Abstract
Spectrins are large, flexible proteins comprised of α-β dimers that are connected head-to-head to form the canonical heterotetrameric spectrin structure. Spectrins were initially believed to be exclusively found in human erythrocytic membrane and are highly conserved among different species. βII spectrin, the most common isoform of non-erythrocytic spectrin, is found in all nucleated cells and forms larger macromolecular complexes with ankyrins and actins. Not only is βII spectrin a central cytoskeletal scaflolding protein involved in preserving cell structure but it has also emerged as a critical protein required for distinct physiologic functions such as posttranslational localization of crucial membrane proteins and signal transduction. In the heart, βII spectrin plays a vital role in maintaining normal cardiac membrane excitability and proper cardiac development during embryogenesis. Mutations in βII spectrin genes have been strongly linked with the development of serious cardiac disorders such as congenital arrhythmias, heart failure, and possibly sudden cardiac death. This review focuses on our current knowledge of the role βII spectrin plays in the cardiovascular system in health and disease and the potential future clinical implications.
Keywords: Spectrin, βII spectrin, Ankyrin, Cytoskeleton
1. Introduction
The spectrin family of proteins was first discovered in 1968 by Marchesi and Steers [1] as components of the human erythrocytic membrane [2], and was initially thought to be exclusively present in red blood cells [3,4]. While many subsequent experimental attempts failed to demonstrate the presence of spectrin in various non-erythroid cells [3,4], notable discoveries in brain did identify new peptides with calmodulin and actin binding properties comprised of 2 subunits (≈ 240 and 235 kDa) and were described as ‘Fodrin’ [5], ‘brain actin-binding protein’ [6] and ‘Calspectin’ [7]. No formal relationship was established between spectrin and these newly discovered brain peptides, yet these new proteins (yet to be termed spectrin) consistently had the same immunological, structural and functional degree of similarity to erythrocyte spectrin [8]. In 1981, Goodman and colleagues discovered that proteins with spectrin-like properties were potentially present in different cells and tissues such as brain, kidney, skeletal muscle, lens, small and large intestines and cardiac muscle [5,8–12]. Non-erythroid spectrins emerged as novel large actin-associated cytoskeletal proteins [2] that maintained cell shape and integrity and formed larger molecular complexes with ankyrins [13].
βII spectrin (the most common member of non-erythroid spectrins) [14] has emerged as a key cytoskeletal protein that is part of a larger macromolecular complex involved in diverse physiologic functions. βII spectrin is critical in posttranslational targeting and localization of essential membrane proteins [15–17], plays a prominent role in signal transduction [18,19], and notably serves as a major scaflolding protein [2]. Defects in βII spectrin have been associated with serious cardiac pathologies such as congenital arrhythmia, acquired and congenital forms of heart failure, and possibly sudden cardiac death [20–23]. This review focuses on the recent advances in defining the role of βII spectrin in the cardiovascular system in physiologic and pathologic states.
2. The spectrin genes and nomenclature
The nomenclature of spectrins has undergone several iterations. Winkelmann and Forget [24] classified spectrins by Roman numerals in the order of their characterization while subtypes were denoted by the Greek symbol sigma (Σ: capital letter, σ: small letter) followed by Arabic numbers.
Spectrin proteins are expressed from numerous genes in metazoans. There are seven genes that code for spectrins in mammalian organisms compared to only three genes in invertebrates. Unlike the three spectrins in invertebrates, the mammalian genome contains two α and five β spectrin genes named in the order of their discovery. SPTA1 gene [25] codes for αI that is expressed in erythroid cells, while SPTAN1 [26] codes for at least four and possibly up to eight different αII isotypes that are present in all non-erythroid cells [27]. The conventional βI-IV-spectrins are encoded by SPTB, SPTBN1, SPTBN2 and SPTBN4, respectively and SPTBN5 encodes a heavy βV-spectrin [2,28]. βI spectrin is the only form expressed in erythrocytes. The spectrin product of these genes can be modiffed via extensive alternative processing of pre-mRNA giving rise to a wide diversity of spectrin spliceoforms. This is particularly important with respect to regulating the interactive and modulatory characteristics of spectrins [2,26–29].
3. Structural domains of spectrin
Spectrins, believed to have evolved from α-actinin [30–33], are formed of two large, similar but non-identical subunits, termed α and β [2,30–36]. Spectrins are flexible rods that have a contour length of approximately 200–260 nm with an actin-binding domain (ABD) on each end [2,37–39]. The α and β subunits are connected side-by-side in an antiparallel fashion via hydrophobic interactions supplemented by electrostatic forces of attraction [40,41] to form a heterodimer [2,28,31,39–41]. This involves an interaction between two repeats near the NH2-terminus of one α spectrin chain and the COOH-terminal region of the antiparallel β subunit. Each of the 2 corresponding dimers is then assembled head-to-head via partial repeats in both α and β subunits to form the final heterotetramer structure of spectrin [2,28,31,42,43]. Owing to the high affinity between α and β chains, spectrins primarily exist as heterotetramers rather than autonomous α or β subunits.
The canonical spectrin subunit is highly conserved among species [2,28], and is comprised of successive repeats of 106 amino acid residues termed spectrin repeats that are folded in a triple α-helical coiled structure. This structural form and interconnection of spectrin repeats are believed to play a role in the flexibility of spectrins [44]. The α and β subunits are comprised of 21 and 17 repeats, respectively [31]. The only exception is βV spectrin which has 30 repeats [45]. The last repeat in each spectrin is an incomplete repeat that mediates end-to-end association between one helix of α spectrin and two helices of β spectrin to form a triple helical bundle [46].
βII spectrin, like all conventional β spectrins, contains 2 tandem calponin homology (CH1 & CH2) domains which both comprise an actin-binding domain (ABD) at the amino-terminal [2,28,31,47,48]. Linked to the ABD domain of βII subunit are 17 successive triple helical motifs and terminates with a carboxyl region. The COOH-terminal region of the 14th repeat and the entire 15th repeat are a prerequisite for ankyrin binding [46,49]. The carboxyl-terminal of βII spectrin is differentially spliced giving origin to long and short βII isoforms (βIIΣ1 and βIIΣ2 respectively) [31,50,51]. The long carboxyl terminus of the last partial repeat of βII spectrin is linked to a pleckstrin homology (PH) domain [50]. The spliced βII isoforms with short C-terminal regions lack this PH domain [52]. The PH domain, a seven stranded antiparallel β-sheet, is comprised of approximately 100–120 amino acids and is located approximately 50–60 amino acid residues before the end of the C-terminus of β chain. This domain serves as a ligand binding site for many phospholipids involved in signal transduction [28,50,53–56]. Immunofluorescence staining shows that βII spectrin is localized in a striated pattern in isolated mouse myocytes [20].
