT | IANT | T6b | NA |
| IHAS.ILAS-Lorr2r | HLC2CO | IHAS-1 | ILAS-1 | IHAS.ILAS-Lorr2r | 729 |
These were used as the working abbreviations during the experiments.
For additional details, see Radcliffe et al., 2009 and Radcliffe et al., 2006.
The Tiia and HLC2CO are available in cryopreserved form at the Rat Resource & Research Center (www.rrrc.us) and can be identified by the indicated numbers.
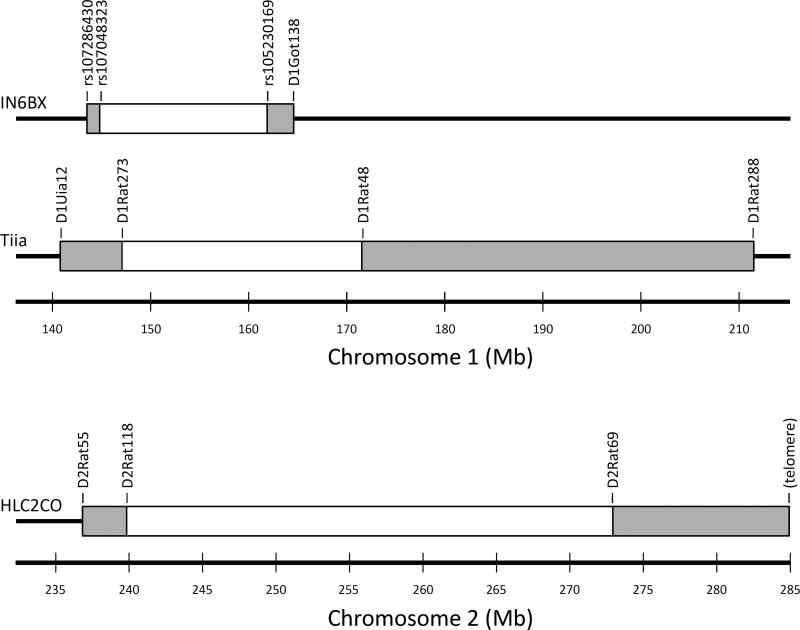
Fig. 1. Locations of the genetic markers that were used to select the IN6BX, Tiia, and HLC2CO congenic rats.
The white boxes indicate the largest known introgressed segment (IANT onto an IAT background for IN6BX and Tiia; ILAS onto an IHAS background for HLC2CO) while the shaded boxes indicate the region of which the recombination point is unknown; i.e., the two markers that flank each shaded box are the most minimally distant informative markers that could be found in that area. Marker locations are from the RGSC Genome Assembly, version 5.0.
All rats were males and were at least 60 days of age at testing inception. Only males were used because the congenics tended to breed poorly and females were needed for breeding purposes. Rats were bred at the University of Colorado Anschutz Medical Campus (UCAMC) and weaned at 21 days of age. LORR testing was conducted at the UCMAC and separate cohorts of rats were transported to the University of Colorado Denver (UCD) for the SA and IA experiments. Rats had ad libitum access to food (2920X, Teklad Extruded Diet, Envigo Laboratories, Madison, WI) and water, and were maintained in a constant 22°C and 40% humidity environment. The housing consisted of Multi-Species Ventilated Caging (Allentown, New Jersey; W12" × D17.5" × H8"; 50 ACH) with autoclaved Aspen Sani-Chips (7090A.BK, Envigo Laboratories, Madison, WI) for the bedding. The rats were either group-housed on a 14:10-h light/dark cycle for LORR or group-housed on a 12-h light/dark cycle for SA (lights on at 0700 hours). The 14:10-h and 12-h light cycles are routinely used at the UCAMC and UCD facilities, respectively. Rats were individually housed on a reversed 14:10-h light/dark cycle for IA (lights off at 10:00 AM). Rats were placed on the reverse light cycle and in individual housing within one day after arriving at the UCD vivarium for the IA experiments; testing commenced one week later. For all experiments, strains were tested randomly due the vagaries of breeding, although controls and congenics were always included in each testing group; this effectively controlled for batch effects. All animal care and use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the CU Denver Institutional Animal Care and Use Committee.
Loss of righting response
LORR was tested as previously described (Radcliffe et al., 2006, Radcliffe et al., 2009). To induce LORR, rats were administered 2.25 g/kg (IHAS/ILAS-related rats) or 3.2 g/kg (IAT/IANT-related rats) EtOH, i.p. (15% in saline, w/v). At the regain of the righting response (i.e., the ability to right themselves three times within one minute in a V-shaped Plexiglas tray), two 40-microliter blood samples were taken from the retro-orbital sinus. The second sample was taken as a backup in case the assay for the first sample failed. LORR testing produced two sensitivity scores: “sleep time” (ST; i.e., LORR duration) and BECRR. BECRR was determined using a reliable enzymatic assay (Lundquist, 1959).
Self administration training
EtOH solutions were prepared from 95% EtOH (v/v; Decon Labs, King of Prussia, PA) in tap water. Rats were trained to self-administer (SA) 10% EtOH (v/v) using an established sucrose-fade procedure (e.g., Files et al., 1996, Samson, 1986) in operant conditioning chambers (29 × 24 × 21 cm; Med Associates, St. Albans, VT, USA) housed within sound-attenuating cabinets. The chambers had two retractable levers on the front wall with stimulus lights positioned 6 cm above each lever. A tone presentation speaker (2900 Hz) and a white noise speaker (90 dB) were mounted 12 cm above the floor on the wall opposite the levers. A houselight (100 mA) was mounted 6 cm above the tone speaker, and a computer-controlled syringe pump delivered liquid infusions into a dual-cup liquid receptacle with infrared head-entry detection (Med Associates). The liquid was always delivered into the same cup, but cups were counterbalanced to control for a side preference. Cups were checked at the end of each session to determine whether there was liquid remaining, and if there was, that volume and the associated number of infusions were controlled for in the data. Rats were weighed prior to each operant session for calculations of g/kg EtOH consumed. Rats were never fluid restricted and had ad libitum access to food and water except during the actual operant sessions. All behavioral events were monitored and controlled using MED-PC for Windows (Med Associates).
SA sessions began with the extension of the retractable levers, white noise activation, and illumination of the stimulus light above the active lever. One second after session initiation, a single liquid priming infusion was delivered, which was always the same as the self-administered solution. During all infusions, the stimulus light over the active lever was turned off, and a tone-house light stimulus complex was activated for 15 s coinciding with a “time-out” period. All SA sessions were 30 min in duration and testing was conducted five days a week. During acquisition, responses on the active lever were reinforced with a 20% (w/v) sucrose solution (0.2 ml/infusion) according to a fixed ratio 1 (FR1) schedule of reinforcement. Responses emitted on the active lever during the sucrose infusion and 15 s stimulus complex presentation were not reinforced, and were recorded separately from reinforced responses. Responses on the inactive lever were recorded, but had no programmed consequence. The inactive lever was counterbalanced across subjects to control for a potential side preference. Rats were given 15 sessions in which to acquire stable responding for 20% sucrose: rats that acquired SA were advanced to the sucrose fade procedure. All reinforced responses during the sucrose-fade, dose-consumption, and progressive ratio (PR) testing resulted in delivery of 0.1 ml of solution. It should be noted that delivery of a solution did not necessarily equal consumption of the solution; however, in the infrequent occurrence of this event, responses were adjusted to reflect the actual amount of solution that was consumed.
