Abstract
Higher-order cognitive training has shown to enhance performance in older adults, but the neural mechanisms underlying performance enhancement have yet to be fully disambiguated. This randomized trial examined changes in processing speed and processing speed-related neural activity in older participants (57–71 years of age) who underwent cognitive training (CT, N=12) compared to wait-listed (WLC, N=15) or exercise-training active (AC, N=14) controls. The cognitive training taught cognitive control functions of strategic attention, integrative reasoning, and innovation over 12 weeks. All three groups worked through an fMRI processing speed task during three sessions (baseline, mid-training, and post-training). Although all groups showed faster reaction times (RT) across sessions, the CT group showed a significant increase and the WLC and AC groups showed significant decreases across sessions in the association between RT and BOLD signal-change within left prefrontal cortex (PFC). Thus, cognitive training led to a change in processing speed-related neural activity where faster processing speed was associated with reduced PFC activation, fitting previously identified neural efficiency profiles.
ClinicalTrials.gov, NCT# 00977418
Keywords: Aging, Cognitive Training, Reasoning, Processing Speed, Neuroplasticity, fMRI, DSVT
1. Introduction
Across a range of cognitive functions, cognitive decline in normal aging has been shown to begin relatively early and progress more rapidly with advancing age (e.g., Baltes & Lindenberger, 1997; Park et al., 2002; Salthouse, 1991, 2009). Evidence is emerging, however, suggesting that higher-order cognitive training and the transfer of cognitive training to lower-order cognitive functions (e.g., Anand et al., 2011; Basak et al., 2008) can mitigate senescence-related cognitive declines. The potential transfer of higher-order cognitive training to lower-order cognitive functions raises the possibility that higher-order cognitive training might benefit either lower-order supporting cognitive functions or mediating higher-order common functions.
Processing speed has been shown to underlie and mediate senescence-related decreases in a host of higher-order cognitive processes (Earles & Salthouse, 1995; Salthouse, 1992, 1996). Measures of processing speed have been designed to be simple enough to minimize the influence of memory and strategy on performance but complex enough to assess more than mere sensorimotor function, with processing speed indexed by the time taken to correctly make perceptual/cognitive decisions (Buckhalt, 1991; Salthouse, 1992) and the total number of correct decisions made within a limited amount of time (Ekstrom, French, & Harman, 1979; Wechsler, 2008). Senescence-related declines in higher-order cognitive functions have been proposed to result from cascading failures originating in lower-order operations slowing overall processing speed (Jensen, 1992; Salthouse, 1996). The proposed essential role of processing speed in senescence-related change in higher-order cognitive functions (Salthouse, 1996) and previously shown transfer of higher-order cognitive training to supporting lower-order cognitive functions (Baniqued et al., 2015; Basak et al., 2008; Mudar et al., 2016; Motes et al., 2014; Vas et al., 2016, Venza et al., 2016), however, raises the possibility that effective forms of higher-order cognitive training might affect processing speed in the elderly.
Although research suggests that failure cascades in lower-order processes contribute to senescence-related declines in processing speed and higher-order cognitive functions (Salthouse, 1996), fMRI research suggests that prefrontal cortex (PFC) resources can be used by older adults to compensate for lower-order processing failures which increase processing speed (Motes, Biswal, & Rypma, 2011; Rypma & D’Esposito, 2000). For example, faster working memory retrieval has been associated with lower PFC activation in younger adults but higher PFC activation in older adults (Rypma & D’Esposito, 2000). Furthermore, on a computer-adapted measure of Digit-Symbol Coding (Wechsler, 2008), a measure of processing speed, faster processing speed has been associated with reduced PFC activation for younger adults but greater PFC activation for older adults (Motes et al., 2011). The results from these studies suggest that faster processing speed in younger adults is associated with reduced involvement of PFC; whereas faster processing speed in older adults is associated with greater involvement of PFC, suggesting that faster processing speed among older adults requires the use of PFC resources to compensate for lower-order processing failures.
In addition to comparing age-differences in processing speed-related neural activity, intra-individual dynamics in processing speed-related neural activity has been investigated using fMRI (Rao, Motes, & Rypma, 2014). Examination of associations between reaction time (RT) and fMRI blood-level oxygen dependent (BOLD) signal-change across trials when completing a computer-adapted version of Digit Symbol Coding (Wechsler, 2008) has revealed positive correlations. Specifically, faster trial RTs were associated with reduced BOLD signal-change, within medial and lateral PFC, parietal, occipital, subcortical, and insula brain regions. Thus, across a broad set of brain regions, including PFC, faster processing speed has been associated with reduced brain activation, suggesting that minimal PFC recruitment is required when subprocesses, mediated by other brain regions, operate efficiently in handling cognitive demands.
The present fMRI study examined the effects of a higher-order cognitive training program, the Strategic Memory Advanced Reasoning Training (SMART©) program (Chapman et al., 2016; Vas et al., 2015) on processing speed-related neural activity in older adults. Participants in cognitive training (CT), wait-listed control (WLC), and physical exercise active control (AC) groups worked through the previously used computer-adapted version of Digit-Symbol Coding (Wechsler, 2008), that is, the Digit-Symbol Verification Task (DSVT; Biswal, Eldreth, Motes, & Rypma, 2010; Motes et al., 2011; Rao et al., 2014; Rypma et al., 2006), while fMRI data were collected during three assessment sessions: pre-training, mid-training and post-training for the CT and AC groups and comparable durations for the WLC group. Performance on the DSVT has been shown to correlate with Digit-Symbol Coding performance (DSVT RT r = −.48, p < .05, and DSVT proportion correct r = .35, p < .05; Rypma, et al., 2006). Additionally, other computerized measures of processing speed similar to the DSVT have been shown to correlate with standardized measures of processing speed (.35 < r < .90) and age (.35 < r < .60) and to account for age-related variance in a host of measures of cognitive abilities (Salthouse, 1996). The correlations provide convergent validation for DSVT and similar computerized tasks as measures of processing speed.
Prior work supports the potential for higher-order cognitive training and SMART (Chapman et al., 2016; Vas et al., 2015), in particular, to be linked to changes in lower-order processes. SMART teaches cognitive control strategies that require integrative processes as well as specialized component processes. The training has been shown to facilitate focused learning, deeper encoding of meaning, and the flexible ability to derive a multitude of interpretations and solutions (Chapman et al., 2015, 2016). Additionally, findings have shown transfer of SMART to lower-order processes, including inhibition, nonverbal reasoning, working memory, immediate and delayed memory, and switching (Mudar et al., 2016; Motes et al., 2014; Vas et al., 2016, Venza et al., 2016), in both healthy and patient samples. Other forms of higher-order cognitive training also have been shown to transfer to executive processes (Basak, Boot, Vas, & Kramer, 2008) and even processing speed (Baniqued et al., 2015; but see Mackey, Hill, Stone, & Bunge, 2011). The distal cognitive functions to which SMART previously has been shown to transfer, particularly, executive processes (Anand et al., 2011; Motes et al., 2014; Vas et al., 2011), also have been shown to be associated with processing speed (e.g., Jensen, 1992; Kail, 1991; Salthouse, 1996) and associated with PFC structure and function (e.g., Alvarez & Emory, 2006; Buchsbaum, Greer, Chang, & Berman, 2005; Motes & Rypma, 2010; Rypma & D’Esposito, 2000; Yuan & Raz, 2014).