The alternatively spliced short variants of βII spectrin called ELF (embryonic liver fodrin) share some similarities with the long βII isoform. ELF-3, a 200 kDa β spectrin and the longest form among other ELFs, is a short βII isoform with an ABD, a 17 repeat domain and COOH terminal lacking the PH domain [52]. On the contrary, ELF-1, a 27 kDa β spectrin, shares no degree of homology in domain 2 of the long βII isoform [57], but has a sole CH1 domain similar to that of the other β spectrins and a C-terminus similar to the short βII isoform [2,52,57].
4. Role of βII spectrin in the heart
The cardiac cytoskeleton has recently emerged as a crucial player for maintaining the cardiac membrane integrity with respect to structure and function in physiologic and pathologic states. Disorders in cardiac cytoskeletal components have been strongly associated with cardiac myopathies, dystrophies, aortopathies and electrical conduction abnormalities [20,58–61]. While βII spectrin has a prominent role in many organ systems (Table 1), it has also emerged as a pivotal protein in maintaining normal cardiac membrane excitability, mechanical function [20] and proper embryogenesis [62].
Table 1.
The physiologic roles of βII spectrin in different organ systems.
| Organ system | Physiologic roles of βII spectrin | Ref. |
|---|---|---|
| Pulmonary system | De novo synthesis and stabilization of lateral membrane of bronchial epithelial cells. | [17] |
| Maintaining polarity of E-cadherin and Na+-K+-ATPase. | [16,63,64] | |
| Nervous system | Binding to small synaptic vesicles via synapsin-I involved in neurotransmission. | [65,66] |
| Important component of paranodal junctions involved in saltatory conduction. | [67,68] | |
| Maintaining structural integrity of neurons. | [68,69] | |
| Molecular partner to α-synuclein that regulate neurite growth during synaptogenesis. | [70] | |
| Hepatic system | Hepatocellular carcinoma suppression mainly via serving as an adaptor protein for Smad3 and Smad4 involved in TGF-β signaling pathway. | [19,71] |
| Involved in the regenerative process following partial hepatectomy. | [72–74] | |
| A mediator and effector protein in acetaminophen-induced liver injury. | [14] | |
| ELF-3 has a role in intrahepatic bile duct formation and hepatic cells differentiation and polarization | [52] | |
| Renal system | Maintaining polarity of Na+-K+-ATPase in renal tubular cells | [75,76] |
5. Role in embryonic heart development
Emerging data suggests that βII spectrin plays an important role in embryonic heart development [62]. Data in mice demonstrate that complete deletion of βII spectrin results in intrauterine death with multiple defects including hepatic, neural, gastrointestinal and angiogenesis abnormalities [77]. Notably loss of βII spectrin is a possible cause for the development of congenital heart defects [62,78,79]. Furthermore, a study performed on homozygous mutant embryos for βII spectrin gene demonstrated that there was a significant difference in heart size between the wild-type embryos and the homozygous mutant embryos with the latter having smaller heart size at embryonic day 15.5 (E15.5). Further histologic studies revealed failure of ventricular wall thickening and blood vessel formation in the homozygous mutant group [62]. Moreover, the embryonic cardiomyocytes of βII spectrin conditional knockout mice displayed an aberrant distribution of tropomyosin and a significant down-regulation in the expression of α-smooth muscle actin (α-SMA), cardiac homeobox protein (NKx2.5) and dystrophin [80] which are muscle differentiation markers [62]. These defects subsequently have an s adverse effect on the contractile ability of cardiomyocytes in vivo. In addition, loss of βII spectrin in homozygous mutant embryos interferes with cardiac cell differentiation and induces extensive apoptosis at E16.5 [62]. It is noteworthy that genetic mutations in dystrophin leads to congenital muscular dystrophies such as Duchenne muscular dystrophy and Becker muscular dystrophy, and many of these patients develop dilated cardiomyopathy and ventricular arrhythmias [81,82]. Hence the aforementioned data strongly suggests that βII spectrin is critical for proper cardiac development.
6. Role in cardiac membrane excitability
Until recently, arrhythmogenic cardiomyopathies were mainly linked to disorders in ion channels [83], however, little was known about arrhythmia resulting from defects in cytoskeletal-associated proteins [20]. Recent data reveal that defects in the βII spectrin-based cytoskeleton disrupt normal electrical conduction in the heart [20], potentially leading to congenital as well as acquired human arrhythmia [21].
Recent data has shown that at the T-tubule in cardiac myocytes, the αII/βII tetramer (linked to actin) recruits ankyrin-B [18] and associates via its ankyrin-binding domain with the NH2 terminal ZU5 (Zu5N) domain of ankyrin-B [84]. This molecular complex interacts with other membrane-associated proteins such as Na+-K+-ATPase and Na+-Ca2 +exchanger (known ankyrin-binding partners) and is important for their localization [20]. A newly identified human variant of ankyrin-B showed a substantial decrease in binding with βII spectrin in heart [20]. Using primary neonatal cardiomyocytes of ankyrin-B conditional knockout (cKO) mice the authors were able to elucidate the relationship between βII spectrin and ankyrin-B, and demonstrated that after transfection the human variant failed to localize Na+-Ca2 + exchanger when compared with the wild-type ankyrin-B which rescued Na+-Ca2+ ion channel localization [20]. Notably previously identified ankyrin-B variants (DAR976AAA and A1000P) [18] which also lacked spectrin-binding activity also showed similar results. Together these data demonstrate that βII spectrin and ankyrin-B are molecular partners in heart, and importantly they form an important macromolecular complex. Interestingly, while ankyrin-B is critical for βII spectrin targeting in the neonatal period [18], βII spectrin plays the predominant role in the mature cells and is crucial for proper ankyrin-B expression and localization [20]. Consistent with these findings, βII spectrin protein expression in right atrial samples of patients with atrial fibrillation (AF) was significantly decreased compared with patients in sinus rhythm [85–87]. Likewise, patients with loss-of-function genetic mutations in ankyrin-B also developed AF. The ankyrin-B protein levels in these patients were markedly decreased in their right atria as well [85]. This is consistent with the recently identified congenital human arrhythmia that arose due to a dysregulation in the ankyrin-B/βII spectrin pathway [20].
Furthermore, telemetry studies performed on cardiac-specific βII spectrin knockout mice showed aberrant cardiomyocyte electrical activity [20]. ECG readings of these mice showed bradycardia, atrioventricular nodal block, and increased rate variability. Notably, pro-arrhythmic patterns were observed, such as widened QRS complexes and prolonged QT interval at baseline and ventricular arrhythmia and mortality was observed in several animals after catecholaminergic induced stress. However, there were no notable differences between βII spectrin knockout mice and the wild-type littermates in terms of cardiac output and left ventricular ejection fraction [20].