Sucrose-fade procedure
To be able to compare our results to past studies of the HAS and LAS rats, we followed the same sucrose-fade and dose-consumption testing procedures as the Files et al. (1996) study. Rats were gradually transitioned from SA of 10% sucrose (w/v; 10S) to SA of 10% EtOH (v/v; 10E) over the course of seven weeks. Sucrose-fade solutions were presented in the following order: 10S; 10% sucrose/2% EtOH (v/v; 10S2E); 10% sucrose/5% EtOH (v/v; 10S5E); 10% sucrose/10% EtOH (v/v; 10S10E); 5% sucrose/10% EtOH (v/v; 5S10E); 2% sucrose/10% EtOH (v/v; 2S10E); and 10E (Samson, 1986, Files et al., 1996). Rats self-administered 10S2E for two consecutive sessions before self-administering the next solution, but self-administered each subsequent solution for three sessions before advancing. At the completion of the fade procedure, the schedule of reinforcement was gradually increased from a FR1 to a FR4. Rats self-administered 10E at each new response requirement for three sessions until they reached a FR4 schedule of reinforcement, at which time they were given five sessions; these sessions served as the starting point for EtOH dose-consumption testing.
Post-fade analyses
During dose-consumption testing, rats self-administered increasing EtOH concentrations in the following order: 10E, 15% EtOH (v/v; 15E), 20% EtOH (v/v; 20E), 30% EtOH (v/v; 30E), and 10E. Rats self-administered each concentration for five consecutive sessions before self-administering the next concentration. At the completion of dose-consumption testing, rats were tested for SA of 10E under a PR schedule of reinforcement for three sessions. PR sessions were 5 h in duration and the response requirement increased progressively according to the following schedule: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, and 737 for infusions 1–25, respectively (Richardson and Roberts, 1996). Break point (BP) was defined as the last response ratio completed before 1 h without earned reinforcement or the end of 5 h. Inactive lever responses were recorded, but had no programmed consequences. At the completion of PR testing for 10E, rats were given three PR sessions during which responding was not reinforced (i.e., extinction sessions).
Intermittent access drinking procedure
Separate groups of rats were tested for EtOH consumption using an intermittent access (IA) two-bottle choice procedure which has been shown to produce high levels of drinking in rat lines that do not normally drink excessively (Simms et al., 2008). Briefly, rats were given 24-h access to one bottle containing 20E and one bottle containing tap water three days a week (Monday, Wednesday, and Friday). The bottles were 8oz high temp French square water bottles with rubber stoppers and open tip sipper tubes (Allentown, Inc., Allentown, NJ) and were weighed before placement in the cage and again 24 hours later when they were removed from the cage; consumption was determined as the difference in bottle weights. Rats had access to two bottles containing tap water on non-testing days. Rats were weighed five days a week (Monday to Friday) and their weekly average body-weight was used to calculate EtOH intake (g/kg/24 hours). EtOH preference was calculated as the ratio of the volume consumed from the EtOH bottle to the total volume consumed. EtOH and water bottle placement were alternated each session, and EtOH access began 30-min into the dark cycle (i.e., at 1030). Rats were given 20 continuous drinking sessions (i.e., three days a week for ~7 weeks) followed by a one-week withdrawal period and final test. The withdrawal period was included to test for the possibility of an enhanced or differential alcohol deprivation effect (reviewed in Becker and Ron, 2014). One bottle each of EtOH and water were placed in an empty cage on each of the racks on which rats were housed. Non-specific fluid loss was recorded from these bottles and subtracted from the final consumption values in a rack-specific manner.
Data analysis
All statistical analyses were conducted using PASW Statistics, version 21.0 (IBM Corp., Somers, NY, USA). Separate one-way ANOVAs were used to compare congenics to control rats for LORR and BECRR. SA data were analyzed with two-way repeated measures ANOVA (RMANOVA). Corresponding strain (IHAS-1 vs. HLC2CO or IAT/IANT vs. IN6BX and Tiia) and session (within-subjects variable) were treated as independent variables; responses, EtOH intake, or BPs were treated as the dependent variables. EtOH and water intake during the IA experiment were analyzed with two-way RMANOVA. Corresponding strain (IHAS-1 vs. HLC2CO or IAT/IANT vs. IN6BX and Tiia) and session (within-subjects variable) were treated as independent variables, and consumption was treated as the dependent variable. When main or interaction effects were revealed, independent samples t-test’s, one-way ANOVA, or one-way RMANOVA with Tukey’s HSD was used for post-hoc analysis. When the assumption of sphericity was violated for a particular repeated-measures analysis, as revealed by Mauchly’s test statistic, tests of significance were based on the more conservative Huynh-Feldt corrected degrees of freedom. The symbol, a, indicates Huynh-Feldt corrected values throughout the text. Data are presented as mean values ± SEM, and the level of statistical significance was set at p < 0.05. In total, four of the 109 rats used in the SA and IA studies were excluded from final analysis: three for health concerns (two HLC2CO and one IHAS-1 rat), and one because it did not acquire SA. In addition, rats that failed to lose or regain the righting response (after 180 minutes) were excluded from the study. In all, 10 rats from the HLC2CO study and 8 rats from the Tiia study failed to lose the righting response and were excluded; 4 rats from the Tiia study did not regain the righting response and were also excluded; no rats from the IN6BX study were excluded.
Results
LORR and BECRR in HLC2CO, IN6BX, and Tiia rats
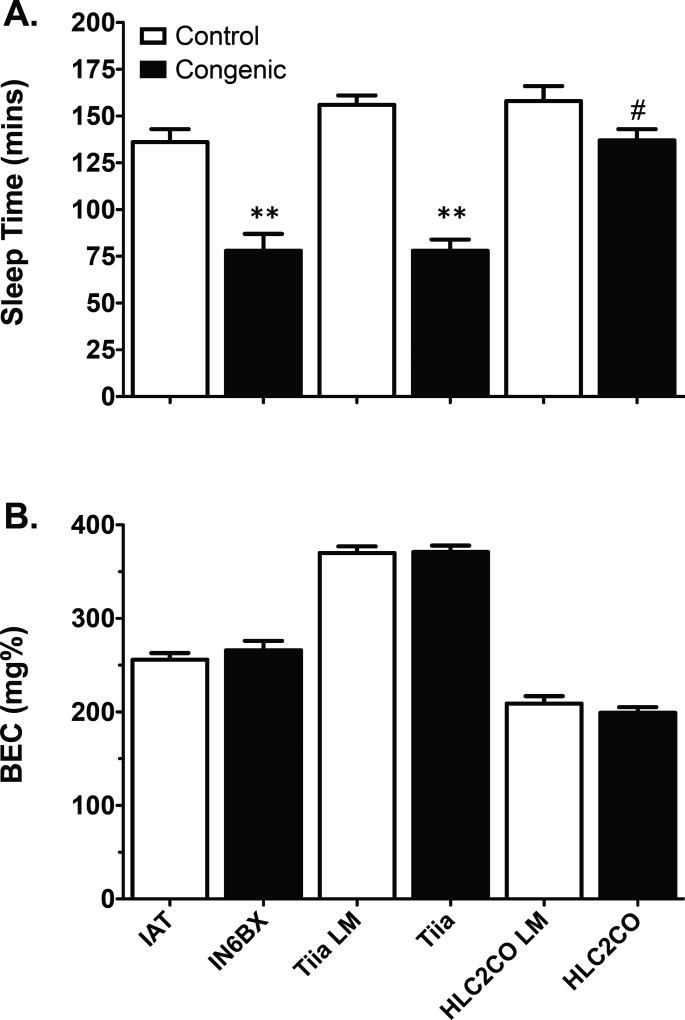
One-way ANOVA revealed that ST was significantly reduced in IN6BX [F1, 35 = 24.9, p < 0.001], Tiia [F1, 171 = 105.1, p < 0.001], and HLC2CO [F1, 108 = 4.6, p = 0.034] congenic rats compared to their respective controls (Figure 2A). None of the congenic rats differed from controls for BECRR (Figure 2B).