Although research has shown that higher-order cognitive training in older adults can transfer to lower-order processes, no known study has examined transfer to processing speed-related neural activity in a randomized trial. Thus, the present study examined the potential for higher-order cognitive training, as represented by SMART, to affect neural mechanisms underlying processing speed. In particular, based on prior evidence that SMART and other forms of higher-order cognitive training seem to recruit and strengthen PFC-mediated executive functions and that PFC function has been shown to mediate age-related change in processing speed, the study allowed for testing the prediction that higher-order cognitive training would transfer to processing speed-related neural activity within PFC. Aerobic exercise training served as an AC condition for the study in that aerobic exercise training has been shown to lead to improvements in processing speed (Colcombe & Kramer, 2003; Smith et al., 2010; but see Young, Angevaren, Rusted, & Tabet, 2015) and functional changes within frontal and other brain regions (Hillman, Erikson, & Kramer, 2008; Voelcker-Rehage & Niemann, 2013), including, BOLD signal-change increases within PFC while working on a computer-adapted measure of Digit-Symbol Coding (Rosano et al., 2010).
2. Material and Methods
2.1. Participants
A total of 57 cognitively normal older adults (M= 63.2; 56–71 years of age) were randomly assigned to a higher-order CT (n=19), WLC (n=19), or AC (n=19) group. All participants underwent (see summaries below) Telephone Interview of Cognitive Status-Modified (TICS-M) to prescreen for dementia, Montreal Cognitive Assessment (MoCA) to detect early cognitive impairment in person, Beck Depression Inventory-II (BDI) to screen for depressive symptoms, and complete medical, physical, and laboratory assessments by a physician to ensure good general health. Inclusion criteria included: no history of neurological or psychiatric conditions, IQ within the normal range, English as the native language, and minimum of a high school education. Exclusionary criteria included: MR scanning contraindications, cognitive status impairment (TICS-M < 28 and MoCA < 26), depression indication (BDI > 14), left-handedness, elevated body mass (BMI>40, ), abnormal electrocardiographic response, significant hypertensive blood pressure response to exercise, or inability to reach 85% of age-related maximum predicted heart rate. Additionally, participants were excluded if they reported regular aerobic activity of more than twice a week for 20 min or more or if they reported exercising regularly for at least 3 months prior to enrolling in the study. The experiment was conducted according to the principles expressed in the Declaration of Helsinki, and written informed consent was obtained from all subjects in accordance with the Institutional Review Board (IRB) of our academic institutions: The University of Texas at Dallas and the University of Texas Southwestern Medical Center. During consenting, participants were told that they would be randomly assigned to receive cognitive training following the baseline assessment session or to be given the opportunity to receive CT following the third assessment session (i.e., be in the WLC or AC groups).
Although all participants completed the cognitive measurements at the baseline assessment session, some participants in the groups either did not complete all assessment sessions (CT n=4; WLC n=1; AC n=3), had high motion during scanning session (> 3mm and > 3°; CT n=2; WLC n=2; AC n=0), or poor normalization in preprocessing (CT n=1; WLC n=1; AC n=2). As a result, the final behavioral and MRI data analyses were conducted on 12 (6 females) participants in CT group, 15 (11 females) in WLC group, and 14 (10 females) in the AC group. No significant between-group differences were noted in any of the demographic or cognitive screening measures (all ps>0.08).
2.2. Procedure
2.2.1. Strategic Memory Advance Reasoning Training (SMART) Program
The cognitive training was delivered by a trained instructor in small groups (n≤5) of one 1-h session per week for 12 weeks (hours = 12). Initially, the SMART strategies (Table 2) were overviewed, with an emphasis on the use of the strategies on mental tasks throughout one’s daily routine. The sessions then focused on the use of strategic attention, integrative reasoning and innovation cognitive control functions (Table 2 Function) via integrated practice with a wide range of everyday type tasks, such as, reading newspaper articles, conversing about movies or discussing investments with a financial planner (Chapman, 2014). Overall, SMART teaches metacognitive strategies (a) to enable better time and cognitive resource management by prioritizing goal setting, blocking distractions and inhibiting irrelevant information, (b) to engage deeper level synthesis of incoming information by “boiling the meaning down to its essence,” and (c) to encourage fluid and flexible thinking (Chapman et al., 2015, 2016).
Table 2.
SMART strategies emphasized over 12 sessions.
| Sessions | Function | Strategy | Illustration |
|---|---|---|---|
| 1–3 | Strategic attention: Reduce the load of incoming details by inhibiting less relevant information | Filter/single - task/mental breaks | Tackle mental tasks without distractions, set fixed time (~30 min) to focus attention while consciously blocking extraneous stimulation. Example: Read article, and delete “unimportant” information. |
|
| |||
| 4–5 | Integrative Reasoning: Combine ideas to form condensed meanings | Synthesize | Create synthesized abstracted meanings |
| Interpret in a broader context | Zoom Out | Devise broader viewpoints/solutions based on acquired new knowledge | |
| Apply to real life contexts | Zoom Deep & Wide | Construct interpretive messages of application to current contexts/problems. Example: Write sentence that synthesizes important information in an article into one’s own words. Give an alternative interpretation and application to a current context |
|
|
| |||
| 6–8 | Innovation: Derive multiple ways to approach mental tasks and minimize fear of failure or unknown | Infinite | Fluidly generate a multitude of alternative solutions/perspectives |
| 9–10 | Paradox/Unknown | Identify daily low performance on tasks and find ways to push new approaches; seek new tasks/contexts/frontiers Example: Given a problem and solution or scenario and outcome, create alternative solutions or paths to alternative outcomes. Consider ways to encourage thinking of and using the alternatives. |
|
|
| |||
| 11–12 | Booster Sessions | Review Strategies | Discuss applications of strategies to real life scenarios. |
In addition to the once a week group session, participants also completed personally selected homework assignments related to each session. These homework activities involved two additional 1-h sessions per week for 12 weeks (total hours logged = 24). Record logs of time and notation of assignment completion were kept for the homework to chart compliance. The amount of time spent on homework varied across participants, and although all completed the homework, not all participants required investing the full 24 hours. Trainers provided feedback to reinforce understanding and utilization of the strategies.