7. Role in heart failure and cardiac remodeling
Dysregulation of βII spectrin has been linked with the development of acquired forms of heart failure (HF) in both human and animal models [21]. Following transverse aortic constriction (TAC), βII spectrin cKO mice suffered severe and accelerated cardiac failure after 6 weeks characterized by left ventricular free wall degeneration and interventricular septal vacuolation. Furthermore, βII spectrin levels were found to be significantly decreased in the left ventricular tissues of ischemic and non-ischemic heart failure in murine and canine myocardial failure models [21].
It is critical to note that abnormal and selective cardiac remodeling occurs with the loss of βII spectrin in heart [20]. In accordance with earlier studies in avian erythrocytes (whose erythrocytic spectrin is more similar to mammalian non-erythroid spectrin [24,88,89]) where α spectrin levels were decreased in the absence of erythrocytic β spectrin [90–92], βII spectrin cKO mice also displayed a sharp decline in αII levels in the heart [20] (Table 2). The levels of select proteins in the heart, namely βI spectrin, α and β tubulin, were all upregulated in βII spectrin cKO mice, likely as a compensatory process [20]. These data are consistent with previous reports of elevated tubulin expression and remodeling in failing myocardium [93]. The levels of actin and desmin proteins in βII spectrin cKO mice were not altered which further support that the remodeling process is selective [20].
Table 2.
Cardiac expression and localization of different proteins linked to βII spectrin loss.
| Protein | Expression levels | Proper localization | Ref. |
|---|---|---|---|
| αII spectrin | ↓ | No | [20] |
| βI spectrin | ↑ | Not available | [20] |
| Actin | – | Yes | [20] |
| Desmin | – | Yes | [20] |
| α tubulin | ↑ | Not available | [20] |
| β tubulin | ↑ | Not available | [20] |
| Ankyrin-B | ↓ | No | [20] |
| Ankyrin-G | ↑ | Not available | [20] |
| Ankyrin-R | – | Not available | [20] |
| Ryanodine receptor 2 | ↓ | No | [20] |
| Na+-K+-ATPase | ↓ | No | [20,101] |
| Na+-Ca2 +-exchanger | ↓ | No | [20,101] |
| Inositol triphosphate receptor | ↓ | No | [101,102] |
| L-type calcium channel (Cav1.2) | Not available | Yes | [20] |
| Voltage-gated Na channel (Nav1.5) | – | Not available | [20] |
| Sarco/endoplasmic reticulum Ca2 +-ATPase (SERCA2) | – | Yes | [20] |
In human heart failure the levels of βII spectrin protein were significantly decreased in patients with non-ischemic [21] and ischemic heart failure [94], as were the levels of ankyrin-B protein [21,94] and Na+-K+-ATPase [94]. Contrarily, the levels of βII spectrin mRNA transcripts as well as those of ankyrin-B and Na+-K+-ATPase were not dramatically different between diseased and normal groups [21,94]. This observation is likely due to post-translational process induced by Ca2+- and calpain-dependent proteolysis [21]. In avian erythrocytes, remodeling at the cellular level is associated with spectrin-based cytoskeletal cleavage [90–92], and this proteolytic process is induced by calpains [95–97]. Similarly, the heart and brain of adult mice showed that cleavage of the βII spectrin based cytoskeleton occurs following exposure to pathological concentrations of Ca2+ [21]. It is important to note that the degradation of βII spectrin and ankyrin-B was prevented by the calpain inhibitor MDL-28170 [21,95]. In addition, previous data show that reactive oxygen species (ROS) has been linked with apoptosis of cardiomyocytes through various signaling pathways including activation of cardiac calpain proteases [21,98,99], and this is another mechanism that could lead to breakdown of the spectrin cytoskeleton. As the failing heart exhibits dysregulation in Ca2 + homeostasis and a rise in the concentration of ROS, this likely [100] explains decreased protein expression of βII spectrin and ankyrin-B proteins in non-ischemic heart failure patients [21].
8. Future directions and clinical implications
8.1. New therapeutic targets
Several molecules underlying biogenesis and regulation of the ryanodine receptor 2 (RyR2) have been identified, including calstabin, calmodulin, protein kinase A (PKA), and junctophilin to name a few [103–105]. Unfortunately there has been a failure of past ion channel specific therapies for heart disease—hence it is critical that we find new molecules that can be targeted to regulate contractility/excitability beyond ion channels and receptors, and the calcium-induced calcium release (CIRC) pathway is an ideal target. As the cytoskeletal protein βII spectrin is a critical node linked to local RyR2 regulation [20], it might be exploited as a potential diagnostic and therapeutic target for heart failure and arrhythmia.
It is well known that mislocalization or dysfunction of RyR2 is associated with the development of arrhythmia, heart failure and sudden cardiac death in animals and humans [105–110]. In βII spectrin cKO mice, the levels of RyR2, Na+-Ca2 + exchanger and Na+-K+-ATPase were dramatically decreased (Table 2) [20]. There were no differences observed in T-tubule L-type calcium channel localization or T-tubule morphology between wild type and βII spectrin cKO mice, but there were heterogeneity, reduced size and intensity of the remaining RyR2 clusters [20]. In addition, the ventricular cardiac cells of βII spectrin cKO mice exhibited recurrent spontaneous Ca2 +-induced after-depolarizations (Fig. 1). These afterdepolarizations were eliminated with ryanodine which is known to inhibit Ca2 + release. Compared with cardiac cells of the wild-type mice, the βII spectrin cKO mice showed almost a threefold increase in spontaneous Ca2 + waves that are known to provoke arrhythmia [20]. It is also noteworthy that βII spectrin controls RyR2 localization independently of ankyrin-B as studies with ankyrin-B haploinsufficient mice showed no abnormalities in RyR2 localization [111]. Thus, βII spectrin plays an integral role in properly targeting and localizing important cardiac membrane-associated proteins, and abnormalities with spectrin localization could lead to arrhythmias induced by calcium overload.
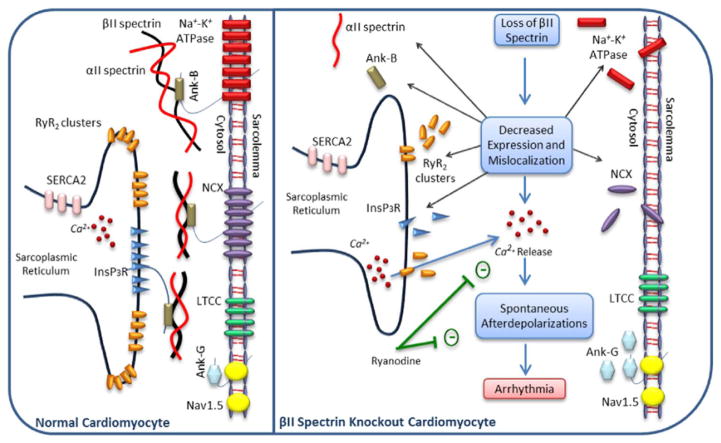
Fig. 1.
