Abstract
Purpose
LRBA (lipopolysaccharide-responsive beige like anchor protein) and CTLA4 (cytotoxic T lymphocyte antigen 4) deficiencies give rise to overlapping phenotypes of immune dysregulation and autoimmunity, with dramatically increased frequencies of circulating T Follicular helper (cTFH) cells. We sought to determine the mechanisms of cTFH cell dysregulation in LRBA deficiency and the utility of monitoring cTFH cells as a correlate of clinical response to CTLA4-Ig therapy.
Methods
cTFH cells and other lymphocyte subpopulations were characterized. Functional analyses included in vitro TFH cell differentiation and cTFH/naïve B cell co-cultures. Serum soluble IL-2 receptor alpha chain (sIL-2Rα), and in vitro immunoglobulin production by cultured B cells were quantified by ELISA.
Results
cTFH cell frequencies in patients with LRBA or CTLA4 deficiency sharply declined with CTLA4-Ig therapy, in parallel with other markers of immune dysregulation including sIL-2R α, CD45RO+CD4+ effector T cells and auto-antibodies, and predictive of favorable clinical responses. cTFH cells in patients with LRBA deficiency were biased towards a TH1-like cell phenotype, which was partially reversed by CTLA4-Ig therapy. LRBA-sufficient but not -deficient regulatory T (Treg) cells suppressed in vitro TFH cell differentiation in a CTLA4-dependent manner. LRBA deficient TFH cells supported in vitro antibody production by naïve LRBA-sufficient B cells.
Conclusions
cTFH cell dysregulation in LRBA deficiency reflects impaired control of TFH differentiation due to profoundly decreased CTLA4 expression on Treg cells, and probably contributes to autoimmunity in this disease. Serial monitoring of cTFH cell frequencies is highly useful in gauging the clinical response of LRBA deficient patients to CTLA4-Ig therapy.
Keywords: Autoantibodies, LRBA, CTLA4, T Regulatory Cells, T Follicular Helper Cells, T Follicular Regulatory Cells
Graphical abstract
Introduction
Follicular helper T (TFH) cells are a distinct subset of T cells that have a crucial role in humoral adaptive immunity 1. This specific T helper (TH) lineage has a unique phenotype, expressing high levels of CXCR5, PD-1, ICOS and CD40L2, 3. TFH cells constitutively express high levels of B cell lymphoma 6 (Bcl-6), whereas other T helper cell populations including TH1, TH2 and TH17 cells express high levels of the antagonizing transcription factor Blimp-1 4. Various signaling pathways have been implicated in TFH cell differentiation including signals from dendritic cells, B cells, cytokines (IL-6 and IL-21 in mice; IL12, 1L23 and TGF-β in human) and surface molecules (ICOS, CD28, CD40L, PD-1, BTLA, and SAP) 1. TFH cells are essential for germinal center formation and B cell differentiation into long-lived memory B cells and plasma cells within secondary lymphoid tissues. Up-regulation of CXCR5 and down-regulation of CCR7 guide TFH cell migration into the B cell follicle 1. The interaction between TFH cells and their cognate B cells provides fundamental signals for high-affinity antibodies production through affinity maturation and class switch recombination. The importance of the TFH cell lineage in promoting antibody responses was established by the observation of defective antibodies class switching in mice lacking TFH cells 5, whereas unbridled TFH cell responses trigger humoral autoimmunity due to generation of autoantibodies by autoreactive B cells 5–10. Circulating TFH (cTFH) cells were recently identified in human subjects as reflective of TFH cells 3, 11. Increased cTFH cells have been reported in several human autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjogren’s syndrome and autoimmune thyroid disease 12–16. Reciprocally, decreased cTFH have been found in several monogenic immunodeficiency disorders associated with humoral deficiency 17.
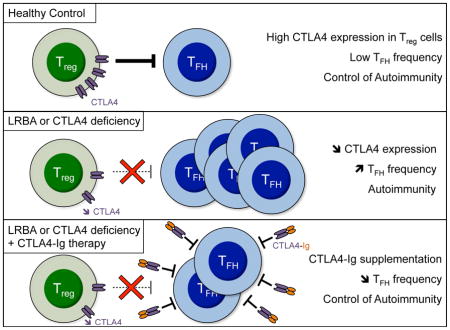
T follicular regulatory (TFR) cells, that originate from FOXP3+ T cells and express high levels of CXCR5, play a pivotal role in controlling immune dysregulation by regulating TFH and activated B cell responses through the inhibitory effect of cytotoxic T lymphocyte associated antigen 4 (CTLA4) 18–22. CTLA4 deletion in mice results in exaggerated B cell response and increased TFH cells frequencies 21–24. A number of inherited disorders of immune dysregulation that potentially impact TFR function result in high cTFH cell frequencies, which positively correlate with autoantibodies production. The best characterized of these are monogenic defects involving the LRBA-CTLA4 pathway 25–30. LPS-responsive beige-like anchor (LRBA) deficiency is a primary immunodeficiency characterized by recurrent infections with hypogammaglobulinemia, giving rise to a common variable immunodeficiency-like phenotype, and immunodysregulation with autoimmunity, including inflammatory bowel disease, autoimmune endocrinopathies and cytopenias 25, 26, 29–31. LRBA regulates the intracellular trafficking of CTLA4 30. Its deficiency results in dysregulation of the TFH cell response and is associated with intense immune dysregulation and auto-antibody production 29. The precise mechanism for TFH cell dysregulation in LRBA deficiency, whether it involves enhanced TFH cell differentiation, impaired TFR regulation or both, remains unclear. CTLA4-Ig therapy has emerged as an effective treatment for the immune dysregulation associated with both gene defects 30. The capacity to rapidly and sensitively monitor the immune status of these patients and their response to CTLA4-Ig therapy would be of great clinical utility in optimizing their care.