2.2.2 Active Control Exercise
The active control physical exercise program trained participants to meet 2008 physical activity guidelines of 150 min per week. Training consisted of three aerobic exercise training session per week, 60 minutes each, over 12 weeks. Aerobic exercise alternated between exercise bike and treadmill sessions. Biking sessions consisted of a 5 min warm up at 43 watts, cycling for 50 min at a speed that increased their heart rate to 50–75% of their maximum achieved heart rate on VO2max testing, and a 5 min cool down at 43 watts. Treadmill sessions consisted 5 min warm up at 2 mph, walking on treadmill for 50 min at a speed that increased their heart rate to 50–75% of their maximum achieved heart rate on VO2max testing, and a 5 min cool down at 2 mph. An exercise physiologist and a nurse practitioner assessed whether participants reached their target heart rate at each session.
2.2.3. Test of Strategic Learning as a Proximal Assessment of Cognitive Training
The Test of Strategic Learning (TOSL) served as a proximal assessment of SMART (Chapman et al., 2015; Chapman & Mudar, 2014; Vas et al., 2016). TOSL was developed to assess the ability to synthesize abstract meanings from complex information. Participants read complex passage (approximately 600 words) and were then instructed to generate a high-level summary of the text. A TOSL Abstraction Score was computed based on a manualized objective scoring system where each abstracted idea in the summary received one point and verbatim or paraphrased ideas did not receive any points. The final Abstraction Score then reflected the total number of accurately abstracted meanings from the text. Three different versions of the TOSL were constructed and administered at the three assessment periods in a counter-balanced order across participants.
2.2.4. Processing Speed Task
Participants completed the Digit Symbol Verification Task (DSVT; Rypma, et al., 2006) as a measure of processing speed while in the scanner. A table containing nine digit-symbol pairs and a single digit-symbol probe (Figure 1) appeared simultaneously on each trial for 3.5 s, and participants had full 3.5 s to indicate whether the probe-pair matched a symbol-number pair in the key or not. The symbols paired with the nine digits in the key varied across trials to discourage the use of memory-based strategies, and the symbol paired with the probe number and the probe number itself also varied across trials. There were 52 trials all completed in one scanning run. The probe-pair matched one of the pairs in the key on half of the trials, and the probe-pair did not match one of the pairs in the key on the other half of the trials. On trials when the probe pair did not match a pair in the key, the probe symbol was present in the key, but it was paired with a different number than in the probe-pair. Inter-stimulus intervals varied from .5 to 16.5 s, constituting baseline, rest periods in the jittered, rapid, event-related design. RT, as an index of processing speed, was measured from the onset of a trial to the time of the response (right thumb button-press “yes” and left for “no”). Three versions of the task were constructed with the trial order randomized across the versions.
Figure 1.
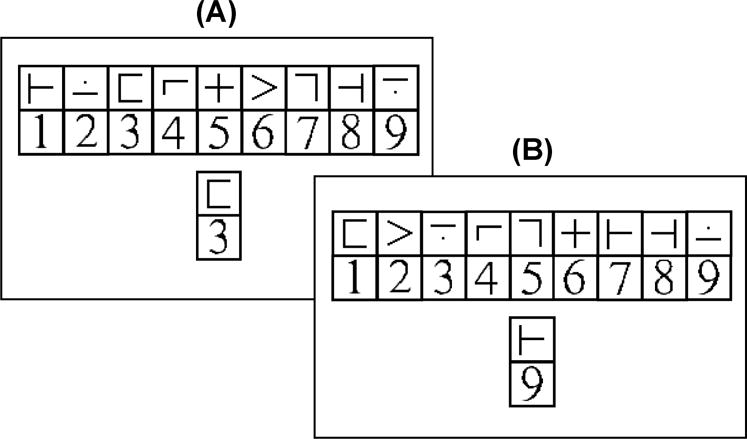
Example of stimuli from the Digit-Symbol Verification Task. Participants in the present study completed the digit-symbol verification task while fMRI data were collected. On each trial, a key containing nine digit-symbol pairs and a single digit-symbol probe-pair appeared simultaneously for 3.5 s, and participants were to judge whether the probe-pair was in the key (A) or not (B). The digit-symbol pairings in the key and the probe-pair varied across trials. On half of the trials, the probe-pair matched a digit-symbol pair in the key, and on half of the trials, the probe-pair did not match a digit-symbol pair in the key. There were 52 trials in a run, and inter-stimulus intervals varied from .5 to 16.5 s.
The rear-projected trials were viewed by the participants using an angled mirror sitting above the receiving coil (≈12 cm above the participant’s eye). Black key and probe symbols and digits each appeared within white squares on a black background. Each symbol or number square measured 0.40 × 0.40 cm at the mirror (≈ 1.95° visual angle), with the full key measuring approximately 4.00 × 0.85 cm (≈ 18.6° × 4.05° visual angle) and the top of the key to the bottom of the probe measuring 1.75 cm (≈ 8.3° visual angle).
2.2.5. Neuroimaging Acquisition Parameters and Data Processing Pipeline
Imaging data were collected at three assessment sessions: baseline, mid (6-weeks for the CT group), and post (12-weeks for the CT group). The imaging data were collected on a Philips Achieva 3T scanner equipped with an 8-element, SENSE, receive-only head coil. High-resolution anatomical images (MPRAGE; 1 mm3; sagittal; TE = 3.7 ms; flip angle = 12°) and functional images using EPI (voxel = 3.5 × 3.5 × 4 mm; 36 slices/volume; 150 volumes/run; TR = 2000 ms, TE = 30 ms; flip angle = 70°; matrix = 64×64; axial; inferior to superior interleaved) were collected. Six discarded scans occurred at the beginning of the functional run to remove T1 saturation effects.
Previous research on the present sample of participants showed cognitive training related increases in global and regional resting-state cerebral blood flow (CBF; Chapman et al., 2015) with the increases linked to improved executive function. Additionally, age-related reductions in cerebrovascular reactivity (CVR) have been observed in PFC (Lu et al., 2010; Yezhuvath et al., 2012), and CVR has been shown to be associated with BOLD signal-change on the DSVT (Kannurpatti, Motes, Rypma, & Biswal, 2011). During the imaging session, pseudo-continuous arterial spin labeling (pCASL) (Aslan, et al., 2010) and cerebrovascular reactivity data (CVR) (Yezhuvath, et al., 2012), based on hypercapnia using BOLD fMRI, were also collected during resting-state runs separate from the DSVT fMRI run. These data allowed for assessing whether training-related fMRI changes were associated with possible training-related changes in resting blood flow and vascular reactivity. Imaging parameters for pCASL experiments were: single-shot gradient-echo EPI, field-of-view (FOV)=240×240, matrix=80×80, voxel size=3×3 mm2, 27 slices acquired in ascending order, slice thickness=5 mm, no gap between slices, labeling duration=1650 ms, time interval between consecutive slice acquisitions=35.5 ms, TR/TE=4020/14 ms, SENSE factor 2.5, number of controls/labels=30 pairs, RF duration=0.5 ms, pause between RF pulses=0.5 ms, labeling pulse flip angle=18°, bandwidth=2.7 kHz, echo train length=35, and scan duration 4.5 minutes. The hypercapnia BOLD imaging parameters were: single shot gradient echo EPI sequence, TR/TE/flip=2000ms/25ms/80°, 43 axial slices, slice thickness=3.5 mm, FOV=220×220 mm2, matrix size=64×64 and scan duration 7 minutes 18 seconds. Hypercapnia was administered using a Douglas bag with a two-way valve to switch between blocks of 5% CO2-breathing (mixed with 21% O2 and 74% N2) and air-breathing. Physiologic parameters, including end-tidal (Et) CO2, breathing rate, heart rate, and arterial oxygenation (sO2), were recorded during the scan (MEDRAD, Pittsburgh, PA and Novametrix Medical Systems, Wallingford, CT).