A schematic view of a normal cardiomyocyte and a βII spectrin knockout cardiomyocyte. In the absence of βII spectrin protein, the expression levels of ryanodine receptor 2 (RyR2), Na+-K+-ATPase and Na+-Ca2 +-exchanger (NCX), Ankyrin-B (Ank-B), αII spectrin are significantly decreased and showed defective localization [20]. The expression of both sarco/endoplasmic reticulum Ca2 +-ATPase (SERCA2) and voltage-gated Na+ channel (Nav1.5) are not impacted by the loss of βII spectrin [20]. Additionally, both SERCA2 and L-Type Ca2 + channel (LTCC) are properly localized. The levels of Ankyrin-G (Ank-G) are increased in the absence of βII spectrin, likely as a compensatory response to decrease ankyrin-B expression [20]. Defects in ryanodine receptors result in aberrant Ca2 +-dependent release and subsequent spontaneous afterdepolarizations which eventually lead to the development of arrhythmias. In a murine model of a cKO of βII spectrin, ryanodine was shown to inhibit Ca2+ release and abolishes these spontaneous afterdepolarizations. It is important to note that further research is warranted to demonstrate the localization of Nav1.5 and the expression of LTCC in βII spectrin deficiency. In addition, no data are currently available that directly links the expression and localization of inositol triphosphate receptor (InsP3R), a known ankyrin-B binder, to βII spectrin protein. However, mislocalization and decreased expression of InsP3R is expected due to decreased ankyrin-B expression [101,102].
As previously mentioned, dysregulation in Ca2 + homeostasis and subsequent activation of calpain enzymes occur in heart failure and consequently βII spectrin-based cytoskeleton degradation and remodeling ensue. This degradation was prevented by the calpain inhibitor MDL-28170 [20]. Thus, calpain inhibition could serve as a future therapeutic intervention to prevent destabilization of the spectrin cytoskeleton. As βII spectrin-based cytoskeletal cleavage and cellular remodeling are tightly linked with Ca2+-dependent calpain proteases, it is reasonable to hypothesize that malignant proteolysis of βII spectrin plays a direct role in local RyR2 regulation, hence calpain inhibition could have a role in preserving RyR2 integrity. Further research aimed at unveiling the complexity of calpain substrate specificity and the mechanisms involved in off- and on-target effects of calpain inhibitors is needed.
8.2. Breakdown products and biomarkers
Spectrin breakdown products (SBDPs) are produced by calpain- and caspase-3-mediated mechanisms and can be detected in the cerebrospinal fluid (CSF) [112–114] and serum [115]. In brain, αII spectrin is degraded by calpain into 2 main fragments (150 kDa and 145 kDa) while caspase-3-induced proteolysis results in the formation of a 150 kDa fragment which is further degraded yielding a 120 kDa spectrin breakdown product [116–120]. Caspase-3-mediated proteolysis is believed to be an indication of apoptosis, while calpain-induced proteolytic cleavage is proposed to be an indicator of excitotoxic and necrotic neuronal death [116–122]. In rat models, four main βII spectrin degradation products (110, 108, 85, 80 kDa) accumulated in the cortical cells due to activation of calpain-2 and caspase-3 following brain injury. The 110 kDa and 85 kDa fragments are calpain specific BDPs while the 108 kDa and 80 kDa are caspase specific products [119,123]. These recent reports showed that both αII and βII SBDPs could serve as potential biomarkers that would aid in the clinical diagnosis and outcome as well as correlate with the severity of neurologic insults [113,114,124] including traumatic and ischemic brain injuries and neurodegenerative diseases [120] such as Alzheimer’s Dementia. Likewise, it has been demonstrated in the heart of adult mice that cleavage of the βII spectrin based cytoskeleton occurs following Ca2+-triggered calpain activation (Fig. 2) [21]. One could hypothesize that βII spectrin breakdown products could serve as an early diagnostic biomarker for acute cardiovascular diseases as cardiac and myocyte insult typically leads to calcium release and ROS activation, which inevitably will lead to cleavage of spectrins [21].
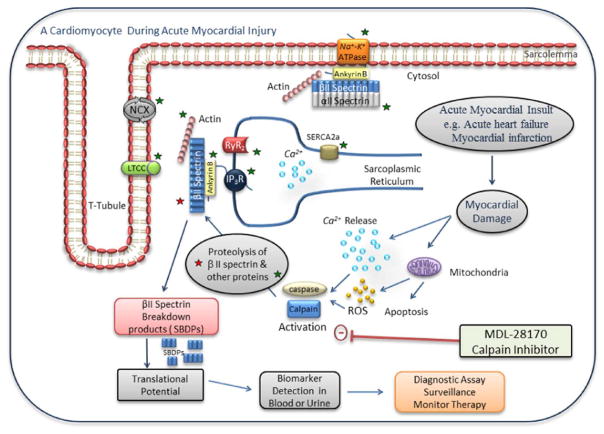
Fig. 2.