Fig. 2. Characterization of LORR in IN6BX, Tiia, and HLC2CO congenic rats.
ST (A) was significantly reduced in IN6BX, Tiia, and HLC2CO congenic rats compared to their respective control rats (LM = littermates). In contrast to ST, BECRR (B) was not significantly different between any of the congenic strains and their respective controls. Data are mean values ± SEM. N values for the IN6BX, Tiia, and HLC2CO congenic rats and their respective controls are given in Table 2. **p < 0.001; # p < 0.05.
EtOH SA in IHAS-1 and HLC2CO rats
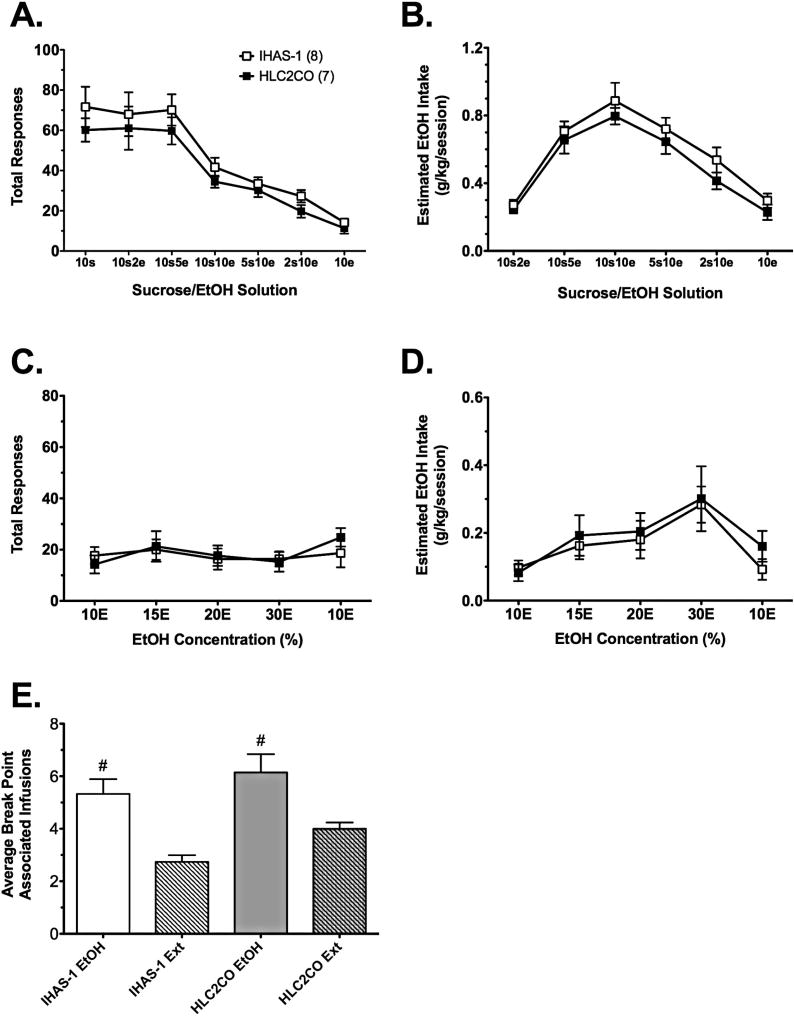
Analysis of responding during the fade procedure in IHAS-1 and HLC2CO rats with two-way RMANOVA revealed an effect of EtOH/sucrose concentration [aF(6, 78) = 47.5, p < 0.001], but no other significant effects or interactions. Relative to 10S alone, responding significantly decreased regardless of strain beginning with SA of 10S10E (Figure 3A). Analysis of EtOH intake during the fade procedure with two-way RMANOVA also revealed an effect of EtOH/sucrose concentration [F(5, 65) = 66.3, p < 0.001], but no other significant effects or interactions. EtOH intake followed an inverted-U function, with peak intake occurring at 10S10E (Figure 3B).
Fig. 3. Operant EtOH SA in IHAS/ILAS parent and congenic rats.
Responding (A) and estimated EtOH intake (B) in IHAS and HLC2CO rats during the sucrose-fade procedure. Responding (C) and estimated EtOH intake (D) in IHAS and HLC2CO rats during dose-consumption testing. E) Break points in IHAS and HLC2CO rats during SA of 10E (3-session average) or extinction (3-session average) on a PR schedule of reinforcement. Data are mean values ± SEM. N values are given in parentheses. # p < 0.05 vs. extinction.
Analysis of responding during dose-consumption testing in IHAS-1 and HLC2CO rats with two-way RMANOVA revealed an effect of EtOH concentration [aF(4, 52) = 3.5, p = 0.028], but no other significant effects or interactions. Tukey’s pairwise comparisons did not reveal significant differences in responding for any of the EtOH concentrations; there was no effect of strain at any of the concentrations (Figure 3C). Analysis of EtOH intake during dose-consumption testing with two-way RMANOVA also revealed an effect of EtOH concentration [aF(4, 52) = 12.7, p < 0.001], but no other significant effects or interactions. Tukey’s pairwise comparisons revealed that relative to baseline intake of 10E, rats consumed significantly more 15E, 20E, and 30E (Figure 3D). Similar to responding, there was no effect of strain on consumption at any concentration.
At the completion of dose-consumption testing, we assessed motivation to respond for 10E on a PR schedule of reinforcement. Analysis of BP-associated infusions in IHAS-1 and HLC2CO rats with two-way RMANOVA revealed an effect of EtOH [F(1, 13) = 45.1, p < 0.001], but no other significant effects or interactions. BP-associated infusions were significantly higher during SA of 10E than they were during the extinction sessions (Figure 3E). During the EtOH PR sessions, the final response requirements associated with these infusions were 9 and 12 for the IHAS-1 and HLC2CO strains, respectively. During the extinction PR sessions, the final response requirements associated with these infusions were 2 and 6 for the IHAS-1 and HLC2CO strains, respectively.