The fMRI BOLD data were analyzed using AFNI (Cox, 1996). The data for individual participants were corrected for slice-timing offset and motion. The time series was then spatially smoothed with an iterative Gaussian kernel to a final FWHM smoothness value of 8 mm (based on the residual maps generated following an initial deconvolution). The smoothed time-series data were then deconvolved using voxel-wise linear regression to obtain task-related and RT-related signal-change estimates. A task-related regressor was constructed by convolving a task-reference delta function for correct responses by the hemodynamic response model (HRF, a gamma-variate function; Cohen [1997] parameters b = 8.6, c = 0.547; max amplitude = 1.0). A second regressor was created to obtain RT-related effects (i.e., trial-level processing speed effects). For the RT-related regressor, the task-related regressor was proportionally scaled based on the corresponding trial RT, with
where t=time in the time-series, k=trial for the condition, ak = reaction time for the kth trial, ā =mean reaction time, τk=time of the onset of the kth trial for the condition, and h(t − τk) = t8.6exp(−[t − τk]/0.547). The task-related regressor was then regressed from the RT-scaled model, removing the canonical HRF component and leaving orthogonalized RT-related and task-related regressors for the deconvolution. Thus, regression allowed for obtaining estimates of task-related and RT-related BOLD signal-change from the baseline rest periods for each participant for each assessment session. Nuisance regressors for incorrect responses and long RTs (i.e., RT>2.5 SD from the participant’s mean RT), motion correction parameters, and for linear, quadratic, and cubic trends also were also included in the deconvolution design matrix. At each voxel, the smoothed data were then expressed in terms of percent signal-change relative to the mean (i.e., 100 * yt/My, t = time point), and the final deconvolution was performed on these smoothed, scaled data. The parameter estimate matrices for the task-related and RT-related regressors were spatially normalized to MNI space by first registering the MPRAGE for each participant to an MNI template via a set of linear and nonlinear spatial transformations and then applying the transformation parameters to the task-related and RT-related parameter estimate maps.
The primary aim of the study was to evaluate training-related effects, so separate voxel-wise linear interaction contrasts were used to test for group differences in change from the baseline assessment session in the behavioral data and the task-related and RT-related BOLD signal change parameter estimates, with contrast coding allowing for bi-directional differences. Group status (i.e., CT, WLC, or AC) was a between-groups factor, and assessment session (i.e., baseline, mid, and post) was a within groups factor. Cluster thresholding was used to control family wise error (FWE) rates. Separately for CT versus WLC and AC combined, CT versus WLC, and CT versus AC comparisons, cluster thresholding involved computing residuals for the Group × Assessment Session linear interaction contrast, generating a null distribution by randomizing the signs of the residuals per subject, iteratively repeating t-tests on these residual matrices 10,000 times, and then using the 10,000 matrices to determine false positive probabilities of clusters of a given size with different voxel-wise p-value thresholds (as implemented in 3dClustSim; Cox, Reynolds, & Taylor, 2016). The permutation tests were implemented to address inflated false positive rate concerns raised regarding fMRI studies (Eklund, Nichols, & Knutsson, 2016). Cox et al. showed accurate to slightly conservative false-positive rates using this non-parametric approach to cluster thresholding with degrees of smoothness varying FWHM=4–10 mm, voxel-wise thresholds from p=.01 to p=.001, and for various task designs, including rapid event-related designs like the DSVT. Based on this approach, minimum cluster sizes were k=86 for CT comparisons to the combined WLC and AC groups, k=116 for CT comparisons to the WLC group, and k=126 for CT comparisons to the AC group voxels with a voxel-wise α=.001 to meet a cluster-wise α=.05.
After identifying regions in the BOLD data showing significant Group × Assessment Session linear interaction effects, data from the coordinates of the peak voxel for the Group × Assessment Session interaction effect within the cluster were extracted from each participant’s BOLD, CBF and CVR maps, and tested for both Group × Assessment Session linear interaction effects and for the association of the fMRI BOLD effects with the behavioral data. CBF and CVR maps were generated as follows. The pCASL MRI data underwent routine processing (Aslan, et al., 2010; also see Supplementary Materials S1). PCASL image series were realigned to the first volume for motion correction (SPM’s realign function). All datasets were within the applied motion threshold of 3 mm translation and 3 degrees rotation. An in-house MATLAB (Mathworks, Natick, MA) program was used to calculate the difference between averaged control and label images. Then, the difference image was corrected for imaging slice delay time to yield CBF-weight image, which was normalized to the brain template in standard space using HAMMER. Last, the absolute CBF was estimated in the units of mL blood/min/100g of brain tissue (Aslan, et al., 2010). CVR data analysis followed protocols established previously (Yezhuvath, et al., 2012; also see Supplementary Materials S1). Briefly, BOLD data was motion corrected (two datasets had motion over the selected threshold of 3 mm translation and 3 degrees rotation) and smoothed (6 mm FWHM). A linear regression was performed between the EtCO2 trace (extracted using in-house MATLAB scripts) and the BOLD signal time-course to generate CVR maps in units of %BOLD/mmHg CO2. Normalization of CVR maps to standard space followed a similar pipeline as the CBF data (for details on CBF and CVR calculations, see Chapman et al., 2015, 2016).
3. Results
3.1. Training-Related Change in TOSL Abstraction Score
Training-related change TOSL Abstraction Score served a proximal measure of SMART efficacy. As previously reported on this sample of participants (Chapman et al., 2015), the Group × Assessment Session linear interaction contrast revealed significant group differences in change in TOSL Abstraction over the assessment sessions when comparing the CT group to the combined control groups, t(39)=2.36, p=0.023,1 and to the WLC group, t(25)=2.54, p=0.018, with a marginally significant effect for the comparison to the AC group t(24)=1.84, p=0.078 (Figure 2A). Thus, the increase from baseline in TOSL Abstraction for the CT group significantly differed from the decrease in baseline for the WLC and AC group, providing validation for CT actually training higher-order cognitive processing in the form of abstracting meaning or essential gist from texts.
Figure 2.