In this report, we examined the mechanisms of TFH dysregulation in human subjects with LRBA deficiency and the utility of using cTFH cell frequencies as a clinical indicator of response to treatment with CTLA4-Ig. Serial cTFH cell analysis was carried out on LRBA deficient patients before and after starting CTLA4-Ig therapy. Our results show that TFH dysregulation in LRBA deficiency was not due to an intrinsic abnormality in TFH cells. Rather, it reflects failure of LRBA-deficient Treg cells to control TFH cell differentiation. cTFH cells were found to be a sensitive marker of the clinical response to CTLA4-Ig therapy. Our finding supports the use of cTFH cell frequencies as a marker for monitoring the clinical status of LRBA deficient patients and their response to therapy.
Materials and Methods
Patients
Patients P1 and P2 are two previously described Saudi Arabian siblings with LRBA deficiency due to a homozygous deletion in the BEACH domain of LRBA that abolished protein expression (patients P5 and P6; Family C, in our original report) 29. Patient P3 is an 11 year old girl with chronic immune dysregulation who was diagnosed with LRBA deficiency due to exon 57 deletion as confirmed by genomic analysis, cDNA sequencing and absent LRBA protein on flow cytometry and immunoblotting (Fig. E1 in this article’s Online Repository). Her clinical presentation is detailed in the Online Repository Text. Patient P4 is a 6 year old Saudi Arabian boy who developed severe Crohn’s-like illness and type 1 diabetes and was found on whole exome sequencing to have a 4 base pair (bp) deletion in LRBA (c.4757_4760del, p.L1586fs). LRBA deficiency was confirmed by absent protein expression on flow cytometry (Fig E2 in the online repository). Patients P5 and P6 are newly diagnosed Turkish brothers with LRBA deficiency due to a single bp deletion in exon 54 of LRBA, as confirmed by genomic analysis, leading to a frame shift and premature stop codon (c.7885delA, p.R2629fs). LRBA deficiency was confirmed by near absent protein expression on flow cytometry (Fig E3 in the online repository). Patients P7–P9 were found to have heterozygous mutations in CTLA4 (Fig E4 in the online repository). Their clinical presentations are detailed in the Online Repository Text. A patient with IPEX due to an A384T amino acid substitution in FOXP3 was identified by exome analysis followed by Sanger sequencing of the mutation site in the FOXP3 gene. Subject with the diagnosis of SLE was identified at the Rheumatology clinic at the Boston children’s Hospital. Control subjects were age group-matched. All study participants were recruited using written informed consent approved by the local Institutional Review Boards. Studies at the Boston Children’s Hospital were conducted under approved protocol #04-09-113R.
Antibodies and flow cytometry
Information on the antibodies employed is provided in the Online Repository Text. Whole blood was incubated with mAbs against surface markers for 30 min on ice. Intracellular staining with FOXP3 and CTLA4, was performed using eBioscience Fixation/Permeabilization according to the manufacturer’s instructions. Indirect intracellular staining for LRBA was performed on freshly isolated peripheral blood mononuclear cells (PBMCs) using BD Biosciences Fixation/Permeabilization buffer with polyclonal rabbit anti-LRBA antibodies (Sigma-Aldrich, St. Louis, MO) or Rabbit IgG XP (R) isotype control (cell signaling), followed by secondary detection with Brilliant Violet 421™ Donkey anti-Rabbit IgG (Biolegend). For chemokine receptor staining in cTFH cells, PBMCs were isolated by Ficoll-Paque Plus gradient and surface staining for PD1, CXCR5, CXCR3 and CCR6 was performed for 30 min on ice. For cytokine detection among TFH cells, PBMCs were stimulated in vitro for 4h in complete RPMI medium with Golgi plug (1/1000), PMA (50ng/mL) and ionomycin (500 ng/mL). Cells were washed and incubated with mAbs against surface markers for 30 min on ice, permeabilized using BD Biosciences Fixation/Permeabilization buffer and intracellular staining with mAbs against cytokines was performed. For characterization of circulating B cell subsets, previously frozen PBMCs were stained for surface antigens CD19, CD27 and IgD. Data were collected with a Fortessa cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Whole exome sequencing (WES)
WES was performed on genomic DNA of the probands P3, P4, P5 and P9 as described in earlier reports 25, 29.
Sanger sequencing analysis
LRBA and CTLA4 sequences were amplified from genomic and complimentary (c)DNA by the polymerase chain reaction and sequenced bidirectionally using dye-terminator chemistry 29. Primers used in amplification reactions are available upon request.