EtOH SA in IAT, IANT, IN6BX, and Tiia rats
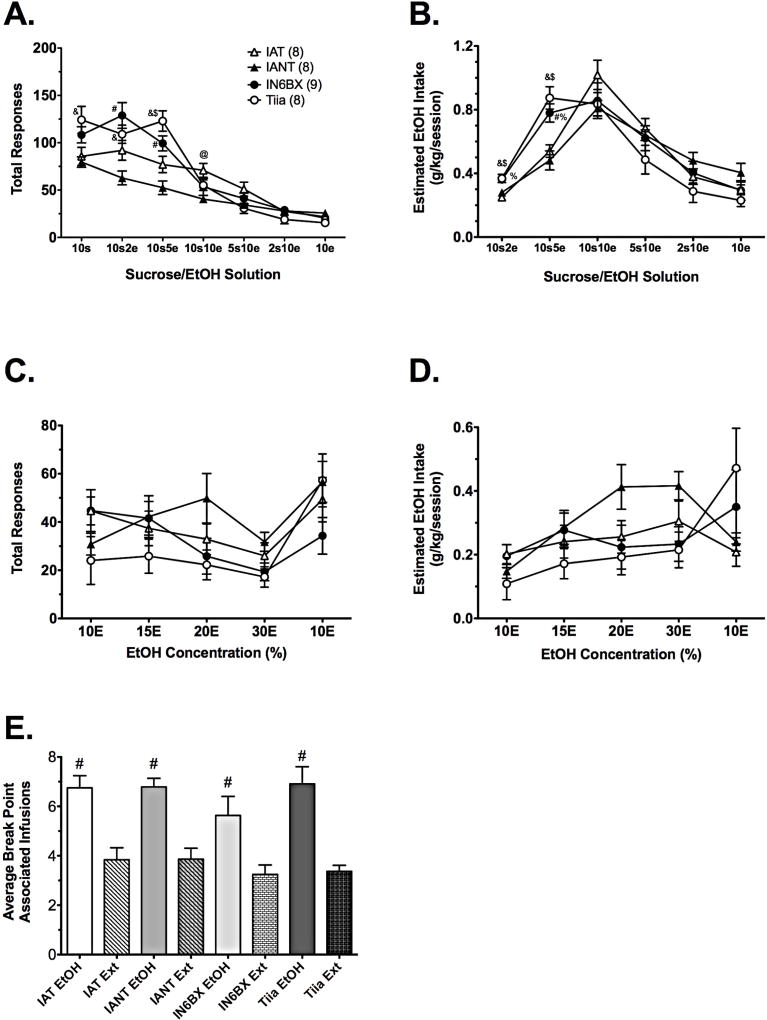
Analysis of responding during the fade procedure in IAT, IANT, IN6BX, and Tiia strains with two-way RMANOVA revealed a strain x concentration interaction [aF(18, 174) = 6.9, p < 0.001]. Tukey’s pairwise comparisons revealed significant differences between strains during SA of 10S up to 10S10E, but no significant differences between strains thereafter (Figure 4A): IANT rats responded significantly less than IAT rats for 10S10E; IN6BX rats responded significantly more than IANT rats for 10S2E and 10S5E; and Tiia rats responded significantly more than IANT rats for 10S, 10S2E, and 10S5E, and IAT rats for 10S5E. There were no significant differences between IN6BX and Tiia rats. Relative to 10S alone, responding in all strains significantly decreased beginning with the SA of 10S10E. Analysis of EtOH intake during the fade procedure with two-way RMANOVA also revealed a strain x concentration interaction [aF(15, 145) = 5.4, p < 0.001]. Tukey’s pairwise comparisons revealed significant differences between strains during SA of 10S2E to 10S5E, but no significant differences between strains thereafter (Figure 4B). IN6BX rats consumed significantly more EtOH than IAT rats at 10S2E and 10S5E, and more EtOH than IANT rats at 10S5E; Tiia rats consumed significantly more EtOH than IAT and IANT rats at both concentrations (Figure 4B).
Fig. 4. Operant EtOH SA in IAT/IANT parent and congenic rats.
Responding (A) and estimated EtOH intake (B) in IAT, IANT, IN6BX, and Tiia rats during the sucrose-fade procedure. Responding (C) and estimated EtOH intake (D) in IAT, IANT, IN6BX, and Tiia rats during dose-consumption testing. E) Break points in IAT, IANT, IN6BX, and Tiia rats during SA of 10E (3-session average) or extinction (3-session average) on a PR schedule of reinforcement. Data are mean values ± SEM. N values are given in parentheses. Symbols in (A) and (B) denote p < 0.05 for the following comparisons: @ IAT vs. IANT; # IN6BX vs. IANT; % IN6BX vs. IAT; & Tiia vs. IANT; $ Tiia vs. IAT. Symbols in (E) denote # p < 0.05 vs. extinction.
Analysis of responding during dose-consumption testing in IAT, IANT, IN6BX, and Tiia rats with two-way RMANOVA revealed a strain x concentration interaction [F(12, 116) = 3.8, p < 0.001]. However, Tukey’s pairwise comparisons did not reveal significant differences in responding between strains at any EtOH concentration (Figure 4C). Analysis of EtOH intake during dose-consumption testing with two-way RMANOVA also revealed a strain x concentration interaction [aF(12, 116) = 4.4, p < 0.001], but again, Tukey’s pairwise comparisons did not reveal significant differences between strains at any EtOH concentration (Figure 4D). Analysis of BP-associated infusions for 10E in IAT, IANT, IN6BX, and Tiia rats with two-way RMANOVA revealed an effect of EtOH [F(1, 24) = 217.6, p < 0.001], but no other significant effects or interactions. BP-associated infusions were significantly higher during SA of 10E than they were during extinction in all strains (Figure 4E). During the EtOH PR sessions, the final response requirements associated with these infusions were 12, 12, 9, and 12 for the IAT, IANT, IN6BX, and Tiia strains, respectively. During the extinction PR sessions, the final response requirement associated with these infusions was 4 for each of the strains.
EtOH drinking during IA
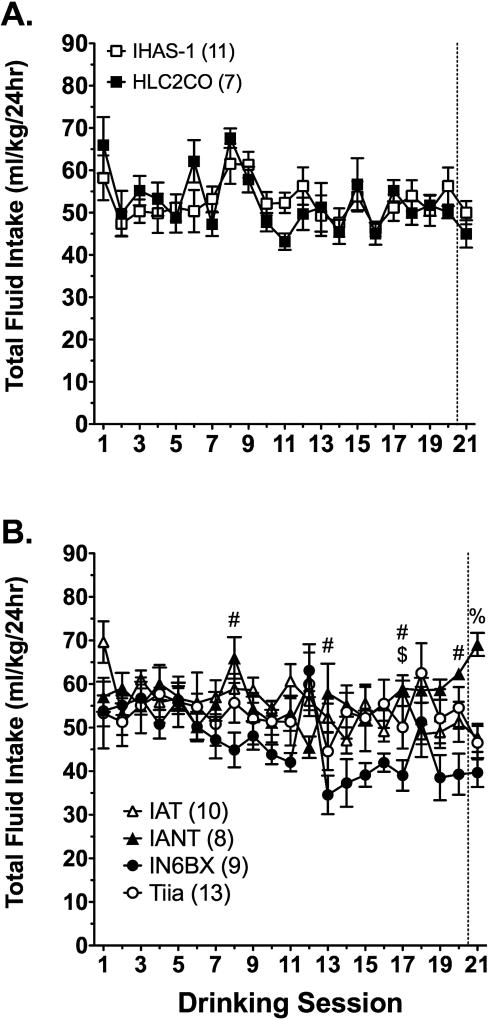
Analysis of total fluid intake in IHAS-1 and HLC2CO rats during the IA procedure with two-way RMANOVA did not reveal any significant effects or interactions (Figure 5A). In contrast, two-way RMANOVA analysis of total fluid intake in IAT, IANT, IN6BX, and Tiia rats revealed a strain x session interaction [aF(20, 720) = 3.6, p < 0.001]. Tukey’s pairwise comparisons revealed that IANT rats drank significantly more total fluid than IN6BX rats on sessions 8, 13, 17, 20, and 21 and significantly more total fluid than IAT and Tiia rats on session 21 (Figure 5B). In addition, IAT rats drank more total fluid than IN6BX rats on session 17. Analysis of total fluid consumption over the course of testing with separate one-way RMANOVAs revealed an effect of session in IANT [F(20, 140) = 4.5, p = 0.004] and IN6BX [F(20, 160) = 6.4, p = 0.001] rats. Relative to the second session, IANT rats displayed a significant decrease in intake on sessions 6 and 12, and and a significant increase on session 21. IN6BX rats significantly decreased their total fluid drinking relative to the second session on sessions 4, 8, 10–11, 13–17, and 19–21.
Fig 5. Total fluid drinking in IHAS/ILAS and IAT/IANT parent and congenic rats.
Total fluid intake in IHAS and HLC2CO rats (A) and IAT, IANT, IN6BX, and Tiia rats (B) during the IA procedure. Data are mean values ± SEM. N values are given in parentheses. Symbols in (B) denote p < 0.05 for the following comparisons: # IANT vs. IN6BX; $ IAT vs. IN6BX; % IANT vs IAT, IN6BX, and Tiia.