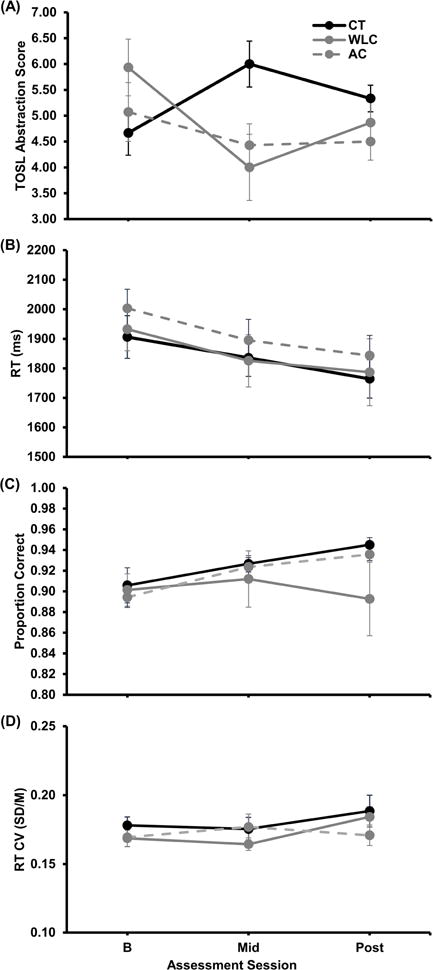
(A) TOSL Abstraction Score and DSVT (B) RT, (C) proportion correct, and (D) RT coefficient of variation (CV) as functions of group and assessment session. For assessment session, B=baseline, Mid=6 weeks into the training or waiting period, and Post=post-training or post-waiting period. Black=Cognitive Training (CT) group, gray=Wait Listed Control (WLC) group, and gray dashed=Active Control (AC) group. Errors bars show SEM.
3.3. Training-Related Change in DSVT performance
Training-related change in RT (for correct responses), accuracy, and RT variability (coefficient of variation, CV, RT SD/M) were examined for transfer of cognitive training to performance on the DSVT (see Figure 2B, 2C, & 2D, respectively). Although the decrease in RT across assessment sessions was significant when averaging over groups, t(40)=−4.70, p<.001, and the increase in proportion correct and RT CV were marginally significant, t(40)= −1.77, p=0.085, and t(40)=1.88, p=0.067, respectively, the group differences in change from baseline were not significant, all combined control group ts(39)<1.00, WLC ts(25)≤1.31, ps≤0.201, and AC ts(24)<1.00.
3.4. Training-Related Change in fMRI BOLD Signal-Change
Task-related signal-change and RT-related signal-change for each group at each assessment session were consistent with previously reported findings (see Supplementary Figure S3.1; Biswal, et al., 2010; Motes et al., 2011; Rao et al., 2014; Rypma, et al., 2006). However, Group × Assessment Session linear interaction contrasts on the RT-related regression coefficients comparing the CT and combined WLC and AC groups (B) revealed a single significant cluster (k=210 voxels; cluster-wise α=.05 requiring k=82 voxels at a voxel-wise Z=3.28 and α=.001, based on the above described non-parametric approach to cluster thresholding to control for false positive rates, Cox et al., 2016) within left PFC (Brodmann Area 9; Figure 3A, statistical parameter map). In separate analyses, contrasts for CT versus WLC and for CT versus AC groups also revealed significant clusters (k=139 and k=126 voxels, respectively; cluster-wise α=.05 requiring k=116 and k=126, respectively, voxels at a voxel-wise Z=3.28 and α=.001) within left PFC (see Figures 3B and 3C, statistical parameter maps, respectively). For the CT versus AC group contrasts, significant differences also were observed bilaterally within cerebellum (see Supplementary Materials S3). Group × Assessment Session interaction contrasts on the task-related BOLD signal-change did not reveal any clusters meeting the FWE cluster thresholds. For the left PFC cluster showing significant Group × Assessment Session effect on the RT-related BOLD signal-change, the increase in the correlation between RT and BOLD signal-change for the CT group significantly differed from the decrease in the correlation for the WLC and AC groups (illustrated in Figure 3A, B, and C, line graph; CT versus combined WLC and AC group peak t[39]=5.19, p<0.001, MNI coordinates −44L 26A 24S; CT versus WLC group peak t[25]=4.65, p<0.001, and AC group peak t[24]=4.65, p<0.001, MNI coordinates −44L 26A 26S for both peaks).2 Thus, across sessions and within left PFC, the CT group showed an increase in the association between trial-level RT and BOLD signal-change, with faster trial RT associated with less BOLD signal-change; whereas the WLC and AC groups showed decreases in the association between trail-level RT and BOLD signal-change.
Figure 3.
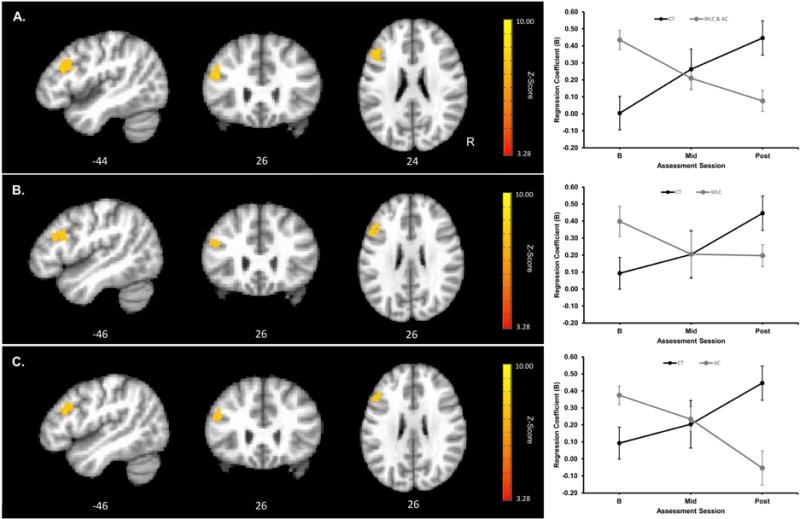
Cluster showing significant Group × Assessment Session interaction contrast of RT-related parameter estimates and mean RT-related parameter estimates as a function of assessment session and group extracted from the peak voxel within the cluster (peak voxel MNI coordinates shown below the images). Upper panel (A) shows a significant cluster in left prefrontal cortex (PFC) for the CT versus combined WLC and AC group contrasts (k=210 voxels; cluster-wise α=.05 requiring k=82 voxels at a voxel-wise Z=3.28 and α=.001); middle panel (B) shows significant cluster in left PFC for the CT versus WLC group contrast (k=139 voxels; cluster-wise α=.05 requiring k=116 voxels at a voxel-wise Z=3.28 and α=.001); and lower panel (C) shows significant cluster in left PFC for the CT versus AC group contrast (k=126 voxels; cluster cluster-wise α=.05 requiring k=126 voxels at a voxel-wise Z=3.28 and α=.001). Image orientations are in neurological convention indicated with R=Right. Red to yellow indicates increasing strength of the Group × Assessment Session interaction contrast. For assessment session, BL=baseline, Mid=6 weeks into the training or waiting period, and Post=post-training or post-waiting period. Black=Cognitive Training (CT) group, gray=combined Wait Listed Control (WLC) and Active Control (AC) group in A, gray=WLC group in B, and gray=AC group in C. Errors bars show SEM.