Immunoblotting
Protein lysates derived from lymphocytes were resolved on SDS-PAGE gels and transferred to nitrocellulose filters as described. Immunoblots were carried out using polyclonal rabbit anti-LRBA antibody (Sigma-Aldrich, St. Louis, MO). The blots were reprobed with polyclonal rabbit anti-DOCK8 antibody (Sigma-Aldrich, St. Louis, MO).
TFH and naïve B cell co-culture
cTFH and naïve B cells were isolated from the peripheral blood of patient P3 and her human leukocyte antigen (HLA) fully matched healthy sister by fluorescent activated cell sorting (FACS). The cells were mixed as indicated and cultured for 12 days in the presence of endotoxin-reduced staphylococcal enterotoxin B (SEB) at 1μg/ml in RPMI 1640 complete medium supplemented with 10% heat-inactivated fetal bovine serum 32. At the end of the incubation period, the IgM and IgG concentrations in the culture supernatants were measured by ELISA.
In vitro generation of induced TFH
CD4+ T cells were isolated from PBMCs by negative selection using magnetic beads (Miltenyi). Naïve CD3+CD4+CCR7+CD45RA+ cells were purified from CD4+ T cells by cell sorting on a FACS ARIA (purity > 98%). Naïve CD4 T cells were seeded at a concentration of 5×104 per well of a 96-well plate and stimulated with recombinant human IL-12, 1L-23 and TGF-β1 in the presence or absence of Treg cells, CTLA4-Ig and anti-CTLA4 33. The cells were cultured for 4 days, at the end of which they were stained for TFH cell markers.
Autoantibody assays
For autoantibody detection, plasma aliquots from patient and control subjects were analyzed using microarrays spotted with 84 autoantigens (University of Texas Southwestern Medical Center, Genomic and Microarray Core Facility), as described 34. Data was normalized to healthy controls.
Statistical Analysis
Comparison between groups was carried out using Student’s unpaired two tailed t test and 2-way ANOVA with Bonferroni post-test analysis, as indicated. Differences in mean values were considered significant at a p < .05.
Results
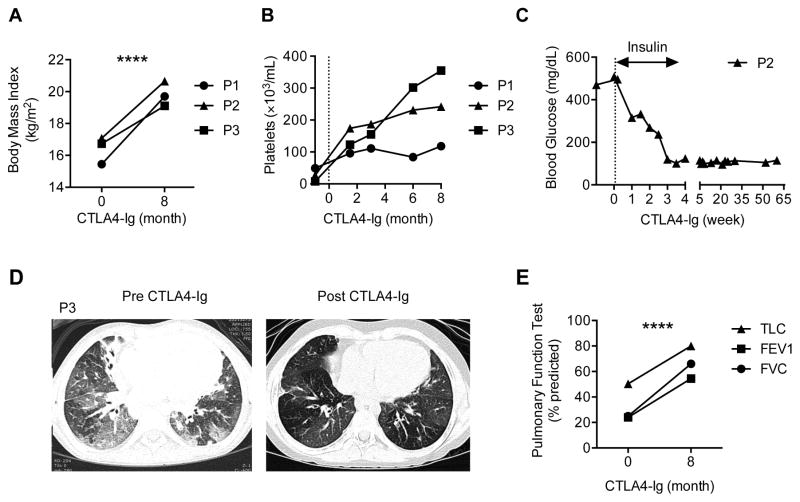
Three patients with definitive LRBA deficiency were studied for their response to CTLA4 therapy. Details of the clinical and immunological findings of the three patients are shown in Table E1 and Table E2, respectively, in the Online Repository. The three patients exhibited severe immune dysregulation with autoimmune cytopenias, chronic end organ inflammation and damage, especially affecting the lungs (P1, P3) and the gut (P1, P2). All patients showed marked clinical response to CTLA4-Ig therapy with decreased disease symptomatology, weight gain and resolution of their thrombocytopenia (Fig 1, A and B). Patients P1 and P2, who suffered from colitis, showed good clinical response with decreased diarrhea. Patient P2, who developed type 1 diabetes shortly before initiation of therapy, had resolution of her insulin dependence and normalization of her blood glucose (Fig 1, C). Patient P3, who suffered from severe lung disease and was oxygen dependent, responded very favorably to CTLA4-Ig therapy with marked improvement in her lung imaging and function and resolution of her oxygen dependency (Fig 1, D and E).
Figure 1. Clinical response of LRBA-deficient subjects to CTLA4-Ig therapy.
A and B, Body mass index (Fig 1, A) and platelet counts (Fig 1, B) of LRBA deficient subjects pre- and post-treatment with CTLA4-Ig. C, Serum glucose levels and insulin use in patient P2 status post CTLA4-Ig therapy. D and E, High-resolution chest CT scans (Fig 1, D) and Pulmonary function tests (Fig 1, E) of patient P3 pre and post treatment with CTLA4-Ig; FVC, forced vital capacity; FEV1, forced expiratory volume; TLC, total lung capacity. ****P < .0001, 2-way ANOVA with post-test analysis.