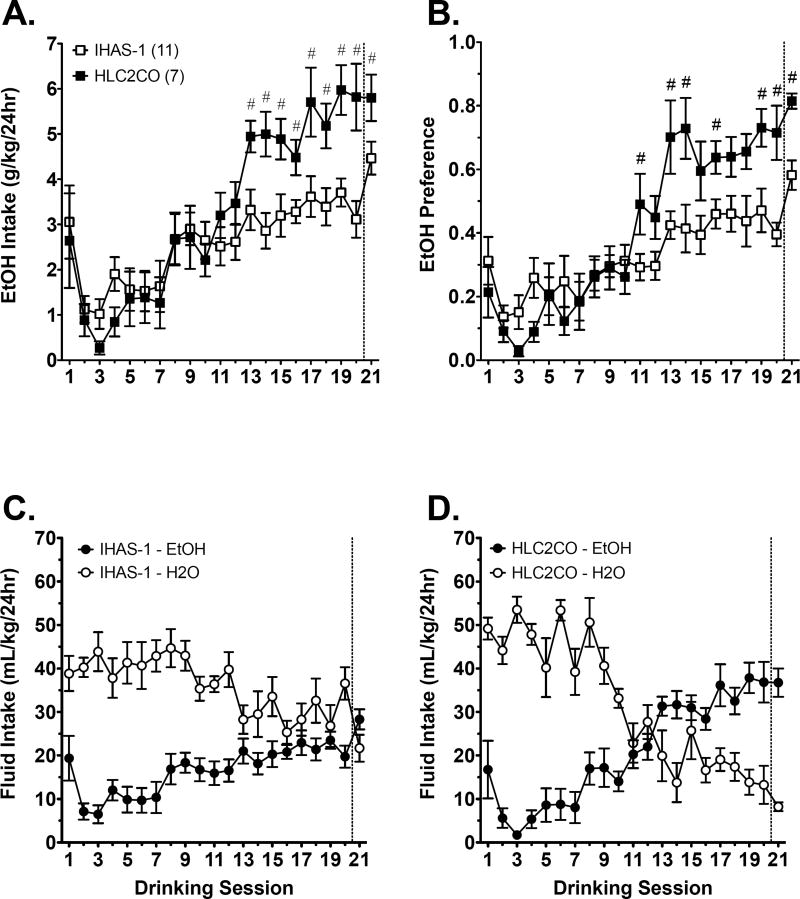
Analysis of EtOH consumption in IHAS-1 and HLC2CO rats with two-way RMANOVA revealed a strain x session interaction [F(20, 320) = 3.2, p < 0.001]. Post hoc analysis with independent samples t-test’s revealed that HLC2CO rats consumed significantly more EtOH than IHAS-1 rats from sessions 13 to 21 (Figure 6A). Analysis of EtOH intake over the course of testing with separate one-way RMANOVAs revealed an effect of session in both IHAS-1 [F(20, 200) = 5.1, p < 0.001] and HLC2CO [F(20, 120) = 13.2, p < 0.001] rats. Because EtOH presentation on the first test was novel, we opted to define initial consumption as the second EtOH presentation for all within-subjects post hoc comparisons. Relative to the second session, IHAS-1 and HLC2CO rats displayed sustained significant increases in EtOH consumption beginning on sessions eight and 11, respectively (Figure 6A).
Fig. 6. IA EtOH drinking in IHAS/ILAS parent and congenic rats.
EtOH intake (A) and preference (B) in IHAS and HLC2CO rats during the IA procedure. Water and EtOH fluid intake over the course of IA testing in IHAS (C) and HLC2CO (D) rats. Data are mean values ± SEM. N values are given in parentheses. # p < 0.05, HLC2CO vs. IHAS.
Analysis of EtOH preference in IHAS-1 and HLC2CO rats also revealed a strain x session interaction [F(20, 320) = 4.5, p < 0.001]. Post hoc analysis with independent samples t-test’s revealed that HLC2CO rats also displayed a significantly greater preference for EtOH than IHAS-1 rats beginning on session 11, and for the majority of testing (Figure 6B). Analysis of EtOH preference over the course of testing with separate one-way RMANOVAs revealed an effect of session in both IHAS-1 [F(20, 200) = 6.1, p < 0.001] and HLC2CO [F(20, 120) = 21.3, p < 0.001] rats. Relative to the second session, both strains displayed sustained significant increases in EtOH preference beginning with the eighth session (Figure 6B).
Analysis of fluid intake in IHAS-1 rats with separate one-way RMANOVAs revealed main effects of session for consumption of both EtOH [F(20, 140) = 3.8, p < 0.001] and water [F(20, 120) = 2.4, p = 0.002]; EtOH consumption significantly increased over the course of testing, whereas water consumption significantly decreased (Figure 6C). This effect also was revealed for HLC2CO rats. Analysis of fluid intake with separate one-way RMANOVAs again revealed main effects of session for consumption of both EtOH [F(20, 120) = 13.2, p < 0.001] and water [F(20, 80) = 11.3, p < 0.001]. Again, EtOH consumption significantly increased over the course of testing, whereas water consumption significantly decreased (Figure 6D).
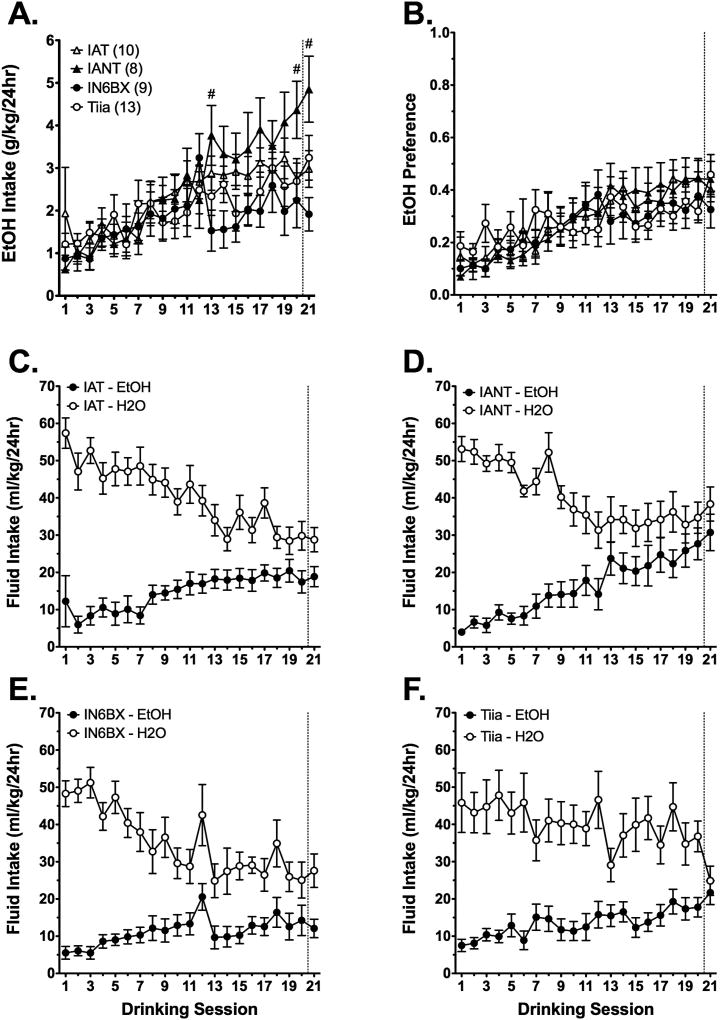
Analysis of EtOH consumption in IAT, IANT, IN6BX, and Tiia rats with two-way RMANOVA revealed a strain × session interaction [aF(20, 720) = 2.3, p < 0.001]. Tukey’s pairwise comparisons revealed that IANT rats consumed significantly more EtOH than IAT rats on session 20, and significantly more EtOH than IN6BX rats on sessions 13 and 21 (Figure 7A). Analysis of EtOH consumption over the course of testing with separate one-way RMANOVAs revealed an effect of session in IAT [F(20, 180) = 3.9, p < 0.001], IANT [F(20, 140) = 14.1, p < 0.001], IN6BX [F(20, 160) = 2.3, p = 0.002], and Tiia [F(20, 240) = 5.1, p < 0.001] rats. Relative to the second session, IAT and IANT rats displayed significant and sustained increases in EtOH consumption beginning on session 8, whereas significant and sustained increases in EtOH consumption did not occur in Tiia rats until session 12. IN6BX rats significantly increased EtOH consumption relative to the second session only on sessions 12, 16, 18, and 21.