Covariate Analyses of Training-Related Change in fMRI BOLD Signal-Change
CBF and CVR data were extracted from the peak voxel within the significant left PFC cluster. Some participants in the groups did not have useable CBF (CT, n=1; WLC, n=0; AC, n=0) or CVR (CT, n=2; WLC, n=6; AC, n=4) data. As a result, the analyses were conducted 11 CBF and 10 CVR datasets for participants in the CT group, 15 CBF and 9 CVR in the WLC group, and 14 CBF and 10 CVR in the AC group. Group differences in CBF or CVR change from baseline from the peak voxel for the Group × Assessment Session BOLD interaction effect within the left PFC cluster were not significant. Step-wise, hierarchical linear modeling was used to test for associations between cognitive training related linear change in RT-related BOLD signal change within the left PFC cluster (i.e., data extracted from the peak-voxel within the cluster) and change in CBF within the cluster, CVR within the cluster, RT, RT CV, proportion correct, and TOSL Coherence. In a series of seven separate step-wise, hierarchical linear models, the linear change in RT-related B was regressed on (1) group (dummy coded as combined WLC and AC=0 and CT=1), followed by (2) group and physiological or performance covariate of interest (i.e., change CBF, CVR, RT, RT CV, proportion correct, or TOSL Abstraction), and followed by (3) group, physiological or performance covariate of interest, and a group × covariate interaction term (i.e., Group * Predictor). Significant change in R2 was computed to determine whether the covariate or interaction added to or reduced the Group × Assessment Session linear contrast. However, none of the hierarchical models revealed significant change in R2 from the initial effect of Group on the linear contrast, with the original R2=0.47 (CBF subgroup R2=0.42; CVR subgroup R2=0.48), for all covariates R2change≤0.019, Fchange≤1.37, and all full models with interaction terms R2change≤0.012, Fchange≤0.89. Thus, the cognitive training related linear change RT-related BOLD signal change was not found to be significantly accounted for by change in CBF, CVR, DSVT performance, or TOSL Abstraction.
4. Discussion
The results provided evidence that higher-order cognitive training can affect processing speed-related neural activity within PFC. Within left PFC, Brodmann Area 9, the correlation between RT and BOLD signal-change while working on the DSVT increased across the assessment sessions for the CT group but decreased for the WLC and AC groups. After cognitive training, faster RT for the cognitive training group was associated with lower BOLD signal-change. This post-cognitive training pattern is consistent with previously noted neural efficiency profiles (Motes et al., 2011; Rypma et al., 2006), particularly, intra-individual neural efficiency profiles observed in young adults (Rao et al., 2014). The results suggest that following cognitive training, minimal PFC recruitment was necessary on trials when lower-order processes could handle task demands (i.e., trials in which trained participants were faster) but also suggest that PFC-mediated cognitive functions were available and could be recruited when cascading lower-order process failures were slowing processing speed (i.e., trials in which trained participants were slower).
The observed transfer of higher-order cognitive training to processing speed adds to previously reported findings on higher-order cognitive training effects on PFC function. Reasoning training, for example, has been associated with mean decreased left PFC activation on fMRI when performing other untrained reasoning tasks (Mackey, Miller Singley, Wendelken, & Bunge, 2015), at least among young adults, suggesting more efficient use of PFC resources (i.e., reduced PFC activation) following training. The present study, however, demonstrates cognitive training related transfer to PFC mediation of trial-level processing speed, with faster processing speed across trials (i.e., faster RT) associated with lower PFC activation (i.e., BOLD signal change), rather than a reduction in PFC activation following cognitive training. As noted above, previous research (Motes et al., 2011) has shown that faster processing speed among older adults was associated with greater use of PFC-mediated resources (i.e., greater PFC BOLD signal-change amongst faster older adults than among slower older adults and faster younger adults), suggesting that processing speed for older adults depends on the availability of PFC resources for coordination of sub-process timing and output and to preserve cognitive functions in general (Park & Reuter-Lorenz, 2009; Rypma & Prabhakaran, 2009). However, the results from the present randomized training trial support the notion that cognitive training targeting higher-order cognitive processes can alter PFC involvement in processing speed and may serve to mitigate age-related changes in PFC function, in general.
The transfer of higher-order cognitive training to only PFC mediated processing speed-related neural activity, and not to other brain regions associated with processing speed (Rao et al., 2014), suggests that transfer of higher-order cognitive training to lower-order cognitive functions (Baniqued et al., 2015; Basak et al., 2008; Mudar et al., 2016; Motes et al., 2014; Vas et al., 2016, Venza et al., 2016) might occur through higher-order cognitive training indirectly benefiting mediating higher-order common functions served by PFC rather than indirectly benefiting a host of lower-order supporting functions. Across CT, WLC, and AC groups and assessment periods in the present study (Supplementary Figure 1) and in a study of healthy young adults examining intra-individual dynamics in RT-BOLD association on the DSVT (Rao et al., 2014), faster processing speed was associated with reduced brain activation across a host of brain regions, including medial and lateral PFC, parietal, occipital, subcortical, and insula brain regions. However, in the present study, the observed CT versus WLC and AC group differences in change in intra-individual dynamics in processing speed-related neural activity were confined to left lateral PFC and not other regions associated with processing speed. The localization of cognitive training effects to only PFC suggests that higher-order cognitive training indirectly benefits PFC function in processing speed, and at this point, cognitive training does not appear to show transfer to lower-order supporting functions in processing speed, at least in older adults. Thus, the previously observed transfer of higher-order cognitive training to other lower-order cognitive functions (Baniqued et al., 2015; Basak et al., 2008; Mudar et al., 2016; Motes et al., 2014; Vas et al., 2016, Venza et al., 2016) also might result from cognitive training effects on common functions served by PFC.