A schematic view of a cardiomyocyte during acute myocardial injury. In the event of acute myocardial damage, Ca2 + dysregulation occurs leading to Ca2 + leak from the sarcoplasmic reticulum and subsequent activation of calpain and caspase enzymes. Increased levels of reactive oxygen species (ROS) released within the cardiomyocyte also activate Ca2 +dependent proteases. These proteases then cleave βII spectrin (denoted by red star) and other potential protein substrates (denoted by green stars) producing βII spectrin breakdown products (SBDPs) which could have translational potential as a biomarker of cardiac damage via detection in blood or urine. SBDPs could serve as early biomarkers in acute cardiac injury that would aid in the diagnosis, surveillance, and monitoring of patients who have acute cardiac syndromes. Of note the calpain inhibitor MDL-28170 prevents the degradation of βII spectrin, and this could serve as a therapeutic intervention for the stabilization of βII spectrin following acute cardiac injury [20]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Cardiovascular disease remains the number one cause of death in the U.S. Further, dilated cardiomyopathies, HF and arrhythmias are a significant health burden. Despite advancement in medical therapies, cardiac resynchronization therapies, and left ventricular assist devices, HF remains a global epidemic with most deaths ultimately caused by arrhythmias [125]. Additional insight and research into the molecular underpinnings of the cardiac cytoskeleton could lead to new diagnostic and therapeutic targets for therapies and warrants further investigation.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Marchesi VT, Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968;159:203–204. doi: 10.1126/science.159.3811.203. http://dx.doi.org/10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 3.Hiller G, Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977;266:181–183. doi: 10.1038/266181a0. http://dx.doi.org/10.1038/266181a0. [DOI] [PubMed] [Google Scholar]
- 4.Painter RG, Sheetz M, Singer SJ. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975;72:1359–1363. doi: 10.1073/pnas.72.4.1359. http://dx.doi.org/10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–642. doi: 10.1083/jcb.90.3.631. http://dx.doi.org/10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimo-Oka T, Watanabe Y. Stimulation of actomyosin Mg2+-Atpase activity by a brain microtubule-associated protein fraction. High-molecular-weight actin-binding protein is the stimulating factor. J Biochem. 1981;90:1297–1307. doi: 10.1093/oxfordjournals.jbchem.a133595. http://dx.doi.org/10.1093/oxfordjournals.jbchem.a133595. [DOI] [PubMed] [Google Scholar]
- 7.Kakiuchi S, Sobue K, Fujita M. Purification of a 240 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1981;132:144–148. doi: 10.1016/0014-5793(81)80449-8. http://dx.doi.org/10.1016/0014-5793(81)80449-8. [DOI] [PubMed] [Google Scholar]
- 8.Goodman SR, Zagon IS. Brain spectrin: a review. Brain Res Bull. 1984;13:813–832. doi: 10.1016/0361-9230(84)90239-9. http://dx.doi.org/10.1016/0361-9230(84)90239-9. [DOI] [PubMed] [Google Scholar]
- 9.Burridge K, Kelly T, Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982;95:478–486. doi: 10.1083/jcb.95.2.478. http://dx.doi.org/10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenney JR, Jr, Glenney P, Fodrin Is. The general spectrin-like protein found in most cells whereas spectrin and the Tw protein have a restricted distribution. Cell. 1983;34:503–512. doi: 10.1016/0092-8674(83)90383-5. http://dx.doi.org/10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- 11.Repasky EA, Granger BL, Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982;29:821–833. doi: 10.1016/0092-8674(82)90444-5. http://dx.doi.org/10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- 12.Goodman SR, Zagon IS, Kulikowski RR. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981;78:7570–7574. doi: 10.1073/pnas.78.12.7570. http://dx.doi.org/10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. http://dx.doi.org/10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Baek HJ, Lee YM, Kim TH, Kim JY, Park EJ, Iwabuchi K, Mishra L, Kim SS. Caspase-3/7-mediated cleavage of beta2-spectrin is required for acetaminophen-induced liver damage. Int J Biol Sci. 2016;12:172–183. doi: 10.7150/ijbs.13420. http://dx.doi.org/10.7150/ijbs.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman MJ, Mohler PJ. Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ Res. 2010;107:457–465. doi: 10.1161/CIRCRESAHA.110.224592. http://dx.doi.org/10.1161/CIRCRESAHA.110.224592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+)Atpase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. http://dx.doi.org/10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 17.Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. http://dx.doi.org/10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 18.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. http://dx.doi.org/10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 19.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L. Tgf-beta signaling in development. Sci STKE. 2007;2007:cm1. doi: 10.1126/stke.3992007cm1. http://dx.doi.org/10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- 20.Smith SA, Sturm AC, Curran J, Kline CF, Little SC, Bonilla IM, Long VP, Makara M, Polina I, Hughes LD, Webb TR, Wei Z, Wright P, Voigt N, Bhakta D, Spoonamore KG, Zhang C, Weiss R, Binkley PF, Janssen PM, Kilic A, Higgins RS, Sun M, Ma J, Dobrev D, Zhang M, Carnes CA, Vatta M, Rasband MN, Hund TJ, Mohler PJ. Dysfunction in the betaII spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation. 2015;131:695–708. doi: 10.1161/CIRCULATIONAHA.114.013708. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SA, Hughes LD, Kline CF, Kempton AN, Dorn LE, Curran J, Makara M, Webb TR, Wright P, Voigt N, Binkley PF, Janssen PM, Kilic A, Carnes CA, Dobrev D, Rasband MN, Hund TJ, Mohler PJ. Dysfunction of the beta2-spectrin-based pathway in human heart failure. Am J Physiol Heart Circ Physiol. 2016;310:H1583–1591. doi: 10.1152/ajpheart.00875.2015. http://dx.doi.org/10.1152/ajpheart.00875.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, Zouhairi ME, Mendelson J, Shetty K, Kallakury B, Berry DL, Shin KH, Mishra B, Reddy EP, Kim SS, Mishra L. Transforming growth factor-beta adaptor, beta2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology. 2011;53:1676–1684. doi: 10.1002/hep.24128. http://dx.doi.org/10.1002/hep.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K, Tian X, Marshall JL, Byers SW, He AR. BetaII-spectrin (Sptbn1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598–612. doi: 10.1002/hep.27558. http://dx.doi.org/10.1002/hep.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81:3173–3185. [PubMed] [Google Scholar]
- 25.Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, Leto TL, Linnenbach AJ, Winkelmann JC, Speicher DW, Marchesi VT, et al. The complete Cdna and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem. 1990;265:4434–4443. [PubMed] [Google Scholar]
- 26.Moon RT, McMahon AP. Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of Cdnas encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990;265:4427–4433. [PubMed] [Google Scholar]
- 27.Cianci CD, Zhang Z, Pradhan D, Morrow JS. Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry. 1999;38:15721–15730. doi: 10.1021/bi991458k. http://dx.doi.org/10.1021/bi991458k. [DOI] [PubMed] [Google Scholar]