Fig. 7. IA EtOH drinking in IAT/IANT parent and congenic rats.
EtOH intake (A) and preference (B) in IAT, IANT, IN6BX, and Tiia rats during the IA procedure. Water and EtOH fluid intake over the course of IA testing in IAT (C), IANT (D), IN6BX (E), and Tiia (F) rats. Data are mean values ± SEM. N values are given in parentheses. The # in panel (A) denotes p < 0.05 for IANT vs. IN6BX.
Analysis of EtOH preference with two-way RMANOVA also revealed a strain × session interaction [aF(20, 720) = 1.4, p = 0.046]. In contrast to EtOH intake, however, Tukey’s pairwise comparisons did not reveal significant differences in EtOH preference between any of the strains at any point during testing (Figure 7B). Analysis of EtOH preference over the course of testing with separate one-way RMANOVAs revealed an effect of session in IAT [F(20, 180) = 5.4, p < 0.001], IANT [F(20, 140) = 11.3, p < 0.001], IN6BX [F(20, 160) = 2.9, p < 0.001], and Tiia [F(20, 240) = 3.3, p < 0.001] rats. Relative to the second session, IAT, IANT, IN6BX, and Tiia rats displayed significant and sustained increases in EtOH preference beginning on sessions 8, 13, 15, and 13, respectively.
Analysis of fluid intake with separate one-way RMANOVAs revealed main effects of session for both EtOH and water in IAT [F(20, 120) = 10.4, p < 0.001 and F(20, 100) = 7.9, p < 0.001, respectively], IANT [F(20, 140) = 14.1, p < 0.001 and F(20, 140) = 7.6, p < 0.001, respectively], IN6BX [F(20, 160) = 2.3, p = 0.002 and F(20, 120) = 5.3, p < 0.001, respectively], and Tiia [F(20, 240) = 4.8, p < 0.001 and F(20, 220) = 2.7, p < 0.001, respectively] rats. In IAT rats, EtOH consumption significantly increased from session nine onward, whereas water consumption significantly decreased from session 13 onward (Figure 7C). In IANT rats, EtOH consumption significantly increased from session 13 onward, whereas water consumption significantly decreased from session nine onward (Figure 7D). In IN6BX rats, EtOH consumption was significantly increased only on sessions 12, 16, 18, and 21, whereas water consumption significantly decreased from session 13 onward (Figure 7E). In Tiia rats, EtOH consumption significantly increased from session 12 onward, whereas water consumption was significantly decreased only on sessions 13, 19, and 21 (Figure 7F).
Discussion
This study examined the relationship of QTLs that modify variation in initial EtOH sensitivity and EtOH consummatory behaviors in congenics derived from rats that were selectively bred for differences in initial EtOH sensitivity. Although we did not find clear differences in EtOH SA behavior between any of the parent or congenic rats, we did find differences in EtOH drinking during the IA procedure: HLC2CO rats consumed significantly more EtOH and showed a significantly greater EtOH preference than IHAS-1 rats. We did not find similar increases in EtOH drinking in congenic rats derived from the IAT/IANT parent strains. The difference in EtOH drinking in HLC2CO and IHAS-1 rats did not emerge until approximately halfway into the procedure suggesting the possibility of a gene x environment interaction at this locus; i.e., sufficient EtOH exposure may be required before this particular sensitivity QTL affects drinking behavior. Overall, this study suggests there may be a relationship between drinking and acute LORR sensitivity for the one QTL that was captured in the HLC2CO rats.
ST testing in this current group of congenics is consistent with our past results: the congenics were less sensitive to alcohol than their respective background strain as measured by a reduction in ST (Radcliffe et al., 2006, Radcliffe et al., 2009). Male HLC2CO congenics showed a significant decrease in ST; however, we previously observed a significant effect only in females. This may be the result of more generations of backcrossing, which would have eliminated all ILAS-1 alleles elsewhere in the genome. In addition, more than twice as many rats were used in the current study; i.e., congenics in the previous study did have a shorter ST than the control line, but it was not statistically significant (Radcliffe et al., 2006). The Tiia and IN6BX rats showed remarkably similar results to the recombinant congenic strains from which they were derived (Radcliffe et al., 2009). The result with the IN6BX rats confirms that the interval estimated from our recombinant congenic analysis contains the QTL, and it also reduces the minimum possible size from approximately 23 Mb containing 314 genes to approximately 17 Mb containing 246 genes.
In previous studies, progenitors for the current congenics showed significant increases in BECRR, although only in females for the HLC2CO rats (Radcliffe et al., 2006, Radcliffe et al., 2009). In the current study, however, there was not a significant difference in BECRR between any of the congenics and their controls suggesting that these particular QTLs mediate a metabolic effect, but not necessarily neuronal sensitivity. One possibility is that there are two “sub-QTLs” for neuronal sensitivity and alcohol metabolism, and only the metabolism QTLs have remained in the current congenics. The results are especially puzzling for the Tiia and IN6BX rats because of the many other related congenic strains that showed a significant effect for both ST and BECRR (see Radcliffe et al., 2009). Although the mechanism of decreased ST in the congenics remains unclear (i.e., neuronal sensitivity or alcohol metabolism), it is important to note that the most well-known genetic effects on drinking behavior in humans involve alcohol metabolism genes (Edenberg, 2007). Interestingly, an alcohol dehydrogenase (ADH) gene cluster that occurs in the HLC2CO QTL is orthologous to the human ADH cluster that has been implicated in human drinking behavior (Edenberg et al., 2006); however, no obvious EtOH metabolism genes are in either the Tiia or IN6BX QTL regions.
EtOH SA has been assessed previously in HAS/LAS parent rats prior to inbreeding, and it was found that only LAS, and not HAS, rats were successfully trained to self-administer 10E (Files et al., 1996). Thus, we followed the same sucrose-fade and dose-consumption testing procedures as the Files et al. (1996) study, hypothesizing that any increase in EtOH SA behavior in HLC2CO rats relative to IHAS rats should be apparent. Similar to the Files et al. study (1996), we found that IHAS rats displayed very low levels of EtOH intake during SA which likely would have had minimal pharmacological effects. Although intake was low, it should be noted that none of the rats ceased responding despite the long duration of post-fade testing (~9 weeks) and that responding on the PR schedule was significantly greater for EtOH than it was during extinction, suggesting that a combination of EtOH and associated cues (e.g., chemosensory or conditioned) did indeed maintain operant behavior. Regardless, the important finding from these data is that SA did not differ at any point between HLC2CO and IHAS rats. Thus, the QTL conferring decreased initial alcohol sensitivity in the HLC2CO does not appear to increase EtOH SA, at least under these conditions. It should be noted that the number of subjects was based on power analyses from previous studies (e.g., Files et al., 1996), and although appropriate for the estimated large effect size tested in this study (and correspondingly more meaningful differences in drinking behavior), small differences in drinking behavior might have gone undetected because of the relatively small sample size. In fact, based on the variances from the experiment, 8 rats would have been sufficient to detect a difference of 30% or more, and a sample size of greater than 50 in each group would have been required to observe a significant difference of 10%.