Aerobic exercise training served as the AC condition in the present study, because aerobic training previously has been associated with improvements in processing speed (Colcombe & Kramer, 2003; Smith et al., 2010) and functional change within PFC and other frontal regions (Hillman, Erikson, & Kramer, 2008; Voelcker-Rehage & Niemann, 2013). Aerobic exercise, however, did not show processing speed-related neural activity benefits compared to CT in the present cohort. Meta-analyses on the effects of aerobic exercise training on processing speed have shown relatively weak effect-sizes (Hedge’s g=0.27 in Colcombe & Kramer, 2003; Hedge’s g =0.16 Smith et al., 2010), and aerobic exercise training alone (Hedge’s g =0.10) has been shown to produce weaker effects on processing speed than aerobic exercise combined with other training (e.g., strength training or yoga; Hedge’s g =0.25; Smith et al., 2010; see also Colcombe & Kramer, 2003). Further, meta-analysis using more restrictive study inclusion criteria (i.e., randomized controlled trials with a minimum age requirement of 55 years and excluding studies on MCI, dementia, stroke, and depression) failed to show significant effects of aerobic exercise training compared to active or WLC groups (Young et al., 2015). Additionally, meta-analysis assessing association between aerobic fitness and cognitive performance, including processing speed, in older adults failed to show a significant relationship in cross-sectional designs and instead showed that increases in fitness were associated with decreases in cognitive performance in pre-post designs (Etnier, Nowell, Langers, & Sibley, 2006; see also Colcombe & Kramer, 2003). Functional brain imaging studies suggest that exercise interventions up to a year might be required before cognitive changes are observed in the older groups. In a two year follow-up study of a 12-month exercise intervention in elderly participants, that included aerobic training, participants who completed and maintained their regimen after the intervention showed faster processing speed and increased BOLD signal-change within PFC in a computerized measure of Digit-Symbol Coding compared to subjects who remained sedentary following the intervention (Rosano et al., 2010). Additionally, 12 months of aerobic exercise training, but not six months or training or 12 months of nonaerobic stretching, in elderly participants was associated with increased functional connectivity within PFC and parts of the default mode network (Voss et al, 2010). In summary, the present study showed evidence of transfer of higher-order CT, but not aerobic exercise, to processing speed-related neural activity within PFC, raising questions about the efficacy of short-term aerobic exercise to affect processing speed. However, more research is needed, particularly on protocol and participant characteristics, to elucidate the contribution of aerobic exercise on cognition, processing speed, and brain function.
5. Limitations
Whereas a number of studies are beginning to investigate the neural correlates of training in older adults (Brehmer et al., 2011; Heinzel et al., 2016), this investigation represents one of the first attempts to study the effects of higher-order cognitive training on processing speed-related neural activity. This evidence builds on prior findings showing corresponding enhanced cognitive and neural gains with cognitive training training (i.e., SMART) in older adults (Chapman et al., 2015). Nonetheless, the current findings need to be interpreted cautiously in light of a few limitations. First, the sample sizes were relatively small and require replication in larger sample sizes. Still, the pattern of separate trends on processing speed-related neural activity for the CT versus WLC and AC randomized groups offers promise for achieving training-related benefits. Second, although we observed cognitive training related change in processing speed-related neural activity, we did not observe task-related performance differences between the CT, WLC, and AC groups. There might be limits to performance on the DSVT that prevented detecting training-related change, and given the improvement in the WLC group, there are clearly practice effects. Third, the population was homogeneous in relative high educational attainment, and the results may not generalize to samples consisting of adults with more varied educational backgrounds. The homogenization of educational achievement, however, might have restricted the range of processing speed variability in the present sample, and thus, the effects observed in the present study might be stronger in a more varied sample. Fourth, the cognitive training related change in processing speed-PFC activation profiles was not correlated with change in TOSL Abstraction, the most proximal measure to the cognitive training. With the additional processes required for TOSL Abstraction compared DSVT processing speed performance, however, weaker correlations would be expected between the two measures, and therefore, larger sample sizes might be required. Finally, there was an imbalance the proportion of males to females across groups (CT Females=6, Males=6; WLC Females=11, Males=4; AC Females=4, Males=10). However, the difference was not statistically significant, X2(N=41)=1.91, p=0.385, and previous studies on SMART in adults did not show sex differences.
Conclusions
In sum, the present results suggest that higher-order cognitive training in older adults can lead to PFC mediation of processing speed. Similar to previous observations in younger adults (Rao et al., 2014), after higher-order cognitive training, faster processing speed among older adults was associated with lower PFC activation. These results suggest that cognitive training can lead to improvement in PFC-mediated coordination of sub-process timing and output. Furthermore, evidence of increased PFC-mediation of processing speed with higher-order cognitive training raises the possibility that such cognitive training might also lead to general increases in PFC function and resource availability and thus increases in a broad spectrum of neuro-cognitive functions in the elderly and across the lifespan. Increased PFC-mediation of processing speed and resource availability might be mechanisms contributing to the previously shown transfer of higher-order cognitive training to improvements in executive functions (Anand et al., 2011; Chapman et al., 2015, 2016; Motes et al., 2014; Vas, et al., 2011).
Supplementary Material
Table 1.
Participant characteristics (Mean ± S.D.)
| CT (n=12) | WLC (n=15) | AC (n=14) | |
|---|---|---|---|
| Age | 63.1±3.1 | 63.8±3.4 | 62.9±3.3 |
| IQ | 121.7±8.9 | 119.5±11.4 | 117.3±9.7 |
| MoCA | 28.4±1.2 | 28.1±1.4 | 28.1±1.4 |
| TICS-M | 29.8±2.5 | 29.6±2.0 | 31.0±1.8 |
IQ=Intelligence Quotient, MoCA=Montreal Cognitive Assessment, TICS-M=Telephone Interview of Cognitive Status-Modified.
Highlights.
Higher-order cognitive training in older adults was found to affect PFC neural efficiency.
Higher-order cognitive training in older adults was found to affect PFC resting-state blood flow.
PFC efficiency and resource availability offer candidate mechanisms mediating training transfer.
Acknowledgments
This work was supported by a grant from the National Institutes of Health [RC1-AG035954, 2009; R01-NS067015, 2010; R01-AG033106; 2009] and by grants from the Lyda Hill Foundation, T. Boone Pickens Foundation, and the Dee Wyly Distinguished University Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no competing financial interests.
Comparisons of group differences at baseline were not significant. Additionally, the difference on TOSL Abstraction Scores at the post assessment session, with the baseline assessment data used as a covariate, between CT and the combined WLC and AC groups was significant, F(1,38)=4.37, p<0.05, between CT and WLC groups was marginally significant, F(1, 24)=2.93, p<0.10, and between CT and AC groups was significant, F(1, 23)=5.04, p<0.05.
Comparisons of group differences at baseline did not reveal any clusters meeting the FWE cluster threshold. Additionally, Group × Assessment Session contrasts on RT-related BOLD signal-change estimates separately comparing baseline to mid and mid to post sessions did not reveal any cluster meeting the FWE cluster threshold.