- 28.Machnicka B, Grochowalska R, Boguslawska DM, Sikorski AF, Lecomte MC. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci. 2012;69:191–201. doi: 10.1007/s00018-011-0804-5. http://dx.doi.org/10.1007/s00018-011-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. BetaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. http://dx.doi.org/10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual J, Castresana J, Saraste M. Evolution of the spectrin repeat. BioEssays. 1997;19:811–817. doi: 10.1002/bies.950190911. http://dx.doi.org/10.1002/bies.950190911. [DOI] [PubMed] [Google Scholar]
- 31.Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37:796–803. doi: 10.1042/BST0370796. http://dx.doi.org/10.1042/BST0370796. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GH, Newbern EC, Korte CC, Bales MA, Muse SV, Clark AG, Kiehart DP. Intragenic duplication and divergence in the spectrin superfamily of proteins. Mol Biol Evol. 1997;14:1285–1295. doi: 10.1093/oxfordjournals.molbev.a025738. http://dx.doi.org/10.1093/oxfordjournals.molbev.a025738. [DOI] [PubMed] [Google Scholar]
- 33.Viel A. Alpha-actinin and spectrin structures: an unfolding family story. FEBS Lett. 1999;460:391–394. doi: 10.1016/s0014-5793(99)01372-1. http://dx.doi.org/10.1016/S0014-5793(99)01372-1. [DOI] [PubMed] [Google Scholar]
- 34.Pascual J, Pfuhl M, Walther D, Saraste M, Nilges M. Solution structure of the spectrin repeat: a left-handed antiparallel triple-helical coiled-coil. J Mol Biol. 1997;273:740–751. doi: 10.1006/jmbi.1997.1344. http://dx.doi.org/10.1006/jmbi.1997.1344. [DOI] [PubMed] [Google Scholar]
- 35.Dubreuil Ronald R, Byers TJ, Sillman AL, Bar-Zvi D, Goldstein LS, Branton D. The complete sequence of drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989;109:2197–2205. doi: 10.1083/jcb.109.5.2197. http://dx.doi.org/10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byers TJ, Husain-Chishti A, Dubreuil Ronald R, Branton D, Goldstein LS. Sequence similarity of the amino-terminal domain of drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989;109:1633–1641. doi: 10.1083/jcb.109.4.1633. http://dx.doi.org/10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett V, Davis J, Fowler WE. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982;299:126–131. doi: 10.1038/299126a0. http://dx.doi.org/10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- 38.Glenney JR, Jr, Glenney P, Osborn M, Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982;28:843–854. doi: 10.1016/0092-8674(82)90063-0. http://dx.doi.org/10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- 39.Shotton DM, Burke BE, Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and Electron Microscopic Studies. 1979;131:303–329. doi: 10.1016/0022-2836(79)90078-0. http://dx.doi.org/10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Zhang C, Zhao Q, Li D. Spectrin: structure, function and disease. Sci China Life Sci. 2013;56:1076–1085. doi: 10.1007/s11427-013-4575-0. http://dx.doi.org/10.1007/s11427-013-4575-0. [DOI] [PubMed] [Google Scholar]
- 41.Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragon A, Speicher DW. Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood. 2010;115:4843–4852. doi: 10.1182/blood-2010-01-261396. http://dx.doi.org/10.1182/blood-2010-01-261396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ungewickell E, Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978;88:379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. http://dx.doi.org/10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- 43.Cherry L, Menhart N, Fung LW. Interactions of the alpha-spectrin N-terminal region with beta-spectrin. Implications for the spectrin tetramerization reaction. J Biol Chem. 1999;274:2077–2084. doi: 10.1074/jbc.274.4.2077. http://dx.doi.org/10.1074/jbc.274.4.2077. [DOI] [PubMed] [Google Scholar]
- 44.Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999;98:523–535. doi: 10.1016/s0092-8674(00)81980-7. http://dx.doi.org/10.1016/S0092-8674(00)81980-7. [DOI] [PubMed] [Google Scholar]
- 45.Stabach PR, Morrow JS. Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem. 2000;275:21385–21395. doi: 10.1074/jbc.C000159200. http://dx.doi.org/10.1074/jbc.C000159200. [DOI] [PubMed] [Google Scholar]
- 46.Davis L, Abdi K, Machius M, Brautigam C, Tomchick DR, Bennett V, Michaely P. Localization and structure of the ankyrin-binding site on beta2-spectrin. J Biol Chem. 2009;284:6982–6987. doi: 10.1074/jbc.M809245200. http://dx.doi.org/10.1074/jbc.M809245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djinovic Carugo K, Banuelos S, Saraste M. Crystal structure of a calponin homology domain. Nat Struct Biol. 1997;4:175–179. doi: 10.1038/nsb0397-175. http://dx.doi.org/10.1038/nsb0397-175. [DOI] [PubMed] [Google Scholar]
- 48.Banuelos S, Saraste M, Djinovic Carugo K. Structural comparisons of calponin homology domains: implications for actin binding. Structure. 1998;6:1419–1431. doi: 10.1016/s0969-2126(98)00141-5. http://dx.doi.org/10.1016/S0969-2126(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. http://dx.doi.org/10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, Kordeli E, Baines AJ. Identification of a novel C-terminal variant of Beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci. 2000;113(Pt 11):2023–2034. doi: 10.1242/jcs.113.11.2023. [DOI] [PubMed] [Google Scholar]
- 51.Tang Y, Katuri V, Iqbal S, Narayan T, Wang Z, Lu RS, Mishra L, Mishra B. Elf a beta-spectrin is a neuronal precursor cell marker in developing mammalian brain; structure and organization of the Elf/beta-G spectrin gene. Oncogene. 2002;21:5255–5267. doi: 10.1038/sj.onc.1205548. http://dx.doi.org/10.1038/sj.onc.1205548. [DOI] [PubMed] [Google Scholar]
- 52.Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–364. doi: 10.1038/sj.onc.1202313. http://dx.doi.org/10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- 53.Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. http://dx.doi.org/10.1016/S0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- 54.Grzybek M, Chorzalska A, Bok E, Hryniewicz-Jankowska A, Czogalla A, Diakowski W, Sikorski AF. Spectrin-phospholipid interactions. Existence of multiple kinds of binding sites? Chem Phys Lipids. 2006;141:133–141. doi: 10.1016/j.chemphyslip.2006.02.008. http://dx.doi.org/10.1016/j.chemphyslip.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 55.An X, Guo X, Gratzer W, Mohandas N. Phospholipid binding by proteins of the spectrin family: a comparative study. Biochem Biophys Res Commun. 2005;327:794–800. doi: 10.1016/j.bbrc.2004.12.063. http://dx.doi.org/10.1016/j.bbrc.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 56.Diakowski W, Ozimek L, Bielska E, Bem S, Langner M, Sikorski AF. Cholesterol affects spectrin-phospholipid interactions in a manner different from changes resulting from alterations in membrane fluidity due to fatty acyl chain composition. Biochim Biophys Acta. 2006;1758:4–12. doi: 10.1016/j.bbamem.2005.11.009. http://dx.doi.org/10.1016/j.bbamem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 57.Mishra L, Cai T, Levine A, Weng D, Mezey E, Mishra B, Gearhart J. Identification of Elf1, a beta-spectrin, in early mouse liver development. Int J Dev Biol. 1998;42:221–224. [PubMed] [Google Scholar]