In contrast to findings in the HLC2CO rats, we did observe significant differences in SA between the IAT/IANT parent and congenic lines during the sucrose-fade procedure. If initial sensitivity is important for EtOH drinking, we might expect to see differences during the fade procedure, as this is the rats’ first exposure to EtOH. Congenic rats displayed both increased responding and EtOH intake relative to the parent strains during the initial stages of the fade procedure (Figures 4A and B). These differences were not sustained once the EtOH concentration reached 10S10E, however, nor were there differences between strains during dose-consumption or PR testing. Regardless, it appears that the IAT/IANT QTL conferring variance in alcohol sensitivity did increase the reinforcing effectiveness of EtOH for the congenics, but only during the earliest stages of exposure. It also should be noted, however, that the IN6BX and Tiia showed a greater number of responses than the IAT when offered sucrose without EtOH (10s; Figure 4A) which may be indicative of a possible difference in taste preference. Further testing with other tastants such as saccharin or quinine could clarify this issue.
Unlike the observed differences between the congenics and the IAT for EtOH intake during the sucrose-fade procedure, the IAT and IANT parental strains were found to have no differences (figure 4b). This suggests a lack of pleiotropy between SA and the TPT, the selection trait of the original AT and ANT. In addition, this is a clear case of transgressive segregation for SA in the congenics; i.e., increaser and decreaser alleles for SA were balanced in the IAT and IANT leading to similar phenotypes, and differences were revealed only when the relevant introgressed region was isolated in the congenics. One might conclude that the TPT may not be a good model for examining the relationship between acute sensitivity and drinking behavior, though it may be the case that there were no relevant genes with variant alleles present in this population of rats. Interestingly, this conclusion is similar to that of an earlier study assessing a free choice drinking behavior in F2 hybrid crosses of AT/ANT rats, which did not find a correlation between drinking behavior and motor impairment on the TPT (Sarviharju and Korpi, 1993).
In contrast to the SA results, the IA procedure revealed clear differences in both EtOH intake and preference between IHAS parent and HLC2CO congenic rats: HLC2CO rats consumed significantly more EtOH and displayed a significantly greater EtOH preference than IHAS rats (Figures 5A and B). These findings suggest that the low sensitivity allele of the QTL in the IHAS/ILAS derived lines caused a robust increase in EtOH drinking. Although these findings differ from the SA findings for IHAS/ILAS derived rats, there are important differences between the two procedures that could contribute to the discrepancy. For example, total EtOH exposure was relatively low per 30-min SA session compared to EtOH exposure per 24-h IA drinking session. Although rats consumed lower levels of EtOH through the first half of the IA procedure, they were still exposed to more EtOH than during SA. Further, the increase in EtOH consumption and preference took time to develop and was not present until about halfway through testing. It is therefore possible that the decreased sensitivity ILAS allele requires sufficient EtOH exposure to exert its effect on EtOH drinking which may be indicating an effect on tolerance. A similar pre-exposure to EtOH prior to SA may reveal differences in operant responding not revealed presently. As noted above, this QTL seems to confer a metabolic rather than neuronal sensitivity effect. However, it is not possible to know what effect, if any, this difference in metabolism had on the IA outcome in the HLC2CO since the metabolic effect was related to acute administration and the IA effect did not reveal itself until after chronic exposure. Moreover, the congenics drank nearly twice as much as the controls at some of the time points, which is unlikely to be a completely metabolic effect. It is also possible that the genes affecting LORR, regardless of the underlying mechanism, are unrelated to the genes influencing any drinking responses; i.e., the effect was a result of linkage rather than pleiotropy. This is a possibility for any QTL that influences two given traits, but it is particularly relevant in this case because the QTL interval is so large.
Although there were some significant differences in EtOH drinking during the IA procedure in IAT/IANT parent and congenic rats, these results were contrary to our hypothesis. If the LORR QTL had any effect on EtOH drinking, it was actually opposite to our hypothesis; i.e., the low sensitivity allele was associated with a decrease in EtOH intake. In contrast to a previous drinking study in AT/ANT rats (Sarviharju and Korpi, 1993), we found that IANT rats exhibited increased EtOH consumption compared to IAT rats. This may be explained by differences in testing conditions, as we used both a higher EtOH concentration (i.e., 20% vs. 10%) and intermittent, rather than continuous, access. It is also possible that genetic drift or inbreeding that occurred over the intervening 20+ years sufficiently modified the genetic architecture of the lines to produce the discordant results or that the current or original observation was a false positive. Our results are consistent with the previous study in that none of the strains showed EtOH preferences above 50% (Figure 4B; Sarviharju and Korpi, 1993). Thus, IAT/IANT congenics that show decreased initial alcohol sensitivity do not appear to increase EtOH drinking during an IA procedure.
Interestingly, the IANT rats displayed the highest levels of EtOH intake out of any of the IAT/IANT derived lines. This effect was not due to an overall increase in fluid intake, because although IANT rats did significantly increase total fluid intake over the course of testing (Figure 5B), this increase was associated with a selective increase in EtOH intake (Figure 6D). IANT rats, which have the higher sensitivity on the TPT, actually have decreased sensitivity when measured with LORR and BECRR (Radcliffe et al., 2009). Thus, in our hands, LORR may be a better measure of initial sensitivity than the TPT as it relates to EtOH drinking behavior in the IAT and IANT.
To our knowledge, no other LORR QTLs have been mapped to rat chromosome 1, but a single suggestive mouse LORR QTL has been mapped to the region that is syntenic to the chromosome 2 QTL (Bennett et al., 2006). Regarding EtOH drinking behaviors, a significant EtOH consumption QTL has been mapped in rat to the same locus as the chromosome 2 LORR QTL (Terenina-Rigaldie et al., 2003) and two separate studies have mapped suggestive EtOH consumption and preference QTLs in the mouse region that is syntenic to the chromosome 2 QTL (Peirce et al., 1998; Tarantino et al., 1998). Similarly, suggestive QTLs for EtOH consumption and preference in both rat and mouse have been mapped in the region of the chromosome 1 QTL (Gill et al., 1998; Vanderlinden et al., 2014) and, as noted above, human GWAS investigating alcohol dependence or consumption have implicated the region containing the ADH cluster that is syntenic to the HLC2CO introgressed region (Edenberg et al., 2006). These studies support that there is a gene or genes in these regions that modulate drinking behavior and that possibly may be pleiotropic to the LORR gene(s) found within the chromosome 1 and 2 QTLs that we have confirmed.
Overall, the experiments presented herein provide support for the hypothesis that QTLs that mediate variance in initial EtOH sensitivity as measured with LORR duration are associated with variation in EtOH consummatory behaviors. This was most clear in the HLC2CO with the use of the IA procedure and to some extent in the Tiia and IN6BX which both showed a modest effect in the predicted direction in the SA procedure, though the extent to which taste preference played a role needs to be explored. In general, the results are consistent with other genetic studies in rodents that have observed an inverse relationship between EtOH drinking and acute sensitivity, including LORR, though the relationship does not always hold up (e.g.: Bowers et al., 1999; Crabbe et al., 2006; Crabbe et al., 2010a; Hodge et al., 1999; Kurtz et al., 1996; Mcbride and Li, 1998; Radcliffe et al., 2013). Various experimental factors may have contributed to the negative findings including genetic background, the tests employed and the specific testing procedure, previous exposure to EtOH, etc. In addition, it is extremely unlikely that all genes involved in a given response to acute alcohol are also involved in drinking behavior and vice versa. In the current study, it is interesting that the congenics for one QTL showed an effect exclusively in the SA procedure (Tiia and IN6BX) while the congenic for the other QTL showed an effect exclusively in the IA procedure (HLC2CO). Regardless of whether or not the findings represent true pleiotropy, the results suggest that the two drinking models are under independent genetic regulation, at least in part.