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Anand R, Chapman SB, Rackley A, Keebler M, Zientz J, Hart J., Jr Gist reasoning training in cognitively normal seniors. International Journal of Geriatric Psychiatry. 2001;26:961–8. doi: 10.1002/gps.2633. [DOI] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine. 2010;63:765–71. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Baniqued PL, Allen CM, Kranz MB, Johnson K, Sipolins A, Dickens C, Ward N, Geyer A, Kramer AF. Working memory, reasoning, and task switching training: Transfer effects, limitations, and great expectations? PLoS ONE. 2015;10:e0142169. doi: 10.1371/journal.pone.0142169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology of Aging. 2008;23:765–77. doi: 10.1037/a0013494. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Eldreth DA, Motes MA, Rypma B. Task-dependent individual differences in prefrontal connectivity. Cereb Cortex. 2010;20:2188–97. doi: 10.1093/cercor/bhp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Rieckmann A, Bellander M, Westerberg H, Fischer H, Backman L. Neural correlates of training-related working-memory gains in old age. NeuroImage. 2011;58:1110–20. doi: 10.1016/j.neuroimage.2011.06.079. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhalt JA. Reaction time measures of processing speed: Are they yielding new information about intelligence? Personality and Individual Differences. 1991;12:683–8. [Google Scholar]
- Carroll JB, Maxwell SE. Individual differences in cognitive abilities. Annual Review of Psychology. 1979;30:603–40. doi: 10.1146/annurev.ps.30.020179.003131. [DOI] [PubMed] [Google Scholar]
- Chapman SB. Make Your Brain Smarter: Increase Your Brain’s Creativity, Energy, and Focus. 1. Simon & Schuster; 2014. [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Hart JJ, Jr, Bartz EK, Didehbani N, Keebler MW, Gardner CM, Strain JF, DeFina LF, Lu H. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cerebral Cortex. 2015;25:396–405. doi: 10.1093/cercor/bht234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Keebler MW, DeFina LF, Didehbani N, Perez AM, Lu H, D’Esposito M. Distinct brain and behavioral benefits from cognitive vs. physical training: A randomized trial in aging adults. Frontiers in Human Neuroscience. 2016;18 doi: 10.3389/fnhum.2016.00338. doi.org/10.3389/fn-hum.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Mudar RA. Enhancement of cognitive and neural functions through complex reasoning training: evidence from normal and clinical populations. Frontiers in Systems Neuroscience. 2014;8:69. doi: 10.3389/fnsys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9289.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Cox RW. ANFI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Earles JL, Salthouse TA. Interrelations of age, health, and speed. Journal of Gerontology: Psychological Sciences. 1995;50:P33–P41. doi: 10.1093/geronb/50b.1.p33. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH. Cognitive factors: Their identification and replication. Multivariate Behavioral Research Monographs. 1979;79:8–84. [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gamino JF, Chapman SB, Hull EL, Lyon R. Effects of higher-order cognitive strategy training on gist reasoning and fact learning in adolescents. Front Psychology. 2010;1:188. doi: 10.3389/fpsyg.2010.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Lorenz RC, Pelz P, Heinz A, Walter H, Kathmann N, Rapp MA, Stelzel C. Neural correlates of training and transfer effects in working memory in older adults. NeuroImage. 2016;134:236–49. doi: 10.1016/j.neuroimage.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erikson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The importance of intraindividual variation in reaction time. Personality and Individual Differences. 1992;13:869–81. [Google Scholar]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychol Bull. 1991;109:490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Kannuripatti SS, Motes MA, Rypma B, Biswal BB. Increasing measurement accuracy of age-related BOLD signal change: Minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Human Brain Mapping. 2012;32:1125–40. doi: 10.1002/hbm.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral Cortex. 2010;21:1426–34. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AP, Hill SS, Stone SI, Bunge SA. Differential effects of reasoning and speed training in children. Developmental Science. 2011;14:582–90. doi: 10.1111/j.1467-7687.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Miller Singley AT, Wendelken C, Bunge SA. Characterizing behavioral and brain changes associated with practicing reasoning skills. PLoS ONE. 2015;10:e0137627. doi: 10.1371/journal.pone.0137627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motes MA, Biswal BB, Rypma B. Age-dependent relationships between prefrontal cortex activation and processing efficiency. Cognitive Neuroscience. 2011;2:1–10. doi: 10.1080/17588928.2010.512974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motes MA, Gamino JF, Chapman SB, Rao NK, Maguire MJ, Brier MR, Kraut MA, Hart J., Jr Inhibitory control gains from higher-order cognitive strategy training. Brain Cognition. 2014;84:44–62. doi: 10.1016/j.bandc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Motes Ma, Rypma B. Working memory component processes: Isolating BOLD signal-changes. Neuroimage. 2010;49:1933–41. doi: 10.1016/j.neuroimage.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudar RA, Chapman SB, Rackley A, Eroh J, Chaing HS, Parez A, Venza E, Spence JS. Enhancing latent cognitive capacity in mild cognitive impairment with gist reasoning training: A pilot study. International Journal of Geriatric Psychiatry. 2016 doi: 10.1002/gps.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N, Motes MA, Rypma B. Investigating the neural bases for intra-subject cognitive efficiency changes using functional magnetic resonance imaging. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00840. doi.org/10.3389/fnhum.2014.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, Aizenstein H. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. Journal of Gerontology, Series A: Biological Scienes and Medical Sciences. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. NeuroImage. 2006;33:969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–15. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–22. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- Salthouse TA. What do adult age differences in the Digit Symbol Substitution Test reflect? Journal of Gerontology: Psychological Sciences. 1992;47:121–8. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Davatzikos C. HAMMER: Hierarchical attribute matching mechanism for elastic registration. IEEE Transactions on Medical Imaging. 2002;21:1421–1439. doi: 10.1109/tmi.2002.803111. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine: Journal of Behavioral Medicine. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas A, Chapman S, Aslan S, Spence J, Keebler M, Rodriguez-Larrain G, Rodgers B, Jantz T, Martinez D, Rakic J, Krawczyk D. Reasoning training in veteran and civilian traumatic brain injury with persistent mild impairment. Neuropsychological Rehabilitation. 2015:1–30. doi: 10.1080/09602011.2015.1044013. [DOI] [PubMed] [Google Scholar]
- Vas AK, Chapman SB, Cook LG, Elliott AC, Keebler M. Higher-order reasoning training years after traumatic brain injury in adults. J Head Trauma Rehab. 2011;26:224–39. doi: 10.1097/HTR.0b013e318218dd3d. [DOI] [PubMed] [Google Scholar]
- Venza EE, Chapman SB, Aslan S, Zientz JE, Tyler DL, Spence JS. Enhancing executive function and neural health in bipolar disorder through reasoning training. Frontiers in Psychology. 2016;1 doi: 10.3389/fpsyg.2016.01676. doi.org/10.3389/fp-syg.2016.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon PA. Speed of information processing and general intelligence. Intelligence. 1983;7:53–70. [Google Scholar]
- Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neuroscience and Biobehavioral Reviews. 2013;13:2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. https://doi.org/10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erikson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Maily EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervetion trial of exercise training in older adults. Frontiers in Aging Neuroscience. 2010;32 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- Yezhuvath US, Uh J, Cheng Y, Martin-Cook K, Weiner M, Diaz-Arrastia R, van Osch M, Lu H. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiol Aging. 2012;33(1):75–82. doi: 10.1016/j.neurobiolaging.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezhuvath US, Uh J, Cheng Y, Martin-Cook K, Weiner M, Diaz-Arristia R, van Osch M, Lu H. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiology of Aging. 2012;33:75–82. doi: 10.1016/j.neurobiolaging.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2015;4:CD005381. doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2014;42:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