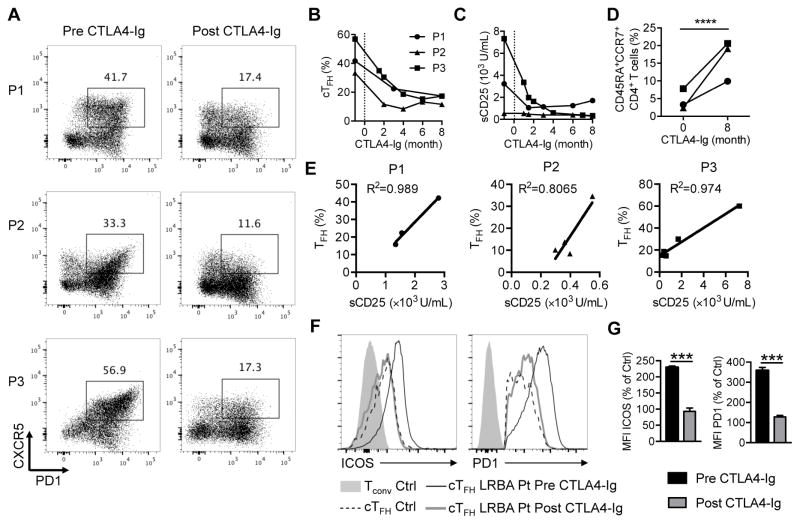
We have previously documented that patients with LRBA deficiency exhibit dysregulated cTFH29, a phenotype related to the profound deficiency of CTLA4 expression by LRBA-deficient Treg cells 21, 22, 29. Flow cytometric analysis of peripheral blood T lymphocyte cells of the three patients demonstrated a markedly increased number of CD4+FOXP3−PD1+CXCR5+ cTFH cells (Fig 2, A). The frequency of cTFH and CTLA4 expression of Treg cells of three other patients with LRBA deficiency (P4–P6) were also analyzed. They also exhibited high frequency of cTFH cells in association with low CTLA4 expression on their Treg cells (Fig E2 and E3). Importantly, the frequency of cTFH cells significantly declined after CTLA4-Ig therapy (Fig 2, A and B). CTLA4-Ig therapy also resulted in the decline of other markers of inflammation, including serum soluble CD25 (sCD25) (Fig 2, C). The frequency of circulating naïve CD4+ T cells (CD4+CD45RA+CCR7+) increased following CTLA4-Ig therapy, indicative of more effective immunoregulation (Fig 2, D). The decline in cTFH cells upon CTLA4-Ig therapy correlated well with that of sCD25, validating the monitoring of these cells as a proxy for the immune dysregulatory status of the patients (Fig 2, E). In addition to their increased frequency, cTFH cells of LRBA-deficient subjects also had increased expression of the cTFH cell markers ICOS and PD1 as compared to those of control subjects, consistent with them being activated 11, 35. The expression levels of both markers were normalized following therapy with CTLA4-Ig (Fig 2, F and G).
Figure 2. TFH cell frequency in LRBA-deficient patients correlates with other markers of immune dysregulation.
A, Flow cytometric analyses of CXCR5 and PD1 expression in CD4+ T cells in LRBA- deficient subjects pre- and post-treatment with CTLA4-Ig. B–D, cTFH cell frequencies (Fig 2, B), serum sCD25 levels (Fig 2, C) and naïve CD4+CD45RA+CCR7+ T cell frequencies (Fig 2, D) in LRBA deficient patients pre and post-treatment with CTLA4-Ig. E, Correlation between cTFH cell frequencies and sCD25 levels in LRBA-deficient patients. F, Flow cytometric analysis of ICOS and PD1 expression on control cTFH cells and on patient cTFH cells before and after CTLA4-Ig therapy. G, Relative mean fluorescence intensity of ICOS and PD1 expression on patient versus control cTFH cells. ***P < .001 and ****P < .0001, by student’s two tailed t test.
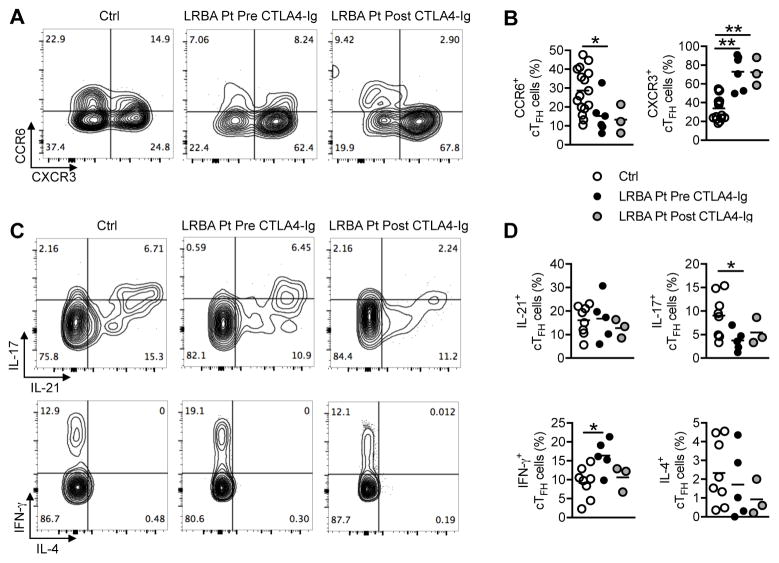
To further characterize the phenotype of cTFH cells in LRBA-deficient patients and also the impact of CTLA4-Ig treatment, we evaluated the expression of chemokine receptors and the capacity of cTFH to secrete cytokines. cTFH cells of LRBA deficient patients highly expressed the TH1-associated chemokine receptor CXCR3 and IFN-γ, markers that define TH1-biased TFH cells (or TFH-1)11, 35. In contrast, expression of the TH17-associated chemokine receptor CCR6 and IL-17, markers that define TH17-biased TFH cells (TFH17) 11, 35, was markedly decreased in patient as compared to control cTFH cells (Fig 3, A–D). Some of these abnormalities, such as increased CXCR3 expression, persisted despite CTLA4-Ig therapy, and probably reflected either attributes intrinsic to LRBA-deficient TFH cells or ongoing TH1 cell inflammation in the hosts. In contrast, expression of IL-21 and IL-4 were similar in patient and control cTFH cells and appeared unaffected by CTLA4-Ig therapy.