Important questions remain for this work, particularly for the high drinking phenotype observed in the HLC2CO rats in the IA study. First is the question of whether it is actually the same genes that mediate acute sensitivity and drinking behavior. The QTL intervals captured in the congenics are rather large which raises the possibility that the genes modulating LORR are independent from those that are affecting drinking behavior. The second important question is what are specific genes that contribute to the co-variance in LORR and drinking. If this is true pleiotropy, knowledge of these genes would be helpful in understanding the relationship between acute sensitivity and drinking that has been consistently observed in humans; however, even if the genes are not pleiotropic, their identity would add to our knowledge about mechanisms of behavioral responses to EtOH. Finally, it would be of interest to determine the roles of EtOH metabolism and tolerance in the observed behaviors. LORR testing implicated pharmacokinetics as the underlying basis of the sleeptime differences in the congenics, yet the latent increase in drinking in the HLC2CO may be indicating the development of tolerance that potentially could be interacting with the pharmacokinetic effect. The genetic questions could be addressed through the continued development of recombinant congenics followed by a variety of genomic studies as we have done in our related mouse research (e.g., Bennett et al., 2015; Dowell et al., 2016; Dumas et al., 2014; Radcliffe et al., 2013). Regarding the question of EtOH metabolism, it would have been helpful to have conducted pharmacokinetic studies in the congenics, including the measurement of BECs during the drinking procedures. Unfortunately, various resource-related issues precluded conducting these studies. Future experiments addressing these issues could provide insight into the nature of the relationship between LORR sensitivity and drinking behavior for these particular rat QTLs.
Highlights.
The objective was to determine if two separate congenic rat lines each carrying a distinct QTL (chromosome 1 and 2) for acute hypnotic sensitivity to ethanol would show a difference in operant or two-bottle choice drinking behavior.
There was very little effect of either QTL on operant self-administration (SA).
Congenics carrying the QTL on chromosome 2 had significantly increased drinking in an intermittent access (IA) two-bottle choice procedure, but only after several weeks into the test.
Congenics carrying the QTL on chromosome 1 did not differ in the IA procedure.
Overall, there was modest support for the hypothesis that the chromosome 2 acute sensitivity QTL contributed to variation in drinking behavior with the low sensitivity congenics drinking more than higher sensitivity controls.
Acknowledgments
This work was supported by NIAAA grant R01AA018120.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker HC, Ron D. Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol. 2014;48:205–208. doi: 10.1016/j.alcohol.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B, Larson C, Richmond PA, Odell AT, Saba LM, Tabakoff B, Dowell R, Radcliffe RA. Quantitative Trait Locus Mapping of Acute Functional Tolerance in the LXS Recombinant Inbred Strains. Alcohol Clin Exp Res. 2015;39:611–620. doi: 10.1111/acer.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in γ-Protein Kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010a;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet. 2010b;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell R, Odell A, Richmond P, Malmer D, Halper-Stromberg E, Bennett B, Larson C, Leach S, Radcliffe RA. Genome characterization of the selected long- and short-sleep mouse lines. Mamm Genome. 2016;27:574–586. doi: 10.1007/s00335-016-9663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draski LJ, Spuhler KP, Erwin VG, Baker RC, Deitrich RA. Selective breeding of rats differing in sensitivity to the effects of acute ethanol administration. Alcohol Clin Exp Res. 1992;16:48–54. doi: 10.1111/j.1530-0277.1992.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Dumas L, Dickens CM, Anderson N, Davis J, Bennett B, Radcliffe RA, Sikela JM. Exome sequencing and arrayCGH detection of gene sequence and copy number variation between ILS and ISS mouse strains. Mamm Genome. 2014;25:235–243. doi: 10.1007/s00335-014-9502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Chen HJ, Tian H, Wetherill LF, Dick DM, Almasy L, Bierut L, Bucholz KK, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Porjesz B, Rice J, Schuckit M, Tischfield J, Begleiter H, Foroud T. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006;15:1539–1549. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT, Deitrich RA, Draski LJ. Initiation of ethanol self-administration by the sucrose-substitution method with HAS and LAS rats. Alcohol Clin Exp Res. 1996;20:677–681. doi: 10.1111/j.1530-0277.1996.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Gill K, Desaulniers N, Desjardins P, Lake K. Alcohol preference in AXB/BXA recombinant inbred mice: gender differences and gender-specific quantitative trait loci. Mamm Genome. 1998;9:929–935. doi: 10.1007/s003359900902. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, et al. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKC. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissue. Method Biochem Anal. 1959;7:217–251. [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JL, Derr R, Shendure J, Kolata T, Silver LM. A major influence of sex-specific loci on alcohol preference in C57Bl/6 and DBA/2 inbred mice. Mamm Genome. 1998;9:942–948. doi: 10.1007/s003359900904. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Bludeau P, Asperi W, Fay T, Deng XS, Erwin VG, Deitrich RA. Confirmation of quantitative trait loci for ethanol sensitivity and neurotensin receptor density in crosses derived from the inbred high and low alcohol sensitive selectively bred rat lines. Psychopharmacology. 2006;188:343–354. doi: 10.1007/s00213-006-0512-2. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG, Bludeau P, Deng X, Fay T, Floyd KL, Deitrich RA. A major QTL for acute ethanol sensitivity in the alcohol tolerant and non-tolerant selected rat lines. Genes Brain Behav. 2009;8:611–625. doi: 10.1111/j.1601-183X.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG, Draski L, Hoffmann S, Edwards J, Deng X-S, Bludeau P, Fay T, Lundquist K, Asperi W, Deitrich RA. Quantitative trait loci mapping for ethanol sensitivity and neurotensin receptor density in an F2 intercross derived from inbred high and low alcohol sensitivity selectively bred rat lines. Alcohol Clin Exp Res. 2004a;28:1796–1804. doi: 10.1097/01.alc.0000148106.71801.d7. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Hoffmann SE, Deng XS, Asperi W, Fay T, Bludeau P, Erwin VG, Deitrich RA. Behavioral characterization of alcohol-tolerant and alcohol-nontolerant rat lines and an F(2) generation. Behav Genet. 2004b;34:453–463. doi: 10.1023/B:BEGE.0000023650.32243.39. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Larson C, Bennett B. Genetic studies of acute tolerance, rapid tolerance, and drinking in the dark in the LXS recombinant inbred strains. Alcohol Clin Exp Res. 2013;37:2019–2028. doi: 10.1111/acer.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sarviharju M, Korpi ER. Ethanol sensitivity and consumption in F2 hybrid crosses of ANT and AT rats. Alcohol. 1993;10:415–418. doi: 10.1016/0741-8329(93)90030-r. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiat. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn JA. The patterns of drug and alcohol use and associated problems over 30 years in 397 men. Alcohol Clin Exp Res. 2014;38:227–234. doi: 10.1111/acer.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino LM, McClearn GE, Rodriguez LA, Plomin R. Confirmation of quantitative trait loci for alcohol preference in mice. Alcohol Clin Exp Res. 1998;22:1099–1105. [PubMed] [Google Scholar]
- Terenina-Rigaldie E, Moisan MP, Colas A, Beauge F, Shah KV, Jones BC, Mormede P. Genetics of behaviour: phenotypic and molecular study of rats derived from high- and low-alcohol consuming lines. Pharmacogenetics. 2003;13:543–554. doi: 10.1097/01.fpc.0000054120.14659.8c. [DOI] [PubMed] [Google Scholar]
- Vanderlinden LA, Saba LM, Printz MP, Flodman P, Koob G, Richardson HN, Hoffman PL, Tabakoff B. Is the alcohol deprivation effect genetically mediated? Studies with HXB/BXH recombinant inbred rat strains. Alcohol Clin Exp Res. 2014;38:2148–2157. doi: 10.1111/acer.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]