- 58.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin a/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. http://dx.doi.org/10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moulik M, Vatta M, Witt SH, Arola AM, Murphy RT, McKenna WJ, Boriek AM, Oka K, Labeit S, Bowles NE, Arimura T, Kimura A, Towbin JA. Ankrd1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54:325–333. doi: 10.1016/j.jacc.2009.02.076. http://dx.doi.org/10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 61.Stroud MJ, Banerjee I, Veevers J, Chen J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ Res. 2014;114:538–548. doi: 10.1161/CIRCRESAHA.114.301236. http://dx.doi.org/10.1161/CIRCRESAHA.114.301236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim JA, Baek HJ, Jang MS, Choi EK, Lee YM, Lee SJ, Lim SC, Kim JY, Kim TH, Kim HS, Mishra L, Kim SS. Loss of beta2-spectrin prevents cardiomyocyte differentiation and heart development. Cardiovasc Res. 2014;101:39–47. doi: 10.1093/cvr/cvt222. http://dx.doi.org/10.1093/cvr/cvt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. http://dx.doi.org/10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins PM, Vasavda C, Hostettler J, Davis JQ, Abdi K, Bennett V. E-cadherin polarity is determined by a multifunction motif mediating lateral membrane retention through ankyrin-G and apical-lateral transcytosis through clathrin. J Biol Chem. 2013;288:14018–14031. doi: 10.1074/jbc.M113.454439. http://dx.doi.org/10.1074/jbc.M113.454439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sikorski AF, Terlecki G, Zagon IS, Goodman SR. Synapsin I-mediated interaction of brain spectrin with synaptic vesicles. J Cell Biol. 1991;114:313–318. doi: 10.1083/jcb.114.2.313. http://dx.doi.org/10.1083/jcb.114.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sikorski AF, Sangerman J, Goodman SR, Critz SD. Spectrin (betaspiisigma1) is an essential component of synaptic transmission. Brain Res. 2000;852:161–166. doi: 10.1016/s0006-8993(99)02253-2. http://dx.doi.org/10.1016/S0006-8993(99)02253-2. [DOI] [PubMed] [Google Scholar]
- 67.Susuki K, Rasband MN. Spectrin and ankyrin-based cytoskeletons at polarized domains in myelinated axons. Exp Biol Med (Maywood) 2008;233:394–400. doi: 10.3181/0709-MR-243. http://dx.doi.org/10.3181/0709-MR-243. [DOI] [PubMed] [Google Scholar]
- 68.Zhang C, Susuki K, Zollinger DR, Dupree JL, Rasband MN. Membrane domain organization of myelinated axons requires betaII spectrin. J Cell Biol. 2013;203:437–443. doi: 10.1083/jcb.201308116. http://dx.doi.org/10.1083/jcb.201308116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. http://dx.doi.org/10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HJ, Lee K, Im H. Alpha-synuclein modulates neurite outgrowth by interacting with Sptbn1. Biochem Biophys Res Commun. 2012;424:497–502. doi: 10.1016/j.bbrc.2012.06.143. http://dx.doi.org/10.1016/j.bbrc.2012.06.143. [DOI] [PubMed] [Google Scholar]
- 71.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. http://dx.doi.org/10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 72.Arthur HM, Bamforth SD. Tgfbeta signaling and congenital heart disease: insights from mouse studies. Birth Defects Res A Clin Mol Teratol. 2011;91:423–434. doi: 10.1002/bdra.20794. http://dx.doi.org/10.1002/bdra.20794. [DOI] [PubMed] [Google Scholar]
- 73.Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. http://dx.doi.org/10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thenappan A, Shukla V, Abdul Khalek FJ, Li Y, Shetty K, Liu P, Li L, Johnson RL, Johnson L, Mishra L. Loss of transforming growth factor beta adaptor protein beta-2 spectrin leads to delayed liver regeneration in mice. Hepatology. 2011;53:1641–1650. doi: 10.1002/hep.24111. http://dx.doi.org/10.1002/hep.24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piepenhagen PA, Nelson WJ. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell. 1998;9:3161–3177. doi: 10.1091/mbc.9.11.3161. http://dx.doi.org/10.1091/mbc.9.11.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-Atpase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989;108:455–465. doi: 10.1083/jcb.108.2.455. http://dx.doi.org/10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baek HJ, Lim SC, Kitisin K, Jogunoori W, Tang Y, Marshall MB, Mishra B, Kim TH, Cho KH, Kim SS, Mishra L. Hepatocellular cancer arises from loss of transforming growth factor beta signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128–1137. doi: 10.1002/hep.22460. http://dx.doi.org/10.1002/hep.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in Elf beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. http://dx.doi.org/10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 79.Yao ZX, Jogunoori W, Choufani S, Rashid A, Blake T, Yao W, Kreishman P, Amin R, Sidawy AA, Evans SR, Finegold M, Reddy EP, Mishra B, Weksberg R, Kumar R, Mishra L. Epigenetic silencing of beta-spectrin, a Tgf-beta signaling/scaflolding protein in a human cancer stem cell disorder: Beckwith-Wiedemann Syndrome. J Biol Chem. 2010;285:36112–36120. doi: 10.1074/jbc.M110.162347. http://dx.doi.org/10.1074/jbc.M110.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaprielian RR, Severs NJ. Dystrophin and the cardiomyocyte membrane cytoskeleton in the healthy and failing heart. Heart Fail Rev. 2000;5:221–238. doi: 10.1023/A:1009805419285. http://dx.doi.org/10.1023/A:1009805419285. [DOI] [PubMed] [Google Scholar]
- 81.Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. 2011;4:67–76. doi: 10.1161/CIRCIMAGING.110.960740. http://dx.doi.org/10.1161/CIRCIMAGING.110.960740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. http://dx.doi.org/10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. http://dx.doi.org/10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ipsaro JJ, Mondragon A. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. doi: 10.1182/blood-2009-11-255604. http://dx.doi.org/10.1182/blood-2009-11-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, Karck M, Schott JJ, Probst V, Le Marec H, Anderson ME, Dobrev D, Wehrens XH, Mohler PJ. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation. 2011;124:1212–1222. doi: 10.1161/CIRCULATIONAHA.111.023986. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, Wang G, Skapura D, Voigt N, Dobrev D, Wehrens XH. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res. 2014;103:178–187. doi: 10.1093/cvr/cvu123. http://dx.doi.org/10.1093/cvr/cvu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leto TL, Fortugno-Erikson D, David Barton TL, Yang-Feng U, Francke Alan Harris, Morrow JS, Marchesi VT, Jr, Benz Edward. Comparison of Nonerythroid A-Spectrin Genes Reveals Strict Homology among Diverse Species. 1988;8:1–9. doi: 10.1128/mcb.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glenney JR, Jr, Glenney P. Comparison of spectrin isolated from erythroid and non-erythroid sources. Eur J Biochem. 1984;144:529–539. doi: 10.1111/j.1432-1033.1984.tb08498.x. http://dx.doi.org/10.1111/j.1432-1033.1984.tb08498.x. [DOI] [PubMed] [Google Scholar]
- 90.Moon RT, Lazarides E. Biogenesis of the avian erythroid membrane skeleton: receptor-mediated assembly and stabilization of ankyrin (goblin) and spectrin. J Cell Biol. 1984;98:1899–1904. doi: 10.1083/jcb.98.5.1899. http://dx.doi.org/10.1083/jcb.98.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blikstad I, Nelson WJ, Moon RT, Lazarides E. Synthesis and assembly of spectrin during avian erythropoiesis: stoichiometric assembly but unequal synthesis of alpha and beta spectrin. Cell. 1983;32:1081–1091. doi: 10.1016/0092-8674(83)90292-1. http://dx.doi.org/10.1016/0092-8674(83)90292-1. [DOI] [PubMed] [Google Scholar]
- 92.Woods CM, Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985;40:959–969. doi: 10.1016/0092-8674(85)90356-3. http://dx.doi.org/10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]