Figure 3. cTFH cells of LRBA-deficient patients are skewed towards a TH1-like cell phenotype.
A and B, Flow cytometric analyses of CXCR3 and CCR6 expression in cTFH cells of LRBA-sufficient and LRBA- deficient subjects pre- and post-treatment with CTLA4-Ig. C and D, Flow cytometric analyses of IL-17 and IL-21 expression (Fig 3, C, upper panels) or IFN-γ and IL-4 (Fig 3, C, lower panels) and the respective scatter plot representation (Fig 3, D) in cTFH cells of LRBA-sufficient and LRBA- deficient subjects pre- and post-treatment with CTLA4-Ig. *P < .05, **P < .01 by 1-way ANOVA with post-test analysis.
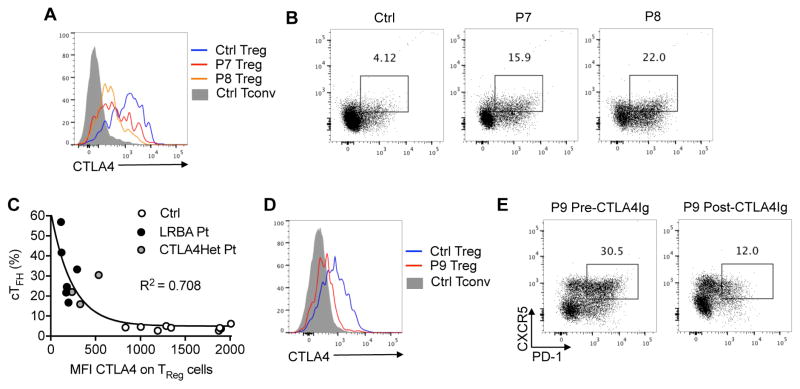
We have previously reported that patients with LRBA deficiency have profound Treg cell abnormalities including decreased Treg cell frequencies and reduced expression of several Treg cell markers, including CTLA4 29. In view of this immune dysregulation, we analyzed the correlation of the magnitude of CTLA4 expression on Treg cells and the frequency of cTFH cells. To that end, we examined the frequency of cTFH cells in three patients with heterozygous loss of function mutations in CTLA4. Their mutational analysis is shown in Fig E4 in the online Repository, and their clinical and immunological findings are detailed in Table E1 and Table E3, respectively, in the Online Repository. Consistent with the role of CTLA4 deficiency in dysregulated cTFH cells in LRBA deficiency, patients with heterozygous loss of function mutations in CTLA4 also manifested increased cTFH cell frequencies (Fig 4, A and B). Overall, cTFH cells frequencies were inversely correlated with the CTLA4 MFI on Treg cells of LRBA and CTLA4-deficient subjects, reflecting the role of CTLA4 in controlling TFH cell differentiation. Subjects with complete LRBA deficiency, associated with near complete absence of CTLA4 expression on Treg cells, manifested the highest cTFH frequencies, while those with heterozygous loss of function CTLA4 mutations had a more moderate increase in cTFH frequencies. (Fig 4, C). Similar to the case of CTLA4-Ig-treated LRBA-deficient subjects, treatment of a patient with CTLA4 deficiency with CTLA4-Ig resulted in the decline of cTFH cells, consistent with the role of CTLA4 deficiency in cTFH dysregulation in both disorders (Fig 4, D and E).
Figure 4. TFH cell frequency in CTLA4 deficiency and its response to CTLA4-Ig therapy.
A and B, Flow cytometric analysis of CTLA4 expression on CD4+FOXP3+ Treg cells (Fig 3, A) and cTFH (Fig 3, B) of control (Ctrl) subjects and patients P7 and P8 with a heterozygous CTLA4 mutation. C, Correlation between cTFH cell frequencies and the mean fluorescence intensity (MFI) of CTLA4 expression in Treg cells of Ctrl (n=8), LRBA-deficient (n=6) and heterozygous CTLA4-mutant subjects (n=3). D, Flow cytometric analysis of CTLA4 expression on CD4+FOXP3+ cells in patient P9 with a heterozygous CTLA4 mutation. E, cTFH cells in patient P9 before and after CTLA4-Ig therapy.