- 93.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. http://dx.doi.org/10.1161/01.RES.86.8.846. [DOI] [PubMed] [Google Scholar]
- 94.Kashef F, Li J, Wright P, Snyder J, Suliman F, Kilic A, Higgins RS, Anderson ME, Binkley PF, Hund TJ, Mohler PJ. Ankyrin-B protein in heart failure: identification of a new component of metazoan cardioprotection. J Biol Chem. 2012;287:30268–30281. doi: 10.1074/jbc.M112.368415. http://dx.doi.org/10.1074/jbc.M112.368415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lofvenberg L, Backman L. Calpain-induced proteolysis of beta-spectrins. FEBS Lett. 1999;443:89–92. doi: 10.1016/s0014-5793(98)01697-4. http://dx.doi.org/10.1016/S0014-5793(98)01697-4. [DOI] [PubMed] [Google Scholar]
- 96.Glantz SB, Cianci CD, Iyer R, Pradhan D, Wang KK, Morrow JS. Sequential degradation of alphaII and betaII spectrin by calpain in glutamate or maitotoxin-stimulated cells. Biochemistry. 2007;46:502–513. doi: 10.1021/bi061504y. http://dx.doi.org/10.1021/bi061504y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shukla M, Rajgopal Y, Babu PP. Activation of calpains, calpastatin and spectrin cleavage in the brain during the pathology of fatal murine cerebral malaria. Neurochem Int. 2006;48:108–113. doi: 10.1016/j.neuint.2005.09.001. http://dx.doi.org/10.1016/j.neuint.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 98.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. http://dx.doi.org/10.1161/01.CIR.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 99.Shah AM. Parsing the role of NADPH oxidase enzymes and reactive oxygen species in heart failure. Circulation. 2015;131:602–604. doi: 10.1161/CIRCULATIONAHA.115.014906. http://dx.doi.org/10.1161/CIRCULATIONAHA.115.014906. [DOI] [PubMed] [Google Scholar]
- 100.Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. http://dx.doi.org/10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K Atpase, Na/ca exchanger, and Insp3 receptor in a cardiac T-tubule/Sr microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. http://dx.doi.org/10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004;279:12980–12987. doi: 10.1074/jbc.M313979200. http://dx.doi.org/10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 103.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel Pka phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. http://dx.doi.org/10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, Uchinoumi H, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca(2+) release in heart failure. Cardiovasc Res. 2010;87:609–617. doi: 10.1093/cvr/cvq108. http://dx.doi.org/10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. http://dx.doi.org/10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. http://dx.doi.org/10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. http://dx.doi.org/10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. http://dx.doi.org/10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 110.Liu N, Denegri M, Dun W, Boncompagni S, Lodola F, Protasi F, Napolitano C, Boyden PA, Priori SG. Abnormal propagation of calcium waves and ultra-structural remodeling in recessive catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;113:142–152. doi: 10.1161/CIRCRESAHA.113.301783. http://dx.doi.org/10.1161/CIRCRESAHA.113.301783. [DOI] [PubMed] [Google Scholar]
- 111.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-Qt cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. http://dx.doi.org/10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 112.Schober ME, Requena DF, Davis LJ, Metzger RR, Bennett KS, Morita D, Niedzwecki C, Yang Z, Wang KK. Alpha II spectrin breakdown products in immature Sprague Dawley rat hippocampus and cortex after traumatic brain injury. Brain Res. 2014;1574:105–112. doi: 10.1016/j.brainres.2014.05.046. http://dx.doi.org/10.1016/j.brainres.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mondello S, Robicsek SA, Gabrielli A, Brophy GM, Papa L, Tepas J, Robertson C, Buki A, Scharf D, Jixiang M, Akinyi L, Muller U, Wang KK, Hayes RL. AlphaII-spectrin breakdown products (Sbdps): diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. http://dx.doi.org/10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pineda JA, Lewis SB, Valadka AB, Papa L, Hannay HJ, Heaton SC, Demery JA, Liu MC, Aikman JM, Akle V, Brophy GM, Tepas JJ, Wang KK, Robertson CS, Hayes RL. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. http://dx.doi.org/10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 115.Jain P, Spaeder MC, Donofrio MT, Sinha P, Jonas RA, Levy RJ. Detection of alpha II-spectrin breakdown products in the serum of neonates with congenital heart disease*. Pediatr Crit Care Med. 2014;15:229–235. doi: 10.1097/PCC.0000000000000059. http://dx.doi.org/10.1097/PCC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, Cadet JL. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66:118–127. doi: 10.1016/j.biopsych.2009.02.021. http://dx.doi.org/10.1016/j.biopsych.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pike BR, Flint J, Dave JR, Lu XC, Wang KK, Tortella FC, Hayes RL. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. http://dx.doi.org/10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- 118.Zhao X, Newcomb JK, Pike BR, Wang KK, d’Avella D, Hayes RL. Novel characteristics of glutamate-induced cell death in primary septohippocampal cultures: relationship to calpain and caspase-3 protease activation. J Cereb Blood Flow Metab. 2000;20:550–562. doi: 10.1097/00004647-200003000-00014. http://dx.doi.org/10.1097/00004647-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 119.Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (Cpp32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. http://dx.doi.org/10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- 120.Yan XX, Jeromin A, Jeromin A. Spectrin breakdown products (Sbdps) as potential biomarkers for neurodegenerative diseases. Curr Transl Geriatr Exp Gerontol Rep. 2012;1:85–93. doi: 10.1007/s13670-012-0009-2. http://dx.doi.org/10.1007/s13670-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z, Larner SF, Liu MC, Zheng W, Hayes RL, Wang KK. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–1298. doi: 10.1007/s10495-009-0405-z. http://dx.doi.org/10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]
- 122.Weiss ES, Wang KK, Allen JG, Blue ME, Nwakanma LU, Liu MC, Lange MS, Berrong J, Wilson MA, Gott VL, Troncoso JC, Hayes RL, Johnston MV, Baumgartner WA. Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest. Ann Thorac Surg. 2009;88:543–550. doi: 10.1016/j.athoracsur.2009.04.016. http://dx.doi.org/10.1016/j.athoracsur.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobeissy FH, Liu MC, Yang Z, Zhang Z, Zheng W, Glushakova O, Mondello S, Anagli J, Hayes RL, Wang KK. Degradation of betaII-spectrin protein by calpain-2 and caspase-3 under neurotoxic and traumatic brain injury conditions. Mol Neurobiol. 2015;52:696–709. doi: 10.1007/s12035-014-8898-z. http://dx.doi.org/10.1007/s12035-014-8898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farkas O, Polgar B, Szekeres-Bartho J, Doczi T, Povlishock JT, Buki A. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—preliminary observations. Acta Neurochir. 2005;147:855–861. doi: 10.1007/s00701-005-0559-6. http://dx.doi.org/10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- 125.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Committee American Heart Association Statistics, and Subcommittee Stroke Statistics, Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. http://dx.doi.org/10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]