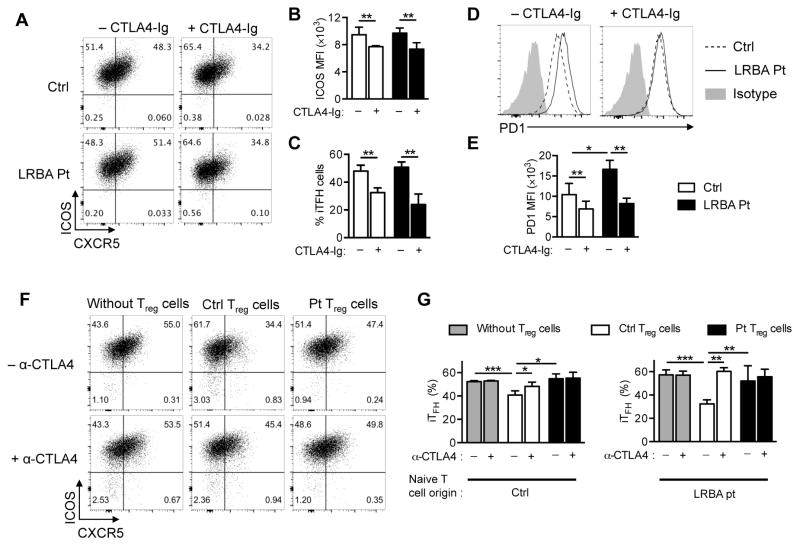
To determine whether LRBA deficiency impaired TFH differentiation, we tested the capacity of LRBA-sufficient and deficient naive CD4+ T cells to differentiate into induced follicular helper (iTFH) cells upon stimulation with IL12, IL23 and TGF-β1 in the presence or absence of CTLA4-Ig 33. CD4+ T cells from patients and control subjects were enriched by magnetic bead separation, and naive CD4+ T cells were purified to greater than 99% purity by cell sorting (Fig E5 in the Online Repository). LRBA-sufficient and -deficient T cells were found to be equivalent in their capacity to differentiate into iTFH cells that expressed similar levels of Inducible T cell Costimulator (ICOS) regardless of the CTLA4 status of naïve CD4+ cells (Fig 5, A–C). However, iTFH cells generated from LRBA-deficient naïve T cells exhibited higher expression of PD1, as compared to those from LRBA-sufficient naïve T cells (Fig 5, D and E). Both iTFH differentiation and expression of ICOS and PD1 were partially inhibited upon treatment of the differentiating T cell cultures with CTLA4-Ig (Fig 5, B–E). Given the normal differentiation of LRBA deficient T cell into iTFH cells, we further analyzed the capability of LRBA-sufficient and deficient Treg cells to control iTFH cells differentiation. Treg cells were isolated from patient and healthy control by means of cell sorting of CD4+CD25+CD127low Treg cells. Equal numbers of patient and control Treg cells were added to an equal number of control naïve CD4+cells that were stimulated with IL12, IL23 and TGF-β in the presence or absence of anti-CTLA4. LRBA-deficient Treg cells failed to suppress iTFH differentiation compared with control Treg cells, indicative of their impaired function (Fig 5, F and G).
Figure 5. Ineffective Treg cells control of TFH Cell differentiation in LRBA deficient subjects.
A, Flow cytometric analysis of CXCR5 and ICOS expression in in vitro-differentiated induced (i)TFH-like cells of control and LRBA-deficient subjects. B and C, MFI of ICOS expression in iTFH-like cells (Fig 5, B), and frequencies of in vitro-differentiated iTFH-like cells (Fig 5, C) derived from control and LRBA-deficient naïve CD4+ T cells in the absence or presence of CTLA4-Ig. D and E, Flow cytometric analysis of PD1 expression (Fig 5, D) and bar graph representation (Fig 5, E) of PD1 expression in iTFH cells of control and LRBA-deficient subjects. F and G, Flow cytometric analysis (Fig 5, F) and frequencies (Fig 5, G) of in vitro-differentiated iTFH-like cells derived from control and LRBA-deficient naïve CD4+ T cells in the absence or presence of anti-CTLA4 mAb and/or patient or control Treg cells. Results are representative of 2 independent experiments. *P < .05, **P < .01 and ***P < .001 2-way ANOVA with post-test analysis.
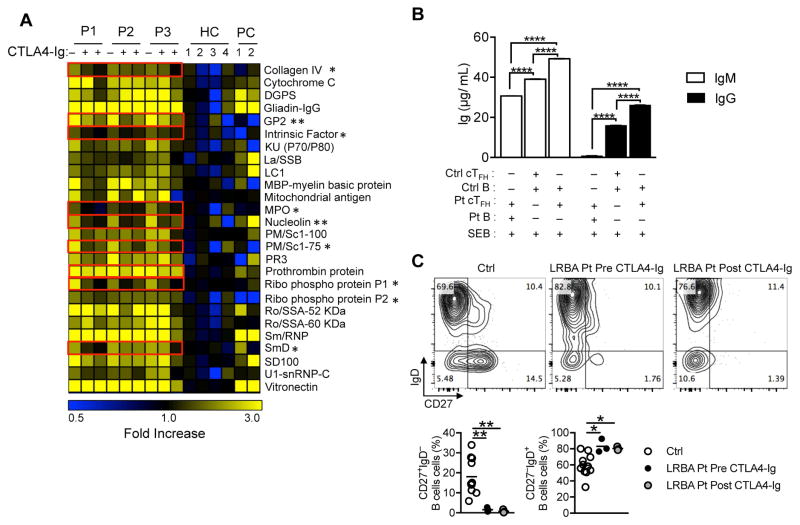
Patients with LRBA deficiency frequently present with hypogammaglobulinemia and defective specific antibody titers, with the majority exhibiting B cell dysfunction and reduced class-switched memory B-cells 31, 36. Nevertheless, most of these patients suffer from autoimmunity with increased circulating autoantibodies 29, 31, 36. To determine whether the production of circulating autoantibodies responded to CTLA4-Ig therapy, we screened our patients for serum autoantibodies before and two time points after CTLA4-Ig therapy using an array of 84 auto-antigens 34. Also included in the analysis were four healthy subjects whose sera were used as negative controls and two positive control sera including one patient with IPEX and another patient with SLE. LRBA patients exhibited a number of circulating IgG autoantibodies whose relative abundance significantly decreased following CTLA4-Ig therapy (Fig 6, A). We next employed in vitro co-culture studies to examine the functional capacity of LRBA-deficient cTFH to support IgM and IgG antibody production by naïve B cells. CD4+CD25−CD127HighPD1+CXCR5+ cTFH cells and CD19+IgD+CD27− naïve B cells were isolated from LRBA-deficient patient P3 and her fully HLA-matched healthy sister by cell sorting and co-cultured in the presence of staphylococcal enterotoxin B (SEB). Results showed that while cTFH cells of both the LRBA-deficient patient and her healthy sibling supported in vitro IgM and IgG antibody production by the sibling’s naïve LRBA-sufficient B cells (Fig 6, B), the LRBA-deficient cTFH cells were more effective in that regard, consistent with their heightened activation state (Fig 2, F and G) 11, 35. In contrast, the patient’s cTFH supported IgM but minimally IgG production by B cells, in agreement with the previous report of defective IgG isotype switching in LRBA-deficient B cells 26. We also evaluated the impact of CTLA4-Ig treatment on B cell phenotype. Whereas LRBA-deficient patients exhibited increased frequencies of naïve (IgD+CD27low) and correspondingly decreased frequencies of switched memory (IgD+CD27low) B cells, CTLA4-Ig treatment did not change the ratio of naïve/memory B cells in circulation (Fig 6, C). Given that expansion of TFH cells promotes auto-antibody production 5–10, these findings indicated that the dysregulated TFH expansion in LRBA deficiency is functionally relevant to the excess auto-antibody production in this disorder.
Figure 6. Autoantibody production and cTFH function in LRBA-deficient subjects.
A, Autoantibody production in LRBA deficient patients before and after CTLA4-Ig therapy. Heat map showing IgG autoantibodies against self-antigens in sera of LRBA-deficient patients, healthy control subjects, patient with IPEX, and a patient with SLE. A value of 1 (black) is equal to the control average + 1 SD. Autoantibody responses affected by CTLA4-Ig therapy are boxed in red. B, IgM and IgG production in co-cultures of cell-sorted cTFH and naïve B cells derived from patient P3 and her HLA fully matched sister in the presence of Staphylococcal enterotoxin B (SEB). C. Flow cytometric analysis (Fig 6, C, upper panels) and scatter plot representation (Fig 6, C, lower panels) of CD27 and IgD expression on circulating B cells of control subjects and LRBA deficient subjects before and after treatment with CTLA4-Ig. *p<0.05 and **p<0.01, Student unpaired 2-tailed t test; ****P < .0001 by 1-way ANOVA with post-test analysis.
Discussion
Patients with deleterious mutations in LRBA gene have dysregulated TFH cell responses, as reflected by the high frequency of cTFH cells, which may play a causative role in disease-related autoimmunity. In this report, we examined the mechanisms of TFH dysregulation in LRBA deficiency and the usefulness of monitoring cTFH cell frequencies as a measure of disease activity and response to therapy. We found that TFH cell dysregulation involved failure of CTLA4-dependent Treg cell to control TFH cell differentiation. Furthermore, the elevated cTFH cell frequencies in LRBA deficiency and the related CTLA4 deficiency dramatically declined following CTLA4-Ig therapy, in concordance with the decline in other markers of disease activity and improved clinical outcome. While cTFH cell frequencies in LRBA-deficient subjects tightly correlated with other markers of immune dysregulation such as sCD25, the former offers the advantage of ease of its measurement by flow cytometry and its sensitive and fast response to CTLA4-Ig therapy. Thus, monitoring cTFH cell frequencies in LRBA and CTLA4 deficiencies maybe particularly useful in tracking disease activity and response to different therapies.
Recent studies identified the critical role of CTLA4 in Treg cells in controlling humoral immunity. In vivo Treg/TFR cells depletion or selective deletion or blockage of CTLA4 in Treg compartment resulted in unrestrained TFH cell differentiation and profoundly increased auto-antibody production 21, 22. In concordance with these studies, we demonstrated that LRBA-deficient naïve CD4+ T cells effectively differentiate into TFH cells. However, LRBA-deficient Treg cells, like normal Treg cells treated with anti-CTLA4 mAb, failed to suppress the expansion of in vitro differentiated TFH cells, pointing to the reduced CTLA4 expression on LRBA-deficient Treg cells as a key mechanism underlying the dysregulated TFH response in LRBA deficiency.
Many patients with LRBA deficiency present with hypogammaglobulinemia and low numbers of switched memory B cells 31, 36. LRBA deficient naïve B cells have an intrinsic defect in their capacity to differentiate into IgM and IgG producing plasma cells 26. Despite their B cell switch defect, LRBA-deficient subjects mount an intense autoantibody response. cTFH cells of LRBA-deficient subjects were activated, as evidenced by their heightened expression of ICOS and PD1, a phenotype that was normalized upon CTLA4-Ig therapy. They were more effective in supporting immunoglobulin production by LRBA-sufficient B cells as compared to control cTFH cells, indicative of their augmented functional capacity. In vitro differentiated iTFH cells also exhibited increased PD1 expression that was reversed by CTLA4-Ig treatment, reflecting the role of CTLA4 deficiency in dysregulating LRBA-deficient TFH cells both in vitro and in vivo. We suggest that the profound dysregulation of the TFH cell compartment in LRBA deficient subjects, precipitated by TFR cell depletion and virtually absent CTLA4 expression, coupled with defective B cell isotype switching and its associated somatic hypermutation, may propel humoral autoimmunity by limiting the pruning of auto-reactive antibodies normally achieved with somatic hypermutation 37. Such a mechanism would be consistent with the reversal of TFH dysregulation and down-regulation of auto-antibody production by CTLA4-Ig therapy. Because CD80 signaling favors B cell isotype switching and immunoglobulin production, we cannot exclude an additional direct effect of CTLA4-Ig treatment on B cells resulting in the reduction of auto-antibody production in LRBA-deficient patients 38. Further studies would be required to validate these predictions.
In summary, these findings support the monitoring of cTFH cells as a sensitive marker for monitoring the response of LRBA and CTLA4-deficient patients to CTLA4-Ig therapy. Its use may help optimize the management of LRBA-deficient subjects to reach sustained clinical improvement and optimal preparation for hematopoietic stem cell transplantation. More broadly, monitoring the TFH/TFR axis may also aid in the diagnosis and treatment of primary immune dysregulatory disorders.
Supplementary Material
Key Messages.
LRBA and CTLA4 deficiencies result in highly elevated frequencies of circulating T follicular helper (cTFH) cells.
cTFH dysregulation in LRBA deficiency reflects impaired CTLA4-dependent suppression of TFH cell differentiation by T regulatory (Treg) cells.
Monitoring cTFH frequencies in LRBA and CTLA4 deficiencies is useful in gauging the clinical response to CTLA4-Ig therapy.
Acknowledgments
This work was supported by the National Institutes of Health 5R01AI065617 to Talal A. Chatila and 4R01AI100315 to Raif S. Geha, and a grant from the Scientific and Technological Research Council of Turkey (1059B191300622) to Sevgi Keles.
Abbreviations
- CTLA4
cytotoxic T lymphocyte associated antigen 4
- CVID
Common variable immunodeficiency
- ELISA
enzyme-linked immunosorbant assay
- FACS
fluorescence-activated cell sorting
- HLA
human leukocyte antigens
- IPEX
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked
- LRBA
LPS-responsive beige-like anchor
- MFI
mean fluorescence intensity
- SLE
systemic lupus erythematosus
- SEB
staphylococcal enterotoxin B
- Treg
T regulatory
- TFH
T Follicular Helper
- TFR
T Follicular Regulatory
- TH1
T helper type 1
- TH17
T helper Type 17
- WES
Whole exome sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. 2016 doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 2.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–81. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185:4042–52. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 6.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–9. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 8.Kim YU, Lim H, Jung HE, Wetsel RA, Chung Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS One. 2015;10:e0120294. doi: 10.1371/journal.pone.0120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proc Natl Acad Sci U S A. 2011;108:7932–7. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 13.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015;67:988–99. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Wu Q, Su D, Che N, Chen H, Geng L, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–50. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 16.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–47. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136:993–1006. e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–82. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271:246–59. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 21.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–25. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–39. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A. 2015;112:524–9. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. 2016;113:E2383–92. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alangari A, Alsultan A, Adly N, Massaad MJ, Kiani IS, Aljebreen A, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130:481–8. e2. doi: 10.1016/j.jaci.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–7. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–6. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135:217–27. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–40. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 31.Alkhairy OK, Abolhassani H, Rezaei N, Fang M, Andersen KK, Chavoshzadeh Z, et al. Spectrum of Phenotypes Associated with Mutations in LRBA. J Clin Immunol. 2016;36:33–45. doi: 10.1007/s10875-015-0224-7. [DOI] [PubMed] [Google Scholar]
- 32.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–9. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–65. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–52. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137:223–30. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A. 2014;111:E2567–75. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.